Abstract
The presence of lipid domains in cellular membranes and their characteristic features are still an issue of dividing discussion. Several recent studies implicate lipid domains in plasma membranes of mammalian cells as short lived and in the submicron range. Measuring the fluorescence lifetime of appropriate lipid analogues is a proper approach to detect domains with such properties. Here, the sensitivity of the fluorescence lifetime of1-palmitoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]-hexanoyl]-sn-glycero-3-phospholipid (C6-NBD-phospholipid) analogues has been employed to characterize lipid domains in giant unilamellar vesicles (GUVs) and the plasma membrane of mammalian cells by fluorescence lifetime imaging (FLIM). Fluorescence decay of C6-NBD-phosphatidylcholine is characterized by a short and long lifetime. For GUVs forming microscopically visible lipid domains the longer lifetime in the liquid disordered (ld) and the liquid ordered (lo) phase was clearly distinct, being ∼7 ns and 11 ns, respectively. Lifetimes were not sensitive to variation of cholesterol concentration of domain-forming GUVs indicating that the lipid composition and physical properties of those lipid domains are well defined entities. Even the existence of submicroscopic domains can be detected by FLIM as demonstrated for GUVs of palmitoyloleoyl phosphatidylcholine/N-palmitoyl-d-sphingomyelin/cholesterol mixtures. A broad distribution of the long lifetime was found for C6-NBD-phosphatidylcholine inserted in the plasma membrane of HepG2 and HeLa cells centered around 11 ns. FLIM studies on lipid domains forming giant vesicles derived from the plasma membrane of HeLa cells may suggest that a variety of submicroscopic lipid domains exists in the plasma membrane of intact cells.
More than three decades ago model studies in binary phospholipid mixtures have already shown that differences of acyl chain lengths can trigger lateral heterogeneities (1–3). Not much later heterogeneities caused by cholesterol have been described for phospholipid membranes (4) and biological membranes (5, 6–8). Later on, the cholesterol-dependent inhomogeneous lateral organization of biological membranes has lead to the concept of so-called “rafts” (9). Rafts resemble domains that are enriched in phospholipids and/or (glyco)sphingolipids with long saturated acyl and alkyl chains. They are segregated from non-raft domains, which are mainly formed by phospholipids with unsaturated acyl chains. Initially, rafts were defined on the basis of their resistance to extraction by Triton X-100 at low temperature (4 °C) (9, 10). However, it has been shown that detergent-resistant fractions correspond to aggregates of raft domains and hence may not resemble the native state of rafts in the plasma membrane of mammalian cells. In particular, such biochemical approaches may even trigger domain formation (11, 12). Being aware of those drawbacks and associated artifacts, other methods have been introduced to visualize rafts. Apart from planar lipid mono- and bilayers, giant unilamellar vesicles (GUVs)2 have become an attractive tool to study lipid domains. GUVs of appropriate lipid mixtures have been shown to form domains with a size in the micrometer scale (13–15). These domains can be visualized by various lipid-like fluorophores, which preferentially enrich either in the ld or in the lo domain (16, 17). Recently, Baumgart et al. (18) have shown that giant vesicles obtained by blebbing of plasma membranes may also form large visible lipid domains allowing studies mimicking the native situation better.
Typically, for cells such large domains have not been observed suggesting that rafts may be organized at the submicroscopic level. Indeed, several attempts to image raft domains in biological membranes suggested that rafts are very small and highly dynamic (19, 20). To unravel the lateral heterogeneity of cellular membranes, in particular of mammalian plasma membranes, at this scale various techniques of fluorescence spectroscopy and microscopy have been applied, among them Förster resonance energy transfer, fluorescence correlation spectroscopy, and fluorescence anisotropy. Based on homo-Förster resonance energy transfer and fluorescence anisotropy measurements glycosylphosphatidylinositol-anchored proteins have been suggested to localize in small clusters with about three to four copies (21–24). Mathematical modeling of these experimental data is consistent with a domain diameter of ∼5 nm, which would contain ∼20 lipid molecules (24, 25).
Very recently, the first efforts have been made to take advantage of the sensitivity of the fluorescence lifetime to local properties of lipid bilayers for studying lipid domains (26–28). The lifetime of the membrane staining fluorophore 1-[2-Hydroxy-3-(N,N-di-methyl-N-hydroxyethyl)ammoniopropyl]-4-[β-[2-(di-n-butylamino)-6-napthyl]vinyl]pyridinium dibromide (di-4-ANEPPDHQ) originally developed for probing membrane voltage was lower in the ld phase than in the lo phase (26). Using headgroup-labeled Rhodamine dioleoylphosphatidylethanolamine (N-Rho-DOPE) de Almeida et al. (27) could distinguish three lipid phases, gel, lo, and ld, in GUVs by fluorescence lifetime imaging microscopy (FLIM). Margineanu et al. (28) have successfully applied a peryleneimide probe to visualize the lo and the ld phase in GUVs by fluorescence intensity and FLIM. They could also show that lifetime was sensitive to cholesterol depletion in the plasma membrane of Jurkat cells. Upon activation of cells, formation of microscopic visible rafts in the plasma membrane with characteristic lifetimes was reported. Although properties of fluorophores such as peryleneimide are valuable for applied methods, for example high quantum yield and photo stability, it would be of particular interest to employ fluorescent phospholipids for biological membranes. Although fluorescent moieties would still interfere with lipid packing in membranes, the use of fluorescent lipid analogues may allow a better approximation of organization and dynamics of endogenous phospholipids. However, analogues with two long fatty acid chains such as N-Rho-DOPE are not well suited for rapid incorporation into intact membranes. In the present study we have used the fluorescent phospholipid analogues C6-NBD-PC and C6-NBD-PS. Those phospholipid analogues with a long fatty acid chain in the sn-1 position and a short chain in the sn-2 position bearing the fluorescence NBD moiety can be readily incorporated into cell membranes (29). Here we show that the NBD fluorescence lifetime of these analogues depends on the lipid composition and, in particular, on lipid-dependent physical properties of bilayers. Specifically, the lifetime is sensitive to domain localization of the lipid analogues being significantly longer in the lo domain in comparison to the ld domain. As shown for GUVs made from synthetic phospholipids, lifetime imaging of analogues enables the detection of lipid domains at submicroscopic scale. Application of this approach to living HeLa and HepG2 cells revealed that, although the fluorescence lifetime is sensitive to the composition of the plasma membrane, distinct lipid domains as found for GUVs are not detected. However, our data on giant vesicles derived from the plasma membrane of HeLa cells rather support the recent hypothesis (19, 30) that an ensemble of lipid domains being of submicroscopic size exist in the plasma membrane.
EXPERIMENTAL PROCEDURES
Materials—Phospholipids and NBD-labeled lipids were obtained from Avanti Polar Lipids (Birmingham, AL), and cholesterol from Sigma-Aldrich. Lipids were used without further purification. Solvents used for vesicle preparation were of the purest available grade. Indium tin oxide (ITO)-coated glass slides were obtained from Präzisions Glas & Optik GmbH (Iserlohn, Germany). Dulbecco's modified Eagle's medium, HBSS, DPBS, and penicillin/streptomycin were obtained from PAN Biotech (Aidenbach, Germany), fetal bovine serum from Invitrogen. Collagen A was from Seromed (Biochrom, Berlin, Germany). HBSS+ refers to HBSS supplemented with 1.25 mm CaCl2 and 0.5 mm MgCl2. Diisopropyl fluorophosphate was obtained from Fluka (Neu-Ulm, Germany).
Preparation of GUVs—GUVs were produced from lipid films dried on ITO-coated glass slides by electroswelling as originally described by Angelova and Dimitrov (31). In short, lipid mixtures were made from stock solutions in chloroform kept at -20 °C. 100 nmol of lipids (including cholesterol) were mixed in 30 μl of chloroform. Single drops of the lipid mixture were spread onto two ITO slides. To obtain homogeneously distributed lipid films the solvent was evaporated on a heater plate at 50–60 °C. To get rid of traces of the solvent glass slides were placed under vacuum (<10 mbar) for 1 h. The electroswelling chamber was assembled from both lipid ITO-coated slides using 1-mm Teflon spacers. The chamber was filled with 1 ml of prewarmed (50–60 °C) sucrose buffer (250 mm sucrose, 15 mm NaN3) with an osmolarity of 280 mosm. Immediately an alternating voltage (rising from 0.02 to 1.1 V over 30 min) with a frequency of 10 Hz was applied. GUVs formed during 2-h incubation at 50–60 °C. To detach the vesicles a voltage of 1.3 V (4 Hz) was applied for 30 min. The vesicles were stored in the dark at room temperature for up to 4 days.
For FLIM, 75 μl of the GUV solution was mixed with 225 μl of a glucose buffer (280 mm glucose, 11.6 mm potassium phosphate, pH 7.2). A drop of the sample was placed on a cell culture dish covered with polylysine. Before imaging GUVs were allowed to settle down to the bottom of the dish for 3 min.
Preparation of Cells and Giant Plasma Membrane Vesicles— HeLa and HepG2 cells were grown in Dulbecco's modified Eagle's medium containing 4.5 g/liter glucose, supplemented with 10% heat-inactivated fetal bovine serum, and penicillin/streptomycin and routinely passaged in 25-cm2 plastic culture flasks (in the case of HepG2 cells coated with collagen A), medium was changed every 3–4 days. Cells were maintained at 37 °C under 5% CO2.
Cholesterol depletion of HeLa cells was performed by incubating cells for 1 min on ice with 5 mm MβCD (Sigma-Aldrich) (32, 33) before labeling. For measuring MβCD-induced cholesterol efflux, HeLa cells were labeled with 1 μCi/ml [3H]cholesterol (GE Healthcare) in Dulbecco's modified Eagle's medium containing 1 mg/ml bovine serum albumin for 24 h. After exposure to MβCD, medium was collected and centrifuged. Cells were washed with DPBS and dissolved in 0.1 m NaOH. Radioactivity in medium and in cells was analyzed by liquid-scintillation counting and calculated as the counts in medium divided by the sum of counts in the medium plus cell lysate. Giant plasma membrane vesicles (GPMVs) were prepared from HeLa cells by the formaldehyde-based induction of plasma membrane blebbing as described by Baumgart et al. (18).
Labeling of Cells—NBD-labeled phospholipids were stored at -20 °C in chloroform or chloroform/methanol (1/1). Aliquots were transferred into a glass tube and dried under nitrogen before being resuspended in DPBS at the desired concentration. HeLa cells (5 × 104) were seeded on 35-mm culture dishes with a glass bottom (MatTek, Ashland, MA) and grown for 1 day. After washing with cold DPBS, cell labeling with C6-NBD-PC was performed for 20 min on ice (final lipid concentration was 0.25 μm in DPBS); cells were then extensively washed with DPBS (25 °C) and immediately analyzed by FLIM to ensure that NBD-lipids localized essentially in the outer leaflet of the plasma membrane. HepG2 cells were prepared according to the same protocol with minor changes: cells were seeded on poly-d-lysine-coated dishes, grown for at least 3–4 days, the final concentration of labeled lipids was 0.5–1 μm, and all washing steps were done with HBSS+.
Fluorescence Microscopy and FLIM—Images were taken by confocal laser scanning microscopy using an inverted Fluoview 1000 microscope (Olympus, Tokyo, Japan) and a 60× (numerical aperture 1.35) oil-immersion objective at 25 °C, if not stated otherwise. Images with a frame size of 512 × 512 pixels were acquired. For confocal images of NBD-labeled cells the green NBD fluorescence was excited with a 488 nm argon ion laser and was recorded between 500 and 530 nm. FLIM images were acquired by a commercial FLIM upgrade kit (PicoQuant, Berlin, Germany). NBD was excited with a pulsed diode laser (pulse width: 60 ps; pulse frequency: 10 MHz; 4 μs/pixel) with a wavelength of 468 nm. Emission was recorded using a 540/40 band-pass filter. Single photons were registered with a single photon avalanche photo diode.
For each image 50–70 frames were acquired; the average photon count rate was ∼2–4 × 104 counts/s. The images were pseudocolor-coded according to the average lifetim e(τav) of the pixels.
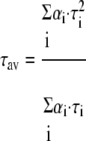 |
(Eq. 1) |
For determination of fluorescence lifetimes (τi) and respective amplitudes (αi) see below.
Determination of Fluorescence Lifetimes—For the analysis of fluorescence lifetime parameters of NBD analogues in GUVs and GPMVs membranes as well as in cell membrane compartments were selected applying an intensity threshold to exclude fluorescence from background or cytoplasm. If necessary the selection was refined manually to exclude regions not associated with the membrane. For the selected regions of interest an overall fluorescence decay curve was generated by summing up the photons registered for that region. From the decay curve only the part not affected by the instrument response function was used (“tail-fit”; approximately from 3 ns after beginning of the pulse). Using a non-linear least squares iterative fitting procedure fluorescence decay curves were fitted as a sum of exponential terms to obtain the fluorescence lifetimes of the NBD-group,
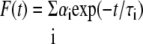 |
(Eq. 2) |
where F(t) is the fluorescence intensity at time t, and αi is a pre-exponential factor representing the intensity of the time-resolved decay of the component with lifetime τi. Typically, appropriate fitting of NBD fluorescence decay in domain-forming GUVs showed three lifetime components. However, for GUVs with homogeneous lipid mixtures (no domain formation) two lifetimes already provided adequate fitting (see Tables 1 and 2, under “Results”). For fitting of fluorescence decays in cellular membrane, i.e. the plasma membrane and intracellular membranes, three lifetimes were required. However, when fitting decays originating only from analogues in the plasma membrane, two lifetimes provided a sufficient quality of fit. Due to the tail fitting method it is likely that the short component τ1 detected for NBD (see “Results”) might be underestimated. Quality of fits was judged by the distribution of the residuals and the χ2 value.
TABLE 1.
Fluorescence lifetimes τ (τ1 omitted) of C6-NBD-PC in DOPC/SSM/Chol GUVs at 25 °C The concentration of C6-NBD-PC corresponded to 1 mol% of total lipids. For estimation and explanation of τ2(ld) and τ2(lo), see Material and Methods and Results. Data are presented as average ± S.E. (n = 8). The presence of lipid domains visible by fluorescence microscopy is indicated in the right column.
| DOPC/SSM/Chol | τ2(ld) | τ2(lo) | Visible domains |
|---|---|---|---|
| mol/mol/mol | ns | ns | |
| 10/0/0 | 7.1 ± 0.1 | No | |
| 8/1/1 | 7.6 ± 0.1 | No | |
| 4/4/2 | 7.3 ± 0.1 | Noa | |
| 1/1/1 | 7.0 ± 0.3 | 12.1 ± 0.4 | Yes |
| 3/3/4 | 7.1 ± 0.4 | 11.6 ± 0.1 | Yes |
| 2/4/4 | 7.4 ± 0.2 | 11.4 ± 0.2 | Yes |
| 1/1/8 | 12.4 ± 0.2 | No |
Typically, GUVs with a lipid composition of 4/4/2 did not show domain formation. Occasionally, small domains detectable by microscopy were observed
TABLE 2.
Fluorescence lifetimes τ (τ1 omitted) and respective amplitudes α of C6-NBD-PC in POPC/PSM/Chol GUVs at 25 °C The concentration of C6-NBD-PC corresponded to 1 mol% of total lipids. For estimation and explanation of τ and α, see “Experimental Procedures” and “Results.” Amplitudes are given as a percentage of total amplitude (α1 (not shown), α2(ld), and α2(lo)). Data are presented as average ± S.E. (n = 8). Note, lipid domains were not visible by fluorescence microscopy (see “Results”).
| POPC/PSM/Chol | τ2(ld) | α(ld) | τ2(lo) | α(lo) |
|---|---|---|---|---|
| mol/mol/mol | ns | % | ns | % |
| 7.5/2/0.5 | 8.0 ± 0.1 | 83 ± 1 | ||
| 7/2/1a | 8.4 ± 0.1 | 85 ± 1 | ||
| 6/2/2a | 7.8 ± 0.1 | 86 ± 1 | ||
| 5/2/3 | 7.9 ± 0.4 | 37 ± 2 | 10.9 ± 0.1 | 52 ± 3 |
| 4/2/4 | 7.5 ± 0.2 | 25 ± 3 | 11.6 ± 0.1 | 69 ± 2 |
| 2/2/6 | 7.0 ± 0.4 | 21 ± 2 | 11.4 ± 0.1 | 73 ± 2 |
Typically, GUVs with a lipid composition of 7/2/1 and 6/2/2 were fitted biexponentially. In some cases a triexponential fit could be applied (not shown)
For construction of lifetime histograms 2 × 2 pixels in the selected regions of interest were binned, and the fitting procedure described above was repeated for each binned pixel using the lifetime parameters obtained from the overall decay curve as starting parameters (see above). In the histograms the intensity weighted frequencies (αi × τi) are plotted.
RESULTS
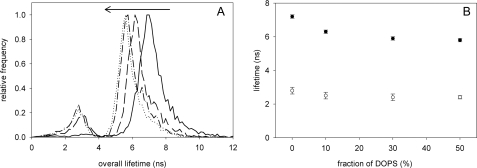
Fluorescence Lifetime of C6-NBD Lipid Analogues in DOPC/DOPS GUVs—Fitting of fluorescence decays of C6-NBD-PC in DOPC GUVs at 25 °C revealed a short and a long lifetime τ1 and τ2 of ∼3 ns and 7 ns, respectively (Fig. 1A). Very similar lifetimes were observed for C6-NBD-PS (data not shown). τ2 represents the major component of the fluorescence decays. Variation of the C6-NBD-PC concentration between 0.1 mol% and 1 mol% had no influence on lifetimes and their amplitudes (data not shown). Quantitatively similar components of C6-NBD-PC were found in cuvette experiments with large DOPC unilamellar vesicles.3
FIGURE 1.
Fluorescence lifetimes of C6-NBD-PC in DOPC and DOPC/DOPS GUVs. A, lifetime histograms for τ1 and τ2 of C6-NBD-PC in GUVs of different mixtures of DOPC/DOPS (mol/mol) (25 °C): Curves are for 0%, 10%, 30, and 50% DOPS along the path of the arrow. Histograms were normalized by setting the maximum to 1. B, averages (±S.E.) of τ1 (open circles) and τ2 (closed circles) of DOPC/DOPS GUVs are shown as a function of the molar fraction of DOPS (n = 7).
With increasing concentration of DOPS τ2 was shifted to lower values (Fig. 1, A and B). This indicated that τ2 is sensitive to physical properties of the membrane depending on the lipid composition.
Fluorescence Lifetime of C6-NBD-PC in SM/Cholesterol-containing GUVs—To assess whether fluorescence decay of C6-NBD-PC is sensitive to physical properties of lipid domains, GUVs of DOPC/SSM/Chol (1/1/1, mol/mol/mol) and of DOPC/DPPC/Chol (1/1/1) mixtures were prepared. GUVs of those lipid mixtures are known to form lo and ld domains at 25 °C (15, 34), which can be visualized by lipid-like fluorophores such as C6-NBD-PC. In Fig. 2A fluorescence lifetime images of GUVs (average fluorescence lifetime) are presented. Note, the brightness reflects the concentration of the analogue, whereas the lifetime is coded by the color. DOPC GUVs (left image) and GUVs of DOPC/SSM/Chol (1/1/8, excess of cholesterol) (right image) show a homogenous distribution of the lipid analogues consistent with the fact that DOPC forms a pure ld phase, whereas the mixture DOPC/SSM/Chol (1/1/8) forms a pure lo phase. However, the lifetime of C6-NBD-PC in the ld phase is much shorter in comparison to the lo phase (see below). GUVs prepared from 1/1/1 mixtures of DOPC/SSM/Chol (middle image) or DOPC/DPPC/Chol (not shown) show a heterogeneous lateral distribution of C6-NBD-PC documenting the domain formation. The bright region corresponds to an ld domain, because it is known that C6-NBD-PC recruits in model membranes preferentially to such domain type (16). Again, the ld domain is characterized by a much shorter lifetime in comparison to the lo domain.
FIGURE 2.
Fluorescence lifetime of C6-NBD-PC in lipid domains. A, average lifetime of C6-NBD-PC in GUVs prepared from DOPC (I, pure ld phase), DOPC/SSM/Chol = 1/1/1 (II, ld and lo phase), and DOPC/SSM/Chol = 1/1/8 (III, pure lo phase) at 25 °C. The average lifetime is longer in lo phase than in ld phase. B, lateral distribution of lifetime components τ2(lo) and τ2(ld) (τ1 is omitted) of C6-NBD-PC in GUVs prepared from DOPC/SSM/Chol = 1/1/1 (mol/mol/mol) at 25 °C. For each pixel the amplitude of the lifetime components τ2(ld) (green, top image, right column) or τ2(lo) (red, bottom image, right column) is presented. Comparison of the overlay of both images (bottom image, left column) with the intensity image (top image, left column) shows that τ2(ld) is associated with the ld domain, whereas τ2(lo) is associated with the lo domain. In addition for each domain the respective lifetime histogram is given (center column). For further details see “Experimental Procedures” and “Results.” White bars correspond to 10 μm.
In Fig. 2B a detailed analysis of domain associated lifetimes of C6-NBD-PC in DOPC/SSM/Chol (1/1/1) GUVs is presented. For this purpose, we have also constructed histograms reflecting the distribution and variability of lifetimes (see “Experimental Procedures”). The analysis revealed that τ1 (2–3 ns) was insensitive to the domain. In contrast, the longer lifetime τ2 was dependent on the lipid domain being significantly shorter for the ld domain (τ2(ld) ∼ 7.0 ± 0.3 ns) than for the lo domain (τ2(lo) ∼ 12.1 ± 0.4 ns). A similar pattern of fluorescence lifetimes was found for DOPC/DPPC/Chol (1/1/1) GUVs (data not shown) providing evidence that the longer lifetime in the lo domain is not due to association of C6-NBD-PC with sphingomyelin but depends essentially on the properties of the phospholipid membrane.
Measurements on DOPC/SSM/Chol GUVs with C6-NBD-PC concentrations in the range from 0.1 to 1 mol% showed that lifetimes did not depend on NBD concentration (data not shown), proving that the differences between τ2(ld) and τ2(lo) do not ensue from self quenching of NBD in the ld domain. As τ1 comprises only a small contribution in each decay curve (relative amplitude < 5%) it was omitted in the presentation of further results (see also “Discussion”).
In Fig. 3 the lifetime histograms of GUVs prepared from different mixtures of DOPC, SSM, and Chol are shown. Representative values of lifetimes are summarized in Table 1. GUVs composed of 10/0/0 or 4/4/2 DOPC/SSM/Chol showed a homogenous distribution of C6-NBD-PC. Domain formation was not detectable by fluorescence microscopy (Table 1). τ2 was ∼7 ns corresponding to τ2(ld). Also, we did not observe any domain formation in 1/1/8 GUV. In this case only a longer τ2 equivalent to τ2(lo) was found. However, both components, τ2(ld) and τ2(lo), were observed for lipid mixtures for which domains were visible by fluorescence microscopy (1/1/1, 3/3/4, and 2/4/4). Notably, τ2(ld) and τ2(lo) were rather insensitive to the lipid mixture. τ2(ld) varied between 7.0 and 7.6 ns, whereas τ2(lo) varied between 11.4 and 12.4 ns. The constant lifetimes of ∼7 ns measured for the ld phase either at low or at high cholesterol concentrations indicate that only minor amounts of cholesterol redistribute to the ld phase formed by DOPC.
FIGURE 3.
Fluorescence lifetime of C6-NBD-PC in DOPC/SSM/Chol GUVs. Lifetime histograms of C6-NBD-PC in GUVs prepared from DOPC/SSM/Chol mixtures (mol/mol/mol) at 25 °C: 4/4/2 (dashed line), 3/3/4 (full line), 2/4/4 (dotted line), and 1/1/8 (dashed-dotted line). Histograms were normalized by setting the maximum to 1. For further details see “Experimental Procedures,” “Results,” and Table 1.
Next, lifetime histograms were constructed from the fluorescence decay of C6-NBD-PC in GUVs of POPC/PSM/Chol mixtures with constant PSM content (20 mol%) at 25 °C (Fig. 4).
FIGURE 4.
Fluorescence lifetime of C6-NBD-PC in POPC/PSM/Chol GUVs. Lifetime histograms of C6-NBD-PC in GUVs prepared from POPC/PSM/Chol mixtures (mol/mol/mol) at 25 °C: 7.5/2/0.5 (dashed line), 6/2/2 (full line), 4/2/4 (dotted line), and 2/2/6 (dashed-dotted line). Histograms were normalized by setting the maximum to 1. For further details see “Experimental Procedures,” “Results,” and Table 2.
The cholesterol content varied between 5 and 60 mol%. Although at 5 mol% we found only the short component, τ2(ld), between 20 and 60 mol% cholesterol we observed both components of τ2, τ2(ld) and τ2(lo) (compare Fig. 4 with Table 2). By raising cholesterol concentration from 20 and 40 mol% the relative fraction of τ2(lo) increased. At high cholesterol concentration (60 mol%) only minor amounts of τ2(ld) could be found. Again, τ2(ld) and τ2(lo) were rather insensitive to the lipid mixture. Although the presence of τ2(ld) and τ2(lo) indicated the existence of lipid domains, neither the fluorescence intensity pattern nor fluorescence lifetime images revealed the existence of domains being of microscopic size. Hence, lipid domains are of submicroscopic size. In contrast, at 10 °C POPC/PSM/Chol mixtures may form domains in the microscopic range as we have observed for a (1/1/1) mixture (see supplemental Fig. S1). However, while for DOPC/SSM/Chol a strong partition of C6-NBD-PC into the ld domain was observed (Fig. 2, see supplemental Fig. S1), the ld domain-specific enrichment is much weaker for the POPC/PSM/Chol mixture. Hence, the partition coefficient for C6-NBD-PC between lipid domains depends on the lipid composition of domains.
Lifetime Analysis of C6-NBD Analogues in Cellular Membranes of HepG2 and HeLa Cells—Upon incubation of HepG2 cells with C6-NBD-PC on ice (see “Experimental Procedures”), essentially the plasma membrane was labeled (Fig. 5A). Intracellular staining was very faint if at all (Fig. 5, A and I). Analysis of fluorescence lifetime measured at 25 °C (Fig. 5A, III and IV) allows distinguishing between several components. Whereas the amplitude of the short lifetime τ1 around 2 ns was very small, we observed the longer lifetime τ2 with two maxima assigned as τ2i and τ2p at 6.4 ns and 11.5 ns, respectively (Fig. 5A, IV). Image analysis revealed that τ2p was essentially found in the plasma membrane while τ2i is associated with analogues in intracellular membranes. The larger contribution of τ2p with respect to τ2i was in agreement with the preferential localization of analogues in the plasma membrane. Notably, if the plasma membrane was selected as the region of interest, then in addition to τ1 only τ2p was found. Essentially the same pattern of lifetimes was observed when cells were incubated for 1 h at 37 °C after labeling (Fig. 5B). The analogue remained mainly confined to the plasma membrane. Intracellular labeling was slightly enhanced as indicated by an increase of the contribution of τ2i. This was due to transport of the analogues along endocytic pathways or some slow redistribution across the plasma membrane.
FIGURE 5.
Fluorescence lifetimes of C6-NBD-PC (A and B) and C6-NBD-PS (C) in HepG2 cells. Fluorescence lifetime of lipid analogues was measured at 25 °C immediately after labeling (A and C) and after 1 h of incubation at 37 °C (B) (see “Experimental Procedures”). I, confocal fluorescence microscopy; II, DIC microscopy; III, average lifetime shown as pseudocolor image (see scale); IV, histogram of lifetime: τ2i (full line) and τ2p (dashed line). For explanation see “Results”. The larger spherical compartment in B corresponds to a canalicular vacuole (see arrow). C6-NBD-PC becomes enriched in this compartment (35).
Similar lifetimes were observed for C6-NBD-PS (Fig. 5C) with a maximum at 6.5 ns and 10.9 ns for τ2i and τ2p, respectively. Again, τ2p essentially originated from analogues in the plasma membrane, whereas τ2i was from intracellular membranes. However, contribution of τ2i was much higher in comparison to C6-NBD-PC reflecting the enhanced intracellular localization of the PS analogue. Intracellular staining was even stronger after 1 h of incubation (not shown), due to an aminophospholipid translocase activity (see “Discussion”).
Cellular distribution and fluorescence lifetime pattern of C6-NBD-PC or C6-NBD-PS was very similar for HeLa cells. Fitting of fluorescence decays at the plasma membrane revealed τ1 and longer lifetimes, which were similar to τ2p of HepG2 cells (Fig. 6, shown for C6-NBD-PC). Upon depletion of cholesterol by MβCD, a shift of the lifetime distribution to lower values was observed (Fig. 6). MβCD extracted ∼20% of total cholesterol from these cells (20.4 ± 1.2%, n = 2) as measured after labeling of the cells with [3H]cholesterol (see “Experimental Procedures”). Lifetime of intracellular membranes was similar to HepG2 cells (not shown).
FIGURE 6.
Fluorescence lifetime of C6-NBD-PC in the plasma membrane of HeLa cells. Left, the average lifetime (images) in the plasma membrane of HeLa cells at 25 °C before (I) and after (II) depletion of cholesterol by treatment of cells with MβCD is shown as pseudocolor image (see scale). White bars correspond to 10 μm. Right, histograms of τ2p of C6-NBD-PC in HeLa cells at 25 °C before (solid line) and after (dashed line) depletion of cholesterol by treatment of cells with MβCD. Histograms were normalized by setting the maximum to 1. For further details see “Experimental Procedures” and “Results.”
Fluorescence Lifetime of C6-NBD-PC in Giant Plasma Membrane Vesicles (GPMVs) of HeLa Cells—Neither fluorescence intensity and lifetime images nor lifetime histograms of C6-NBD analogues in the plasma membrane of HeLa and HepG2 cells revealed the presence of distinct lipid domains as found for GUVs (see above). Recently, Baumgart et al. (18) has shown that giant vesicles can be generated from plasma membranes, which may form lo and ld domains in the micron scale. We applied this procedure successfully to HeLa cells (Fig. 7). Whereas at 25 °C ∼40% of the vesicles showed domain formation, this fraction increased to over 50% at 10 °C. In contrast to GUVs made from mixtures of synthetic lipids (see above), C6-NBD-PC showed some preference to the lo domain at both temperatures (25 °C, see Fig. 7; 10 °C see supplemental Fig. S1), as determined by co-staining with N-Rho-DOPE (not shown), which is known to partition into ld domains of GPMVs (17, 18). However, by selection of regions of interest separate analysis of domains demonstrated lipid domain characteristic values for τ2 as observed for GUVs (Table 3). In Fig. 7 the lifetime histograms for lo and ld domains as well as their envelope are shown (only for 25 °C). We also found domain-forming GPMVs that did not show a domain-specific preference of C6-NBD-PC. In that case domains could be distinguished by fluorescence lifetimes (see arrow in Fig. 7, I and II). Lifetimes of GPMVs were lower with respect to those found in the plasma membranes of intact HeLa cells. Very likely, preparation of GPMVs affects membrane organization, e.g. as interaction with cytoskeleton and transbilayer lipid asymmetry (18).
FIGURE 7.
Partition and fluorescence lifetime of C6-NBD-PC in GPMVs. GPMVs prepared from HeLa cells show only a very weak domain preference of C6-NBD-PC (I, intensity image). ld (white arrow) and lo domains can be characterized by lifetime differences (II, pseudocoloring according to the average lifetime). White bars correspond to 10 μm. Below, the lifetime histograms of τ2 for ld domains (green line) and lo domains (red line) as well as their envelope (full line, black) are shown. The histogram of GPMVs showing no lipid domains (dotted line, black) is very similar to the envelope histogram of GPMVs forming large domains. In the inset the normalized histograms for ld and lo domains are shown. All measurements were made at 25 °C.
TABLE 3.
Fluorescence lifetimes τ2 of C6-NBD-PC in domain-forming GPMVs prepared from HeLa-cells at 10 °C and 25 °C For estimation and explanation of lifetimes see “Experimental Procedures” and “Results.” Data are presented as average ± S.E. (n > 6).
| Temperature | τ2(ld) | τ2(lo) | |
|---|---|---|---|
| ns | |||
| 25 °C | 6.7 ± 0.2 | 9.6 ± 0.3 | |
| 10 °C | 8.4 ± 0.3 | 10.8 ± 0.1 | |
We also analyzed fluorescence decays of C6-NBD-PC in GPMVs for which lipid domains of microscopic size were not observed, neither by intensity nor by lifetime images. Interestingly, the lifetime histogram of those GPMVs was very similar to the envelope histogram of GPMVs forming large domains (Fig. 7).
DISCUSSION
FLIM Is Suitable to Detect Transient Small Lipid Domains— To study domains of submicroscopic dimension methods are required of which the resolution does not interfere with the size and the dynamics of those domains. Due to the high time resolution FLIM should be a very suitable method to detect even very small lipid domains. To resolve two distinct lipid environments by FLIM, lipid analogues must not interchange between the environments during emission, otherwise the two different lifetimes would average out. Thus, domains should be stable at least for about three times the fluorescence lifetime of the analogue, e.g. for ∼30 ns in the case of a C6-NBD-lipid analogue. In this time period, an analogue would diffuse ∼2 nm assuming a lateral lipid diffusion of ∼10-7 cm2/s. Thus, lipid domains as small as ∼10 nm2 should be detectable by FLIM. This corresponds to ∼16 lipid molecules with a surface area of ∼0.6 nm2 per lipid. Taking into account that a lateral diffusion coefficient of 10-7 cm2/s is an upper estimate, domains resolvable by FLIM could be even smaller. Typical values for lateral diffusion of lipid analogues in plasma membranes are between 1 × 10-9 cm2/s and 5 × 10-9 cm2/s as measured for fibroblasts (36) and red blood cells (37), respectively. Hence, FLIM should be appropriate to capture even very small lipid domains as long as they are stable for at least 30 ns. However, these estimates show also the limitations of FLIM in that FLIM is not sensitive to domain sizes and lifetime beyond 12 nm and 30 ns, respectively.
Fluorescence Lifetimes of C6-NBD-PC in a Homogenous Environment—In vesicles with a homogeneous environment such as pure DOPC vesicles we observed two lifetimes for C6-NBD-PC, a short (τ1) and a long one (τ2) around 2–3 ns and 7 ns, respectively. The biexponential decay of C6-NBD-PC in homogenous PC bilayers is in agreement with previous experimental observations (38). Very likely, τ1 originates from the red edge excitation shift of NBD in membranes, as in FLIM imaging the excitation wavelength of the NBD fluorescence is at 468 nm being 3 nm above the excitation maximum (39). Photons emitted early with a small Stokes shift are preselected and give rise to the faster decaying component τ1. In agreement with the small wavelength difference of 3 nm the contribution of τ1 was very small (<5%). We surmise that τ2 is determined by the motional freedom of lipid analogues and, in particular, by that of the NBD moiety. By using 1H NMR measurements we have recently shown a highly dynamic reorientation of the NBD group of C6-NBD-phospholipid analogue in the membrane due to thermal fluctuations (40). A broad distribution of the fluorophore in the lipid bilayer was observed with a preferential location of the NBD group in the upper acyl chain/glycerol region confirming earlier studies (41).
Fluorescence Lifetimes in DOPC/SSM/Chol GUVs Forming Microscopic Lipid Domains—Indeed, by using GUVs of lipid mixtures forming microscopic domains we found that τ2 of C6-NBD-PC was strongly affected by the lipid environment of the fluorophore. In ld domains, the lifetime τ2(ld) was ∼7 ns, similar to that found for pure DOPC GUVs. In contrast, it was shifted to ∼12 ns in lo domains (τ2(lo)) reflecting the higher degree of order in those domains. In line with previous results (40, 42) we surmise that the relocation dynamics of the NBD moiety is sensitive to lipid packing and that the increase in the degree of order when going from a ld to a lo phase causes the large shift of τ2 toward longer lifetimes. We detected only minor amounts if at all of τ2(lo) in the ld domain and no contributions of τ2(ld) in the lo domain. This shows that the lo and ld domains are well separated and do not harbor ld or lo domains of submicroscopic size, respectively. This also clearly demonstrates the identical nature of facing domains in both leaflets confirming previous studies on inter-bilayer coupling (13, 14). Hence, the lateral organization of lipid domains is identical in either monolayer.
At high and low cholesterol concentration we could detect neither microscopic nor submicroscopic domains in DOPC/SSM/Chol GUVs by FLIM in agreement with previous results (43). At low cholesterol concentration we found only lifetimes comparable to those of the ld domain (τ2(ld)) of domain-forming GUVs (see Fig. 3). For high cholesterol concentrations lifetimes similar to those of lo domains were measured. Although detection of small submicroscopic lo domains surrounded by a large ld domain might be hampered by the fact that the partition of C6-NBD-PC into ld domains is favored, this is not the case for the opposite. Hence, the absence of a short lifetime component equivalent to τ2(ld) at high cholesterol concentration strongly suggests that no ld domains even on the submicroscopic scale have been formed.
Fluorescence Lifetimes in POPC/PSM/Chol GUVs Forming Submicroscopic Lipid Domains—To assess whether lifetime measurements of C6-NBD-PC can resolve small lipid domains not detectable by fluorescence microscopy, we prepared GUVs from various mixtures of POPC/PSM/Chol with a constant content of PSM (20 mol%). Indeed, consistent with previous observations (14, 15, 34, 44–46), those GUVs did not form visible domains at 25 °C. However, at cholesterol concentrations between 20 and 60 mol%, we found two different lifetimes components (additional to τ1) similar to τ2(ld) and τ2(lo) observed for DOPC/SSM/Chol GUVs. This indicates that domains in the submicroscopic region dimensions have formed. Previous studies have already suggested that in such POPC/PSM/Chol mixtures with a cholesterol content above ∼35 mol% domains of submicroscopic sizes between 20 nm and 100 nm could be formed (45, 46). The authors surmised that at cholesterol concentrations below 35 mol% domains should be present, although they could not be detected by their approach based on Förster resonance energy transfer between fluorescent lipid analogues. Our FLIM data show that, below 35 mol% cholesterol, domains are indeed present. This is in agreement with our very recent NMR study showing that in POPC/PSM/Chol mixtures with <35 mol% cholesterol, domains in the order of ∼40 to 70 nm2 are formed (47).
To prove that POPC/PSM/Chol mixtures can form micron scale domains, we studied GUVs with a 1/1/1 mixture at 10 °C. Indeed, in agreement with other studies, we found large domains (15). Interestingly, we observed only a weak preference of C6-NBD-PC for the ld domain. This is in contrast to DOPC/SSM/Chol GUVs showing a strong preference of the analogue for the ld domain at either temperature in agreement with Shaw et al. (16). However, the domain-specific lifetime of C6-NBD-PC was not affected by the partition behavior.
Fluorescence Lifetimes of C6-NBD Analogues in Cellular Membranes—We observed a bimodal distribution of the fluorescence lifetime τ2 of C6-NBD-PC and C6-NBD-PS for HeLa and HepG2 cells. FLIM-based image analysis revealed that the shorter component (τ2i) corresponds to intracellular membranes, whereas the longer lifetime (τ2p) is related to the plasma membrane. Support of this conclusion is given by the higher amplitude of the short component in the case of C6-NBD-PS. It is known that PS and respective analogues such as C6-NBD-PS but not PC (analogues) are rapidly transported from the exoplasmic to the cytoplasmic leaflet of the plasma membrane by the aminophospholipid translocase activity in mammalian cells, e.g. for HepG2 (48, 49) and HeLa cells (50). Even at low temperature, an efficient transport occurs, which leads to intracellular staining already during the labeling procedure. Upon translocation to the cytoplasmic leaflet, C6-NBD-PS redistributes among intracellular membranes. We noted that the plasma membrane-associated lifetime of C6-NBD-PS is slightly shorter than that of C6-NBD-PC. This might be explained again by the fact that a significant fraction of the PS analogue has been transported to the cytoplasmic leaflet sensing the reduced lipid packing of this leaflet as previously shown by biochemical and biophysical studies (36, 37).
A similar dependence of lifetime on membrane localization has been made for the peryleneimide chromophore in Jurkat cells (28). The fluorescence decay of this probe was shorter in intracellular membranes with respect to the plasma membrane. Very likely, this difference of lifetimes between intracellular membranes and the plasma membrane is related to the high concentration of cholesterol in the plasma membrane.
As evident from the histograms, the distribution of lifetimes of the plasma membrane of HepG2 cells and HeLa cells was rather broad with a maximum of ∼11 ns for C6-NBD-PC. This lifetime was similar to that of the lo phase of GUVs. The histograms did not reveal resolvable peaks corresponding to different lifetimes as found for lo and ld domains of GUVs. Nevertheless, several studies suggest that in resting cells rather small lipid (raft-like) domains with only transient stability exist in the nanometer dimension (16, 19, 30, 51). Sophisticated biophysical techniques and mathematical modeling of generated experimental data have indeed shown that such domains are between 5 nm and 20 nm in diameter (24, 52). Employing time-resolved Förster resonance energy transfer Sharma et al. (24) concluded that about four glycosylphosphatidylinositol-anchored proteins are localized in small clusters with ∼40 lipids. Single particle tracking experiments indicated that single glycosylphosphatidylinositol-anchored proteins are organized in very small domains (<10 nm) with a short lifetime being in the order of 0.1 ms (52). This short transient stability is in agreement with electron spin resonance measurements revealing that spin-labeled lipids reside within a typical time of ∼100 ns in rafts (53). Based on these characteristic properties of lipid domains and our estimates on the resolution of FLIM measurements (see above), our approach should be suitable to detect even nanometer-sized domains with very short stability. However, we were not able to resolve distinguishable lifetime components in the plasma membrane of cells as those found for domain-forming GUVs. This may support recent skepticism on the existence and relevance of raft domains in biological membranes (54). On the other hand, alternative explanations that are in agreement with other studies may account for our results. First, one should keep in mind that the cholesterol content is typically very high in the plasma membrane. Based on that, one would expect that the plasma membrane would rather consist of a continuous lo phase with embedded possible small ld domains (55, 56). This could explain a lifetime histogram of C6-NBD analogues in the plasma membrane centered at values typically found for lo domains of GUVs. Second, we suppose that the data are compatible with a view of a large variety of lipid domains differing in their composition as well as in their properties such as size and stability. This gives rise to an overlapping histogram of domain-specific lifetimes rather than to well resolvable ones. Indeed, the lifetime histograms of the NBD analogues in the plasma membrane are much broader than that in the lo domain of GUVs. Previous reports have already discussed the presence of an ensemble of domains varying in size and composition (19, 30). Even a very simple model membrane system, which normally only separates into a distinct ld and lo domain, shows a more complex phase behavior if membrane asymmetry is generated (57). Jacobson et al. (30) pointed out that the plasma membrane should be viewed as a lipid-protein composite, rather than a dilute solution of proteins in a lipid bilayer. As recently shown by mathematical modeling, the presence of membrane proteins and their interaction with lipids significantly reduces the ability of lipids to phase separate already at a rather low area fraction (5–10%) covered by proteins (58). Hancock (19) suggested that the two classes of proteins that either can capture and stabilize lo domains and those that are excluded from lo domains represent the two ends of a continuous spectrum of membrane proteins that can give rise to a distribution of intermediate size and stability of domains. Likewise, coupling of the membrane to the actin cytoskeleton may prevent formation of micrometer-scale domains in plasma membranes, which might explain the formation of large domains in GPMVs (18). Hence, apart from lipid-lipid interactions organization and dynamics of biological membranes are determined by protein-lipid and protein-protein interactions giving rise to a complex network of different lateral lipid arrangements. Indeed, our study on GPMVs provides some experimental evidence for the existence of a variety of lipid domains of submicroscopic size in intact plasma membranes. For GPMVs forming large domains, we could extract lifetimes separately for lo and ld domain. Although the lifetimes of both domains could be clearly distinguished from each other, the difference was much lower in comparison to that found for lo and ld domains of GUVs. This is also evident from the fact that the envelope of the two lifetime distributions of domain-forming GPMVs gives rise to a continuous lifetime distribution but not to a bimodal distribution as found for GUVs. Surprisingly, the envelope was almost identical to the lifetime distribution of GPMVs that did not form any visible lipid domains. This may indicate that in those GPMVs the large domains disintegrated into smaller ones below microscopic resolution, whereas essential physical properties, e.g. lipid packing, are preserved. Being aware of the limitations of GPMVs (18) a similar situation may apply to intact plasma membranes supporting the view that a spectrum of lipid domains differing in their physical properties exist in those membranes (19, 30).
Supplementary Material
This work was supported by the Deutsche Forschungsgemeinschaft (Grants FG 475 and SFB 740) and the European Union (Grant MRTN-CT-2004-005330) (to A. H.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: GUV, giant unilamellar vesicle; PSM, N-palmitoyl-d-sphingomyelin; SSM, N-stearoyl-d-sphingomyelin; C6-NBD-PC, 1-palmitoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]-hexanoyl]-sn-glycero-3-phosphatidylcholine; C6-NBD-PS, 1-palmitoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-sn-glycero-3-phosphatidylserine; lo, liquid-ordered; ld, liquid-disordered; DOPC, dioleoylphosphatidylcholine; POPC, palmitoyloleoylphosphatidylcholine; Chol, cholesterol; ITO, indium tin oxide; N-Rho-DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl); HBSS, Hanks' balanced salt solution; FLIM, fluorescence lifetime imaging; DPBS, Dulbecco's modified phosphate-buffered saline; MβCD, methyl-β-cyclodextrin; GPMV, Giant plasma membrane vesicle.
A. Tannert and A. Herrmann, unpublished results.
References
- 1.Shimshick, E. J., and McConnell, H. M. (1973) Biochemistry 12 2351-2360 [DOI] [PubMed] [Google Scholar]
- 2.Mabrey, S., and Sturtevant, J. M. (1976) Proc. Natl. Acad. Sci. U. S. A. 73 3862-3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee, A. G. (1977) Biochim. Biophys. Acta 472 285-344 [DOI] [PubMed] [Google Scholar]
- 4.Recktenwald, D. J., and McConnell, H. M. (1981) Biochemistry 20 4505-4510 [DOI] [PubMed] [Google Scholar]
- 5.Petit, V. A., and Edidin, M. (1974) Science 184 1183-1185 [DOI] [PubMed] [Google Scholar]
- 6.Tillack, T. W., Allietta, M., Moran, R. E., and Young, W. W., Jr. (1983) Biochim. Biophys. Acta 733 15-24 [DOI] [PubMed] [Google Scholar]
- 7.Karnovsky, M. J., Kleinfeld, A. M., Hoover, R. L., and Klausner, R. D. (1982) J. Cell Biol. 94 1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Meer, G., Stelzer, E. H., Wijnaendts-van-Resandt, R. W., and Simons, K. (1987) J. Cell Biol. 105 1623-1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simons, K., and Ikonen, E. (1997) Nature 387 569-572 [DOI] [PubMed] [Google Scholar]
- 10.Brown, D. A., and Rose, J. K. (1992) Cell 68 533-544 [DOI] [PubMed] [Google Scholar]
- 11.Heerklotz, H. (2002) Biophys. J. 83 2693-2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munro, S. (2003) Cell 115 377-388 [DOI] [PubMed] [Google Scholar]
- 13.Korlach, J., Schwille, P., Webb, W. W., and Feigenson, G. W. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 8461-8466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietrich, C., Bagatolli, L. A., Volovyk, Z. N., Thompson, N. L., Levi, M., Jacobson, K., and Gratton, E. (2001) Biophys. J. 80 1417-1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veatch, S. L., and Keller, S. L. (2003) Biophys. J. 85 3074-3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw, J. E., Epand, R. F., Epand, R. M., Li, Z., Bittman, R., and Yip, C. M. (2006) Biophys. J. 90 2170-2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgart, T., Hunt, G., Farkas, E. R., Webb, W. W., and Feigenson, G. W. (2007) Biochim. Biophys. Acta 1768 2182-2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumgart, T., Hammond, A. T., Sengupta, P., Hess, S. T., Holowka, D. A., Baird, B. A., and Webb, W. W. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 3165-3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock, J. F. (2006) Nat. Rev. Mol. Cell. Biol. 7 456-462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silvius, J. R., and Nabi, I. R. (2006) Mol. Membr. Biol. 23 5-16 [DOI] [PubMed] [Google Scholar]
- 21.Varma, R., and Mayor, S. (1998) Nature 394 798-801 [DOI] [PubMed] [Google Scholar]
- 22.Friedrichson, T., and Kurzchalia, T. V. (1998) Nature 394 802-805 [DOI] [PubMed] [Google Scholar]
- 23.Pralle, A., Keller, P., Florin, E. L., Simons, K., and Horber, J. K. (2000) J. Cell Biol. 148 997-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma, P., Varma, R., Sarasij, R. C., Ira, Gousset, K., Krishnamoorthy, G., Rao, M., and Mayor, S. (2004) Cell 116 577-589 [DOI] [PubMed] [Google Scholar]
- 25.Prior, I. A., Muncke, C., Parton, R. G., and Hancock, J. F. (2003) J. Cell Biol. 160 165-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen, D. M., Lanigan, P. M., Dunsby, C., Munro, I., Grant, D., Neil, M. A., French, P. M., and Magee, A. I. (2006) Biophys. J. 90 L80-L82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Almeida, R. F., Borst, J., Fedorov, A., Prieto, M., and Visser, A. J. (2007) Biophys. J. 93 539-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margineanu, A., Hotta, J., Vallee, R. A., Van der Auweraer, M., Ameloot, M., Stefan, A., Beljonne, D., Engelborghs, Y., Herrmann, A., Mullen, K., De Schryver, F. C., and Hofkens, J. (2007) Biophys. J. 93 2877-2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devaux, P. F., Fellmann, P., and Herve, P. (2002) Chem. Phys. Lipids 116 115-134 [DOI] [PubMed] [Google Scholar]
- 30.Jacobson, K., Mouritsen, O. G., and Anderson, R. G. (2007) Nat. Cell Biol. 9 7-14 [DOI] [PubMed] [Google Scholar]
- 31.Angelova, M. I., and Dimitrov, D. S. (1986) Faraday Discuss. Chem. Soc. 81 303-311 [Google Scholar]
- 32.Landry, Y. D., Denis, M., Nandi, S., Bell, S., Vaughan, A. M., and Zha, X. (2006) J. Biol. Chem. 281 36091-36101 [DOI] [PubMed] [Google Scholar]
- 33.Zidovetzki, R., and Levitan, I. (2007) Biochim. Biophys. Acta 1768 1311-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veatch, S. L., and Keller, S. L. (2003) Biophys. J. 84 725-726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wustner, D., Mukherjee, S., Maxfield, F. R., Muller, P., and Herrmann, A. (2001) Traffic 2 277-296 [DOI] [PubMed] [Google Scholar]
- 36.el Hage Chahine, J. M., Cribier, S., and Devaux, P. F. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 447-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrot, G., Cribier, S., Devaux, P. F., Geldwerth, D., Davoust, J., Bureau, J. F., Fellmann, P., Herve, P., and Frilley, B. (1986) Proc. Natl. Acad. Sci. U. S. A. 83 6863-6867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mukherjee, S., Raghuraman, H., Dasgupta, S., and Chattopadhyay, A. (2004) Chem. Phys. Lipids 127 91-101 [DOI] [PubMed] [Google Scholar]
- 39.Chattopadhyay, A., and Mukherjee, S. (1993) Biochemistry 32 3804-3811 [DOI] [PubMed] [Google Scholar]
- 40.Huster, D., Muller, P., Arnold, K., and Herrmann, A. (2001) Biophys. J. 80 822-831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chattopadhyay, A., and London, E. (1987) Biochemistry 26 39-45 [DOI] [PubMed] [Google Scholar]
- 42.Raghuraman, H., Shrivastava, S., and Chattopadhyay, A. (2007) Biochim. Biophys. Acta 1768 1258-1267 [DOI] [PubMed] [Google Scholar]
- 43.Veatch, S. L., and Keller, S. L. (2005) Biochim. Biophys. Acta 1746 172-185 [DOI] [PubMed] [Google Scholar]
- 44.de Almeida, R. F., Fedorov, A., and Prieto, M. (2003) Biophys. J. 85 2406-2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Almeida, R. F., Loura, L. M., Fedorov, A., and Prieto, M. (2005) J. Mol. Biol. 346 1109-1120 [DOI] [PubMed] [Google Scholar]
- 46.Silvius, J. R. (2003) Biophys. J. 85 1034-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bunge, A., Muller, P., Stockl, M., Herrmann, A., and Huster, D. (2008) Biophys. J. 94 2680-2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muller, P., Pomorski, T., Porwoli, S., Tauber, R., and Herrmann, A. (1996) Hepatology 24 1497-1503 [DOI] [PubMed] [Google Scholar]
- 49.Tannert, A., Wustner, D., Bechstein, J., Muller, P., Devaux, P. F., and Herrmann, A. (2003) J. Biol. Chem. 278 40631-40639 [DOI] [PubMed] [Google Scholar]
- 50.Alder-Baerens, N., Muller, P., Pohl, A., Korte, T., Hamon, Y., Chimini, G., Pomorski, T., and Herrmann, A. (2005) J. Biol. Chem. 280 26321-26329 [DOI] [PubMed] [Google Scholar]
- 51.Lagerholm, B. C., Weinreb, G. E., Jacobson, K., and Thompson, N. L. (2005) Annu. Rev. Phys. Chem. 56 309-336 [DOI] [PubMed] [Google Scholar]
- 52.Kusumi, A., Koyama-Honda, I., and Suzuki, K. (2004) Traffic 5 213-230 [DOI] [PubMed] [Google Scholar]
- 53.Kawasaki, K., Yin, J. J., Subczynski, W. K., Hyde, J. S., and Kusumi, A. (2001) Biophys. J. 80 738-748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaw, A. S. (2006) Nat. Immunol. 7 1139-1142 [DOI] [PubMed] [Google Scholar]
- 55.Mukherjee, S., and Maxfield, F. R. (2004) Annu. Rev. Cell Dev. Biol. 20 839-866 [DOI] [PubMed] [Google Scholar]
- 56.Almeida, P. F., Pokorny, A., and Hinderliter, A. (2005) Biochim. Biophys. Acta 1720 1-13 [DOI] [PubMed] [Google Scholar]
- 57.Collins, M. D., and Keller, S. L. (2008) Proc. Natl. Acad. Sci. U. S. A. 105 124-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yethiraj, A., and Weisshaar, J. C. (2007) Biophys. J. 93 3113-3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.