Abstract
The papillomaviruses are small DNA viruses that encode approximately eight genes, and require the host cell DNA replication machinery for their viral DNA replication. Thus papillomaviruses have evolved strategies to induce host cell DNA synthesis balanced with strategies to protect the cell from unscheduled replication. While the papillomavirus E1 and E2 genes are directly involved in viral replication by binding to and unwinding the origin of replication, the E6 and E7 proteins have auxillary functions that promote proliferation. As a consequence of disrupting the normal checkpoints that regulate cell cycle entry and progression, the E6 and E7 proteins play a key role in the oncogenic properties of human papillomaviruses with a high risk of causing anogenital cancers (HR HPVs). As a consequence, E6 and E7 of HR HPVs are invariably expressed in cervical cancers. This article will focus on the E6 protein and its numerous activities including inactivating p53, blocking apoptosis, activating telomerase, disrupting cell adhesion, polarity and epithelial differentiation, altering transcription and reducing immune recognition.
Structure of E6
All papillomaviruses encode an E6 open reading frame immediately downstream of the noncoding region (NCR). E6 proteins are approximately 150 amino acids containing two CX2C-X29-CX2C zinc-like fingers joined by an interdomain linker of 36 amino acids, flanked by short amino (N) and carboxy (C) terminal domains of variable lengths. The E7 protein contains a similarly spaced zinc-like finger leading to the hypothesis that the E6 and E7 genes may have arisen from duplication events of one of these core motifs (Cole and Danos, 1987). A number of papillomavirus E6 and E7 proteins have been shown to bind zinc through the coordination of the cysteine residues (Barbosa, Lowy, and Schiller, 1989; Grossman and Laimins, 1989). Attempts to obtain the crystal structure of E6 have been plagued by the tendancy of E6 to form insoluble aggregates. Recently the solution structure of the C-terminal half of HPV 16E6 was solved by nuclear magnetic resonance (NMR), and a model for the whole protein was proposed (Figure 1) (Nomine et al., 2006). The two zinc finger domains face each other symmetrically in a pseudodimeric fashion with the interdomain forming a helix that contributes to the rigidity of the two domains. Each domain consists of a three stranded beta sheet (S1, S2, S3) and two short helices (H1, H2). The N and C terminal domains are of variable lengths and sequences. The proposed structure indicated that many of the mutations that have been made to map functions of E6 likely disrupted the overall structure, rather than specific protein-protein interactions.
Figure 1.
The Pseudodimeric Model of E6 (taken from Nominé et al, 2006). (A) E6N model and E6C structure positioned symmetrically with exposed hydrophobic patches facing each other. (B) Proposed pseudodimeric arrangement of E6N and E6C. The gray closed line indicates the DNA binding region of E6C, mapped in the present work. (C) Contact map of hydrophobic residues involved in the interface of the pseudodimeric model. (D) Position of the three flanking regions not predicted by the model.(E) A view of the core model highlighting surface residues conserved in all E6 proteins. Previously mutated residues are indicated with underlined red labels.
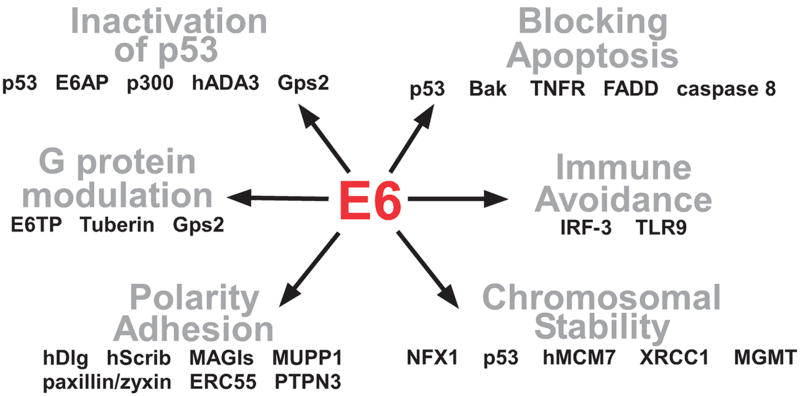
Although E6 may dimerize at high salt and protein concentrations, it is thought to be monomeric at physiologic conditions (Lipari et al., 2001). It has been difficult to study the expression of endogenous E6 proteins as they are expressed at low levels and few sensitive antibodies exist, however E6 is thought to be largely nuclear, though some fraction of E6 may also be cytoplasmic (Lowy and Howley, 2001). Spliced isoforms have been reported for the high risk E6 genes, that would give rise to truncated E6 proteins denoted as E6*, encoding residues 1- 41 of 16E6 (Pim and Banks, 1999). E6* inactivates the functions of full-length E6, which is proposed to be due to its ability to bind to the interface of the N- and C-terminal halves of E6 (Nomine et al., 2006). No enzymatic activities have been reported for E6, and although the HR E6 proteins have been reported to bind specifically to four-way DNA junctions (Ristriani et al., 2001), most of the activities of E6 are thought to be mediated by protein-protein interactions. A summary of the interactions of HR HPV E6 proteins with other proteins and the pathways they impinge upon are shown in Figure 2.
Figure 2.
Binding Partners of E6. E6 alters numerous cellular pathways through the binding of other proteins.
E6 binding motifs
The first protein that was shown to interact with E6 was E6 associated protein (E6AP), an E3 ubiquitin ligase (Huibregtse, Scheffner, and Howley, 1991; Scheffner et al., 1993). The ubiquitin cascade functions to target proteins for proteasomal degradation by means of adding multiple ubiquitin monomers to the protein destined to be destroyed. E1 proteins activate the ubiquitin monomers that are subsequently transferred by E2 conjugating enzymes to an E3 ubiquitin ligase, which confers target specificity (for review see Hershko and Ciechanover, 1992). E6AP is the founding member of the HECT-domain family of ubiquitin ligases, a group of related proteins with homology to E6AP C-terminal (HECT) domain involved in ubiquitination of bound substrates, and divergent N-termini that mediate substrate specificity (Schwarz, Rosa, and Scheffner, 1998). E6AP forms a complex with both E6 and target proteins leading to ubiquitination of the target protein and subsequent proteasome mediated degradation (Scheffner et al., 1993). The most well studied E6/E6AP target is the p53 tumor suppressor (discussed below), though other proteins are also targeted by this complex. Numerous studies have identified residues of the E6 protein that diminishes binding to E6AP (Crook, Tidy, and Vousden, 1991; Gewin et al., 2004; Liu et al., 1999b), but recently most of these mutations have been predicted to disrupt the global integrity of E6 (Nomine et al., 2006). Moreover, mutations of numerous surface exposed residues of E6 did not eliminate its binding to E6AP. However, the motif on E6AP that binds E6 has been extensively characterized (Be et al., 2001; Chen et al., 1998; Elston, Napthine, and Doorbar, 1998) and is referred to as an LXXLL motif. A number of other proteins also bind to E6 by way of LXXLL motif including, ERC-55 (E6BP), IRF3, paxillin and tuberin (Chen et al., 1998; Elston, Napthine, and Doorbar, 1998; Ronco et al., 1998; Tong and Howley, 1997). These motifs are leucine-rich amphipathic helices with limited substitution of hydrophobic residues for leucines and at least one negative charge in an X position. The sequence of E6AP that interacts with E6 is ELQELLGE. Binding to proteins with an LXXLL motif is a conserved property of the E6 proteins of numerous papillomaviruses, as E6AP binds to both high and low risk genus alpha HPVs, at least some genus beta HPVs and bovine papillomavirus type 1 (Bedard et al., 2008; Chen et al., 1998; Kuballa, Matentzoglu, and Scheffner, 2007).
HR HPV E6 proteins all have a motif designated as XT/SXV at their C-termini. This motif on the E6 protein mediates binding to specific domains on cellular proteins known as PDZ proteins. PDZ domains are approximately 90 amino acid stretches found in a wide variety of proteins and are named after the first letters of three proteins which were first discovered to share the domain; post synaptic density protein (PSD95), Drosophila disc large tumor suppressor (DlgA), and zonula occludens-1 protein (ZO-1). Among the PDZ proteins reported to bind E6 are hDlg1 and hDlg4, human homologs of Dlg (Kiyono et al., 1997; Lee, Weiss, and Javier, 1997); hScrib, a homolog of the Drosophilia scribble protein (Nakagawa and Huibregtse, 2000); MAGI 1, MAGI 2 and MAGI 3, Membrane Associated Guanylate kinase homology proteins with an Inverted domain structure (Glaunsinger et al., 2000; Thomas et al., 2001); MUPP1, a multi PDZ protein (Lee et al., 2000); and PTPN3, a membrane associated tyrosine phosphatase (Jing et al., 2007; Spanos et al., 2008b). Mutational analyses have demonstrated the importance of the XS/TV motif in binding, but it is possible that other residues of E6 also bind and confer specificity for which PDZ proteins and which PDZ domains are bound. The concurrent binding of E6 to E6AP and to PDZ proteins can target the PDZ proteins for degradation, though other ubiquitin ligases have also been implicated (Grm and Banks, 2004; Sterlinko et al., 2004).
Other proteins such as p53, Bak, p300/CBP, hADA3, NFX1, Gps2, FADD, and procaspase 8 have been reported to bind various E6 proteins, but they lack both LXXLL and PDZ domains (Degenhardt and Silverstein, 2001b; Filippova et al., 2007; Filippova, Parkhurst, and Duerksen-Hughes, 2004; Gewin et al., 2004; Kumar et al., 2002; Patel et al., 1999; Thomas and Banks, 1999; Zimmermann et al., 2000). They may bind to E6 through as yet undefined motifs, or indirectly through binding to E6AP or other E6 associated proteins.
Inactivation of p53
One of the most well-studied interacting proteins of E6 is the p53 tumor suppressor, a DNA site-specific transcription factor, and one of the key signaling coordinators in the cell following genotoxic or cytotoxic stress (for review see Murray-Zmijewski, Slee, and Lu, 2008). Normally present in low levels and transcriptionally inactive, cellular damage triggers an increase in p53 protein levels and activation via post-translational modifications. Once activated, p53 functions to initiate pathways for DNA repair, cell cycle arrest and/or apoptosis, based upon the type and extent of damage. The importance of p53 in orchestrating the cellular response to cytotoxic agents is exemplified by the observation that approximately one-half of all human cancers harbor mutations in the p53 gene. These mutations impair the ability of p53 to trigger the appropriate signaling pathways to repair the damage or trigger cell death in cases where the damage is beyond repair. This in turn allows for replication of damaged DNA, and survival of cells with deleterious mutations that would normally be eliminated.
Importantly, in addition to genotoxic and cytotoxic damage, p53 is also activated upon improper stimulation of DNA synthesis, such as that induced by HPV infection. Both HR and LR HPVs infect cells in the basal layer of the epithelium, however viral replication occurs only in differentiating cells - which would have normally exited the cell cycle and turned off their cellular DNA synthesis machinery (Doorbar et al., 1997). HPVs must therefore stimulate DNA synthesis in these cells, while concomitantly inhibiting the normal cellular response of activating p53. Importantly, unlike most other cancer cell types, cervical cancers generally harbor wild-type p53 (Crook, Wrede, and Vousden, 1991; Scheffner et al., 1991). HPVs have evolved a number of mechanisms to block p53 function in the infected cell through the actions of the E6 oncoprotein. In this way our understanding of E6 highlights the functions of p53 in normal cells, and demonstrates the mechanisms by which a regulatory checkpoint can be countered during viral infection and/or cancer progression via protein degradation, mislocalization and modification.
The principle mechanism by which HR HPVs inactivate p53 is by inducing its degradation through the ubiquitin-proteasome pathway (Scheffner et al., 1990). Normally p53 protein levels are regulated by the Mdm2 E3 ubiquitin ligase (Honda, Tanaka, and Yasuda, 1997). However Mdm2-mediated degradation of p53 is inhibited during viral infection and other stress conditions, allowing for stabilization of p53 protein levels and subsequent activation (for review see Ashcroft and Vousden, 1999). Instead, HR HPV E6 induces p53 degradation by forming a complex with another E3 ubiquitin ligase, E6AP (Huibregtse, Scheffner, and Howley, 1991; Huibregtse, Scheffner, and Howley, 1993a; Scheffner et al., 1993).
E6AP per se, is unable to bind p53 and induce its degradation. Instead, E6 must first bind to the N-terminal substrate recognition domain of E6AP before p53 can be bound and ubiquitinated (Huibregtse, Scheffner, and Howley, 1993a; Huibregtse, Scheffner, and Howley, 1993b). Interestingly, both HR and LR HPV E6 proteins have been shown to interact with p53, however they differ with respect to the domains of p53 with which they interact. While both HR and LR E6 proteins can bind to the p53 C-terminus, only HR E6 proteins are capable of binding to the core region of p53. It is this binding of the core region that is required for p53 degradation by HR E6 (Crook, Tidy, and Vousden, 1991; Li and Coffino, 1996).
Although stimulation of p53 degradation mediates a considerable block to p53 function, not all of the p53 within E6-expressing cells is degraded (Cooper et al., 1993; Lechner et al., 1992; Lie et al., 1999; Mantovani and Banks, 1999). Moreover, as mentioned above, the LR genus alpha HPV, and the genus beta HPV E6 proteins do not degrade p53, and therefore have evolved different mechanisms of circumventing p53 growth suppression. In part, infection with these papillomaviruses may induce a p53 response less robustly than the HR HPVs. Additionally, some assays have shown that expression of both HR and LR E6 proteins can abolish p53-mediated transcriptional activity (Crook et al., 1994; Giampieri et al., 2004; Lechner et al., 1992; Mietz et al., 1992). It has been shown that E6 can mediate these effects through a number of mechanisms, including inhibition of p53 binding, aberrant p53 localization, and post-translational modifications of the p53 protein.
First, it has been shown that E6 interaction with p53 can inhibit the binding of p53 to its site-specific DNA sequences (Lechner and Laimins, 1994; Thomas et al., 1995). The level of inhibition was found to correlate with the affinity that each E6 protein has for p53; thus 16E6 shows the highest level of site specific binding inhibition, 31E6 and 18E6 show intermediate levels of inhibition, and 11E6 shows the least, albeit still detectable inhibitory effect (Lechner and Laimins, 1994). Moreover, it was later shown that E6 association with p53 can induce a conformational change in the p53 protein, which in turn leads to an inhibition of p53 binding to DNA, or a dissociation between p53-DNA complexes that have already been formed (Thomas et al., 1995). Importantly, these inhibitory effects were shown to correlate with the ability of different E6 proteins to inhibit p53 transactivation, and were shown to be independent of E6/E6AP-mediated p53 degradation.
The second proposed mechanism by which E6 may be able to inhibit p53 signaling independent of protein degradation is by means of sequestration of p53 in the cytoplasm. This has been hypothesized to be a result of either masking of the nuclear localization signal (NLS) on the p53 C-terminus due to E6 binding of p53, or an enhancement of p53 nuclear export (see Mantovani and Banks, 2001). As both HR and LR E6 proteins are able to bind to the C-terminus of p53, masking the p53 NLS is an attractive possibility. Evidence for E6-mediated mislocalization of p53 has been demonstrated from experiments using cervical cell lines in which E6-mediated p53 degradation was blocked. In these cells, even though p53 levels returned to normal, correct nuclear localization of p53 was perturbed (Mantovani and Banks, 1999). Further investigation will be required to determine if either of these mechanisms play a role in E6-mediated mislocalization of p53.
The third mechanism employed by E6 to inhibit p53 activity is its abrogation of the transactivation of p53 responsive genes via interaction with either the CBP/p300 (Patel et al., 1999; Zimmermann et al., 2000) or hADA3 (Kumar et al., 2002) histone acetyltransferases. Following DNA damage, p300 is known to acetylate p53, thus enhancing its ability to bind to specific DNA sequences in the promoters of p53-responsive genes, and in turn upregulating their transcription (Barlev et al., 2001; Gu and Roeder, 1997; Ito et al., 2001; Liu et al., 1999a; Yuan et al., 1999). The E6 proteins have been shown to bind to p300, and this interaction inhibits p53 acetylation at p300-dependent sites, leading to decreased expression from a p53 responsive luciferase reporter (Patel et al., 1999; Zimmermann et al., 2000). Both HR and LR E6 proteins have been shown to bind to p300, although the HR E6 proteins appear to bind with a higher affinity. In-vivo studies have reported that only the HR E6 proteins prevent p300 transactivation of p53 responsive genes (Patel et al., 1999; Zimmermann et al., 2000); however an in-vitro analysis using chromatinized templates demonstrated that LR HPV 11E6 was also capable of inhibiting p53 transactivation (Thomas and Chiang, 2005). This study also demonstrated that while E6 mutants deficient in E6AP binding were capable of inhibiting p53, mutants defective in binding to either p53 or p300 were unable to elicit this response. Thus a complex between E6, p53 and p300 appears to be required to block transactivation.
Similarly, HR E6 proteins are also able to block p53 activation by interacting with another histone acetyltransferasere, hADA3 (Kumar et al., 2002). hADA3 is the human homolog of the yeast transcriptional activator yADA3, which functions as an essential component of the ADA transcriptional coactivator complex (Pina et al., 1993). However, unlike with p300, E6 interaction with hADA3 results in hADA3 degradation (Kumar et al., 2002). This degradation has been shown to abrogate both p53- and retinoic X receptor (RXR) alpha-mediated transactivation (Kumar et al., 2002; Zeng et al., 2002).
Finally, recent evidence suggests that E6 may also inhibit p53 activation by blocking the p14/ARF pathway (Shamanin and Androphy, 2004; Shamanin, Sekaric, and Androphy, 2008). Following most cellular stresses, p53 is activated due to cellular signals that prevent its interaction with and degradation by Mdm2 (see above). However, p53 can also be activated during “oncogenic stress” by a mechanism involving direct inhibition of Mdm2 ubiquitin ligase activity through its association with ARF (Matheu, Maraver, and Serrano, 2008). 16E6 has been shown to inhibit p14/ARF dependent activation of p53 without inactivation of the p53-dependent DNA damage response, and in a manner that is not dependent on E6-mediated p53 degradation (Shamanin and Androphy, 2004). Interestingly, one mechanism by which this has been proposed to be facilitated is through the degradation of hADA3 (Shamanin, Sekaric, and Androphy, 2008).
Modulation of G-protein signaling
As described above, E6 is able to modulate transcription of p53-dependent genes either by degradation of p53 or via interaction with the p300 and hADA3 transactivators. In addition, yeast two-hybrid experiments from a number of groups have demonstrated that E6 is able to modulate transcription from other cellular signaling pathways as well, highlighting the ability of the E6 oncoprotein to act as a diverse modulator of host cell signaling. With respect to G-protein signaling, E6 has been shown to interact with three different proteins. First, HR E6 was shown to bind and degrade a novel protein termed E6-targeted protein 1 (E6TP1) in an E6AP dependent manner (Gao et al., 2002; Gao et al., 1999; Lee, Wooldridge, and Laimins, 2007). E6TP1 has homology to GTPase activating proteins (GAPs) for Rap (Gao et al., 1999), and recent experiments demonstrated that E6TP1 does indeed harbor GAP activity for Rap1 and Rap2 (Singh et al., 2003). Importantly, E6 mediated degradation of E6TP1 enhances the GTP-loading of RAP, thus supporting a role for the RAP small G-protien pathway in E6-mediated oncogenesis (Singh et al., 2003). Another protein with GAP activity, tuberin, was also shown to be bound and degraded by E6 (Lu et al., 2004; Zheng et al., 2008). Tuberin functions in the harmatin-tuberin complex, which exhibits GAP activity toward the small G-protein Rheb, and the complex is a critical negative regulator of mTOR signaling (reviewed in Huang and Manning, 2008). As this pathway is an important mediator of cell growth, it highlights yet another mechanism by which E6 can modulate the regulation of such processes. Finally, E6 from both HR and LR HPVs was shown to bind and degrade Gps2 (Degenhardt and Silverstein, 2001b). Gps2 is involved in suppressing G-protein mediated signaling pathways (Spain et al., 1996), c-Jun N-terminal kinase activity (Jin et al., 1997), and known to stimulate transcriptional activation by the BPV1 E2 protein (Breiding et al., 1997). As seen with BPV, Gps2 was found to stimulate transcription from HPV promoters (Degenhardt and Silverstein, 2001b). Moreover, HR (but not LR) E6-mediated degradation of Gps2 was shown to suppress this transactivation of the HPV early promoter. As Gps2 is known to interact with and positively regulate p300 (Peng et al., 2000), the implications of Gps2 degradation may extend beyond that of transcriptional control of HPV encoded genes, however this remains to be determined.
Modulation of Immune Recognition
E6 has also been shown to modulate transcription of genes whose protein products are involved in innate immunity. HR E6 has been shown to interact with two proteins that are part of the innate immune response to viral infection; Interferon regulatory factor-3 (IFR-3) (Ronco et al., 1998) and Toll-like receptor 9 (TLR9) (Hasan et al., 2007). IRF-3 becomes activated by dsRNA or viral infection, and this activation leads to transcription of Interferon-β (IFN-β) (for review see Hiscott, 2007). 16E6 interaction with IRF-3 has been shown to inhibit its transactivation ability, and thus prevents the induction of IFN-β following viral infection (Ronco et al., 1998). TLR9 becomes activated by viral or bacterial dsDNA derived CpG motifs, and induces cytokine production as a means to defend the cell against the invading organism (Muller, Hamm, and Bauer, 2008). Exogenous expression of 16E6/E7 has been shown to inhibit TLR9 transcription, leading to a functional loss of TLR9 signaling pathways within the cell (Hasan et al., 2007). Similar results were seen in the HPV 16 positive cervical carcinoma cell lines CaSki, SiHa and HeLa, demonstrating that this may indeed contribute to HPV-mediated carcinogenesis.
Blocking apoptosis
One of the main consequences of E6 degrading or blocking p53 function is to inhibit apoptotic signaling that would otherwise eliminate the HPV infected cell. However, p53 independent apoptotic signals can also be employed to eliminate abnormal cells, and E6 has been shown to block apoptosis in cells and mice lacking p53 (Pan and Griep, 1995; Steller et al., 1996). There are two major apoptotic pathways that can be triggered by different stresses – the extrinsic pathway, and the intrinsic pathway (Figure 3). Interestingly, the E6 protein has been shown to disrupt both pathways to facilitate a cytoprotective environment and prevent cell death, thus highlighting the critical signaling events that a cell undergoes following exogenous or endogenous stress.
Figure 3.
Extrinsic and Intrinsic Apoptotic Pathways. The extrinsic pathway is activated through trimerization of death receptors, followed by recruitment of adapter molecules and pro-caspase 8 to form the DISC. Subsequent cleavage of caspase 8 leads to cleavage of downstream executioner caspases, caspase 3 and caspase 7, and subsequent cell death. E6 can block death receptor association with adapter molecules (1) and can induce the degradation of certain adapter molecules as well as caspase 8 (2). Activation of the intrinsic pathway by “intrinsic” cell stresses leads to the formation of pores in the mitochondrial membrane, and subsequent release of mitochondrial inner membrane proteins into the cytosol. These proteins, along with pro-caspase 9 form the apoptosome, which cleaves caspase 9. Activated caspase 9 then goes on to cleave the downstream executioner caspases 3 and 7, leading to cell death. E6 has been shown to degrade Bak, and thus block the release of the mitochondrial inner membrane proteins into the cytosol (3). E6 is also able to inhibit IAPs and thus block the apoptosome and cleavage of the exectutioner caspases (4).
The extrinsic apoptotic signaling pathway can be induced during viral infection as part of the host response, and is triggered by extracellular signals that induce the activation of “death receptors” on the cell surface (for review see Gewies, 2003). These receptors are members of the tumor necrosis factor receptor (TNFR) family and include TNF receptor 1 (TNFR-1), Fas/CD95 and the TNF-related apoptosis inducing ligand (TRAIL) receptors DR-4 and DR-5. Upon binding to their cognate ligand (TNF, Fas-L or TRAIL), these receptors trimerize and recruit adapter molecules such as FADD, TRADD and initiator caspases such as caspase-8 and caspase-10 to their cytoplasmic death domains (DD), thus forming the death inducing signaling complex (DISC). The DISC is involved in activating caspase 8, which in turn cleaves and activates downstream executioner caspases (caspase 3 and caspase 7). The executioner caspases target and cleave downstream substrates such as poly(ADP-ribose) polymerase (PARP) and Lamin B.
E6 has been shown to inhibit extrinsic apoptotic signaling at each of the early stages, by interacting with TNFR-1, FADD and caspase-8. 16E6 was shown to directly bind to the death receptor TNFR-1, an interaction that inhibited TNFR-1 association with the TNF R1-associated death domain (TRADD) adapter molecule, and blocked TNFR-1 DD mediated apoptosis (Filippova et al., 2002) (Figure 3 #1). Importantly, 16E6 has also been shown to block apoptosis normally triggered by TNF, the cognate ligand for TNFR-1 (Duerksen-Hughes, Yang, and Schwartz, 1999). In addition to the TNF pathway, it has also been shown that 16E6 is capable of inhibiting apoptosis stimulated by both the Fas and the TRAIL pathways. This inhibition is mediated by E6 binding to and degradation of both the FADD adapter protein and the effector caspase, caspase-8 (Filippova, Parkhurst, and Duerksen-Hughes, 2004; Garnett, Filippova, and Duerksen-Hughes, 2006) (Figure 3 #2). As FADD and caspase-8 are key components to apoptotic signaling through all of the death receptors, this mechanism of cytoprotection demonstrates how the E6 oncoprotein is able to exploit one or two proteins as a means to block multiple signaling pathways. Importantly, these effects were all seen using the HR 16E6 oncoprotein, and it is unknown whether LR or genus beta HPV E6 proteins function in a similar manner with respect to blocking extrinsic apoptotic pathways. As the binding of FADD is not dependent on the conserved PDZ domain of HR E6, but rather a novel domain (Tungteakkhun et al., 2008), it is possible that other E6 proteins may inhibit these extrinsic pathways.
The intrinsic apoptotic pathway is involved in sensing apoptotic signals that originate from within the cell, such as DNA damage, oxidative stress, starvation and those mediated by chemotherapeutic drugs (Gewies, 2003; Kaufmann and Earnshaw, 2000; Wang, 2001). These stresses activate a number of pathways that converge on the mitochondria, which acts as a hub to sense the balance of pro- and anti-apoptotic signals and facilitate downstream apoptotic signaling when this balance is upset. When the cell senses intrinsic stress, pro-apoptotic BH3-only proteins become activated and abrogate the function of anti-apoptotic proteins. This allows for the formation of pores in the mitochondrial membrane comprised of pro-apoptotic Bax or Bak, and subsequent release of mitochondrial inner membrane proteins such as cytochrome c, apoptosis inducing factor (AIF), endonuclease G, Smac/Diablo and Htr/Omi. These mitochondrial proteins form the “apoptosome”, an apoptotic signaling complex that, like the DISC from the extrinsic pathway, result in the cleavage of caspase 3 and caspase 7, and ultimately cell death.
The E6 oncoproteins from both HR and LR HPVs have been found to block intrinsic apoptotic signaling primarily by interacting with only one protein – Bak (Figure 3 #3). Both HR and LR E6 proteins from genus alpha and beta HPVs have been shown to bind Bak, and induce its proteasomal-dependent degradation (Jackson et al., 2000; Thomas and Banks, 1998; Thomas and Banks, 1999; Underbrink et al., 2008). While E6AP has been shown to play a role in Bak degradation (Thomas and Banks, 1998; Underbrink et al., 2008), it has also been proposed that this may not be a universal mechanism for all of the HPV types (Simmonds and Storey, 2008), thus the involvement of other E3 ubiquitin ligases in this process is an area of interest. Whether Bak degradation is constitutive or induced following cell stress is also a topic of controversy. Recent evidence has shown that Bak degradation is not constitutive, but rather occurs only after apoptotic signals have been initiated, indicating that a Bak conformational change and/or dissociation from its anti-apoptotic partner MCL-1 may be necessary for its interaction with E6 and E6AP (Jackson et al., 2000; Underbrink et al., 2008). Other studies have found constitutively lower levels of total Bak protein in E6 expressing cells (Du et al., 2004; Struijk et al., 2008; Thomas and Banks, 1998; Thomas and Banks, 1999). Regardless of whether Bak degradation is constitutive or induced, the overall effect has been shown to be a block the release of cytochrome c, AIF and Omi from the mitochondria, preservation of mitochondrial integrity, and disruption of the cleavage of effector caspases (Leverrier et al., 2007; Underbrink et al., 2008). A final area of controversy surrounds the ability of E6 to perturb other intrinsic apoptotic mediators. One study has shown that 16E6 expression leads to an increase in the anti-apoptotic Bcl-2 protein (Du et al., 2004). Another group demonstrated that 5E6 and 8E6 expression leads to a decrease in the levels of Bax protein expression (Struijk et al., 2008). A third study examined the levels of Noxa, Puma, Bcl-xL, Mcl-1, Bcl-2 and Bax, and found none of these intrinsic apoptotic signaling proteins to be perturbed in E6-expressing cells (Underbrink et al., 2008).
Importantly the extrinsic and intrinsic pathways are not isolated. Caspase 8 can be activated during intrinsic apoptotic signaling via an amplification loop mediated by caspases 3 and 7. Likewise, mitochondrial signaling can be triggered during activation of the extrinsic cascade, via caspase 8 mediated cleavage of the BH3-only protein Bid. These mechanisms are thought to help amplify apoptotic signaling once signals have been detected (for review see Gewies, 2003). Thus, by E6 targeting both intrinsic and extrinsic signaling, it not only protects an infected cell from multiple apoptotic stimuli, but also protects the cell from cross activation between these two pathways. Moreover, E6 has been shown to interact with proteins that are involved in apoptotic signaling at the crossroads where the intrinsic and extrinsic pathways join, downstream of the effector caspases. HPV 16E6 has been shown to upregulate the expression of two inhibitor of apoptosis (IAP) proteins, c-IAP2 (James, Lee, and Klingelhutz, 2006b; Yuan et al., 2005) and survivin (Borbely et al., 2006) (Figure 3 #4). IAPs are proteins that can bind to and inactivate the exectutioner caspases, caspase-3 and caspase-7 (see Gewies, 2003). c-IAP2 upregulation appears to be due to E6 mediated activation of NF kappa B, a well known anti-apoptotic signaling molecule (James, Lee, and Klingelhutz, 2006b). As NF kappa B has other well known cytoprotective effects, it is possible that other NF kappa B responsive proteins play a role in E6 mediated cytoprotection as well. Finally, 16E6 has also been reported to bind to and degrade c-Myc in a proteasomal and E6AP dependent manner (Gross-Mesilaty et al., 1998). However other studies have reported increased levels of c-Myc in E6 expressing cells (McMurray and McCance, 2003) or no change in the level of c-Myc (Gewin and Galloway, 2001; Veldman et al., 2001), thus the overall role that c-Myc may play in E6-mediated cytoprotection is uncertain.
Induction of telomerase activity
Expression of HR HPV E6 protein in concert with HR HPV E7 protein can immortalize epithelial cells in culture (Hawley-Nelson et al., 1989; Munger et al., 1989), and one critical cellular target is telomerase (Kiyono et al., 1998; Klingelhutz, Foster, and McDougall, 1996; Veldman et al., 2003). Understanding how E6 affects telomerase shines a spotlight on a critical target for oncogenic progression and avoidance of cellular senescence.
As linear DNA is replicated in each cell division, approximately 150-200 nucleotides at the 3′ end of chromosomes is lost due to the directional amplification of DNA (Levy et al., 1992). To avoid losing critical genetic information on chromosomes and to prevent recombination at the termini, their ends are capped with repetitive telomeric DNA approximately 10-12 kilobases in length and proteins collectively named shelterin (de Lange, 2005). The protection of genetic material within chromosomes is important, as is the folding of telomeric DNA into a T-loop (Griffith et al., 1999) to avoid DNA damage signals and senescence or apoptosis signals (Karlseder et al., 1999). As telomeres shorten with each cell division, the age of a cell is clearly marked in time (Allsopp et al., 1992; Harley, Futcher, and Greider, 1990). When telomeres become critically shortened, cells are signaled to senesce (Hayflick, 1965; Hayflick and Moorhead, 1961); if they do not, there can be catastrophic DNA damage, including anaphase bridges and double strand DNA breaks.
Telomerase is a ribonucleoprotein that extends to telomeric ends of linear chromosomes in eukaryotes. Normally quiescent in somatic cells, telomerase is active in stem cells and during embryonic development. The RNA component of telomerase, TERC, is the template for the repetitive DNA in telomeres, TTAGGG in humans. Dyskerin is an additional key protein subunit of telomerase (Cohen et al., 2007), and hTERT is the catalytic subunit of telomerase (Cohen et al., 2007; Kilian et al., 1997; Nakamura et al., 1997). The expression level of hTERT is proportionate to the enzymatic activity of telomerase in cells, and in the majority of immortalized cells and cancers hTERT is detected (Bryan and Cech, 1999; Meyerson et al., 1997; Shay and Bacchetti, 1997). If telomerase is not active in immortalized cells or in cancers, telomeric DNA is extended using the alternative lengthening of telomeres (ALT) pathway of homologous end joining (Bryan et al., 1995; Neumann and Reddel, 2002). So, the protection of telomeric DNA to avoid signals of cellular senescence or catastrophic chromosomal damage is key to cellular dysregulation in cancers and must be controlled in normal, differentiated somatic cells.
In 1996 it was found that HR HPV E6 protein could activate telomerase in epithelial cells (Klingelhutz, Foster, and McDougall, 1996), and less than ten years later it was found that E6AP was critical for hTERT regulation by E6 (Gewin et al., 2004; Liu et al., 2005). HR E6 binds the endogenous E6AP protein and utilizes it in its activation of hTERT and telomerase (Gewin et al., 2004; James, Lee, and Klingelhutz, 2006a; Klingelhutz, Foster, and McDougall, 1996). Knockdown of E6AP decreases the telomerase activity triggered by HPV 16E6, as well as genus beta HPV E6 types 38 and 8 (Bedard et al., 2008; Gewin et al., 2004). In one study, mutants of HPV 16E6 that cannot bind E6AP, when expressed in epithelial cells, were also unable to induce hTERT transcription (Gewin and Galloway, 2001), however, another study using E6 mutants did not find a requirement for E6AP in hTERT induction (Sekaric, Cherry, and Androphy, 2008).
The oncogene c-Myc has been shown to activate hTERT transcription (Wang et al., 1998; Wu et al., 1999). The heterodimer c-Myc/Max binds two E-box sequences in the core proximal promoter of hTERT (Cong, Wen, and Bacchetti, 1999; Lebel et al., 2007; Oh et al., 1999; Oh, Kyo, and Laimins, 2001; Takakura et al., 1999) (Figure 4). The HR E6 protein requires the two proximal E-box sequences to activate hTERT, as mutations in the E-box sequence blunts the activation of hTERT by E6 in luciferase assays (Gewin and Galloway, 2001; Liu et al., 2005; Oh, Kyo, and Laimins, 2001; Veldman et al., 2001). In vivo, E6, c-Myc, and E6AP are found at the hTERT promoter (Liu et al., 2005; Veldman et al., 2003), and although there is no strong evidence for significant total increases in protein levels of c-Myc nor differences in c-Myc levels at the hTERT promoter with E6 expression in keratinocytes (Gewin and Galloway, 2001; Veldman et al., 2001; Veldman et al., 2003; Xu et al., 2008), c-Myc is important in HPV-associated and non-HPV associated activation of hTERT. The interaction of HPV E6 with E6AP and c-Myc helps define the role of c-Myc in hTERT activation in keratinocytes and differentiate the regulation of hTERT in keratinocytes and fibroblasts.
Figure 4.
Model of the hTERT promoter. The catalytic subunit of telomerase, hTERT, is regulated at its core promoter by cis elements that include X1 boxes, E boxes, and GC-rich sequences. Repressors (shown above the promoter as rectangles) include USF1 and 2, which bind to E-boxes, and NFX1-91, which binds to the downstream X1 box sequence and recruits histone deacetylase activity through mSin3A. Activators (shown below the promoter as ovals) include the c-Myc/Max heterodimer, which binds to E box sequences and activates hTERT expression, Sp1, which binds to GC-rich sequences, and histone acetyltransferases, which increase acetylated histones at the hTERT promoter. E6/E6AP affects all of these repressors and activators. NFX1-123 with cytoplasmic poly(A) binding proteins (PABPCs) work in concert with E6/E6AP to augment hTERT activation.
Upstream stimulatory factors (USF1 and USF2) also bind to E-box sites and disrupt binding of c-Myc/Max (McMurray and McCance, 2003) (Figure 4). Although USFs bind with less affinity to E-boxes, USF1 and USF2 are more abundant than cMyc/Max and can compete for these sites. USF1 and USF2 have been reported to occupy the hTERT promoter in keratinocytes, and with E6 expression the amount of USF1 and USF2 at the promoter is reduced (McMurray and McCance, 2003).
GC-rich sequences are founds throughout the hTERT promoter, and Sp1 binds to five GC-rich sites flanked by the two critical E-boxes (Oh, Kyo, and Laimins, 2001; Takakura et al., 1999) (Figure 4). Mutations within these sites reduce the basal expression detected from the hTERT promoter, and the increases in hTERT promoter driven expression are seen in HPV E6 cells (Oh, Kyo, and Laimins, 2001). Therefore, the effects of HPV E6 on hTERT expression point to an additional transcriptional activator of hTERT found commonly in keratinocytes and recruited by HPV E6 for its activation of telomerase.
Transcriptional activators and RNA polymerases all require access to their DNA sequences, like c-Myc/Max to their E-boxes and Sp1 to its GC-rich sites. Acetylation of histones on which DNA is wound opens the structure of chromatin to these proteins. HPV E6 induces histone acetylation at the hTERT promoter, and this acetylation depends on E6AP (James, Lee, and Klingelhutz, 2006a; Xu et al., 2008) (Figure 4). With continued passage of HPV E6 cells in culture, the acetylation of the hTERT promoter increases, and knockdown of E6AP reduces this acetylation (James, Lee, and Klingelhutz, 2006a). Thus HPV E6 with E6AP adds to the evidence of direct transcriptional gene regulation through transcriptional activators or repressors binding to cis elements in promoters, as well as epigenetic regulation of gene expression, all through its effect on hTERT.
As E6/E6AP targets p53 for polyubiquitination and degradation, it also was hypothesized that E6/E6AP may target a yet unknown transcriptional repressor at the hTERT promoter for polyubiquitination and degradation. In 2004, NFX1-91 was identified as a transcriptional repressor of hTERT in a yeast two hybrid screen with HPV 16E6 and E6AP (Gewin et al., 2004) (Figure 4). NFX1, or nuclear factor binds to the X1 box, was originally identified as an MHC class II gene repressor (Song et al., 1994). Two splice variant isoforms are expressed in epithelial cells, with NFX1-91 referring to its kilodalton mass. In keratinocytes, NFX1-91 binds to a X1 box sequence in the hTERT promoter, recruiting the transcriptional co-repressor mSin3A and histone deacetylase activity to shut off hTERT expression (Xu et al., 2008). The protein-protein interaction of NFX1-91 with the heterodimer HPV E6/E6AP identified a new endogenous transcriptional repressor of hTERT important in the regulation of telomerase in epithelial cells.
Like p53, NFX1-91 is polyubiquitinated and targeted for degradation by 16E6/E6AP. The level of NFX1-91 in HPV 16E6 expressing cells is reduced, as is its occupancy at the hTERT promoter (Gewin et al., 2004; Xu et al., 2008). Concomitantly, histone acetylation increases at the hTERT promoter, though the acetylase has yet to be identified (Xu et al., 2008).
To fully induce hTERT expression and telomerase activity in keratinocytes, HPV E6/E6AP requires expression of NFX1-123, the other splice variant of NFX1, (Katzenellenbogen et al., 2007) (Figure 4). Unlike NFX1-91, NFX1-123 augments hTERT promoter driven expression by HPV E6/E6AP (Katzenellenbogen et al., 2007). NFX1-123 interacts with cytoplasmic poly(A) binding proteins, which bind to the poly(A) tail of mRNA and increase protein expression through nuclear-to-cytoplasmic transcript shuttling, translational machinery recruitment, and mRNA stabilization (for review see Kuhn and Wahle, 2004). The PAM2 of NFX1-123 is required for the interaction with cytoplasmic poly(A) binding proteins, and this motif is critical for the increase in hTERT expression when NFX1-123 is overexpressed in E6 expressing keratinocytes (Katzenellenbogen et al., 2007). This interaction between HPV E6/E6AP, NFX1-123, and cytoplasmic poly(A) binding proteins, hints at additional evidence of hTERT regulation beyond transcriptional regulation (Anderson et al., 2006; Cerezo et al., 2002; Emerald et al., 2007; Fujiwara et al., 2004).
Mediating Chromosome Stability
In addition to increasing the expression of hTERT, E6 also interacts with other proteins involved in maintaining chromosomal stability within the HPV infected cell. First, E6 from both HR and LR HPVs have been shown to interact with the human minichromosome maintenace 7 (hMCM7) protein, although binding by HR E6 proteins appears to be stronger than that by LR E6 proteins (Kukimoto et al., 1998). Moreover, 18E6 was shown to mediate MCM7 degradation via E6AP and proteasomal involvement (Kuhne and Banks, 1998). As MCM7 is involved in licensing DNA replication to ensure a single round of DNA replication per cell cycle, it is thought that E6 interaction with and/or degradation of MCM7 may lead to chromosomal abnormalities in HPV infected cells. E6 has also been shown to interact with two proteins involved in single strand DNA break repair – XRCC1 and O(6)-methylguanine-DNA methyltransferase (MGMT) (Iftner et al., 2002; Srivenugopal and Ali-Osman, 2002). XRCC1 was shown to be bound by HPV 1, 8 and 16E6, and this interaction reduced the ability of XRCC1 to repair methyl methane sulfate (MMS) induced DNA damage (Iftner et al., 2002). E6 interaction with MGMT induces its proteasomal-mediated degradation via E6AP, which has been hypothesized to sensitize HPV-infected cells to alkylating DNA damage (Srivenugopal and Ali-Osman, 2002). Finally, HR E6 mediated p53 loss inactivates the G1 checkpoint. Prolonged proliferation in the absence of p53 can lead to the loss of the G2 checkpoint, which can result in aneuploidy (Passalaris et al., 1999). Together, these interactions may lead to increased genomic instability and accelerate the progression to carcinogenesis.
Disrupting cell adhesion, polarity and epithelial differentiation
Basal cells of squamous epithelium attach to the extracellular matrix (ECM) of the basement membrane and receive signals allowing their proliferation. Once daughter cells detach from the basement membrane and migrate to suprabasal layers, proliferative signals cease and markers of epithelial differentiation are expressed. The establishment of cell:ECM adhesion, cell:cell contact, cytoskeletal organization and apicobasal polarity of epithelial cells is tightly regulated to assure regulated proliferation and differentiation (Bilder, 2004). E6 proteins, particularly of HR HPVs, disrupt many of these processes to allow proliferation of differentiated cells and inhibition of terminal differention to support viral replication.
Both paxillin and zyxin are focal adhesion molecules that are involved in tethering the cellular cytoskeleton to the ECM and transmitting signals along the actin network from the ECM to the nucleus. E6 from diverse papillomaviruses have been shown to bind to these proteins (Degenhardt and Silverstein, 2001a; Tong and Howley, 1997) resulting in the disruption of actin fibers and a failure to maintain proper cell structure.
hScrib, a PDZ domain containing protein, is also involved in epithelial tight junctions, mediating the adhesion of basal cells to the ECM. It functions as a tumor suppressor that negatively regulates proliferation. HR HPV E6 proteins bind to hScrib, and at least in some cell types have been shown to mediate its degradation (Nakagawa and Huibregtse, 2000). Similarly, hDlg is a PDZ domain containing protein involved in epithelial tight junctions, cell:cell junctions and epithelial polarity, functioning as a tumor suppresor. hDlg was the first PDZ protein shown to bind to HR E6 proteins (Kiyono et al., 1997; Lee, Weiss, and Javier, 1997). The MAGI proteins and MUPP1 also play a role in maintaining epithelial cell junctions, negatively regulating cell proliferation and in signal transduction from the cell membrane. Likewise they bind HR HPV E6 proteins and disrupt regulation of epithelial proliferation (Glaunsinger et al., 2000; Thomas et al., 2002). Recently it has been shown that PTPN3, a membrane-bound tyrosine phosphatase that regulates growth factor receptors is also a PDZ protein that binds and is disrupted by E6 (Jing et al., 2007; Spanos et al., 2008a).
Whether HR HPV E6 proteins bind and target all those PDZ proteins for degradation in vivo is controversial. When the HPV 31 genome was transfected into keratinocytes Western analysis did not reveal any significant changes in the levels of PDZ proteins and in organotypic raft cultures, immunohistochemical analysis failed to identify substantial changes in the differentiation-dependent membrane localization of hDlg proteins (Lee and Laimins, 2004). Instead, deletion or mutation of PDZ domain-binding motif of E6 impaired the growth rate of cell lines harboring the mutant genomes and reduced the viral copy number compared to cells transfected with wild-type genomes. The results suggested that binding of E6 to PDZ proteins modulates the early viral functions such as proliferation and maintenance of the viral copy number in undifferentiated cells. Additionally, studies that have investigated the mechanism of degradation of the PDZ proteins in-vitro have reached opposite conclusions as to whether E6AP is the ubiquitin ligase that promotes proteosomal degradation (Grm and Banks, 2004; Sterlinko et al., 2004).
Conclusions
The small DNA tumor viruses have provided valuable tools for understanding fundamental processes involved in cellular replication and tumorigenesis. They encode only a small number of genes, which are able to subvert the controls that regulate proliferation, contributing both acutely to transformation and by initiating genetic instability. The E6 protein of papillomaviruses targets numerous cellular pathways to insure viral DNA replication and is a key oncogene in HPV associated neoplasias. E6 from the high risk HPVs has been shown to inactivate p53, block apoptosis, activate telomerase, disrupt cell adhesion, polarity and epithelial differentiation, alter transcription and G-protein signaling, and reduce immune recognition of HPV infected cells. The pathways that are targeted by E6 in HPV associated cancers have provided important insights to identify the critical mutations that are commonly found in other tumors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Allsopp RC, Vaziri H, Patterson C, Goldstein S, Younglai EV, Futcher AB, Greider CW, Harley CB. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89(21):10114–8. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CJ, Hoare SF, Ashcroft M, Bilsland AE, Keith WN. Hypoxic regulation of telomerase gene expression by transcriptional and post-transcriptional mechanisms. Oncogene. 2006;25(1):61–9. doi: 10.1038/sj.onc.1209011. [DOI] [PubMed] [Google Scholar]
- Ashcroft M, Vousden KH. Regulation of p53 stability. Oncogene. 1999;18(53):7637–43. doi: 10.1038/sj.onc.1203012. [DOI] [PubMed] [Google Scholar]
- Barbosa MS, Lowy DR, Schiller JT. Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. Journal of Virology. 1989;63:1404–1407. doi: 10.1128/jvi.63.3.1404-1407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, Berger SL. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell. 2001;8(6):1243–54. doi: 10.1016/s1097-2765(01)00414-2. [DOI] [PubMed] [Google Scholar]
- Be X, Hong Y, Wei J, Androphy EJ, Chen JJ, Baleja JD. Solution structure determination and mutational analysis of the papillomavirus E6 interacting peptide of E6AP. Biochemistry. 2001;40(5):1293–1299. doi: 10.1021/bi0019592. [DOI] [PubMed] [Google Scholar]
- Bedard KM, Underbrink MP, Howie HL, Galloway DA. The E6 oncoproteins from human betapapillomaviruses differentially activate telomerase through an E6AP-dependent mechanism and prolong the lifespan of primary keratinocytes. J Virol. 2008;82(8):3894–902. doi: 10.1128/JVI.01818-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18(16):1909–25. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Murvai M, Konya J, Beck Z, Gergely L, Li F, Veress G. Effects of human papillomavirus type 16 oncoproteins on survivin gene expression. J Gen Virol. 2006;87(Pt 2):287–94. doi: 10.1099/vir.0.81067-0. [DOI] [PubMed] [Google Scholar]
- Breiding DE, Sverdrup F, Grossel MJ, Moscufo N, Boonchai W, Androphy EJ. Functional interaction of a novel cellular protein with the papillomavirus E2 transactivation domain. Mol Cell Biol. 1997;17(12):7208–19. doi: 10.1128/mcb.17.12.7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan TM, Cech TR. Telomerase and the maintenance of chromosome ends. Curr Opin Cell Biol. 1999;11(3):318–24. doi: 10.1016/S0955-0674(99)80043-X. [DOI] [PubMed] [Google Scholar]
- Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. Embo J. 1995;14(17):4240–8. doi: 10.1002/j.1460-2075.1995.tb00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerezo A, Kalthoff H, Schuermann M, Schafer B, Boukamp P. Dual regulation of telomerase activity through c-Myc-dependent inhibition and alternative splicing of hTERT. J Cell Sci. 2002;115(Pt 6):1305–12. doi: 10.1242/jcs.115.6.1305. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Hong Y, Rustamzadeh E, Baleja JD, Androphy EJ. Identification of an alpha helical motif sufficient for association with papillomavirus E6. Journal of Biological Chemistry. 1998;273(22):13537–13544. doi: 10.1074/jbc.273.22.13537. [DOI] [PubMed] [Google Scholar]
- Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315(5820):1850–3. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- Cole ST, Danos O. Nucleotide sequence and comparative analysis of the human papillomavirus type 18 genome. Phylogeny of papillomaviruses and repeated structure of the E6 and E7 gene products. J Mol Biol. 1987;193(4):599–608. doi: 10.1016/0022-2836(87)90343-3. [DOI] [PubMed] [Google Scholar]
- Cong YS, Wen J, Bacchetti S. The human telomerase catalytic subunit hTERT: organization of the gene and characterization of the promoter. Hum Mol Genet. 1999;8(1):137–42. doi: 10.1093/hmg/8.1.137. [DOI] [PubMed] [Google Scholar]
- Cooper K, Herrington CS, Evans MF, Gatter KC, McGee JO. p53 antigen in cervical condylomata, intraepithelial neoplasia, and carcinoma: relationship to HPV infection and integration. J Pathol. 1993;171(1):27–34. doi: 10.1002/path.1711710107. [DOI] [PubMed] [Google Scholar]
- Crook T, Fisher C, Masterson PJ, Vousden KH. Modulation of transcriptional regulatory properties of p53 by HPV E6. Oncogene. 1994;9(4):1225–30. [PubMed] [Google Scholar]
- Crook T, Tidy JA, Vousden KH. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and transactivation. Cell. 1991;67:547–556. doi: 10.1016/0092-8674(91)90529-8. [DOI] [PubMed] [Google Scholar]
- Crook T, Wrede D, Vousden KH. p53 point mutation in HPV negative human cervical carcinoma cell lines. Oncogene. 1991;6(5):873–5. [PubMed] [Google Scholar]
- de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19(18):2100–10. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- Degenhardt YY, Silverstein S. Interaction of zyxin, a focal adhesion protein, with the e6 protein from human papillomavirus type 6 results in its nuclear translocation. J Virol. 2001a;75(23):11791–802. doi: 10.1128/JVI.75.23.11791-11802.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt YY, Silverstein SJ. Gps2, a protein partner for human papillomavirus E6 proteins. J Virol. 2001b;75(1):151–60. doi: 10.1128/JVI.75.1.151-160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorbar J, Foo C, Coleman N, Medcalf L, Hartley O, Prospero T, Napthine S, Sterling J, Winter G, Griffin H. Characterization of events during the late stages of HPV16 infection in vivo using high-affinity synthetic Fabs to E4. Virology. 1997;238(1):40–52. doi: 10.1006/viro.1997.8768. [DOI] [PubMed] [Google Scholar]
- Du J, Chen GG, Vlantis AC, Chan PK, Tsang RK, van Hasselt CA. Resistance to apoptosis of HPV 16-infected laryngeal cancer cells is associated with decreased Bak and increased Bcl-2 expression. Cancer Lett. 2004;205(1):81–8. doi: 10.1016/j.canlet.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Duerksen-Hughes PJ, Yang J, Schwartz SB. HPV 16 E6 blocks TNF-mediated apoptosis in mouse fibroblast LM cells. Virology. 1999;264(1):55–65. doi: 10.1006/viro.1999.9977. [DOI] [PubMed] [Google Scholar]
- Elston RC, Napthine S, Doorbar J. The identification of a conserved binding motif within human papillomavirus type 16 E6 binding peptides, E6AP and E6BP. Journal of General Virology. 1998;79(Pt 2):371–374. doi: 10.1099/0022-1317-79-2-371. [DOI] [PubMed] [Google Scholar]
- Emerald BS, Chen Y, Zhu T, Zhu Z, Lee KO, Gluckman PD, Lobie PE. AlphaCP1 mediates stabilization of hTERT mRNA by autocrine human growth hormone. J Biol Chem. 2007;282(1):680–90. doi: 10.1074/jbc.M600224200. [DOI] [PubMed] [Google Scholar]
- Filippova M, Johnson MM, Bautista M, Filippov V, Fodor N, Tungteakkhun SS, Williams K, Duerksen-Hughes PJ. The large and small isoforms of human papillomavirus type 16 E6 bind to and differentially affect procaspase 8 stability and activity. J Virol. 2007;81(8):4116–29. doi: 10.1128/JVI.01924-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippova M, Parkhurst L, Duerksen-Hughes PJ. The human papillomavirus 16 E6 protein binds to Fas-associated death domain and protects cells from Fas-triggered apoptosis. Journal of Biological Chemistry. 2004;279(24):25729–44. doi: 10.1074/jbc.M401172200. [DOI] [PubMed] [Google Scholar]
- Filippova M, Song H, Connolly JL, Dermody TS, Duerksen-Hughes PJ. The human papillomavirus 16 E6 protein binds to tumor necrosis factor (TNF) R1 and protects cells from TNF-induced apoptosis. J Biol Chem. 2002;277(24):21730–9. doi: 10.1074/jbc.M200113200. [DOI] [PubMed] [Google Scholar]
- Fujiwara M, Kamma H, Wu W, Hamasaki M, Kaneko S, Horiguchi H, Matsui-Horiguchi M, Satoh H. Expression and alternative splicing pattern of human telomerase reverse transcriptase in human lung cancer cells. Int J Oncol. 2004;24(4):925–30. [PubMed] [Google Scholar]
- Gao Q, Kumar A, Singh L, Huibregtse JM, Beaudenon S, Srinivasan S, Wazer DE, Band H, Band V. Human papillomavirus E6-induced degradation of E6TP1 is mediated by E6AP ubiquitin ligase. Cancer Res. 2002;62(11):3315–21. [PubMed] [Google Scholar]
- Gao Q, Srinivasan S, Boyer SN, Wazer DE, Band V. The E6 oncoproteins of high-risk papillomaviruses bind to a novel putative GAP protein, E6TP1, and target it for degradation. Mol Cell Biol. 1999;19(1):733–44. doi: 10.1128/mcb.19.1.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett TO, Filippova M, Duerksen-Hughes PJ. Accelerated degradation of FADD and procaspase 8 in cells expressing human papilloma virus 16 E6 impairs TRAIL-mediated apoptosis. Cell Death Differ. 2006;13(11):1915–26. doi: 10.1038/sj.cdd.4401886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewies A. ApoReview: Introduction to Apoptosis. 2003. [Google Scholar]
- Gewin L, Galloway DA. E box-dependent activation of telomerase by human papillomavirus type 16 E6 does not require induction of c-myc. J Virol. 2001;75(15):7198–201. doi: 10.1128/JVI.75.15.7198-7201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewin L, Myers H, Kiyono T, Galloway DA. Identification of a novel telomerase repressor that interacts with the human papillomavirus type-16 E6/E6-AP complex. Genes & Development. 2004;18(18):2269–82. doi: 10.1101/gad.1214704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampieri S, Garcia-Escudero R, Green J, Storey A. Human papillomavirus type 77 E6 protein selectively inhibits p53-dependent transcription of proapoptotic genes following UV-B irradiation. Oncogene. 2004;23(34):5864–70. doi: 10.1038/sj.onc.1207711. [DOI] [PubMed] [Google Scholar]
- Glaunsinger BA, Lee SS, Thomas M, Banks L, Javier R. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene. 2000;19(46):5270–5280. doi: 10.1038/sj.onc.1203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Comeau L, Rosenfield S, Stansel RM, Bianchi A, Moss H, de Lange T. Mammalian telomeres end in a large duplex loop. Cell. 1999;97(4):503–14. doi: 10.1016/s0092-8674(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Grm HS, Banks L. Degradation of hDlg and MAGIs by human papillomavirus E6 is E6-AP-independent. Journal of General Virology. 2004;85(Pt 10):2815–9. doi: 10.1099/vir.0.80035-0. [DOI] [PubMed] [Google Scholar]
- Gross-Mesilaty S, Reinstein E, Bercovich B, Tobias KE, Schwartz AL, Kahana C, Ciechanover A. Basal and human papillomavirus E6 oncoprotein-induced degradation of Myc proteins by the ubiquitin pathway. Proc Natl Acad Sci U S A. 1998;95(14):8058–63. doi: 10.1073/pnas.95.14.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SR, Laimins LA. E6 protein of human papillomavirus type 18 binds zinc. Oncogene J1 - O. 1989;4:1089–1093. [PubMed] [Google Scholar]
- Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90(4):595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–60. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, Mansour M, Vincent I, Gissmann L, Iftner T, Sideri M, Stubenrauch F, Tommasino M. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol. 2007;178(5):3186–97. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P, Vousden KH, Hubbert NL, Lowy DR, Schiller JT. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. Embo J. 1989;8(12):3905–10. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. The Limited in Vitro Lifetime of Human Diploid Cell Strains. Exp Cell Res. 1965;37:614–36. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 2007;282(21):15325–9. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420(1):25–7. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412(2):179–90. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus type 16 or 18. EMBO Journal. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse JM, Scheffner M, Howley PM. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol. 1993a;13(2):775–84. doi: 10.1128/mcb.13.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse JM, Scheffner M, Howley PM. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol Cell Biol. 1993b;13(8):4918–27. doi: 10.1128/mcb.13.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftner T, Elbel M, Schopp B, Hiller T, Loizou JI, Caldecott KW, Stubenrauch F. Interference of papillomavirus E6 protein with single-strand break repair by interaction with XRCC1. Embo J. 2002;21(17):4741–8. doi: 10.1093/emboj/cdf443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Lai CH, Zhao X, Saito S, Hamilton MH, Appella E, Yao TP. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. Embo J. 2001;20(6):1331–40. doi: 10.1093/emboj/20.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 2000;14(23):3065–73. doi: 10.1101/gad.182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MA, Lee JH, Klingelhutz AJ. HPV16-E6 associated hTERT promoter acetylation is E6AP dependent, increased in later passage cells and enhanced by loss of p300. Int J Cancer. 2006a;119(8):1878–85. doi: 10.1002/ijc.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MA, Lee JH, Klingelhutz AJ. Human papillomavirus type 16 E6 activates NF-kappaB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J Virol. 2006b;80(11):5301–7. doi: 10.1128/JVI.01942-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DY, Teramoto H, Giam CZ, Chun RF, Gutkind JS, Jeang KT. A human suppressor of c-Jun N-terminal kinase 1 activation by tumor necrosis factor alpha. J Biol Chem. 1997;272(41):25816–23. doi: 10.1074/jbc.272.41.25816. [DOI] [PubMed] [Google Scholar]
- Jing M, Bohl J, Brimer N, Kinter M, Vande Pol SB. Degradation of tyrosine phosphatase PTPN3 (PTPH1) by association with oncogenic human papillomavirus E6 proteins. J Virol. 2007;81(5):2231–9. doi: 10.1128/JVI.01979-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283(5406):1321–5. doi: 10.1126/science.283.5406.1321. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen RA, Egelkrout EM, Vliet-Gregg P, Gewin LC, Gafken PR, Galloway DA. NFX1-123 and poly(A) binding proteins synergistically augment activation of telomerase in human papillomavirus type 16 E6-expressing cells. J Virol. 2007;81(8):3786–96. doi: 10.1128/JVI.02007-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256(1):42–9. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- Kilian A, Bowtell DD, Abud HE, Hime GR, Venter DJ, Keese PK, Duncan EL, Reddel RR, Jefferson RA. Isolation of a candidate human telomerase catalytic subunit gene, which reveals complex splicing patterns in different cell types. Hum Mol Genet. 1997;6(12):2011–9. doi: 10.1093/hmg/6.12.2011. [DOI] [PubMed] [Google Scholar]
- Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396(6706):84–8. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophilia discs large tumor supressor protein. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature. 1996;380(6569):79–82. doi: 10.1038/380079a0. [DOI] [PubMed] [Google Scholar]
- Kuballa P, Matentzoglu K, Scheffner M. The Role of the Ubiquitin Ligase E6-AP in Human Papillomavirus E6-mediated Degradation of PDZ Domain-containing Proteins. Journal of Biological Chemistry. 2007;282(1):65–71. doi: 10.1074/jbc.M605117200. [DOI] [PubMed] [Google Scholar]
- Kuhn U, Wahle E. Structure and function of poly(A) binding proteins. Biochim Biophys Acta. 2004;1678(23):67–84. doi: 10.1016/j.bbaexp.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kuhne C, Banks L. E3-ubiquitin ligase/E6-AP links multicopy maintenance protein 7 to the ubiquitination pathway by a novel motif, the L2G box. J Biol Chem. 1998;273(51):34302–9. doi: 10.1074/jbc.273.51.34302. [DOI] [PubMed] [Google Scholar]
- Kukimoto I, Aihara S, Yoshiike K, Kanda T. Human papillomavirus oncoprotein E6 binds to the C-terminal region of human minichromosome maintenance 7 protein. Biochem Biophys Res Commun. 1998;249(1):258–62. doi: 10.1006/bbrc.1998.9066. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zhao Y, Meng G, Zeng M, Srinivasan S, Delmolino LM, Gao Q, Dimri G, Weber GF, Wazer DE, Band H, Band V. Human papillomavirus oncoprotein E6 inactivates the transcriptional coactivator human ADA3. Molecular & Cellular Biology. 2002;22(16):5801–5812. doi: 10.1128/MCB.22.16.5801-5812.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel R, McDuff FO, Lavigne P, Grandbois M. Direct visualization of the binding of c-Myc/Max heterodimeric b-HLH-LZ to E-box sequences on the hTERT promoter. Biochemistry. 2007;46(36):10279–86. doi: 10.1021/bi700076m. [DOI] [PubMed] [Google Scholar]
- Lechner MS, Laimins LA. Inhibition of p53 DNA binding by human papillomavirus E6 proteins. J Virol. 1994;68(7):4262–73. doi: 10.1128/jvi.68.7.4262-4273.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner MS, Mack DH, Finicle AB, Crook T, Vousden KH, Laimins LA. Human papillomavirus E6 proteins bind p53 in vivo and abrogate p53-mediated repression of transcription. Embo J. 1992;11(8):3045–52. doi: 10.1002/j.1460-2075.1992.tb05375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Laimins LA. Role of the PDZ domain-binding motif of the oncoprotein E6 in the pathogenesis of human papillomavirus type 31. Journal of Virology. 2004;78(22):12366–77. doi: 10.1128/JVI.78.22.12366-12377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Wooldridge TR, Laimins LA. Analysis of the roles of E6 binding to E6TP1 and nuclear localization in the human papillomavirus type 31 life cycle. Virology. 2007;358(1):201–10. doi: 10.1016/j.virol.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Lee SS, Glaunsinger B, Mantovani F, Banks L, Javier RT. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J Virol. 2000;74(20):9680–93. doi: 10.1128/jvi.74.20.9680-9693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Weiss RS, Javier RT. Binding of human virus oncoproteins to hDLG/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:6670–6675. doi: 10.1073/pnas.94.13.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverrier S, Bergamaschi D, Ghali L, Ola A, Warnes G, Akgul B, Blight K, Garcia-Escudero R, Penna A, Eddaoudi A, Storey A. Role of HPV E6 proteins in preventing UVB-induced release of pro-apoptotic factors from the mitochondria. Apoptosis. 2007;12(3):549–60. doi: 10.1007/s10495-006-0004-1. [DOI] [PubMed] [Google Scholar]
- Levy MZ, Allsopp RC, Futcher AB, Greider CW, Harley CB. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225(4):951–60. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- Li X, Coffino P. High-risk human papillomavirus E6 protein has two distinct binding sites within p53, of which only one determines degradation. J Virol. 1996;70(7):4509–16. doi: 10.1128/jvi.70.7.4509-4516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie AK, Skarsvag S, Skomedal H, Haugen OA, Holm R. Expression of p53, MDM2, and p21 proteins in high-grade cervical intraepithelial neoplasia and relationship to human papillomavirus infection. Int J Gynecol Pathol. 1999;18(1):5–11. doi: 10.1097/00004347-199901000-00002. [DOI] [PubMed] [Google Scholar]
- Lipari F, McGibbon GA, Wardrop E, Cordingley MG. Purification and biophysical characterization of a minimal functional domain and of an N-terminal Zn2+-binding fragment from the human papillomavirus type 16 E6 protein. Biochemistry. 2001;40(5):1196–204. doi: 10.1021/bi001837+. [DOI] [PubMed] [Google Scholar]
- Liu L, Scolnick DM, Trievel RC, Zhang HB, Marmorstein R, Halazonetis TD, Berger SL. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol Cell Biol. 1999a;19(2):1202–9. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yuan H, Fu B, Disbrow GL, Apolinario T, Tomaic V, Kelley ML, Baker CC, Huibregtse J, Schlegel R. The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein. J Biol Chem. 2005;280(11):10807–16. doi: 10.1074/jbc.M410343200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen JJ, Gao Q, Dalal S, Hong Y, Mansur CP, Band V, Androphy EJ. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. Journal of Virology. 1999b;73(9):7297–7307. doi: 10.1128/jvi.73.9.7297-7307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy DR, Howley PM. Papillomaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Fourth. Lippincott Williams & Wilkins; 2001. pp. 2231–2264. [Google Scholar]
- Lu Z, Hu X, Li Y, Zheng L, Zhou Y, Jiang H, Ning T, Basang Z, Zhang C, Ke Y. Human papillomavirus 16 E6 oncoprotein interferences with insulin signaling pathway by binding to tuberin. J Biol Chem. 2004;279(34):35664–70. doi: 10.1074/jbc.M403385200. [DOI] [PubMed] [Google Scholar]
- Mantovani F, Banks L. Inhibition of E6 induced degradation of p53 is not sufficient for stabilization of p53 protein in cervical tumour derived cell lines. Oncogene. 1999;18(22):3309–15. doi: 10.1038/sj.onc.1202688. [DOI] [PubMed] [Google Scholar]
- Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20(54):7874–87. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- Matheu A, Maraver A, Serrano M. The Arf/p53 pathway in cancer and aging. Cancer Res. 2008;68(15):6031–4. doi: 10.1158/0008-5472.CAN-07-6851. [DOI] [PubMed] [Google Scholar]
- McMurray HR, McCance DJ. Human papillomavirus type 16 E6 activates TERT gene transcription through induction of c-Myc and release of USF-mediated repression. J Virol. 2003;77(18):9852–61. doi: 10.1128/JVI.77.18.9852-9861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q, Bacchetti S, Haber DA, Weinberg RA. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90(4):785–95. doi: 10.1016/s0092-8674(00)80538-3. [DOI] [PubMed] [Google Scholar]
- Mietz JA, Unger T, Huibregtse JM, Howley PM. The transcriptional transactivation function of wild-type p53 is inhibited by SV40 large T-antigen and by HPV-16 E6 oncoprotein. Embo J. 1992;11(13):5013–20. doi: 10.1002/j.1460-2075.1992.tb05608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Hamm S, Bauer S. TLR9-mediated recognition of DNA. Handb Exp Pharmacol. 2008;(183):51–70. doi: 10.1007/978-3-540-72167-3_3. [DOI] [PubMed] [Google Scholar]
- Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63(10):4417–21. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray-Zmijewski F, Slee EA, Lu X. A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol. 2008;9(9):702–12. doi: 10.1038/nrm2451. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Molecular & Cellular Biology. 2000;20(21):8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277(5328):955–9. doi: 10.1126/science.277.5328.955. [DOI] [PubMed] [Google Scholar]
- Neumann AA, Reddel RR. Telomere maintenance and cancer -- look, no telomerase. Nat Rev Cancer. 2002;2(11):879–84. doi: 10.1038/nrc929. [DOI] [PubMed] [Google Scholar]
- Nomine Y, Masson M, Charbonnier S, Zanier K, Ristriani T, Deryckere F, Sibler AP, Desplancq D, Atkinson RA, Weiss E, Orfanoudakis G, Kieffer B, Trave G. Structural and functional analysis of E6 oncoprotein: insights in the molecular pathways of human papillomavirus-mediated pathogenesis. Molecular Cell. 2006;21(5):665–78. doi: 10.1016/j.molcel.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Oh S, Song YH, Kim UJ, Yim J, Kim TK. In vivo and in vitro analyses of Myc for differential promoter activities of the human telomerase (hTERT) gene in normal and tumor cells. Biochem Biophys Res Commun. 1999;263(2):361–5. doi: 10.1006/bbrc.1999.1366. [DOI] [PubMed] [Google Scholar]
- Oh ST, Kyo S, Laimins LA. Telomerase activation by human papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J Virol. 2001;75(12):5559–66. doi: 10.1128/JVI.75.12.5559-5566.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Griep AE. Temporally distinct patterns of p53-dependent and p53-independent apoptosis during mouse lens development. Genes Dev. 1995;9(17):2157–69. doi: 10.1101/gad.9.17.2157. [DOI] [PubMed] [Google Scholar]
- Passalaris TM, Benanti JA, Gewin L, Kiyono T, Galloway DA. The G(2) checkpoint is maintained by redundant pathways. Mol Cell Biol. 1999;19(9):5872–81. doi: 10.1128/mcb.19.9.5872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D, Huang SM, Baglia LA, McCance DJ. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO Journal. 1999;18(18):5061–5072. doi: 10.1093/emboj/18.18.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YC, Breiding DE, Sverdrup F, Richard J, Androphy EJ. AMF-1/Gps2 binds p300 and enhances its interaction with papillomavirus E2 proteins. J Virol. 2000;74(13):5872–9. doi: 10.1128/jvi.74.13.5872-5879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pim D, Banks L. HPV-18 E6*I protein modulates the E6-directed degradation of p53 by binding to full-length HPV-18 E6. Oncogene. 1999;18(52):7403–8. doi: 10.1038/sj.onc.1203134. [DOI] [PubMed] [Google Scholar]
- Pina B, Berger S, Marcus GA, Silverman N, Agapite J, Guarente L. ADA3: a gene, identified by resistance to GAL4-VP16, with properties similar to and different from those of ADA2. Mol Cell Biol. 1993;13(10):5981–9. doi: 10.1128/mcb.13.10.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristriani T, Nomine Y, Masson M, Weiss E, Trave G. Specific recognition of four-way DNA junctions by the C-terminal zinc-binding domain of HPV oncoprotein E6. J Mol Biol. 2001;305(4):729–39. doi: 10.1006/jmbi.2000.4330. [DOI] [PubMed] [Google Scholar]
- Ronco LV, Karpova AY, Vidal M, Howley PM. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes & Development. 1998;12:2061–2072. doi: 10.1101/gad.12.13.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Scheffner M, Munger K, Byrne JC, Howley PM. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991;88(13):5523–7. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–36. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Schwarz SE, Rosa JL, Scheffner M. Characterization of human hect domain family members and their interaction with UbcH5 and UbcH7. J Biol Chem. 1998;273(20):12148–54. doi: 10.1074/jbc.273.20.12148. [DOI] [PubMed] [Google Scholar]
- Sekaric P, Cherry JJ, Androphy EJ. Binding of human papillomavirus type 16 E6 to E6AP is not required for activation of hTERT. J Virol. 2008;82(1):71–6. doi: 10.1128/JVI.01776-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamanin VA, Androphy EJ. Immortalization of human mammary epithelial cells is associated with inactivation of the p14ARF-p53 pathway. Mol Cell Biol. 2004;24(5):2144–52. doi: 10.1128/MCB.24.5.2144-2152.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamanin VA, Sekaric P, Androphy EJ. hAda3 degradation by papillomavirus type 16 E6 correlates with abrogation of the p14ARF-p53 pathway and efficient immortalization of human mammary epithelial cells. J Virol. 2008;82(8):3912–20. doi: 10.1128/JVI.02466-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33(5):787–91. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Simmonds M, Storey A. Identification of the regions of the HPV 5 E6 protein involved in Bak degradation and inhibition of apoptosis. Int J Cancer. 2008;123(10):2260–2266. doi: 10.1002/ijc.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L, Gao Q, Kumar A, Gotoh T, Wazer DE, Band H, Feig LA, Band V. The high-risk human papillomavirus type 16 E6 counters the GAP function of E6TP1 toward small Rap G proteins. J Virol. 2003;77(2):1614–20. doi: 10.1128/JVI.77.2.1614-1620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Krishna S, Thanos D, Strominger JL, Ono SJ. A novel cysteine-rich sequence-specific DNA-binding protein interacts with the conserved X-box motif of the human major histocompatibility complex class II genes via a repeated Cys-His domain and functions as a transcriptional repressor. J Exp Med. 1994;180(5):1763–74. doi: 10.1084/jem.180.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain BH, Bowdish KS, Pacal AR, Staub SF, Koo D, Chang CY, Xie W, Colicelli J. Two human cDNAs, including a homolog of Arabidopsis FUS6 (COP11), suppress G-protein- and mitogen-activated protein kinase-mediated signal transduction in yeast and mammalian cells. Mol Cell Biol. 1996;16(12):6698–706. doi: 10.1128/mcb.16.12.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos WC, Geiger J, Anderson ME, Harris GF, Bossler AD, Smith RB, Klingelhutz AJ, Lee JH. Deletion of the PDZ motif of HPV16 E6 preventing immortalization and anchorage-independent growth in human tonsil epithelial cells. Head Neck. 2008a;30(2):139–47. doi: 10.1002/hed.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos WC, Hoover A, Harris GF, Wu S, Strand GL, Anderson ME, Klingelhutz AJ, Hendriks W, Bossler AD, Lee JH. The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss, which allows anchorage-independent growth and synergizes with ras for invasive growth. J Virol. 2008b;82(5):2493–500. doi: 10.1128/JVI.02188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivenugopal KS, Ali-Osman F. The DNA repair protein, O(6)-methylguanine-DNA methyltransferase is a proteolytic target for the E6 human papillomavirus oncoprotein. Oncogene. 2002;21(38):5940–5. doi: 10.1038/sj.onc.1205762. [DOI] [PubMed] [Google Scholar]
- Steller MA, Zou Z, Schiller JT, Baserga R. Transformation by human papillomavirus 16 E6 and E7: role of the insulin-like growth factor 1 receptor. Cancer Res. 1996;56(21):5087–91. [PubMed] [Google Scholar]
- Sterlinko GH, Weber M, Elston R, McIntosh P, Griffin H, Banks L, Doorbar J. Inhibition of E6-induced degradation of its cellular substrates by novel blocking peptides. Journal of Molecular Biology. 2004;335(4):971–985. doi: 10.1016/j.jmb.2003.10.079. [DOI] [PubMed] [Google Scholar]
- Struijk L, van der Meijden E, Kazem S, ter Schegget J, de Gruijl FR, Steenbergen RD, Feltkamp MC. Specific betapapillomaviruses associated with squamous cell carcinoma of the skin inhibit UVB-induced apoptosis of primary human keratinocytes. J Gen Virol. 2008;89(Pt 9):2303–14. doi: 10.1099/vir.0.83317-0. [DOI] [PubMed] [Google Scholar]
- Takakura M, Kyo S, Kanaya T, Hirano H, Takeda J, Yutsudo M, Inoue M. Cloning of human telomerase catalytic subunit (hTERT) gene promoter and identification of proximal core promoter sequences essential for transcriptional activation in immortalized and cancer cells. Cancer Res. 1999;59(3):551–7. [PubMed] [Google Scholar]
- Thomas M, Banks L. Inhibition of Bak-induced apoptosis by HPV-18 E6. Oncogene. 1998;17(23):2943–54. doi: 10.1038/sj.onc.1202223. [DOI] [PubMed] [Google Scholar]
- Thomas M, Banks L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. Journal of General Virology. 1999;80(Pt 6):1513–7. doi: 10.1099/0022-1317-80-6-1513. [DOI] [PubMed] [Google Scholar]
- Thomas M, Glaunsinger B, Pim D, Javier R, Banks L. HPV E6 and MAGUK protein interactions: determination of the molecular basis for specific protein recognition and degradation. Oncogene. 2001;20(39):5431–5439. doi: 10.1038/sj.onc.1204719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Laura R, Hepner K, Guccione E, Sawyers C, Lasky L, Banks L. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene. 2002;21(33):5088–5096. doi: 10.1038/sj.onc.1205668. [DOI] [PubMed] [Google Scholar]
- Thomas M, Massimi P, Jenkins J, Banks L. HPV-18 E6 mediated inhibition of p53 DNA binding activity is independent of E6 induced degradation. Oncogene. 1995;10(2):261–8. [PubMed] [Google Scholar]
- Thomas MC, Chiang CM. E6 oncoprotein represses p53-dependent gene activation via inhibition of protein acetylation independently of inducing p53 degradation. Mol Cell. 2005;17(2):251–64. doi: 10.1016/j.molcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Tong X, Howley PM. The bovine papillomavirus E6 oncoprotein interacts with paxillin and disrupts the actin cytoskeleton. 1997;94:4412–4417. doi: 10.1073/pnas.94.9.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungteakkhun SS, Filippova M, Neidigh JW, Fodor N, Duerksen-Hughes PJ. The interaction between human papillomavirus type 16 and FADD is mediated by a novel E6 binding domain. J Virol. 2008;82(19):9600–14. doi: 10.1128/JVI.00538-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underbrink MP, Howie HL, Bedard KM, Koop JI, Galloway DA. The E6 proteins from multiple beta HPV types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J Virol. 2008 doi: 10.1128/JVI.00902-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman T, Horikawa I, Barrett JC, Schlegel R. Transcriptional activation of the telomerase hTERT gene by human papillomavirus type 16 E6 oncoprotein. J Virol. 2001;75(9):4467–72. doi: 10.1128/JVI.75.9.4467-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman T, Liu X, Yuan H, Schlegel R. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc Natl Acad Sci U S A. 2003;100(14):8211–6. doi: 10.1073/pnas.1435900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xie LY, Allan S, Beach D, Hannon GJ. Myc activates telomerase. Genes Dev. 1998;12(12):1769–74. doi: 10.1101/gad.12.12.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15(22):2922–33. [PubMed] [Google Scholar]
- Wu KJ, Grandori C, Amacker M, Simon-Vermot N, Polack A, Lingner J, Dalla-Favera R. Direct activation of TERT transcription by c-MYC. Nat Genet. 1999;21(2):220–4. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- Xu M, Luo W, Elzi DJ, Grandori C, Galloway DA. NFX1 interacts with mSin3A/histone deacetylase to repress hTERT transcription in keratinocytes. Mol Cell Biol. 2008;28(15):4819–28. doi: 10.1128/MCB.01969-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Fu F, Zhuo J, Wang W, Nishitani J, An DS, Chen IS, Liu X. Human papillomavirus type 16 E6 and E7 oncoproteins upregulate c-IAP2 gene expression and confer resistance to apoptosis. Oncogene. 2005;24(32):5069–78. doi: 10.1038/sj.onc.1208691. [DOI] [PubMed] [Google Scholar]
- Yuan ZM, Huang Y, Ishiko T, Nakada S, Utsugisawa T, Shioya H, Utsugisawa Y, Yokoyama K, Weichselbaum R, Shi Y, Kufe D. Role for p300 in stabilization of p53 in the response to DNA damage. J Biol Chem. 1999;274(4):1883–6. doi: 10.1074/jbc.274.4.1883. [DOI] [PubMed] [Google Scholar]
- Zeng M, Kumar A, Meng G, Gao Q, Dimri G, Wazer D, Band H, Band V. Human papilloma virus 16 E6 oncoprotein inhibits retinoic X receptor-mediated transactivation by targeting human ADA3 coactivator. J Biol Chem. 2002;277(47):45611–8. doi: 10.1074/jbc.M208447200. [DOI] [PubMed] [Google Scholar]
- Zheng L, Ding H, Lu Z, Li Y, Pan Y, Ning T, Ke Y. E3 ubiquitin ligase E6AP-mediated TSC2 turnover in the presence and absence of HPV16 E6. Genes Cells. 2008;13(3):285–94. doi: 10.1111/j.1365-2443.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Koh CH, Degenkolbe R, O'Connor MJ, Muller A, Steger G, Chen JJ, Lui Y, Androphy E, Bernard HU. Interaction with CBP/p300 enables the bovine papillomavirus type 1 E6 oncoprotein to downregulate CBP/p300-mediated transactivation by p53. Journal of General Virology. 2000;81(Pt11):11–23. doi: 10.1099/0022-1317-81-11-2617. [DOI] [PubMed] [Google Scholar]