Abstract
To maintain genomic integrity, telomeres must undergo switches from a protected state to an accessible state that allows telomerase recruitment. To better understand how telomere accessibility is regulated in fission yeast, we analysed cell cycle-dependent recruitment of telomere-specific proteins (telomerase Trt1, Taz1, Rap1, Pot1 and Stn1), DNA replication proteins (DNA polymerases, MCM, RPA), checkpoint protein Rad26 and DNA repair protein Nbs1 to telomeres. Quantitative chromatin immunoprecipitation studies revealed that MCM, Nbs1 and Stn1 could be recruited to telomeres in the absence of telomere replication in S-phase. In contrast, Trt1, Pot1, RPA and Rad26 failed to efficiently associate with telomeres unless telomeres are actively replicated. Unexpectedly, the leading strand DNA polymerase ɛ (Polɛ) arrived at telomeres earlier than the lagging strand DNA polymerases α (Polα) and δ (Polδ). Recruitment of RPA and Rad26 to telomeres matched arrival of DNA Polɛ, whereas S-phase specific recruitment of Trt1, Pot1 and Stn1 matched arrival of DNA Polα. Thus, the conversion of telomere states involves an unanticipated intermediate step where lagging strand synthesis is delayed until telomerase is recruited.
Keywords: cell cycle, DNA polymerase, DNA replication, pot1, telomerase
Introduction
To maintain genomic integrity, telomeres must fulfill two major functions (Blackburn, 2001). First, telomeres must be able to prevent DNA repair and DNA damage checkpoint proteins from causing uncontrolled fusion and degradation of telomeric DNA and eliciting permanent cell cycle arrest. Second, telomeres must provide access to telomerase, a reverse transcriptase that can extend the GT-rich telomeric repeat sequence by using its RNA subunit as a template. Recruitment of telomerase is essential to prevent loss of telomeric DNA after each round of DNA replication because replicative DNA polymerases cannot fully replicate ends of linear DNA molecules. Thus, telomeres must undergo dynamic switches from a highly protected state that prevents full access to DNA repair and DNA damage checkpoint proteins to a more accessible state that allows recruitment of telomerase, as well as various DNA repair and DNA damage checkpoint proteins (Verdun and Karlseder, 2007). Previous studies have found that such changes in telomere status are likely to occur in a cell cycle-dependent manner (Gilson and Geli, 2007). Specifically, replication of telomeres has been proposed to have an important function in controlling accessibility of telomeres.
Evolutionarily conserved unique structural features of telomeric DNA include species-specific GT-rich repetitive double-stranded DNA (dsDNA) as well as a 3′ single-stranded GT-rich overhang (G-tail). These features are important for both protection and replication associated functions of telomeres as they provide binding sites for telomere-specific proteins and telomerase (Verdun and Karlseder, 2007). The G-tail is required for the extension of telomeric DNA by telomerase, as the telomerase RNA subunit cannot anneal to blunt ends (Lingner and Cech, 1996). Loss of telomere-specific factors, such as the budding yeast G-tail-binding capping protein Cdc13, can lead to degradation of the telomeric CA-rich strand, massive accumulation of DNA repair and checkpoint factors at telomeres, and cell cycle arrest (Melo et al, 2001). The length of the G-tail is cell cycle regulated, and increases during S-phase in both budding yeast Saccharomyces cerevisiae and fission yeast Schizosaccharomyces pombe (Wellinger et al, 1996; Tomita et al, 2004).
Semi-conservative DNA replication of linear chromosomes generates two structurally distinct termini at telomeres (Ohki et al, 2001). The strand replicated by lagging strand synthesis will leave a 3′ single-stranded overhang (G-tail), whereas the strand replicated by leading strand synthesis will likely produce a blunt terminus. Therefore, leading-strand telomeres must be processed to re-generate the G-tail. Evidence for this processing comes from studies showing that both ends of chromosomes terminate in a 3′ overhang in yeasts, ciliates and humans (Wellinger et al, 1993; Makarov et al, 1997; Jacob et al, 2001; Munoz-Jordan et al, 2001). This end processing does not require telomerase, as the 3′ overhang was still present at both ends of chromosomes in the absence of telomerase (Wellinger et al, 1996; Makarov et al, 1997). The existence of two distinct types of end processing mechanisms at telomeres has been supported by studies that observed (1) chromosomal fusions only among leading-strand telomeres in mammalian cells carrying mutant versions of TRF2 or DNA-PKcs (Bailey et al, 2001), (2) preferential loss of lagging-strand telomeres in human cells defective in RecQ DNA helicase WRN (Crabbe et al, 2004) and (3) much longer G-tails on lagging-strand telomeres than leading-strand telomeres in human cells lacking active telomerase (Chai et al, 2006a).
Chromatin immunoprecipitation (ChIP) analyses in S. cerevisiae have shown increased association of the G-tail-binding protein Cdc13 and the telomerase subunit Est1 in late S-phase (Taggart et al, 2002; Schramke et al, 2004). Est2, the catalytic subunit of telomerase, is loaded to telomeres in G1-phase through specific interaction between the Ku70–Ku80 complex and telomerase RNA, but it also shows increased association with telomeres during late S-phase (Fisher et al, 2004). Timing of maximum recruitment for the single-stranded DNA (ssDNA)-binding protein complex RPA (replication protein A) and DNA polymerase ɛ to telomeres coincide with the timing of the maximum recruitment for Est1 and Cdc13, suggesting that Est1 and Cdc13 are recruited to telomeres when the replication fork arrives at telomeres in budding yeast (Schramke et al, 2004; Bianchi and Shore, 2007). However, it is also possible that MRX (Mre11–Rad50–Xrs2)-dependent resection of the CA-strand, rather than arrival of the DNA replication fork at telomeres itself, is responsible for the observed RPA loading to telomeres, as increased binding of the G-tail-binding protein Cdc13 to telomeres during late S-phase requires MRX-dependent generation of long G-tails (Larrivee et al, 2004; Takata et al, 2005; Goudsouzian et al, 2006). Association of budding yeast MRX with telomeres also increases during late S-phase (Takata et al, 2005). Likewise, S-phase specific association of human NBS1 (budding yeast Xrs2 ortholog) with telomeres has been observed (Wu et al, 2000; Zhu et al, 2000). On the other hand, unlike yeast MRX, human MRE11 and RAD50 appear to constitutively associate with telomeres, perhaps through specific interaction with TRF2 (Zhu et al, 2000). This difference might be due to the fact that S. cerevisiae lacks TRF1/TRF2-like proteins.
In fact, S. cerevisiae has diverged significantly in telomere protein components from humans, whereas telomere components in fission yeast S. pombe are more closely related to human telomere proteins. For example, S. cerevisiae lacks orthologs for human telomeric dsDNA-binding proteins TRF1 and TRF2, whereas S. pombe Taz1 shows sequence and functional similarity to both TRF1 and TRF2. In S. cerevisiae, Rap1 directly binds to telomeric dsDNA. In contrast, human and S. pombe Rap1 proteins do not directly bind to telomeric DNA, but they are brought to telomeres through protein–protein interaction with TRF2 and Taz1, respectively (Smogorzewska and de Lange, 2004). Budding yeast Cdc13 also lacks any obvious ortholog in S. pombe or human, although Pot1 proteins from both human and S. pombe are likely to play similar functional roles as Cdc13. It was recently shown that S. pombe Pot1 forms a multi-protein complex that closely resembles the mammalian shelterin complex (Miyoshi et al, 2008; Palm and de Lange, 2008). Therefore, although studies in S. cerevisiae have provided the most detailed molecular description of telomere components to date, S. pombe should provide new insights as a model system more closely resembling the mechanisms of human telomere maintenance.
Fission yeast telomeres are replicated very late in S-phase (Kim and Huberman, 2001). Presumably, late replication of telomeres is the consequence of a local chromatin environment, which is composed of GT-rich repetitive telomeric repeat DNA (both double-stranded and single-stranded with 3′ overhang), telomere-specific dsDNA-binding proteins (Taz1 and Rap1), telomere-specific ssDNA-binding proteins (Pot1–Tpz1–Poz1–Ccq1 and Stn1–Ten1 complexes), various dsDNA break repair proteins (Ku70–Ku80 and Mre11–Rad50–Nbs1 complexes), and DNA damage checkpoint proteins (Rad3–Rad26 and Rad9–Rad1–Hus1 complexes) (Kanoh and Ishikawa, 2001; Nakamura et al, 2002; Martin et al, 2007; Miyoshi et al, 2008). Furthermore, proteins involved in heterochromatin formation might also have a function in establishing late S-phase replication of telomeres (Mickle et al, 2007). All these proteins are expected to contribute in regulating telomere accessibility changes during the cell cycle; however, currently no data are available with regard to how binding of telomere-associated proteins at telomeres is regulated during the fission yeast cell cycle. Therefore, we decided to characterise cell cycle specific changes in telomere protein composition and directly co-relate them with the timing of telomere replication.
Results
Telomere replication occurs in late S-phase and is inhibited by hydroxyurea
To investigate how late S-phase replication of telomeres is established and coordinated with the extension of telomeric repeats by telomerase in fission yeast, we used the temperature sensitive cdc25-22 allele to synchronise cells (Alfa et al, 1993). After incubating cdc25-22 cells at the non-permissive temperature (36°C) for 3 h, late G2-phase arrested cells were shifted to the permissive temperature (25°C) for synchronous cell cycle re-entry, and samples were collected every 20 min and processed for ChIP analysis. On the basis of 5-bromo-2′-deoxyuridine (BrdU) incorporation analysis (Hodson et al, 2003), the early replication origins ars2004 and ars3002 replicated 60–100 min after the temperature shift, whereas telomeres replicated at 100–140 min (Figure 1A and data not shown), confirming the previously established timing of replication for these regions (Kim and Huberman, 2001). In agreement with previous studies, when cells were released from the cdc25-22 arrest in the presence of 15 mM hydroxyurea (HU), BrdU incorporation at telomeres was abolished, whereas BrdU was still efficiently incorporated at ars2004 (Hayashi et al, 2007; Mickle et al, 2007; Supplementary Figure S3). Previous studies have shown that firing of late origins, including telomeres, are extremely delayed and/or inhibited by the addition of HU, and that prevention of origin firing at telomeres in the presence of HU requires S-phase checkpoint proteins, such as Rad3 and Cds1 kinases (Hayashi et al, 2007; Mickle et al, 2007).
Figure 1.
Late S-phase replication of telomeres is reflected in delayed recruitment of MCM and RPA to telomeres compared with early replication origin ars2004. Data are from multiple (n) independent experiments, and error bars represent average deviation (n=2) or standard deviation (n=3). For the definition of relative precipitation (Rel. precipitation), see ‘ChIP analysis' sub-section in the Materials and methods. These values represent background corrected percentage precipitation, normalised to the peak values in minus HU experiments. (A) BrdU incorporation at telomeres and ars2004 for cdc25-22 synchronised cells (n=3). (B) Recruitment of MCM (Mcm2) to telomeres and ars2004 for cdc25-22 synchronised cells (n=2). (C) Recruitment of RPA (Rad11) to telomeres and ars2004 for cdc25-22 synchronised cells (n=2). (D) Comparison of peak percentage precipitation values (see Materials and methods) at telomeres and ars2004 in the absence or presence of 15 mM HU for MCM and RPA. The peak percentage precipitation value ratios (telomere/ars2004) in the absence or presence of HU were calculated for the indicated proteins.
We next monitored the cell cycle-regulated recruitment of the hexameric MCM complex to telomeres and ars2004. MCM is loaded to DNA through the function of the origin recognition complex (ORC), and it is believed to be the replicative helicase required for both initiation and elongation stages of DNA replication (Bochman and Schwacha, 2008). Recent genome-wide ChIP analyses have found that sub-telomeric regions are highly enriched with ORC and MCM (Hayashi et al, 2007). However, kinetics of MCM loading to telomeres has not been compared with other replicating origins. We found that recruitment of Mcm2 (a subunit of the MCM complex) to telomeres was delayed (binding peak at 100 min) compared with ars2004 (binding peak at 80 min) (Figure 1B). At both telomeres and ars2004, recruitment of Mcm2 preceded BrdU incorporation; however, we observed a greater time lag between MCM loading and BrdU incorporation at telomeres than at ars2004 (Supplementary Figure S4A and B). Therefore, we conclude that delayed loading of MCM to telomeres is only partially responsible for establishing the late S-phase replication of telomeric DNA.
In the presence of HU, increased binding of Mcm2 was observed at both telomeres and ars2004 (Supplementary Figure S4C and D). Although MCM dissociated from ars2004 as DNA replication was completed, MCM remained bound to telomeres for the duration of our experiment, correlating well with the observation that DNA replication is inhibited at telomeres in the presence of HU (Supplementary Figures S3, S4C and D). HU had little effect on the initial recruitment timing of MCM to telomeres. Moreover, the ratio of the peak percentage precipitation values at telomeres against ars2004 for MCM was altered only slightly by the addition of HU (Figure 1D). Therefore, it appears that inhibition of DNA replication at telomeres in the presence of HU is caused by inhibition of a step after loading of MCM to telomeres.
Another hallmark of DNA replication is recruitment of the ssDNA-binding protein RPA after the replicative helicase unwinds DNA. Therefore, we next monitored recruitment of RPA to telomeres and ars2004. As shown in Figure 1C, we found that Rad11 (the largest subunit of the RPA complex) is recruited maximally to telomeres at 120 min after release from the cdc25-22 arrest, compared with 80 min at ars2004. RPA binding to telomeres matched closely to the timing of BrdU incorporation at telomeres, whereas RPA binding to ars2004 preceded BrdU incorporation and more closely resembled the timing of Mcm2 recruitment (Supplementary Figure S5).
Interestingly, we also found that RPA was able to precipitate approximately four-fold more telomeric DNA than ars2004 DNA when the peak percentage precipitation values were compared (Figure 1D). Thus, telomeres appear to accumulate significantly more ssDNA than ars2004 when the DNA replication fork moves in. In the presence of HU, the DNA replication-induced peak of RPA binding at telomeres was essentially eliminated, consistent with the observation that telomeres are not replicated in the presence of HU (Supplementary Figure S5C). In contrast, RPA binding to ars2004 was extended and increased approximately five-fold in the presence of HU, and RPA precipitated approximately 2.5-fold more ars2004 DNA compared with telomeric DNA (Figure 1D; Supplementary Figure S5D). Such results are consistent with the notion that the HU-induced stalling/collapse of replication forks results in accumulation of ssDNA at actively replicating regions of the genome.
Telomeres are recognised by checkpoint protein Rad26 and DNA repair protein Nbs1 as damaged DNA
Given that much more RPA (approximately four-fold) was recruited to telomeres than to ars2004 during replication (Figure 1D), and the peak of RPA recruitment to telomeres in the absence of HU (2.47±0.70% precipitation) was comparable to the peak of recruitment of RPA to ars2004 in the presence of HU (3.05±0.50% precipitation), we suspected that replicating telomeres might be recognised as stalled/damaged forks by DNA damage/replication checkpoint proteins. Therefore, we next monitored the cell cycle-regulated recruitment of the checkpoint protein Rad26 (mammalian ATRIP ortholog) to telomeres and ars2004.
Indeed, Rad26 binding to telomeres increased as telomeres were replicated, with the peak of recruitment at 120 min after the release from cdc25-22 arrest, whereas DNA replication-specific Rad26 recruitment to telomeres was largely abolished in the presence of HU (Figure 2A). Very little Rad26 was detected at ars2004 during unperturbed cell cycle progression (0.073±0.038% precipitation), but Rad26 was efficiently recruited and retained at ars2004 in the presence of HU (0.30±0.10% precipitation), consistent with the notion that actively replicating regions are recognised and protected by S-phase checkpoint proteins after addition of HU (Figure 2B). We also found that timing and level of RPA and Rad26 recruitment to telomeres matched well either in the presence or absence of HU (Figures 1D, 2E and F). This is expected, as previous studies have established that Rad26/ATRIP is recruited to ssDNA bound by RPA (Zou and Elledge, 2003). Taken together, these results establish that Rad26 transiently recognises telomeres as stalled forks and/or damaged DNA during unperturbed replication of telomeres in fission yeast.
Figure 2.
Rad26 and Nbs1 recognise telomeres as damaged DNA during S-phase. For explanation of error bars, see Figure 1. (A, B) Recruitment of Rad26 to telomeres (A) or ars2004 (B) in the absence or presence of 15 mM HU for cdc25-22 synchronised cells (n=3). (C, D) Recruitment of Nbs1 to telomeres (C) or ars2004 (D) in the absence or presence of 15 mM HU for cdc25-22 synchronised cells (n=3). (E) Comparison of peak percentage precipitation values (see Materials and methods) at telomeres and ars2004 in the absence or presence of 15 mM HU for Rad26 and Nbs1. The peak percentage precipitation value ratios (telomere/ars2004) in the absence or presence of HU were calculated for the indicated proteins. (F) Recruitment kinetics of Rad26 and RPA to telomeres are very similar in both absence and presence of HU. Data from panel A (Rad26) and Supplementary Figure S3C (Rad11/RPA) are combined.
We next monitored recruitment of the DNA repair protein Nbs1 to telomeres. Nbs1, a component of the Mre11–Rad50–Nbs1 (MRN) complex, is needed to recruit Tel1 (ATM) kinase to DNA damage sites (You et al, 2005). Previously, MRN–Tel1 and the Rad3–Rad26 complexes have been shown to represent two redundant pathways that are essential for fission yeast cells to maintain stable telomeres and to prevent chromosome circularisation (Naito et al, 1998; Nakamura et al, 2002; Chahwan et al, 2003). Similarly, budding yeast MRX–Tel1 and Mec1–Ddc2 (Rad3–Rad26 orthologs) are redundantly required to maintain stable telomeres (Craven et al, 2002), and MRX–Tel1 are recruited to telomeres in late S-phase to promote recruitment of telomerase preferentially to shorter telomeres (Goudsouzian et al, 2006; Hector et al, 2007; Sabourin et al, 2007).
In contrast to budding yeast, we found that Nbs1 in fission yeast shows constitutive binding to telomeres throughout the cell cycle with a broad approximately two-fold increase in binding during S-phase (Figure 2C). Much like RPA and Rad26, the peak percentage precipitation value was approximately five-fold higher at telomeres compared with ars2004 during unperturbed S-phase (Figure 2E). However, in contrast to RPA and Rad26, Nbs1 binding to telomeres still increased as cells enter S-phase in the presence of HU. Therefore, it appears that increased association of Nbs1 to telomeres does not require actual replication of telomeric DNA. Given the role of MRN as a sensor of DNA breaks, the presence of DNA ends at telomeres may be the main cause for Nbs1 recruitment to telomeres. During S-phase, even before actual replication, telomeric DNA ends might become more accessible to Nbs1 due to changes in local chromatin structure. At ars2004, we observed that Nbs1 recruitment is markedly increased when cells enter S-phase in the presence of HU, consistent with the notion that MRN is involved in detection/repair of HU-induced collapsed replication forks (Figure 2D and E).
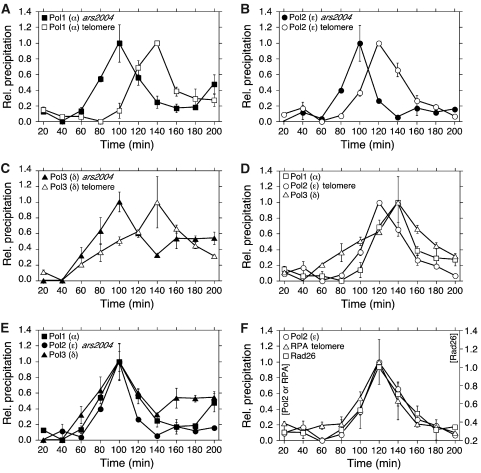
Arrival of lagging strand DNA polymerases and is delayed compared with arrival of leading strand DNA polymerase at telomeres
DNA replication of genomic DNA in eukaryotic cells is performed by DNA polymerases α, δ and ɛ. DNA polymerase α (Polα/Pol1) is in a complex with DNA primase, and thus, it is continually required for synthesis of Okazaki fragments on the lagging strand. Recent studies in budding yeast have established that DNA polymerase ɛ (Polɛ/Pol2) is primarily involved in leading strand synthesis, whereas DNA polymerase δ (Polδ/Pol3) is mostly involved in lagging strand synthesis (Pursell et al, 2007; Nick McElhinny et al, 2008). Interestingly, studies in both budding and fission yeasts have found that mutations in Polɛ lead to shorter telomeres, whereas mutations in DNA Polα and Polδ lead to longer telomeres (Adams-Martin et al, 2000; Ohya et al, 2002; Dahlén et al, 2003). Thus, defects in leading strand synthesis and lagging strand synthesis show opposite effects on telomere maintenance in both yeasts.
When cell cycle-regulated recruitment kinetics of the DNA polymerases were compared (Figure 3A–C; Supplementary Figures S6–S8), we were surprised to find that Polɛ arrived at telomeres significantly earlier (peak at 120 min) than Polα and Polδ (peaks at 140 min) (Figure 3D). As expected, recruitment of all three polymerases to telomeres was essentially abolished when HU was added (Supplementary Figures S6C, S7C and S8C). In contrast, recruitment kinetics for all three polymerases were similar at ars2004 or non-ARS (a region approximately 30 kb from ars2004) (Figure 3E; Supplementary Figure S9A). All polymerases showed increased and extended association with ars2004 in the presence of HU (Supplementary Figures S6D, S7D and S8D).
Figure 3.
Differential arrival of leading strand polymerase (Polɛ) and lagging strand polymerases (Polα and Polδ) at telomeres. For explanation of error bars, see Figure 1. (A–C) Recruitment of Polα (A), Polɛ (B) or Polδ (C) to telomeres and ars2004 for cdc25-22 synchronised cells (n=2). (D) The peak of Polɛ recruitment to telomeres is earlier than those of Polα and Polδ. Data from panels A–C are combined. (E) All three polymerase (Polα, Polδ and Polɛ) are recruited to ars2004 with similar kinetics. Data from panels A–C are combined. (F) Recruitment kinetics of Polɛ, RPA and Rad26 to telomeres are very similar. To better compare S-phase specific recruitment of these factors, Rad26 (right y-axis) was offset from Polɛ and RPA (left y-axis) to match baselines. Data from Figures 1C, 2A and 3B are combined.
We also compared recruitment kinetics of Polα and Polɛ at centromeres to test whether delayed arrival of the lagging strand polymerases at telomeres might be related to the presence of heterochromatin. However, Polα and Polɛ arrived at centromeres with similar timing (Supplementary Figure S9B). Thus, delayed arrival of lagging strand polymerases (Polα and Polδ) compared with leading strand polymerase (Polɛ) is not a general feature of heterochromatin regions, but unique to telomeres.
When recruitment patterns for Polɛ, RPA and Rad26 were aligned, telomere recruitment kinetics matched quite well for these three proteins (Figure 3F). Thus, our observations are consistent with the notion that delayed arrival of Polα/Polδ compared with Polɛ could lead to accumulation of RPA-coated ssDNA on the lagging strand telomeres, which are in turn recognised by the checkpoint protein Rad26. Once Polα/Polδ is recruited and lagging strand synthesis is completed, RPA and Rad26 association would then be reduced, as telomeres no longer carry long ssDNA G-tails.
S-phase specific recruitment of telomerase and Pot1, but not Stn1, to telomeres during S-phase is dependent on telomere replication
Telomeres are bound by various telomere-specific factors. To understand the dynamics of those proteins that are crucial for telomere function and found only at telomeres, we next monitored the recruitment of telomerase, Taz1, Rap1, Pot1 and Stn1 to telomeres during cell cycle progression. The catalytic subunit of telomerase Trt1 (TERT, ortholog of budding yeast Est2) was maximally recruited to telomeres at 140 min after release from the cdc25-22 arrest (Figure 4A). Thus, timing of Trt1 recruitment matches more closely to the recruitment timing of Polα (Figure 4E) than Polɛ. Unlike in budding yeast (Taggart et al, 2002; Fisher et al, 2004), we did not see recruitment of Trt1 to telomeres in G1. This is not surprising, as previous studies in fission yeast have found that the Ku70–Ku80 complex does not associate with Trt1, and Ku70 is dispensable for recruitment of Trt1 to telomeres (Subramanian et al, 2008; Webb and Zakian, 2008).
Figure 4.
Cell cycle-regulated association of telomere specific factors to telomeres. For explanation of error bars, see Figure 1. (A–D) Recruitment of Trt1 (A; n=3), Taz1 and Rap1 (B; n=2), Pot1 (C; n=3) or Stn1 (D; n=3) to telomeres in the absence or presence of 15 mM of HU for cdc25-22 synchronised cells. (E) S-phase specific recruitment kinetics of Polα, Trt1 and Pot1 to telomeres are very similar. To better compare S-phase specific recruitment of these factors, Pot1 (right y-axis) was offset from Polα and Trt1 (left y-axis) to match baselines. Data from Figures 3A, 4A and C are combined. (F) S-phase specific recruitment kinetics of Pot1 and Stn1 to telomeres are very similar. To better compare S-phase specific recruitment of these factors, Stn1 (right y-axis) was offset from Pot1 (left y-axis) to match lowest precipitated DNA values. Data from panels C and D are combined.
In the presence of HU, recruitment of Trt1 to telomeres was greatly reduced (Figure 4A). Therefore, we conclude that maximum recruitment of Trt1 to telomeres requires arrival of the replication fork at telomeres. However, we noticed that a significant amount of Trt1 was still recruited to telomeres in the presence of HU (Figure 4A), even though recruitment of replicative polymerases to telomeres was essentially eliminated by addition of HU (Supplementary Figures S6C, S7C and S8C). Therefore, initial recruitment of telomerase to telomeres might not be strictly dependent on arrival of the DNA replication fork. Rather, other earlier events in G1/S-phase, such as loading of MCM, increased binding of Nbs1, or reduced binding of Taz1, Pot1 and Stn1 (see below) might allow changes in the chromatin environment at telomeres to allow some recruitment of Trt1 to telomeres before replication of telomeric DNA. Conversely, replication-independent extension of telomeres by telomerase might also contribute to loading of Stn1 and Nbs1. Further studies are needed to sort out these possibilities.
We found that the telomere dsDNA-binding protein Taz1 was constitutively bound to telomeres but showed broad approximately two-fold reduction in telomere association throughout S-phase. On the other hand, Rap1, a protein thought to be recruited to telomeric dsDNA by Taz1, showed very little changes in binding during the cell cycle (Figure 4B). Therefore, Rap1 might be able to stay bound to telomeres in the absence of Taz1. In fact, there are evidences that Rap1 has a Taz1-independent role in protection of telomeres despite the fact that Rap1 cannot be detected at telomeres in taz1Δ cells by ChIP assays (Miller et al, 2005; Subramanian et al, 2008). Alternatively, Taz1 might become less accessible to the antibody used for ChIP, due to recruitment of other factors during S-phase. Nbs1, which shows a broad approximately two-fold increase in S-phase association to telomeres, could be one of those factors. In this regard, it is interesting to note that mammalian TRF2 (Taz1 ortholog) has been reported to associate with the MRN complex (Zhu et al, 2000). Previous studies have also reported that budding yeast Rap1 and Rif1 (Smith et al, 2003) and mammalian TRF1 (Verdun et al, 2005) show reduced binding to telomeres during S-phase.
Association of the ssDNA telomere-binding proteins Pot1 and Stn1 to telomeres was reduced in M/G1-phase to 20–30% of maximum binding, followed by increases in association as telomeres are replicated (Figure 4C and D). In fact, the S-phase specific increase in Pot1 and Stn1 association occurred with very similar timing as increase in binding for Polα and Trt1 (Figure 4E and F). Previous studies have found that Polα and Trt1 can be co-immunoprecipitated during S-phase in a DNA-independent manner, and that the Pot1-associated protein Tpz1 can pull down catalytically active telomerase from cell extract (Dahlén et al, 2003; Miyoshi et al, 2008). Furthermore, we found that addition of HU resulted in reduction of telomere binding for Trt1, Pot1 and Polα (Figure 4A and C; Supplementary Figure S6C). Thus, it is possible that recruitment of Trt1, Polα and Pot1 might be influenced by protein–protein interactions among these complexes.
Although Pot1 and Stn1 showed similar cell cycle-regulated recruitment patterns to telomeres in the absence of HU, Pot1 binding was much more severely reduced in the presence of HU than Stn1 binding (Figure 4C and D). As most Stn1 (approximately 80% compared with non-HU culture) could still be recruited to telomeres and stayed bound at an elevated level in the presence of HU, we conclude that recruitment of Stn1 to telomeres is not strictly dependent on actual replication of telomeres. In addition, it appears that there is no strong interaction between the Pot1 complex and the Stn1–Ten1 complex (Martin et al, 2007; Supplementary Figure S10). Thus, although late S-phase recruitment of Stn1 and Pot1 occurs with very similar timing, it is unlikely that Pot1, Stn1 and Ten1 are recruited to telomeres as a stable RPA-like complex, as it has been proposed for the Cdc13–Stn1–Ten1 complex in budding yeast (Gao et al, 2007). Conversely, as both Pot1 and Stn1 have been shown to be essential for protection of telomeres from fusions and checkpoint activation (Baumann and Cech, 2001; Martin et al, 2007), late S-phase recruitment of Pot1 and Stn1 might prevent checkpoint activation by inhibiting the association of Rad3–Rad26 at telomeres either by contributing to the timely arrival of Polα/Polδ to reduce ssDNA or by competing for G-tail binding with RPA.
Discussion
In this study, we analysed the cell cycle-regulated recruitment of various DNA replication proteins (MCM, RPA, Polα, Polδ and Polɛ), the DNA damage checkpoint protein Rad26, the DNA repair protein Nbs1 and various telomere proteins (Trt1/TERT, Taz1, Rap1, Pot1 and Stn1) to telomeres to better understand how semi-conservative DNA replication and telomerase-dependent telomere extension are coordinated in fission yeast. Prior to our study, no information regarding cell cycle-regulated behaviour of these proteins at telomeres was available in fission yeast.
Not all changes in protein association at telomeres during S-phase are coupled strictly to actual replication of telomeric DNA
As summarised in Figure 5A, recruitment of MCM, Nbs1 and Stn1 to telomeres was not inhibited by the addition of HU, a chemical that inhibits DNA replication at telomeres. Thus, it suggests that these factors undergo changes in telomere association during S-phase in a manner independent of the actual arrival of the DNA replication fork. A previous study has shown that Stn1 recruitment to telomeres is increased in cells carrying longer G-tails (Martin et al, 2007). In addition, the MRN complex is known to be involved in the G-tail generation at telomeres (Tomita et al, 2003). Therefore, one interesting possibility is that a gradual increase in telomere association of MRN during S-phase could lead to increased G-tail without DNA replication at telomeres, and allow recruitment of Stn1. Alternatively, Stn1 might be recruited to telomeres by interacting with other factors (such as Nbs1 and MCM) that show increased and sustained recruitment to telomeres without an actual increase in G-tail length at telomeres, as increased binding in RPA and Rad26 was not observed at telomeres during HU arrest. In fact, S-phase specific telomere recruitment of RPA, Rad26 and Pot1 was strongly inhibited by the addition of HU (Figures 2F and 4C), indicating that recruitment of these factors is more strictly coupled to replication of telomeric DNA.
Figure 5.
Summary and model for cell cycle-regulated dynamics of telomere-associated factors. (A) Summary of ChIP data. The timeline for the peak of protein binding at telomeres after release from cdc25-22 arrest are indicated as boxes with protein names. Degrees of reduction in S-phase specific telomere binding by HU are also indicated by different shades on the boxes. (B) A model of telomere replication incorporating the differential arrival of leading and lagging strand DNA polymerases at telomeres. Delayed synthesis of Okazaki fragments on the lagging strand would lead to exposure of extended ssDNA at lagging strand telomeres, which is then bound by RPA and Rad3–Rad26. Thus, RPA and Rad3–Rad26 are proposed to be especially important for controlling the accessibility of lagging strand telomeres to telomerase. On the other hand, MRN–Tel1 and Dna2, likely to be involved in re-generation of the G-tail on leading strand telomeres, are expected to be more important in controlling accessibility of leading strand telomeres to telomerase.
Telomerase catalytic subunit Trt1 was maximally recruited to telomeres significantly after DNA Polɛ arrived at telomeres (Figures 3B and 4A). Therefore, it appears that arrival of the DNA replication fork precedes the recruitment of Trt1 to telomeres. However, we note that residual Trt1 recruitment in S-phase was observed even in the presence of HU (Figure 4A). Thus, there may be a mechanism of telomerase recruitment that is coupled to S-phase but independent of telomere replication in fission yeast.
Delayed arrival of lagging strand DNA polymerases can lead to transient formation of long G-tails on the lagging strand telomeres
We were surprised to find that arrival of lagging strand DNA polymerases (Polα and Polδ) at telomeres is significantly delayed (∼20 min) compared with the arrival of the leading strand DNA polymerase (Polɛ) (Figure 3D). To our knowledge, such a large delay has not been observed before, and our data suggest that replication forks may carry an unusually large ssDNA loop on the lagging strand as they approach telomeres, due to a long delay in synthesis of new Okazaki fragments. The transient presence of extended ssDNA on the lagging strand telomere is also supported by our observation that the ssDNA-binding protein complex RPA and the DNA damage/replication checkpoint protein Rad26/ATRIP accumulate at telomeres when Polɛ arrives at telomeres, and dissociate once the lagging strand polymerases and telomerase arrive at telomeres (Figures 3D, F and 5B).
On the basis of a previous in vitro study, leading strand synthesis can replicate linear DNA fully to the end, whereas lagging strand synthesis gradually halts near the end to leave an approximately 500 bp region as ssDNA (Ohki et al, 2001). Therefore, our observation might simply reflect the fact that DNA Polα-primase is less efficient in initiating lagging strand synthesis near the end of linear DNA. In addition, repetitive sequences found at telomeres and telomere-proximal regions (Sugawara, 1988), heterochromatin structure at telomeric and sub-telomeric regions (Kanoh et al, 2005; Mickle et al, 2007), and/or binding of telomere-specific factors and various DNA repair/checkpoint proteins (Nakamura et al, 2002) could also contribute to delaying the arrival of the lagging strand polymerases at telomeres.
Fission yeast cells lacking the telomerase catalytic subunit Trt1 are estimated to lose only approximately three bases of telomeric DNA per cell division (Nakamura et al, 1997). Thus, native telomeres appear to possess telomerase-independent mechanism(s) that promote initiation of lagging strand synthesis very close to the ends of parental telomeres, rather than leaving large gaps. Efficient and timely synthesis of Okazaki fragments very close to the ends of lagging strand telomeres may also be critical in reducing ssDNA-bound RPA to attenuate checkpoint responses at telomeres. As the budding yeast telomere capping proteins Cdc13 and Stn1 interact with the Polα–primase complex (Qi and Zakian, 2000; Grossi et al, 2004), fission yeast Pot1 and Stn1 might also interact with the Polα–primase complex and promote efficient recruitment of Polα near telomeric ends. Taz1, previously shown to promote replication of telomeric repeats (Miller et al, 2006) and to prevent long G-tail formation at telomeres (Tomita et al, 2003) in fission yeast, may also have an important function in regulating the arrival of leading and lagging strand polymerases at telomeres.
ATR recruitment to telomeres may be especially important for telomere length regulation on the lagging strand telomeres
As mutations in RPA and Rad3–Rad26 (ATR–ATRIP) lead to substantial telomere shortening, cell cycle-regulated accumulation of Rad3–Rad26 and RPA to telomeres is very important for telomere maintenance in fission yeast (Nakamura et al, 2002; Ono et al, 2003). On the basis of our observations, differential arrival of DNA polymerases is likely to be the major cause for accumulation of RPA and Rad3–Rad26 to lagging strand telomeres. RPA and Rad3–Rad26 could also be significantly recruited to leading strand telomeres after a post-replicative resection of the CA-rich strand by the MRN complex and Dna2 nuclease (Tomita et al, 2003, 2004). However, as mutations in MRN or Dna2 lead to little to no telomere shortening, we believe that RPA and Rad3–Rad26 most likely contribute to telomere length regulation primarily on the lagging strand telomeres in fission yeast (Figure 5B). On the other hand, as previous genetic analyses have suggested that Rad3–Rad26 and Tel1–MRN pathways contribute redundantly to telomere capping in fission yeast (Nakamura et al, 2002), Rad3–Rad26 may contribute to leading strand telomere maintenance in strains deficient for Tel1–MRN functions.
Although simultaneous loss of the ATR–ATRIP pathway (Rad3–Rad26 in fission yeast and Mec1–Ddc2 in budding yeast) and the ATM–MRN pathway (Tel1–MRN in fission yeast and Tel1–MRX in budding yeast) has been shown to cause catastrophic loss of telomere stability in both budding and fission yeasts, the relative importance of the ATR and ATM pathways in telomere length maintenance is reversed between these two yeast species (Naito et al, 1998; Craven et al, 2002; Nakamura et al, 2002). However, we expect that the ATM and ATR pathways provide evolutionarily conserved functions to telomere maintenance in both yeasts. Thus, one very intriguing model that could explain this difference is that budding yeast primarily regulates telomere length by controlling Tel1–MRX activity on the leading strand, whereas fission yeast primarily regulates telomere length by controlling Rad3–Rad26 activity on the lagging strand. Although further studies are clearly needed to directly test our hypothesis on the strand specific actions of the ATR–ATRIP and ATM–MRN pathways in budding and fission yeasts, studies in mammalian cells have previously suggested that telomerase and the MRN complex are involved in preferentially extending leading strand telomeres (Chai et al, 2006a, 2006b).
Materials and methods
Yeast strains
The fission yeast strains used in this study were constructed by standard techniques (Alfa et al, 1993) and are listed in Supplementary Table S1. Sources and construction details of strains are also available in Supplementary data online.
ChIP analysis
Cells were processed for ChIP analysis as previously described (Nakamura et al, 2002) with minor modifications. Dynabeads Protein G (Invitrogen) were added to 2 mg whole cell extracts pre-incubated with monoclonal anti-myc (9B11; Cell Signaling), anti-HA (12CA5; Roche) or anti-FLAG (M2-F1802; Sigma) antibodies. For BrdU incorporation analysis (see Supplementary data online), heat denatured genomic DNA was pre-incubated with monoclonal anti-BrdU antibody (B44; Becton-Dickinson). After extensive washes, bead-bound DNA was recovered using Chelex-100 resin (Bio-Rad) (Nelson et al, 2006). Recovered DNA was analysed by triplicate SYBR Green-based real-time PCR (Bio-Rad) using primers listed in Supplementary Table S3. Raw percent precipitated DNA values (percentage raw-precipitation) were then calculated based on ΔCt between Input and IP samples. ChIP analyses were also performed using strains expressing untagged proteins to obtain percentage background-precipitation values, and they were subtracted from percentage raw-precipitation values to obtain percentage precipitation values. To compare ChIP data for different proteins and for different loci, we then converted the percentage precipitation values to relative precipitation values by setting the maximum percentage precipitation values from non-HU experiments to be 1.
Supplementary Material
Supplementary Information
Acknowledgments
We thank JP Cooper, TR Cech, SL Forsburg, F Ishikawa, J Kanoh, P Russell and VA Zakian for strains and plasmids. This work was supported by UIC start-up fund, Sidney Kimmel Scholar Program and the NIH grant GM078253 to TMN. LS is supported by a pre-doctoral fellowship from the American Heart Association. EN is currently supported by the NIH grant GM077604 and was previously supported by Leukemia Research Foundation.
References
- Adams-Martin A, Dionne I, Wellinger RJ, Holm C (2000) The function of DNA polymerase α at telomeric G tails is important for telomere homeostasis. Mol Cell Biol 20: 786–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfa C, Fantes P, Hyams J, McLoed M, Warbrick E (1993) Experiments with Fission Yeast. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Bailey SM, Cornforth MN, Kurimasa A, Chen DJ, Goodwin EH (2001) Strand-specific postreplicative processing of mammalian telomeres. Science 293: 2462–2465 [DOI] [PubMed] [Google Scholar]
- Baumann P, Cech TR (2001) Pot1, the putative telomere end-binding protein in fission yeast and humans. Science 292: 1171–1175 [DOI] [PubMed] [Google Scholar]
- Bianchi A, Shore D (2007) Early replication of short telomeres in budding yeast. Cell 128: 1051–1062 [DOI] [PubMed] [Google Scholar]
- Blackburn EH (2001) Switching and signaling at the telomere. Cell 106: 661–673 [DOI] [PubMed] [Google Scholar]
- Bochman ML, Schwacha A (2008) The Mcm2−7 complex has in vitro helicase activity. Mol Cell 31: 287–293 [DOI] [PubMed] [Google Scholar]
- Chahwan C, Nakamura TM, Sivakumar S, Russell P, Rhind N (2003) The fission yeast Rad32 (Mre11)-Rad50-Nbs1 complex is required for the S-phase DNA damage checkpoint. Mol Cell Biol 23: 6564–6573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai W, Du Q, Shay JW, Wright WE (2006a) Human telomeres have different overhang sizes at leading versus lagging strands. Mol Cell 21: 427–435 [DOI] [PubMed] [Google Scholar]
- Chai W, Sfeir AJ, Hoshiyama H, Shay JW, Wright WE (2006b) The involvement of the Mre11/Rad50/Nbs1 complex in the generation of G-overhangs at human telomeres. EMBO Rep 7: 225–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe L, Verdun RE, Haggblom CI, Karlseder J (2004) Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science 306: 1951–1953 [DOI] [PubMed] [Google Scholar]
- Craven RJ, Greenwell PW, Dominska M, Petes TD (2002) Regulation of genome stability by TEL1 and MEC1, yeast homologs of the mammalian ATM and ATR genes. Genetics 161: 493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlén M, Sunnerhagen P, Wang TS (2003) Replication proteins influence the maintenance of telomere length and telomerase protein stability. Mol Cell Biol 23: 3031–3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher TS, Taggart AK, Zakian VA (2004) Cell cycle-dependent regulation of yeast telomerase by Ku. Nat Struct Mol Biol 11: 1198–1205 [DOI] [PubMed] [Google Scholar]
- Gao H, Cervantes RB, Mandell EK, Otero JH, Lundblad V (2007) RPA-like proteins mediate yeast telomere function. Nat Struct Mol Biol 14: 208–214 [DOI] [PubMed] [Google Scholar]
- Gilson E, Geli V (2007) How telomeres are replicated. Nat Rev Mol Cell Biol 8: 825–838 [DOI] [PubMed] [Google Scholar]
- Goudsouzian LK, Tuzon CT, Zakian VA (2006) S. cerevisiae Tel1p and Mre11p are required for normal levels of Est1p and Est2p telomere association. Mol Cell 24: 603–610 [DOI] [PubMed] [Google Scholar]
- Grossi S, Puglisi A, Dmitriev PV, Lopes M, Shore D (2004) Pol12, the B subunit of DNA polymerase α, functions in both telomere capping and length regulation. Genes Dev 18: 992–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Katou Y, Itoh T, Tazumi A, Yamada Y, Takahashi T, Nakagawa T, Shirahige K, Masukata H (2007) Genome-wide localization of pre-RC sites and identification of replication origins in fission yeast. EMBO J 26: 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector RE, Shtofman RL, Ray A, Chen BR, Nyun T, Berkner KL, Runge KW (2007) Tel1p preferentially associates with short telomeres to stimulate their elongation. Mol Cell 27: 851–858 [DOI] [PubMed] [Google Scholar]
- Hodson JA, Bailis JM, Forsburg SL (2003) Efficient labeling of fission yeast Schizosaccharomyces pombe with thymidine and BUdR. Nucleic Acids Res 31: e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob NK, Skopp R, Price CM (2001) G-overhang dynamics at Tetrahymena telomeres. EMBO J 20: 4299–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh J, Ishikawa F (2001) spRap1 and spRif1, recruited to telomeres by Taz1, are essential for telomere function in fission yeast. Curr Biol 11: 1624–1630 [DOI] [PubMed] [Google Scholar]
- Kanoh J, Sadaie M, Urano T, Ishikawa F (2005) Telomere binding protein Taz1 establishes Swi6 heterochromatin independently of RNAi at telomeres. Curr Biol 15: 1808–1819 [DOI] [PubMed] [Google Scholar]
- Kim SM, Huberman JA (2001) Regulation of replication timing in fission yeast. EMBO J 20: 6115–6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrivee M, LeBel C, Wellinger RJ (2004) The generation of proper constitutive G-tails on yeast telomeres is dependent on the MRX complex. Genes Dev 18: 1391–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingner J, Cech TR (1996) Purification of telomerase from Euplotes aediculatus: requirement of a primer 3′ overhang. Proc Natl Acad Sci USA 93: 10712–10717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov VL, Hirose Y, Langmore JP (1997) Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell 88: 657–666 [DOI] [PubMed] [Google Scholar]
- Martin V, Du LL, Rozenzhak S, Russell P (2007) Protection of telomeres by a conserved Stn1-Ten1 complex. Proc Natl Acad Sci USA 104: 14038–14043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo JA, Cohen J, Toczyski DP (2001) Two checkpoint complexes are independently recruited to sites of DNA damage in vivo. Genes Dev 15: 2809–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickle KL, Ramanathan S, Rosebrock A, Oliva A, Chaudari A, Yompakdee C, Scott D, Leatherwood J, Huberman JA (2007) Checkpoint independence of most DNA replication origins in fission yeast. BMC Mol Biol 8: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Ferreira MG, Cooper JP (2005) Taz1, Rap1 and Rif1 act both interdependently and independently to maintain telomeres. EMBO J 24: 3128–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KM, Rog O, Cooper JP (2006) Semi-conservative DNA replication through telomeres requires Taz1. Nature 440: 824–828 [DOI] [PubMed] [Google Scholar]
- Miyoshi T, Kanoh J, Saito M, Ishikawa F (2008) Fission yeast Pot1-Tpp1 protects telomeres and regulates telomere length. Science 320: 1341–1344 [DOI] [PubMed] [Google Scholar]
- Munoz-Jordan JL, Cross GA, de Lange T, Griffith JD (2001) t-loops at Trypanosome telomeres. EMBO J 20: 579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito T, Matsuura A, Ishikawa F (1998) Circular chromosome formation in a fission yeast mutant defective in two ATM homologues. Nat Genet 20: 203–206 [DOI] [PubMed] [Google Scholar]
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR (1997) Telomerase catalytic subunit homologs from fission yeast and human. Science 277: 955–959 [DOI] [PubMed] [Google Scholar]
- Nakamura TM, Moser BA, Russell P (2002) Telomere binding of checkpoint sensor and DNA repair proteins contributes to maintenance of functional fission yeast telomeres. Genetics 161: 1437–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JD, Denisenko O, Bomsztyk K (2006) Protocol for the fast chromatin immunoprecipitation (ChIP) method. Nat Protoc 1: 179–185 [DOI] [PubMed] [Google Scholar]
- Nick McElhinny SA, Gordenin DA, Stith CM, Burgers PM, Kunkel TA (2008) Division of labor at the eukaryotic replication fork. Mol Cell 30: 137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki R, Tsurimoto T, Ishikawa F (2001) In vitro reconstitution of the end replication problem. Mol Cell Biol 21: 5753–5766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya T, Kawasaki Y, Hiraga S, Kanbara S, Nakajo K, Nakashima N, Suzuki A, Sugino A (2002) The DNA polymerase domain of polɛ is required for rapid, efficient, and highly accurate chromosomal DNA replication, telomere length maintenance, and normal cell senescence in Saccharomyces cerevisiae. J Biol Chem 277: 28099–28108 [DOI] [PubMed] [Google Scholar]
- Ono Y, Tomita K, Matsuura A, Nakagawa T, Masukata H, Uritani M, Ushimaru T, Ueno M (2003) A novel allele of fission yeast rad11 that causes defects in DNA repair and telomere length regulation. Nucleic Acids Res 31: 7141–7149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm W, de Lange T (2008) How shelterin protects mammalian telomeres. Annu Rev Genet 42: 301–334 [DOI] [PubMed] [Google Scholar]
- Pursell ZF, Isoz I, Lundstrom EB, Johansson E, Kunkel TA (2007) Yeast DNA polymerase ɛ participates in leading-strand DNA replication. Science 317: 127–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Zakian VA (2000) The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase α and the telomerase-associated Est1 protein. Genes Dev 14: 1777–1788 [PMC free article] [PubMed] [Google Scholar]
- Sabourin M, Tuzon CT, Zakian VA (2007) Telomerase and Tel1p preferentially associate with short telomeres in S. cerevisiae. Mol Cell 27: 550–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramke V, Luciano P, Brevet V, Guillot S, Corda Y, Longhese MP, Gilson E, Geli V (2004) RPA regulates telomerase action by providing Est1p access to chromosome ends. Nat Genet 36: 46–54 [DOI] [PubMed] [Google Scholar]
- Smith CD, Smith DL, DeRisi JL, Blackburn EH (2003) Telomeric protein distributions and remodeling through the cell cycle in Saccharomyces cerevisiae. Mol Biol Cell 14: 556–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smogorzewska A, de Lange T (2004) Regulation of telomerase by telomeric proteins. Annu Rev Biochem 73: 177–208 [DOI] [PubMed] [Google Scholar]
- Subramanian L, Moser BA, Nakamura TM (2008) Recombination-based telomere maintenance is dependent on Tel1-MRN and Rap1 and inhibited by telomerase, Taz1, and Ku in fission yeast. Mol Cell Biol 28: 1443–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara NF (1988) DNA sequences at the telomeres of the fission yeast S. pombe. Ph.D. Thesis Cambridge, Massachusetts: Harvard University
- Taggart AK, Teng SC, Zakian VA (2002) Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science 297: 1023–1026 [DOI] [PubMed] [Google Scholar]
- Takata H, Tanaka Y, Matsuura A (2005) Late S phase-specific recruitment of Mre11 complex triggers hierarchical assembly of telomere replication proteins in Saccharomyces cerevisiae. Mol Cell 17: 573–583 [DOI] [PubMed] [Google Scholar]
- Tomita K, Kibe T, Kang HY, Seo YS, Uritani M, Ushimaru T, Ueno M (2004) Fission yeast Dna2 is required for generation of the telomeric single-strand overhang. Mol Cell Biol 24: 9557–9567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita K, Matsuura A, Caspari T, Carr AM, Akamatsu Y, Iwasaki H, Mizuno K, Ohta K, Uritani M, Ushimaru T, Yoshinaga K, Ueno M (2003) Competition between the Rad50 complex and the Ku heterodimer reveals a role for Exo1 in processing double-strand breaks but not telomeres. Mol Cell Biol 23: 5186–5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdun RE, Crabbe L, Haggblom C, Karlseder J (2005) Functional human telomeres are recognized as DNA damage in G2 of the cell cycle. Mol Cell 20: 551–561 [DOI] [PubMed] [Google Scholar]
- Verdun RE, Karlseder J (2007) Replication and protection of telomeres. Nature 447: 924–931 [DOI] [PubMed] [Google Scholar]
- Webb CJ, Zakian VA (2008) Identification and characterization of the Schizosaccharomyces pombe TER1 telomerase RNA. Nat Struct Mol Biol 15: 34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellinger RJ, Ethier K, Labrecque P, Zakian VA (1996) Evidence for a new step in telomere maintenance. Cell 85: 423–433 [DOI] [PubMed] [Google Scholar]
- Wellinger RJ, Wolf AJ, Zakian VA (1993) Saccharomyces telomeres acquire single-strand TG1−3 tails late in S phase. Cell 72: 51–60 [DOI] [PubMed] [Google Scholar]
- Wu G, Lee WH, Chen PL (2000) NBS1 and TRF1 colocalize at promyelocytic leukemia bodies during late S/G2 phases in immortalized telomerase-negative cells. Implication of NBS1 in alternative lengthening of telomeres. J Biol Chem 275: 30618–30622 [DOI] [PubMed] [Google Scholar]
- You Z, Chahwan C, Bailis J, Hunter T, Russell P (2005) ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol Cell Biol 25: 5363–5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XD, Kuster B, Mann M, Petrini JH, de Lange T (2000) Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat Genet 25: 347–352 [DOI] [PubMed] [Google Scholar]
- Zou L, Elledge SJ (2003) Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300: 1542–1548 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information