Abstract
Retention of bile acids within the liver is a primary factor in the pathogenesis of cholestatic liver disorders, which are more common in human infants. The objective of this study was to evaluate developmental changes in mitochondrial factors involved in bile acid-induced hepatocyte injury. Hepatic mitochondria from adult rats (aged 9 weeks) underwent a mitochondrial permeability transition (MPT) and release of cytochrome c upon exposure to glycochenodeoxycholic acid (GCDC). In contrast, mitochondria from young rats (age 6–36 days) were resistant to MPT induction and cytochrome c release. Neither mitochondrial levels of MPT-associated proteins (voltage-dependent anion channel, cyclophilin D, or adenine nucleotide translocase), Bcl-2 family proteins, nor antioxidant enzymes explained this resistance. Mitochondria from young rats contained 2–3-fold higher α-tocopherol (α-TH). In vivo α-TH enrichment of adult hepatic mitochondria increased their MPT resistance. Tetra-linoleoyl cardiolipin (TL-CL), the primary molecular species of cardiolipin (CL), was reduced in mitochondria of the young rat; however, enrichment with CL and TL-CL only modestly increased their MPT susceptibility. In conclusion, we observed an unexpected resistance in young rats to bile acid induction of mitochondrial cell death pathways, which may be related to developmental differences in membrane composition.
Keywords: Mitochondria, Development, Permeability transition, α-tocopherol, Cholestasis, Cardiolipin
Despite recent advances in elucidating the mechanisms of liver injury in cholestatic disorders, the development of effective therapies has been hampered by a lack of potential therapeutic targets. For these reasons, cholestatic diseases are among the leading indications for liver transplantation. The human neonate is exceptionally prone to the development of cholestasis (1). Characteristic features of neonatal cholestasis include histological evidence of hepatocyte swelling (2) and elevated circulating hepatocellular enzymes (AST and ALT), both of which suggest oncotic necrosis in the pathogenesis of cell injury. It is not well understood why the neonatal hepatocyte has this increased susceptibility to cholestatic injury, although, proposed factors include immaturity of synthesis, hepatocyte uptake, and canalicular transport of bile acids (1), reduced antioxidant defense systems (3,4), or other developmental factors.
The accumulation of hydrophobic bile acids in hepatocytes, such as GCDC, is a primary factor in the pathogenesis of cholestatic liver injury. While the precise mechanisms contributing to bile acid-induced toxicity are unknown, several lines of evidence suggest that these compounds directly target hepatocyte mitochondria perturbing mitochondrial homeostasis, culminating in apoptotic and necrotic cell death. For example, liver mitochondria exposed to hydrophobic bile acids have reduced electron transport and impaired mitochondrial respiration (5,6), findings, which have also been, demonstrated the bile duct-ligated model of cholestatic injury (7). Hepatocytes exposed to bile acids demonstrate changes in mitochondrial function (induction of MPT) that trigger cell death (8). The MPT is characterized by mitochondrial swelling and outer membrane permeabilization, followed by cytochrome c release, a pivotal event during apoptotic cell death. Furthermore, several laboratories have implicated oxidative stress as a primary factor in bile acid-induced MPT (9,10), which are ameliorated with antioxidants (9). In addition to direct effects on mitochondria, bile acids can also promote necrotic or apoptotic cell death through activation and/or translocation of critical cell signaling proteins. Bile acid effects on death receptors (Fas, TRAIL) facilitate the translocation of the pro-apoptotic Bcl-2 regulatory protein Bax from cytosol to mitochondria, wherein the protein inserts itself into the outer membrane inducing the MPT and activating downstream caspases (11). Although bile acid-mediated toxicity acts, in part, through death receptor-mediated hepatocyte cell death (12), this signaling pathway alone does not account for the full extent of cholestatic injury (13).
The objective of this study was to evaluate developmental effects on the function and composition of hepatic mitochondria in relation to bile acid induction of mitochondrial pathways of cell death. Isolated hepatic mitochondria were used as the experimental model in this study in preference to cultured hepatocytes for several reasons. First, culturing primary hepatocytes from suckling rats poses experimental problems including: a) technical difficulties in isolating sufficient quantities of hepatocytes from suckling rats for use as the experimental model, and b) the developmentally regulated marked reduction in bile acid uptake by suckling rat hepatocytes (14). Thus, inducing toxicity by incubating hepatocytes with bile acids, the standard experimental model, would not be possible because of severely impaired bile acid uptake by neonatal hepatocytes. Second, mitochondrial cell death pathways can be directly evaluated in purified mitochondria, which allow for in vitro manipulation of membrane composition that can be used to delineate the effects of membrane components. In this study, isolated mitochondria from young, suckling rats exhibited marked resistance to bile acid-induced MPT, which may be related to mitochondrial membrane composition.
MATERIALS AND METHODS
Materials
(See Supplemental Materials online at www.pedresearch.org)
Isolation of Rat Liver Mitochondria
Rat liver mitochondria were isolated from adult and young rats by differential centrifugation (Supplemental Materials online at www.pedresearch.org). All animals received humane care in compliance with the Committee on Laboratory Animal Research of the University of Colorado Denver Health Sciences Center
MPT Measurements
The MPT was determined spectrophotometrically at 540nm (Supplemental Materials online).
Glutathione, Glutathione-dependent Enzyme Activity and Vitamin E Levels
Mitochondrial glutathione (GSH) concentrations were determined using a commercial spectrophotometric-based kit (QuantiChrom Glutathione Assay Kit: DIGT-250, Bioassay Systems, Hayward, CA). Glutathione peroxidase (GSH-Px) activity and glutathione reductase (GSSG-R) were determined according to the methods of Lawrence and Burk (15), and Carlberg and Mannervik (16), respectively, and expressed as units/mg pro. α-TH and γ-tocopherol (γ–TH)) was determined in liver homogenate and mitochondrial fractions by HPLC with electrochemical detection (17,18).
Measurement of ROS Generation
Generation of reactive oxygen species (ROS) was determined by dichlorofluorescin (DCF) fluorescence, as previously described (19).
SDS-PAGE and Immunoblotting
Proteins were subject to SDS-PAGE and immunoblotting as previously described (Supplemental Materials online).
Enrichment of Liver Mitochondrial α-TH in Adult Rats
Adult rats were treated with subcutaneous injections of Vital E-300 (20). Vital E-300 is a veterinary injectable form of d-α-TH containing 300 IU/ml of d-α-TH compounded with 20% ethanol, 1% benzyl alcohol in an emulsifiable base. Adult rats were administered a single subcutaneous injection of Vital E-300 (2.5, 5.0, or 10mg/100g body weight) or saline to achieve a range of anticipated α-TH levels in hepatic mitochondria. After 48 hrs, mitochondria were subject to GCDC-induced MPT and α-TH determination.
Isolation and Analysis of Cardiolipin and Cholesterol
Total and individual CL molecular species were quantified from rat liver mitochondria by electrospray ionization mass spectrometry (21). The quantities of individual CL species were calculated based upon the relative ratios of peak areas of the specific molecular species to the area of the internal standard and expressed as nmoles of individual fatty acyl side chain CL species (carbon number:number of double bonds)/mg protein, using a standard curve.
Mitochondrial cholesterol was measured fluorimetrically using an Amplex Red assay kit (Invitrogen/Molecular Probes, Eugene, OR).
Enrichment of Cardiolipin into Young Rat Liver Mitochondria
To enrich mitochondria from livers of young rats, the amount of added CL required to achieve levels in the range of that measured in the adult was calculated. To compensate for the poor permeability of phospholipids into mitochondrial membranes (22), a 10-fold (11.7 nmol/ml mitochondria) and 100-fold (117 nmol/ml mitochondria) excess was utilized. The appropriate amount of bovine liver CL, containing approximately 50% TL-CL, dissolved in ethanol, or ethanol alone (solvent control), was added to flasks and the solvent removed by evaporation under a N2 stream. To the dried lipid, 10 ml hepatic mitochondria suspended in MPT buffer (approx. 1mg pro/ml) was added and the samples were incubated for 30 minutes at 28°C. After the incubation period, the mitochondria were centrifuged at 10,000g for 10 minutes and the enriched mitochondrial pellets were resuspended to the original volume and subjected to the MPT assay. Aliquots were also stored at −70°C for analysis of total and individual CL subspecies.
Statistical Analysis
Statistical comparisons among experimental groups were conducted by either an ANOVA or student t-test. For analyzing the association between mitochondrial α-TH levels and mitochondrial swelling, a least squared regression curve was calculated. A p-value of <0.05 was considered statistically significant.
RESULTS
Rat liver mitochondria exhibit age-related resistance to GCDC-induced MPT
Mitochondria from young rat livers exposed to 100µM GCDC demonstrated significant resistance to MPT induction compared with adult rats (representative MPT tracing shown in Figure 1A). This age-related resistance to GCDC-induced mitochondrial swelling was also concentration-dependent (Figure 1B). Resistance to GCDC-induced swelling in young rat mitochondria was also confirmed morphologically by electron microscopy (Figure 1C). In addition, mitochondria from young rats maintained their cytochrome c content when exposed to 25–100µM GCDC, whereas adult liver mitochondria released cytochrome c at 100µM GCDC (Figure 2). Cyclosporin A (CsA) prevented GCDC-induced swelling in mitochondria and cytochrome c release at all ages (data not shown), indicating that these effects were caused specifically by the MPT.
Figure 1.
(A) A representative tracing demonstrating age-related resistance to GCDC-induced MPT in 6-day old (●), 10-day old (○), 21-day old (▲), 36-day old (△), and adult (□) rat mitochondria exposed to 100µM GCDC (added at 5 min). Note that y-axis values do not begin at “0”. (B) Mean values ± SEM of rats of different ages during the time course of MPT induction by 0µM (■), 25µM (□), 50µM ( ) and 100µM GCDC (
) and 100µM GCDC ( ). *p< 0.0001 between adult and all other rat ages for 25–100µM GCDC by ANOVA analysis. Results were from 3 separate experiments. (C) Transmission electron microscopy of mitochondria isolated from rats of different ages at end of MPT experiment. Bar in 36-day panel exposed to GCDC represents 1.0µm.
). *p< 0.0001 between adult and all other rat ages for 25–100µM GCDC by ANOVA analysis. Results were from 3 separate experiments. (C) Transmission electron microscopy of mitochondria isolated from rats of different ages at end of MPT experiment. Bar in 36-day panel exposed to GCDC represents 1.0µm.
Figure 2.
GCDC-induced cytochrome c release from mitochondria. Liver mitochondria from all rat ages were exposed to 0–100µM GCDC during the MPT assay and analyzed for mitochondrial cytochrome c content by immunoblot analysis as described in Supplemental Materials, online. COX II represents subunit of cytochrome c oxidase, which shows equal loading of all lanes. Shown is a representative immunoblot obtained from 3 independent experiments.
To investigate the factors causing MPT resistance in young rats, in subsequent experiments we compared mitochondria from 10 day-old rats (representative of the young rats) with those of adult rats.
Mitochondria from young rats are resistant to other MPT inducers
In these experiments, adult mitochondria incubated with 100µM GCDC underwent an MPT that was 4-fold greater than that of the 10-day old (Figure 3). Adult mitochondria incubated with the thiol-modifying reagent phenylarsine oxide (PhASO), or the peroxides, t-butyl hydroperoxide (t-BOOH) and hydrogen peroxide (H2O2), also underwent significant swelling, which were inhibited by CsA (data not shown). Mitochondria from 10-day old rats underwent similar swelling with PhASO, as did the mitochondria from adult rats, however, the 10-day old mitochondria responded with <50% of the adult response to the peroxides. Thus, the resistance of the 10-day old rat to undergo an MPT was greatest with GCDC, substantial with peroxides, and absent with PhASO.
Figure 3.
Effect of MPT inducers on swelling of liver mitochondria isolated from adult (□) and 10-day old (■) rats. MPT was induced by incubation with 100µM GCDC, 100µM PhASO, 10mM H2O2 or 100µM t-BOOH. CsA (5µM) was preincubated for 5 minutes before the addition of 100µM GCDC. * p <0.01 between adult and 10-day old mitochondria using a student t-test analysis. Results were from at least 5 separate experiments.
Mitochondrial Redox Status and Protein Expression in Adult and Young Rat Liver
ROS generation in the presence of GCDC was similar in liver mitochondria from 10-day old and adult rats (Figure 4A). Despite greater GSH-Px and GSSG-R activities in adult rats (Figure 4B), GSH concentrations were similar in young and adult mitochondria, suggesting that GSH-dependent enzymes were capable of reducing excessive peroxide generation. Among the Bcl-2 family proteins examined, levels of Bak and Bcl-2 were similar between adult and young rat mitochondria (Figure 4C). In contrast, expression of Bcl-xL was higher in mitochondria from the adult rat, whereas 10-day old mitochondria had greater Bax content. These alterations in Bcl-2 proteins would have the combined effect to increase sensitivity, rather than the observed decrease of sensitivity, to MPT induction and cytochrome c release in the 10-day old rat. Mitochondrial levels of adenine nucleotide translocase (ANT), voltage-dependent anion channel (VDAC) and cyclophilin D (CpD) were similar between the 10-day old and adult rat liver, as were the antioxidant enzymes manganese-superoxide dismutase (MnSOD), thioredoxin (Trx) or thioredoxin reductase (Trx-R) (Figure 1S, supplementary material online at www.pedresearch.org). Thus, neither differences in Bcl-2 proteins and MPT pore proteins, nor antioxidant defenses explained the MPT resistance of young rat mitochondria.
Figure 4.
Comparison between adult and 10-day old rat liver mitochondria for (A) GCDC-induced ROS generation, (B) GSH levels, GSH-Px and GSSG-R activities, and, (C) expression of Bcl-2 proteins. In panel A, young (solid symbol) and adult (open symbol) rat mitochondria were exposed to 0µM (circle), 50µM (triangle), 100µM GCDC (square), or 100µM GCDC + 5µM CsA (diamond). * p <0.05 between adult and 10-day old hepatic mitochondria; ** p<0.005 between adult and 10-day old hepatic mitochondria using a student t-test. Results were from at least 4 separate experiments.
Relationship between MPT induction and α-TH levels
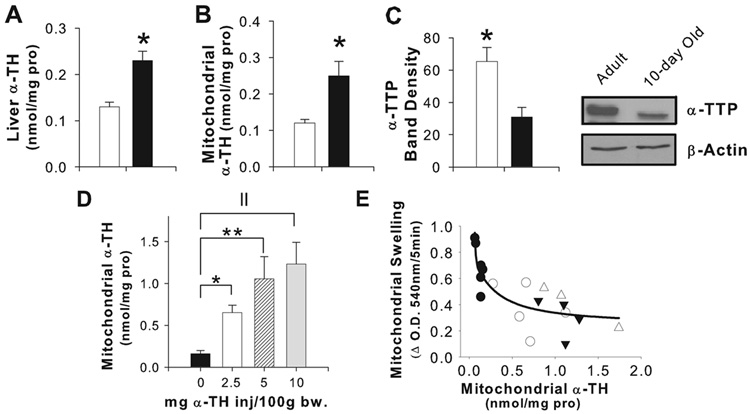
Significantly increased α-TH concentrations were present in liver homogenate (Figure 5A) and hepatic mitochondria (Figure 5B) from 10-day old compared to adult rats. Neither γ-TH nor cholesterol differed between adult and young rat mitochondria (data not shown). Ontogenic differences were also observed in expression of α-TH transfer protein (α-TTP), a primary regulator of liver α-TH secretion, with liver homogenates from the 10-day old containing 63% less α-TTP compared to the adult (Figure 5C).
Figure 5.
Liver (A) and mitochondrial (B) α-TH were significantly higher in 10-day old rats (□) compared with adults (■); * p<0.05 between adult and 10-day old hepatic mitochondria. (C) α-TTP levels by immunoblot in liver homogenate were significantly increased in adult rats compared with the 10-day old. * p<0.05 between adult and 10-day old hepatic mitochondria using a student t-test. In vivo α-TH administration resulted in elevated mitochondrial α-TH (D). * p <0.05, **p<0.005, || p<0.0005 by ANOVA analysis (E) Rats receiving a single subcutaneous injection of Vital E-300 (saline-● 2.5-○, 5-▲, or 10mg/100 g body weight-△) exhibited an inverse curvilinear relationship between mitochondrial α-TH concentrations and mitochondrial swelling (r=−0.83, p=0.0007) using a regression analysis. Results were from at least 3 separate experiments (panels A–C) or at least 3 animals receiving each SQ dose of α-TH.
To determine if α-TH content was responsible for the age-related differences in MPT susceptibility, we administered subcutaneous α-TH to achieve in vivo mitochondrial α-TH levels in adult rats in the range observed in the young rat. Administration of a single subcutaneous injection of Vital-E 300 resulted in approximately 4, 7, and 8-fold increases of mitochondrial α-TH concentrations (Figure 5D), with an inverse correlation observed between MPT induction and mitochondrial α-TH concentrations (Figure 5E). These results suggest that higher mitochondrial α-TH in young rats may be a factor providing resistance to bile acid-induced MPT.
Cardiolipin Levels and Relationship to Bile Acid-Induced MPT
Total mitochondrial CL concentrations did not significantly differ between adult and 10 day-old rats (Table 1); however, there were significant differences in individual molecular species of CL between the adult and 10-day old. The predominant CL species in mammalian mitochondria, TL-CL, was >1.5-fold greater in adult mitochondria compared with the 10-day old, and as a percentage of the total CL pool, TL-CL accounted for 52% and 37% of the CL present in adult and young rat mitochondria, respectively.
Table 1.
Total Cardiolipin (CL) and Individual CL Molecular Species in Hepatic Mitochondria
| Rat Age | CL Species Fatty acyl chain) |
nmol/mg pro (mean ± SEM) |
p-Value |
|---|---|---|---|
| Adult 10-day old |
18:2-18:2-16:1-16:1 | 0.028 ± 0.004 0.069 ± 0.002 |
0.0001 |
| Adult 10-day old |
18:2-18:2-16:1-16:0 | 0.032 ± 0.004 0.074 ± 0.007 |
0.002 |
| Adult 10-day old |
18:2-18:1-16:1-16:0 | 0.021 ± 0.003 0.033 ± 0.003 |
0.019 |
| Adult 10-day old |
18:2-18:2-18:2-16:1 | 0.240 ± 0.04 0.143 ± 0.01 |
0.038 |
| Adult 10-day old |
18:2-18:2-18:2-16:0 18:2-18:2-18:1-16:1 |
0.152 ± 0.02 0.149 ± 0.01 |
0.47 |
| Adult 10-day old |
18:2-18:1-18:1-16:1 18:2-18:2-18:1-16:0 |
0.066 ± 0.009 0.104 ± 0.008 |
0.017 |
| Adult 10-day old |
18:2-18:2-18:2-18:2 | 3.007 ± 0.128 1.922 ± 0.141 |
0.001 |
| Adult 10-day old |
18:2-18:2-18:2-18:1 | 1.283 ± 0.110 1.415 ± 0.085 |
0.220 |
| Adult 10-day old |
18:2-18:2-18:2-20:4 | 0.132 ± 0.013 0.180 ± 0.015 |
0.040 |
| Adult 10-day old |
18:1-18:2-18:2-20:4 | 0.294 ± 0.038 0.299 ± 0.016 |
0.459 |
| Adult 10-day old |
18:2-18:2-18:2-20:2 | 0.343 ± 0.035 0.404 ± 0.019 |
0.117 |
| Adult 10-day old |
18:2-18:2-18:2-22:6 | 0.128 ± 0.011 0.237 ± 0.019 |
0.003 |
| Adult 10-day old |
22:5-18:2-18:2-18:2 | 0.072 ± 0.011 0.207 ± 0.010 |
<0.0001 |
| Adult 10-day old |
Total CL | 5.80 ± 0.30 5.24 ± 0.33 |
0.158 |
CL levels were determined by electrospray mass spectrometry as described in Methods. Results were obtained from 4 separate preparations and expressed as nmol Total CL or individual species/mg protein. Acyl group composition of individual species are listed as carbon number:double bonds. Two acyl groups are listed when they could not be separated. Statistically significant differences (p<0.05) in content of individual molecular species of CL between adult and 10-day old rat liver mitochondria are shown in bold.
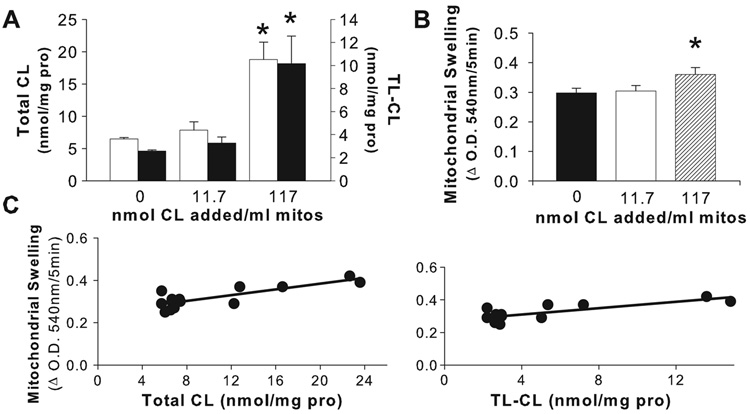
To determine whether young rat MPT resistance was related to their CL concentrations, we increased the CL content of young rat hepatic mitochondria. Mitochondria from young rats incubated with 11.7 nmol CL/ml mitochondria did not result in enrichment of either CL or TL-CL (Figure 6A), or change in GCDC-induced MPT (Figure 6B). In contrast, significant enrichment occurred with 117 nmol CL/ml mitochondria; mitochondria from young rats achieved a 2.9-fold increase in total CL and a 3.9-fold increase in TL-CL concentration resulting in only a modest increase in GCDC-induced mitochondrial swelling (Figure 6B). Although a positive correlation was demonstrated between both total CL and TL-CL content in young rat mitochondria (Figure 6C), overall these data indicate that elevating CL content in young rat hepatic mitochondria to levels measured in adult mitochondria only slightly increased their susceptibility toward bile acid-induced MPT.
Figure 6.
In vitro CL enrichment in mitochondria from young rats resulted in elevated concentrations of total CL (■) and TL-CL (□) (A). Incubation of 117 nmol CL/ml mitochondria increased total CL, (p=0.0002 for 0 vs. 117 nmol CL/ml mitochondria) and TL-CL, (p=0.0012 for 0 vs. 117 nmol CL/ml mitochondria). Incubation of 11.7 nmol CL/ml mitochondria failed to significantly increase total CL or TL-CL in mitochondria. (B) Young rat mitochondria incubated with 117 nmol CL/ml mitochondria showed a small increase in bile acid-induced MPT compared with control treatment (*p≤0.05). Significance was determined by ANOVA analysis. (C) Linear regression analysis revealed a positive relationship between GCDC-induced MPT and total CL concentration (r=0.82, p<0.001) and TL-CL concentration (r=0.79, p<0.001) in hepatic mitochondria of 10-day old rats. Results were from at least 4 separate preparations for each CL treatment.
DISCUSSION
The human neonate is particularly susceptible to cholestatic liver injury by mechanisms not well understood. In this study, we used purified mitochondrial fractions to investigate developmental differences in mitochondrial properties and pathways involved in bile acid-induced cell death. We observed that hepatic mitochondria from young rats were resistant to alterations in permeability induced by bile acids that are retained in cholestasis, and that differences in mitochondrial membrane lipid composition between young and adult rats were related to these findings. It is unclear why purified mitochondria from the young rat, which typically exhibits “physiologic cholestasis” at birth (23), would be resistant to bile acid toxicity. One possible explanation may be, in fact, that this represents a protective response that prevents the normally elevated concentrations of bile acids (related to the immature bile acid secretory pathways that are present in the neonate (24,25) from initiating hepatocellular apoptosis or necrosis during a period of rapid growth of the developing liver.
Hydrophobic bile acids directly promote mitochondrial dysfunction or promote cell death by several interrelated pathways, which are regulated by Bcl-2 family proteins. In the current study, mitochondria from young rats were extremely resistant to bile acid-induced MPT, despite having greater levels of pro-apoptotic Bax and reduced amounts of anti-apoptotic Bcl-xL. Thus, the balance of Bcl-2 proteins in young rat liver mitochondria would favor bile acid induction of the MPT and cytochrome c release, which was in contrast to the observed MPT resistance in the 10-day old mitochondria (Figure 1 and Figure 2). Although an earlier study reported no Bcl-2 expression in normal rat hepatocytes (26), we demonstrated the presence of the protein in our purified mitochondria, a finding supported by other groups (27,28). Since Bcl-2 proteins did not explain our MPT findings, we analyzed other factors that could contribute to MPT induction.
Mitochondria from 10-day old rats contained amounts of ANT, VDAC and CpD, putative components of the MPT megapore, similar to adult rat mitochondria. Thus, the structural capability for pore formation was present in the developing rat and MPT resistance was not explained by expression differences in MPT proteins. Inasmuch as bile acid toxicity is associated with significant mitochondrial ROS generation (29,30), it would follow that developmental differences in antioxidant defenses within mitochondria could affect MPT susceptibility. Mitochondria from the 10-day old contained significantly lower GSH-Px and GSSG-R activities, raising the possibility that young rats might not be capable of neutralizing excess hydroperoxides. However, it is unlikely that this reduced activity contributes to their resistance to bile acid-induced MPT, particularly in the presence of adequate GSH concentrations. Furthermore, the young and adult rat mitochondria generated equivalent ROS suggesting that the GSH-dependent enzyme systems functioned similarly in the young and adult rat. Adult rat liver mitochondria contained half of the α-TH concentration of young rat liver mitochondria, which corresponded with reduced hepatic expression of α-TTP, supporting a previous finding (31).
Since α-TTP is responsible for transfer of α-TH out of the hepatocyte via VLDL secretion (32), lower expression of TTP could be responsible for higher levels of α-TH in the liver of the young rat, providing increased protection in the developing animal to hepatic-derived oxidative stress. In vivo enrichment of adult rats with subcutaneous α-TH increased resistance to GCDC-induced MPT. This finding supports a possible role of higher mitochondrial α-TH concentrations in the protection of the young rat against bile acid toxicity.
Due to its abundance and essential role in mitochondrial function, CL has been investigated in apoptotic cell death (33,34). In one previous study, elevated levels of total CL were associated with reduced liver injury and MPT susceptibility in bile duct-ligated rats (35). Although the current study revealed no difference in total CL between young and adult rat hepatic mitochondria, alterations in the overall profile of individual CL subspecies were observed. In particular, differences in TL-CL concentrations could affect several mitochondrial-dependent processes involved in cell signaling or mitochondrial structure and function. For example, reduced TL-CL in the young rat could alter the ability of CL to bind cytochrome c, a high affinity ligand for CL (36); less TL-CL could provide fewer anchor points for hydrophobic and electrostatic binding between CL and cytochrome c. The interaction of CL with cytochrome c also promotes a peroxidative activity, which in turn facilitates the release of pro-apoptotic factors from mitochondria (37,38). Finally, a reduction of TL-CL in the young rat may generate less CL hydroperoxides, (particularly in the presence of the elevated α-TH observed in young rat mitochondria), which have been shown to promote mitochondrial swelling and cytochrome c release from mitochondria (39). In the current study, enrichment of CL and TL-CL into liver mitochondrial membranes of young rats only slightly increased their susceptibility to bile acid-induced MPT, suggesting that MPT resistance in the young rat was not the result of lower basal TL-CL content. It is also possible that other minor CL subspecies could modulate MPT induction through interactions with mitochondrial pore proteins.
In conclusion, we have demonstrated an unexpected resistance of hepatic mitochondria in the young, developing rat to bile acid-induced MPT and cytochrome c release. This phenomenon may be a novel adaptive response that allows the developing hepatocyte to cope with the increased bile acid levels to which it is exposed physiologically, providing protection against the MPT and release of cytochrome c, which induce hepatocyte death in the adult rat. Membrane compositional differences between adult and developing young rats are related to this MPT resistance. Further elucidation of the factors responsible for this MPT resistance could potentially be exploited as candidate targets for treatment of cholestasis in older patients, considering the important role of mitochondrial perturbations in the pathogenesis of cholestatic liver injury. Whether similar resistance to MPT is developmentally regulated in other organ systems awaits investigation.
Supplementary Material
Supplemental materials, which accompany this article, are available online at www.pedresearch.org.
Acknowledgments
Financial Support: Supported in part by grants to RJS from the NIH (RO1DK038446); The Children’s Hospital Research Institute and the Abby Bennet Liver Research Fund, and grants to MGT from the NIH (RO1DK59576) and the Environmental Health Sciences Center at Oregon State University (NIEHS P30 ES00210).
ABBREVIATIONS
- α-TH,
alpha-tocopherol
- α-TTP
alpha-tocopherol transfer protein
- CL
cardiolipin
- GSH-Px
glutathione peroxidase
- GSSG-R
glutathione reductase
- GCDC
glycochenodeoxycholic acid
- MPT
mitochondrial permeability transition
- ROS
reactive oxygen species
- TL-CL
tetra-linoleoyl CL
Footnotes
Publisher's Disclaimer: Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the 56th Annual Meeting of the American Association for the Study of Liver Diseases, San Francisco, CA., November 2005, and published in abstract form (Hepatology 2005; 777A); and at the 58th Annual Meeting of the American Association for the Study of Liver Diseases, Boston, MA., November 2007 and published in abstract form (Hepatology 2007: 516A)
REFERENCES
- 1.Arrese M, Ananthananarayanan M, Suchy FJ. Hepatobiliary transport: molecular mechanisms of development and cholestasis. Pediatr Res. 1998;44:141–147. doi: 10.1203/00006450-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Phillips MJ. Mechanisms and morphology of cholestasis. In: Suchy FJ, editor. Liver Disease in Children. Moseby: St. Louis; 1994. pp. 129–144. [Google Scholar]
- 3.Pittschieler K, Lebenthal E, Bujanover Y, Petell JK. Levels of Cu-Zn and Mn superoxide dismutases in rat liver during development. Gastroenterology. 1991;100:1062–1068. doi: 10.1016/0016-5085(91)90283-q. [DOI] [PubMed] [Google Scholar]
- 4.Bohles H. Antioxidative vitamins in prematurely and maturely born infants. Int J Vitam Nutr Res. 1997;67:321–328. [PubMed] [Google Scholar]
- 5.Rolo AP, Oliveira PJ, Moreno AJ, Palmeira CM. Bile acids affect liver mitochondrial bioenergetics: possible relevance for cholestasis therapy. Toxicol Sci. 2000;57:177–185. doi: 10.1093/toxsci/57.1.177. [DOI] [PubMed] [Google Scholar]
- 6.Krahenbuhl S, Talos C, Sven F, Reichen J. Toxicity of bile acids on the electron transport chain in isolated rat liver mitochondria. Hepatology. 1994;19:471–479. doi: 10.1002/hep.1840190228. [DOI] [PubMed] [Google Scholar]
- 7.Krahenbuhl S, Talos C, Reichen J. Mechanisms of impaired hepatic fatty acid metabolism in rats with long-term bile duct ligation. Hepatology. 1994;19:1272–1281. doi: 10.1002/hep.1840190528. [DOI] [PubMed] [Google Scholar]
- 8.Palmeira CM, Rolo AP. Mitochondrially-mediated toxicity of bile acids. Toxicology. 2004;203:1–15. doi: 10.1016/j.tox.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Sokol RJ, Straka MS, Dahl R, Devereaux MW, Yerushalmi B, Gumpricht E, Elkins N, Everson G. Role of oxidant stress in the permeability transition induced in rat hepatic mitochondria by hydrophobic bile acids. Pediatr Res. 2001;49:519–531. doi: 10.1203/00006450-200104000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues CM, Fan G, Wong PY, Kren BT, Steer CJ. Ursodeoxycholic acid may inhibit deoxycholic acid-induced apoptosis by modulating mitochondrial transmembrane potential and reactive oxygen species production. Mol Med. 1998;4:165–178. [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues CM, Ma X, Linehan-Stieers C, Fan G, Kren BT, Steer CJ. Ursodeoxycholic acid prevents cytochrome c release in apoptosis by inhibiting mitochondrial membrane depolarization and channel formation. Cell Death Differ. 1999;6:842–854. doi: 10.1038/sj.cdd.4400560. [DOI] [PubMed] [Google Scholar]
- 12.Sodeman T, Bronk SF, Roberts PJ, Miyoshi H, Gores GJ. Bile salts mediate hepatocytes apoptosis by increasing cell surface trafficking of fas. Am J Physiol Gastrointest Liver Physiol. 2000;278:G992–G999. doi: 10.1152/ajpgi.2000.278.6.G992. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi H, Rust C, Roberts PJ, Burgart LJ, Gores GJ. Hepatocyte apoptosis after bile duct ligation in the mouse involves Fas. Gastroenterology. 1999;117:669–677. doi: 10.1016/s0016-5085(99)70461-0. [DOI] [PubMed] [Google Scholar]
- 14.Cheng X, Buckley D, Klaassen CD. Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem Pharmacol. 2007;74:1665–1676. doi: 10.1016/j.bcp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 16.Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 17.Lang JK, Gohil K, Packer L. Simultaneous determination of tocopherols, ubiquinols, and ubiquinones in blood, plasma, tissue homogenates, and subcellular fractions. Anal Biochem. 1986;157:106–116. doi: 10.1016/0003-2697(86)90203-4. [DOI] [PubMed] [Google Scholar]
- 18.Podda M, Weber C, Traber MG, Packer L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J Lipid Res. 1996;37:893–901. [PubMed] [Google Scholar]
- 19.Gumpricht E, Dahl R, Devereaux MW, Sokol RJ. Licorice compounds glycyrrhizin and 18beta-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J Biol Chem. 2005;280:10556–10563. doi: 10.1074/jbc.M411673200. [DOI] [PubMed] [Google Scholar]
- 20.Gumpricht E, Devereaux MW, Traber M, Sokol RJ. Enrichment of rat hepatic organelles by vitamin E administered subcutaneously. Free Radic Biol Med. 2004;37:1712–1717. doi: 10.1016/j.freeradbiomed.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 21.Sparagna GC, Johnson CA, McCune SA, Moore RL, Murphy RC. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J Lipid Res. 2005;46:1196–1204. doi: 10.1194/jlr.M500031-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Hackenbrock CR, Chazotte B. Lipid enrichment and fusion of mitochondrial inner membranes. Methods Enzymol. 1986;125:35–45. doi: 10.1016/s0076-6879(86)25006-5. [DOI] [PubMed] [Google Scholar]
- 23.Belknap WM, Balistreri WF, Suchy FJ, Miller PC. Physiologic cholestasis II: serum bile acid levels reflect the development of the enterohepatic circulation in rats. Hepatology. 1981;1:613–616. doi: 10.1002/hep.1840010608. [DOI] [PubMed] [Google Scholar]
- 24.Rippin SJ, Hagenbuch B, Meier PJ, Stieger B. Cholestatic expression pattern of sinusoidal and canalicular organic anion transport systems in primary cultured rat hepatocytes. Hepatology. 2001;33:776–782. doi: 10.1053/jhep.2001.23433. [DOI] [PubMed] [Google Scholar]
- 25.Gao B, St Pierre MV, Stieger B, Meier PJ. Differential expression of bile salt and organic anion transporters in developing rat liver. J Hepatol. 2004;41:201–208. doi: 10.1016/j.jhep.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 26.Kurosawa H, Que FG, Roberts LR, Fesmier PJ, Gores GJ. Hepatocytes in the bile duct-ligated rat express Bcl-2. Am J Physiol. 1997;272:G1587–G1593. doi: 10.1152/ajpgi.1997.272.6.G1587. [DOI] [PubMed] [Google Scholar]
- 27.Janiak F, Leber B, Andrews DW. Assembly of Bcl-2 into microsomal and outer mitochondrial membranes. J Biol Chem. 1994;269:9842–9849. [PubMed] [Google Scholar]
- 28.Motoyama S, Kitamura M, Saito S, Minamiya Y, Suzuki H, Saito R, Terada K, Ogawa J, Inaba H. Bcl-2 is located predominantly in the inner membrane and crista of mitochondria in rat liver. Biochem Biophys Res Commun. 1998;249:628–636. doi: 10.1006/bbrc.1998.9205. [DOI] [PubMed] [Google Scholar]
- 29.Yerushalmi B, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology. 2001;33:616–626. doi: 10.1053/jhep.2001.22702. [DOI] [PubMed] [Google Scholar]
- 30.Sokol RJ, Dahl R, Devereaux MW, Yerushalmi B, Kobak GE, Gumpricht E. Human hepatic mitochondria generate reactive oxygen species and undergo the permeability transition in response to hydrophobic bile acids. J Pediatr Gastroenterol Nutr. 2005;41:235–243. doi: 10.1097/01.mpg.0000170600.80640.88. [DOI] [PubMed] [Google Scholar]
- 31.Kim HS, Arai H, Arita M, Sato Y, Ogihara T, Tamai H, Inoue K, Mino M. Age-related changes of alpha-tocopherol transfer protein expression in rat liver. J Nutr Sci Vitaminol (Tokyo) 1996;42:11–18. doi: 10.3177/jnsv.42.11. [DOI] [PubMed] [Google Scholar]
- 32.Traber MG, Burton GW, Hamilton RL. Vitamin E trafficking. Ann N Y Acad Sci. 2004;1031:1–12. doi: 10.1196/annals.1331.001. [DOI] [PubMed] [Google Scholar]
- 33.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 34.Matsko CM, Hunter OC, Rabinowich H, Lotze MT, Amoscato AA. Mitochondrial lipid alterations during Fas- and radiation-induced apoptosis. Biochem Biophys Res Commun. 2001;287:1112–1120. doi: 10.1006/bbrc.2001.5696. [DOI] [PubMed] [Google Scholar]
- 35.Lieser MJ, Park J, Natori S, Jones BA, Bronk SF, Gores GJ. Cholestasis confers resistance to the rat liver mitochondrial permeability transition. Gastroenterology. 1998;115:693–701. doi: 10.1016/s0016-5085(98)70149-0. [DOI] [PubMed] [Google Scholar]
- 36.Basova LV, Kurnikov IV, Wang L, Ritov VB, Belikova NA, Vlasova II, Pacheco AA, Winnica DE, Peterson J, Bayir H, Waldeck DH, Kagan VE. Cardiolipin switch in mitochondria: shutting off the reduction of cytochrome c and turning on the peroxidase activity. Biochemistry. 2007;46:3423–3434. doi: 10.1021/bi061854k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayir H, Fadeel B, Palladino MJ, Witasp E, Kurnikov IV, Tyurina YY, Tyurin VA, Amoscato AA, Jiang J, Kochanek PM, DeKosky ST, Greenberger JS, Ahvedova AA, Kagan VE. Apoptotic interactions of cytochrome c: redox flirting with anionic phospholipids within and outside of mitochondria. Biochim Biophys Acta. 2006;1757:648–659. doi: 10.1016/j.bbabio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 39.Petrosillo G, Casanova G, Matera M, Ruggiero FM, Paradies G. Interaction of peroxidized cardiolipin with rat-heart mitochondrial membranes: induction of permeability transition and cytochrome c release. FEBS Lett. 2006;580:6311–6316. doi: 10.1016/j.febslet.2006.10.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental materials, which accompany this article, are available online at www.pedresearch.org.