Abstract
The faces of birds and mammals exhibit remarkable morphologic diversity, but how variation arises is not well-understood. We have previously demonstrated that a region of facial ectoderm, which we named the Frontonasal Ectodermal Zone (FEZ), regulates proximo-distal extension and dorso-ventral polarity of the upper jaw in birds. In this work, we examined the equivalent ectoderm in murine embryos and determined that the FEZ is conserved in mice. However, our results revealed that fundamental differences in the organization and constituents of the FEZ in mice and chicks may underlie the distinct growth characteristics that distinguish mammalian and avian embryos during the earliest stages of development. Finally, current models suggest that neural crest cells regulate size and shape of the upper jaw, and that signaling by Bone morphogenetic proteins (Bmps) within avian neural crest helps direct this process. Here we show that Bmp expression patterns in neural crest cells are regulated by signals from the FEZ. The results of our work reconcile how a conserved signaling center that patterns growth of developing face may generate morphologic diversity among different animals. Subtle changes in the organization of gene expression patterns in the FEZ could underlie morphologic variation observed among and within species, and at extremes, variation could produce disease phenotypes.
Keywords: Sonic Hedgehog, Fibroblast growth factor 8, Bone morphogenetic protein, Bmp-2, Bmp-4, Bmp-7, Frontonasal Ectodermal Zone (FEZ), mouse-chick chimera
Introduction
Formation of the face in vertebrates occurs through development of homologous facial primordia. In part, the initial divergence of facial morphologies in birds and mammals results from unique patterns of growth within the facial primordia that will form the upper jaw. In chicks the middle and upper face is derived from the frontonasal process (FNP). In mice, initial growth is enhanced in lateral regions of the face. Instead of an FNP, this part of the mammalian face is comprised of paired median nasal processes (MNP). In mice, as the right and left median nasal processes grow, the mesenchymal tissues forming the medial edge of each MNP merge and form the FNP. Therefore, understanding mechanisms that regulate the establishment of these different growth zones will illuminate the regulation of patterning and variation in the shape of the upper jaw.
A series of reciprocal signaling interactions among the forebrain, the neural crest, and the surface ectoderm controls morphogenesis of the upper jaw. For example, signals from the brain and neural crest regulate gene expression within the adjacent surface ectoderm (Marcucio et al., 2005; Schneider and Helms, 2003). In turn, the ectoderm signals back to the mesenchyme. In the cephalic ectoderm of avian embryos, we defined a boundary between cells expressing Sonic hedgehog (Shh) and Fibroblast growth factor 8 (Fgf8), and we named this tissue Frontonasal Ectoderm Zone (FEZ; (Hu et al., 2003)). We showed that transplantation of the FEZ to ectopic locations within the FNP induces expression of down-stream targets of the SHH pathway (i.e., Ptc and Gli1 (Dahmane et al., 1997; Goodrich and Scott, 1998; Lee et al., 1997)) in neural crest cells, and induces duplications of the upper beak. Interestingly, this work also revealed that the FEZ induces duplications of the lower jaw when transplanted onto the mandibular process, but does not affect the morphology of the hyoid arch (Hu et al., 2003). These results indicate that the FEZ does not specify regional anatomical identity of the underlying neural crest cells. Rather, the FEZ controls dorso-ventral polarity and proximo-distal extension of the FNP by evoking intrinsic responses from the mesenchyme in chicks. However, the extent to which the FEZ is a conserved signaling center that could participate in generating unique avian and mammalian growth zones is not known.
Growth of facial primordia is regulated by a variety of signaling molecules including: Bone morphogenetic proteins (Bmps), Fibroblast Growth Factors (Fgfs), Sonic Hedgehog (Shh), Retinoids, and Wingless family members (Wnts) (Depew and Simpson, 2006; Hu and Helms, 1999; Lee et al., 2001; Mina et al., 1994; Mina et al., 2002; Richman et al., 2006; Richman et al., 1997; Song et al., 2004; Wilke et al., 1997). Of these pathways, the role that Bmp signaling plays in regionalizing domains of cell proliferation in the upper jaw anlagen has been characterized. Differences in proliferation between avian species are associated with Bmp signaling in neural crest mesenchyme in the FNP (Wu et al., 2006). Furthermore, differential expression of Bmp-2 and -4 in neural crest mesenchyme correlates with variation in shape and size of the upper beak observed among Darwin's finches (Abzhanov et al., 2004), and activation of Bmp signaling creates localized growth zones that transform the shape of the cartilage elements that comprise the upper beak (Abzhanov et al., 2004; Wu et al., 2006; Wu et al., 2004). Together, these results underscore the importance of signaling by Bmps in neural crest mesenchyme.
In this work we examined the ectoderm covering the median nasal processes for evidence of a FEZ in mice. While many studies have examined the expression patterns of Fgf8 and Shh in the mouse (e.g., (Jeong et al., 2004; Kawauchi et al., 2005)), the distinct relationship between these two genes and other molecules expressed by the mouse FEZ has not been illustrated. Further, there has been no direct comparison of these expression patterns between mammals and birds. Therefore, we compared the molecular constituents of the FEZ between mice, chicks, and ducks, and then we performed a functional analysis of the mouse FEZ. We demonstrate that the FEZ is conserved between mice and chicks. Thus, the FEZ may be a fundamental signaling center that participates in regulating development of the upper jaw in vertebrates, in part by regulating expression patterns of Bmps in neural crest mesenchyme. Overall, our results indicate that the unique molecular organization of the FEZ in mice and chicks correlates with the divergent facial characteristics that are apparent during the earliest stages of facial development in birds and mammals. The FEZ appears to be a source of patterning information that could contribute to variation in facial form, and at extremes, variation produced by the FEZ could create disease phenotypes such as those observed in formes frustes of Holoprosencephaly (HPE) or cleft lip and palate.
Materials and Methods
Preparation of embryos and engraftment of the mFEZ
Fertilized chicken eggs (Gallus gallus, Petaluma Farms, Petaluma, CA) were prepared for surgical manipulations as follows. Embryos were incubated to Hamburger and Hamilton stage 10 (HH 10 (Hamburger and Hamilton, 1951)) and then a small hole was made in the shell directly over the embryo after removing 1.0ml of albumin. Embryos were returned to the incubator until HH 21 or 25. At this time the ectoderm covering the dorsal region of the FNP was removed with a sharpened tungsten needle. Grafts were prepared from mouse embryos at e10 as described (Hu et al., 2003). Briefly, mouse embryos were dissected from uteri of euthanized dams and placed in ice cold PBS. Facial tissues were dissected from embryos and were placed in dispase (2.5U/ml in PBS) for 20 mins. Then putative FEZ ectoderm was removed, and the graft was transferred to the host and positioned to replace the removed ectoderm. The graft was secured with glass pins (Supplemental Figure 1, and see (Hu et al., 2003)). Mouse embryos were collected at e9.5, e10, and e10.5 for in situ hybridization analysis. Animal procedures were approved by the UCSF IACUC.
Histology
Twenty-four hours after engraftment and at days 9, 12, and 13 days of development embryos were collected, fixed in 4% paraformaldehyde, stained with ethidium bromide, and then photographed using epifluorescent or brightfield illumination on a Leica MZFLIII microscope connected to a computer. After documentation, chimeras were dehydrated, embedded in paraffin, and sectioned (10μm). Sections were stained with Safranin-O/Fast Green to visualize cartilage (Lu et al., 2005), modified Milligan's Trichrome to visualize bone (Lu et al., 2005). Sections were imaged using a Leica DM5000B and Adobe Photoshop.
In situ hybridization
Patterns of gene expression in chimeras and normal mouse, chick, and duck embryos were analyzed on tissue sections and/or in whole mount via in situ hybridization using radiolabeled or digoxigenin-labeled riboprobes as previously described (Lu et al., 2005). Subclones of mouse B2 SINE (Bollag et al., 1999), Shh, Fgf8, Bmp-2, Bmp4, and Bmp-7, and chick Bmp2, Bmp4, Bmp7, Shh, Fgf8, Msx1, and Msx2 were linearized to transcribe riboprobes. Images of in situ hybridization assays performed on tissue sections are pseudo-colored superimpositions of the in situ hybridization signal and a blue nuclear stain (bis-benzimide; Sigma). Whole mount in situ hybridizations were photographed using a Leica MFLZIII dissecting microscope.
Results
Ontogeny of the murine FEZ
We initially identified the FEZ in chick embryos based on the presence of a boundary between Shh and Fgf8-expressing cells in the ectoderm covering the FNP at HH 20 (Hu et al., 2003). To compare the relationship between Shh and Fgf8 expression domains in the FEZ in birds and mammals, we performed whole mount in situ hybridization. In mice and chicks, Fgf8 transcripts were detected in the ectoderm that spans the medio-lateral axis of the developing middle and upper face (Fig. 1A,B). However, expression of Shh in these animals is unique. In chick embryos Shh and Fgf8 expressing cells form a boundary in the ectoderm covering the neural crest cells that comprise the avian FNP at Hamburger and Hamilton Stage 20 ((HH20) (Hamburger and Hamilton, 1951)), and Shh expression is continuous across the medio-lateral axis of the FNP (Fig. 1C,E (Hu et al., 2003)). This same pattern is also observed in duck embryos at (Supplemental Figure 2A). In contrast to the avian FEZ, the murine FEZ is not a single signaling center. Rather Shh is expressed in domains on the right and left side of the mouse face creating left and right FEZs (Fig. 1D,F). In these regions neural crest cells are present, but these cells are absent or greatly reduced at the midline. A bilateral pattern of Shh expression is also observed in human embryos in this region and is accompanied by reduced mesenchymal cells in this region (Odent et al., 1999) and the presence of median nasal processes rather than a Frontonasal Process. Thus, these unique patterns of Shh expression in the FEZ correlate with the morphology that distinguishes avian and mammalian embryos at these early times.
Figure 1. Distinct organization of the FEZ in chicks and mice.
(A) Fgf8 expression in the ectoderm covering the middle part of the upper jaw in chick and (B) mouse embryos are similar. Transcripts are detected across the medio-lateral axis of this region of the face. (C,E) In chick embryos a single domain of Shh expression spans the medio-lateral axis of the FNP (arrow). (D,F) In mice, Shh expression is restricted to lateral domains of ectoderm (arrows). Shh expression in the basal forebrain is circled. (G) The FEZ and mice in chicks are organized differently. In chicks a single boundary between Fgf8 and Shh expressing cells spans the medio-lateral axis of the FNP. In mice a Fgf8/Shh boundary is formed on the left and right side of the face. Scale bars: A-E=500μm, F=250μm.
Our next step in characterizing the murine FEZ was to examine the ontogeny of Shh and Fgf8 expression prior to and after outgrowth of the median nasal processes had begun. At e9.5 (n=3) Shh expression was detected in the forebrain epithelium but not in the surface ectoderm in either medial or lateral domains (Fig. 2A,B and see supplemental Figure 2). In contrast, Fgf8 transcripts were detected in the forebrain, and in medial and lateral domains of the surface ectoderm (Fig. 2G,H). By e10.0 (n=6) Shh transcripts were not present in the medial region of the face (Fig. 2C, Supplemental Figure 2), and concomitantly there were no neural crest cells in this region of the face. In contrast, Shh transcripts were present in the ectoderm adjacent to neural crest cells that formed the median nasal processes (Fig. 2D), and this domain forms a boundary with cells expressing Fgf8 (Fig. 2I,J). The boundary between Shh and Fgf8-expressing cells persists in the left and right median nasal processes at e10.5 (Fig. 2F,L,K, n=10), but no Shh transcripts were detected in ectodermal cells located between the median nasal processes (Fig. 2E). Again, no neural crest cells were present in the midline of the mouse face.
Figure 2. Ontogeny of the FEZ in mice.
(A) In situ hybridization to detect Shh transcripts in a sagittal section through the midline of the mouse face at e9.5 reveals that Shh (red) expression is limited to the ventral neural tube (arrows). No expression is detected in the ectoderm of the face (arrowhead). (B) In a lateral section through this same embryo, Shh is still not expressed in the stomodeal ectoderm (arrowhead). Shh transcripts are detected in the endoderm of the mandible (mn) and in the ventral neural tube (arrow). (C) At e10.0 Shh transcripts are restricted to the neural tube in the facial midline (arrows). Shh expression is not observed in the stomodeal ectoderm (arrowhead). (D) In contrast, in a section from a more lateral region of this same embryo, Shh expression is detected in the stomodeal ectoderm (arrowhead). (E) At e10.5 Shh transcripts are still restricted to the ventral neural tube in the midline, but (F) expression of Shh in the stomodeal ectoderm (arrowhead) extends to the ventral edge of the growing tip of the upper jaw in lateral regions. (G) In contrast to Shh, Fgf8 transcripts (yellow) are detected in the stomodeal ectoderm (arrowheads) in medial and (H) lateral regions at e9.5, (I, J) e10.0, and (K, L) e10.5. Bis-benzimide was used as a counterstain so nuclei appear blue. rp=Rathke's pouch. Scale bars: 200μm.
In addition to Shh and Fg8, Bmps are also expressed in the FEZ of birds and mice. In chick embryos at HH 22 (n=10) Bmp-2 (Fig. 3A), Bmp-4 (Fig. 3B), and Bmp-7 (Fig. 3C) transcripts were present in the FEZ. Again, the expression of these genes spanned the entire medio-lateral axis of the FNP. In mice, Bmp-2 expression was not evident in the FEZ during the times we examined (e9-5,n=3; e10.0, n=6; e10.5,n=10, Fig. 3D,G and data not shown). At e10.5 Bmp-4 transcripts were detected in the lateral areas of the FEZ (Fig. 3H), but not in medial regions (Fig. 3E). Bmp-7 transcripts were present throughout the FEZ (Fig. 3F,I). Collectively, the results of this gene expression analysis suggest a FEZ is present in ectoderm covering the developing mouse face, but the spatial arrangement and some of the molecular constituents of the FEZ are different in mice and birds (Fig. 1G).
Figure 3. Expression of Bmps in the FEZ.
(A) In situ hybridization on a section through the middle of a HH22 chick face reveals the presence of Bmp-2 transcripts (purple) in the proximal FEZ (arrow) and in the diencephalon (asterisk) at this time. (B) Similarly, Bmp-4 and (C) Bmp-7 transcripts are present in the FEZ at HH 22. (D) In sections through medial regions of the mouse face Bmp-2 and (E) Bmp-4 are not expressed in the ectoderm covering the medial part of the upper jaw (arrowhead). Bmp-4 transcripts are detected in the anterior region of Rathke's pouch (arrows) and in the telencephalon. (F) Bmp-7 transcripts (yellow) are present in the ectoderm in this region of the mouse face (arrow) and in the forebrain. (G) Bmp-2 transcripts are not detected in ectoderm covering lateral regions of the mouse face (arrowhead). (H) Bmp-4 and (I) Bmp-7 are expressed in similar spatial domains in lateral regions of murine ectoderm (arrows) and the adjacent neural crest cells. Scale bars=250μm.
FEZ function is conserved across species
Based on our gene expression analysis, the FEZ appears to be present in mouse embryos. We assessed the functional capacity of the murine FEZ by replacing the chick FEZ at HH 21 with the equivalent ectoderm derived from e10.0 mouse embryos, or as a control, with flank ectoderm from the mouse. Transplantation of flank ectoderm to the avian FEZ did not produce morphologic alterations (Fig. 4D, n=5) which agrees with our previous observations (Hu et al., 2003). However, when we grafted the mouse FEZ onto the developing FNP of chicks, we observed morphologic alterations. The grafted mouse FEZ altered the morphology of the avian FNP even thought the grafted tissue was very small and occupied up to about one-third of the FNP (Supplemental Fig.1). In these mouse-chick chimeras we observed that the distal portion of the upper jaw was duplicated at day 9 (Fig. 4B). There were two egg teeth present on the upper beak, and there was a cleft between the two tips (n=5). We performed whole mount in situ hybridization to detect the mouse-specific repetitive DNA element B2SINE. As expected, we observed that one of the tips was associated with the transplanted mouse ectoderm while the other was associated with chick ectoderm (Fig. 4C). Thus, by transplanting the mouse FEZ onto the FNP of developing chick embryos, the avian FEZ was altered. In these chimeras, there were two FEZ's located side-by-side, and we observed a duplication of the upper beak.
Figure 4. The mouse FEZ can partially replace the chick FEZ.
(A) Dorsal view of a normal chick embryos at day 9 of development. The egg tooth (black arrow) is clearly visible on the dorsal surface of the upper beak. (B) At day 9, a dorsal view of a mouse-chick chimera clearly demonstrates the presence of a duplication of the distal tip of the upper beak. The autochthonous egg tooth (black arrow) and the ectopic egg tooth (red arrow) are visible. (C) In situ hybridization with the antisense SINEB2 probe illustrates the location of the transplanted mouse ectoderm (red bracket). (D) Transplantation of flank ectoderm to the avian FNP does not alter the morphology of the upper beak. Scale bars: A,B, D=2mm, C=1mm.
In order to examine changes in the mesenchyme in response to the grafted ectoderm, we transplanted the putative FEZ ectoderm from mouse embryos at e10.0 to the dorsal surface of the FNP of HH 25 chick embryos (Hu et al., 2003). Using this approach we could position the small graft adjacent to responsive mesenchyme located at a distance from the autochthonous FEZ, and then we could observe changes exclusively due to the presence of the murine ectoderm. We examined mouse-chick chimeras at various times after engraftment. At day 9 (n=3, Fig. 5A-C), 12 (n= 3, Fig. 5D-F), and 13 (n=1, Fig. 5G-I) ectopic growths were evident on the upper beaks (Fig. 5A,D,G). These outgrowths were comprised of skeletal elements that were integrated with the host skeleton, and the bone and cartilage developed with the same dorso-ventral polarity as in the autochthonous beak (Fig. 5B,H). Furthermore, an egg tooth is normally formed in ectoderm covering the dorsal surface of the distal tip of the upper beak, and an egg tooth was also observed on the dorsal surface of each duplicated beak (Fig. 5E,H). Again, we used in situ hybridization to detect the mouse-specific repetitive DNA element B2SINE. The ectopic egg tooth was formed exclusively by host ectoderm (Fig. 5F); in this embryo, the mouse ectoderm was not apparent in sections containing the egg tooth. Nonetheless, these results indicate that in addition to patterning the skeleton, the FEZ induces differentiation of the egg tooth in birds. Importantly, we never observed hair, or hair follicles, in mouse-chick chimeras supporting the idea that specification of ectodermal appendages arises from signals provided by the mesenchyme (Schneider, 2005).
Figure 5. Function of the mFEZ.
(A) At day 9 of development an ectopic outgrowth (arrow) was observed on the upper jaw of chimeras. (B) Safranin-O fast green staining of a section through the outgrowth reveals the presence of a cartilage element (red). Inset outlines regions shown in C. (C) In situ hybridization with B2SINE indicates that grafts were comprised exclusively of ectoderm. (D) At day 12 of development chimeras had an outgrowth (arrow) on the upper jaw. (E) These outgrowths were covered by ectoderm that had formed an egg tooth (arrow). The cartilage in this outgrowth is stained red (asterisk). (F) In situ hybridization with B2SINE reveals that the egg tooth is derived from the host ectoderm and indicates the transplanted murine ectoderm did not form an avian-specific structure in response to host signals. (G) An ectopic outgrowth (arrow) is present on the upper beak of chimeric embryos at day 13. (H) Low and (I) high magnification through the upper beak of this chimera reveals the presence of bifurcated cartilage (asterisk) and ectopic bone (arrow). Scale bars A,D, G=5mm , B,C,I=500μm , E,F=100μm, H,=2mm.
Response of neural crest mesenchyme to the FEZ
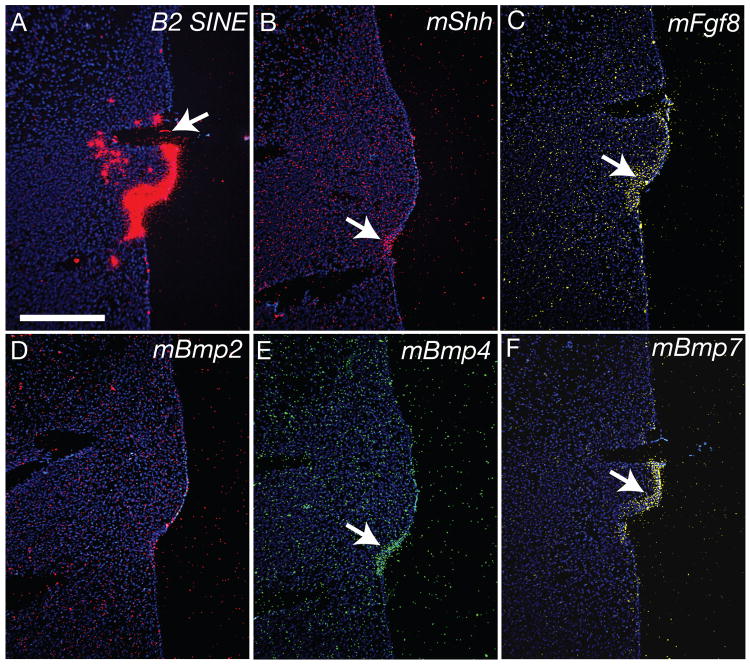
Our results demonstrate that mouse ectoderm covering the median nasal processes contains patterning information that regulates morphogenesis of the upper jaw. Next, we examined mechanisms responsible for patterning. Twenty-four hours after engraftment (n=5/5) Shh (Fig. 6B) and Fgf8 (Fig. 6C) transcripts were still present in the donor ectoderm (Fig. 6A). Additionally, using mouse-specific in situ hybridization probes we observed mBmp-4 (Fig. 6E), and mBmp-7 (Fig. 6F) expression in the transplanted ectoderm, but mBmp-2 expression was not induced in the grafted ectoderm (Fig.6D).
Figure 6. Gene Expression in the Transplanted FEZ is Maintained.
(A) In situ hybridization for B2SINE illustrates the location of the donor ectoderm. The section is adjacent to those in B-F. The arrow indicates the location of the pin that was used to hold the graft in place, and the internalized mouse tissue is ectoderm as judged histologically. (B) Twenty-four hours after engraftment, the donor ectoderm expressed murine Shh (red; n=5) and (C) mFgf8 (yellow; n=5). (D) We observed no evidence of induction of mBmp-2 in the grafted mouse ectoderm, but (E) mBmp-4 and (F) mBmp-7 expression (green; n=5) was maintained in the grafted ectoderm at this time. Scale bar=250μm.
In our previous work, we demonstrated that Msx-1 was up-regulated in response to grafted avian ectoderm (Hu et al., 2003). We assessed whether the chick mesenchyme had a similar response to the grafted mouse ectoderm. Indeed, we observed ectopic expression of Msx-1 and Msx-2 in mesenchyme adjacent to the grafted mouse ectoderm (data not shown). We also examined expression of Bmps in mesenchyme beneath the grafted tissue, because these molecules regulate morphogenesis of the upper jaw (Abzhanov et al., 2004; Wu et al., 2006; Wu et al., 2004). Normally, at this time (∼HH28) Bmp-2, Bmp-4, and Bmp-7 expression patterns become restricted to the mesenchyme in the tip of the growing beak (Fig. 7A-H). Bmp-2 (Fig. 7B) was expressed in the dorsal region, Bmp-7 (Fig. 7D) was expressed in the ventral region, and Bmp-4 (Fig. 7C) was expressed throughout the growing FNP. In mesenchyme located in the region of the grafted ectoderm, Bmp-2 (Fig. 7F), Bmp-4 (Fig. 7G), and Bmp-7 (Fig. 7H) transcripts were not detected. Adjacent to grafted ectoderm in chimeras we observed strong up-regulation of Bmp-2 (Fig. 7I) and Bmp-7 (Fig. 7L), and weak up-regulation of Bmp-4 at this time in each chimera (n=5/5), Fig. 7K and Supplemental Figure 3). These results indicate that signals from the FEZ regulate expression of patterning genes in neural crest cells that will form the skeleton of the upper jaw.
Figure 7. Response of the Avian Mesenchyme to the mFEZ.
(A) Box indicates location of sections in B-D. (B) Bmp-2 (red, n=4) is expressed in the mesenchyme in the dorsal region of the expanding FNP (arrow). (C) Bmp-4 expression (green, n=4) is restricted to a large domain within the tip of the expanding FNP (arrows), and (D) Bmp-7 (yellow, n=4) is expressed in the mesenchyme in the ventral region of the growing FNP (arrow). (E) Box indicates area seen in F-H. (F) In controls Bmp-2, (G) Bmp-4, and (H) Bmp-7 transcripts were not detected in the dorsal region of the FNP embryos near where the grafts were positioned. (I) In situ hybridization for the mouse-specific B2SINE (red) on the section nearly adjacent to those in J-L was used to assess the distribution of donor ectoderm in chimeras. Positive cells inside the mesenchyme is herniated ectoderm (See Figure 6A). (J) Bmp-2 transcripts (red, arrow; n=5) are up-regulated in the dorsal region of the ectopic outgrowth (bracket). (K) At this time, a small domain of ectopic Bmp-4 expression (green, arrows; n=5) is evident in the middle region of the ectopic outgrowth (bracket). (L) Bmp-7 transcripts (yellow, arrow; n=5) are up-regulated in the ventral region of the ectopic outgrowth (bracket). Scale bar, A, E, I=500μm, B-D, F-H, J-L= 250μm.
Discussion
In the developing head multiple epithelia produce morphogenetic signals that participate in regulating facial development. For example, the brain is an important signaling center that controls facial development (DeMyer, 1964; Marcucio et al., 2005; Schneider et al., 2001). In our previous work we isolated the role of SHH signaling within the brain (Marcucio et al., 2005), and we demonstrated that the brain participates in regulating the onset of Shh expression in the FEZ. However, the lack of appropriate genetic models to remove expression of signaling molecules exclusively within the cephalic ectoderm at the correct times has precluded analysis of the role that this tissue plays during development of the upper jaw in mammals. To overcome this problem we created mouse-chick chimeras to assess the function of the murine FEZ, and we determine that the FEZ is conserved in mouse and chick embryos. Further, our results revealed that the FEZ controls morphogenesis of the medial region of the upper jaw by regulating molecules that are involved in growth (Ashique et al., 2002b; Foppiano et al., 2007) and patterning (Ashique et al., 2002a; Barlow et al., 1999; Barlow and Francis-West, 1997; Masayoshi Kawakami, 2006) of facial primordia. Collectively, these results demonstrate that the FEZ regulates patterned growth of the upper jaw anlagen.
Conservation of function between mFEZ and cFEZ
Our experiments suggest that mechanisms mediating function of the FEZ are highly conserved between mice and chicks. For example, when transplanted ectopically the FEZ from the mouse and the chick induce duplications of the distal portion of the upper beak that exhibit dorso-ventral polarity. Similarly, Msx-1 is induced in the mesenchyme in response to either murine (not shown) or avian (Hu et al., 2003) ectoderm. Additionally, ectodermal responses to the FEZ are similar; transplanted mouse and chick (Hu et al., 2003) ectoderm induced egg tooth formation in host ectoderm. These observations suggest that molecular signals emanating from the FEZ are shared between the signaling centers in these two species, and that these signals evoke intrinsic responses from underlying mesenchymal cells. While we are unable to replace the mouse FEZ with the equivalent avian tissue or remove the mouse FEZ to directly test the necessity of this signaling center for development of the mouse jaw, the similarity of function between the chick and mouse FEZ in our chimeric assay suggests that the FEZ is an important signaling center that patterns the developing primordia of the upper jaw in mammals.
How does the FEZ form?
Neural crest cells and the brain participate in regulating the onset of Shh expression in the FEZ. When duck neural crest cells were replaced with crest from the more precocious quail, Shh expression in the duck host was accelerated (Schneider and Helms, 2003). Similarly, Shh expression in the FEZ was delayed by ablating presumptive neural crest cells. In these experiments the cut edge of the neural tube regenerated neural crest cells, and Shh expression began once the regenerated cells reached the FNP (Marcucio et al., 2005). In zebrafish Shh expression in the oral ectoderm requires the presence of neural crest cells (Eberhart et al., 2008). Based on these observations, the establishment of unique organization of the FEZ in mice and chicks may reflect the intrinsic differences that are apparent in the distribution of neural crest cells in this region of the face. In chicks, neural crest cells are present across the entire mediolateral axis of the FNP, but in mice, these cells are located in lateral regions adjacent to the areas of Shh expression. Additionally, SHH signaling within the forebrain is required for Shh expression in the FEZ (Marcucio et al., 2005; Eberhart et al., 2006), and unique signals from this tissue may also regulate spatial organization of the FEZ.
Variation in FEZ organization is associated with early facial form
Our findings suggest that the initial growth patterns that generate Median Nasal Processes in mammals and Frontonasal Processes in birds result from distinct organization of the FEZ between these classes of animals. In chicks and ducks the FEZ is a single unit that spans the FNP, but in mice and humans, the FEZ is divided into left and right halves. Accordingly, the FNP initially extends as a unified entity, but growth in the homologous region in mammalian embryos occurs in the left and right median nasal processes which merge in the midline as development proceeds. We did not observe an avian-to-mammalian transformation when we replaced the avian FEZ with a transplanted murine FEZ. Rather, we observed a duplication of the distal tip of the upper beak. We determined that the transplanted mouse ectoderm was associated with one of the duplicated structures, while chick ectoderm was associated with the other. The grafted mouse ectoderm was very small and spanned less that one third of the avian FNP. Hence, any spatial patterning information contained in the mouse ectoderm was likely diluted in the presence of the large amount of mesenchyme in the host. In fact, the presence of neural crest cells in the midline of the chick FNP and their absence in the homologous region of the mouse indicates that the distribution of cells that can respond to the FEZ are also unique between these animals, and these differences are also likely to contribute to the distinct morphologies that are observed. Alternatively, the transplanted mouse FEZ may have been reprogrammed by the chick mesenchyme. For instance, when we examined Shh expression in the transplanted tissue we never observed a gap in expression of Shh. This observation suggests that the avian neural crest cells may have induced Shh expression in the grafted FEZ. However, this must be interpreted cautiously since we were not able to examine the transplanted mouse ectoderm in its entirety, and we may have missed the Shh-negative region.
The extent that the FEZ regulates species-specific morphology is unknown. However, our data suggest that the FEZ participates in the dialogue that patterns the developing uppe jaw. The FEZ appears to evoke species-specific responses from the adjacent mesenchyme. This agrees with previous data indicating that neural crest cells autonomously control shape and size of skeletal elements in the head (Schneider and Helms, 2003; Tucker and Lumsden, 2004). Unique signals from the brain and other adjacent signaling centers as well as information contained in the neural crest cells themselves are likely to elaborate species-specific morphologies. For instance, regulation of Bmp-4 expression by Fibroblast growth factors produced in the nasal pit has been suggested to regulate the widening of the bill that occurs in duck embryos (Szabo-Rogers et al., 2008). Nonetheless, the mouse and chick FEZ appear to use similar molecular signals to establish different growth zones in the upper jaw, and these growth zones correspond to the morphological features that distinguish the earliest developmental features of the avian and mammalian face (Fig. 8).
Figure 8. Organization of the FEZ Regulates Patterning of the Upper Jaw.
(A) Diagrammatic view of the front of the HH 20 chick face. The various primordia are indicated by color. The FNP is blue, the Lateral nasal processes (LNP) are yellow, the maxillary process (MXP) is green, and the mandible (mn) is pink. A red box on the FNP represents the FEZ. (B) A diagrammatic illustration of the mouse face at e10.5. Colors correspond to the homologous region of the chick face shown in A, and the median nasal processes are blue. The FEZ (red boxes) in mice are located on each of the median nasal processes. We propose that signaling by each FEZ generates the unique facial morphologies observed in (C) chicks and (D) mice in part by regulating Bmp expression patterns and controlling growth.
In addition to tissues that regulate morphogenesis of the upper jaw, the role of specific signaling molecules have been described. For instance, species-specific patterning of the upper jaw in birds has been shown to rely partly on signaling by BMP-2 and BMP-4 (Abzhanov et al., 2004). Further, BMP-4 has been shown to establish dual growth zones in the mesenchyme of the developing upper jaw mesenchyme of duck embryos (Wu et al., 2006; Wu et al., 2004). Our data indicate that the FEZ participates in regulating expression of Bmp-2, Bmp-4, and Bmp-7 in neural crest mesenchyme. Previous work has demonstrated that combinatorial signaling by FGF8 and SHH induces Bmp-4 expression in neural crest mesenchyme at late stages of avian development. In these embryos, ectopic cartilaginous outgrowths were observed (Abzhanov and Tabin, 2004). These observations support our conclusion that signals from the FEZ act upstream of molecules that regulate morphogenesis of the skeleton of the upper jaw.
Regulation of variation by the FEZ may have important clinical implications as well, because extremes in morphologic variation could create birth defects. Disruptions to SHH signaling (Ahlgren and Bronner-Fraser, 1999; Chiang et al., 1996; Jeong et al., 2004) or the FEZ (Cordero et al., 2004) produce catastrophic structural defects in the face. For example, altered SHH signaling creates defects in the middle of the upper jaw and face in individuals with Holoprosencephaly (HPE; reviewed in: (Dubourg et al., 2007)). A hallmark of HPE is variable presentation of the disease phenotype (McKusick, 2000; Ming and Muenke, 2002). At extremes, fetuses exhibit severe forebrain defects and cyclopia characterized by a single median eye located beneath a proboscis. Microforms of HPE include midfacial hypoplasia, a single central incisor, and hypotelorism, while some individuals with mutations in HPE genes are clinically unaffected. However, there is no clear phenotype-genotype correlation that adequately explains the spectrum of phenotypes in patients with HPE (Marini et al., 2003; Ming and Muenke, 2002; Nanni et al., 1999; Roessler et al., 1996) indicating the multifactorial etiology of the disease. These observations inspired studies to identify multiple mutations in patients with HPE and correlate these mutations with severity of phenotype (Ming and Muenke, 2002). Our results suggest that genes involved in regulating formation or function of the FEZ may be candidates for “second hit genes.” These molecules may participate in generating facial defects associated with HPE by contributing to variation in morphogenesis of the upper jaw and mid-face. Therefore, understanding how the FEZ forms, and in particular how the spatial domain of Shh expression is regulated, could provide significant insight into mechanisms of facial patterning and disease phenotypes.
In conclusion, results of our work illustrate a complex set of tissue interactions among forebrain, neural crest, and stomodeal ectoderm that regulate the earliest morphogenetic events during development of the upper jaw. Previously we showed that the brain is an important signaling center that participates in regulating facial development, in part by controlling the onset of FEZ activity (Marcucio et al., 2005). In addition, neural crest cells (Marcucio et al., 2005; Schneider and Helms, 2003) and Bmps (Foppiano et al., 2007) participate in regulating Shh expression in the FEZ. The FEZ then regulates expression of Bmps in neural crest mesenchyme and establishes growth patterns in the upper jaw anlagen. These mechanisms appear conserved between mice and birds, but our results illustrate that differences in the earliest patterns of growth within the upper jaw may be generated through a relatively simple mechanism. The spatial organization pattern of genes that are expressed in the FEZ correspond to either median or frontonasal processes. Signals from the brain or migration of neural crest patterns may be different between mammals and birds and could establish unique organization of the FEZ, but further work is required to identify the regulatory differences that control these unique patterns. Nonetheless, these results reconcile how similar molecular signals may generate morphological diversity in the face. These molecular differences are apparent during the earliest stages of facial development, and they presage outgrowth of facial primordia. Thus, distinct patterns of facial morphogenesis in birds and mammals is apparent before there is gross evidence of divergent facial morphology. In fact, formation of the distinct FEZ in mammals and birds is currently the earliest known feature of the developing upper jaw that distinguishes these organisms. Future exploration of the interactions that regulate formation and organization of the FEZ is likely to yield evidence of even earlier differences and will allow us to identify avenues by which variation in facial morphology can be influenced.
Supplementary Material
(A) Image of an e10 mouse (B) HH21 chick, and (C) HH25 chick embryo that were used in grafting experiments. The graft was derived from the developing upper jaw of the mouse (A, red box) and transplanted either in place of the avian FEZ (B, HH21), or onto the dorsal surface of the FNP (C, HH25). The red box in A shows the location of where the graft was derived from, but the shape of the graft was not square due to the three-dimensional morphology in this region of the mouse face. The red box in B and C shows the placement of each graft. The magnification of each image and the size of the red box are identical in each picture. Scale bar=500μm.
(A)At e9.0 Shh is not expressed in ectoderm spanning the medial portion of the developing murine upper jaw anlagen. (B) Higher magnification of A showing expression of Shh in the forebrain (arrows), but not in the ectoderm (arrowhead). (C) Even higher magnification of B, demonstrating the absence of Shh transcripts in the medial ectoderm. (D,E,F) Similarly, successively higher magnifications through the medial portion of the upper jaw of embryos at e10. 0 demonstrate that Shh transcripts are present in the brain (arrows), but not in the ectoderm (arrowhead). (G,H,I) In contrast, in lateral portions of the upper jaw anlagen the ectoderm of e10.0 embryos does express Shh (arrowhead). Scale bar: A,D,G=250μm, B,E,H=200μm, C,F,I=100μm.
(A) In situ hybridization for B2SINE illustrates the position of the grafted ectoderm in chimeras 24 hours after transplantation. (B) Higher magnification of section in A. (C) Low magnification in section nearly adjacent to that in A illustrates up-regulation of Bmp-4 in mesenchyme. (D) High magnification of section in C illustrates small number of cells that are expressing Bmp-4 in response to transplanted FEZ (arrows). In situ hybridization signal is perinuclear and is not artifact due to background. (E) In contrast to Bmp-4, Bmp-7 is strongly up-regulated in the mesenchyme adjacent to the grafted ectoderm. (F) High magnification of E illustrates perinuclear localization of the in situ hybridization signal. Scale bar, A, C,E=250μm, B,D,F=100μm.
Acknowledgments
We would like to thank Drs Richard Schneider, Silvia Foppiano, Benedikt Halgrimsson, Melanie McCollum, Andrew Jehong, and Nathan Young for critical reading of the manuscript and discussions of the research project. We would like to acknowledge the efforts of Gina Baldoza, as well as, all of the other members of Dr. Marcucio's laboratory. We would also like to thank Stan “the Eggman” Keena from Petaluma Farms for providing farm fresh eggs. This work was funded by the UCSF Research Evaluation and Allocation Committee and NIDCR (R03-DE015901 and R01-1DE018234) to R.M.
Abbreviations
- Bmp
Bone morphogenetic protein
- FEZ
Frontonasal Ectodermal Zone
- Fgf
Fibroblast growth factor
- FNP
Frontonasal process
- HPE
Holoprosencephaly
- MNP
median nasal process
- Shh
Sonic hedgehog
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. Bmp4 and morphological variation of beaks in Darwin's finches. Science. 2004;305:1462–5. doi: 10.1126/science.1098095. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Tabin CJ. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev Biol. 2004;273:134–48. doi: 10.1016/j.ydbio.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Ahlgren SC, Bronner-Fraser M. Inhibition of sonic hedgehog signaling in vivo results in craniofacial neural crest cell death. Current Biology. 1999;9:1304–14. doi: 10.1016/s0960-9822(00)80052-4. [DOI] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002a;129:4647–60. doi: 10.1242/dev.129.19.4647. [DOI] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Signalling via type IA and type IB bone morphogenetic protein receptors (BMPR) regulates intramembranous bone formation, chondrogenesis and feather formation in the chicken embryo. Int J Dev Biol. 2002b;46:243–53. [PubMed] [Google Scholar]
- Barlow AJ, Bogardi JP, Ladher R, Francis-West PH. Expression of chick Barx-1 and its differential regulation by FGF-8 and BMP signaling in the maxillary primordia. Developmental Dynamics. 1999;214:291–302. doi: 10.1002/(SICI)1097-0177(199904)214:4<291::AID-AJA2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Barlow AJ, Francis-West PH. Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development. 1997;124:391–8. doi: 10.1242/dev.124.2.391. [DOI] [PubMed] [Google Scholar]
- Bollag RJ, Crawford KB, Stadt H, Kumiski D, Zdanowicz M, Baptista C, Herlea V, Kirby ML. Use of a repetitive mouse B2 element to identify transplanted mouse cells in mouse-chick chimeras. Exp Cell Res. 1999;248:75–8. doi: 10.1006/excr.1999.4401. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA. Temporal perturbations in sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J Clin Invest. 2004;114:485–494. doi: 10.1172/JCI19596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmane N, Lee J, Robins P, Heller P, Ruiz I Altaba A. Activation of the transcription factor Gli 1 and the Sonic hedgehog signalling pathway in skin tumors. Nature. 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- DeMyer W. The face predicts the brain: diagnostic significance of median facial anomialies for holoprosencephaly (arhinencephay) Pediatrics. 1964 August;:256–263. [PubMed] [Google Scholar]
- Depew MJ, Simpson CA. 21st century neontology and the comparative development of the vertebrate skull. Dev Dyn. 2006;235:1256–91. doi: 10.1002/dvdy.20796. [DOI] [PubMed] [Google Scholar]
- Dubourg C, Bendavid C, Pasquier L, Henry C, Odent S, David V. Holoprosencephaly. Orphanet J Rare Dis. 2007;2:8. doi: 10.1186/1750-1172-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK, He X, Swartz ME, Yan YL, Song H, Boling TC, Kunerth AK, Walker MB, Kimmel CB, Postlethwait JH. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat Genet. 2008;40:290–8. doi: 10.1038/ng.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK, Swartz ME, Crump JG, Kimmel CB. Early Hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development. 2006;133:1069–77. doi: 10.1242/dev.02281. [DOI] [PubMed] [Google Scholar]
- Foppiano S, Hu D, Marcucio RS. Signaling by bone morphogenetic proteins directs formation of an ectodermal signaling center that regulates craniofacial development. Dev Biol. 2007;312:103–114. doi: 10.1016/j.ydbio.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich LV, Scott MP. Hedgehog and patched in neural development and disease. Neuron. 1998;21:1243–57. doi: 10.1016/s0896-6273(00)80645-5. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- Hu D, Helms JA. The role of sonic hedgehog in normal and abnormal craniofacial morphogenesis. Development. 1999;126:4873–84. doi: 10.1242/dev.126.21.4873. [DOI] [PubMed] [Google Scholar]
- Hu D, Marcucio R, Helms JA. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development. 2003;130:1749–58. doi: 10.1242/dev.00397. [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev. 2004;18:937–951. doi: 10.1101/gad.1190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi S, Shou J, Santos R, Hebert JM, McConnell SK, Mason I, Calof AL. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132:5211–23. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–52. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- Lee SH, Fu KK, Hui JN, Richman JM. Noggin and retinoic acid transform the identity of avian facial prominences. Nature. 2001;414:909–12. doi: 10.1038/414909a. [DOI] [PubMed] [Google Scholar]
- Lu C, Miclau T, Hu D, Hansen E, Tsui K, Puttlitz C, Marcucio RS. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–7. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D, Helms JA. Molecular interactions coordinating the development of the forebrain and face. Dev Biol. 2005;284:48–61. doi: 10.1016/j.ydbio.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Marini M, Cusano R, De Biasio P, Caroli F, Lerone M, Silengo M, Ravazzolo R, Seri M, Camera G. Previously undescribed nonsense mutation in SHH caused autosomal dominant holoprosencephaly with wide intrafamilial variability. Am J Med Genet. 2003;117A:112–5. doi: 10.1002/ajmg.a.10163. [DOI] [PubMed] [Google Scholar]
- Kawakami Masayoshi, I M, Richman Joy M. Cell dissociation experiments reveal that positional information operates in the chicken frontonasal mass. genesis. 2006;44:105–114. doi: 10.1002/gene.20191. [DOI] [PubMed] [Google Scholar]
- McKusick VA. Online Mendelian Inheritance in Man, OMIM (TM) Vol. 2002. McKusick-Nathans Institute for Genetic Medicine, Johns Hopkins University; Baltimore, MD: National Center for Biotechnology Information, National Library of Medicine; Bethesda, MD: 2000. [Google Scholar]
- Mina M, Upholt WB, Kollar EJ. Enhancement of avian mandibular chondrogenesis in vitro in the absence of epithelium. Archives of Oral Biology. 1994;39:551–62. doi: 10.1016/0003-9969(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Mina M, Wang YH, Ivanisevic AM, Upholt WB, Rodgers B. Region-and stage-specific effects of FGFs and BMPs in chick mandibular morphogenesis. Dev Dyn. 2002;223:333–52. doi: 10.1002/dvdy.10056. [DOI] [PubMed] [Google Scholar]
- Ming JE, Muenke M. Multiple hits during early embryonic development: digenic diseases and holoprosencephaly. Am J Hum Genet. 2002;71:1017–32. doi: 10.1086/344412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni L, Ming JE, Bocian M, Steinhaus K, Bianchi DW, Die-Smulders C, Giannotti A, Imaizumi K, Jones KL, Campo MD, Martin RA, Meinecke P, Pierpont ME, Robin NH, Young ID, Roessler E, Muenke M. The mutational spectrum of the sonic hedgehog gene in holoprosencephaly: SHH mutations cause a significant proportion of autosomal dominant holoprosencephaly. Hum Mol Genet. 1999;8:2479–88. doi: 10.1093/hmg/8.13.2479. [DOI] [PubMed] [Google Scholar]
- Odent S, Atti-Bitach T, Blayau M, Mathieu M, Aug J, Delezo de AL, Gall JY, Le Marec B, Munnich A, David V, Vekemans M. Expression of the Sonic hedgehog (SHH ) gene during early human development and phenotypic expression of new mutations causing holoprosencephaly. Hum Mol Genet. 1999;8:1683–9. doi: 10.1093/hmg/8.9.1683. [DOI] [PubMed] [Google Scholar]
- Richman JM, Buchtova M, Boughner JC. Comparative ontogeny and phylogeny of the upper jaw skeleton in amniotes. Dev Dyn. 2006;235:1230–43. doi: 10.1002/dvdy.20716. [DOI] [PubMed] [Google Scholar]
- Richman JM, Herbert M, Matovinovic E, Walin J. Effect of fibroblast growth factors on outgrowth of facial mesenchyme. Developmental Biology. 1997;189:135–47. doi: 10.1006/dbio.1997.8656. [DOI] [PubMed] [Google Scholar]
- Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, Tsui LC, Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nature Genetics. 1996;14:357–60. doi: 10.1038/ng1196-357. [DOI] [PubMed] [Google Scholar]
- Schneider RA. Developmental mechanisms facilitating the evolution of bills and quills. J Anat. 2005;207:563–73. doi: 10.1111/j.1469-7580.2005.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:565–568. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Hu D, Rubenstein JL, Maden M, Helms JA. Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development. 2001;128:2755–67. doi: 10.1242/dev.128.14.2755. [DOI] [PubMed] [Google Scholar]
- Song Y, Hui JN, Fu KK, Richman JM. Control of retinoic acid synthesis and FGF expression in the nasal pit is required to pattern the craniofacial skeleton. Developmental Biology. 2004;276:313–329. doi: 10.1016/j.ydbio.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Szabo-Rogers HL, Geetha-Loganathan P, Nimmagadda S, Fu KK, Richman JM. FGF signals from the nasal pit are necessary for normal facial morphogenesis. Dev Biol. 2008;318:289–302. doi: 10.1016/j.ydbio.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Lumsden A. Neural crest cells provide species-specific patterning information in the developing branchial skeleton. Evol Dev. 2004;6:32–40. doi: 10.1111/j.1525-142x.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- Wilke TA, Gubbels S, Schwartz J, Richman JM. Expression of fibroblast growth factor receptors (FGFR1, FGFR2, FGFR3) in the developing head and face. Developmental Dynamics. 1997;210:41–52. doi: 10.1002/(SICI)1097-0177(199709)210:1<41::AID-AJA5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Shen JY, Widelitz RB, Chuong CM. Morphoregulation of avian beaks: comparative mapping of growth zone activities and morphological evolution. Dev Dyn. 2006;235:1400–12. doi: 10.1002/dvdy.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Jiang TX, Suksaweang S, Widelitz RB, Chuong CM. Molecular shaping of the beak. Science. 2004;305:1465–6. doi: 10.1126/science.1098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Image of an e10 mouse (B) HH21 chick, and (C) HH25 chick embryo that were used in grafting experiments. The graft was derived from the developing upper jaw of the mouse (A, red box) and transplanted either in place of the avian FEZ (B, HH21), or onto the dorsal surface of the FNP (C, HH25). The red box in A shows the location of where the graft was derived from, but the shape of the graft was not square due to the three-dimensional morphology in this region of the mouse face. The red box in B and C shows the placement of each graft. The magnification of each image and the size of the red box are identical in each picture. Scale bar=500μm.
(A)At e9.0 Shh is not expressed in ectoderm spanning the medial portion of the developing murine upper jaw anlagen. (B) Higher magnification of A showing expression of Shh in the forebrain (arrows), but not in the ectoderm (arrowhead). (C) Even higher magnification of B, demonstrating the absence of Shh transcripts in the medial ectoderm. (D,E,F) Similarly, successively higher magnifications through the medial portion of the upper jaw of embryos at e10. 0 demonstrate that Shh transcripts are present in the brain (arrows), but not in the ectoderm (arrowhead). (G,H,I) In contrast, in lateral portions of the upper jaw anlagen the ectoderm of e10.0 embryos does express Shh (arrowhead). Scale bar: A,D,G=250μm, B,E,H=200μm, C,F,I=100μm.
(A) In situ hybridization for B2SINE illustrates the position of the grafted ectoderm in chimeras 24 hours after transplantation. (B) Higher magnification of section in A. (C) Low magnification in section nearly adjacent to that in A illustrates up-regulation of Bmp-4 in mesenchyme. (D) High magnification of section in C illustrates small number of cells that are expressing Bmp-4 in response to transplanted FEZ (arrows). In situ hybridization signal is perinuclear and is not artifact due to background. (E) In contrast to Bmp-4, Bmp-7 is strongly up-regulated in the mesenchyme adjacent to the grafted ectoderm. (F) High magnification of E illustrates perinuclear localization of the in situ hybridization signal. Scale bar, A, C,E=250μm, B,D,F=100μm.