Abstract
Adult human mesenchymal stromal or stem cells (MSC) can differentiate into a variety of cell types and are candidate cellular therapeutics in regenerative medicine. Surprisingly, these cells also display multiple potent immunomodulatory capabilities, including allosuppression, making allogeneic cell therapy a possibility. The exact mechanisms involved in regulatory T cell induction by allogeneic human MSC was examined, using purified CD4+ populations and well-characterized bone marrow-derived adult human MSC. Allogeneic MSC were shown to induce forkhead box P3 (FoxP3)+ and CD25+ mRNA and protein expression in CD4+ T cells. This phenomenon required direct contact between MSC and purified T cells, although cell contact was not required for MSC induction of FoxP3 expression in an unseparated mononuclear cell population. In addition, through use of antagonists and neutralizing antibodies, MSC-derived prostaglandins and transforming growth factor (TGF)-β1 were shown to have a non-redundant role in the induction of CD4+CD25+FoxP3+ T cells. Purified CD4+CD25+ T cells induced by MSC co-culture expressed TGF-β1 and were able to suppress alloantigen-driven proliferative responses in mixed lymphocyte reaction. These data clarify the mechanisms of human MSC-mediated allosuppression, supporting a sequential process of regulatory T cell induction involving direct MSC contact with CD4+ cells followed by both prostaglandin E2 and TGF-β1 expression. Overall, this study provides a rational basis for ongoing clinical studies involving allogeneic MSC.
Keywords: cell contact, mesnchymal stem cell, PGE2, regulatory T cell, TGF-β1
Introduction
Mesenchymal stem cells or mesenchymal stromal cells (MSC) have the capacity to differentiate into cell types of the mesenchymal lineage [1,2], and contribute to haematopoiesis [3,4] and the resolution of physiological insult [5]. These characteristics have prompted the clinical use of MSC in regenerative and cell therapies [6–8]. However, MSC possess another attribute attractive for cell-based therapy – they display potent immunosuppressive qualities [9–11]. Consequently, MSC-based approaches for graft-versus-host disease (GVHD) and other immune pathologies are being explored. Extensive research has focused upon the mechanisms behind MSC-mediated immunosuppressive activity. Although these mechanisms remain to be elucidated fully, it is now clear that MSC can suppress allogeneic responses [10,12–14]. In effect, MSC modulate different aspects of the rejection process, including the preservation of dendritic cell (DC) immaturity [15], skewing of CD4+ T helper population phenotypes and modulation of CD8+ cytotoxic T lymphocyte and natural killer cell functions [16,17]. These attributes provide a rationale for potential allogeneic therapies against diabetes, autoimmune diseases and organ transplant rejection.
Modulation of regular allorejection processes by MSC is multi-factorial, requiring different contact-dependent and -independent signals under different circumstances. It is now clear that modulation of DC maturation by MSC requires interleukin (IL)-6 and a contact-dependent signal [12,15], whereas full suppression of T cell function by MSC (both human and murine) involves some degree of MSC activation or ‘licensing’ thought to involve interferon (IFN)-γ in conjunction with IL-1α, IL-1β or tumour necrosis factor-α[18]. Non-specific suppression of T cell proliferation is mediated by soluble factors such as transforming growth factor (TGF)-β, kynurenine, [9,19,20] prostaglandin E2 (PGE2) [19–21], nitric oxide [22], haem oxygenase products [23] and insulin-like growth factor binding protein [24]. In addition to these mechanisms, there is increasing evidence that MSC modulation of T cell responses is more subtle than simple induction of global suppression. For example, MSC inhibit proliferation but not all effector functions of T cells [11,25], a condition similar to that of division arrest anergy [26] and also reminiscent of the split suppression seen in regional mucosal immune responses [27]. However, there is insufficient understanding of how MSC induce the expansion of regulatory T cells (Treg) [21,28].
Peripheral tolerance is an active phenomenon that involves regulatory CD4+ T cells [29] including regulatory T Treg that co-express surface CD4 and CD25. The mechanisms that generate Treg are not elucidated fully; however, the transcription factor forkhead box P3 (FoxP3) is known to play a role in both natural and induced Treg differentiation from non-committed precursors [30,31]. The selective induction of Tregs can be promoted by a number of factors, including DC maturation status or small molecules such as IL-10, TGF-β and PGE2[32,33].
The present study sought to determine if human MSC exert their immunosuppressive activity by manipulating the immune system through the selective induction of regulatory CD4+ T cells, and to define the mechanisms essential for this to occur. The capacity of MSC to induce CD4+ CD25+ FoxP3+ Treg was examined, and the requirement for different MSC-derived soluble factors characterized through neutralization or chemical antagonism. Finally, MSC-induced regulatory CD4+ T cells were isolated and examined for their functional capability to modulate alloresponses in vitro.
Material and methods
Cell isolation, purification and culture
Human MSC were isolated and expanded from aspirates of bone marrow by direct plating, as described previously [2,34]. Cultures were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (v/v) fetal calf serum and 1% (v/v) penicillin/streptomycin (Invitrogen-Gibco, Paisley, Scotland, UK). Contamination in MSC populations is a key confounding variable, therefore rigorous quality control was adopted to ensure that MSC were not contaminated with haematopoietic or other cell types, and that cells retained differentiation capacity as described previously [2,34]. Human CD4+ T cells were isolated from peripheral blood mononuclear cells (PBMC) using a MagCellect CD4+ T cell negative selection, isolation kit according to the manufacturer's instructions (R&D Systems, Abingdon, Oxon, UK). CD4+ T cells were co-cultured with MSC at a ratio of 3:1 (CD4+ : MSC), a ratio determined to be the optimal dose for MSC modulation of T cells and DC. Similarly, for transwell experiments, MSC were cultured in the tissue culture insert incorporating a 0·4 µm membrane placed in the wells of six- or 24-well plates (Greiner Bio-one, Stonehouse, Glos, UK), while CD4+ T cells were cultured in the well beneath the insert. Study design and use of human mesenchymal stem cells was approved by the bioethics committees of the National University of Ireland Maynooth and the National University of Ireland Galway.
Characterization of human MSC surface marker expression and differentiation potential
Human MSC were detached from substrate with trypsin/ethylenediamine tetraacetic acid (EDTA), washed and resuspended in phosphate-buffered saline (PBS) with 1% (v/v) bovine serum albumin. Cells (1 × 105) were incubated with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibodies for 15 min at 4°C, washed and then analysed by flow cytometry. Antibodies recognizing the following human antigens or appropriate isotype controls were used: human leucocyte antigen (HLA)-ABC, HLA-DR, CD106, CD117 (eBioscience, San Diego, CA, USA), CD11b, CD29, CD31, CD34, CD44, CD45, CD54, CD105 (Immunotools, Friesoythe, Germany) and CD90 (BD Biosciences, Oxford, UK).
Human MSC were examined for their ability to differentiate into adipocytes, osteoblasts and chondrocytes. Adipogenic differentiation was induced by 1 µM dexamethasone, 200 µM indomethacin, 10 µg insulin and 500 µM 3-isobutyl-methyl-xanthine, and osteogenic differentiation was induced by 100 nM dexamethasone, 10 mM β-glycerolphosphate and 50 µM ascorbic acid-2-phosphate. For chondrogenic differentiation a pellet culture system was used. Some 2 × 105 cells were centrifuged in a 15 ml polypropylene tube. The pellet was cultured at 37°C in 5% CO2 in 500 µl chondrogenic media containing high-glucose DMEM supplemented with 10 ng/ml human TGF-B3 (TS:beta) (R&D Systems), 100 nM dexamethasone, 50 ug/ml ascorbic acid-2-phosphate, 40 ug/ml proline, 1 mM sodium pyruvate and (1:99) ITS + supplement (BD Biosciences). Differentiation cultures were harvested after 21 days. Oil red O and Alizarin red S were used to identify adipocytes and osteoblasts respectively. Chondrogenic pellets were harvested on day 21 for glycosaminoglycan (GAG) quantitation. Briefly, chondrogenic pellets were washed with Dulbecco's PBS and digested with papain solution (1 mg/ml in 50 mM sodium phosphate, pH 6·5 containing 2 mM N-acetyl cysteine and 2 mM EDTA) for 16 h at 65°C. GAG was measured by reaction with 1,9-dimethylmethylene blue using shark chondroitin sulphate as standard. DNA quantitation was carried out using the PicoGreen® dsDNA Quantitation Kit (Invitrogen) with phage lambda DNA as standard as described previously [35]. Data was expressed as a ratio of GAG/DNA.
Analysis of FoxP3 and TGF-β1 mRNA by reverse transcription–polymerase chain reaction (RT–PCR) and real-time PCR
Characterization of FoxP3 and TGF-β1 mRNA expression by CD4+ T cells was performed using semi-quantitative and quantitative (qRT–PCR). Purified CD4+ T cells (3 × 105/ml) and MSC (1 × 105/ml) were co-cultured in 24-well plates (Nunc, Roskilde, Denmark). In additional studies, anti-TGF-β (R&D Systems) or indomethacin (Sigma-Aldrich, Dublin, Ireland) were added to cultures at concentrations of 4 µg/ml and 40 µM respectively. After 24 h, non-adherent CD4+ T cells were removed by aspiration leaving the adherent MSC monolayer untouched using a simple method described previously [15]. Purification of total RNA from CD4+ T cell cultures was performed using the RNeasy® Plus Mini kit (Qiagen, Crawley, UK) according to manufacturer's instructions, reverse-transcribed to cDNA and analysed for the expression of human FoxP3 and TGF-β1 by a hot-start RT–PCR reaction (Promega, Southampton, UK). Glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) was detected as an internal control. Primer sequences were as follows: GAPDH forward 5′-GGTGAAGGTCGGAGTCAACG-3′ and reverse 5′-CAAAGTTGTCATGGATGACC-3′, with an annealing temperature of 55°C; TGF-β1, annealing at 54°C: forward 5′-CAGATCCTGTCCAAGCTG-3′; reverse 5′- TCGGAGCTCTGATGTGTT-3′; and FoxP3 annealing at 57°C: forward 5′-AGGTGGCAGGATGGTTTCT-3′; reverse 5′-AACAGCACATTCCCAGAGTTC-3′. Quantitative real-time PCR analysis was performed using a QuantiTect™ SYBR Green PCR Kit (Qiagen) with a DNA Opticon™ quantitative thermocycler (MJ Research, Waltham, MA, USA) [20].
Standard curves for each target gene were generated by amplifying 10-fold serial dilutions of known quantities of target gene PCR product standards. Quantification of target gene expression was obtained using sequence detector system software (MJ Research, Inc.). In this study the resultant target mRNA concentrations were expressed as fg/500 ng cDNA.
Analysis of CD25 and FoxP3 protein expression by flow cytometry
CD4+ T cells or PBMC were co-cultured with MSC in 2 ml volumes in six-well plates (Nunc) or in transwells for 72 h as described above. CD4+ T cells or PBMC were incubated at 0·5 × 106 cells/ml and MSC at 1·5 × 105/ml. Cells were labelled for surface CD4 and CD25 and intracellular FoxP3. Briefly, cells were incubated with CD4-PE or CD4-PE/Cy5 and CD25-PE or CD25-FITC or isotype-matched control antibodies (eBioscience) for 15 min at 4°C then washed and fixed by 2% (v/v) paraformaldehyde/PBS. Cells were permeabilized using cold PBS and 0·2% (v/v) Tween20/PBS then blocked with normal rat serum. After blocking, cells were incubated with fluorochrome-conjugated anti-human FoxP3 (eBioscience) or isotype control for at least 30 min at 4°C in the dark. At the end of the incubation period, cells were washed and resuspended in PBS containing 1% v/v formaldehyde. Analysis was performed within 4 h of preparation using BD fluorescence activated cell sorter (FACS)Calibur and CellQuest software (BD Biosciences).
CD4+CD25+ functional assay
After 72 h co-culture of purified CD4+ cells and MSC, CD4+ T cells were removed by aspiration and CD4+CD25+ cells isolated using CD25 microbeads according to the manufacturer's instructions (Miltenyi Biotech, Bisley, Surrey, UK). CD4+CD25+ T cells were evaluated for their ability to suppress allodriven proliferation in mixed lymphocyte reaction (MLR) by co-culturing PBMC from two MHC mismatched donors in 96-well plates, as described previously [20]. Proliferation was measured using [3H]-thymidine incorporation for the final 6 h of culture represented as the incorporated radioactivity in counts per minute (cpm). Results are expressed as the mean of triplicate values ± standard error (s.e.).
Statistical methods
Statistical significance was assessed using Prism3 software (GraphPad Software, San Diego, CA, USA). Paired data were analysed by paired t-test and three or more data sets were compared using one-way anova, with Tukey's multiple comparison with measure significance. Data are presented as the mean ± s.e. P-values of P < 0·05 (*), P < 0·01 (**) or P < 0·001 (***) were considered statistically significant.
Results
Characterization of human bone marrow-derived MSC
Human MSC derived from bone marrow displayed a fibroblastoid morphology and expressed surface markers typical of adult MSC [36,37], including HLA-ABC, CD44, CD90 and CD106 (Fig. 1a). Human MSC did not express HLA-DR, the haematopoietic cell markers CD34, CD117 or the co-stimulatory molecules CD40, CD80 or CD86 (Fig. 1a). Human MSC did not express CD29, CD54, CD105 or CD154 (Fig. 1a). Human MSC had the ability to differentiate along the chondrogenic, osteoblastic or adipocytic pathways after culture in the appropriate conditions (Fig. 1b and c).
Fig 1.

Characterization and differentiation potential of human mesenchymal stromal cells (MSC). (a) Cell surface markers expressed by human MSC were determined by flow cytometry. Isotype controls are represented by open histograms, specific cell surface markers by closed histograms. The capacity of MSC to differentiate along mesenchymal lineages was also determined by: (b) phase-contrast microscopy (magnification × 200) of (i) control, undifferentiated MSC; (ii) adipogenic differentiated MSC, determined by oil red O staining; (iii) osteogenic differentiated MSC determined by alizarin red S staining; or (c) glycosaminoglycan content, an indicator of chondrogenic differentiation, determined using a 1,9-dimethylmethylene blue assay and a picogreen DNA assay for undifferentiated and differentiated MSC (n = 2). Differentiation conditions are described in Methods. Data are represented as the mean ± standard error ratio of GAG/DNA (µg/µg). *P < 0·05 compared with undifferentiated MSC.
Allogeneic MSC induce human CD4+CD25HighFoxP3+ T cells
Previous studies have suggested that MSC induce T cells with a regulatory phenotype when co-cultured with PBMC. However, these data are difficult to interpret, as multiple cell types (CD8+ T cells, etc.) are present in such systems. Therefore, this study sought to investigate whether allogeneic MSC induced T cells with suppressive/regulatory activity using defined purified populations of human CD4+ T cells. MSC were co-cultured with MHC mismatched CD4+ T cells and qRT–PCR used to quantify expression of the Treg transcription factor, FoxP3. CD4+ T cells were cultured in the presence or absence of MSC for 24 h. FoxP3 expression could be observed in CD4+ T cells, cultured in the presence or absence of MSC (Fig. 2a); however, there was a significant increase (**P < 0·01) of FoxP3 in purified CD4+ T cells cultured previously in the presence of allogeneic MSC compared with CD4+ T cells cultured alone (Fig. 2b).
Fig 2.
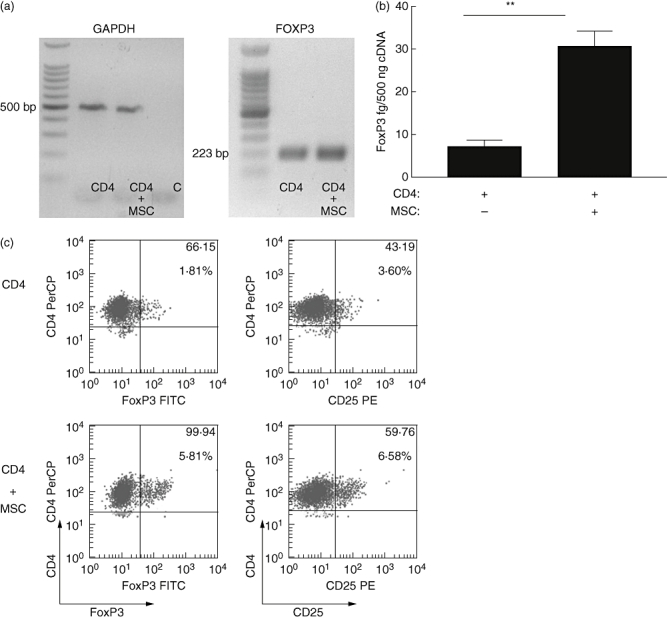
Exposure to mesenchymal stromal cells (MSC) increases CD4+ T cell expression of CD25 and forkhead box P3 (FoxP3). CD4+ T cells were cultured in the absence (CD4) or presence of MSC (CD4+MSC), purified and examined by either (a) semi-quantitative reverse transcription– polymerase chain reaction (RT–PCR) for the expression of glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) or FoxP3 mRNA after 24 h co-culture; or (b) quantitative real-time PCR for FoxP3 mRNA. **P < 0·01 compared with CD4+ T cells alone. Data representative of three independent determinations. Intracellular expression of FoxP3 or surface CD25 from parallel experiments were determined by flow cytometry from purified CD4+ cell populations (c). CD4+FoxP3+ or CD4+CD25High cells were determined from purified CD4+ T cells following 72 h culture in the absence (top panel) or presence of MSC (lower panel). Dot plots are representative of three independent experiments. The numbers in the upper right quadrants indicate the percentage of double-positive cells (large font) with mean fluorescent intensity (MFI) above (small font).
mRNA concentration does not always correlate with protein levels; therefore, FoxP3 and CD25 protein expression were determined by flow cytometry. Purified CD4+ T cells were cultured in the presence or absence of MSC for 72 h. In the absence of MSC, small numbers of CD4+ T cells expressed FoxP3 (Fig. 2c) or CD25 (Fig. 2c). In contrast, there was a consistent increase in FoxP3 and CD25High expression after co-culture with MSC (Fig. 2c). Thus MSC induce human CD4+CD25HighFoxP3+ expression in allogeneic T cells.
Allogeneic MSC induction of FoxP3 and CD25High expression by human T cells involves a cell contact-dependent mechanism
In other systems, MSC-mediated immunomodulation involves both cell contact-dependent and -independent mechanisms mediated through the release of soluble factors. In order to probe the role of cell contact, transwell experiments were performed that prevented direct contact between purified CD4+ T cells and allogeneic MSC. FoxP3 mRNA expression was examined after 24 h using qRT–PCR. Separation of MSC from CD4+ T cells prevented the induction of FoxP3 mRNA (Fig. 3a). To investigate the correlation at the protein level, CD4+ cells were recovered from the lower chamber after 72 h of culture, and both FoxP3 and CD25 expression were analysed. When cell–cell contact was prevented, allogeneic MSC did not induce FoxP3+ CD25+ expression in purified CD4+ cells (Fig. 3b). Consistently fewer CD4+ T cells expressed FoxP3 and CD25High when compared with cultures where cell contact was permitted (Fig. 3a and b). In separate experiments, the importance of other immune cells in MSC induction of FoxP3 expression was examined. Unseparated PBMC were cultured in the presence or absence of MSC in regular cultures or in a transwell system that prevented cell contact. PBMC were recovered after 72 h and FoxP3 and CD25 expression analysed by flow cytometry. Although co-culture of PBMC with MSC resulted in increased expression of FoxP3 and CD25, prevention of cell contact did not interfere with MSC induction of FoxP3 and CD25, indicating that in the presence of other immune cells, cell contact is not required (Fig. 3c). Taken together, these data show that cell–cell contact contributes to MSC-driven Treg induction in purified CD4+ T cells, but that in a multi-cell system such as PBMC additional factors can substitute for cell contact-derived signals.
Fig 3.
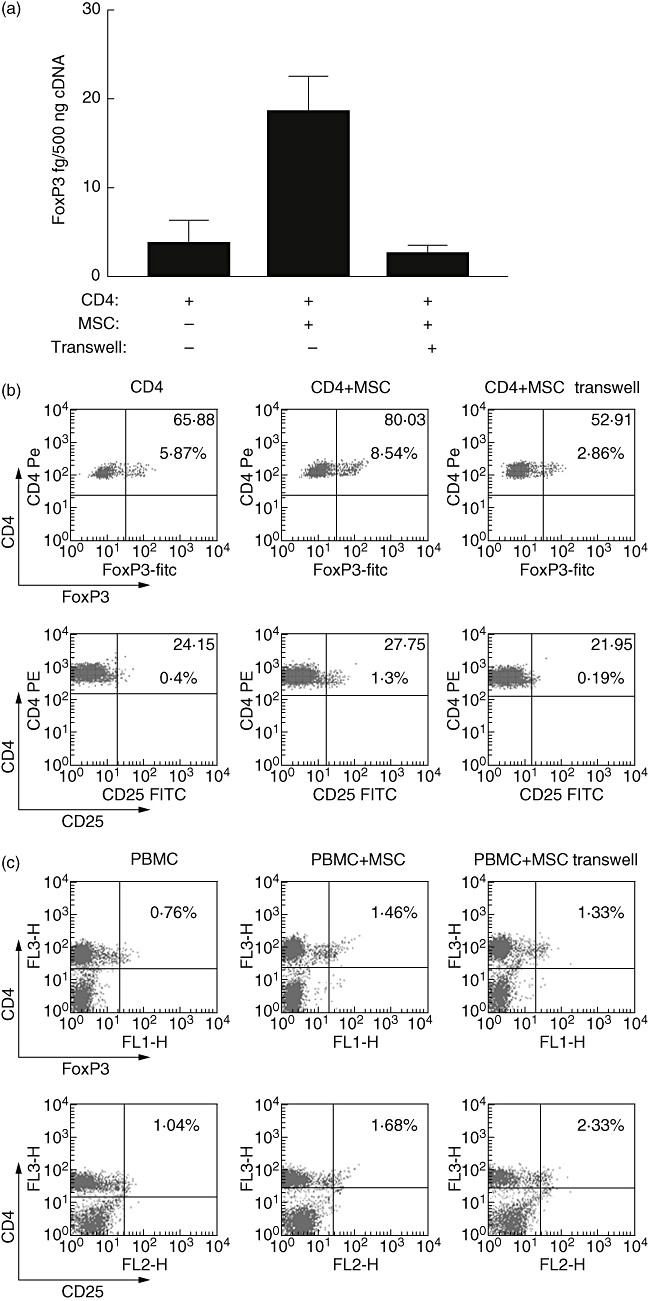
Prevention of T cell–mesenchymal stromal cells (MSC) contact reduced forkhead box P3 (FoxP3) and CD25High induction in CD4+ T cells. CD4+ T cells or unseparated peripheral blood mononuclear cells (PBMC) were cultured with MSC for 72 h or separated in co-culture using a transwell system with MSC in the upper chamber and CD4+ T cells or unseparated PBMC in the lower. FoxP3 mRNA concentration in CD4+ cells was determined by quantitive reverse transcription–polymerase chain reaction (qRT–PCR) (a). Intracellular FoxP3 or surface CD25 protein expression by CD4+ T cells (b) or unseparated PBMC (c) was determined by flow cytometry. CD4+ T cells (CD4) and unseparated PBMC cultured alone are shown for comparison. Data are representative of three experiments. The numbers in the upper right quadrants indicate the percentage of double-positive cells (large font) with MFI above (small font).
The TGF-β1 and PGE2 play a non-redundant role in MSC induction of human CD4+ CD25High FoxP3+ T cells
The observed role for cell contact in Treg induction in CD4+ T cells does not exclude the possibility that soluble factors play a non-redundant role in Treg induction, and indeed the data in Fig. 3c support an important role for soluble factors in MSC induction of Treg. In other systems, PGE2 contributes to the induction of FoxP3 [33], and we have demonstrated previously that PGE2 secreted by human MSC was required for modulation of allodriven proliferation in MLR [20]. TGF-β1 is also known to be involved in Treg induction [38], and moreover is up-regulated in MSC ‘licensed’ by IFN-γ stimulation [20]. Therefore, the effects of blocking both factors in allogeneic MSC : purified CD4+ T cell co-cultures were examined. Cyclooxygenase activity and therefore PGE2 induction was ablated using the antagonist indomethacin, whereas TGF-β1 was neutralized by a well-characterized antibody. The presence of indomethacin or anti-TGF-β1 alone resulted in a significant decrease in FoxP3 mRNA expression by CD4+ T cells cultured with allogeneic MSC (Fig. 4a). However, a combination of both reduced FoxP3 mRNA to background levels (Fig. 4a). Neutralization of TGF-β resulted in decreased protein expression of both intracellular FoxP3 and surface CD25High by purified CD4+ cells following co-culture (Fig. 4b and c) and antagonism of PGE2 production produced a similar effect (Fig. 4b and c). This indicates that although cell contact is involved in MSC induction of human CD4+ CD25High FoxP3+ T cells, both TGF-β1 and PGE2 play non-redundant roles in this process. In effect, both cell contact and soluble factors are required for MSC induction of CD4+ Treg cells. The transwell experiments show that cell contact is required and soluble factors are not sufficient to achieve this. The neutralization study which allowed cell contact demonstrated that TGF-β1 and PGE2 are the effector molecules involved in the induction of Treg in the absence of other immune cells.
Fig 4.

Transforming growth factor (TGF)-β1 and prostaglandin E2 (PGE2) play a non-redundant role in mesenchymal stromal cells (MSC) induction of forkhead box P3 (FoxP3) and CD25High expression by CD4+ T cells. (a) FoxP3 mRNA expression determined by real-time polymerase chain reaction (PCR) of CD4+ T cells purified from culture alone (CD4) or in the presence of MSC and/or the cyclooxygenase-2 antagonist (Indo) (40 µM) and/or neutralizing specific antibody against TGF-β1 (αTGF-β1) (4 µg/ml). MSC induced significant FoxP3 expression in CD4+ T cells. Addition of anti-TGF-β1 or indomethacin (40 µM) to CD4+ T cells co-cultured with MSC significantly reduced FoxP3 mRNA expression). Statistical analysis was performed by one-way anova with Tukey's multiple comparisons; results are expressed as mean concentration ± standard error. *P < 0·05; **P < 0·01). Analysis of intracellular FoxP3 (b) and CD25High surface expression (c), by purified CD4+ T cell from parallel co-cultures. Data are representative of three experiments. The numbers in the upper right quadrants indicate the percentage of double-positive cells (large font) with MFI given above (small font).
Human CD4+CD25+ T cells induced by allogeneic MSC act as conventional Treg
Blockade of cytokine production from CD4+ T cell/MSC co-cultures is a straightforward technique to determine a role for cytokines in Treg induction; however, this method does not provide information of the functional activity of the induced T cell. Therefore CD4+ T cells, cultured in previously the presence or absence of MSC for 24 h, were re-isolated from the adherent monolayer of MSC. TGF-β1 mRNA was significantly higher in CD4+ T cells that had been co-cultured with MSC (Fig. 5a and b), supporting hypotheses that MSC-derived signals condition the local tissue environment to promote induction of T cells secreting this immunosuppressive cytokine [16,39]. Nevertheless, the induction of CD4+ cells secreting TGF-β1 is insufficient to describe these cells as Tregs. Similarly, the induced expression of CD25 does not necessarily define these as Tregs, because CD25 is also expressed by recently activated T cells. To ascertain whether MSC generate functional Tregs, CD4+CD25+ T cells induced by allogeneic MSC were purified and examined for their suppressive activity in MLR. After 72 h MSC co-culture, CD4+CD25+ cells were retrieved using CD25+ microbeads and were added subsequently to MLR containing PBMC from mismatched donors. CD4+CD25+ cells induced by allogeneic MSC secreted TGF-β1 (Fig. 5a and b) and reduced allodriven proliferation significantly (Fig. 5c). Thus CD4+CD25+ T cells induced by allogenic MSC displayed conventional suppressive activity consistent with definitions of Tregs.
Fig 5.

Purified CD4+ CD25+ T cells induced by mesenchymal stromal cells (MSC), expressed transforming growth factor (TGF)-β1 and suppressed alloresponses. TGF-β1 mRNA was increased in purified CD4+ T cells cultured previously in the presence of MSC determined by reverse transcription–polymerase chain reaction (RT–PCR) (a) and quantified by real-time PCR for TGF-β1 mRNA (b). Real-time PCR showed a significant (***P < 0·001) increase in TGF-β1 expression by CD4+ T cells co-cultured previously with MSC for 24 h compared with CD4+ T cells alone. Results are representative of three independent experiments each performed in duplicate, expressed as mean concentration ± standard error (s.e.). Purified CD4+CD25+ T cells induced by MSC co-culture suppressed alloantigen-driven proliferation in mixed lymphocyte reaction (MLR) (c). Microbead purified CD4+CD25+ T cells induced from co-cultured with MSC were then cultured with or without MHC mismatched donor cells (P1 or P2) in two-way MLR at a 1:1 ratio for 96 h. Allodriven proliferation was reduced (*P = 0·034) significantly in the presence of CD4+CD25+ T cells measured by [3H]-thymidine incorporation and expressed as mean cpm ± s.e. Results are representative of three independent experiments each performed in triplicate.
Discussion
An understanding of the mechanisms by which MSC modulate allogeneic reactivity will be critical to the approval process for new therapies involving regenerative medicine. The current study examined the mechanisms of MSC induction of human Tregs relevant to peripheral tolerance. Allogeneic MSC co-cultured with purified CD4+ T cells resulted in a significant increase in FoxP3 expression. Direct MSC–T cell contact was required for FoxP3 and CD25High expression by CD4+ T cells; however, soluble MSC-derived factors also played a non-redundant role. In particular, both TGF-β1 and PGE2 derived from MSC contributed to allogeneic MSC induction of FoxP3+CD25+CD4+ T cells. CD4+CD25+ T cells induced by encounter with MSC suppressed alloantigen-driven proliferation. Therefore, MSC induce CD4+ T cell populations with all the characteristics of Treg through a process involving MSC derived PGE2 and TGF-β1, subsequent to the prerequisite of cell contact.
The MSC have attracted attention by virtue of their differentiation capacity and therapeutic potential, offering a new frontier in regenerative medicine [16]. The immunosuppressive properties of MSC have great significance with respect to the potential clinical application of MSC. A variety of studies have shown beneficial effects of MSC therapy in a number of disease models, including osteoarthritis [6], diabetes [40] and myocardial infarction [41], and successful outcomes from transplantation studies using MSC for treating GVHD [42,43], autoimmune disorders [44,45], metachromatic leucodystrophy and Hurler syndrome [46] and stroke [47], as well as repair of damaged tissue [48,49]. Many of these studies encourage the potential use of allogeneic MSC in regenerative medicine.
The realization that direct MSC–T cell interaction induces T cells with regulatory properties has emerged from a number of reports examining the broader question of MSC immunomodulation. Initial studies by Beyth et al.[50] and Maccario et al.[28] noted the induction of cells with phenotypic characteristics of Treg but did not involve functional studies of suppression. Li et al. have shown similar findings using placental-derived multi-potent stem cells [51]; this observation is interesting, as it again highlights the similarities between immunomodulation at the feto–maternal interface [39] and those operating during repair [52–54]. Aggarwal et al. extended these observations by showing that MSC induce a glucocorticoid-induced T cell receptor (GITR)+ T cell and demonstrated suppressor activity in a PBMC co-culture system; however, the mechanism remained unclear [21]. The present study supports those findings and clarifies an important non-redundant contribution to PGE2 and TGF-β1 in the MSC induction of FoxP3+CD4+CD25+ T cells with direct suppressor activity.
These findings are in contrast with studies of Provosto et al.; although that group showed CD25+ and GITR+/cytotoxic T lymphocyte associated antigen-4+ T cell induction with suppressor activity, no role for PGE2, TGF-β or IL-10 was observed [55]. These contradictory data relating to TGF-β and PGE2 are difficult to resolve, but may be due to differences in MSC isolation. The human MSC used in this study are well characterized [20], and do not express HLA-DR. Alternatively, as the readout for suppression used by Prevosto et al. involved a multi-cell system, whereas the present work examined only the suppressive effect of a purified CD4+CD25+ population, it might well be that in a more complex readout system the specific role of individual mediators could be masked by other mechanisms [55]. This is indeed likely, as we have shown previously in murine systems that multiple independent pathways modulate different aspects of suppression [15,19].
Very recently, Selmani et al. have demonstrated a role for HLA-G5 in MSC mediated suppression using an MSC–PBMC system, and demonstrating an important role for cell–cell contact in Treg functional suppression [56]. The data herein are consistent with that study. Using a different approach involving purified cells, we show that direct cell contact is required between the MSC and the CD4+ T cell to achieve maximum induction of FoxP3, CD25+ CD4+ T cells. Thus a model is emerging where MSC directly modulate T cell polarization involving a cell contact step, and MSC production of HLA-G, followed by PGE2 and TGF-β1 signalling to the CD4+ T cell. In summary, both contact-dependent and -independent interactions are required, most probably in a sequential manner, and this phenomenon is analogous to T cell help for B cells requiring both cell contact and soluble factors [57]. In this study we also examined the importance of other immune cells in MSC induction of CD4+CD25+FoxP3+ Treg cells using unseparated PBMC. This complex system of MSC co-culture with PBMC might reflect a situation in a complex immunological milieu and be informative. The only difference to the above system is that while cell contact is required for the MSC-driven induction of FoxP3 in CD4+ T cells, in the unseparated PBMC system there is no such requirement for cell contact for MSC induction of FoxP3 expression (Fig. 3c). Presumably in such an environment contact signals are substituted by other soluble factors not present in the two-cell system.
This study has focused upon the direct induction of Treg by MSC; however, MSC can also induce Tregs via an indirect route, through modulation of DC maturation. Previously, using murine and human systems we and others have demonstrated that MSC veto DC maturation marker expression, chemokine receptor switch, antigen display and antigen presentation function, while preserving DC expression of tissue-anchoring E-cadherin [12,15,58–60]. This can lead to Treg induction via an indirect route that requires IL-6 [15]. Furthermore, specific aspects of immune function can be modulated by kynurenine concentration [20], IDO [61], haem oxygenase, insulin-like growth factor binding protein and other MSC-derived factors [22–24]. The major point is that MSC modulate immunity by multiple inter-related pathways, but that clarification of the mechanisms involved relies on highly defined populations and systems.
The aim of this study was to determine if MSC mediate their suppressive effect by inducing CD4+ T cells to a regulatory phenotype. This study showed clearly that allogeneic human MSC have the ability to modulate CD4+ T cell function directly. Herein it is shown that the immunosuppressive function of MSC is conducive to the development of a suppressor or Treg phenotype, with a corresponding increase in TGF-β1 expression, which is known to suppress the development of an effector T cell response. These findings suggest that MSC induce CD4+CD25HighFoxP3+ T cells to modulate alloresponses from an effector to a suppressive or regulatory response and thereby interfere with the immune response to alloantigen. This hypothesis is amenable to testing and is supported by human clinical studies of MSC in the prevention of allograft rejection, GVHD, chronic inflammatory disease and autoimmunity [62–64]. Recent reports have suggested a broad role for TGF-β1 and PGE2 in the generation and expansion of Treg from CD4+CD25− precursors [33,38,65–67]. A report by Fu et al. demonstrated that TGF-β signalling is a key regulator of the signalling pathway that initiates and maintains FoxP3 expression and suppressor function [38]. Interestingly, human MSC express constitutively both TGF-β1 and PGE2[20], but both are significantly up-regulated upon ‘licensing’ by inflammatory mediators [19,20]. Therefore, this study proceeded to determine if MSC-derived soluble factors were responsible for Treg induction by blocking PGE2 and TGF-β1 with indomethacin and anti-TGF-β respectively. Several studies have demonstrated the ability of CD4+CD25+ regulatory cells to suppress alloreactivity and can attenuate GVHD [68,69]. Consistent with this role for Tregs, CD4+CD25+ T cells that were retrieved from MSC/CD4+ T cell co-cultures displayed allosuppressive function when added to an MLR containing allogeneic PBMC.
Djouad and colleagues have demonstrated that MSC induction of CD8+ Treg was responsible for certain immunosuppressive activities of MSC in vitro[70]. In contrast to these data, Krampera et al. showed that CD4+CD25+ regulatory cells were not required for the inhibitory activity mediated by MSC [10]. However, this does not contradict the present study; we have demonstrated previously that immunomodulation occurs by multiple redundant pathways of which CD4+ Treg induction is only one [15,19,20]. Furthermore, we have shown previously that some discrepancies may be explained by differences between murine and human MSC, in particular in our hands unlicensed human MSC, unlike murine MSC, do not express IL-10 constitutively [19,20].
The discovery of MSC modulation of allogeneic responses was greeted with some scepticism by immunologists; however, it is now clear that MSC modulate immunity by multiple different factors. This process involves cross-talk or licensing from immune and inflammatory signals, and may be a fundamental aspect of peripheral tolerance induction to neo-antigens during adult tissue repair [71]. The present study demonstrates a novel non-redundant role for cell contact and both TGF-β1 and PGE2 in MSC induction of regulatory CD4+ T cells expressing FoxP3. This definition of the precise mechanisms involved in allosuppression support the proposed use of allogeneic MSC in human clinical trials and provides a rational scientific basis for the observations seen in the use of allogeneic MSC as a novel therapy against GVHD.
Acknowledgments
K. E. and J. M. R. contributed equally to this work. K. E., J. M. R. and L. T. performed the experimental procedures, F. B. and M. J. M. advised on stem cell purification, B. P. M. directed this study, led the experimental design and edited the manuscript. All authors contributed to writing the paper. This work was funded by a Science Foundation Ireland, Centre for Science Engineering and Technology award (CSET) in Regenerative Medicine. B. M. is a Wellcome Trust/HRB ‘New Blood’ Fellow. L. T. is funded by the Irish Health Research Board PhD Scholars programme in immunology.
References
- 1.Barry FP. Mesenchymal stem cell therapy in joint disease. Novartis Found Symp. 2003;249:86–96. discussion 96–102, 170–4, 239–41. [PubMed] [Google Scholar]
- 2.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJP, Petrokova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–90. [PubMed] [Google Scholar]
- 4.Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20:161–71. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair – current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 6.Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–74. doi: 10.1002/art.11365. [DOI] [PubMed] [Google Scholar]
- 7.Le Blanc K, Samuelsson H, Gustafsson B, et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733–8. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- 8.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 9.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 10.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 11.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–7. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 12.Djouad F, Charbonnier LM, Bouffi C, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an Il-6-dependent mechanism. Stem Cells. 2007;25:2025–32. doi: 10.1634/stemcells.2006-0548. [DOI] [PubMed] [Google Scholar]
- 13.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 14.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 15.English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunol Lett. 2008;115:50–8. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm (Lond) 2005;2:8. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–73. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 18.Polchert D, Sobinsky J, Douglas G, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–55. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.English K, Barry FP, Field-Corbett CP, Mahon BP. IFN-gamma TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 2007;110:91–100. doi: 10.1016/j.imlet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353–63. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–34. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 23.Chabannes D, Hill M, Merieau E, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110:3691–4. doi: 10.1182/blood-2007-02-075481. [DOI] [PubMed] [Google Scholar]
- 24.Gieseke F, Schutt B, Viebahn S, et al. Human multipotent mesenchymal stromal cells inhibit proliferation of PBMCs independently of IFNgammaR1 signaling and IDO expression. Blood. 2007;110:2197–200. doi: 10.1182/blood-2007-04-083162. [DOI] [PubMed] [Google Scholar]
- 25.Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–6. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 26.Otten GR, Germain RN. Split anergy in a CD8+ T cell: receptor-dependent cytolysis in the absence of interleukin-2 production. Science. 1991;251:1228–31. doi: 10.1126/science.1900952. [DOI] [PubMed] [Google Scholar]
- 27.Holt PG, McMenamin C. Defence against allergic sensitization in the healthy lung: the role of inhalation tolerance. Clin Exp Allergy. 1989;19:255–62. doi: 10.1111/j.1365-2222.1989.tb02380.x. [DOI] [PubMed] [Google Scholar]
- 28.Maccario R, Podesta M, Moretta A, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–25. [PubMed] [Google Scholar]
- 29.Roncarolo MG, Levings MK. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr Opin Immunol. 2000;12:676–83. doi: 10.1016/s0952-7915(00)00162-x. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills KH. Regulatory T cells friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–55. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 33.Baratelli F, Lin Y, Zhu L, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–90. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 34.Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002;46:704–13. doi: 10.1002/art.10118. [DOI] [PubMed] [Google Scholar]
- 36.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–17. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 37.Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–5. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 38.Fu S, Zhang N, Yopp AC, et al. induces Foxp3+ T-regulatory cells from CD4+ CD25– precursors. Am J Transpl. 2004;4:1614–27. doi: 10.1111/j.1600-6143.2004.00566.x. [DOI] [PubMed] [Google Scholar]
- 39.Barry FP, Murphy JM, English K, Mahon BP. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev. 2005;14:252–65. doi: 10.1089/scd.2005.14.252. [DOI] [PubMed] [Google Scholar]
- 40.Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–43. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007;30:97–104. doi: 10.1007/s10753-007-9025-3. [DOI] [PubMed] [Google Scholar]
- 42.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 43.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–7. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Li Y, Chen J, et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Exp Neurol. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175–86. doi: 10.1002/art.22511. [DOI] [PubMed] [Google Scholar]
- 46.Koc ON, Day J, Nieder M, Gerson SL, Lazarus HM, Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and Hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–22. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 47.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–82. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 48.Awad HA, Butler DL, Boivin GP, et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267–77. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 49.Ringden O, Uzunel M, Sundberg B, et al. Tissue repair using allogeneic mesenchymal stem cells for hemorrhagic cystitis, pneumomediastinum and perforated colon. Leukemia. 2007;21:2271–6. doi: 10.1038/sj.leu.2404833. [DOI] [PubMed] [Google Scholar]
- 50.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–19. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 51.Chang CJ, Yen ML, Chen YC, et al. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells. 2006;24:2466–77. doi: 10.1634/stemcells.2006-0071. [DOI] [PubMed] [Google Scholar]
- 52.Makwana M, Jones LL, Cuthill D, et al. Endogenous transforming growth factor beta 1 suppresses inflammation and promotes survival in adult CNS. J Neurosci. 2007;27:11201–13. doi: 10.1523/JNEUROSCI.2255-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patil KA, Bellner L, Cullaro G, Gotlinger K, Dunn MW, Schwartzman ML. Heme oxygenase-1 induction attenuates corneal inflammation and accelerates wound healing after epithelial injury. Invest Ophthalmol Vis Sci. 2008;49:3379–86. doi: 10.1167/iovs.07-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peranteau WH, Zhang L, Muvarak N, et al. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128:1852–60. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 55.Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell–lymphocyte interaction. Haematologica. 2007;92:881–8. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 56.Selmani Z, Naji A, Zidi I, et al. HLA-G5 secretion by human mesenchymal stem cells is required to suppress T-lymphocyte and NK function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2007;26:212–22. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 57.Croft M, Swain SL. B cell response to fresh and effector T helper cells. Role of cognate T–B interaction and the cytokines IL-2, IL-4, and IL-6. J Immunol. 1991;146:4055–64. [PubMed] [Google Scholar]
- 58.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–6. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 59.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–7. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 60.Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–71. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 61.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 62.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 63.Bach JF. Regulatory T cells under scrutiny. Nat Rev Immunol. 2003;3:189–98. doi: 10.1038/nri1026. [DOI] [PubMed] [Google Scholar]
- 64.Juedes AE, Von Herrath MG. Using regulatory APCs to induce/maintain tolerance. Ann NY Acad Sci. 2003;1005:128–37. doi: 10.1196/annals.1288.014. [DOI] [PubMed] [Google Scholar]
- 65.Horwitz DA, Zheng SG, Gray JD. The role of the combination of IL-2 and TGF-beta or IL-10 in the generation and function of CD4+ CD25+ and CD8+ regulatory T cell subsets. J Leukoc Biol. 2003;74:471–8. doi: 10.1189/jlb.0503228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25– precursors. J Immunol. 2002;169:4183–9. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 67.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–9. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 68.Sakaguchi S, Sakaguchi N, Shimizu J, et al. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 69.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–44. doi: 10.1182/blood-2003-04-1193. [DOI] [PubMed] [Google Scholar]
- 71.Waldmann H, Adams E, Fairchild P, Cobbold S. Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol Rev. 2006;212:301–13. doi: 10.1111/j.0105-2896.2006.00406.x. [DOI] [PubMed] [Google Scholar]


