Abstract
Many lines of evidence indicate that GABA and GABAA receptors make important contributions to human sleep regulation. Pharmacological manipulation of these receptors has differential effects on sleep onset and sleep maintenance insomnia. Here we show that sleep is regulated by GABA in Drosophila and that a mutant GABAA receptor, RdlA302S, specifically decreases sleep latency. The drug carbamazepine (CBZ) has the opposite effect on sleep; it increases sleep latency as well as decreasing sleep. Behavioral and physiological experiments indicated that RdlA302S mutant flies are resistant to the effects of CBZ on sleep latency and that mutant RDLA302S channels are resistant to the effects of CBZ on desensitization, respectively. These results suggest that this biophysical property of the channel, specifically channel desensitization, underlies the regulation of sleep latency in flies. These experiments uncouple the regulation of sleep latency from that of sleep duration and suggest that the kinetics of GABAA receptor signaling dictate sleep latency.
Insomnia is the most common sleep problem and affects approximately one third of the adult American population1. Patients with insomnia are generally subdivided into three categories: sleep onset insomnia, sleep maintenance insomnia and terminal insomnia (early-morning awakening coupled with an inability to return to sleep)2. The biological basis for these insomnia classifications remains unknown. Nonetheless, a single class of drugs, agonistic modulators of GABAA receptors, effectively ameliorates these diverse symptoms2,3. GABAA receptors are a family of pentameric ligand-gated Cl- channels4 and are a major source of inhibitory currents throughout the CNS5,6. These receptors are also an important target for pharmacologic treatment of many other neurological disorders in addition to sleep7.
The fruit fly Drosophila melanogaster is an ideal model for dissecting the relationships between molecules and behaviors, as well as between different sleep states8,9. As in mammals, it has been shown that the sleep-like state of Drosophila is associated with reduced sensory responsiveness and reduced brain activity10,11, and is subject to both circadian and homeostatic regulation12,13. Researchers have also identified a number of genes14,15, circuits16,17 and biological processes18 that affect fly sleep. However, there is no reported role for GABA and no reported manipulation of GABA receptors in fly sleep studies.
The first GABAA receptor mutant was isolated from pesticide-resistant Drosophila and the locus was named Resistant to dieldrin (Rdl)19,20. Similar to mammalian GABAA receptors, RDL channels mediate fast inhibitory neurotransmission21 and are expressed in the CNS22. Notably, the mutation that causes the insecticide resistance phenotype (A302S)20 specifically decreases the rate of RDL desensitization with little or no effect on other channel properties23. As a consequence, the mutant receptor has a longer single channel open duration and, therefore, increased channel current, at least under certain conditions (see below). Because of these characteristics, and because this mutation does not have obvious effects on health or viability, we decided to establish the importance of GABAergic transmission to sleep in flies and to examine the effects of the Rdl mutation.
Interestingly, flies with this mutant GABAA receptor subunit slept more, primarily because of decreased sleep latency. CBZ, which accelerates RDL desensitization, had the opposite effect and increased sleep latency. Behavioral and electrophysiological experiments indicated that the Rdl mutant was largely resistant to the effects of CBZ, both its ability to alter desensitization and to decrease sleep latency. These results suggest that these two phenomena are mechanistically linked. To account for these observations, we propose that sleep is initiated by fast-firing GABAergic neurons.
RESULTS
To determine whether GABAergic transmission is important for sleep in Drosophila, we first used the GAL4/UAS system24 to decrease GABA release by expressing the hyperpolarizing potassium channel Shaw25 in GABAergic neurons. Total sleep was reduced, due in turn to an increase in mean wake-episode duration and to a decrease in sleep-bout duration. There was also an increase in the night-time sleep latency, consistent with a role for GABAergic tone in regulating both the initiation and maintenance of sleep (Fig. 1).
Figure 1.
GABAergic neurons control sleep in Drosophila. Sleep parameters are shown for flies expressing a hyperpolarizing potassium channel (UAS-Shaw) under the control of GAD-GAL4. Single transgene sibling controls (UAS only and GAL4 only) are also shown. (a) Conventional sleep plot showing sleep in a 12-h light:dark cycle. (b) Total sleep duration (24 h) and total sleep during the light and dark periods. (c) Maximum sleep-episode duration. (d) Sleep latency after lights off. (e) Mean wake duration for light and dark periods. Values and error bars for b-e indicate mean ± s.e.m. (* indicates P < 0.05, ** indicates P < 0.005 and *** indicates P < 0.0005 for the comparison of wild type and mutant by one-way ANOVA with post hoc test; n = 32 for GAL4 alone, n = 31 for UAS alone and n = 62 for GAD-GAL4;UAS-Shaw).
To investigate the potential role of Rdl, the sleep pattern of an extensively outcrossed homozygous RdlA302S strain, RdlMDRR (see Methods), was assayed under standard light-dark (12-h:12-h light-dark cycles) conditions (Fig. 2). As GABAA currents in RdlA302S fly neurons have longer single channel open durations23, we surmised that these flies might have increased sleep, as a result of either decreased sleep latency and/or increased sleep duration. Indeed, RdlMDRR flies had decreased sleep latency (compare the beginning of dark period in the control and RdlMDRR genotypes in Fig. 2a; see Fig. 2c, left for quantification). They also had a small, but significant, increase in total daily (24 h) sleep (P < 0.005; Fig. 2b, left).
Figure 2.
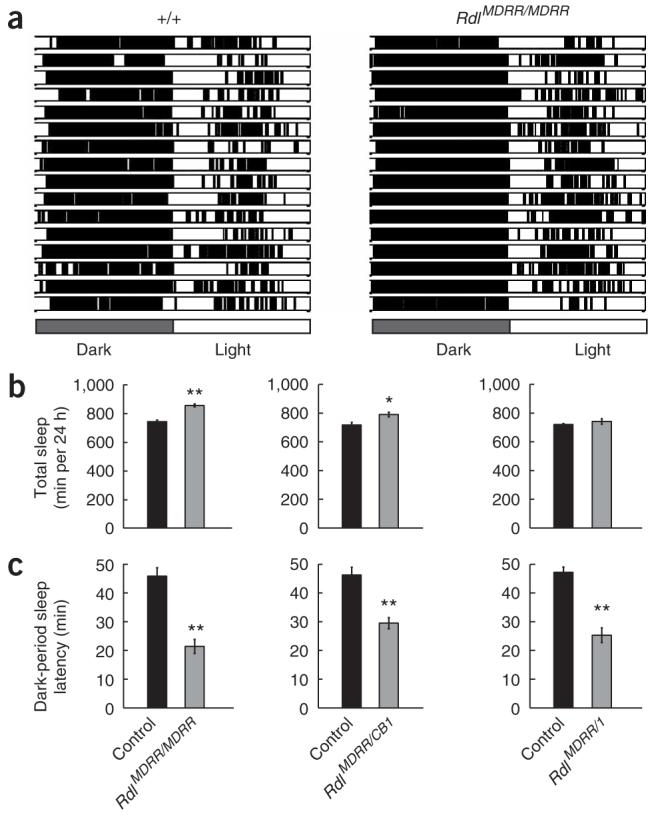
RdlA302S flies show decreased sleep latency and increased sleep-episode duration. RdlMDRR and RdlCB1 are independently isolated A302S point mutations in the Rdl gene. Wild-type sibling controls for each experimental genotype are shown. (a) Raster plots showing the sleep/wake pattern of 16 individual wild-type (+/+, left) and RdlMDRR (right) flies during a 24-h (12 h:12 h dark:light) period. White areas represent periods of wakefulness (periods of movement, or inactivity <5 min), whereas the black areas show sleep periods (inactive periods >5 min). (b) Quantification and comparison of total 24-h sleep between wild-type (n = 135) and RdlMDRR(n = 155, left) flies, wild-type (n = 51) and RdlMDRR/RdlCB1(n = 49, middle) flies, and wild-type (n = 277) and RdlMDRR/Rdl1 (n = 50, right) flies. (c) Sleep latency after lights off for same genotypes. Values and error bars for b and c indicate mean ± s.e.m. (* indicates P < 0.005, ** P < 0.0005 for the comparison of wild type and mutant by two-tailed Student’s t-test).
Despite the extensive outcrossing of the RdlMDRR A302S mutant chromosome, we made two additional chromosome combinations to validate these results. We assayed an RdlMDRR/Rdl1 combination, which creates a hemizygous RdlA302S allele because of the Rdl1 null mutation26. We also assayed an independent homozygous RdlA302S combination, made by crossing our outcrossed RdlMDRR chromosome with an independent RdlA302S mutant chromosome, RdlCB1. The latter was previously identified in another ethyl methanesulfonate screen on a different background chromosome. Both of the new combinations had a very similar sleep latency phenotype (Fig. 2c, middle and right), and the RdlA302S/Rdl1 combination had little effect on total sleep duration (Fig. 2b, right). None of these genotypes had substantial increases in locomotor activity during active periods, indicating that these sleep phenotypes are not the result of simple hyperactivity (data not shown). We conclude that the most consistent effect of the RdlA302S mutation is to decrease sleep latency.
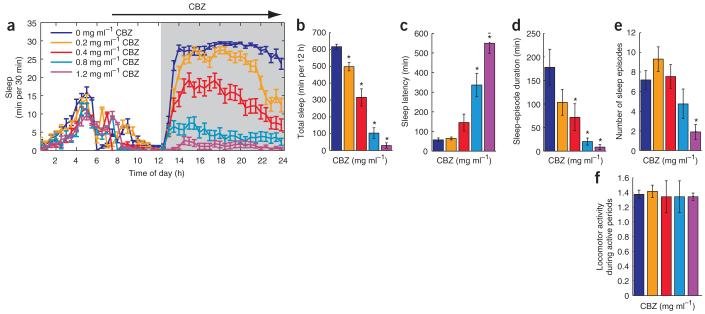
In a search for a complementary approach to better understand the effect of the RdlA302S mutation, we first assayed the sleep effects of a number of drugs on wild-type flies. Only the human anticonvulsant CBZ had a marked dose-dependent effect, with the highest dose (1.2 mg ml-1) almost completely inhibiting night-time sleep (Fig. 3a,b). This was the result of both an increase in sleep latency (Fig. 3c) and a decrease in sleep-episode duration (Fig. 3d). The number of sleep episodes appeared to show a biphasic response, with an increase at a low dose and a prominent, significant decrease at a high dose (P < 0.05; Fig. 3e). Locomotor activity during active periods was unchanged (Fig. 3f), indicating that the sleep effects were not the result of hyperactivity.
Figure 3.
CBZ markedly decreases fly sleep by increasing sleep latency and decreasing episode duration (a) Conventional sleep plot showing the effect of different concentrations of CBZ on the sleep pattern of wild-type (Canton-S) flies during the first day of drug treatment. Values indicate the average sleep per 30 min of groups of flies fed with either solvent (blue, 0 mg ml-1, n = 27) or various doses of CBZ (orange, 0.2 mg ml-1, n = 26; red, 0.4 mg ml-1, n = 24; turquoise, 0.8 mg ml-1, n = 28, 1.2 purple, mg ml-1, n = 27). White and gray areas are the dark and light periods, respectively. Black arrow on the top part of the graph indicates CBZ application. (b-f) Effect of CBZ on total sleep (b), sleep latency after lights off (c), sleep-episode duration (d), number of sleep episodes (e) and locomotion during wake periods (f) on the first night of drug application. Values represent mean ± s.e.m. for all panels. For panels b-f, * indicates P < 0.05 compared with vehicle control using one-way ANOVA factor ‘CBZ concentration’ and Tukey-Kramer post hoc test.
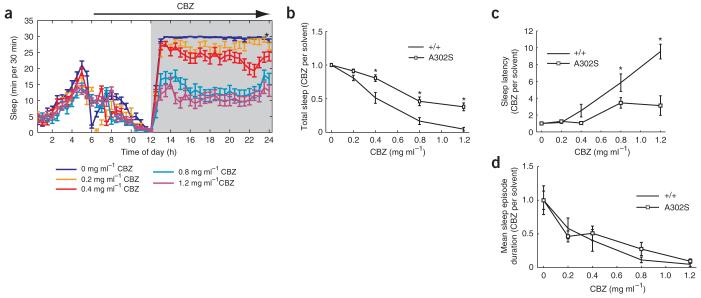
To relate these CBZ effects to the RdlA302S phenotypes described above, we assayed the sleep of the homozygous RdlA302S strain after CBZ feeding (Fig. 4). The drug effects were less potent than in wild-type flies (Fig. 4a,b; also compare Fig. 4a with Fig. 3a). This was because the sleep latency of RdlA302S flies was almost completely resistant to CBZ; wild-type flies showed a large dose-dependent increase in sleep latency, whereas RdlA302S latency remained unaffected, even at high levels of the drug (Fig. 4c). This suggests that the latency effects of CBZ and the RdlA302S mutation share a common mechanism. In contrast, the mutant flies were equally sensitive to the sleep-duration effects of the drug (Fig. 4d). These data pharmacologically uncouple sleep latency from sleep duration.
Figure 4.
RdlA302S flies are resistant to CBZ effects on sleep latency, but not to its effects on sleep-episode duration. (a) Conventional sleep plot showing the effect of different concentrations of CBZ on the sleep pattern of RdlA302S mutants during the first day of drug treatment. (b-d) CBZ dose-response curves comparing the different sleep parameters between wild-type (+/+) and RdlA302S flies during the fist night of drug treatment. The behavioral response to each CBZ concentration was divided by the average response of the respective control group (0 mg ml-1) to normalize for genotype differences on the various sleep parameters. Significant differences in CBZ sensitivity were observed between wild type and RdlA302S in total sleep (0.4, 0.8 and 1.2 mg ml-1; b) and sleep latency (0.8 and 1.2 mg ml-1; c), but not mean sleep-episode duration (d).* indicates P < 0.05 by two-way ANOVA with ‘genotype’ and ‘CBZ concentration’ as factors. In order of increasing dose, n = 27, 26, 24, 28 and 27 for the A302S mutant RdlMDRR.
The experiments also shed light on the mechanisms underlying sleep homeostasis. A decrease in sleep-episode duration with CBZ should normally increase sleep pressure, resulting in a compensatory increase in sleep-episode number. The CBZ-dependent block of sleep initiation, as well as sleep duration, in wild-type flies apparently affected this compensation by preventing an increase in the number of sleep episodes (Fig. 5a). In contrast, RdlA302S flies were able to increase the number of sleep episodes and, therefore, compensate for the decreased sleep duration, presumably because their ability to fall asleep was less affected by the drug (Fig. 5a). This was also apparent in the marked difference in short 5-15 min sleep episodes between genotypes; only RdlA302S flies were able to initiate more short episodes despite increasing drug concentrations (Fig. 5b,c). These data further support the idea that sleep latency and duration are separable processes in flies and suggest that the Rdl GABAA receptor is the target responsible for the sleep latency effects of CBZ.
Figure 5.
The RdlA302S mutation rescues sleep homeostasis. (a) Significant differences in CBZ sensitivity were observed between wild type and RdlA302S in the number of sleep episodes at 0.4, 0.8 and 1.2 mg ml-1, showing that the RdlA302S mutant can respond homeostatically to sleep deprivation. * indicates P < 0.05 by two-way ANOVA with ‘genotype’ and ‘CBZ concentration’ as factors. (b,c) Sleep-episode bout length distribution in wild-type (b) and RdlA302S (c) flies with increasing amounts of CBZ.
To assay whether CBZ might directly affect the RDL channel, we expressed RDL in Xenopus oocytes and measured current in the presence of increasing concentrations of CBZ. CBZ specifically increased RDL desensitization without affecting peak amplitude after a single GABA pulse (Fig. 6a,b). However, an increase in desensitization might alter the peak current amplitude of GABA pulse trains if they were sufficiently frequent to prevent channel recovery before the next pulse. Indeed, CBZ had a peak amplitude effect with this kind of GABA pulse-train stimulation protocol (Fig. 6c). We concluded that the GABA application frequency determines the effect of CBZ on current amplitude. Importantly, the CBZ effect on RDL desensitization was completely blocked by the A302S mutation, which has a potent effect on desensitization without drug (ref. 23 and Fig. 6d).
Figure 6.
CBZ specifically increases RDL desensitization and the A302S mutation prevents CBZ effects (a) Response to 90-s application of 100 μM GABA with variable doses of CBZ, recorded from oocytes expressing RDL held at -60 mV under voltage clamp. (b) Comparison of current amplitudes with (1 mM CBZ) and without (control) drug perfusion. Change in peak amplitude was not statistically significant (left, P > 0.9, paired t-test), whereas steady state amplitude was significantly decreased (P < 0.005, paired t-test). Steady state amplitude was calculated by normalizing the peak current amplitude to 1 and fitting to a single exponential equation. (c) Current evoked by successive pulses of 50 μM GABA with and without CBZ. (d) Response of oocytes expressing A302S mutant channel to 100 μM GABA under the same conditions as in a. Traces in c and d were normalized to the first peak amplitude.
These data imply that the ability of the Rdl channel to desensitize is critical for sleep onset and provide a coherent, biophysical explanation for the drug’s, as well as the mutant’s, effects on sleep latency. Because desensitization is expected to have more significant effects on total current at synapses receiving high frequency input (Fig. 6), we propose a model (Supplementary Fig. 1 online) in which fast-firing GABAergic (sleep initiation) neurons induce sleep onset by inhibiting wake-promoting neurons. The desensitization-induced synaptic current reduction at sleep initiation-wake promoting synapses is enhanced by CBZ, which leads to a faster current decrease and increased sleep latency. The A302S mutant channel shows reduced desensitization, as well as reduced CBZ-sensitivity, consistent with the observed in vivo effects in these flies. The lack of a differential (A302S versus wild type) CBZ effect on sleep duration suggests that other, slower-firing GABAergic inputs maintain sleep (sleep maintenance neurons) and that the CBZ effects on sleep duration may also involve effects on other sleep-relevant molecules.
DISCUSSION
Here we show that GABA and GABAA receptors are important for sleep in Drosophila. To the best of our knowledge, this is the first demonstration in an animal model that sleep onset and maintenance are differentially regulated by GABAA receptors, as well as by a pharmacological agent. The ability of Rdl mutant flies to mount a homeostatic response to the sleep-depriving drug CBZ further suggests that the sleep-regulating neurons that express this receptor are part of the core sleep circuit. These findings further support the use of Drosophila melanogaster as a model for understanding the mechanisms and function of mammalian sleep.
Our results suggest that GABAergic inputs control both the onset and maintenance of sleep, as both latency and duration of sleep episodes were affected by suppressing GABAergic transmission. Our finding that fast-desensitizing GABAA receptors preferentially controlled sleep initiation suggests that there are fast-spiking GABAergic sleep-initiation neurons that synapse onto RDL-expressing postsynaptic wake-promoting neurons (Supplementary Fig. 1). In this model, RDL channel desensitization should decrease wake-promoting hyperpolarization. The virtue of desensitization in this context is that it helps prevent spurious and/or unwanted sleep episodes. Sleep drive must be sufficient for sleep-initiation firing to occur for a sufficiently long time to achieve wake-promoting silencing. Only under conditions that sustain high-frequency sleep-initiation neuron firing does the animal fall asleep. Once wake-promoting neurons are quiescent, putative sleep-maintenance neurons are released from inhibition and act to maintain wake-promoting neurons below their action potential threshold. Other GABA receptor subunits (and/or other transmitter systems) are presumably more important than RDL for sleep-maintenance neurons. At the end of the night, extrinsic inputs, regulated by circadian as well as by homeostatic factors, presumably inhibit the sleep-promoting regions (sleep maintenance and sleep initiation) and the fly wakes up.
The latency assay, the time it takes flies to fall asleep once the lights have turned off at Zeitgeber Time 12, presumably reflects sleep pressure, which influences sleep-initiation neuronal firing. The importance of desensitization to sleep latency (or sleep initiation) is emphasized by the RdlA302S phenotype, the kinetics of RDLA302S currents, the effect of CBZ on fly sleep and the effect of CBZ on wild-type and mutant receptor physiology. The slow decay kinetics of RDLA302S currents suggest that hyperpolarization of wake-promoting neurons occurs more easily in mutant flies. The stronger and more consistent mutant effect on sleep latency, as compared to sleep duration, indicates that the output of the sleep-initiation neurons is affected more strongly by the mutant receptor than that of sleep-maintenance neurons. Although this could reflect the presence of other GABA receptor subunits in sleep-maintenance neurons, it could also just reflect a slower firing rate. CBZ acts specifically on RDL desensitization and this effect is blocked by the RDLA302S mutation, which explains why the drug effect on sleep latency is substantially reduced in the RdlA302S genotype.
There was an additional, prominent CBZ effect on sleep-episode duration, which was not ameliorated by the RdlA302S mutation. This suggests that there are additional CBZ targets, which affect wake-promoting activity either directly or indirectly (Supplementary Fig. 1). The multiple effects of CBZ on the Drosophila sleep circuit are not surprising, as its pharmacology in humans27 indicates that it interacts with many targets, including sodium channels, GABAA receptors28 and adenosine receptors.
Nonetheless, the decrease in fly sleep is the result, in part, of the unexpected CBZ effects on fly GABAA receptor kinetics. CBZ is widely used in the treatment of multiple neurological disorders, including epilepsy, bipolar disorder and trigeminal neuralgia29, and therapeutic drugs for these disorders might be expected to increase sleep. Indeed, several studies have reported that patients and healthy volunteers receiving CBZ therapy experience sleep problems, which normally disappear with long-term treatment30. It is intriguing that the CBZ effects on fly sleep also decrease with chronic drug treatment (data not shown). Our observations, along with others, further illustrate the potential utility of using Drosophila models combined with pharmacological approaches to better understand complex behaviors and disease states.
The similarities between fly and mammalian sleep suggest that altering subtle aspects of GABAA receptor function in mammals will also lead to discrete effects on sleep structure. Indeed, a human mutation altering desensitization and deactivation of a GABAA receptor subunit causes a familial type of insomnia31. Taken together with our data on the Drosophila RDL channel, this suggests that targeting specific aspects of GABAA receptor function such as desensitization will give rise to more specific and effective sleep therapeutics.
METHODS
Animals
Fly cultures were kept at 25 °C with a 12-h light/dark cycle on cornmeal, yeast, sucrose and agar food. The original Rdl A302S allele, RdlMDRR, which was isolated from the wild20, and Rdl1/TM326 flies were obtained from the Bloomington Stock Center (Bloomington). RdlCB1 was isolated in a modified dominant F1 screen for insecticide resistance in which Canton-S flies were ethyl methanesulfonate mutagenized using previously described methods32. For all strains, the third chromosome was crossed into Canton-S background; for RdlMDRR the strain was subsequently outcrossed over six generations and isolated using PCR-ren33.
Behavioral assays and drug administration
Behavioral assays and analysis were carried out as previously described12. For all genotypes, the locomotor activity of 5-7-day-old flies loaded with 5% sucrose in 2% agar was monitored using the Trikinetics system (Waltham) in 24-h light-dark cycles. Data were collected in 1-min bins and a sliding window was applied. Sleep was defined as 5 consecutive min of inactivity12,34; sleep latency was measured from the time of lights off to the onset of the first sleep episode. For episodes flanking a given period, quantifications for sleep duration were done with episodes truncated to start and end in the respective periods. Only data from female flies are shown34. In all cases where we assayed male flies, we observed qualitatively similar results on sleep latency.
For experiments involving CBZ, a stock solution (20 mg ml-1) was solubilized in 45% (2-hydroxypropyl)-β-cyclodextrin (Sigma) and mixed into the standard agar medium. Experiments were carried out by recording 5-6 baseline d on standard medium, and then switching to CBZ/sucrose/agar 6 h before the onset of the dark period.
Electrophysiological recording
The wild-type Drosophila Rdl clone (GH019619) was obtained from the Drosophila Genome Collection 1 (DGC1). This clone lacks TM4 and has more stable desensitization kinetics (J.C.C. and J.A., unpublished results). Oocyte collection, injection and electrophysiological recordings were carried out as described35,36. Two electrode voltage-clamp experiments were performed 1-5 d post-injection. An oocyte chamber of ∼200 μl was perfused with a flow rate of ∼10-15 ml min-1 and recording were made at a holding potential of -60 mV in Barth’s solution (2 mM KCl, 5 mM HEPES, pH 7.6, 1 mM MgCl2, 1.8 mM CaCl2, 100 mM NaCl). CBZ was dissolved in 45% (2-hydroxypropyl)-β-cyclodextrin (Sigma). Because cyclodextrin forms a complex with these drugs37,38, the effective concentration of these agents is expected to be much lower that what is indicated in the experiments. We used GABA concentrations that were close to the saturated region of the dose-response curve because we observed that CBZ effects were essentially independent of GABA and that desensitization is more prominent in this range.
Statistical Analysis
Data were analyzed as described in the figure legends using JMP software version 5.0.1.2 for the PC and Macintosh (SAS Institute).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to E. Marder, R. Allada and R. Greenspan for comments on the manuscript. This work was funded by a grant from the US Army (W81XWH-04-1-0158) to M.R. and L.C.G., and MH 067284 to L.C.G.
References
- 1.Roth T. Prevalence, associated risks, and treatment patterns of insomnia. J. Clin. Psychiatry. 2005;66(Suppl 9):10–13. quiz 42-43. [PubMed] [Google Scholar]
- 2.Rosenberg RP. Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies. Ann. Clin. Psychiatry. 2006;18:49–56. doi: 10.1080/10401230500464711. [DOI] [PubMed] [Google Scholar]
- 3.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225–233. [PMC free article] [PubMed] [Google Scholar]
- 4.Smith GB, Olsen RW. Functional domains of GABAA receptors. Trends Pharmacol. Sci. 1995;16:162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- 5.Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol. Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- 6.Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111:231–239. doi: 10.1016/s0306-4522(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 7.Lancel M. Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. 1999;22:33–42. doi: 10.1093/sleep/22.1.33. [DOI] [PubMed] [Google Scholar]
- 8.Greenspan RJ, Dierick HA. ‘Am not I a fly like thee?’ From genes in fruit flies to behavior in humans. Hum. Mol. Genet. 2004;13(Spec No 2):R267–273. doi: 10.1093/hmg/ddh248. [DOI] [PubMed] [Google Scholar]
- 9.Hendricks JC, Sehgal A, Pack AI. The need for a simple animal model to understand sleep. Prog. Neurobiol. 2000;61:339–351. doi: 10.1016/s0301-0082(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 10.Nitz DA, van Swinderen B, Tononi G, Greenspan RJ. Electrophysiological correlates of rest and activity in Drosophila melanogaster. Curr. Biol. 2002;12:1934–1940. doi: 10.1016/s0960-9822(02)01300-3. [DOI] [PubMed] [Google Scholar]
- 11.van Swinderen B, Nitz DA, Greenspan RJ. Uncoupling of brain activity from movement defines arousal states in Drosophila. Curr. Biol. 2004;14:81–87. [PubMed] [Google Scholar]
- 12.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 13.Hendricks JC, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 14.Bushey D, Huber R, Tononi G, Cirelli C. Drosophila Hyperkinetic mutants have reduced sleep and impaired memory. J. Neurosci. 2007;27:5384–5393. doi: 10.1523/JNEUROSCI.0108-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirelli C, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 16.Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 17.Pitman JL, McGill JJ, Keegan KP, Allada R. A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature. 2006;441:753–756. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- 18.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Waking experience affects sleep need in Drosophila. Science. 2006;313:1775–1781. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 19.Stilwell GE, Rocheleau T, Ffrench-Constant RH. GABA receptor minigene rescues insecticide resistance phenotypes in Drosophila. J. Mol. Biol. 1995;253:223–227. doi: 10.1006/jmbi.1995.0547. [DOI] [PubMed] [Google Scholar]
- 20.Ffrench-Constant RH, Rocheleau TA, Steichen JC, Chalmers AE. A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature. 1993;363:449–451. doi: 10.1038/363449a0. [DOI] [PubMed] [Google Scholar]
- 21.Lee D, Su H, O’Dowd DK. GABA receptors containing Rdl subunits mediate fast inhibitory synaptic transmission in Drosophila neurons. J. Neurosci. 2003;23:4625–4634. doi: 10.1523/JNEUROSCI.23-11-04625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aronstein K, Auld V, Ffrench-Constant R. Distribution of two GABA receptor-like subunits in the Drosophila CNS. Invert. Neurosci. 1996;2:115–120. doi: 10.1007/BF02214114. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HG, Ffrench-Constant RH, Jackson MB. A unique amino acid of the Drosophila GABA receptor with influence on drug sensitivity by two mechanisms. J. Physiol. (Lond.) 1994;479:65–75. doi: 10.1113/jphysiol.1994.sp020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brand AH, Dormand EL. The GAL4 system as a tool for unraveling the mysteries of the Drosophila nervous system. Curr. Opin. Neurobiol. 1995;5:572–578. doi: 10.1016/0959-4388(95)80061-1. [DOI] [PubMed] [Google Scholar]
- 25.Hodge JJ, Choi JC, O’Kane CJ, Griffith LC. Shaw potassium channel genes in Drosophila. J. Neurobiol. 2005;63:235–254. doi: 10.1002/neu.20126. [DOI] [PubMed] [Google Scholar]
- 26.Ffrench-Constant RH, Mortlock DP, Shaffer CD, MacIntyre RJ, Roush RT. Molecular cloning and transformation of cyclodiene resistance in Drosophila: an invertebrate gamma-aminobutyric acid subtype A receptor locus. Proc. Natl. Acad. Sci. USA. 1991;88:7209–7213. doi: 10.1073/pnas.88.16.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambrosio AF, Soares-Da-Silva P, Carvalho CM, Carvalho AP. Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2-093, and BIA 2-024. Neurochem. Res. 2002;27:121–130. doi: 10.1023/a:1014814924965. [DOI] [PubMed] [Google Scholar]
- 28.Granger P, et al. Modulation of the gamma-aminobutyric acid type A receptor by the antiepileptic drugs carbamazepine and phenytoin. Mol. Pharmacol. 1995;47:1189–1196. [PubMed] [Google Scholar]
- 29.Spina E, Perugi G. Antiepileptic drugs: indications other than epilepsy. Epileptic Disord. 2004;6:57–75. [PubMed] [Google Scholar]
- 30.Bazil CW. Effects of antiepileptic drugs on sleep structure: are all drugs equal? CNS Drugs. 2003;17:719–728. doi: 10.2165/00023210-200317100-00003. [DOI] [PubMed] [Google Scholar]
- 31.Buhr A, et al. Functional characterization of the new human GABA(A) receptor mutation beta3(R192H) Hum. Genet. 2002;111:154–160. doi: 10.1007/s00439-002-0766-7. [DOI] [PubMed] [Google Scholar]
- 32.Greenleaf AL, Borsett LM, Jiamachello PF, Coulter DE. α-amanitin-resistant D. melanogaster with an altered RNA polymerase II. Cell. 1979;18:613–622. doi: 10.1016/0092-8674(79)90116-8. [DOI] [PubMed] [Google Scholar]
- 33.Aronstein K, Ode P, Ffrench-Constant R. Direct comparison of PCR-based monitoring for cyclodiene resistance in Drosophila populations with insecticide bioassay. Pestic. Biochem. Physiol. 1994;48:229–233. [Google Scholar]
- 34.Andretic R, Shaw PJ. Essentials of sleep recordings in Drosophila: moving beyond sleep time. Methods Enzymol. 2005;393:759–772. doi: 10.1016/S0076-6879(05)93040-1. [DOI] [PubMed] [Google Scholar]
- 35.Boileau AJ, Baur R, Sharkey LM, Sigel E, Czajkowski C. The relative amount of cRNA coding for gamma2 subunits affects stimulation by benzodiazepines in GABA(A) receptors expressed in Xenopus oocytes. Neuropharmacology. 2002;43:695–700. doi: 10.1016/s0028-3908(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 36.Priestley CM, Williamson EM, Wafford KA, Sattelle DB. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABA(A) receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br. J. Pharmacol. 2003;140:1363–1372. doi: 10.1038/sj.bjp.0705542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brewster ME, Anderson WR, Estes KS, Bodor N. Development of aqueous parenteral formulations for carbamazepine through the use of modified cyclodextrins. J. Pharm. Sci. 1991;80:380–383. doi: 10.1002/jps.2600800420. [DOI] [PubMed] [Google Scholar]
- 38.Masson M, Sigurdardottir BV, Matthiasson K, Loftsson T. Investigation of drug-cyclodextrin complexes by a phase-distribution method: some theoretical and practical considerations. Chem. Pharm. Bull. (Tokyo) 2005;53:958–964. doi: 10.1248/cpb.53.958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.