Abstract
The optic tectum is central for transforming incoming visual input into orienting behavior. Yet it is not well understood how this behavior is organized early in development and how it relates to the response properties of the developing visual system. We designed a novel behavioral assay to study the development of visually guided behavior in Xenopus laevis tadpoles. We found that, during early development, visual avoidance—an innate, tectally mediated behavior—is tuned to a specific stimulus size and is sensitive to changes in contrast. Using in vivo recordings we found that developmental changes in the spatial tuning of visual avoidance are mirrored by changes in tectal receptive field sharpness and the temporal properties of subthreshold visual responses, whereas contrast sensitivity is affected by the gain of the visual response. We also show that long- and short-term perturbations of visual response properties predictably alter behavioral output. We conclude that our assay for visual avoidance is a useful functional measure of the developmental state of the tectal circuitry. We use this assay to show that the developing visual system is tuned to facilitate behavioral output and that the system can be modulated by neural activity, allowing it to adapt to environmental changes it encounters during development.
INTRODUCTION
One of the central goals of contemporary neurobiology is to understand how behavioral output arises from the response properties of individual neurons within a neural circuit. The Xenopus tadpole visual system has been used as a model to understand the anatomical and electrophysiological development of a neural circuit (Cline 1991; Ruthazer and Cline 2004) and to elucidate the role of neural activity in shaping the response properties of this circuit (Aizenman et al. 2003; Engert et al. 2002; Tao and Poo 2005). However, relatively little is known either about the functional significance of these developmental findings or about the role they might play in shaping visually guided behavior during development.
In Xenopus, the optic tectum is the primary area involved in visual processing and it receives a topographically organized projection from the retina. This projection requires visual experience to refine into its adult form and this refinement is thought to occur via N-methyl-d-aspartate receptor (NMDAR) –mediated synaptic plasticity (Cline and Constantine-Paton 1989; Cline et al. 1990). Local circuitry within the tectum is also refined by visual experience (Pratt et al. 2008). Refinement of these local second-order projections may be important for facilitating the integration of multiple sensory modalities and ultimately shaping visually guided orienting behavior (Grusser and Grusser-Cornhels 1976; Nakagawa et al. 1997). The developing Xenopus visual system shows great flexibility, in that its constituent neurons can adapt multiple cellular properties in response to changes in visual input. For example, tectal cells will adjust their own intrinsic excitability by modulating voltage-gated Na+ currents as a result of long- and short-term changes in synaptic drive (Aizenman et al. 2003; Pratt and Aizenman 2007) and the growth rate of tectal neuron dendritic arbors is sensitive to changes in input level (Haas et al. 2006; Sin et al. 2002). At the circuit level, tectal neurons can alter their direction selectivity and spatial location of their receptive fields (RFs) in response to patterned visual input (Engert et al. 2002; Mu and Poo 2006; Vislay-Meltzer et al. 2006). Patterned input from the retina can also sculpt the temporal response properties of the intratectal circuitry (Pratt et al. 2008). By probing how the development of different tectal response properties mediates the emergence of visually guided behavior, we can begin to understand some of the functional significance of these changes.
Frogs and other amphibians exhibit visually guided behaviors that are specifically tuned to the characteristics of the visual stimulus. Adult frogs will orient toward small objects within their visual field, which they identify as prey, and will perform an avoidance response when presented with looming or approaching objects (Ewert 1997). The tuning of these behaviors to specific characteristics of the visual stimuli has been correlated to specific response properties of retinal ganglion cells (RGCs). For example, movement-sensitive RGCs have been described in the toad retina that respond to similar visual stimuli to those that elicit prey-catching or avoidance behavior (Ewert and Hock 1972). However, the response properties of RGCs cannot fully account for the neural coding of behaviorally relevant stimuli and it has been shown that the response properties of neurons downstream in the visual pathway, such as in the optic tectum, are also closely correlated with visually guided behavior (Grusser and Grusser-Cornhels 1976; Ingle 1976). It is not known how these behaviors emerge in the tadpole and how they relate to the emerging response properties of its developing visual system. We will focus on the early development of the Xenopus tadpole, during developmental stages (st) 44–49, when the visual system is known to undergo dramatic anatomical and physiological changes (Akerman and Cline 2006; Cline 2001; Pratt and Aizenman 2007; Tao and Poo 2005). During these developmental stages, tadpoles filter feed and do not catch prey (Hoff et al. 1999) and therefore do not orient toward small objects, but do show an avoidance response. Thus we will use avoidance behavior to design a behavioral test to probe visual function in tadpoles and compare developmental changes in behavior to developmental changes in tectal neuron response properties. We will then experimentally alter specific response properties and test whether we can observe predictable changes in behavioral output.
METHODS
Electrophysiology
All animal experiments were done in accordance to IACUC standards. Wild-type Xenopus laevis tadpoles were raised on a 12:12-h light:dark cycle at 21°C in 10% Steinberg's solution. Developmental stages of tadpoles were determined according to Nieuwkoop and Faber (1956). Under our rearing conditions, tadpoles generally reach st 44–46 at 9–12 days postfertilization (dpf) and st 48–49 at 18–20 dpf. Animals were prepared for in vivo recording by transferring MS-222 (0.02%) anesthetized tadpoles to a recording chamber with HEPES buffer extracellular media (containing in mM: 115 NaCl, 2 KCl, 3 CaCl2, 3 MgCl2, 5 HEPES, 10 mM glucose; pH 7.2, osmolarity 255 mOsm), in the presence of 0.1 mM tubocurarine, and stabilized with dissecting pins (after Aizenman et al. 2003). The tectal lobes were exposed by removing the overlying skin. Cells were visualized using a ×60 water-immersion objective. Recordings were limited to the central third of the tectum to reduce any potential variability introduced by the developmental gradient along the rostrocaudal axis. Whole cell voltage-clamp recordings as well as cell-attached recordings were made at room temperature (20–22°C) using glass micropipettes (8–12 MΩ) filled with intracellular saline (containing in mM: 100 K-gluconate, 8 KCl, 5 NaCl, 1.5 MgCl, 20 HEPES, 10 EGTA, 2 ATP, 0.3 GTP; pH 7.2, osmolarity 255 mOsm). Signals were measured with a Multiclamp 700B amplifier and digitized using a Digidata 1440A A-D board (Axon Instruments). Traces were recorded using pClamp 10 software, digitized at 10 kHz, and analyzed off-line. MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d]cyclohepten-5,10-imine maleate] was obtained from Tocris; all other chemicals were from Sigma.
Whole-field visual stimulation and receptive field mapping and analysis
To record visually evoked spiking, whole-field flashes of light were presented via a fiber-optic cable (Edmund Scientific) attached to a green light-emitting diode (LED; λ-max 567 nm, Allied Electronics, Fort Worth, TX). Light at this wavelength is known to evoke robust visual responses (Aizenman et al. 2003). The fiber-optic cable was positioned directly in front of the contralateral eye to stimulate the entire monocular visual field. Visual stimuli were triggered by computer and stimulus intensity was controlled by varying the voltage across the LED. Responses were recorded to 1-s on stimuli, presented every 10–20 s and over multiple trials. Visually evoked action potentials were counted over the 1-s stimulus period. To map receptive fields (RFs), we used a custom-built blue-green LED array consisting of a 3 × 4 grid (based on Xu et al. 2008). Each LED element had a 50-μm diameter with a center-to-center distance of 100 μm. The array was coupled to a 30,000-pixel, multicore image fiber (FIGH-30-650 S from Fujikura, Tokyo, Japan) with a 600-μm image-area diameter. This allowed us to faithfully project the image of the array to the contralateral eye. The projecting end of the optical fiber was placed near the eye such that the center-to-center spacing between the LEDs in the array was about 33–38° of arc. RFs were mapped by illuminating individual LEDs for 1 s during whole cell recording. Stimuli were presented at 6-s intervals and every LED was illuminated at least three times during the duration of the procedure. Whole cell currents resulting from the on response were averaged for every spatial location and the integrated synaptic charge was calculated over a period of 1 s.
Results were plotted as image maps, where the brightest color corresponds to the spatial location with the largest response. We defined the largest response as the peak of the RF. Overall peak response magnitudes did not vary significantly between stages (st 44–46: 94 ± 26 nA·s vs. st 48–49: 114 ± 24 nA·s). To calculate sharpness, we averaged the responses of all the spatial locations immediately adjacent to the peak, including diagonally, and averaged the responses two spatial locations away from the peak. This provided the average rate of decay per distance away from peak. This means that for every receptive field we have a minimum of three data points for the immediately adjacent locations and a minimum of five data points for locations two spaces away from the peak. We saw no correlation between RF sharpness and location of the RF peak, suggesting that this is a useful metric to assess the spatial decay of tectal RFs regardless of location. Values were normalized to the peak value and then fit with an exponential decay using the least-squares method. Curves obtained from this method were compared using an F-test. To calculate temporal decay, we used peak responses and integrated the charge over 200-ms bins for the first 600 ms. The decay was best fit to a line and the slopes were compared using an F-test. To calculate the spike latency range of visual responses we pooled all the spike latencies from a given cell in response to a visual stimulus of a given luminance. We calculated the range of evoked spikes, eliminating the first and last 10% of the spikes to prevent outlying spikes from skewing the distribution. Statistics and curve fitting were done using Prism 5 from GraphPad Software.
In vivo manipulations
For in vivo visual stimulation of freely swimming tadpoles, tadpoles were placed in 5-ml wells with rearing media inside a custom-made chamber with green LEDs along the ceiling of the chamber (after Aizenman et al. 2003). Individual rows of LEDs were flashed in sequence for 1-s intervals, to simulate a moving bar stimulus. Tadpoles were stimulated for 4 h and immediately prepared for electrophysiological recording or for behavioral testing. For MK-801 treatments, tadpoles were reared in solution containing MK-801 (25 μM) starting from st 46 for 8–10 days, until they reached st 49. The drug-containing media was replaced every 3–4 days.
Behavioral testing
Tadpoles were tested in a clear-bottomed rectangular tank (16 × 10 cm) filled to an approximate depth of 1.5 cm with rearing media at room temperature (20–22°C). Tadpoles were maintained in a 12:12-h light:dark cycle in which lights were turned on at 6 am. Experiments were performed between 1 and 4 pm. The sides and top of the tank were darkened. The tank sat atop a CRT monitor screen (maximum luminance 57 cd/m2, minimum luminance 0.3 cd/m2; Dell Ultrascan 1600 SH Series). The behavioral test sequence for the avoidance response was as follows: 1) A group of tadpoles was placed in the center of the tank over a black screen and the tank was covered for 30 s. 2) After this period the stimulus was presented for 30 s such that the stimulus pattern in one half of the tank was drifting, whereas it remained stationary on the other side. The direction of drift was orthogonal to the length of the tank (see Fig. 1). 3) At the end of the stimulus period the number of tadpoles on the stationary side was noted. Different stimulus conditions were presented in random order to avoid accommodation. The stimulus consisted of randomly placed dots of varying diameter and luminance (see results). Both white dots on a black background and black dots on a white background were tested. Testing with stationary stimuli on both sides of the tank resulted in random distribution of tadpoles on either side. The radius of the visual stimuli varied from 4 to 0.5 mm, whereas the size of Xenopus tadpole body varied between about 2 and 4 mm at these developmental stages. Thus the relative size of the visual stimuli ranges from smaller to bigger than the tadpoles. To test for the optomotor response (OMR), a similar procedure was followed except that alternating black and white bars drifted along the length of the tank and the number of tadpoles on the side toward which the bars drifted was counted. The stimulus patterns were generated using a custom-written MATLAB (The MathWorks) script using the Psychophysics Toolbox extensions (Brainard 1997).
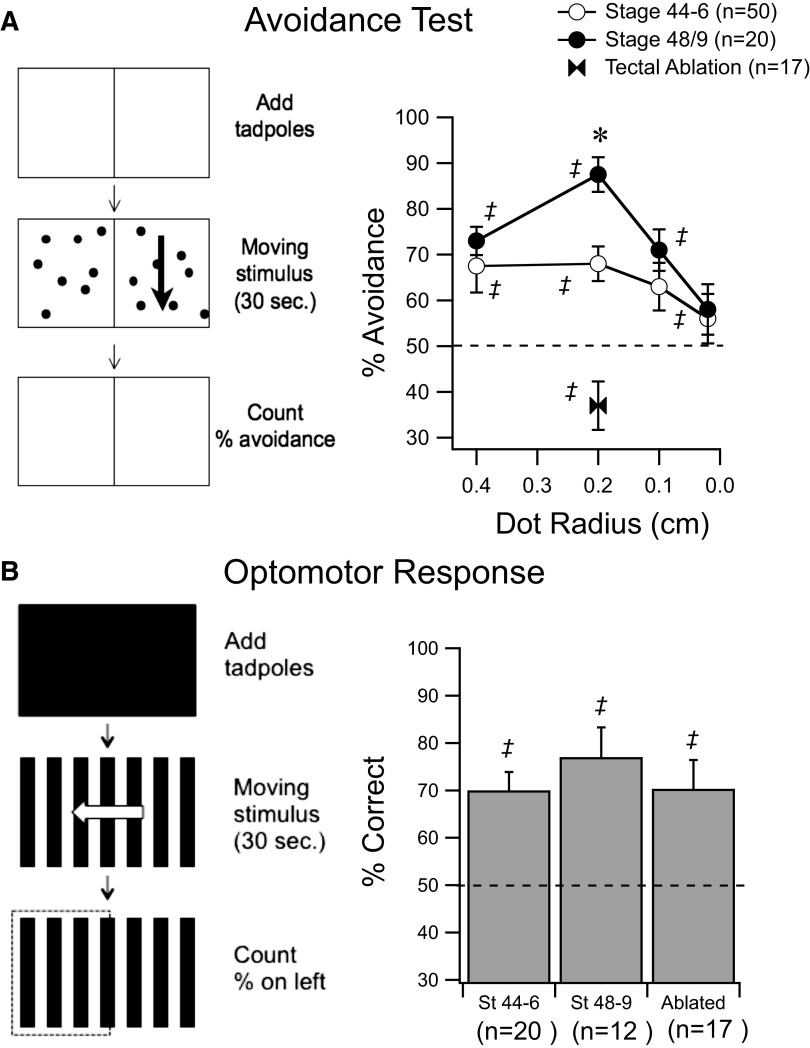
FIG. 1.
Behavioral tests for visual function in Xenopus tadpoles. A, left diagram: the protocol for testing avoidance behavior. Tadpoles are placed in the center of a tank atop a blank screen for 30 s. The stimulus is presented and drifts on one side of the tank for 30 s. The number of tadpoles that end up in the stationary side is counted to calculate the percentage avoidance. Graph on right shows behavioral responses of stage (st) 48–49 tadpoles (dark circles) and st 44–46 tadpoles (open circles) in response to dots of different diameters. Dashed line represents random performance. Bow ties show performance of tectotomized tadpoles. B, left diagram: the protocol for testing the optomotor response (OMR), a task that does not require an intact tectum. Tadpoles are placed in the center of a tank atop a blank screen for 30 s. A drifting grating is presented for 30 s. The number of tadpoles that end up in the side corresponding to the direction of the drift is counted to calculate the percentage correct. Data from st 44–46, st 48–49, and tectotomized tadpoles show that the OMR remains consistent. ‡Represents performance that is significantly different (P < 0.05) from random. *Represents significantly different performance between developmental stages (P < 0.05). Error bars are SE.
To remove the tectum, a fine-gauge syringe needle was used to aspirate the optic tecta bilaterally (Ruthazer et al. 2003). Alternatively, tecta were severed from surrounding areas with the edge of a fine-gauge syringe needle but left in place. With either method, behavioral results were identical. Tadpoles were allowed to recover for several hours or overnight before testing.
Tadpoles were tested in groups of four and any given tadpole was tested only once for each stimulus condition. The one exception was in tectotomized tadpoles, where tadpoles were tested twice due to a lower number of available animals. Each group of four tadpoles was counted as one trial and the ratio of tadpoles on the stationary versus moving side was calculated for each trial, providing a single value for each trial. The percentage avoidance is the average percentage of tadpoles across trials that end up in the stationary side. To test for significant behavior for a given stimulus condition, the ratios of tadpoles on the stationary side versus the moving side were compared across trials and a Wilcoxon signed-rank test (a nonparametric equivalent of a t-test) was used to test whether performance was different from random. This design allowed us to test for significance for individual groups under individual experimental conditions and better reflected the trial-to-trial variability. To test performance across experimental groups we used a Mann–Whitney test to compare the performance ratios. All error values are SE. Behavior was equally robust when white dots were used on a black background than when black dots were used on a white background, although all the data reported come from tests using white dots.
RESULTS
Behavioral tests to assay the development of visual function in tadpoles
Like adult frogs, Xenopus tadpoles show an innate tendency to avoid large moving objects within their visual field. Based on this behavior, we designed a behavioral test in which tadpoles were placed on a clear-bottomed tank atop a CRT computer monitor. A stimulus pattern consisting of random dots was presented such that in half of the tank the pattern was moving, whereas in the other half it was stationary. Within 30 s, most tadpoles reliably swam to the stationary side (Fig. 1A and methods). By changing characteristics of the pattern (e.g., contrast, size, etc.) it is possible to determine to what extent the tadpoles perceive the stimulus, allowing us to systematically measure different aspects of visual function.
Relationship between in vivo response properties of developing tectal neurons and visually guided behavior
Stage 49 tadpoles were presented with a background image consisting of randomly placed moving dots (2-mm dot radius, 1.4 dots/cm2, drifting at 2 cm/s; see methods) and, within 30 s, significantly more tadpoles were on the stationary side (Fig. 1A, 88 ± 3.8% correct, n = 20 trials, P < 0.0001). To determine whether this behavior was tuned to a specific stimulus size, we varied the diameter of the dots. Performance on the task predictably decreased as the diameter of the dots was reduced until performance was not significantly above chance (Fig. 1A). Presenting the tadpoles with larger dots (4 mm) also resulted in decreased performance, indicating a preference for a specific stimulus size (Fig. 1A). Next, we surgically ablated the optic tectum to test whether performance of this avoidance task required an intact optic tectum (see methods). Tectotomized tadpoles did not perform the avoidance task (Fig. 1A, 37 ± 5.3% correct, n = 17 trials) and, in fact, showed a significant bias toward the moving side (P = 0.021), perhaps unmasking an underlying phototactic behavior (Copp and McKenzie 1984). To rule out that tectal ablation did not nonspecifically alter all visual processing and all visually guided behavior, we tested tectotomized tadpoles for an OMR, a visual task that is known not to require an intact optic tectum (Roeser and Baier 2003). To test the OMR, tadpoles were presented with a background of moving alternating black and white bars (0.2 cycle/cm drifting at 2 cm/s; see methods). Both embryonic zebrafish and tadpoles will swim in the direction of the moving bars (Pronych et al. 1996; Roeser and Baier 2003). The majority of control tadpoles performed this task correctly (Fig. 1B, 77 ± 6.3%, n = 12 trials, P = 0.004). Tectotomized tadpoles also performed significantly above chance in this task (70.3 ± 6.1%, n = 17 trials, P = 0.008) and their performance was not significantly different from that of controls (P = 0.58). This suggests that visual avoidance is a good measure of a visually guided behavior which requires tectal processing. This does not rule out the possibility that this behavior is also supported by processing in other visual nuclei or the retina.
We then tested whether performance of these visually guided behaviors changes over development. Between developmental st 44–46 and st 48–49 the response properties of tectal neurons change significantly (Pratt and Aizenman 2007) and tectal RF size is known to decrease (Tao and Poo 2005). If visual avoidance was correlated with RF properties, we would predict that behavioral responses in younger tadpoles, with larger tectal RFs, would be more broadly tuned than those in older tadpoles. St 44–46 tadpoles performed significantly above chance in the avoidance test (Fig. 1A, 68 ± 3.8%, n = 50 trials, P = 0.0001). However, under optimal stimulus conditions (2-mm spots, full contrast), they performed significantly worse than did st 48–49 tadpoles (P = 0.006, MW). Interestingly, st 44–46 tadpoles performed similarly in response to 4- and 2-mm spots and also performed at levels similar to those of st 48–49 tadpoles in response to larger spots. Since behavioral performance in younger tadpoles does not appear to vary much in response to stimuli of different sizes, this suggests that in younger tadpoles, visual avoidance is more broadly tuned to stimulus size. This observation is consistent with the larger tectal RFs observed in younger tadpoles (Tao and Poo 2005). St 44–46 tadpoles performed comparably to st 48–49 tadpoles in the OMR (Fig. 1B, 70 ± 3.9%, n = 20), indicating that at these earlier stages tadpoles can still see, orient themselves, and swim normally. This observation is consistent with the hypothesis that the differences in performance seen during development are due to differences in tectal function.
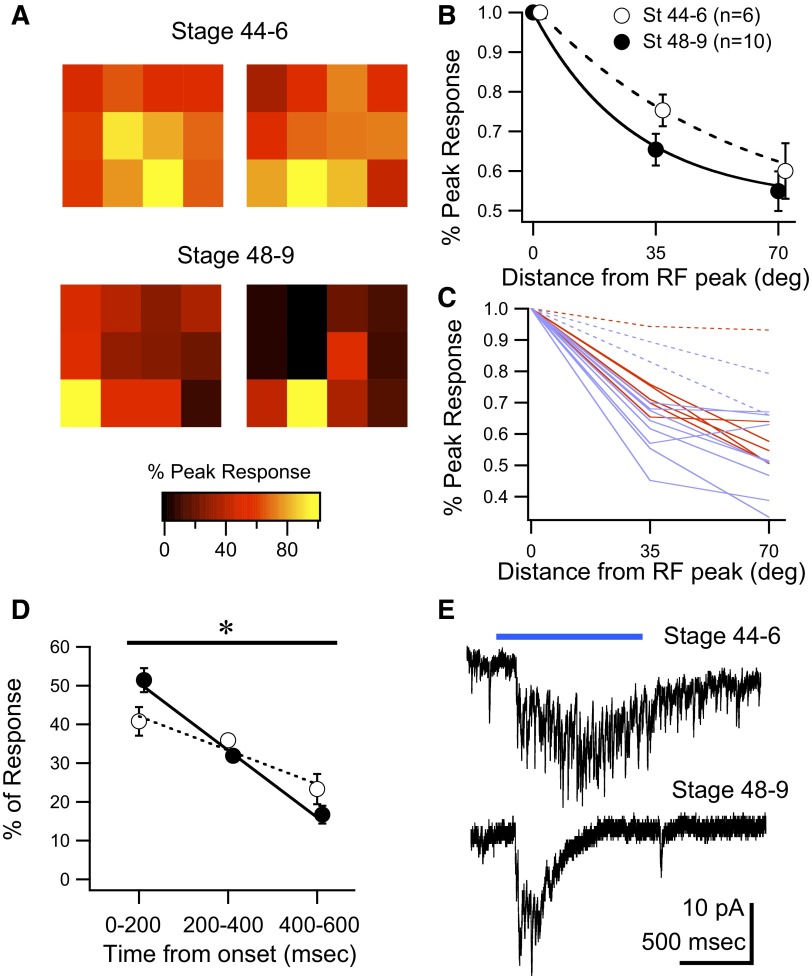
To test the response properties of tectal cells directly, we performed whole cell recordings in vivo while presenting patterned visual stimuli to the eye. The stimuli were generated by a 4 × 3 micro-LED array and delivered to the eye via a fiber-optic bundle (Xu et al. 2008; see methods). We generated RF maps for st 44–46 and st 48–49 tectal neurons (Fig. 2A) and analyzed the “sharpness” of the RF as well as the temporal profiles of the visual responses. RF sharpness was calculated by measuring the average decay in response size (measured as total integrated synaptic charge) as a function of distance from the RF peak. Consistent with prior observations (Tao and Poo 2005) we found that st 48–49 RFs tended to be sharper than those in st 44–46 tadpoles (Fig. 2A). At one LED element away from the RF peak (∼35° of arc), the average response size in st 44–46 tadpoles had decreased to 75 ± 4% of peak (n = 6), whereas by st 48–49, the responses decreased to 65 ± 4% (n = 10; Fig. 2B). However, in contrast to prior studies (Tao and Poo 2005), in our hands this difference was not statistically significant. We attribute this difference to the large variability in RF sharpness observed within groups. At both stages, we found a subset of cells (∼20%) with broad RFs, with a <15% decrease in amplitude over a 35° distance. The proportion of these broad RF neurons was similar in younger and older animals (st 44–46: 1/6; st 48–49: 2/10). This variability is illustrated in Fig. 2C in which individual RFs are shown. If these cells are analyzed separately, then the differences in sharpness between the remaining cells with normal RF structure across developmental groups becomes significant (P = 0.036). Nevertheless, since it remains unclear whether these broad RF neurons constitute a separate cell type—and the detailed description of their response properties remains beyond the scope of this study—we did not eliminate them from any of the figures. Finally, although our method allows us to generate an estimate of the spatial extent of the RFs, a stimulus spacing of 35° does not allow us to generate high-resolution RF maps, which may account for some of the observed differences between our results and those of previously published studies.
FIG. 2.
Development of tectal cell visual response properties. A: representative examples of receptive field (RF) maps from 2 individual tectal cells from st 44–46 and st 48–49 tadpoles obtained using a 4 × 3 LED array and whole cell voltage-clamp recordings (see methods). Maps show that the drop-off in response size, relative to the peak response, decays faster in the more mature animals. The center-to-center distance between squares is roughly 35° of arc. B: average decrease in response size at 35 and 70° of arc away from the RF peak for both developmental groups (see methods). RFs from the older group tend to decay at a faster rate than those from the younger group. C: decays of all RFs in the data set illustrate a great degree of variability. RFs from st 44–46 tadpoles are red, whereas those from st 48–49 tadpoles are in blue. Dotted lines indicate “wide” receptive fields, which are found in roughly 20% of the cells. D: averaged temporal decay of visual responses show that responses in st 48–49 tadpoles decay significantly faster than responses from st 44–46 tadpoles. Decay was calculated by integrating the response over 200-ms time bins (see methods). E: representative whole cell visual responses to illustrate the development of temporal properties. Responses are individual traces taken from the peak of the RF. Horizontal bar represents visual stimulus. Data points in B and C have been offset slightly in the x-axis to facilitate data visualization. *Represents P < 0.05 and error bars are SE.
The temporal profiles of the visual responses also changed over development. To calculate the temporal profiles of the responses, we integrated the synaptic charge in three 200-ms bins and expressed these as a percentage of total charge over the total 600-ms analysis window. St 44–46 tadpoles showed a fairly flat temporal profile in which there was little decay in response size over the first two bins (Fig. 2, D and E; n = 6). On the other hand, st 48–49 had a significantly faster decay of the visual response (Fig. 2, D and E; n = 10). The integrated charge values were fit using linear regression across the different bins to calculate the decay of the response and the slopes of the lines were compared between groups. We found that the decay rate was significantly faster in the older group (P = 0.003, F-test). This is consistent with prior observations that had shown that the temporal properties of tectal neuron responses evoked by direct optic nerve stimulation are known to become more temporally coherent and less variable over development as a result of refinement of local intratectal connections and a consequent reduction in recurrent tectal activity (Pratt et al. 2008).
Tectal neurons are known to alter their intrinsic excitability in response to changes in global synaptic input (Aizenman et al. 2003; Pratt and Aizenman 2007). Between st 44–46 and st 48–49, as retinal input becomes stronger, voltage-gated-Na+ currents decrease in tectal neurons, resulting in reduced spiking during depolarization. As a result, the input–output function of tectal neurons remains stable, maximizing the dynamic range of neuronal responses. From this we would predict that st 44–46 and st 48–49 tectal cells would fire a similar amount of spikes in response to a whole-field visual stimulus. Here we use the number of spikes evoked by a visual stimulus to define the “gain” of the visual response. Using cell-attached recordings, we determined that there is no significant difference in the gain and onset latency of the spiking response to visual stimuli between groups (st 44–46: response gain = 2.9 ± 0.5 spikes, onset = 137 ± 7 ms; st 48–49: response gain = 4.2 ± 0.6 spikes, onset = 150 ± 14 ms; P = 0.19 for gain and P = 0.75 for onset). A further prediction would be that, if the gain of the response is stable over development, then a behavior that might be dependent on response gain, such as contrast sensitivity, is also expected to remain stable.
To test contrast sensitivity, we presented tadpoles with moving dots (0.2 cm) over a range of luminance levels. Luminance of the dots was decreased to 25, 2.5, and 1.25% of maximum and, as the dots became dimmer, performance on the avoidance task decreased to random levels (Fig. 3C). St 44–46 tadpoles reached random performance at 2.5% maximum luminance (53.8 ± 4.5% correct, n = 22), whereas st 48–49 tadpoles reached random levels at 1.25% maximum luminance (57 ± 4% correct, n = 26). Since initial performance at full luminance was already decreased in the younger tadpoles (see Fig. 1), we used two different strategies to compare contrast sensitivity more accurately between both groups. To compare contrast sensitivity directly between groups, we tested st 48–49 tadpoles using smaller-diameter (0.1-cm) dots because their behavioral output under these conditions was experimentally determined to be similar to that of the st 44–46 tadpoles with the larger (0.2-cm) dots. At maximum luminance st 44–45 tadpoles (with 0.2-cm dots) and st 48–49 tadpoles (with 0.1-cm dots) performed equally on the task (Fig. 3D; st 44–45: 71 ± 4.8%, n = 22; st 48–49: 71 ± 4.5%, n = 20). Under these conditions both groups reached random performance at 2.5% maximum luminance and the performance curves are not significantly different (Fig. 3D). As a further comparison, we normalized the data from Fig. 3C to account for the initial differences in performance. We expressed behavioral performance as a percentage of the performance using maximum luminance for each group. This allows us to quantitate the relative decrease in performance as contrast is reduced. Using this measure, performance of both groups was nearly identical (Fig. 3E). Thus we conclude that contrast sensitivity remains stable during this period in development.
FIG. 3.
Relationship between the gain of the visual response and contrast sensitivity. A: representative cell-attached spiking responses from tectal neurons from both developmental groups. For each group, 3 individual traces are superimposed. Arrow indicates the onset of the visual stimulus. Responses are evoked by whole-field visual stimuli (see methods). B: summary of response gain, as measured by the number of evoked spikes, and onset latency of the first spike show no significant change over development. C: behavioral responses of st 48–49 tadpoles (dark circles) and st 44–46 tadpoles (open circles) in response to dots (0.2 cm) of decreasing luminance. D: since immature tadpoles show decreased performance at even the highest luminance levels, behavioral performance of st 44–46 tadpoles was compared with performance of st 48–49 tadpoles using smaller dots (0.1 cm, triangles) such that initial performance at the highest luminance was matched. E: normalized performance for different luminance values, relative to performance at maximum luminance, from data in C. Gray bars are st 44–46 tadpoles and black bars are st 48–49. ‡Represents performance that is significantly different (P < 0.05) from random. Error bars are SE.
Modulation of visual response properties and behavior by activity
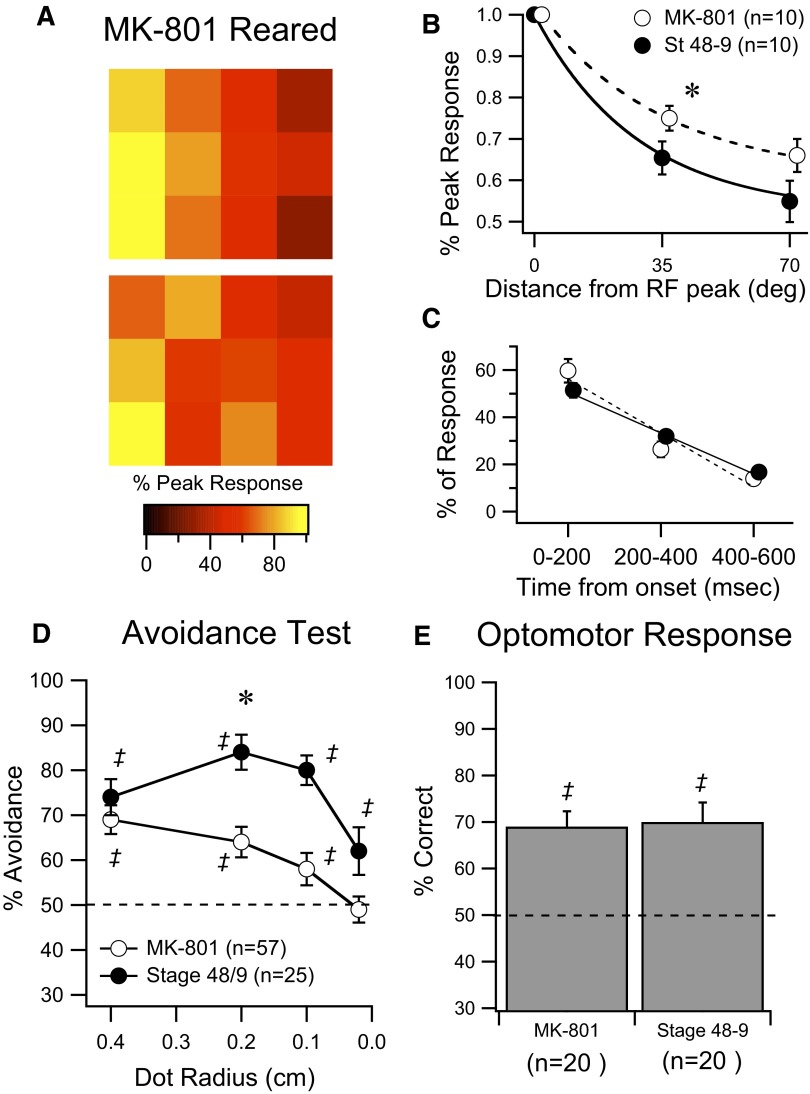
From these observations, we would predict that experimental manipulations that alter the sharpness of the RF or the temporal profile of the visual responses would also alter performance on the visual avoidance test. Manipulations that alter the gain of the visual response—that is, alter spike output—are expected to affect contrast sensitivity. To test the first prediction we reared tadpoles from st 46 to st 49 (8–10 days) in the presence of NMDAR blocker MK-801 (25 μM). MK-801 has been shown to prevent stabilization of RGC axons and therefore prevent refinement of the retinotectal map (Cline and Constantine-Paton 1989; Ruthazer et al. 2003). Due to a very permeable blood–brain barrier, drugs introduced into the rearing media are known to permeate into the tadpole brain (Rajan and Cline 1998). To ensure that the MK-801 remained active it was replaced every 3–4 days. In our hands, MK-801–reared tadpoles had expanded RFs relative to controls (Fig. 4, A and B; P = 0.02, F-test), suggesting that the drug was active during this time. In contrast, temporal properties of visual responses of drug-reared animals were comparable to those of controls (Fig. 4C). Visual avoidance behavior in MK-801–reared tadpoles was predictably more broadly tuned than that in matched clutchmates, consistent with expanded RFs (Fig. 4D). Since MK-801 was bath applied, we cannot rule out the possibility that MK-801 was having more widespread effects by blocking NMDAR in other areas of the brain such as the retina. However, drug-reared tadpoles had a normal OMR that was comparable to that of controls (Fig. 4E) and would avoid the largest spots at the same level as that in controls (Fig. 3D), suggesting that drug rearing does not affect—in a nonspecific manner—their ability to swim, orient their bodies, or perceive visual stimuli.
FIG. 4.
Developmental blockade of N- methyl-d-aspartate receptor (NMDAR) causes tectal RF expansion and perturbs spatial tuning of avoidance behavior. A: representative examples of RF maps from 2 individual tectal cells from st 48–49 tadpoles reared in NMDAR blocker MK-801 since st 46. B: average decrease in response size at 35 and 70° of arc away from the RF peak for MK-801–reared (open circles) and control st 48–49 animals (dark circles). RFs from the drug-treated group are significantly expanded, compared with controls. C: averaged temporal decay of visual responses show that drug rearing does not affect development of temporal response properties. D: summary of behavioral responses of MK-801–reared tadpoles and untreated clutchmates. Dots of decreasing size were tested as in Fig. 1 and drug-treated animals show significant deficits in performance. E: averaged results from the OMR show that this nontectal-dependent behavior is not affected by MK-801 rearing. Data points in B and C have been offset slightly in the x-axis to facilitate data visualization. ‡Represents performance that is significantly different (P < 0.05) from random. *Represents significance between groups (P < 0.05). Error bars are SE.
As a second test, we performed an experimental manipulation that would alter both the temporal response properties and gain of tectal cells. Prior studies showed that enhanced visual stimulation over a period of 4–5 h results in a persistent increase in tectal neuron intrinsic excitability and increased spiking in response to visual stimuli, without fatigue of visual responses (Aizenman et al. 2003). We tested the effects of this manipulation on visually guided behavior, in an attempt to link these experience-dependent cellular changes to behavior. Freely swimming st 48–49 tadpoles were presented with patterned visual stimulation for a period of 4 h (see methods) and subsequently tested behaviorally. Visually stimulated tadpoles exhibited a marked decrease in visual avoidance (2-mm spots, full contrast) compared with that in controls (Fig. 5A, 66 ± 2%; n = 144; P < 0.0007 vs. controls), although the OMR remained normal (Fig. 5B; 82 ± 3.6%; n = 20). Visual avoidance of larger (4-mm) spots also remained comparable to that of controls. Tectal RF sharpness of visually stimulated tadpoles was comparable to that of controls (Fig. 5, C and D), although we did observe a change in the temporal profile of visual responses after visual stimulation. Visually stimulated tadpoles had a significantly slower decay of their visual responses compared with that of controls (Fig. 5, E and F; n = 7, P = 0.02, F-test) and were more similar to st 44–46 tadpoles in that respect. The change in temporal profile was likely due to increased recurrent activity within the tectum resulting from enhanced intrinsic excitability of tectal cells (Aizenman 2003). Taken together, these results suggest that both the RF sharpness and the temporal profile of visual responses are important for modulating the spatial tuning of the visual avoidance response.
FIG. 5.
Prolonged exposure to patterned visual stimulation alters temporal properties of visual responses and perturbs spatial tuning of avoidance behavior. A: summary of performance on the avoidance test of control tadpoles (dark circles) and tadpoles that underwent 4 h of patterned visual stimulation (see methods). This manipulation is known to increase excitability of tectal neurons (Aizenman et al. 2003). Visually stimulated tadpoles show impaired avoidance behavior and impaired sensitivity to dot size. B: visual stimulation does not affect the OMR. C: representative RF maps from 2 visually stimulated tadpoles. D: summary of the effects of visual stimulation on RF sharpness. RF sharpness is not significantly altered by visual stimulation. E: summary of the effects of visual stimulation on the temporal properties of visual responses. Visual stimulation significantly slows the decay of visual responses. F: sample traces showing visual responses in control and visually stimulated tadpoles illustrate the effect of visual stimulation on the temporal properties of visual responses. Horizontal bars represent the visual stimulus. Data points in A have been offset slightly in the x-axis to facilitate data visualization. ‡Represents performance that is significantly different (P < 0.05) from random. *Represents significance between groups (P < 0.05). Error bars are SE.
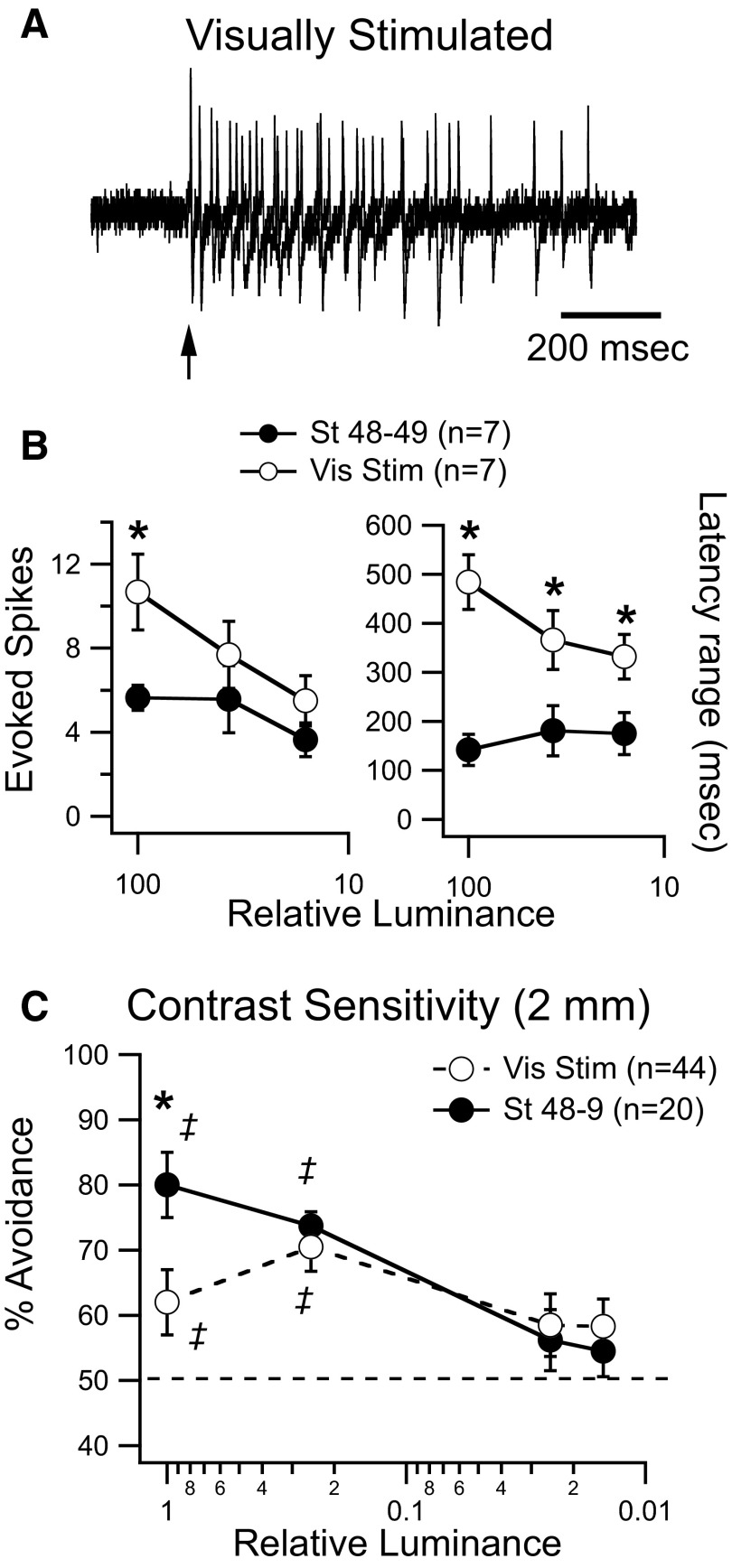
We next tested the relationship between response gain and contrast sensitivity in visually stimulated tadpoles. Visually stimulated tadpoles generated severalfold more spikes in response to a maximal-luminance visual stimulus than controls (Fig. 6, A and B; vis stim: 10.7 ± 1.8 spikes, n = 7; controls: 5.6 ± 0.6 spikes, n = 7; P = 0.017), but showed no difference in the onset latency of the response (not shown). We also measured the duration of the spiking response by calculating the range of spike latencies evoked by visual stimulation over several trials. Visually stimulated tadpoles also had longer-lasting visual spiking responses (Fig. 6, A and B; vis stim: 484 ± 56 s, n = 7; controls: 142 ± 32 s, n = 7; P = 0.0012). This is consistent with the observed alterations in the temporal profile of visually evoked synaptic responses (Fig. 5, E and F) and with the observation that the temporal profile of the spiking response is an accurate reflection of the temporal profile of the underlying subthreshold responses (Pratt et al. 2008). At dimmer luminance levels, both the number of evoked spikes and the duration of the spiking response from both groups became more nearly similar to each other, although the visually stimulated tadpoles still had longer-lasting responses (Fig. 6B). Only a small range of luminances was tested for this experiment and it is difficult to directly compare the effect of changes in luminance here and in the behavioral assay because the nature of the stimulus is fundamentally different. In one case we are using a fiber-optic bundle to carry an LED-generated whole-field visual stimulus to the eye, whereas in the other, a freely moving tadpole approaches a moving spot on the horizontal plane. Nonetheless, this result suggests that the alterations in gain and temporal profile, induced by 4 h of visual stimulation, are significantly more pronounced at maximum luminance levels and that visual responses become more nearly similar to each other with dimmer visual stimuli. Based on these observations, we would predict that at maximum luminance, avoidance behavior would be significantly worse in visually stimulated tadpoles than that in controls, but the differences in performance between groups would be reduced at lower luminance levels. At maximum luminance, visually stimulated tadpoles performed markedly worse than controls on the avoidance task, as described previously (Fig. 5). However at dimmer luminance levels, performance improved and was comparable to that of control values, (Fig. 6C, vis stim: 70.5 ± 3.8% (n = 44), controls: 73.7 ± 2.2% (n = 20) at 25% luminance), indicating a preference for dimmer contrast over maximum contrast. One interpretation is that at dimmer contrast levels, visual inputs recruit less recurrent activity, facilitating performance on the visual avoidance task (see discussion). This experiment further suggests a correlation between the gain of the visual response and contrast sensitivity, although this relationship may not be entirely straightforward since alterations in gain will also affect the temporal profile of visual responses. Ultimately, testing the gain of visual response to moving stimuli of varying contrasts, similar to those encountered during behavior, will allow us to better understand the relationship between response gain and contrast sensitivity.
FIG. 6.
Prolonged exposure to patterned visual stimulation alters the gain of visual responses and improves detection of dimmer contrast. A: representative cell-attached spiking responses from tectal neurons of visually stimulated tadpoles at maximal luminance. Three superimposed traces are shown. B: response gain and response duration measured at different luminance levels in control and visually stimulated tadpoles. At maximum luminance, both gain and duration are altered after 4 h of visual stimulation. Notice that both parameters become more nearly similar between groups at lower luminance levels. C: behavioral responses of stage control (dark circles) and visually stimulated tadpoles (open circles) in response to dots (0.2 cm) of decreasing luminance. Notice the preference for dimmer luminance of the visually stimulated group. ‡ Represents performance that is significantly different (P < 0.05) from random. Error bars are SE.
DISCUSSION
To summarize, these experiments show that the innate visual avoidance response of Xenopus tadpoles can be used to assess the developmental state of the retinotectal system and correlates with the functional properties of tectal neuron responses. This is the first quantitative test that measures tectum-dependent visual behavior in tadpoles. We show that the spatial tuning of visual avoidance improves over development and this correlates with changes in tectal RF sharpness and the temporal profile of visual responses. We further show that relative contrast sensitivity remains stable over development and correlates with the stability of the gain of visual responses. Experimental modification of tectal responses results in predictable changes in behavior. MK-801–induced blockade of NMDARs over development decreases RF sharpness and disrupts the spatial tuning of visual avoidance. Four hours of in vivo visual stimulation results in altered temporal response properties, also correlating with decreased tuning. Visual stimulation also increases response gain, improving the relative sensitivity to dimmer contrast. Taken together our data suggest that specific cellular response properties can be linked to specific behavioral sensitivities and that these can be modulated by activity. They also suggest that these neuronal properties are dynamically adjusted over development to fine-tune visual function in the face of massive changes in visual circuit architecture and levels of sensory input that occur during development.
Origin of behaviorally relevant visual response properties
We describe three tectal cell response properties that appear to be behaviorally relevant: 1) receptive field sharpness, 2) temporal profile of the visual response, and 3) response magnitude or gain. The first two appear to be correlated with spatial tuning of visual avoidance behavior, whereas the third is correlated with contrast sensitivity. Each of these response properties can be associated with known changes in tectal circuitry or properties of tectal neurons. Developmental changes in RF sharpness are caused by anatomical refinement of the terminal arbors of retinotectal axons and reflect the input pattern of RGCs onto tectal neurons. Refinement of these inputs is known to be activity dependent and to require activation of NMDARs (Cline et al. 1990). RF size may also reflect the lateral spread of activity among tectal neurons via intratectal circuits. The temporal response profile is mediated, at least in part, by activation of local recurrent circuitry within the tectum and reflects the level of network activity that ultimately results in output to premotor areas in the hindbrain. Between st 45 and st 49 local excitatory intratectal circuits are refined in an activity-dependent manner, such that tectal neuron responses become more temporally coherent (Pratt et al. 2008). In addition, intrinsic excitability of tectal cells also peaks during st 44–46 (Pratt and Aizenman 2007), likely increasing the amount of recurrent intratectal activity recruited during these stages, and maturation of inhibitory circuits may also alter the temporal profile of the tectal responses (Akerman and Cline 2006; Tao and Poo 2005). Finally, response gain is determined by both the strength of retinotectal synapses and the intrinsic excitability of tectal neurons. Tectal neurons are known to decrease their intrinsic excitability by down-regulating voltage-gated Na+ currents in response to developmental increases in retinotectal transmission (Pratt and Aizenman 2007; Pratt et al. 2008). Experimental decreases in synaptic transmission result in increased Na+ currents and increased excitability. This reciprocal regulation of synaptic and intrinsic properties results in a stable input–output relationship and explains why the gain of the visual responses does not change over development. Our data suggest that the input pattern from the retina to the tectum, the intrinsic excitability of tectal neurons, and the resulting recurrent network activity in the tectum all interact to optimize behavioral responses to visual stimuli.
Modulation of visual responses and behavior
The experimental manipulations carried out alter tectal cell response properties in different ways and have predictable effects on behavior. Rearing tadpoles in MK-801 is known to impair normal development of RGC axon terminal arbors (Ruthazer et al. 2003). This would result in an improperly refined retinotectal map and enlarged tectal cell RFs (Fig. 4), thus impairing spatial tuning of tectal neuron responses, consistent with our observations. Although behavioral responses appear more broadly tuned during the early developmental stages, there is still an overall decrease in performance relative to that of the older tadpoles at medium and small spot sizes. One possibility is that, due to weaker and more widely dispersed retinotectal inputs in immature animals (Pratt and Aizenman 2007), the spots simply do not evoke sufficiently large responses to trigger an avoidance behavior as often as in the older animals. This view is supported by developmental studies of RFs in the mouse superior colliculus—the mammalian homologue of the optic tectum. One of these studies indicates that the summed response size over the extent of the RF remains constant during development, a process that has been termed “response homeostasis” (Chandrasekaran et al. 2007). Thus early in development, RFs are large and individual retinal inputs are more widely dispersed, whereas later in development RFs are smaller, but individual inputs are more focused. Based on this model, if a spot entered the visual field of a tectal cell with enlarged RFs, it would cause a much weaker response than that of the same-sized dot entering the visual field of a tectal cell with a small RF and would therefore be less likely to trigger a behavioral response. In more mature animals, the largest spots may evoke some form of surround inhibition, resulting in decreased tectal cell activation and decreased behavioral performance. Together, these data suggest that avoidance behavior is sensitive to tectal RF size and can be indicative of the maturational state of the retinotectal inputs.
How do the temporal profile and gain of visual responses influence behavior? Both st 44–46 tadpoles and visually stimulated st 48–49 tadpoles have flatter temporal response profiles than those of control st 48–49 tadpoles and both show poorer performance in the visual avoidance test. One explanation for this is that increased cellular excitability and increased network activity found in younger or visually stimulated tadpoles (Aizenman et al. 2003; Pratt and Aizenman 2007) result in lateral spread of excitation, creating a larger apparent RF that extends beyond the area directly activated by retinal afferents, therefore disrupting spatial tuning of visual avoidance. This is supported by the observation that RFs appear broader if measured at the tail end of the visual response, where activity comes mostly from intratectal connections (not shown). A second explanation is that the temporal profile of visual responses is specifically tuned to optimally recruit downstream motor circuitry responsible for generating the avoidance behavior. Altering the timing of this response may impair activation of these downstream circuits, which are only now beginning to be well described in similar preparations (Fetcho et al. 2008; Orger et al. 2008). The gain of the response appears to be important for mediating contrast sensitivity. Visual stimulation increases the intrinsic excitability of tectal neurons, increasing the gain of the visual response and decreasing the signal-to-noise ratio (Aizenman et al. 2003). This would allow the tadpole to distinguish relatively small changes in contrast in a visually noisy environment by selectively suppressing behavioral responses to the highest contrasts. Thus relative sensitivity for dimmer contrast is improved after visual stimulation, even at the expense of spatial tuning. It also shows that the largest visual responses do not necessarily result in the most efficient behavioral output.
It is important to emphasize that these observations linking visual response properties to behavior are purely correlative. For example, although RF size and temporal response properties can track with the tuning of avoidance behavior, they cannot be the sole determinants of this tuning since alterations in either one of these properties will disrupt the behavior. Likewise, changes in response gain can result in changes in the temporal response properties, suggesting that the relationship between these neuronal properties and behavior is likely to be more complex. Furthermore, visual response properties were obtained from single neurons with static visual stimuli, whereas behavior is likely to result from the activity of large networks of neurons in response to dynamic visual stimuli. Although ours is a useful first step toward probing the nature of visual responses over development and correlating them to behavior, further studies of visual responses to more complex behaviorally relevant visual stimuli will provide further insight into the nature of these correlations. Furthermore, our results do not rule out the possibility that maturation in other visual nuclei or the retina may also play an important part in the maturation of behavioral responses.
Visually guided behavior in frogs and fish
Using visually guided behavior to assess visual function has been a fruitful approach to understand visual system development and to screen for abnormalities in visual processing. For example, the visual water task and a virtual optomotor test have been successfully used to study the development of cortical and subcortical vision in mice (Prusky and Douglas 2003; Prusky et al. 2004). Similar tests have been developed to measure the grating acuity of cats (Hall and Mitchell 1991). In zebrafish, a large-scale optomotor test was used to analyze visual behavior in visual mutants (Muto et al. 2005). To the best of our knowledge, ours is the first behavioral test to probe tectally mediated, visually guided behavior in Xenopus tadpoles. The OMR has been used successfully in Xenopus tadpoles to measure circadian modulation and spectral sensitivity of retinal responses (Cronly-Dillon and Muntz 1965; Solessio et al. 2004) and vestibular and visual integration after spaceflight (Pronych et al. 1996). However, as in zebrafish embryos (Roeser and Baier 2003), ablation of the optic tectum does not eliminate the OMR, suggesting it is not tectally dependent. Fish are known to orient toward small moving objects, such as prey, and avoid large objects; this behavior is known to require intact tectal function (Gahtan et al. 2005). Thus prey-catching behavior has been successfully used to measure development of tectal circuits in embryonic zebrafish (Smear et al. 2007). These studies have shown that mutant blumenkohl zebrafish embryos, which have enlarged tectal RFs, also have deficits in their ability to capture small prey, a behavior that requires an intact tectum. This has been interpreted as a deficit in visual acuity caused by enlarged tectal RFs. However, visual avoidance and prey capture have been shown to be mechanistically distinct behaviors in adult anurans (Ingle 1976). Furthermore, a similar test is not possible in Xenopus because Xenopus tadpoles are filter feeders and do not catch prey (Hoff et al. 1999). Therefore we believe that avoidance behavior is ideally suited for testing tectal development in this species. The fact that the OMR remains unchanged during the various experimental interventions, including tectotomy, suggests that changes observed in the avoidance behavior are likely to reflect changes in tectal function and not changes in overall visual function or swimming ability. In fish, avoidance of approaching objects is mediated by feedback cholinergic input to the tectum from the nucleus isthmus (Gallagher and Northmore 2006). Although it is unclear how much influence the nucleus isthmus has on tectal function during the early developmental stages studied here, it will be intriguing to test how this pathway modulates avoidance behavior at later developmental stages.
One interesting question arising from a behavioral perspective is: What advantage is conveyed to the tadpole by having avoidance behavior tuned to best respond to 2-mm-size objects? If the avoidance response is a mechanism important for avoiding predators, perhaps having a more broadly tuned avoidance behavior might be more beneficial. However, there might be a trade-off in that if approaching objects are too large, the tectum might not be able to efficiently extract information regarding their size, position, and velocity, resulting in degraded output to premotor areas. Therefore the tectum might be optimized for mediating avoidance behavior to smaller objects, whereas other visually guided behavior, such as the OMR, may be involved in avoiding larger objects such as predators. The neuroethological implications of these different visually guided behaviors still remain to be elucidated.
Significance of the correlations between tectal cell properties and behavior
One important open question in this study is whether the cells recorded from are the cells directly mediating the behavior. From ablation experiments, we know that the tectum is required for visual avoidance and that the various manipulations that affect avoidance behavior do not affect downstream motor areas since the OMR is equally robust among all experimental groups. However, we cannot determine from our current study whether populations of tectal neurons we record from are directly the ones responsible for visual avoidance. This is complicated by the fact that, although the adult tectum is a layered structure containing multiple cell types with various response characteristics, during the stages studied here the tectum has not yet differentiated into its various cell classes and layers (Aizenman et al. 2003; Lazar 1973). Nonetheless, the response characteristics measured in this study are a reflection of the activity of the whole tectal network. The majority of the synaptic input to tectal neurons has been suggested to originate from within the tectum (Pratt et al. 2008) and visual responses result from activation of both retinal and local tectal inputs. Thus RF size is likely a reflection of the degree of convergence not only of retinal input but also that of convergence from cells in other parts of the tectum. Likewise, the temporal response pattern mostly reflects recurrent activity within the tectum (Pratt et al. 2008). The gain of tectal cells is determined by the intrinsic properties of individual tectal cells, but even this is determined by the amount of background synaptic activity the tectal cell receives (Pratt and Aizenman 2007). This suggests that the response properties of individual neurons are determined by their interactions with other tectal neurons and are thus a good readout of the overall state of the entire tectal network. This supports our view that understanding how these responses change over development is important for understanding avoidance behavior, even if we cannot conclusively identify the tectal cells directly responsible for triggering behavior.
Conclusions
These experiments use a novel behavioral assay to show that during early development visual avoidance becomes increasingly tuned to the spatial characteristics of the visual stimulus. This occurs during a period in development in which major refinement of the retinotectal projection and local tectal circuitry occurs. This assay allows us to correlate behavioral output with observed changes in visual function. Spatial tuning is sensitive to developmental changes in RF size and the temporal pattern of recurrent activity, whereas contrast sensitivity is sensitive to the gain of tectal responses. These results are the first step toward providing an important and long sought-after functional framework in which to place a wide range of developmental studies of the retinotectal system in tadpoles. Furthermore, our experiments show that the Xenopus tadpole visual system is remarkably dynamic. By altering specific cellular properties, both in a short timescale and over development, the visual system is able to adapt to long- and short-term changes in neural activity and circuit architecture. This would help optimize its response properties to facilitate visually guided behavior. Similar principles have been described in mammalian primary visual areas, where RF properties are known to be rapidly modulated. For example, the stimulus selectivity of visual cortical neurons can be modulated and shaped by their intrinsic cellular properties (Priebe and Ferster 2008). This suggests that activity-dependent modulation of response properties may be a general principle by which visual circuits can remain sufficiently flexible to accommodate the large amount of change that occurs during development, yet retain the ability to function robustly throughout this time period and to adapt to changes in the visual environment.
GRANTS
This work was supported by funds from the National Science Foundation and the Klingenstein Foundation. W. Dong was supported by a Fox Postdoctoral Fellowship, Brown University. R. H. Lee received funding from the Brown University Undergraduate Teaching and Research Award program and the Rhode Island Space Grant from the National Aeronautics and Space Administration. K. G. Pratt was supported by a National Research Service Award from the National Eye Institute.
Acknowledgments
We thank A. Nestor for help in generating the MATLAB scripts, I. Sears for technical support, and R. Burwell, D. Sheinberg, and members of the Aizenman lab for helpful discussions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Aizenman et al. 2003.Aizenman CD, Akerman CJ, Jensen KR, Cline HT. Visually driven regulation of intrinsic neuronal excitability improves stimulus detection in vivo. Neuron 39: 831–842, 2003. [DOI] [PubMed] [Google Scholar]
- Akerman and Cline 2006.Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J Neurosci 26: 5117–5130, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran et al. 2007.Chandrasekaran AR, Shah RD, Crair MC. Developmental homeostasis of mouse retinocollicular synapses. J Neurosci 27: 1746–1755, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline 1991.Cline HT Activity-dependent plasticity in the visual systems of frogs and fish. Trends Neurosci 14: 104–111, 1991. [DOI] [PubMed] [Google Scholar]
- Cline 2001.Cline HT Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol 11: 118–126, 2001. [DOI] [PubMed] [Google Scholar]
- Cline and Constantine-Paton 1989.Cline HT, Constantine-Paton M. NMDA receptor antagonists disrupt the retinotectal topographic map. Neuron 3: 413–426, 1989. [DOI] [PubMed] [Google Scholar]
- Cline et al. 1990.Cline HT, Debski EA, Constantine-Paton M. The role of the NMDA receptor in the development of the frog visual system. Adv Exp Med Biol 268: 197–203, 1990. [DOI] [PubMed] [Google Scholar]
- Copp and McKenzie 1984.Copp NH, McKenzie T. Effects of light-deprivation on development of photopositive behavior in Xenopus laevis tadpoles. J Exp Zool 230: 219–228, 1984. [DOI] [PubMed] [Google Scholar]
- Cronly-Dillon and Muntz 1965.Cronly-Dillon JR, Muntz WR. The spectral sensitivity of the goldfish and the clawed toad tadpole under photopic conditions. J Exp Biol 42: 481–493, 1965. [DOI] [PubMed] [Google Scholar]
- Engert et al. 2002.Engert F, Tao HW, Zhang LI, Poo MM. Moving visual stimuli rapidly induce direction sensitivity of developing tectal neurons. Nature 419: 470–475, 2002. [DOI] [PubMed] [Google Scholar]
- Ewert 1997.Ewert JP Neural correlates of key stimulus and releasing mechanism: a case study and two concepts. Trends Neurosci 20: 332–339, 1997. [DOI] [PubMed] [Google Scholar]
- Ewert and Hock 1972.Ewert JP, Hock F. Movement-sensitive neurones in the toad's retina. Exp Brain Res 16: 41–59, 1972. [DOI] [PubMed] [Google Scholar]
- Fetcho et al. 2008.Fetcho JR, Higashijima S, McLean DL. Zebrafish and motor control over the last decade. Brain Res Rev 57: 86–93, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahtan et al. 2005.Gahtan E, Tanger P, Baier H. Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum. J Neurosci 25: 9294–-9303, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher and Northmore 2006.Gallagher SP, Northmore DP. Responses of the teleostean nucleus isthmi to looming objects and other moving stimuli. Vis Neurosci 23: 209–219, 2006. [DOI] [PubMed] [Google Scholar]
- Grusser and Grusser-Cornhels 1976.Grusser O, Grusser-Cornhels U. Neurophysiology of the anuran visual system. In: Frog Neurobiology: A Handbook, edited by Precht W, Llinás R. New York: Springer-Verlag, 1976, p. 297–385.
- Haas et al. 2006.Haas K, Li J, Cline HT. AMPA receptors regulate experience-dependent dendritic arbor growth in vivo. Proc Natl Acad Sci USA 103: 12127–12131, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall and Mitchell 1991.Hall SE, Mitchell DE. Grating acuity of cats measured with detection and discrimination tasks. Behav Brain Res 44: 1–9, 1991. [DOI] [PubMed] [Google Scholar]
- Hoff et al. 1999.Hoff K, Blaustein A, McDiarmid R, Altig R. Behavior: interactions and their consequences. In: Tadpoles: The Biology of Anuran Larvae, edited by McDiarmid R, Altig R. Chicago, IL: Univ. of Chicago Press, 1999, p. 215–239.
- Ingle 1976.Ingle D Behavioral correlates of central visual function in anurans. In: Frog Neurobiology: A Handbook, edited by Precht W, Llinás R. New York: Springer-Verlag, 1976, p. 435–451.
- Lazar 1973.Lazar G The development of the optic tectum in Xenopus laevis: a Golgi study. J Anat 116: 347–355, 1973. [PMC free article] [PubMed] [Google Scholar]
- Mu and Poo 2006.Mu Y, Poo MM. Spike timing-dependent LTP/LTD mediates visual experience-dependent plasticity in a developing retinotectal system. Neuron 50: 115–125, 2006. [DOI] [PubMed] [Google Scholar]
- Muto et al. 2005.Muto A, Orger MB, Wehman AM, Smear MC, Kay JN, Page-McCaw PS, Gahtan E, Xiao T, Nevin LM, Gosse NJ, Staub W, Finger-Baier K, Baier H. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet 1: e66, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa et al. 1997.Nakagawa H, Miyazaki H, Matsumoto N. Principal neuronal organization in the frog optic tectum revealed by a current source density analysis. Vis Neurosci 14: 263–275, 1997. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop and Faber 1956.Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin). New York: Garland Publishing, 1956.
- Orger et al. 2008.Orger MB, Kampff AR, Severi KE, Bollmann JH, Engert F. Control of visually guided behavior by distinct populations of spinal projection neurons. Nat Neurosci 11: 327–333, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt and Aizenman 2007.Pratt KG, Aizenman CD. Homeostatic regulation of intrinsic excitability and synaptic transmission in a developing visual circuit. J Neurosci 27: 8268–8277, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt et al. 2008.Pratt KG, Dong W, Aizenman CD. Development and spike timing-dependent plasticity of recurrent excitation in the Xenopus optic tectum. Nat Neurosci 11: 467–475, 2008. [DOI] [PubMed] [Google Scholar]
- Priebe and Ferster 2008.Priebe NJ, Ferster D. Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron 57: 482–497, 2008. [DOI] [PubMed] [Google Scholar]
- Pronych et al. 1996.Pronych SP, Souza KA, Neff AW, Wassersug RJ. Optomotor behaviour in Xenopus laevis tadpoles as a measure of the effect of gravity on visual and vestibular neural integration. J Exp Biol 199: 2689–2701, 1996. [DOI] [PubMed] [Google Scholar]
- Prusky et al. 2004.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci 45: 4611–4616, 2004. [DOI] [PubMed] [Google Scholar]
- Prusky and Douglas 2003.Prusky GT, Douglas RM. Developmental plasticity of mouse visual acuity. Eur J Neurosci 17: 167–172, 2003. [DOI] [PubMed] [Google Scholar]
- Rajan and Cline 1998.Rajan I, Cline HT. Glutamate receptor activity is required for normal development of tectal cell dendrites in vivo. J Neurosci 18: 7836–7846, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeser and Baier 2003.Roeser T, Baier H. Visuomotor behaviors in larval zebrafish after GFP-guided laser ablation of the optic tectum. J Neurosci 23: 3726–3734, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthazer et al. 2003.Ruthazer ES, Akerman CJ, Cline HT. Control of axon branch dynamics by correlated activity in vivo. Science 301: 66–70, 2003. [DOI] [PubMed] [Google Scholar]
- Ruthazer and Cline 2004.Ruthazer ES, Cline HT. Insights into activity-dependent map formation from the retinotectal system: a middle-of-the-brain perspective. J Neurobiol 59: 134–146, 2004. [DOI] [PubMed] [Google Scholar]
- Sin et al. 2002.Sin WC, Haas K, Ruthazer ES, Cline HT. Dendrite growth increased by visual activity requires NMDA receptor and Rho GTPases. Nature 419: 475–480, 2002. [DOI] [PubMed] [Google Scholar]
- Smear et al. 2007.Smear MC, Tao HW, Staub W, Orger MB, Gosse NJ, Liu Y, Takahashi K, Poo MM, Baier H. Vesicular glutamate transport at a central synapse limits the acuity of visual perception in zebrafish. Neuron 53: 65–77, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solessio et al. 2004.Solessio E, Scheraga D, Engbretson GA, Knox BE, Barlow RB. Circadian modulation of temporal properties of the rod pathway in larval Xenopus. J Neurophysiol 92: 2672–2684, 2004. [DOI] [PubMed] [Google Scholar]
- Tao and Poo 2005.Tao HW, Poo MM. Activity-dependent matching of excitatory and inhibitory inputs during refinement of visual receptive fields. Neuron 45: 829–836, 2005. [DOI] [PubMed] [Google Scholar]
- Vislay-Meltzer et al. 2006.Vislay-Meltzer RL, Kampff AR, Engert F. Spatiotemporal specificity of neuronal activity directs the modification of receptive fields in the developing retinotectal system. Neuron 50: 101–114, 2006. [DOI] [PubMed] [Google Scholar]
- Xu et al. 2008.Xu H, Davitt KM, Dong W, Song YK, Patterson WR, Aizenman CD, Nurmikko AV. Combining multicore imaging fiber with matrix addressable blue/green LED arrays for spatiotemporal photonic excitation at cellular level. IEEE J Select Topics Quantum Electronics 14: 167–170, 2008. [Google Scholar]