Abstract
In leukemia cells, hyperthermia enhances tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. The phenomenon is caspase-dependent and results in membrane changes leading to an increased recognition of TRAIL death receptors by TRAIL. Because either caspase-2 or an apical proteolytic event has been recently proposed to act as an initiator of the cell death mechanism induced by heat shock, we have investigated the hierarchy of caspase activation in cells exposed to the combined heat shock plus TRAIL treatment. We report here that caspases-2, -3, and -8 were the first caspases to be activated. As expected, caspase-8 is required and indispensable during the initiation of this death signaling. Caspase-2 may also participate in the phenomenon but, in contrast to caspase-8, its presence appears dispensable because its depletion by small interfering RNA is devoid of effects. Our observations also suggest a role of caspase-3 and of a particular cleaved form of this caspase during the early signals of heat shock plus TRAIL-induced apoptosis.
Keywords: TRAIL, Apoptosis, Caspase, Heat shock, siRNA, bVAD-fmk, Nucleofection
Introduction
The heat shock (HS) response, characterized by the induction of HS proteins (Hsps), has been extensively studied; however, the prime intracellular signal that triggers the pathway of induction of HS genes is still a matter of discussion between the classical stress-sensing principle based on protein denaturation (Morimoto 1998) and the membrane-sensor approach based on heat-induced changes in the lipid composition and architecture of membranes (Nagy et al. 2007; Vigh et al. 2007). Moreover, the molecular mechanism leading to heat-induced cell death is not better understood (Beere 2004; Lindquist 1986; Milleron and Bratton 2007). Although a mild stress, by inducing Hsps synthesis, can protect cells against heat-induced injuries (Gerner and Schneider 1975), in various cancer cells, hyperthermia has been described to enhance apoptosis induced by anticancer agents (Kampinga 2006).
Caspases (cysteinyl aspartate-specific proteases), essential in the apoptotic machinery, are synthesized as inactive enzyme precursors (zymogens) (Alnemri et al. 1996). Initiator caspases (caspase-2, -8, -9, -10) activate downstream caspase effectors also named executioners (caspase-3, -6, -7), which execute the death program through the destruction of numerous subcellular components (Timmer and Salvesen 2007). Caspase substrates share a tetrapeptide motif often composed of an aspartate in the last position of the cleavage site. Viral and cellular inhibitors of caspases have been described, such as CrmA, Flip (FLICE-inhibitory protein), and XIAP, a member of the inhibitor of apoptosis protein (Callus and Vaux 2007; Ekert et al. 1999). To block apoptosis, various synthetic peptide inhibitors have been developed based on the cleavage site motif of the different caspases (Lavrik et al. 2005; Thornberry et al. 1992), such as the cell permeable and irreversible Pan caspase inhibitor z-Val-Ala-Asp-fluoromethylketone (z-VAD-fmk).
Two main death pathways exist that are characterized by specific caspase initiators. On one hand, the extrinsic apoptosis pathway is initiated subsequent to death receptor signaling by the activation of the initiator caspase-8 and -10 through the death-inducing signaling complex (DISC) formation (Ho and Hawkins 2005). On the other hand, the intrinsic apoptosis pathway depends on mitochondria and causes initiator caspase-9 activation through apoptosome formation (Ho and Hawkins 2005). The last caspase initiator that has been described in the literature, caspase-2, is implicated in various death stimuli and could be recruited to different large multiproteic complexes (Ho and Hawkins 2005). For example, an initiator role of caspase-2, through the formation of the PIDDosome (p53-Induced Death Domain-containing protein) (Tinel and Tschopp 2004), has been described after genotoxic stress. Moreover, Tu et al. (2006) have suggested that caspase-2 is the initiator caspase that triggers HS-induced apoptosis. In contrast, Milleron and Bratton (2006) recently reported that hyperthermia-mediated cell death is independent of any known initiator caspase-activating complex. Moreover, these authors have suggested that an apical protease is activated by HS and subsequently triggers caspase-3 processing.
We have previously shown that hyperthermia combined with the cytokine tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) treatment strongly stimulated the apoptosis of leukemia cells, including cells from chronic lymphocytic leukemia patients (Moulin and Arrigo 2006; Moulin et al. 2007b). Additionally, this cotreatment was not toxic for normal T-lymphocytes. We have also shown that the apoptosis stimulation was caspases and DISC formation-dependent, but protein neosynthesis (i.e., newly made Hsps) -independent. Moreover, the phenomenon was correlated with an enhanced recognition of TRAIL death receptors DR4 and DR5 by the cytokine, a phenomenon linked to ceramide up-regulation and membrane fluidization (Moulin et al. 2007a).
Because the mechanism responsible for HS-induced apoptosis is unclear (Milleron and Bratton 2007), we analyzed whether caspase-2 or an apical proteolytic event was implicated in cell death induced by the cotreatment TRAIL plus HS. Different approaches were used, including small interfering RNAs (siRNAs), inhibitors that target caspases and in situ trapping of activated caspases. We show here that caspase-8 is required for TRAIL and HS cotreatment-induced apoptosis. Moreover, a proteolytic event of unknown origin processed caspase-3 to an intermediate cleavage form different from p17/p12 subunits. These observations suggest an initiator role of caspase-3 in HS combined with TRAIL-treated cells.
Materials and methods
Reagents, treatments, and cell culture
Soluble human recombinant TRAIL (extracellular domain, 169 AA) was from PeproTech (Rocky Hill, NJ, USA). Caspase inhibitors (z-VAD-fluoromethylketone, VDVAD-fmk, DEVD-fmk, IETD-fmk, LEHD-fmk) were purchased from Alexis Biochemicals-Qbiogen (Illkirch, France). Heat treatment was performed at 42 ± 0.1°C in a thermostatic Huber water bath (Fisher Bioblock Scientific, Illkirch, France). Jurkat human T-lymphocytes line has already been described (Manero et al. 2004; Moulin et al. 2007a). Cells were grown at 37°C in the presence of 5% CO2 in Roswell Park Memorial Institute medium supplemented with 10% fetal calf serum and 50 U/ml penicillin/streptomycin (Invitrogen, Cergy Pontoise, France).
Cell death analysis
AnnexinV-FITC and propidium iodide (PI) staining (Roche, Meylan, France) was performed as already described (Moulin and Arrigo 2006).
siRNA-mediated knock-down of specific caspases
RNAi-mediated knockdown of caspase-2, -3, -8, and -9 was performed using 19-nucleotide-specific RNA duplex oligonucleotides (Eurogenetec, Angers, France). Transient transfection (nucleofection) of Jurkat cells was performed using the Amaxa system (Amaxa, Köln, Germany). In 100 μL Nucleofector™ solution containing the different RNA duplexes, 7.5 × 106 cells were resuspended and subjected to pulse I-010 according to the manufacturer’s instructions. Twenty-four and 48 h after nucleofection, the efficiency of siRNA-mediated knockdown of the different caspases was estimated by Western blot using specific antibodies.
Western blot analysis
Gel electrophoresis and immunoblots were performed as already described (Moulin and Arrigo 2006). Anti-actin antibody (clone C4) was from Roche (Indianapolis, IN, USA). Anti-caspase-3 antibody (clone 65906E, pro- and active-forms) was from Pharmingen (SanDiego, CA, USA). Anti-caspase-2 antibody was from BD Transduction Laboratories (Le Pont de Claux, France). Anti-caspase-8 (C15), anti-caspase-9 pro-form was from Alexis Biochemicals-Qbiogen (Illkirch, France) and p37 cleaved form of caspase-9 was from Chemicon (Temecula, CA, USA). Goat anti-mouse antibody was from Caltag (Burlingame, CA, USA). The ECL kit from Amersham Biosciences Europe (Orsay, France) was used to reveal antibody-probed immunoblots. Autoradiographs were recorded onto X-Omat LS films (Eastman Kodak, Rochester, NY, USA).
Caspase activation analysis
ApoStat staining (R&D System, Lille, France) was performed as already described (Moulin and Arrigo 2006). Fluorometric assay kit (R&D Sytems, Lille, France) was used for the determination of VDVAD-AFC-dependent caspase-2-like activities, DEVD-AFC-dependent caspase-3-like activities, IETD-AFC dependent caspase-8-like activities, and LEHD-AFC dependent caspase-9-like activities, and analysis was performed as already described (Moulin and Arrigo 2006). After treatment, each sample was divided in four parts to simultaneously analyze caspase-2, -3, -8, and -9 activities.
In situ trapping of activated caspases
For initiator caspase trapping, the method described by Tu et al. was used (Tu et al. 2006). For 1 h and 45 min, 5 × 107 cells were preincubated with 50 μM of biotin-VAD-fmk (bVAD-fmk) from MP Biomedicals (Illkirch, France). Cells were then either untreated or treated with 100 ng/ml of TRAIL and/or incubated for 1 h at 42°C and allowed to recover for 3 h at 37°C. For effector caspase trapping, the method described by Shin et al. was used (Shin et al. 2005). Before being incubated for 1 h with 10 μM of bVAD-fmk, 2.5 × 107 cells were either untreated or treated with TRAIL and exposed to HS as above. Subsequent to caspase trapping, cells were lysed in CHAPS lysis buffer (KCl 150 mM, Hepes 50 mM, CHAPS 0.5%, glycerol 5%, and a cocktail of protease inhibitors from Roche, (Indianapolis, IN, USA). Active caspases were precipitated using 50 μL of streptavidin-agarose overnight. After five washings, the presence of the precipitated caspases was detected in immunoblots probed with specific antibodies. After each treatment, caspases present in total cellular lyzate, as well as total cellular lyzate depleted of bVAD-fmk recognized caspases (i.e., unbound fractions), were also analyzed.
Results
Analysis of caspase processing after TRAIL and HS cotreatment
We have previously shown that HS is a potent stimulator of TRAIL-induced apoptosis in leukemia cells (Moulin and Arrigo 2006; Moulin et al. 2007b). The death stimulation, characterized by the enhanced recognition of TRAIL death receptor by the cytokine, results from changes in membrane fluidity and ceramide (Moulin et al. 2007a). Recently, two different research groups have suggested the involvement of specific proteases in heat-induced apoptosis. Tu and coworkers (2006) have proposed that caspase-2 is the initiator caspase, whereas Milleron and Bratton (2006, 2007) favor the activation of an unknown apical protease. Here, we have analyzed the caspases activation hierarchy during the apoptotic process induced by the HS + TRAIL cotreatment. We first analyzed whole caspases activation. Jurkat cells were either kept untreated (NT) or treated with 100 ng/ml of the cytokine TRAIL during 4 h. This was combined or not, during the first hour of TRAIL treatment, to a 42°C HS challenge, which was followed by a 3-h recovery period at 37°C. After 4 h of treatment, total caspase activation was analyzed using a cell permeable FITC conjugated caspase inhibitor (ApoStat™). Figure 1a shows that TRAIL induced an 11-fold increase in the activation of total caspases, while the HS treatment only induced a modest activation (1.75-fold). In contrast, the combined treatment induced a 19-fold stimulation of caspase activity, which illustrates the synergistic effect of hyperthermia on TRAIL.
Fig. 1.
Caspase activation by TRAIL and HS cotreatment. a Jurkat cells resuspended in fresh culture medium were either kept untreated (NT) or treated with 100 ng/ml of the cytokine TRAIL during 4 h (TRAIL). This was combined (HS + TRAIL) or not (HS), during the first hour of TRAIL treatment, to a 42°C HS, which was followed by a 3-h recovery period at 37°C. Immediately after the different 4-h treatments, cells were stained with cell-permeable FITC-conjugated caspase inhibitor Apostat-FITC (FL1H) and analyzed by flow cytometry. b Immunoblot analysis of initiator caspase-9, -8, and -2 and executioner caspase-3. Cells were treated 5, 15, 30, or 60 min or 4 h with 100 ng/ml of TRAIL combined or not with heat treatment. Then, protein samples from total cell extracts were analyzed by gel electrophoresis. Autoradiographs of the immunoblots probed with specific antibodies (as described in “Materials and Methods”) are presented
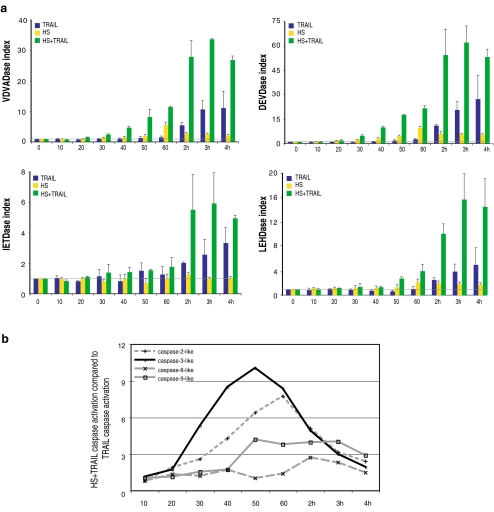
Next, analysis of the kinetics of activation of the different caspases was performed by Western immunoblots and fluorometric assays. In this respect, we analyzed the cleavage process of caspase initiators-2, -8, and -9 and of the main caspase executioner, caspase-3. As seen in the kinetic analysis presented in Fig. 1b, the cleavage of caspases already occurred after 1 h of cotreatment, while it was not detectable in cells treated with TRAIL only. After 4 h of treatment, caspase activation was far more intense in cells exposed to the combined HS + TRAIL treatment than with TRAIL only. This analysis also suggested that the cleavage of the major initiator caspases (-2, -8, -9) occurred at about the same time period as caspase-3. A more precise kinetic analysis was performed by analyzing the activation of the different caspases by fluorometric quantitation assays every 10 min during the first hour of the different treatments and then every each hour up until 4 h. Figure 2a confirms that TRAIL does not significantly induce the activities corresponding to caspase-2, -3, -8, and -9 during the first hour of treatment. In contrast, in cells exposed to HS alone, caspase-2-like (VDVADase), caspase-3-like (DEVDase), and caspase-9-like (LEHDase) activities were slightly stimulated during the first hour of the treatment, whereas IETDase-dependent caspase-8-like activity was not. The activation was maximal at the end of the HS treatment and then decreased during the recovery period at 37°C. In cells exposed to the combined HS + TRAIL treatment, caspases-2-, -3-, and -8-like activities began to be stimulated after 30 min of treatment, while caspase-9-like activity was detectable only 20 min later (Fig. 2a). The activities of the different caspases reached a maximal intensity after 3 to 4 h of treatment.
Fig. 2.
Time course analysis of caspase activation. a Fluorometric caspase activation assays. Jurkat cells were treated for 10 min, 20 min, 30 min, 40 min, 50 min, 60 min, 2 h, 3 h, or 4 h with 100 ng/ml of TRAIL. The 10- to 60-min incubation periods with TRAIL were also performed at 42°C (HS + TRAIL). VDVADase, DEVDase, IETDase, and LEHDase activities in extracts of treated cells were measured as described in “Materials and Methods”. The caspase index corresponds to the ratio of the fluorescence read in treated cells to that read in nontreated cells (time 0). The horizontal gray line corresponds to an index value of 1. The histogram plots are representative of three identical experiments; standard deviations are presented (n = 3). b HS + TRAIL caspase activation compared to that induced by TRAIL. This was calculated as the ratio of the caspase activation index in HS + TRAIL-treated cells to that observed in cells exposed only with TRAIL using data presented in a. Gray lines are used to represent VDVADase, IETDase, and LEHDase activities (initiator caspases) and the black line for DEVDase activity (executioner caspase-3)
We then compared the activity of the different caspases induced by the HS + TRAIL treatment to that observed in TRAIL-only treated cells. As seen in Fig. 2b, during the first hour of the cotreatment, caspase-3-like and caspase-2-like activities were highly stimulated (10-fold and 7.5-fold, respectively) compared to the activation induced by TRAIL only. The maximal stimulations were reached after 50 min (caspase-3) and 60 min (caspase-2) of cotreatment. Thereafter, the stimulations declined with similar kinetics and were less than threefold after 3 h of cotreatment. Concerning caspase-9-like activity, the stimulation (fourfold) was observed after 50 min and was maintained until 3 h of cotreatment. Surprisingly, during the first hour of the cotreatment, no significant stimulation of caspase-8-like activity was observed due to the fact that TRAIL and HS + TRAIL treatments stimulated this activity with a similar intensity. Two hours of cotreatment was necessary to detect a caspase-8-like activity that was more important (threefold) than that induced by TRAIL only. Nevertheless, caspase-8 was indispensable to initiate the death signaling, as its absence (by silencing or analysis of caspase-8−/− negative cells) abolished TRAIL- and HS + TRAIL-induced cell death (see Fig. 3 and Moulin and Arrigo 2006). Hence, the early and intense stimulation of the initiator caspase-2 and the executioner caspase-3 is a specific and initial event that occurs during the HS + TRAIL cotreatment.
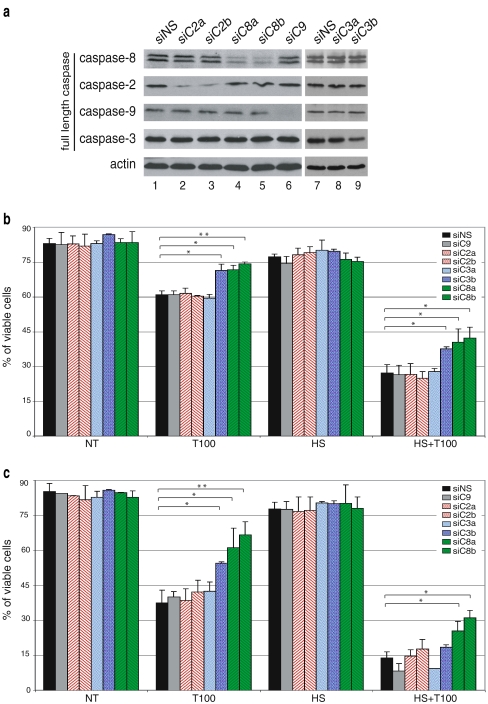
Fig. 3.
Effects of single caspase siRNA treatment on cell death induced by TRAIL and HS. a Jurkat cells were transfected with different caspase siRNA duplex and control siRNA devoid of known target called siNS for nonsilencing sequence (Amaxa nucleofection, see “Materials and Methods”). Immunoblot analysis of the total cellular content of caspase-9, -8, -3, and -2 was performed 48 h after nucleofection. Protein samples were prepared and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Thereafter, the immunoblots were probed with specific antibodies (as described in “Materials and Methods”). Actin was used as a loading control. b Twenty-four hours after nucleofection, transfected cells were treated with 100 ng/ml of TRAIL combined (HS + T100) or not (T100) to a 1-h HS treatment at 42°C. After 4 h of TRAIL treatment, cells were stained with AnnexinV-FITC/PI and analyzed by flow cytometry. The histogram plots are representative of two identical experiments; standard deviations are presented (n = 2). Data were subjected to a one-way analysis of variance. Significant differences are denoted as single asterisks, P < 0.05, and double asterisks, P < 0.01. c Same as b, but in this case, cells were analyzed 48 h after nucleofection using 100 ng/ml of TRAIL combined or not to a 1-h HS treatment at 42°C
Silencing of caspases reveals the predominant role of caspase-8 in HS + TRAIL-induced apoptosis
To further elucidate the role of the different caspases in HS + TRAIL-induced apoptosis, we used siRNA technology to down-regulate the intracellular level of caspase-2, -8, -9, and -3. siRNA sense sequences were as described previously by various research groups (Table 1). The siRNA control (devoid of targets) was called siRNA nonsilencing sequence (siNS) for nonsilencing sequence. Jurkat cells were efficiently transfected up to 80% using Amaxa nucleofection (data not shown). Immunoblot analysis of transfected cells revealed that the level of initiator caspases-2 and -8 was decreased using two independent nonoverlapping siRNAs (siC2a and siC2b in the case of caspase 2 and siC8a and siC8b in the case of caspase 8) (Fig. 3a, lines 2, 3, 4, and 5). A strong reduction in the level of caspase-9 was obtained using siC9 specific siRNA (Fig. 3a, line 6). Concerning the executioner caspase-3, a decrease in the level of this caspase was observed using siC3b but not siC3a (Fig. 3a, lines 8, 9). Nevertheless, the efficiency of siC3b was less pronounced than that observed using the siRNAs targeting the other caspases. This may be due to the high level of caspase-3 expressed in lymphocytes, such as Jurkat cells (Fernandes-Alnemri et al. 1994), and could explain the reduced protective activity of this siRNA (see below Fig. 3c).
Table 1.
Caspase targets, siRNA name, sense sequence, and references of the 19-nucleotide siRNA used in this study
| Target | siRNA name | siRNA sense sequence (5′–>3′) | Source |
|---|---|---|---|
| 0 | siNS | CCGAGACAAUCGCGAACAU | Cuchet (not published) |
| Caspase-8 | siC8a | CUACCAGAAAGGUAUACCU | Chun et al. 2002 |
| siC8b | GGGUCAUGCUCUAUCAGAU | Wagner et al. 2004 | |
| Caspase-2 | siC2a | ACAGCUGUUGUUGAGCGAA | Lassus et al. 2002 |
| siC2b | CUUCCAGCUGGCAUAUAGG | Wagner et al. 2004 | |
| Caspase-9 | siC9 | CCAGGCAGCUGAUCAUAGA | Allan and Clarke 2007 |
| Caspase-3 | siC3a | UGGAUUAUCCUGAGAUGGG | Carlile et al. 2004 |
| siC3b | UGACAUCUCGGUCUGGUAC | Wurzer et al. 2003 |
We then tested if the siRNAs described above interfered with the death induced by either TRAIL or the combined HS + TRAIL treatment. Twenty-four hours after nucleofection, cells were treated with 100 ng/ml of TRAIL combined or not with a HS treatment, and viability was analyzed by flow cytometry using FITC-Annexin-V/PI staining (Fig. 3b). It is seen in this figure that, after 4 h of incubation with 100 ng/ml of TRAIL, about 60% of control transfected Jurkat cells (siNS) were still viable. The two independent nonoverlapping siRNAs targeting caspase-8 increased cell viability up to 75%. The gain of viability in cells treated with siRNAs targeting caspase-8 was still observed in cells exposed to HS + TRAIL treatment. Except for the siRNA-targeting caspase-3, the other siRNAs were devoid of significant effect. The siRNA-mediated silencing of caspase-3 using siC3b was as efficient to increase cell viability as the silencing of caspase-8. Similar protective activity of caspase-8 siRNAs was observed when the analysis was performed 48 h after nucleofection (Fig. 3c). However, in these conditions, the efficiency of siC3b caspase-3 siRNA was reduced, particularly in the HS + TRAIL condition. This phenomenon may result from the reduced efficiency of this siRNA to decrease caspase-3 level (see Fig. 3a).
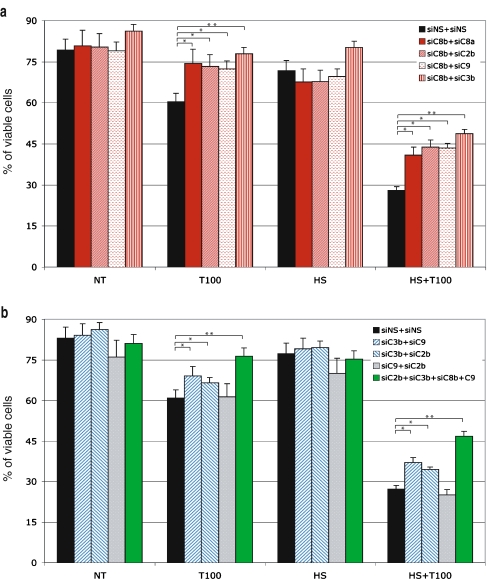
Next, we tried concomitant transfection with multiple siRNAs. Transfection using siRNAs targeting two different caspases was performed. The following combinations were tested: caspase-8 together with caspases-2 or -9, caspase-3 together with caspases-2 or -8, and caspase-9 together with caspases-2 or -3. Unexpectedly, silencing of caspase-8 with a mixture containing the two independent nonoverlapping siRNAs did not increase the efficiency to reduce apoptosis induced by TRAIL only or the combined treatment obtained when using each caspase-8 siRNA alone (Fig. 4a). Using siRNAs that are efficient to decrease the level of expression of their target polypeptides, we tested the following combinations: siC8b with siC2b or siC9 or siC3b. As seen in Fig. 4a, the protection against apoptosis induced by these different combinations of siRNAs was comparable to that observed using siC8a + siC8b. Of interest, the combination of siC8b and siC3b did increase the protection against TRAIL, HS, and HS + TRAIL treatments. We noticed that caspase-3 silencing by siC3b alone or in combination with other caspase siRNA increased viability in nontreated cells. This may be due to a role of caspase-3 in the death induced by the nucleofection. Other combinations of siRNA cotransfections targeting caspase-2 and -9 had no effect on cell death induced by TRAIL or the cotreatment, as the viability was the same as that observed using siRNA control (Fig. 4b). In contrast, the cotranfection of caspase-3 siRNA with caspase-2 or -9 siRNA increased viability significantly by more than 10% in the case of TRAIL or HS + TRAIL treatments with respect to the control (Fig. 4b).
Fig. 4.
Effects of multiple caspase siRNAs on cell death induced by TRAIL and HS. Jurkat cells were cotransfected with two or four siRNA duplex targeting caspases by Amaxa nucleofection. Analysis was performed after nucleofection with the different mixtures of siRNAs as indicated in A and B. The different siRNAs were transfected, and 24 h later, cells were treated with 100 ng/ml of TRAIL combined or not to a 1-h HS treatment at 42°C. After 4 h of TRAIL treatment, cells were stained with AnnexinV-FITC/PI and analyzed by flow cytometry. The histogram plots are representative of two identical experiments; standard deviations are presented (n = 2). Data were subjected to a one-way analysis of variance. Significant differences are denoted as single asterisks, P < 0.05, and double asterisks, P < 0.01
Lastly, we combined the four siRNAs targeting caspases. For an unknown reason, the decrease in the level of the four caspases was less than that observed using single or double transfection (not shown). However, the viability in presence of TRAIL or HS + TRAIL treatment was increased and resembled that observed in the case of the double transfection assays, which include the siRNAs targeting caspase-8 and caspase-3 (Fig. 4b). Even if the efficiency of caspase silencing was not maximal, these results suggest that caspase-8 and, to a lesser extent, caspase-3 are crucial factors of the death stimulation induced by HS + TRAIL cotreatment. This result is in accordance with our previous observations showing that Jurkat cells devoid of caspase-8 are completely resistant to TRAIL and HS + TRAIL-induced apoptosis (Moulin and Arrigo 2006). In contrast, no significant effect of caspase-2 was noticed.
Inhibition of caspase activation reveals intermediate cleavage forms of caspase-3 and -2 in cells exposed to the HS + TRAIL cotreatment
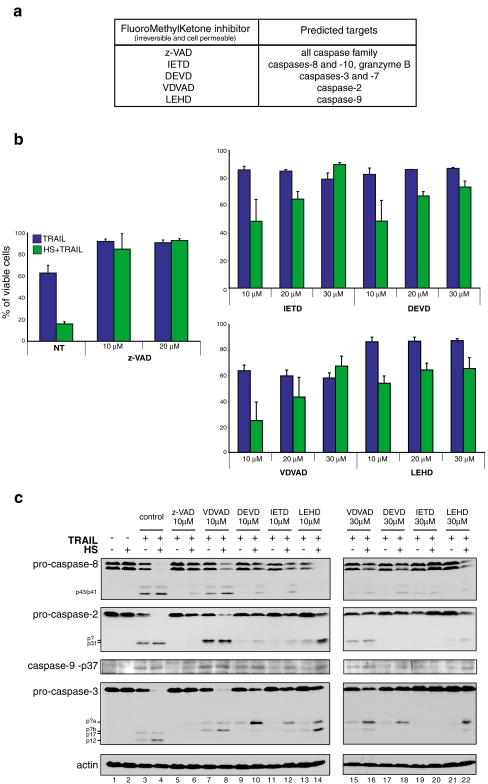
We next analyzed the effects mediated by various inhibitors of caspases on TRAIL and HS + TRAIL apoptosis. Jurkat cells were pretreated for 45 min with various concentrations of pan caspase inhibitor z-VAD-fmk, caspase-8 inhibitor IETD-fmk, caspase-3 inhibitor DEVD-fmk, caspase-2 inhibitor VDVAD-fmk, and caspase-9 inhibitor LEHD-fmk (Fig. 5a). Following the pretreatments, cells were treated for 4 h with 100 ng/ml of TRAIL combined or not with a 1-h HS at 42°C (performed during the first hour of the TRAIL treatment). Immediately after completing the 4 h of treatment, viability was measured by flow cytometry using FITC-AnnexinV/PI staining (Fig. 5b) and caspase processing was analyzed by Western blot (Fig. 5c). After TRAIL treatment, 60% of cells were viable. In the presence of 10 to 30 μM of the different caspase inhibitors, the level of viable cells raised to more than 80%. One exception was the caspase-2 inhibitor VDVAD-fmk that had absolutely no effect towards TRAIL-induced cell death (Fig. 5b). After HS + TRAIL cotreatment, only 20% of the cells were still viable. At low concentration of the inhibitors, only z-VAD-fmk was efficient to block apoptosis induced by the combined treatment (more than 80% of viable cells). At a concentration of 10 μM, caspase-8, -9, and -3 inhibitors were only partially efficient to prevent apoptosis induced by HS + TRAIL cotreatment (Fig. 5b). At higher a concentration (i.e., 30 μM), the caspase-8 inhibitor IETD-fmk completely inhibited apoptosis induced by the combined treatment, whereas the effect was less intense in the case of caspase-3 (DEVD-fmk) and caspase-9 (LEHD-fmk) inhibitors. Caspase-2 inhibitor had no effect towards TRAIL apoptosis at any concentration we used. In contrast, this inhibitor interfered, when used at a concentration of 30 μM, with HS + TRAIL apoptosis similarly to DEVD-fmk and LEHD-fmk inhibitors (Fig. 5b).
Fig. 5.
Effects of caspase inhibitors on cell death and caspase activation induced by TRAIL and HS. A Targets of caspase inhibitors used in this study, according to the manufacturer’s data sheet. B Cells were pretreated for 45 min with either 10 or 20 μM of z-VAD-fmk a pan caspase inhibitor. Similarly, cells were treated with either 10 or 30 μM of inhibitors against caspase-2 (VDVAD-fmk), caspase-3 (DEVD-fmk), caspase-8 (IETD-fmk), and caspase-9 (LEHD-fmk). Cells were then treated for 4 h with 100 ng/ml of TRAIL or the combined treatment HS (1 h at 42°C) plus TRAIL before being stained by AnnexinV and PI. Control experiments where cells were either kept untreated or exposed to a HS treatment of 1 h at 42°C are also presented. The percentage of viable cells is represented as histogram plots and is representative of three identical experiments; standard deviations are presented (n = 3). C Cells were pretreated for 45 min with either 10 μM of the pan caspase inhibitor z-VAD-fmk or 10 to 30 μM of the different caspase inhibitors presented above in A. Cells were then treated for 4 h with 100 ng/ml of TRAIL or the combined treatment HS (1 h at 42°C) and TRAIL. Then, protein samples from total cell extracts were analyzed by gel electrophoresis and immunoblots were probed with specific antibodies (as described in “Materials and Methods”). Autoradiographs are presented
As synthetic caspase inhibitors have been described to be more or less specific and efficient (Callus and Vaux 2007; Ekert et al. 1999; Lavrik et al. 2005), we next analyzed caspase processing after TRAIL and HS + TRAIL treatments in the presence of inhibitors (Fig. 4b). After 4 h of treatment with 100 ng/ml of TRAIL, Western blot analysis revealed efficient cleavage of caspase-8, -2, -3, and -9 (Fig. 5c, line 3). This process was more intense in response to the combined treatment resulting in the nondetection of the full-length pro-caspases (line 4). The pretreatment with z-VAD-fmk prevented all caspase cleavage after TRAIL or HS + TRAIL treatments (lines 5 and 6). Caspase-8 is known as the apical initiator caspase with death receptor pathway. In this respect, 10 μM of the caspase-8 inhibitor IETD-fmk was efficient to block the processing of caspase-8 induced by TRAIL only (line 11). Nevertheless, at this concentration, the inhibitor did not fully inhibit pro-caspase-8 cleavage (line 12) and apoptosis (see Fig. 5b) after HS + TRAIL treatment. However, at a concentration of 30 μM of the inhibitor, the cleavage of the full-length caspase-8 was not detected (line 20), and in this condition, the processing of the other caspases was inhibited, confirming the upstream location of caspase-8. This result is in accordance with the data obtained from experiments aimed at siRNA targeting caspase-8 (Figs. 3 and 4).
In TRAIL- or HS + TRAIL-treated cells, caspase-2 was cleaved leading to the appearance of a p31 cleaved form. The phenomenon was more intense in HS + TRAIL-treated cells (line 4) than in cells exposed to TRAIL only (line 3). In presence of 10 μM of caspase-2 inhibitor VDVAD, this caspase was still processed (lines 7 and 8) with the same intensity as in cells not treated with the inhibitor, but in these conditions, a new cleaved form (indicated p? in Fig. 5c) was observed that migrated slower than the p31 fragment observed in TRAIL- or HS + TRAIL-treated cells not exposed to inhibitors. At a concentration of 30 μM of VDVAD or LEHD-fmk, caspase-2 was less cleaved, particularly in HS + TRAIL-treated cells, but the new cleaved polypeptide was still detectable. This observation points to the low efficiency of these inhibitors. In cells treated with 10 μM of DEVD-fmk or IETD-fmk, the processing of caspase-2 was reduced, but the new cleaved form was still detectable.
Concerning caspase-9, the level of the p37 cleaved form of this caspase is presented (Fig. 4b). At a concentration of 10 μM, the broad-range caspase inhibitor z-VAD-fmk efficiently abolished the cleavage of caspase-9 induced by TRAIL and HS + TRAIL treatments. Similarly, caspase-8 (IETD-fmk) and caspase-9 (LEHD-fmk) inhibitors also abolished the appearance of p37 in response to TRAIL. In contrast, in HS + TRAIL-treated cells, these inhibitors had no effect. The other inhibitors were also not effective at this concentration. At a higher concentration (30 μM), most inhibitors decreased the processing of caspase-9 leading to the appearance of p37.
Following HS + TRAIL cotreatment, caspase-3 underwent cleavage, resulting in the appearance of p17 and p12 polypeptides (Fig. 5c, lines 3 and 4). In the presence of 10 μM of z-VAD-fmk or 30 μM of IETD-fmk, no caspase-3 cleavage (Fig. 5c, lines 5, 6, 19, and 20) and no apoptosis were observed (Fig. 4a). What is more striking were the effects induced by the other inhibitors (except IETD-fmk at 30 μM): indeed, in the presence of these inhibitors, caspase-3 was still processed, but new cleavage forms (noted p?a and p?b in Fig. 5c) were detectable (lines 8, 10, 12, 14, 16, 18, and 22) instead of the classical p17 and p12 forms (lines 3 and 4). These new forms migrated slightly faster than the 20-kDa molecular weight marker (not shown). A concentration of 30 μM of the inhibitors favored the appearance of the p?a form (excepted in the case of IETD-fmk treated cells where it was not detectable). These new processed forms of caspase-3, which were only weakly detectable in samples from TRAIL-only treated cells, correlated with a percentage of cell death ranging from 30 to 70 depending on the inhibitor that was used (Fig. 5b). We then analyzed the effects of 10 μM of the different caspase inhibitors on caspase-3-like activity in cells treated with TRAIL or HS + TRAIL (same conditions as those described in Fig. 5c, lines 1 to 14). As determined by fluorometric assays, TRAIL and HS + TRAIL treatments increased caspase-3-like activity by 11- and 37-fold, respectively, compared to the background activity detected in untreated cells (data not shown). This high level of activity was not detectable in cells pretreated with z-VAD-fmk, DEVD-fmk, or IETD-fmk. However, in cells pretreated with VDVAD-fmk and LEHD-fmk, a slight caspase-3-like activity (2.4-fold increase compared to basal activity) was detectable in HS + TRAIL-cotreated cells (data not shown). In VDVAD-fmk- and LEHD-fmk-treated cells, immunoblot analysis revealed that the signal at the level of p?b cleavage product was more intense than that of p?a polypeptide (lines 8 and 14 in Fig. 5c). This observation, together with the fact that no caspase-3 activity is detected in IETD-fmk-treated cells, which contains mainly p?a polypeptide, suggests that the 2.4-fold increase in caspase-3-like activity may be related to p?b polypeptide.
Cleavage of caspase-3 in a particular intermediate form in HS + TRAIL-induced apoptosis
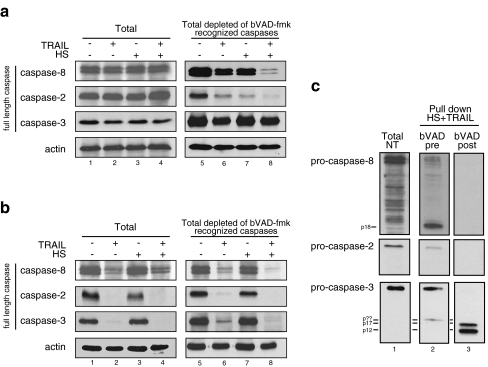
Finally, we expanded our study using a cell-permeable biotinylated caspase inhibitor VAD-fmk (bVAD-fmk) that binds covalently and irreversibly to active caspases (Mohr and Zwacka 2007; Tu et al. 2006). In this respect, bVAD-fmk was used for in situ trapping of activated initiator caspases when it was applied before death treatment. Hence, we treated Jurkat cells with 50 μM bVAD-fmk for 1 h and 45 min before treatments. Figure 6a (lines 1–4) revealed that, in the presence of bVAD-fmk, full-length caspases were not processed in response to TRAIL and HS + TRAIL treatments, a phenomenon that correlated with the inhibition of apoptosis (data not shown). bVAD-fmk-bound activated caspases were precipitated using streptavidin-agarose, as described in “Materials and Methods”. The proteins present in the supernatant fractions devoid of bVAD-fmk recognized caspases were then analyzed in immunoblots probed with specific anti-caspase antibodies. It can be seen in Fig. 6a that, after TRAIL treatment, full-length caspase-8 and caspase-2 were strongly depleted. The depletion was more intense and almost complete in cells exposed to HS + TRAIL cotreatment (line 8) compared to cells exposed to TRAIL only (line 6). A small decrease in caspase-3 level was also detectable, but the effect was of similar intensity in cells exposed to TRAIL or HS + TRAIL. A decrease in caspase-2 was also observed in HS-treated cells; this phenomenon may be reminiscent of the proposed role of this caspase to trigger heat-activated apoptosis (Tu et al. 2006). In a similar experiment, bVAD-fmk was added to cells after TRAIL, HS, or HS + TRAIL treatments to detect caspase executioners. Figure 6b (lines 1–4) reveals that, in response to TRAIL and HS + TRAIL, caspase-8, -3, and -2 were well processed because their full-length forms had disappeared. It is of interest that the effect was not observed in cells exposed to HS only. Analysis of the supernatant fractions devoid of bVAD-fmk-recognized caspases revealed that the profile of remaining full-length caspases was identical to that observed in the total fraction.
Fig. 6.
In situ trapping of initiator and effector caspases in response to TRAIL and HS cotreatment. A bVAD-fmk pretreatment. For 1 h and 45 min, 2.5 × 107 Jurkat cells, preincubated with 50 μM of bVAD-fmk, were either kept untreated or treated with 100 ng/ml of TRAIL during 4 h (TRAIL). Cells were also incubated 1 h at 42°C and allowed to recover 3 h at 37°C (HS) or were exposed to the combined treatment. Immediately after the different 4-h treatments, cells were lysed in CHAPS lysis buffer and active caspase were precipitated using 50 μL of streptavidin-agarose overnight (see “Materials and Methods”). Full-length caspase-8, -3, and -2 were immuno-detected using specific antibodies in the total cell lyzate (Total) and in the unbound fraction (Total lyzate depleted of bVAD-fmk recognized caspases). Autoradiographs are presented. B bVAD-fmk posttreatment. Cells were treated as above (in A) excepted that bVAD-fmk treatment was performed immediately after (instead of prior to) the treatments. In this case, the treatment was for 1 h with 10 μM. Full-length caspase-8, -3, and -2 were immuno-detected with specific antibodies in the total cell lyzate and the unbound fraction (defined above in A). Autoradiographs are presented. C) The bVAD-fmk biotinylated-caspase inhibitor was used either before or after the HS + TRAIL cotreatment (as described in A and B). Active caspases (full-length and cleaved forms) recognized by bVAD-fmk were pulled down and analyzed in immunoblots probed with specific antibodies (as described in “Materials and Methods”)
We next analyzed the pulled-down caspases in bVAD-fmk-pretreated cells that were subsequently HS + TRAIL-treated. It can be seen in Fig. 6c that a small amount of full-length caspase-8 was pulled down together with a high level of the cleaved p18 form (Fig. 6c, line 2). Full-length caspase-2 was also pulled down. Concerning the executioner caspase-3, a striking finding was the detection of the full-length pro-caspase together with a cleaved form (p??), which migrated in the gel similarly to the p?b polypeptide (described above in Fig. 5c). These results first suggest that caspase-8 and -2 are initiator caspases in response to HS + TRAIL treatment. Moreover, we show that an apical cleavage of caspase-3 generating an intermediate form was recognized by bVAD-fmk pretreatment. We also analyzed Hsp90 as a cytoplasmic non-caspase control protein, and this protein was never pulled down whatever the treatment used was (data not shown). Analysis of the pulled-down caspases in bVAD-fmk posttreated cells that were previously HS + TRAIL-treated revealed no signals at the level of the full-length or processed form of caspase-2 and -8, while the cleaved and active forms of caspase-3 (p17–p12) were clearly detected (Fig. 6c, line 3). Caspase-9 was not pulled down in either bVAD-fmk pretreatment or posttreatment (data not shown). In summary, caspase-3 appears to be activated in two different ways: we confirm that caspase-3 is an executioner caspase, but we also show that its full-length and an intermediate form are detected in bVAD-fmk-pretreated cells. This suggests that caspase-3 may also be involved in the mechanism of HS + TRAIL apoptosis initiation.
Discussion
We have previously shown that, in leukemic lymphocytes, HS induces a strong and caspase-dependent stimulation of TRAIL-mediated apoptosis. This phenomenon resulted from cell surface modifications enhancing TRAIL receptors recognition by the cytokine TRAIL (Moulin and Arrigo 2006; Moulin et al. 2007b). Our interest here was to search for the apical proteolytic signal that triggers the intense apoptosis induced by TRAIL in HS-treated cells. In this respect, the recent reports concerning the apical signaling leading to heat-induced cell death are contradictory. On one hand, the caspase initiator caspase-2 has been suggested to act as a proximal mediator of HS-induced apoptosis (Tu et al. 2006), and on the other hand, a novel apical protease bearing caspase-like activity has been suggested (Milleron and Bratton 2006). In the present study and as an approach to better understand which proteolytic event is activated first, we focused on caspase activation upon the combined treatment vs TRAIL treatment alone. As a first step of the analysis, we have determined the hierarchical order of the processing caspase events in Jurkat cells exposed to HS + TRAIL death stimulation. Unexpectedly, a very early event that characterizes the HS + TRAIL stimulus is the activation of the executioner caspase-3 together with the initiator caspase-2 and caspase-8. Caspase-9, implicated in the apoptosome formation, is activated subsequently. These results confirm that the mitochondrial pathway does not seem essential to the HS + TRAIL signaling of apoptosis, as we have previously shown that Bcl-2 overexpression only partially blocked death induced by the cotreatment (Moulin et al. 2007b).
We then used siRNAs to down regulate caspases levels in cells. The observations were that caspase-2 or caspase-9 silencing had no effect on the apoptotic events induced by either TRAIL or HS + TRAIL cotreatment. As expected, the siRNAs targeting caspase-8 strongly protected cells from HS + TRAIL-induced apoptosis. This is in accordance with our previous observations using caspase-8-deficient Jurkat cell lines (Moulin and Arrigo 2006). It is clearly established that caspase-8 is the initiator caspase upon death receptor stimulation whether it be Fas or TRAIL (Walczak and Krammer 2000). In spite of the fact that caspase-2 has been found in the DISC induced by Fas, this caspase does not appear to play an initiating role in Fas death signaling (Lavrik et al. 2006). It is of interest that, using an siRNA approach, we were unable to detect a role of caspase-2 in the death treatment induced by HS + TRAIL. Indeed, we observed that the cosilencing of caspase-2 and -8 did not increase the death protection generated by caspase-8 silencing alone. Caspase-3 silencing was effective but to a lesser degree than caspase-8 silencing, a result probably due to the poor silencing efficiency that was obtained using siRNAs targeting caspase-3.
Using caspase inhibitors, we concluded that 10 μM of the pan caspase inhibitor zVAD completely inhibited the processing of all caspases, as well as the cell death induced by the HS + TRAIL combined treatment. In the presence of caspase-2, -3, -8, and -9 inhibitors, some protection against HS + TRAIL induced cell death, particularly in the case of caspase-8 inhibitor, was observed, but at least 30 μM of the different caspase inhibitors had to be used. Hence, we cannot exclude some nonspecificity of these inhibitors when they are used at such high concentrations (Bang et al. 2004). Analysis of the cleaved fragments revealed that the processing of the different caspases was not completely blocked. An unexpected finding was that the inhibition of the processing of caspase-3 induced by DEVD-fmk caspase-3 inhibitor, as well as by the other caspase inhibitors, was different after TRAIL or HS + TRAIL treatment. The normal cleavage of pro-caspase-3 by an initiator caspase is at Asp175, leading to two caspase-3 subunits p20 and p12. The p20 subunit still contains the prodomain that is removed by Asp28 cleavage leading to the p17 subunit (Han et al. 1997). Such p17/p12 subunits were observed in response to TRAIL and HS + TRAIL treatments. However, this process was not detected in the presence of caspase inhibitors and was replaced by two intermediate caspase-3 forms that displayed different levels depending on the inhibitor and the HS or HS + TRAIL conditions that were used. Unusual caspase-3 fragments have been previously reported (Deveraux et al. 1998; Martin and Panja 2002; Martin et al. 1996), but the proteases responsible for this processing are still unknown.
Using in situ trapping of caspase initiators by pretreatment with b-VAD-fmk, we observed the full-length pro-caspase-2, suggesting that this caspase participates early during the HS + TRAIL activation process. However, its presence may not be essential because depletion by an RNAi approach was devoid of effect. We also detected the p18 cleaved form of caspase-8. This observation concerning the detection of the cleaved form of caspase-8 is reminiscent of controversial studies performed in Jurkat cells exposed to Fas (Mohr and Zwacka 2007; Tu et al. 2006). For example, Mohr and Zwacka (2007) have shown that processed forms of initiator caspase-8 rather than its full length, has described by Tu et al. 2006, were precipitated with this method. The divergent results concerning the caspase initiator trapped by bVAD-fmk could not be explained by the cell lines used (both with Jurkat) but may have arisen from the type of Fas reagent used; in one case it was CH11 agonist antibody and in the other case it was Fas-ligand. More surprising was the finding by in situ trapping of the presence of the caspase-3 pro- and intermediate forms in response to HS + TRAIL treatment. These observations suggest that this polypeptide has an initiator role and accumulates when further processing by other caspases is inhibited. Of interest, Yang et al. (2006) have already suggested an apical role for caspase-3 (Yang et al. 2006).
Hence, we have examined caspase implication in HS + TRAIL-induced apoptosis. We showed, using siRNA method, in situ trapping, and caspase inhibitors, that caspase-8 is essential in the death stimulation mediated by the combined treatment. Moreover, our findings favor the hypothesis that caspase-3 could play an apical role through a particular processing by an unknown protease.
Acknowledgments
We would like to thank Dominique Guillet for excellent technical assistance. We wish to thank Dr. Delphine Cuchet (Lyon, France) for helpful advice with the siRNA experiments and for providing control sequence (siNS).
Abbreviations
- TRAIL
TNF-related apoptosis-inducing ligand
- FADD
Fas-associated death domain
- DISC
death signaling inducing complex
- FlipL
long forms of FADD-like ICE inhibitory protein
- TNF
tumor necrosis factor
- Hsp
heat shock protein
- HS
heat shock
- PI
propidium iodide
- siNS
siRNA nonsilencing sequence
- z-VAD-fmk
z-Val-Ala-Asp-fluoromethylketone
Footnotes
Grant support: The Région Rhône-Alpes (Thématique Prioritaire Cancer and Cible 06).
References
- Allan LA, Clarke PR (2007) Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell 26(2):301–310 [DOI] [PubMed]
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J (1996) Human ICE/CED-3 protease nomenclature. Cell 87:171 [DOI] [PubMed]
- Bang B, Baadsgaard O, Skov L, Jaattela M (2004) Inhibitors of cysteine cathepsin and calpain do not prevent ultraviolet-B-induced apoptosis in human keratinocytes and HeLa cells. Arch Dermatol Res 296:67–73 [DOI] [PubMed]
- Beere HM (2004) “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci 117:2641–2651 [DOI] [PubMed]
- Callus BA, Vaux DL (2007) Caspase inhibitors: viral, cellular and chemical. Cell Death Differ 14:73–78 [DOI] [PubMed]
- Carlile GW, Smith H, Weidman M (2004) Caspase-3 has a non apoptotic function in erythroid maturation. Blood 103:4310–4316 [DOI] [PubMed]
- Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel R, Dale JK, Puck J, Davis J, Hall CG, Skoda-Smith S, Atkinson P, Straus SE, Lenardo MJ (2002) Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature 419:395–399 [DOI] [PubMed]
- Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC (1998) IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J 17:2215–2223 [DOI] [PMC free article] [PubMed]
- Ekert PG, Silke J, Vaux DL (1999) Caspase inhibitors. Cell Death Differ 6:1081–1086 [DOI] [PubMed]
- Fernandes-Alnemri T, Litwack G, Alnemri ES (1994) CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem 269:30761–30764 [PubMed]
- Gerner EW, Schneider MJ (1975) Induced thermal resistance in HeLa cells. Nature 256:500–502 [DOI] [PubMed]
- Han Z, Hendrickson EA, Bremner TA, Wyche JH (1997) A sequential two-step mechanism for the production of the mature p17:p12 form of caspase-3 in vitro. J Biol Chem 272:13432–13436 [DOI] [PubMed]
- Ho PK, Hawkins CJ (2005) Mammalian initiator apoptotic caspases. FEBS J 272:5436–5453 [DOI] [PubMed]
- Kampinga HH (2006) Cell biological effects of hyperthermia alone or combined with radiation or drugs: a short introduction to newcomers in the field. Int J Hypertherm 22:191–196 [DOI] [PubMed]
- Lassus P, Opitz-Araya X, Lazebnik Y (2002) Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science 297:1352–1354 [DOI] [PubMed]
- Lavrik IN, Golks A, Baumann S, Krammer PH (2006) Caspase-2 is activated at the CD95 death-inducing signaling complex in the course of CD95-induced apoptosis. Blood 108:559–565 [DOI] [PubMed]
- Lavrik IN, Golks A, Krammer PH (2005) Caspases: pharmacological manipulation of cell death. J Clin Invest 115:2665–2672 [DOI] [PMC free article] [PubMed]
- Lindquist S (1986) The heat-shock response. Annu Rev Biochem 55:1151–1191 [DOI] [PubMed]
- Manero F, Ljubic-Thibal V, Moulin M, Goutagny N, Yvin JC, Arrigo AP (2004) Stimulation of Fas agonistic antibody-mediated apoptosis by heparin-like agents suppresses Hsp27 but not Bcl-2 protective activity. Cell Stress Chaperones 9:150–166 [DOI] [PMC free article] [PubMed]
- Martin CA, Panja A (2002) Cytokine regulation of human intestinal primary epithelial cell susceptibility to Fas-mediated apoptosis. Am J Physiol Gasterointest Liver Physiol 282:G92–G104 [DOI] [PubMed]
- Martin SJ, Amarante-Mendes GP, Shi L, Chuang TH, Casiano CA, O’Brien GA, Fitzgerald P, Tan EM, Bokoch GM, Greenberg AH, Green DR (1996) The cytotoxic cell protease granzyme B initiates apoptosis in a cell-free system by proteolytic processing and activation of the ICE/CED-3 family protease, CPP32, via a novel two-step mechanism. EMBO J 15:2407–2416 [PMC free article] [PubMed]
- Milleron RS, Bratton SB (2006) Heat shock induces apoptosis independently of any known initiator caspase-activating complex. J Biol Chem 281:16991–17000 [DOI] [PubMed]
- Milleron RS, Bratton SB (2007) ‘Heated’ debates in apoptosis. Cell Mol Life Sci 64:2329–2333 [DOI] [PMC free article] [PubMed]
- Mohr A, Zwacka RM (2007) In situ trapping of initiator caspases reveals intermediate surprises. Cell Biol Int 31:526–530 [DOI] [PubMed]
- Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12:3788–3796 [DOI] [PubMed]
- Moulin M, Arrigo AP (2006) Long lasting heat shock stimulation of TRAIL-induced apoptosis in transformed T lymphocytes. Exp Cell Res 312:1765–1784 [DOI] [PubMed]
- Moulin M, Carpentier S, Levade T, Arrigo AP (2007a) Potential roles of membrane fluidity and ceramide up-regulation in the mechanism of hyperthermia- and alcohol-stimulated TRAIL apoptosis. Apoptosis 12:1703–1720 [DOI] [PubMed]
- Moulin M, Dumontet C, Arrigo AP (2007b) Sensitization of chronic lymphocytic leukemia cells to TRAIL-induced apoptosis by hyperthermia. Cancer Lett 250:117–127 [DOI] [PubMed]
- Nagy E, Balogi Z, Gombos I, Akerfelt M, Bjorkbom A, Balogh G, Torok Z, Maslyanko A, Fiszer-Kierzkowska A, Lisowska K, Slotte PJ, Sistonen L, Horvath I, Vigh L (2007) Hyperfluidization-coupled membrane microdomain reorganization is linked to activation of the heat shock response in a murine melanoma cell line. Proc Natl Acad Sci U S A 104:7945–7950 [DOI] [PMC free article] [PubMed]
- Shin S, Lee Y, Kim W, Ko H, Choi H, Kim K (2005) Caspase-2 primes cancer cells for TRAIL-mediated apoptosis by processing procaspase-8. EMBO J 24:3532–3542 [DOI] [PMC free article] [PubMed]
- Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J et al (1992) A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature 356:768–774 [DOI] [PubMed]
- Timmer JC, Salvesen GS (2007) Caspase substrates. Cell Death Differ 14:66–72 [DOI] [PubMed]
- Tinel A, Tschopp J (2004) The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science 304:843–846 [DOI] [PubMed]
- Tu S, McStay GP, Boucher LM, Mak T, Beere HM, Green DR (2006) In situ trapping of activated initiator caspases reveals a role for caspase-2 in heat shock-induced apoptosis. Nat Cell Biol 8:72–77 [DOI] [PubMed]
- Vigh L, Horvath I, Maresca B, Harwood JL (2007) Can the stress protein response be controlled by ‘membrane-lipid therapy’. Trends Biochem Sci 32:357–363 [DOI] [PubMed]
- Wagner KW, Engels IH, Deveraux QL (2004) Caspase-2 can function upstream of bid cleavage in the trail apoptosis pathway. J Biol Chem 279:35047–35052 [DOI] [PubMed]
- Walczak H, Krammer PH (2000) The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp Cell Res 256:58–66 [DOI] [PubMed]
- Wurzer WJ, Planz O, Ehrhardt C, Giner M, Silberzahn T, Pleschka S, Ludwig S (2003) Caspase-3 activation is essential for efficient influenza virus propagation. EMBO J 22:2717–2728 [DOI] [PMC free article] [PubMed]
- Yang S, Thor AD, Edgerton S, Yang X (2006) Caspase-3 mediated feedback activation of apical caspases in doxorubicin and TNF-alpha induced apoptosis. Apoptosis 11:1987–1997 [DOI] [PubMed]