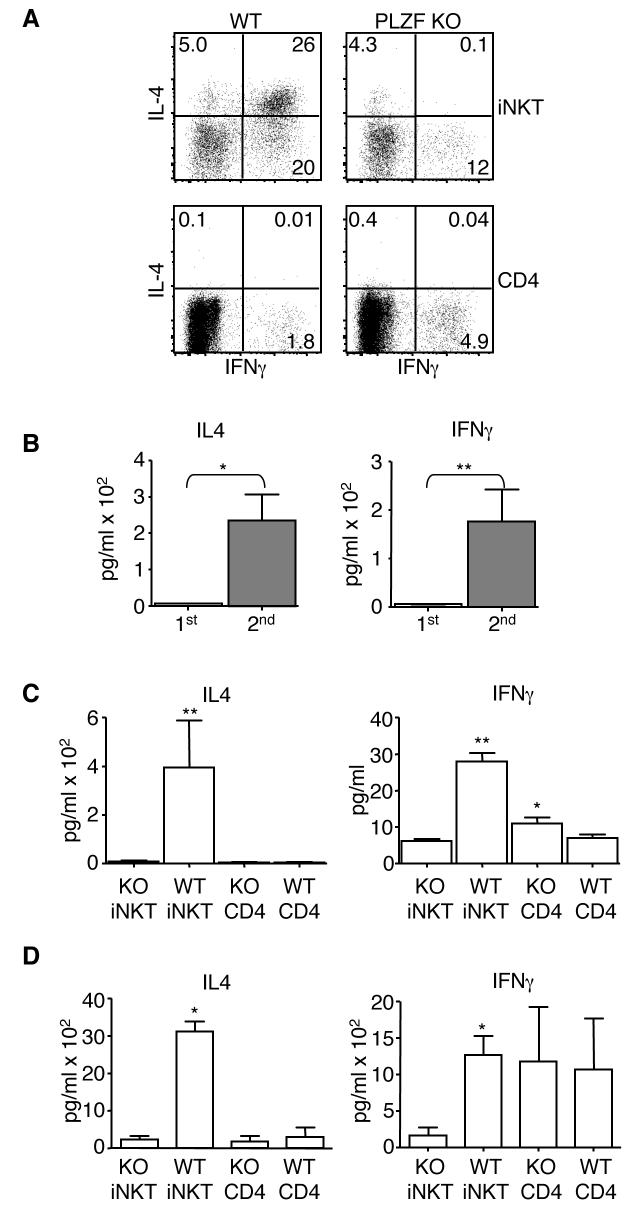
Fig. 7. PLZF deficient iNKT present an altered cytokine secretion pattern.
(A) Cells were collected by cell sorting and then activated with 50 ng/ml PMA and 500 ng/ml ionomycin for a total of five hours. Brefeldin A was added for the last four hours of the incubation. Cells were then fixed and permeabilized with BD's perm/fix reagents. Permeabilized cells were then stained with antibodies against IL-4 and IFNγ. (B) Single cell suspensions of combined lymph node and spleen cells were depleted of CD8 and MHC class II expressing cells and then stained with the CD1d tetramer and an antibody against TCR Cβ. Tetramer+,TCR Cβ+ cells then were collected by FACS. Approximately 1×104 of each T cell type was activated with 5 μg/ml plate bound anti-CD3 and 5 μg/ml soluble anti-CD28 in the presence of IL-2 and IL-15. Supernatants from the primary activation were collected after 24 hours. Cells were then fed with fresh media containing IL-2 and IL-15. After a total of five days, supernatant was removed and discarded. The cells, in fresh media, were reactivated with plate bound anti-CD3 and soluble anti-CD28. Supernatants were again collected after 24 hours. Supernatants were analyzed for the presence of IL-4 and IFNγ by the use of BD Biosciences Cytokine Bead Arrays. Supernatants were collected and analyzed as described above from sorted T cells that were (C) activated and then (D) reactivated as described above. Each T cell type was set up in duplicate or triplicate depending upon the number of cells collected. Repeats from each experiment were averaged. A total of five independent experiments were done. Means and standard deviations for the five experiments are shown. The value for the PLZF deficient iNKTs (KO iNKT) was compared to the other three cell types. P values are marked as * <0.05; ** <0.005. P values were derived using a two-tailed, unparied Mann-Whitney U-test. Choices of statistical tests are explained in the methods section.