Summary
Inhibition of NF-κB activity leads to a reduction in numbers of CD8+ single positive (SP) thymocytes suggesting a selective role for NF-κB in these cells. To further explore the role of NF-κB in SP thymocytes we utilized transgenic models that allow either inhibition or activation of NF-κB. We now show that activation of NF-κB plays an important role in the selection of MHC class I restricted, CD8 T-cells. Surprisingly NF-κB is not activated in positively selected CD4 thymocytes, and inhibition of NF-κB does not perturb positive or negative selection of CD4 cells. However, enforced activation of NF-κB using a constitutively active IκB kinase transgene leads to nearly complete deletion of CD4 cells by pushing positively selecting CD4 cells into negative selection. These studies therefore reveal a surprising difference of NF-κB activation in CD4 and CD8 thymocytes and suggest that NF-κB contributes to the establishment of thresholds of signaling that determine positive-negative selection of thymocytes.
Introduction
The development of T-cells in the thymus is an ordered progression of differentiation and proliferation events (von Boehmer, 1992). Gene knockout experiments have shown that this developmental pathway is regulated by several transcription factors and signaling pathways (Rothenberg and Taghon, 2005). The majority of these regulators play ongoing roles throughout T cell development, however their specific effects can vary from one stage to another.
The various stages of thymocyte differentiation have been characterized through the expression of specific cell surface markers. For T-cells the co-receptors CD4 and CD8 provide the most convenient markers to separate distinct developmental stages (von Boehmer, 1992). The most immature thymocytes, which lack expression of both CD4 and CD8 (CD4−CD8− double negative, DN cells), develop into an intermediate CD4+CD8+ double positive (DP) stage, before maturing into CD4+ or CD8+ single positive (SP) cells. In a process termed as “negative selection”, DP thymocytes which express rearranged receptors that recognize peptides derived from self-antigens bound to self-MHC, are deleted (Palmer, 2003). In contrast, thymocytes with receptors that fail to recognize self-MHC die by a process termed as death by neglect (Egerton et al., 1990). However thymocytes that bear receptors that associate with peptide-bound self-MHC with moderate affinity are selected for further development through the process of positive selection (Hollander et al., 2006). The different fates of the developing thymocytes is believed to be determined by the strength (Hogquist, 2001) and/or duration (Brugnera et al., 2000) of signal from the T-cell receptor and hence understanding the molecular events that determine these signal attributes, and subsequent transcriptional events in developing thymocytes, is crucial for further understanding T-cell development. Indeed, recent studies have identified a specific group of T cell transcription factors and regulatory proteins that are used in the late stages of thymocyte development and lineage choice including GATA-3 (Hernandez-Hoyos et al., 2003); Runx-1 and Runx-3 (Taniuchi and Littman, 2004; Taniuchi et al., 2002; Woolf et al., 2003); Th-POK (He et al., 2005; He and Kappes, 2006); the Notch proteins and their effector molecule RBPSuH (Tanigaki et al., 2004); E2A, HEB (bHLH factors) and their antagonists Id2 and Id3 (Barndt et al., 2000); and possibly members of the Ikaros family (Georgopoulos, 2002). However, the exact mechanism by which these regulators may function and interact during thymocyte development still remains elusive.
What is clear however is that engagement of T-cell antigen receptors leads to activation of several transcription factors including AP-1, NFAT and NF-κB, which influence this key immuno-developmental process (Serfling et al., 1995). To explore the role of NF-κB in developing thymocytes we had previously generated a transgenic mouse strain in which activation of NF-κB leads to the expression of a luciferase reporter gene. Examination of the expression of luciferase activity in thymocytes from these mice revealed significant NF-κB activation in DN cells undergoing β-selection, and in CD8 SP thymocytes. Further characterization indicated that NF-κB is activated in DN cells in response to signaling from the pre-T-cell receptor acts as an anti-apoptotic factor that helps provide a survival advantage to β-selected cells. To further demonstrate the biological role of NF-κB in thymocytes, we transgenically expressed either a non-degradable form of IκBα that inhibits NF-κB, or an active form of the IκB kinase that leads to constitutive activation of NF-κB. Consistent with a pro-survival role for NF-κB in DN thymocytes, expression of the mutant IκBα led to a reduction in the number of DN cells, whereas expression of the active IKK had the reverse effect, namely an increase in the number of DN cells (Voll et al., 2000). The anti-apoptotic role of NF-κB has been well documented in multiple systems and hence a similar function for NF-κB in thymocytes is consistent with its known biological role. However the role of NF-κB in CD8 cells appeared to be more intriguing and complex.
Prior studies had shown that inhibition of NF-κB in transgenic mice using mutant IκBα led to significant reduction in the number of single positive CD8 cells, both in the thymus and the periphery (Hettmann and Leiden, 2000; Mora et al., 1999). More recently conditional deletion of IKKβ or NEMO/IKKγ has also been shown to lead to a dramatic loss of CD8 cells (Schmidt-Supprian et al., 2003). While these results are consistent with a pro-survival role for NF-κB in CD8 cells, another study reported that double positive (CD4+CD8+; DP) thymocytes from transgenic mice expressing the mutant IκB were resistant to anti-CD3-mediated apoptosis in vivo, although they remained sensitive to apoptosis induced by γ-irradiation (Hettmann and Leiden, 2000). Therefore, NF-κB appears to be required for anti-CD3-mediated apoptosis of DP thymocytes through a pathway distinct from that used for survival of β-selected cells or CD8 cells. Hence NF-κB might play both a pro-apoptotic or anti-apoptotic role in thymocytes, depending on the stage of development.
Our previous study on the regulation of NF-κB in thymocytes using the κB-luciferase transgenic mice had revealed that CD8 SP thymocytes had significantly higher levels of active NF-κB in comparison to CD4 SP thymocytes. The differential expression of NF-κB in these subsets was therefore consistent with results showing that inhibition of NF-κB activity in thymocytes led to a disproportionate` loss of CD8 cells (Hettmann and Leiden, 2000). In addition, the level of the anti-apoptotic factor Bcl-2, whose expression in early DN thymocytes is mutually antagonistic to NF-κB, was highly expressed in CD4 cells but not in CD8 cells (Voll et al., 2000). Therefore active NF-κB in CD8 thymocytes might facilitate their survival, similar to the role of NF-κB in β-selected thymocytes. However, an important question that remained unanswered was whether the CD8-specific activation of NF-κB also contributed to the specific differentiation events that lead to the generation of CD8 cells.
To more carefully examine the role of NF-κB in generation and survival of CD4 and CD8 thymocytes, we used the transgenic mice expressing either the super-repressor form (SR) of IκBα or the constitutively active form of IKKβ for further analysis. We report that NF-κB plays an important role in determining the threshold of signaling that determines positive and negative selection, but only in class I restricted CD8 T-cells. The loss of CD8 cells seen upon inhibition of NF-κB probably reflects a combination of fewer DP cells differentiating into the CD8 lineage, as well as decreased survival, since CD8 cells have significantly lower amounts of the pro-survival factor Bcl-2 in comparison to CD4 cells. Therefore the studies in this paper reveal a surprising difference in the regulation of NF-κB in CD4 and CD8 thymocytes, and suggest that NF-κB activation contributes to both the establishment of thresholds of signaling that determine positive-negative selection and survival of CD8 thymocytes.
RESULTS
Generation and characterisation of IKKβ transgenic mice
To examine the role of NF-κB in thymocyte maturation in vivo, we analyzed transgenic mice expressing a constitutively active form of IKKβ (IKKβEE) under the control of a proximal lck promoter. We obtained 3 transgenic lines (#11, 14 and 17), two of which (#11 and 17) showed relatively high levels of expression of the transgene as revealed by immunoblot analysis of thymocytes (data not shown). We further increased the amount of transgene expression by crossing the mouse lines 11 and 17 (Figure 1A, upper panel). The IKK kinase activity markedly increased in the thymus from IKKβEE transgenic mice (Figure 1A, lower panel). Moreover, expression of the transgene was observed in DN, DP, CD4SP and CD8SP cell populations, as determined by both Western blot and FACS analysis for the FLAG epitope on the transgene (data not shown). The constitutive, nuclear NF-κB DNA-binding activity increased in a transgene dose dependent-manner (Figure 1B, upper panel). As a control, Oct-1 DNA binding activity did not change significantly (Figure 1B, lower panel). Antibody supershift experiments demonstrated that the DNA-protein complex contained predominantly p50/p65 heterodimers (Figure 1B, right panel).
Figure 1. Generation of transgenic mice expressing a constitutively active IKKβ (IKKβEE) in thymocytes.
(A) Protein expression level (upper panel) and kinase activity (lower panel) of the IKKβEE transgene in thymus in IKKβEE transgenic line #11x#17.
(B) Electrophoretic mobility shift assay of NF-κB and Oct-1 DNA binding activity in thymocytes from IKKβEE transgenic line #11x#17 (left panel). Supershift analysis of thymic nuclear extracts from IKKβEE homozygous (Tg+/+) mice (right panel).
(C) Expression levels of NF-κB and IκB family members in Wt and IKKβEE thymocytes were examined by immunoblotting (left panel). Total RNA was isolated from thymoctes treated with or without PMA for 4hrs from wild-type (Wt) and IKKβEE (Tg+/+) mice and then analyzed the expression of IκBα mRNA by Northen blot (right panel).
(D) Single cell suspensions of thymocytes from Wt, heterozygous (Tg+/−) and homozygous (Tg+/+) mice were stained with anti-CD8-PE and anti-CD4-Quantum red (QR) antibodies and analysed by flow cytometry (upper panel). Single cell suspensions of splenocytes from Wt, heterozygous (Tg+/−) and homozygous (Tg+/+) mice were stained with anti-TCRαβ-FITC and anti-B220-PE antibodies and analysed by flow cytometry.
(E) Absolute numbers of DP, CD4SP, and CD8SP thymocytes from Wt, heterozygous (Tg+/−) and homozygous (Tg+/+) mice (upper panels). Absolute numbers of CD8 SP cells in TCR high and TCR low thymocytes from Wt, heterozygous and homozygous mice is shown in the lower panel.
(F) (G) Histograms show expression of TCRβ in CD4SP, CD8 SP or DP thymocytes (F), CD5 and CD69 in DP thymocyte (G) in IKKβEE #11x#17. The blue thin lines represent of Wt and thick lines represent IKKβEE thymocytes, respectively.
Because NF-κB regulates the expression of various members of the NF-κB/IκB family of proteins, we compared the level of NF-κB/IκB proteins in thymocytes from WT and IKKβEE transgenic mice using immunoblotting analysis. The expression levels of only RelB and IκBε were significantly increased in IKKβEE mice (data not shown and Figure 1C, left panel) probably because the promoters of these genes are regulated by NF-κB. Surprisingly, IκBα, whose gene promoter is also regulated by NF-κB, was barely detectable in the IKKβEE mice (Figure 1C, left panel). We therefore examined the expression levels of the mRNA for IκBα in IKKβEE thymocytes. The basal level of IκBα mRNA in the IKKβEE thymocytes was significantly elevated and could only be further increased slightly after the treatment with the NF-κB inducer PMA (Figure 1C, right panel). These data therefore demonstrate that although the IκBα mRNA is synthesized in increased amounts in the IKKβEE transgenic thymocytes, the synthesized IκBα protein is most likely continuously hyper-phosphorylated and degraded.
We next examined whether the increased activity of NF-κB in the transgenic animals resulted in an altered thymic phenotype. Thymic atrophy, characterised by 30% average loss of organ weight and a similar reduction in the number of thymocytes, was observed in IKKβEE mice. The formation of thymic medulla was more affected than the cortex (data not shown). Flow cytometric analysis indicated that the number of DP cells was reduced in a transgene-dependent manner (Figure 1D). In contrast to IκBα super repressor (SR) mice, as described previously (Hettmann and Leiden, 2000), the number of CD4SP cells was most severely affected in the IKKβEE mice (Figure 1D and 1E, upper panels). This effect was much more apparent in homozygous transgenic animals. The effect of the transgene was independent of TCR levels as shown for CD8 SP cells (Fig. 1E, lower panel). It has been reported previously that upon TCR signalling, DP thymocytes initially become CD4+,CD8low intermediate thymocytes, and then if signalling from the TCR ceases, these cells further differentiate into CD8 SP cells (Yasumoto K et al. 2000). However, if TCR signal persists the cells differentiate into CD4SP cells. In IKKβEE heterozygous animals, although we detected CD4+,CD8low intermediate thymocytes, their numbers were significantly reduced (Figure 1D, boxed-regions). It is therefore possible that the decrease in the number of the intermediate thymocyte population leads to the decreased number of SP cells in the IKKβEE homozygous animals (Figure 1E).
While T cell subsets are established during the differentiation of immature precursors in the thymus, the ultimate outcome of T cell development is establishment of normal T-cell populations in the periphery (von Boehmer, 1992). It has been shown previously that the number of peripheral CD8 cells is reduced by 50% in IκBαSR mice. In contrast, in the IKKβEE mice the numbers of both mature CD4 and CD8 T cells decreased dramatically in the periphery, although the total number of B cells was unaltered (Figure 1D, lower panel).
The transition of thymocytes from DP to SP cells is associated with upregulation of TCR expression. DP thymocytes normally express relatively low levels of TCR but during positive selection, TCR levels are upregulated and SP cells ultimately express high levels of TCR (Ohashi et al., 1990). There is however a subpopulation with intermediate levels of TCR expression that corresponds to cells that have initiated positive selection and are transitional between DP cells and SP cells. Flow-cytometric analysis showed that SP thymocytes expressing high levels of TCR were reduced in the transgenic animals, while thymocytes expressing low or intermediate levels of the TCR remained (Figure 1F). It is well recognised that induction of positive selection by TCR signalling in DP thymocytes also leads to upregulation of CD5 and CD69 expression (Azzam et al., 1998; Moore et al., 1995) IKKβEE DP cells express higher levels of CD5 than normal and have a higher percentage of cells expressing an intermediate level of TCRαβ (Figure 1F and G). In contrast, the expression level of CD69 in IKKβEE DP cells was almost comparable (Figure 1G). In IKKβEE mice, a subpopulation of CD4+ cells with intermediate/low CD8 expression that express high levels of CD5 and TCR was also observed (data not shown). These cells likely represent an intermediate population of cells that have initiated selection in response to TCR engagement (Lucas and Germain, 2000; Suzuki et al., 1995). Therefore the pattern of expression levels of CD5, CD69 and TCR, and the existence of a subpopulation of CD4+ cells with intermediate/low CD8 expression, on normal and IKKβEE DP cells, suggests that IKKβEE DP thymocytes initiate positive selection.
To exclude the possibility that loss in thymic cellularity was due lineage re-direction, in particular to γδ T cells, the number of γδ T cells in both the thymus and lymph nodes were examined from both WT and IKKβEE mice. FACS analysis showed that the number of γδ T cells was not significantly different between IKKβEE and WT mice (Supplementary figures S1a and S1b).
Neither Bcl-2 transgenic mice nor Faslpr mice rescue the reduction of SP T cells in IKKβEE mice
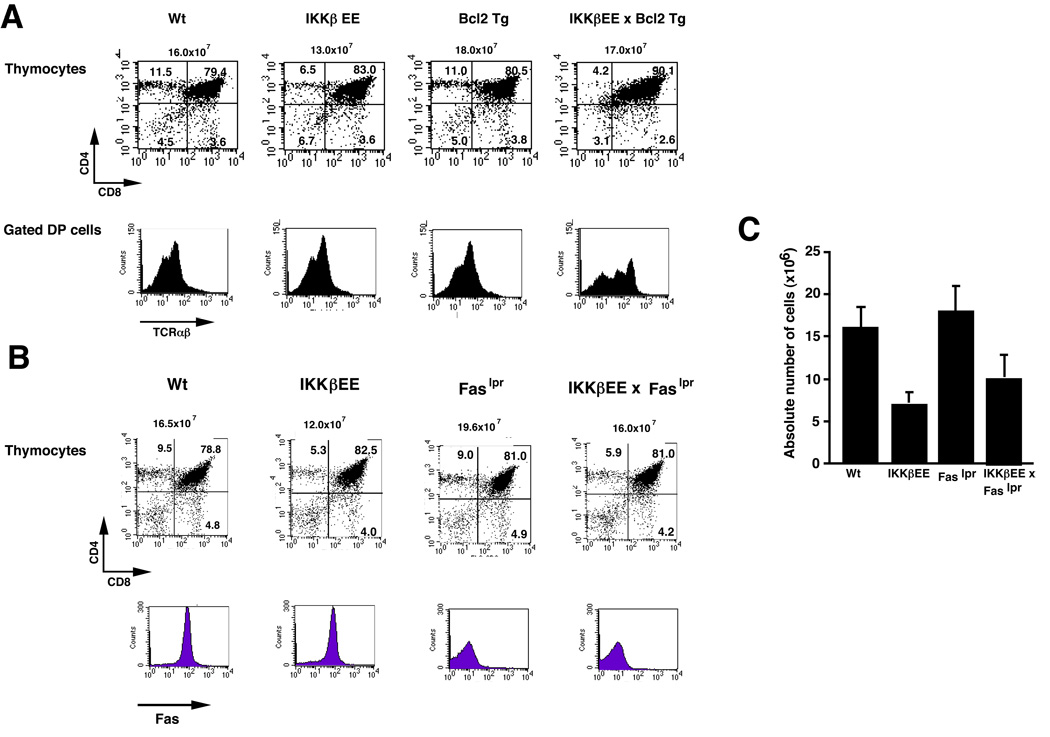
The Bcl-2 family of proteins is composed of multiple members that function either as pro-or anti-apoptotic proteins. Bcl-2 is expressed at high levels in immature DN thymocytes (Sentman et al., 1991; Strasser et al., 1991). Its expression is downregulated in DP cells and it is then re-expressed in mature SP cells, with significantly greater expression in CD4 cells. On the other hand, Bcl-xL is highly expressed in DP cells, and is then downregulated in SP cells (Hettmann et al., 1999). The ectopic expression of Bcl-2 transgene rescues some thymocytes from deletion due to apoptosis, particularly those that die by neglect (Hare et al., 1998). Indeed, a block in thymocyte development caused by loss of survival signals can be restored by the ectopic expression of Bcl-2 protein (Kondo et al., 1997). To test whether Bcl-2 may rescue the DP cells that are lost in the IKKβEE mice, we bred the IKKβEE mice onto Eµ-Bcl-2 transgenic mice. Although normal numbers of total thymocytes were observed in these mice, the population distribution was somewhat altered as compared to IKKEE or WT controls. These thymi contained a very large DP population (approximately 90%), but very few DN, CD4SP and CD8SP cells (Figure 2A). Interestingly, unlike DP cells from IKKβEE or Bcl-2 mice, DP thymocytes expressing high levels of TCRαβ could be detected in IKKβEE/Bcl-2 mice (Figure. 2A). Therefore although Bcl-2 transgene enhances the survival of DP cells of IKKβEE mice, it does not restore the differentiation into SP cells even though some part of DP thymocytes in IKKβEE/Bcl-2 mice have increased TCR levels and are midway in the transition from DP cells to SP cells.
Figure 2. Neither Bcl2 TG mice nor Faslpr mice rescue T lymphocytes differentiation in thymus and in spleen.of IKKβEE mice.
Composition of thymocyte and splenocyte subsets were analysed in IKKβEE, Eμ-Bcl-2 Tg, IKKβ/Bcl-2 mice (A) and in IKKβEE, Faslpr, IKKβEE/Faslpr mice (B) by flow cytometry. Single cells from thymocytes from these mice were stained with anti-TCRβ-FITC or anti-Fas-FITC, anti-CD8-PE and anti-CD4-QR and analysed by flow cytometry.
(C) Absolute numbers of CD4SP thymocytes from Wt, IKKβEE, Faslpr, IKKβEE/Faslpr mice.
It has been shown previously that NF-κB is an important mediator of activation-induced FasL expression and subsequent cell death when T cells are treated with cross-linking anti-TCR antibodies (Kasibhatla et al., 1998). To test whether NF-κB-dependent upregulation of FasL contributes to the loss of thymocytes in IKKβEE mice, the IKKβEE mice were crossed with Faslpr mice and then the CD4/CD8 profile of thymocytes and splenocytes was analyzed (Watanabe-Fukunaga et al., 1992). The transcription of the gene encoding Fas is impaired in lpr mice by the insertion of the transposable element into the intron of the gene. The CD4/CD8 profile (Figure 2B) shows that inhibition of Fas expression does not rescue thymocyte differentiation defects in IKKβEE mice although it does lead to an increase in the total number of thymocytes (Figure 2B, C).
It has been reported that Nur77, an orphan member of the steroid nuclear receptor superfamily plays a central role in thymocyte negative selection (Cho HJ et al., 2003) and Nur77 expression was implicated in FasL gene expression (Matsui K et al., 1997). Therefore, we compared the level of Nur77 expression in thymocytes from both IKKβEE and Wt mice by FACS analysis. Nur77 levels did not change significantly in thymocytes both IKKβEE and Wt thymocytes (data not shown) suggesting that induction of Nur77 expression was independent of NF-κB activation.
These results therefore suggest that increased levels of FasL in IKKβEE mice might affect overall thymocyte numbers by affecting cell death but is not responsible for the block in differentiation.
NF-κB transcriptional activity increases during positive selection and in CD8+, TCRhigh thymocytes
Previous studies have shown that increased NF-κB DNA binding activity is observed in CD69+ thymocytes undergoing positive selection (Moore et al., 1995). However, these CD69+ cells include both DP and CD4SP cells. We therefore first isolated DP cells and then further fractionated them according to the expression level of CD69. Whereas the NF-κB DNA binding activity was significantly greater in nuclear extracts of CD69+DP thymocytes (Figure 3A upper panel), the binding of the constitutive Oct-1 transcription factor did not exhibit the same difference between these CD69+ and CD69− DP thymocytes (Figure 3A lower panel). To determine whether the difference in NF-κB DNA-binding activity was reflected in the transcriptional activity of NF-κB, we used the κB-luciferase reporter transgenic mice to isolate CD69−DP, CD69+DP, CD4+,TCRhigh and CD8+,TCRhigh thymocytes. Consistent with the DNA-binding activity, transcriptional activity of NF-κB increased during the transition from CD69− to CD69+DP thymocytes. These results suggest that activation of NF-κB can be correlated with the activation of DP thymocytes undergoing selection. NF-κB activity was greater in the CD8+TCRhigh population. Little luciferase activity was detected in CD4SP cells (Figure 3B). These results show that NF-κB activity correlates with the initial transition of DP cells into CD8SP cells, but not CD4SP cells.
Figure 3. NF-κB Activity in during positive and negative selection.
(A) Single cell suspension of total thymocyte was stained with anti-TCRβ-FITC, anti-CD69-PE, anti-CD8-QR, and anti-CD4-APC. Nuclear extracts of CD69−DP and CD69+DP cells were analyzed by electrophoretic mobility shift assay (EMSA) for NF-κB and Oct-1.
(B) Luciferase activity of thymocyte population during selection. Total cell extracts were prepared from thymocyte populations and then measured luciferase activity.
Modest impairment of thymocyte positive selection in an IKKβEE mice, MHC class I-restricted TCR transgenic mice
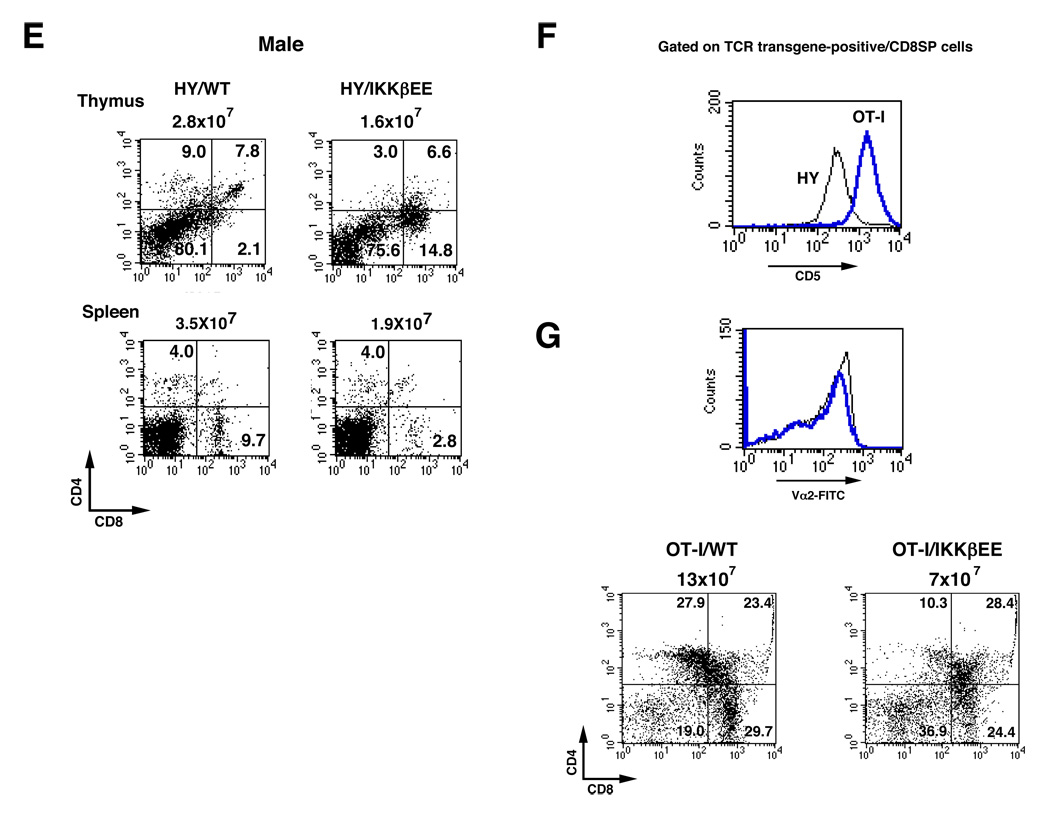
A possible explanation for the dramatic reduction in the number of mature thymocytes in IKKβEE mice, in particular CD4 cells, might be dysregulated positive/negative selection. However, it is difficult to explore the complex process of thymocyte selection in mice with a diverse T cell repertoire. To overcome this difficulty, transgenic mice expressing TCRs of defined specificity have been used. We examined the effect of IKKβEE on MHC class I-restricted positive selection using the H-Y TCR transgenic mice. Thymocytes expressing the H-Y specific TCR are positively selected along the CD8 lineage in female, but are deleted in male mice (Teh et al., 1988). As shown in Figure 4A, total number of thymocytes in H-Y/IKKβEE mice was significantly reduced (~40%) compared to H-Y/Wt thymocytes. We observed a significant increase in the number of Annexin V+ thymocytes in H-Y/IKKβEE mice (Figure 4A, lower panel). The decrease in the number of the different thymocyte populations is demonstrated as a graph in Figure 4B. Interestingly, although the number of DP and SP thymocytes decreases dramatically upon expression of IKKβEE, the number of DN cells is significantly increased due to the anti-apoptotic role of NF-κB during β-selection. Flow cytometric analysis showed that the expression of the transgenic, clonotypic TCR was uniform and increased in the H-Y/IKKβEE mice relative to H-Y/Wt thymocyte (Figure 4C, upper panel). Additionally, when total thymocytes were gated on H-Y TCRhigh cell population, the proportion of CD8SP cells decreased relative to the DN and DP thymocytes in H-Y/IKKβEE mice (Figure 4C lower panel). To determine whether the change in the percentage of CD8SP cells is confined to phenotypically mature CD8SP cells, we examined the expression of cell surface markers that are developmentally regulated during the DP to SP transition, such as CD5, CD69 and HSA, again gated on the H-Y TCRhigh cell population. Expression of CD5 was increased in both DP and CD8SP cells of H-Y/IKKβEE mice (Figure 4D, left panel), whereas CD69 was mainly increased in DP cells (Figure 4D, middle panel). The expression of HSA is downregulated in mature SP thymocyte (Marodon and Rocha, 1994). Although the number of HSA negative cells in CD8SP was decreased, most of CD8SP cells are HSA negative in IKKβEE mice (Figure 4D, right panel). Hence, these results suggest that although the number of CD8SP cells was reduced in the H-Y/IKKβEE mice, the CD8SP cells that remained were normal, because the expression of CD5 was higher, both CD69 and HSA were even lower than these of CD8SP cells in HY/WT mice. Although we do not exactly know why the number of CD8 SP cells is decreased, a likely possibility is that hyperactivation of NF-κB pushes a portion of positively selecting CD8 cells to negative selection.
Figure 4. Impaired thymocyte positive selection in IKKβEE mice, MHC class I-restricted TCR transgenic mice.
(A) Total thymocyte number from 6-week old female H-Y/Wt and H-Y/IKKβEE mice (upper panel). Histograms show AnnexinV-FITC staining in total thymocyte from HY/WT and HY/IKKβEE mice (lower panel).
(B) Absolute numbers of DP, CD4SP, CD8SP and DN thymocytes from H-Y/Wt and H-Y/IKKβEE mice.
(C) Histograms show the expression level of H-Y TCR (T3.70) in total thymocyte (upper panel). Analysis of CD4 and CD8 population in gating on H-Y TCRhigh cells in female H-Y/Wt and H-Y/IKKβEE mice (lower panel). Thymocytes were stained with anti-H-Y TCR (T3.70)-FITC, anti-CD8-PE and anti-CD4-QR antibodies.
(D) Histograms show the expression level of developmental markers, CD5, CD69, and HSA, on both DP and CD8SP in gating on H-Y TCRhigh cells. The thin black lines represent Wt and thick blue lines represent IKKβEE thymocytes, respectively.
(E) Analysis of CD4 and CD8 population in male H-Y/Wt and H-Y/IKKβEE mice. Thymocytes and splenocytes were stained with anti-H-Y TCR (T3.70)-FITC, anti-CD8-PE and anti-CD4-QR antibodies.
(F) Comparison of CD5 expression on thymocyte from H-Y and OT-I TCR transgenic mice. Histogram represents CD5 staining in gating on transgenic TCRhighCD8SP cells. The thin line represents H-Y TCR transgenic thymocytes and thick blue line represents OT-I TCR transgenic thymocytes, respectively.
(G) Histogram shows the expression level of OT-I TCR (Vα2) in total thymocyte (upper panel). The thin line represents Wt and thick blue line represents IKKβEE thymocytes, respectively. Analysis of CD4 and CD8 population in gating on OT-I TCRhigh cells in OT-I/Wt and OT-I/IKKβEE mice (lower panel). Thymocytes were stained with anti-Vα2-FITC, anti-CD8-PE and anti-CD4-QR antibodies.
Negative selection in H-Y male mice is characterised by markedly reduced thymocyte numbers and near absence of DP and SP thymocytes, a profile that may be characteristic of negative selection that occurs early in the DP stage of development. Activation of NF-κB did not rescue H-Y TCR transgenic DP thymocytes from negative selection in male mice, and both total thymocyte and DP cell number were almost comparable in the H-Y/Wt and H-Y/IKKβEE mice (Figure 4E).
The H-Y TCR transgenic mouse is believed to be a model for a weakly selecting TCR (The et al., 1988). To test whether NF-κB affects MHC class I positive selection for a strongly selecting TCR, we used the OT-I TCR transgenic mice, which is specific for ovalbumin (OVA) peptide in the context of H-2b (Clarke et al., 2000). We first compared the expression level of CD5 by gating on transgenic TCRhighCD8SP cells of OT-I TCR transgenic mice and H-Y TCR transgenic mice, since the differences in CD5 levels reflects differences in the avidity of the positively selecting interaction (Azzam et al., 1998). Although the natural selecting ligands for these TCRs are unknown, their relative avidity can be inferred by the efficiency of positive selection (Jameson et al., 1995). The expression of CD5 on transgenic TCRhighCD8SP cells of OT-I TCR transgenic mice is higher than that of H-Y TCR female transgenic mice, suggesting that OT-I TCR displays a greater affinity for its ligand than the HY-TCR (Figure 4F). As shown in Figure 4G, the expression of the clonotypic TCR in OT-I/IKKβEE mice is almost comparable to OT-I/WT mice. However, total cell number is reduced about 50% (13×107 vs 7×107) with the most dramatic effect being confined to CD4SP cells (Figure 4G lower, right panel). The constitutive activation of NF-κB however has a much milder effect on CD8 positive selection in these mice. Therefore overall the effect of the IKKβEE transgene on positive selection is similar in two different class I restricted TCR transgenic mice that express TCRs of different affinities.
Inhibition of NF-κB activity alters both positive and negative selection in MHC class I-restricted TCR transgenic mice
As has been reported previously, the numbers of CD8SP cells are significantly decreased in IκBαSR mice (Hettmann and Leiden, 2000). To determine whether inhibition of NF-κB alters positive/negative selection in MHC class I-restricted TCR transgenic mice, we bred IκBαSR mice onto HY-TCR transgenic mice. The H-Y TCR expression was comparable in both H-Y/Wt and H-Y/IκBαSR female mice, although the number of H-Y TCRhigh population decreased slightly (Figure 5A, upper panel). When total thymocytes were gated on H-Y TCRhigh cells, proportion of DP and CD8SP cells decreased and greater percentage of DN thymocytes was observed (Figure 5A, lower panel). Among the differentiation cell surface markers, HSA expression is still higher in gating on H-Y TCR high CD8SP of H-Y/IκBαSR mice (figure 5B lower right panel). Downregulation of HSA expression normally occurs as a late event during SP thymocyte maturation, and low level of HSA expression correlates with increased maturation. Taken together, these results suggest that the inhibition of NF-κB inhibits the maturation of CD8SP cells (Figure 5B).
Figure 5. Inhibition of NF-κB alters both positive and negative selection in MHC class I-restricted TCR transgenic mice.
(A) Histograms show the expression level of H-Y TCR (T3.70) in total thymocyte (upper panel). Analysis of CD4 and CD8 population in gating on H-Y TCRhigh cells in female H-Y/Wt and H-Y/IκBαDN mice (lower panel). Thymocytes were stained with anti-H-Y TCR (T3.70)-FITC, anti-CD8-PE and anti-CD4-QR antibodies.
(B) Histograms show the expression level of developmental markers, CD5, CD69, and HSA, on both DP and CD8SP in gating on H-Y TCRhigh cells. The thin lines represent Wt and blue thick lines represent IκBαDN thymocytes, respectively.
(C) Analysis of CD4 and CD8 population in male H-Y/Wt and H-Y/IκBαSR mice. Thymocytes were stained with anti-H-Y TCR (T3.70)-FITC, anti-CD8-PE antibodies and anti-CD4-QR.
(D) OT-I mice were bred onto either IκBαSR (B6 background:H-2b) (left panel) or IκBαDN (B10.BR background:H-2k) (right panel) mice. Histograms show the expression level of OT-I TCR (Vα2) in total thymocyte. The thin line represents Wt and thick blue line represents IκBαDN thymocytes, respectively. Analysis of CD4 and CD8 population in gating on OT-I TCRhigh cells in OT-I/Wt and OT-I/ IκBαSR mice. Thymocytes were stained with anti-Vα2-FITC, anti-CD8-PE and anti-CD4-QR antibodies.
Hettmann et al have shown that inhibition of NF-κB prevents anti-CD3 antibody induced DP thymocyte deletion (Hettmann and Leiden, 2000). Although injection of anti-CD3 antibody is not likely to be a very physiological model for negative selection, it may be good model to examine early-stage negative selection because injection of anti-CD3 antibody causes the death of most DP thymocytes. To examine the role of NF-κB in early-stage negative selection, we analysed H-Y/IκBαSR male mice. The H-Y TCR expression was comparable in both H-Y/Wt and H-Y/IκBαSR male mice (Figure 5C, upper panel). Inhibition of NF-κB caused a significant (approximately 4 to 5-fold) rescue of DP thymocytes in H-Y male mice (Figure 5C, lower panel). The increased DP thymocytes in H-Y/IκBαSR male mice reflects impaired negative selection rather than enhanced positive selection, or any general inhibition of apoptosis, because the positive selection was impaired and total cell number in H-Y/IκBαSR female mice did not change compared with H-Y/Wt female mice (Figure 5A).
The OT-I TCR is positively selected on H-2b, whereas negative selection predominates in H-2bxk heterozygotes due to alloreactivity to H-2k molecule (Clarke et al., 2000). Inhibition of NF-κB exerted a relatively modest effect on the development of CD8SP cells under conditions of positive selection (Figure 5D, left panel). In contrast, inhibition of NF-κB led to a slight increase in total cell number in H-2bxk heterozygotes (Figure 5E, right lower panel). The distribution of thymocytes according to high vs. low TCR expression is distinctly different in OT-I/WT and OT-I/IκBαSR mice (Figure 5E right, upper panel). Moreover, the prevalence of mature clonotype-positive CD8SP cells was increased significantly (Figure 5D, right lower panel). Flow-cytometric analysis showed there are few Vα2 high/CD8SP cells in OT-I/WT mice expressing H-2bxk. Thus, the inhibitory transgene IκBαSR imposes a significant block to negative selection of a TCR by endogenous MHC class I molecules.
Positive selection of MHC class II-restricted TCR transgenic mice is completely abolished in IKKβEE mice
To further examine the effect of constitutively activated NF-κB on CD4 positive selection, IKKβEE mice were crossed with two TCR transgenic mice, which express MHC class II-restricted TCRs of defined specificity. The AND TCR and the 1H3.1 TCR are both MHC class II-restricted TCRs and are used as models for a strongly and a weakly selecting TCR, respectively (Kaye et al., 1989; Viret et al., 2001). The AND TCR is strongly positively selected in the H-2k and H-2b background, as demonstrated by the pronounced accumulation of CD4SP thymocytes. As seen in Figure 6A, there was a dramatic reduction in the total number of thymocytes in AND/IKKβEE transgenics compared with wild-type or mice expressing the AND TCR or the IKKβEE transgenes alone. Consistent with this, we observed a significant increase in Annexin V+ staining of thymocytes (Figure 6A, lower panel), the majority of which were in the DN and DP population (data not shown). Most strikingly however, positive selection of CD4SP thymocytes was severely impaired in the AND/IKKβEE mice (Figure 6B and C). A similar reduction of peripheral T cells also observed in the spleen of AND/IKKβEE (data not shown). We next examined the expression levels of AND specific TCR (Vβ3). Figure 6C showed a significant reduction of AND specific TCR expression in AND/IKKβEE mice. Thus, several features of the AND/IKKβEE transgenics were strikingly reminiscent of the thymus of mice that express self-reactive transgenic TCRs, such as the reduction in the total number of thymocytes, decreases in CD4 population and predominance of the DN population (Figure 6B and C). To address the possibility whether constitutively activated NF-κB converts MHC class II positive selection to negative selection even in a model for weakly selecting TCR, we used the 1H3.1 TCR transgenic mice, which is specific for residues 52–68 of the α-chain of I-E MHC (Viret et al., 2001). The efficiency of positive selection was judged on the basis of clonotypic TCR and CD5 expression in transgenic TCRhigh CD4SP cells. Positive selection of 1H3.1 thymocytes was less efficient compared to AND mice (Figure 6D). When IKKβEE mice were bred onto 1H3.1 transgenic mice, we once again observed massive reduction of total thymocytes and CD4SP population and predominance of the DN population (Figure 6E and F). To exclude the possibility that the IKKβEE transgene caused a disruption of maturation such that cells did not develop beyond the transition from DN into DP stage rather than due to effects on positive/negative selection, we bred the IKKβEE mice onto AND mice on a MHC II-deficient background. In the absence of a TCR ligand an excess of DP thymocytes were generated suggesting that the transition of DN into DP thymocyte was not affected in the AND/IKKβEE mice. The total number of thymocyte was not significantly different between AND/Wt and AND/IKKβEE in MHC II-deficient background (Figure 6G, upper panel). We also observed accumulation of DP thymocyte in both AND/Wt and AND/IKKβEE in MHC II-deficient background (Figure 6G, lower panel). Furthermore, previous data from our laboratory demonstrated that expression of the IKKβEE transgene allowed differentiation to the DP stage in a RAG-1-deficient mouse that cannot assemble the pre-TCR. Consequently, these results demonstrate that constitutive activation of NF-κB in Class II-restricted TCR background leads to loss of thymocytes by probably converting positive selection to negative selection.
Figure 6. Positive selection of MHC class II-restricted TCR transgenic mice is completely abolished in IKKβEE mice.
(A) Total thymocyte number from 6-week old AND/Wt and AND/IKKβEE mice (upper panel). Histograms show AnnexinV-FITC staining in total thymocyte from AND/WT and AND/IKKβEE mice (lower panel).
(C) Absolute numbers of DP, CD4SP, CD8SP and DN thymocytes from AND/Wt and AND/IKKβEE mice.
(B) Histogram shows the expression level of AND TCR (Vβ3) in total thymocytes (upper panel). The thin line represents AND/Wt and thick lines represent AND/IKKβEE thymocytes, respectively. Analysis of CD4 and CD8 population in AND/Wt and AND/IKKβEE mice (lower panel). Thymocytes and splenocytes were stained with anti-AND TCR (Vβ3)-FITC, anti-CD8-PE and anti-CD4-QR antibodies.
(D) Comparison of CD5 expression on thymocyte from AND and 1H3.1 TCR transgenic mice. Histogram represents CD5 staining in gating on transgenic TCRhighCD4SP cells. The thin line represents AND TCR transgenic thymocytes and thick blue line represents 1H3.1 TCR transgenic thymocytes, respectively.
(E) Comparison of total thymocyte number from 6-week old Wt or IKKβEE mice bred onto some MHC class II restricted transgenic mice. AND (I-Ek) mice were bred onto either Wt or IKKβEE (I-Ek) mice. AND (I-Ab), or 1H3.1 mice were bred onto either Wt or IKKβEE (I-Ab) mice.
(F) Histograms show the expression level of 1H3.1 TCR (Vβ6) in total thymocyte (upper panel). Analysis of CD4 and CD8 population in gating on 1H3.1 TCR positive cells in OT-II/Wt and OT-II/IKKβEE mice (lower panel). Thymocytes were stained with anti-Vβ6-FITC, anti-CD8-PE and anti-CD4-QR antibodies.
(E) Total thymocyte number from 6-week old AND/Wt, AND/IKKβEE, AND/Wt/MHCII−/− and AND/IKKβEE/MHCII−/− mice. (upper panel). Analysis of CD4 and CD8 population in AND/Wt/MHCII−/− and AND/IKKβEE/MHCII−/− mice. Thymocytes were stained with anti-Vβ3-FITC, anti-CD8-PE and anti-CD4-QR antibodies.
Inhibition of NF-κB does not affect MHC class-II restricted positive/negative selection
As reported previously, number of CD4SP thymocytes did not change in IκBαSR mice, suggesting that either NF-κB activity is not necessary for maintenance of these cells or that NF-κB is dispensable for MHC class-II restricted positive/negative selection. The differential expression of NF-κB activity in CD8 vs. CD4 thymocytes (Figure 3B, Voll et al.) is also consistent with the hypothesis that while NF-κB activation contributes to the threshold of signalling necessary to achieve positive and negative selection in the CD8 lineage, NF-κB does not contribute to the establishment of signalling thresholds necessary to achieve positive/negative selection in CD4 thymocytes. The dramatic loss of CD4 thymocytes in AND and 1H3.1 Class II-restricted transgenics when crossed to the IKKβEE transgenics is fully consistent with this hypothesis. To further test this model, we crossed IκBαSR mice with the AND TCR transgenic mice. Although we observed the expected reduction of CD8low population, which reflects IκBαSR mouse phenotype, dramatic accumulation of CD4SP cells was still observed in AND/IκBαSR mice (Figure 7A). Similar results were obtained when the IκBαSR mice were bred onto 1H3.1 TCR transgenic mice (Figure 7B).
Figure 7. Inhibition of NF-κB does not have any influence for positive/negative selection of MHC class II-restricted TCR transgenic mice.
(A) Analysis of CD4 and CD8 population in AND/Wt and AND/IκBαSR mice. Histogram shows the expression level of AND TCR (Vβ3) in total thymocytes. The thin line represents AND/Wt and thick line represents AND/IκBαSR thymocytes, respectively. Thymocytes were stained with anti-AND TCR (Vβ3)-FITC, anti-CD8-PE and anti-CD4-QR antibodies.
(B) Analysis of CD4 and CD8 population in 1H3.1/Wt and 1H3.1/IκBαSR mice. Thymocytes were stained with anti-1H3.1 TCR (Vβ6)-FITC, anti-CD8-PE and anti-CD4-QR antibodies.
(C) AND/Wt and AND/IκBαDN mice were crossed with B10.S mice (H-2s). Thymocytes and splenocytes were stained with anti-AND TCR (Vβ3)-FITC, anti-CD8-PE and anti-CD4-QR antibodies.
(D) 1H3.1/Wt and 1H3.1/IκBαSR mice were crossed with B10.A(5R) mice. Thymocytes and splenocytes were stained with anti-1H3.1TCR (Vβ6)-FITC, anti-CD8-PE and anti-CD4-QR antibodies.
Since the inhibition of NF-κB significantly inhibits negative selection of Class I restricted TCR-expressing thymocytes, we wanted to examine whether expression of the IκBαSR transgene also imposes a block in negative selection of class II-restricted TCR-expressing thymocytes. Negative selection of AND TCR predominates in I-Abxs heterozygotes due to allo-reactivity to the I-As molecule (Vasquez et al., 1992). AND/Wt mice or AND/IκBαSR mice were bred onto B10S mice to generate I-Abxs heterozygotes. Thymocytes from both strains of transgenic mice, were found to lack significant CD4SP population and accumulates DN cells (Figure 7B). Also, because the B10A(5R) strain assembles the I-Ab:Eα52–68 complex, when 1H3.1 mice were bred onto B10A(5R) mice, 1H3.1 TCR-expressing thymocytes were deleted ((Viret et al., 2001) and Figure 7D). Dramatic reduction of DP cells was observed in both 1H3.1/Wt and 1H3.1/IκBαSR mice (Figure 7D). Thus these results provide strong support to the proposal that positive and negative selection of CD4 thymocytes is independent of NF-κB.
Discussion
It is now well established that transcription factors of the NF-κB family play important roles in the proliferation and function of mature T-cells. However the role of these transcription factors in T-cell development remains to be fully elucidated. We have used transgenic expression of inhibitors and activators of the NF-κB in thymocytes to analyze the role of NF-κB at different stages of thymocyte development. We previously reported that NF-κB is activated in response to signaling from the pre-TCR in β-selected thymocytes, thereby providing a survival advantage to these cells (Voll et al., 2000). We have continued to utilize these transgenic models to explore the role of NF-κB in later stages of thymocyte development. In this manuscript we report that NF-κB plays a critical role in positive selection of CD8 thymocytes and the activation of NF-κB helps to establish the threshold of signaling required for positive/negative selection of these cells. Our current data shows that constitutive activation of NF-κB leads to nearly complete loss of CD4 SP cells, probably because the signaling threshold for positive selection in CD4 cells is normally established in the absence of NF-κB. Therefore, these findings implicate NF-κB activation in DP thymocyte lineage decision and in the molecular control mechanisms that shape the T-cell repertoire during development.
Previous studies from our laboratory that used transgenic expression of mutant IκBα to inhibit NF-κB demonstrated a loss of CD8 thymocytes (Voll et al., 2000). Those data also corroborated observations by Boothby et al (Boothby et al., 1997) and Hettmann et al (Hettmann and Leiden, 2000), who also utilized a mutant IκBα super-repressor system to inhibit NF-κB activity in thymocytes. More recently, conditional knock-outs of the IKKβ and IKKγ/NEMO also revealed a specific reduction in the numbers of CB8 thymocytes (Schmidt-Supprian et al., 2003). Such CD8-specific effect of NF-κB can probably be explained by our demonstration, using κB-luciferase transgenic reporter mice, that active NF-κB can only be detected in CD8+ SP thymocytes. It is likely that NF-κB contributes to the survival of these CD8 SP cells similar to its role in β-selected thymocytes since the level of the survival factor Bcl-2 is significantly lower in CD8 cells compared to CD4 cells. A similar mutually antagonistic relationship between NF-κB and Bcl-2 was observed in DN cells where subpopulations that had high levels of NF-κB had very low levels of Bcl-2 and vice versa. Therefore, a simple explanation for the selective loss of CD8 thymocytes in this scenario would be a pro-survival role for NF-κB in these cells.
An alternative explanation for the decrease in thymic cellularity in our transgenic TCR experiments might be lineage diversion. Indeed, it is known that premature expression of a transgenic TCR-αβ can result in redirection of thymocyte development to gamma delta, DN-TCRαβ, TCRαβ-CD8αα and NKT sub-types (Egawa et al., 2008; Kreslavsky et al., 2008). Terrence et al reported that the thymi of mice with the HY or AND TCR contained no γδ cells and that this was due, at least in part, to suppression of V-γ gene rearrangements, the implication being that there was an outgrowth of “γδ-like” cells within the DN population (Terrence et al., 2000). However, as shown in the supplementary figure S1, we do not detect any increase in γδ cells in the IKKEE transgenic mice, even though they show a drastic reduction in CD4 cells, indicating that at least for the endogenous repertoire, expression of IKKEE transgene does not lead to significant lineage diversion. Also, if lineage diversion were responsible for the dramatic loss in thymic cellularity in our study, we would expect to see it for both CD8 and CD4 populations and not as an almost exclusively CD4-restricted phenomenon. Similarly, increasing NF-κB activity at the same time as the expression of the transgenic TCR (both the IKKEE transgene and the transgenic TCR used in this study were under the control of an lck promoter) should have led to a reduction in both CD4 and CD8 populations, which is not what we observe. Therefore, taken together, these data provide strong evidence that a post-DN stage, NF-κB-dependent process governs the phenomenon described in this paper.
The activation of NF-κB seen in DP cells expressing CD69, a marker for positive selection raises the possibility that NF-κB might be playing an important role in earlier differentiation events from DP to SP cells. To explore this question more deeply we utilized a number of defined transgenic TCR models, H-Y and OT-I for Class I MHC restricted TCRs, and AND and 1H3.1 for MHC Class II restricted TCRs, and crossed them with transgenic mice expressing IκBαSR or IKKβEE, to artificially inhibit or activate NF-κB respectively. These studies revealed a consistent pattern where, in agreement with previous findings, inhibition of NF-κB blocked positive selection in CD8 cells but not CD4 cells (Hettmann and Leiden, 2000). In contrast, forced activation of NF-κB under conditions of positive selection led to almost a complete loss of the CD4 SP thymocyte population, but only a modest decrease in CD8 SP cells. Under conditions of negative selection however, inhibition of NF-κB activity led to a significant block in negative selection of CD8 cells but not CD4 cells, in agreement with the study performed by Mora et al (Mora et al., 2001). However, these results are in contrast to a prior study that failed to see significant block in negative selection in a H-Y model when NF-κB was inhibited by expression of a mutated IκBα protein (Hettmann and Leiden, 2000). One possibility for this apparent difference might lie in the nature of the mutant IκBα used in the two experiments. We have used a super-repressor form of IκBα where in addition to the two sites of phosphorylation, residues that have been implicated for ubiquitination, stability and tyrosine phosphorylation have also been mutated. Since tyrosine phosphorylation is an integral part of signaling from the T-cell receptor, mutating the site of tyrosine phosphorylation may have influenced the effectiveness of the mutant IκB protein. An alternative possibility may be the higher expression of the IκBα super-repressor in our transgenic animals, resulting in a more complete inhibition of NF-κB activity. Therefore, based on our results we conclude that NF-κB is activated during positive/negative selection, but primarily in cells that are destined to differentiate into CD8 SP cells.
As reported previously, forced activation of NF-κB led to an increase in the number of DN cells in the DNIV stage. A candidate target gene responsible for mediating this survival effect of NF-κB in DN thymocytes is Bfl-1/A1 (Mandal et al., 2005), and the involvement of NF-κB in promoting survival is consistent with its well-characterized role as an anti-apoptotic factor. Hence, the ability of hyper-activated NF-κB, in the IKKβEE transgenic mice, to promote apoptosis in later stages of development, particularly in CD4 cells, is quite surprising. The loss of thymus-derived cells was even more pronounced in the periphery where the number of mature, peripheral T-cells was reduced by nearly 10-fold. The expression of the active IKK did not significantly alter the proliferative capacity of thymocytes or their ability to respond to stimulation (data not shown), suggesting that the effect of hyper-activating NF-κB on thymocyte survival was more complex. To overcome the difficulty of following differentiation events with a complex repertoire of T-cell receptors, we used defined TCR transgenics to follow the effect of inhibiting or hyper-activating NF-κB. Our results clearly showed that NF-κB played an important role in positive and negative selection of CD8 SP cells.
It is known that stimulation of T-cells through the T-cell receptor leads to the activation of three major, effector transcription factors, namely AP-1, NFAT and NF-κB (Crabtree and Clipstone, 1994). Therefore it is likely that the activation state of a T-cell is determined by the degree of activation of these transcription factors. While the basic principle that links differentiation of DP thymocytes to the strength of signaling is quite well established, the molecular mechanism underlying this process remains unclear. Various other signaling molecules, besides those directly activated by the TCR, have been suggested to participate in the generation of signaling thresholds including E47 (Engel et al., 2001), Ikaros (Winandy et al., 1999), rlk, and itk (Schaeffer et al., 2000). Recent studies have also suggested a more specialized role for ERK (Fischer et al., 2005) and a putative regulator RasGRP, a guanine nucleotide exchange factor for Ras (Dower et al., 2000). However, in all cases the link between the activation of these pathways and apoptotic/survival pathways remains unclear. While NF-κB is not directly regulated by these molecules (e.g. the ERK pathway is more likely to upregulate AP-1 and NFAT), our studies clearly show an important role for NF-κB in setting the threshold for selection, but only in cells that differentiate into CD8 cells.
An intriguing finding in our study is the demonstration that CD8 SP thymocytes have constitutively activated NF-κB whereas their CD4 counterparts do not. One potential explanation might be an heretofore uncharacterized link between NF-κB and those signaling pathways and transcription factors recently reported to influence thymocyte lineage choice and commitment. These include the Runx factors, GATA-3 and the newly described regulator Th-POK/c-Krox. Runx factors promote CD8 expression in the DN to DP transition and during the selection and maturation of CD8 SP cells. More specifically, it is Runx-1 that is responsible for CD4 silencing in late DN thymocytes and before β-selection, whereas, it is Runx-3, that is responsible for the CD4 silencing in CD8 SP thymocytes after positive selection (Taniuchi et al., 2002). Indeed, Sato et al demonstrated by chromatin immunoprecipitation analysis (CHIP) that Runx proteins bind to the stage-specific CD8 enhancer, as well as the CD4 silencer, in CD8 SP thymocytes (Sato et al., 2005). Therefore while it is possible that NF-κB might influence, or be influenced by, regulators such as Runx proteins in late stage thymocyte development, demonstration of such links remain to be provided.
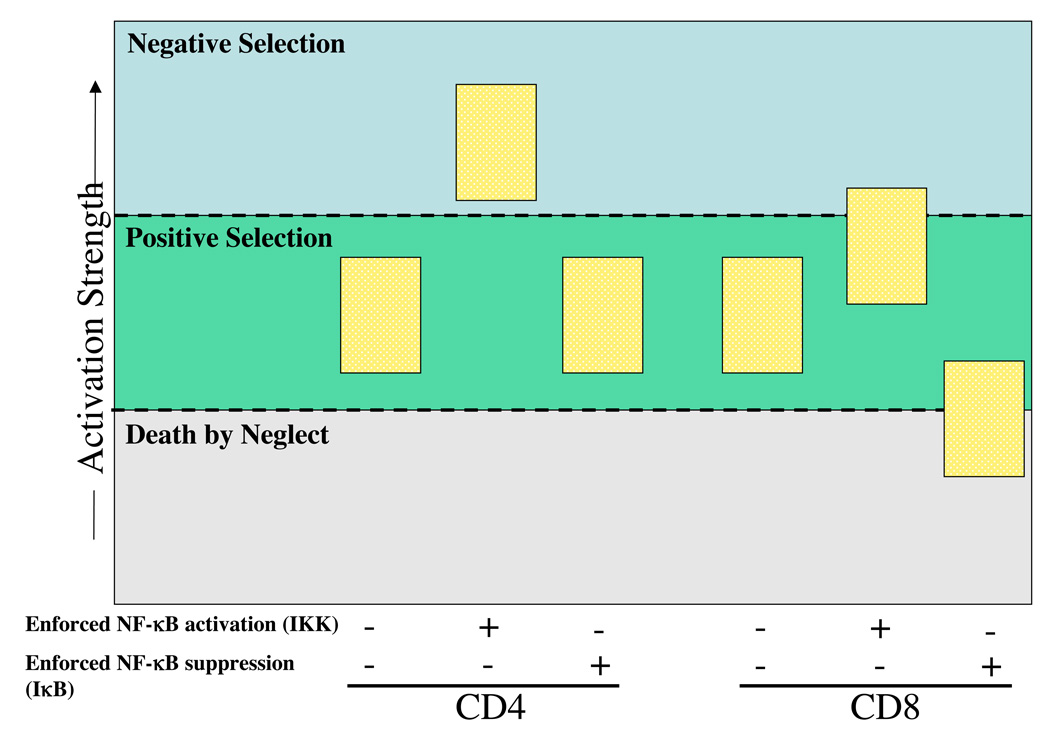
In summary the results described in this paper reveal an important role for NF-κB in differentiation of DP cells to CD8 SP cells, most likely by helping to establish the threshold of signaling necessary for positive/negative selection. We therefore propose the following model that we have summarized in Figure 8. The signal determining MHC Class-I selection consists of AP-1, NFAT and NF-κB. Additional NF-κB activation by expression of IKKβ-EE transgene increases TCR signaling above the threshold for positive selection and pushes some of the cells into negative selection. However, if NF-κB activity in these cells is suppressed through expression of the IκB-SR transgene, a significant number of the cells fail to reach the signaling threshold for positive selection, and die from death by neglect. In contrast, TCR signaling during MHC Class-II selection does not lead to NF-κB activation, and hence comprises of only AP-1 and NFAT components. Enforced expression of NF-κB by expression of IKKβ–EE has a more drastic consequence in these cells as the activation of this transcription factor provides enough additional signal to push most cells into negative selection, which we refer to as "pseudo-negative" selection. However, because NF-κB is not normally activated in these cells, inhibition of NF-κB by IκBαSR in these cells does not affect the selection process. Whether this activation also helps in lineage choice into CD4 or CD8 cells remains to be determined. Additionally, the ability of hyperactivated NF-κB CD4 cells to undergo apoptosis provides a model system to help determine how NF-κB, which in most other cases functions as an anti-apoptotic factor, can link TCR signaling to death pathways that are active in negative selection.
Figure 8. A model describing the involvement of NF-κB in thymocyte selection.
This model also illustrates the proposed inter-relationship between AP-1, NFAT and NF-κB signaling during the CD4 and CD8 thymic selection process.
Materials and Methods
Mice
The IKKβEE, IκBαSR and κB-luciferase mice have been described previously. These mice were backcrossed with either B6 or B10.BR strain more than 10 generations. The H-Y and Abβ(MHCII−/−) mice were purchased from Taconic Laboratory. The Faslpr, AND (I-Ek), OT-I, B10.A(5R), and B10S mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The Eμ-Bcl2 mice, AND (I-Ab), and 1H3.1 transgenic mice were kindly provided by Drs. Kim Bottomly and Christophe Viret (Yale University), respectively.
Antibodies
Anti-TCRβ-FITC (H57–597), anti-Vα2-FITC (B20.1), anti-Vβ3-FITC (KJ25), anti-Vβ6-FITC (RR4.7), anti-CD5-PE (53-7.3), anti-CD8a-PE, -QR (53-6.7), anti-HSA-PE (M1/69), anti-CD69-PE (H1.2F3), anti-CD45R(B220)-PE (RA3-6B2) and anti-CD4-QR, -APC (GK1.5) were purchased from BD Pharmingen. The biotin-labelled T3.70 antibody against H-Y TCR was kindly provided by Dr Leonid Gorelik (Yale University). Antibodies against p50, p52, p65, RelB and c-Rel for supershifs and IκBα, IκBβ, and IκBε for immunoblot were purchased from Santa Cruz Biotechnology.
Flow Cytometry and cell sorting
Single cell suspension of lymphocytes were centrifuged and resuspended in phosphate buffered saline (PBS) containing 1% fetal calf serum (FCS). For sorting of CD4SP or CD8SP thymocytes, cells were stained with TCRβ-FITC, anti-CD69-PE, anti-CD8a-QR and anti-CD4-APC for 30 min. Cells were washed and resuspended in PBS/1% FCS and sorted on a FACS Vantage flow cytometer (Becton Dickinson). For cytoflourometric analysis, thymocytes were stained with individual antibody for 30 min. on ice. Cells were washed and analyzed FACS Calibur flow cytometer (Becton Dickinson). FITC-Annexin V (Pharmingen) was used according to the manufacture’s instructions. Cells were stained and analysed by flow cytometry using standard procedures.
Luciferase assay
Preparation of cell lysate from thymocyte of κB-luciferase mice and carrying out of luciferase analysis were described previously (Voll et al, 2000).
Electric mobility shift assay
EMSAs were performed as described previously (Voll.et al, 200). Samples contained 5 µg of nuclear extracts and for supershifts, 1 µl of each antibody. Samples were incubated (1 hr, 4C) and then electrophoresed and visualized by autoradiography.
Immunoblotting and kinase assay
All imuunoblotting was performed as described by Voll et al. (2000). Cells were lysed in TNT buffer (20 mM Tris/HCl [pH 8.0], 0.2 M NaCl, 0.1% Triton X-100) containing 1 mM DDT, protease inhibitors (pepstatin, aprotinin, leupeptin, and PMSF) and phosphatase inhibitors (β-glycerophosphate, sodium fluoride, and sodium orthovanadate. For kinase assays, the extracts from indicated thymocytes were immunoprecipitated with antibodies against NEMO (IKKγ) (SnataCruz), and the precipitates were used kinase assays as previously described (Voll et al.2000). GST-IκBα was used as a substrate.
Northern blot analysis
Total RNA was extracted thymocytes of Wt or IKKβEE mice treated with or without PMA for 4h. The total RNA (10µg) was electrophoresed in 1.0% agarose-formaldehyde gels and RNA was transferred on nylon membrane filter. Hybridization was performed by the manufacture’s protocol of Northern Max (Ambion).
Supplementary Material
Acknowledgements
The work in this manuscript was supported by the NIH (R37-AI33443 and RO1-AI68977) (S.G) and Mochida Memorial Foundation for Medical and Pharmaceutical Research (E.J). We would like to thank Dr. David Schatz, Dr. Xiao-Hong Sun and Mr. Matthew Hayden for carefully reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barndt RJ, Dai M, Zhuang Y. Functions of E2A-HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB. Mol Cell Biol. 2000;20:6677–6685. doi: 10.1128/mcb.20.18.6677-6685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby MR, Mora AL, Scherer DC, Brockman JA, Ballard DW. Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-kappaB. J Exp Med. 1997;185:1897–1907. doi: 10.1084/jem.185.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnera E, Bhandoola A, Cibotti R, Yu Q, Guinter TI, Yamashita Y, Sharrow SO, Singer A. Coreceptor reversal in the thymus: signaled CD4+8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity. 2000;13:59–71. doi: 10.1016/s1074-7613(00)00008-x. [DOI] [PubMed] [Google Scholar]
- Clarke SR, Barnden M, Kurts C, Carbone FR, Miller JF, Heath WR. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol Cell Biol. 2000;78:110–117. doi: 10.1046/j.1440-1711.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- Crabtree GR, Clipstone NA. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, Stone JC. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- Egawa T, Kreslavsky T, Littman DR, von Boehmer H. Lineage diversion of T cell receptor transgenic thymocytes revealed by lineage fate mapping. PLoS ONE. 2008;3:e1512. doi: 10.1371/journal.pone.0001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerton M, Scollay R, Shortman K. Kinetics of mature T-cell development in the thymus. Proc Natl Acad Sci U S A. 1990;87:2579–2582. doi: 10.1073/pnas.87.7.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel I, Johns C, Bain G, Rivera RR, Murre C. Early thymocyte development is regulated by modulation of E2A protein activity. J Exp Med. 2001;194:733–745. doi: 10.1084/jem.194.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Georgopoulos K. Haematopoietic cell-fate decisions, chromatin regulation and ikaros. Nat Rev Immunol. 2002;2:162–174. doi: 10.1038/nri747. [DOI] [PubMed] [Google Scholar]
- Hare KJ, Wilkinson RW, Jenkinson EJ, Anderson G. Identification of a developmentally regulated phase of postselection expansion driven by thymic epithelium. J Immunol. 1998;160:3666–3672. [PubMed] [Google Scholar]
- He X, Dave VP, Zhang Y, Hua X, Nicolas E, Xu W, Roe BA, Kappes DJ. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- He X, Kappes DJ. CD4/CD8 lineage commitment: light at the end of the tunnel? Curr Opin Immunol. 2006;18:135–142. doi: 10.1016/j.coi.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Hernandez-Hoyos G, Anderson MK, Wang C, Rothenberg EV, Alberola-Ila J. GATA-3 expression is controlled by TCR signals and regulates CD4/CD8 differentiation. Immunity. 2003;19:83–94. doi: 10.1016/s1074-7613(03)00176-6. [DOI] [PubMed] [Google Scholar]
- Hettmann T, DiDonato J, Karin M, Leiden JM. An essential role for nuclear factor kappaB in promoting double positive thymocyte apoptosis. J Exp Med. 1999;189:145–158. doi: 10.1084/jem.189.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettmann T, Leiden JM. NF-kappa B is required for the positive selection of CD8+ thymocytes. J Immunol. 2000;165:5004–5010. doi: 10.4049/jimmunol.165.9.5004. [DOI] [PubMed] [Google Scholar]
- Hogquist KA. Signal strength in thymic selection and lineage commitment. Curr Opin Immunol. 2001;13:225–231. doi: 10.1016/s0952-7915(00)00208-9. [DOI] [PubMed] [Google Scholar]
- Hollander G, Gill J, Zuklys S, Iwanami N, Liu C, Takahama Y. Cellular and molecular events during early thymus development. Immunol Rev. 2006;209:28–46. doi: 10.1111/j.0105-2896.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu Rev Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- Kasibhatla S, Brunner T, Genestier L, Echeverri F, Mahboubi A, Green DR. DNA damaging agents induce expression of Fas ligand and subsequent apoptosis in T lymphocytes via the activation of NF-kappa B and AP-1. Mol Cell. 1998;1:543–551. doi: 10.1016/s1097-2765(00)80054-4. [DOI] [PubMed] [Google Scholar]
- Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- Kondo M, Akashi K, Domen J, Sugamura K, Weissman IL. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity. 1997;7:155–162. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- Kreslavsky T, Garbe AI, Krueger A, von Boehmer H. T cell receptor-instructed alphabeta versus gammadelta lineage commitment revealed by single-cell analysis. J Exp Med. 2008;205:1173–1186. doi: 10.1084/jem.20072425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas B, Germain RN. Opening a window on thymic positive selection: developmental changes in the influence of cosignaling by integrins and CD28 on selection events induced by TCR engagement. J Immunol. 2000;165:1889–1895. doi: 10.4049/jimmunol.165.4.1889. [DOI] [PubMed] [Google Scholar]
- Mandal M, Borowski C, Palomero T, Ferrando AA, Oberdoerffer P, Meng F, Ruiz-Vela A, Ciofani M, Zuniga-Pflucker JC, Screpanti I, et al. The BCL2A1 gene as a pre-T cell receptor-induced regulator of thymocyte survival. J Exp Med. 2005;201:603–614. doi: 10.1084/jem.20041924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marodon G, Rocha B. Generation of mature T cell populations in the thymus: CD4 or CD8 down-regulation occurs at different stages of thymocyte differentiation. Eur J Immunol. 1994;24:196–204. doi: 10.1002/eji.1830240131. [DOI] [PubMed] [Google Scholar]
- Moore NC, Girdlestone J, Anderson G, Owen JJ, Jenkinson EJ. Stimulation of thymocytes before and after positive selection results in the induction of different NF-kappa B/Rel protein complexes. J Immunol. 1995;155:4653–4660. [PubMed] [Google Scholar]
- Mora AL, Chen D, Boothby M, Rubin DH. Lineage-specific differences among CD8+ T cells in their dependence of NF-kappa B/Rel signaling. Eur J Immunol. 1999;29:2968–2980. doi: 10.1002/(SICI)1521-4141(199909)29:09<2968::AID-IMMU2968>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Mora AL, Stanley S, Armistead W, Chan AC, Boothby M. Inefficient ZAP-70 phosphorylation and decreased thymic selection in vivo result from inhibition of NF-kappaB/Rel. J Immunol. 2001;167:5628–5635. doi: 10.4049/jimmunol.167.10.5628. [DOI] [PubMed] [Google Scholar]
- Ohashi PS, Pircher H, Burki K, Zinkernagel RM, Hengartner H. Distinct sequence of negative or positive selection implied by thymocyte T-cell receptor densities. Nature. 1990;346:861–863. doi: 10.1038/346861a0. [DOI] [PubMed] [Google Scholar]
- Palmer E. Negative selection--clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- Rothenberg EV, Taghon T. Molecular genetics of T cell development. Annu Rev Immunol. 2005;23:601–649. doi: 10.1146/annurev.immunol.23.021704.115737. [DOI] [PubMed] [Google Scholar]
- Sato T, Ohno S, Hayashi T, Sato C, Kohu K, Satake M, Habu S. Dual functions of Runx proteins for reactivating CD8 and silencing CD4 at the commitment process into CD8 thymocytes. Immunity. 2005;22:317–328. doi: 10.1016/j.immuni.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Schaeffer EM, Broussard C, Debnath J, Anderson S, McVicar DW, Schwartzberg PL. Tec family kinases modulate thresholds for thymocyte development and selection. J Exp Med. 2000;192:987–1000. doi: 10.1084/jem.192.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Courtois G, Tian J, Coyle AJ, Israel A, Rajewsky K, Pasparakis M. Mature T cells depend on signaling through the IKK complex. Immunity. 2003;19:377–389. doi: 10.1016/s1074-7613(03)00237-1. [DOI] [PubMed] [Google Scholar]
- Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Serfling E, Avots A, Neumann M. The architecture of the interleukin-2 promoter: a reflection of T lymphocyte activation. Biochim Biophys Acta. 1995;1263:181–200. doi: 10.1016/0167-4781(95)00112-t. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Punt JA, Granger LG, Singer A. Asymmetric signaling requirements for thymocyte commitment to the CD4+ versus CD8+ T cell lineages: a new perspective on thymic commitment and selection. Immunity. 1995;2:413–425. doi: 10.1016/1074-7613(95)90149-3. [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Tsuji M, Yamamoto N, Han H, Tsukada J, Inoue H, Kubo M, Honjo T. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20:611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- Taniuchi I, Littman DR. Epigenetic gene silencing by Runx proteins. Oncogene. 2004;23:4341–4345. doi: 10.1038/sj.onc.1207671. [DOI] [PubMed] [Google Scholar]
- Taniuchi I, Osato M, Egawa T, Sunshine MJ, Bae SC, Komori T, Ito Y, Littman DR. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- Teh HS, Kisielow P, Scott B, Kishi H, Uematsu Y, Bluthmann H, von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988;335:229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- Terrence K, Pavlovich CP, Matechak EO, Fowlkes BJ. Premature expression of T cell receptor (TCR)alphabeta suppresses TCRgammadelta gene rearrangement but permits development of gammadelta lineage T cells. J Exp Med. 2000;192:537–548. doi: 10.1084/jem.192.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez NJ, Kaye J, Hedrick SM. In vivo and in vitro clonal deletion of double-positive thymocytes. J Exp Med. 1992;175:1307–1316. doi: 10.1084/jem.175.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viret C, Sant'Angelo DB, He X, Ramaswamy H, Janeway CA., Jr A role for accessibility to self-peptide-self-MHC complexes in intrathymic negative selection. J Immunol. 2001;166:4429–4437. doi: 10.4049/jimmunol.166.7.4429. [DOI] [PubMed] [Google Scholar]
- Voll RE, Jimi E, Phillips RJ, Barber DF, Rincon M, Hayday AC, Flavell RA, Ghosh S. NF-kappa B activation by the pre-T cell receptor serves as a selective survival signal in T lymphocyte development. Immunity. 2000;13:677–689. doi: 10.1016/s1074-7613(00)00067-4. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Thymic selection: a matter of life and death. Immunol Today. 1992;13:454–458. doi: 10.1016/0167-5699(92)90075-I. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Winandy S, Wu L, Wang JH, Georgopoulos K. Pre-T cell receptor (TCR) and TCR-controlled checkpoints in T cell differentiation are set by Ikaros. J Exp Med. 1999;190:1039–1048. doi: 10.1084/jem.190.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf E, Xiao C, Fainaru O, Lotem J, Rosen D, Negreanu V, Bernstein Y, Goldenberg D, Brenner O, Berke G, et al. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci U S A. 2003;100:7731–7736. doi: 10.1073/pnas.1232420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.