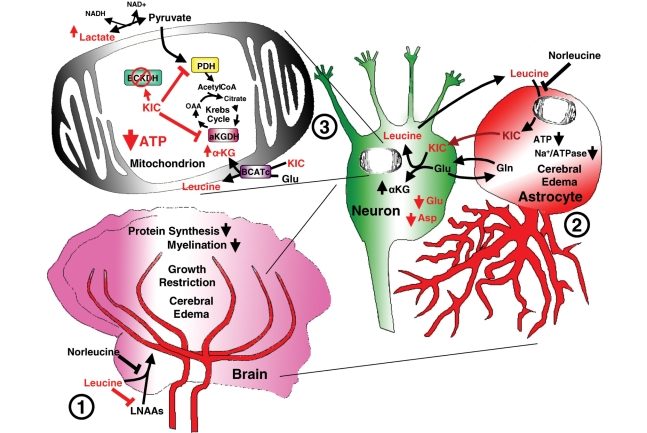
Figure 10.
Proposed model of brain injury and treatment effect in MSUD. (1) Loss of branched-chain ketoacid dehydrogenase function results in accumulation of branched-chain amino acids (mostly leucine) in blood and tissues including muscle, liver and brain. Rapid accumulation of leucine competes with other essential large neutral amino acids using the same transporter (LAT1) for brain access. Reduced levels of these essential amino acids inhibit protein synthesis and deplete dopamine and serotonin by limiting available precursors. Furthermore, loss of branched-chian ketoacid dehydrogenase impairs use of leucine for myelin synthesis, combined with impaired protein synthesis leads to dysmyelination. (2) Accumulation of branched-chain ketoacid (αKIC) within astrocytes and neurons may drive reverse transamination toward leucine resulting in increased αKG/glutamate ratios. (3) An increase in αKG/glutamate ratios may inhibit the malate/aspartate shuttle resulting in increased NADH/NAD+ ratios preventing conversion of lactate to pyruvate. Alternatively, at the mitochondrial level, accumulation of αKIC has been previously shown to inhibit oxidative metabolism through inhibition of pyruvate dehydrogenase (PDH) and α-ketogutarate dehydrogenase (αKGDH) resulting in Krebs cycle dysfunction. Energy failure results in loss of Na+/ATPase function (2) leading to cell swelling and cerebral oedema. Norleucine attenuates leucine transport across the blood–brain barrier (1) and may also limit transamination to αKIC at the astrocyte level (2) and mitochondrial leucine transport (3).