Abstract
Gene therapy aims to revert diseased phenotypes by the use of both viral and nonviral gene delivery systems. Substantial progress has been made in making gene transfer vehicles more efficient, less toxic, and nonimmunogenic and in allowing long-term transgene expression. One of the key issues in successfully implementing gene therapies in the clinical setting is to be able to regulate gene expression very tightly and consistently as and when it is needed. The regulation ought to be achievable using a compound that should be nontoxic, be able to penetrate into the desired target tissue or organ, and have a half-life of a few hours (as opposed to minutes or days) so that when withdrawn or added (depending on the regulatable system used) gene expression can be turned “on” or “off” quickly and effectively. Also, the genetic switches employed should ideally be nonimmunogenic in the host. The ability to switch transgenes on and off would be of paramount importance not only when the therapy is no longer needed, but also in the case of the development of adverse side effects to the therapy. Many regulatable systems are currently under development and some, i.e., the tetracycline-dependent transcriptional switch, have been used successfully for in vivo preclinical applications. Despite this, there are no examples of switches that have been employed in a human clinical trial. In this review, we aim to highlight the main regulatable systems currently under development, the gene transfer systems employed for their expression, and also the preclinical models in which they have been used successfully. We also discuss the substantial challenges that still remain before these regulatable switches can be employed in the clinical setting.
Introduction
Gene therapy has evolved into a promising therapeutic modality to treat and manage a diverse array of diseases. The technology for using genes to provide a desired treatment has become an effective strategy due to advances in viral vector engineering and improved gene regulatory systems to facilitate and control tightly therapeutic gene expression. These transcriptional regulatory systems have been encoded within several viral vectors to facilitate gene expression and further improve the kinetics of gene regulation. Among the existing inducible transcriptional gene regulatory systems, the Tet-regulatable system is the most widely exploited tool for controlling gene expression, as it has many advantages. Tetracyclinebased regulatable systems can facilitate regulatable targeted gene expression with the use of cell-type-specific promoters. In addition, this system is nontoxic to mammalian cells and exhibits no pleiotropic consequences for other cellular metabolic pathways. The Tet regulatory system has been encoded within lentiviruses, adeno-associated viruses, first-generation and high-capacity helper-dependent adenoviruses (HC-Ad), and retroviruses, yielding successful gene regulation. This review will concentrate on the studies that have utilized viral vectors engineered with Tet-regulatable systems and also on new developments for generating superior inducible regulatory systems to optimize gene therapy strategies. Akin to drug treatment, for gene therapy to be used as a clinical treatment, gene therapists must also achieve an adequate understanding of the short- and long-term safety issues involved in the use of regulatable gene expression systems. This includes assessing long- and short-term applications of viral vectors and regulatory systems to produce safe and efficient levels of regulatable transgene expression and the immune impact to the host. With the development of safer viral and nonviral vectors, the current challenge for gene therapists, in conjunction with combating vector-mediated immune responses, is the translation of the concept of efficiently regulating therapeutic gene expression into the clinic. This is critical for chronic diseases, such as neurodegenerative disorders; activating and silencing therapeutic genes will be essential to alleviating recurrent symptoms of the disease in a timely manner and avoiding side effects due to overexpression of these genes. Stringent regulation of therapeutic gene expression within specific cells or in a localized anatomical region by means of cell-type-specific and/or targeted vector systems coupled to efficient transcriptional switches will significantly decrease the potential safety risks. This is especially relevant for chronic neurological diseases like multiple sclerosis and Alzheimer and Parkinson diseases, which exhibit progressive symptoms over time. The goal of clinical gene therapy utilizing emerging Tet-regulatable systems is to manage patients’ symptoms successfully and efficiently by administering an exogenous drug that could turn the therapeutic gene expression on and off as required. There are several other promising drug/hormone-regulatable systems that have been encoded within different vector systems; these include rapamycin and progesterone analogue-inducible systems. Some of the challenges still remaining in all regulatable gene expression systems include leaky gene expression in the off state, which is crucial to address, especially in the case of proteins that could exert untoward toxic effects if expressed long term; lack of penetration of the inducer to the target tissue/organ; and untoward immune effects of the transcriptional activator proteins.
Delivery Systems Available for Gene Therapy
The aim of gene therapy is to introduce therapeutic genes into target cells, leading to efficient and stable expression of the therapeutic molecules and minimizing any putative adverse inflammatory or cytotoxic side effects. This can be achieved using viral and nonviral vectors. Important parameters to be considered when choosing a gene therapy vector include: (1) size limitations for insertion of transgenes, (2) purity and titer of the vector, (3) transduction efficiency, (4) ability to infect dividing and/or quiescent cells, (5) long-term expression of transgenes, (6) integration into the host genome, (7) the need for cell-type specificity or targeted delivery, and (8) vector-associated toxicity and immunogenicity.
Viral Vectors
Viruses can easily enter cells and deliver their genetic material into the nucleus of target cells; therefore, they are in most cases more efficient than nonviral delivery systems (Fig. 1). Most vectors used for gene delivery are derived from human viral pathogens that have been made nonpathogenic by deleting essential viral genes. They usually have a broad tropism; therefore they can infect and deliver their encoded transgenes to a wide spectrum of cells and/or tissues. The most commonly used viral vectors for gene therapy are adenovirus (Ad), adeno-associated virus (AAV), herpes simplex virus type 1-derived vectors (HSV-1), and retrovirus/lentivirus vectors (Table 1). We will briefly discuss the main groups of viral vectors currently being developed for gene therapy applications. These vectors have also been used to encode regulatable gene expression systems.
FIG. 1.
“Gutless” viral vectors for gene transfer/therapy applications. The helper viral DNA contains viral genes encoding structural proteins and proteins for viral genome replication. The vector genome contains the therapeutic gene expression cassette, which is flanked by the inverted terminal repeats (ITR) and cis-elements that are required for genome encapsidation. Upon cotransfection or infection, the vector and helper virus genome are introduced into a packaging cell line. The helper viral genome lacks the packaging signal, rendering the helper virus DNA unpackageable, but it provides the viral functions that are required for replication of the vector DNA, producing viral structural proteins and packaging the vector DNA into virions. Once released from the packaging cells, the viral vector is ready for further purification and titration.
TABLE 1.
Viral vectors used in gene therapy applications
| Vector | Viral genome |
Cloning capacity |
Tropism | Inflammation | Vector genome forms |
Main limitations | Main advantages |
|---|---|---|---|---|---|---|---|
| Enveloped Retrovirus |
RNA | 8 kb | Dividing cells only |
Low | Integrated | Integration might induce oncogenesis in some applications |
Stable gene expression Transduces dividing cells; integration might induce insertional mutagenesis and oncogenesis |
| Lentivirus | RNA | 8 kb | Broad | Low | Integrated | Toxic and inflammatory; transient transgene expression in target tissues except neurons |
Stable gene expression in replicating cells |
| HSV-1 | dsDNA | 40 kba 150 kbb |
Neurons/ broad |
High | Episomal | Stable gene expression in most tissues |
|
| Nonenveloped | Large cloning packaging capacity; broad cell tropism and strong tropism for neurons |
||||||
| AAV | ssDNA | <5 kb | Broad | Low | Episomal (>90%), Integrated (<10%) |
Small cloning capacity | Broad cell tropism; noninflammatory and nonpathogenic |
| Adenovirus | dsDNA | 7.9 kba 30 kbc |
Broad | High | Episomal | Capsid mediates an inflammatory response; preexisting anti-Ad antibodies in most humans |
Highly efficient transduction of most tissues; large cloning capacity; high titer and long-term expressionc |
HSV-1, herpes simplex type 1 recombinant vector; AAV, adeno-associated viral vector; ssDNA, single-stranded DNA; dsDNA, double-stranded DNA.
Replication defective.
Amplicon.
Helper-dependent high capacity adenoviral vectors.
Adenoviral vectors
Adenoviruses are a family of DNA viruses characterized by a capsid containing a linear double-stranded DNA genome of 36 kb. Adenovirus infection is initiated when the fiber protein binds to the coxsackievirus and adenovirus receptor on the cell surface [1]. The penton then binds to integrins αvβ3 and αvβ5 on the cell surface, which facilitates viral internalization by endocytosis [2]. Once inside the cell, the Ad virion disassembles, during which the adenovirus hexon capsid protein remains at the nuclear membrane while the viral DNA is released into the nucleus and remains as an episome [3].
First-generation adenoviral vectors (RAd) have been developed for gene therapy based on human adenovirus type 2 and human adenovirus type 5 and were made replication defective through deletions in the E1 and E3 regions. The transcriptional cassette can be inserted into the E1 region, yielding a recombinant E1/E3-deleted Ad vector. In one of the most frequent systems to produce these RAds the viral genomes are transfected into human 293 cells that express the E1 proteins in trans, allowing for E1-deleted Ad vector replication and packaging. However, these RAd vectors have residual expression of viral genes that leads to a strong host immune response, resulting in the generation of a high titer of neutralizing anti-capsid antibodies that inhibit reinfection with the same serotype of Ad vector [4-6]. At high viral doses, this viral gene expression leads to immune-mediated cellular cytotoxicity, which results in an immune-mediated loss of the Ad vector-transduced cells [7,8]. To overcome this limitation, a series of Ad vectors with multiple deletions has been developed, eliciting reduced toxicity in animal models [9-11]. More recently, a newer generation of helper-dependent high-capacity adenoviral vectors (also known as high-capacity, “gutless” or “gutted” vectors), which are devoid of all viral coding sequences [12-16] have been developed. These vectors have a minimum requirement for the extreme termini of the linear adenoviral genome, containing only those cis-acting elements needed for viral DNA replication and packaging, mainly the inverted terminal repeat sequences (ITR) and the packaging signal (Ψ). Since these elements are contained approximately 500 bp from the ends of the genome [17], these helper-dependent vector genomes have the potential to carry from a few hundred base pairs up to approximately 36 kb of foreign DNA, which is close to the size of the wild-type Ad genome. HC-Ads are copropagated with an E1-deleted helper virus, which provides in trans all the proteins required for the propagation of the vector. Since the 293 cell line used to propagate HC-Ad also expresses Cre or Flpe recombinase, it causes the excision of the Ψ in the helper genome, rendering it unpackageable. HC-Ad vectors can efficiently transduce a wide variety of cell types from numerous species in a cell-cycle-independent manner. HC-Ad vectors have the added advantage of an increased cloning capacity compared to other viral vectors, and due to their reduced immune responses and toxicity, they elicit stable transgene expression in vivo both in peripheral organs and in the central nervous system [4,8,18-23]. The limitations of HC-Ad vectors include difficulty in large-scale production and helper virus contamination. If these obstacles can be surmounted by improving viral vector production techniques, the HC-Ad vectors will become one of the key viral vectors for gene therapy applications.
Adeno-associated virus vectors
The adeno-associated virus is a linear, single-stranded DNA parvovirus, which is currently being developed as a gene therapy vector for the treatment of numerous diseases. During AAV recombinant vector (rAAV) production, cap and rep sequences are provided by a helper plasmid, and a recombinant vector is easily rescued by co-infection with adenovirus [24].
Initially, AAV vectors were developed by replacing viral genes with transgene sequences. The second generation of rAAV vectors consists of only a transgene-expressing cassette flanked by ITRs, which prevents the formation of wild-type AAV during vector production [25]. Recently, a new rAAV production system completely free of Ad helper virus has been developed. This system consists of a plasmid containing a mini-Ad genome capable of propagating rAAV in the presence of AAV rep and cap genes. Transfection of 293 cells with the new mini-Ad helper and AAV packaging plasmids results in high-titer rAAV vectors [26]. The most significant limitation of rAAVs is their relatively small packaging capacity of approximately 4.7 kb. Currently, the packaging capacity of these vectors has been increased by exploiting the fact that rAAV genomes concatemerize after transduction [27]. This means that two vectors can be used, one encoding the first half of a protein and the other encoding the second half. When both are transduced into cells, head-to-tail stitching of the viral genomes results in the reconstitution of a functional gene, effectively increasing the size of the gene that can be delivered [28,29]. Usually, rAAVs remain within the target tissues as episomal entities, although it has been demonstrated that they can also integrate into the host cell’s genome [25].
Retroviral and lentiviral vectors
Retrovirus- and lentivirus-derived vectors constitute a group of RNA viral vectors that can integrate into the host cellular genome unlike many other viruses that remain episomal. This ability to integrate into host DNA makes these vectors an attractive choice for gene therapy applications. Once the vector integrates, the transgene of interest will be copied during DNA replication of the host cell, allowing for prolonged (up to 2 years) transgene expression, which is essential for chronic therapeutic applications [30]. However, there have been documented cases in which transgene expression was gradually silenced over time [31].
Theoretically, the integrated viral DNA would be inherited and this may have beneficial effects for the treatment of genetic diseases, in which inheritance of a therapeutic transgene may be a promising form of preventive therapy. However, this approach is not currently being developed. Also, integration of a gene within random chromatin sites could have the added side effects of oncogene activation, tumor suppressor gene inactivation, or insertional mutagenesis, leading to possible oncogenic transformation in the target cells [32-34].
The most widely used retroviral vectors for gene therapy applications have been the oncoretroviruses. These viruses can transduce only dividing cells, which poses a problem for gene therapy applications for nondividing cells such as those found in the CNS. One of the most commonly used oncoretroviruses is Moloney murine leukemia virus (MMLV), and pseudotyping was first performed using these viral vectors. The vesicular stomatitis virus G protein (VSV-G)was used to pseudotype the MMLV envelope glycoprotein. This modification confers higher stability to the vector particles and allows for viral preparations with higher titers.
Lentiviral vectors are members of the retrovirus family that can transduce nondividing as well as dividing cells [35-39]. Many lentiviral vectors are derived from the human immunodeficiency virus (HIV) and are modified such that less than 5% of the parental genome is retained in the vector and less than 25% of the viral genome is incorporated into packaging constructs. These small percentages minimize the probability of replication-competent revertants. Lentiviral vectors are often pseudotyped with VSV-G. The biosafety of these retroviral vectors has been further increased through the development of self-inactivating vectors that contain deletions of the regulatory elements in the downstream long terminal repeat sequence, thus preventing the transcription of the packaging signal that is required for vector mobilization [40]. Another safety precaution for preventing lentiviruses from becoming replication-competent was the cloning of the viral genes needed for virus packaging and replication in two separate plasmids [41]. Lentiviral vectors have been proposed for treating a wide range of diseases such as hematopoietic disorders and CNS diseases [42-44].
Herpes simplex type 1 vectors
Herpes simplex virus type 1 is a human pathogen and has probably infected around 80% of the population. Once infection occurs in the epithelium, the virus travels to the CNS ganglia via retrograde axonal transport, where it establishes latency as an episome; under reactivation conditions, the virus spreads from the ganglia via anterograde transport to the epithelium (recurrence) where virus replication is active [45]. HSV establishes latency in the nervous system and can therefore exist in an individual for his or her entire lifetime. HSV contains a double-stranded DNA molecule with two unique sequences, which are linked by internal repeat sequences and flanked by terminal repeats. There are about 80 genes in the viral genome, half of which are nonessential. These nonessential genes are deleted during vector development allowing for approximately 50 kb of foreign DNA to be inserted [46]. HSV vectors are rendered recombination deficient to ensure that the stringent biosafety requirements necessary for gene transfer and gene therapy applications are met. Recombination deficiency is achieved by deleting the immediate early genes, such as ICP0, ICP4, ICP22, ICP27, and ICP 47, which are needed for lytic infection and expression of all other viral proteins. The ICP0 is needed for viral replication and is also essential for long-term, high-level transgene expression. Therefore, the production of multiple immediate early gene-deleted HSV-1 vectors is a balance between efficiency, persistent transgene expression, and cytotoxicity. Mutations in the Vmw65 and IE3 genes are also used to prevent viral replication [47,48]. Some viral gene deletions can lead to cytotoxicity in the brain [49]. The use of multiple deletions, including Vmw65, IE1, and IE3, results in reduced cytotoxicity and is therefore a promising vector manipulation for gene therapy [50-56]. The herpesviruses are nonintegrative vectors and, therefore, they remain episomal.
Herpesviral vectors have been used in CNS gene therapy due to their ability to persist in a latent state in neurons. Long-term transgene expression is achieved by using a neuron-specific latency-activated promoter [57-59]. HSV-based vectors are either recombinant viruses containing the gene of interest [60,61] or plasmid-based constructs encompassing certain viral sequence elements that allow for the replication and packaging of the plasmid DNA into HSV capsids in the presence of a defective helper virus [62,63]. Using replication-defective HSV vectors, highly encouraging results have emerged from recent preclinical studies in models of neurological disease including glioma, peripheral neuropathy, chronic pain, and neurodegeneration [64].
Some of the drawbacks of these vectors include the immune response that develops upon their administration and their cytotoxicity [56,65-68]. Another disadvantage to HSV vectors is that a large percentage of the human population has been exposed to herpesviruses and therefore has antibodies to the virus that would hamper the use of HSV vectors for gene therapy applications. However, it has been shown that the use of HSV vectors in the CNS in the presence of a preexisting immune response did not significantly affect transgene expression [69].
Nonviral Vector-Mediated Gene Delivery
In trying to circumvent safety issues inherent to the use of viral vectors, development of numerous nonviral vectors is actively being pursued. The underlying principle of nonviral vector systems is to complex DNA that carries a therapeutic gene with molecules that will facilitate DNA entry into the cells of interest. Complexed DNA binds to the cell membrane, triggering either nonspecific or receptor-mediated endocytosis. Upon entry into the cell these complexes are contained in endosomes. The ability of these complexes to escape from endosomes before lysosomal enzymes destroy them is an essential characteristic of a successful nonviral vector. Once released from the endosomes, these complexes must enter the nucleus to undergo transcription. To be successfully transcribed, complexed DNA must be released from its carrier molecules and stably express RNA. Aside from avoiding the issues of viral safety, nonviral vector DNA remains episomal, allowing long-term and also high levels of gene expression, i.e., in muscle cells for Duchenne muscle dystrophy [70], glial cells and primary neurons [71], fibroblasts for lysosomal storage disorders [72], glial cells for cerebral ischemic diseases [73], and glioblastoma cells [74]. There are several types of nonviral vector systems being explored to find optimal carrier systems. Although uncomplexed DNA has been successfully used to transfect skeletal muscle [75], systemic administration has been unsuccessful due to clearance of DNA by serum nucleases [76,77].
The majority of nonviral vector systems use cationic lipids, polymers, or both as carriers. In these systems negatively charged DNA is strongly attracted to the lipid or polymer, and this interaction causes condensation of the DNA [78,79]. Cationic lipids, which self-assemble into vesicles that adsorb DNA, are known as lipoplexes. The stability of these lipoplexes is dependent on the lipid:DNA ratio [80]. Since lipoplexes are electrostatically, but nonspecifically, attracted to cellular membranes, the specificity of this carrier is low.
Polymers like poly-L-lysine (PLL) and polyethylenimine (PEI) have been used in vitro to introduce DNA more efficiently into cells. The PLL:DNA ratio is essential for successful condensation and efficient intracellular release from endosomes [80]. However, the use of PLL as a carrier agent is limited due to its toxicity. To circumvent this toxicity and prevent rapid clearance from serum, modifications to the basic PLL—DNA complex via conjugation to other substances have been explored. Conjugation to polyethylene glycol (PEG) chains, 2-hydroxypropyl methacryl amide polymer, low-density lipoprotein, and palmitoyl—PEG have all produced more efficient transduction of target cells [81-86]. The targeting specificity of PLL complexes can be increased through the use of specific receptors, antibodies, sugars, peptides, or growth factors [87-95].
PEI polymers are highly branched molecules with an excellent buffering capacity, making efficient the release of DNA from endosomes. As with other polymer systems, the PEI:DNA ratio is an essential consideration for the toxicity and stability of the vector. Conjugation, as with PLL, can improve cell targeting and uptake. Carbohydrates, integrin-binding peptides, transferrin, and antibodies have been successfully used to increase uptake and targeting specifically for PEI [96-100]. While PLL and PEI have been extensively studied, several other cationic polymers are under investigation, including dendrimers [101], Polybrene [102], gelatin [103], tetraminofullerene [104], poly-L-histidine—graft—poly-L-lysine [105], and chitosan [106].
While nonviral vectors are easy to produce and have low immunogenicity, issues of toxicity, specificity, and transfection efficiency must be addressed before wide clinical implementation will be possible.
Regulatable Gene Expression Systems for Gene Therapy Applications
Gene therapy’s success hinges on several factors including site, duration, and levels of gene expression. Regulatory systems have been developed to control the temporal expression of a target gene in vitro and in vivo. Currently, the tetracycline regulatory system [107] is the most widely used and versatile system. We will review this system and will also briefly discuss other regulatory systems that have potential for gene therapy applications.
Initially, promoters that were responsive to a variety of environmental or physiological changes, including heat shock [108], metal ions [109], interferons or double-stranded RNA [110], and steroids [111], were developed as putative regulatable gene expression systems. More recently, a lac operator—IPTG-based system and an FKB12—rapamycin-associated protein/FK106 binding protein were tested in vitro and in vivo [112,113]. Many of these systems suffer from limitations and are currently unsuitable for use in clinical gene therapy. However, the steroid hormone receptor regulatory system is promising for gene therapy applications and will be discussed further in this section.
Steroid hormone receptors are the largest group of transcription factors in the mammalian proteome. The endogenous ligands of steroid receptors cross epithelial barriers and plasma membranes with ease. Ligands bind to their receptors in the cytoplasm and these ligand—receptor complexes can then be translocated to the nucleus where they regulate gene expression [114]. However, a number of drawbacks are associated with steroid hormone receptor regulatory systems. Inducers or repressors of target genes may also modulate endogenous gene expression in cells. Conversely, physiological changes in natural ligand expression may affect expression of the target gene. Several groups have successfully designed strategies to surmount some of the problems associated with steroid hormone receptor regulatory systems [115].
An elegant example of this is the progesterone receptor regulatory system. The progesterone receptor is an attractive regulatory system to be further developed for gene therapy. In addition, the effects of several synthetic progesterone receptor agonists and antagonists have been characterized [116]. This system also contains a gene that encodes the progesterone receptor with a C-terminal truncation, which prevents binding with progesterone, yet the truncated receptor retains the ability to bind with the antagonist mifepristone (RU 486). Furthermore, mifepristone acts as an agonist, promoting transcription of reporter genes containing progesterone-responsive elements [117]. To improve the specificity of this regulatory system, the truncated progesterone receptor was fused with the Gal4 DNA binding domain and VP16 activation domain, a eukaryotic transactivator derived from HSV-1. This chimeric protein induces transcription more than 10-fold in vivo at a dose of mifepristone well below the threshold required to induce abortions in women. However, concentrations of mifepristone used to induce this activation are similar to estrogen replacement therapies currently used during menopause [118]. This system reduces many problems associated with steroid hormone regulatory systems. A modified version of this regulatory system has been tested in high-capacity adenoviral vectors. This vector induced potent liver-specific expression of human growth hormone (hGH) in vitro and in vivo only in the presence of mifepristone. In addition, only slight decreases in transgene expression were evident in animals, even after 4 weeks of continuous mifepristone administration [119]. An advantage of steroid hormone receptors is that the majority of the system comprises modified human proteins and should not be a potent activator of the immune system. However, inducers of the steroid hormone receptor system are generally able to activate native steroid hormone receptors in cells in addition to regulating transgene expression. As a consequence, such systems may have significant side effects or may not be suitable for use in all individuals.
A strategy designed to overcome the limitations of steroid hormone receptor regulatory systems was the utilization of a nonmammalian steroid hormone receptor. Ecdysone receptor (EcR) is a steroid hormone receptor involved in triggering metamorphosis in Drosophila melanogaster. A different class of steroid ligands called ecdysteroids bind to EcR, and the ecdysone-responsive element is also unique [120]. EcR offers a number of advantages over mammalian steroid receptors. Ecdysteroids have short half-lives, which aids in precise, potent gene induction. In addition, studies indicate that ecdysteroids are relatively nontoxic and nonteratogenic in mammals and do not appear to affect mammalian physiology [121]. Systemic delivery of an adenoviral vector with an ecdysone reporter system in mice showed in vivo favorable baseline and rapid and robust induction of reporter gene expression in tumor xenografts [122]. Since steroids diffuse through the blood—brain barrier, the EcR regulatory system was successfully used in a gene therapy study to regulate neuronal excitation using adenoviral vectors [123]. Also, mutation of EcR amino acids critical for 20-hydroxyecdysone binding led to a steroid-insensitive EcR that responds to nonsteroidal ligands [124]. The combination of this mutant with the wild-type EcR could be useful to regulate the expression of two genes in the same cell. This system may eliminate many of the side effects of mammalian steroid hormone receptors, because the DNA binding site of EcR is unique. However, expression of insect proteins in vivo may induce an immune response eliminating transgene expression. Also, the lipid solubility of steroid hormones results in slower metabolism and clearance from the body than highly hydrophilic drugs. This may affect the ability to reduce transgene expression rapidly should an adverse reaction to the therapy result.
Preclinical Applications of Regulatable Gene Expression Systems
Regulatable gene expression systems are an attractive gene therapy development, and potential applications have been assessed in a wide variety of preclinical laboratory models of disease. In this section we highlight some important advances and promising strategies in the most commonly studied diseases, namely cancer, diabetes, arthritis, and ischemia, that make use of inducible gene expression systems. The tetracycline-dependent regulatable system is not included in this section but will be reviewed on its own later in this article.
Cancer
The majority of research into applications for regulatable gene expression vectors is in models of cancer. This is due, in part, to inadequacies of current therapies, including radiotherapy and chemotherapy. One strategy commonly employed is to regulate expression of a cytotoxic gene with a promoter that can be activated by ionizing radiation. The first example of successfully utilizing radiation to induce gene expression in a tumor model was described more than a decade ago. It was found that TNF-α expression, under the control of the Egr-1 promoter, could be increased in response to ionizing X-ray radiation and this was associated with an improved control of tumor growth in comparison with X-ray radiation alone [125]. Since then, other radiation-sensitive promoters, including VEGF, Rec-A, and WAF-1 promoters, have been investigated in preclinical tumor models [126-128]. Induction of gene expression by ionizing radiation offers a number of advantages over other inducible systems for treating cancer. These include the ability to control temporal and spatial gene expression within the ionizing radiation field, reducing damage to adjacent, healthy tissue. In addition, certain genes, including TNF-α and inducible nitric oxide synthase, have synergistic cytotoxic functions when utilized in combination with ionizing radiation [125,129].
The anatomy of growing tumors has been exploited by another commonly employed inducible gene therapy strategy. As a consequence of relatively poor vascularization, the center of most tumors is usually a nutrient-starved and hypoxic environment. Promoters activated by hypoxia or nutrient deficiency have been utilized to drive expression of tumoricidal genes. The glucose-regulated protein 78 promoter was used to express HSV-1 thymidine kinase (TK) and successfully eliminate murine fibrosarcomas [130]. HSV-1-TK was also used successfully to treat hepatocellular carcinoma when expressed by a hypoxic-responsive element/α-fetoprotein promoter [131]. Inducible promoters such as radiation-sensitive or hypoxia-sensitive promoters are highly specific, and ease of use makes them valuable tools for managing certain diseases. Unfortunately, the application of these promoters is limited and they are not suitable for treating the majority of diseases.
Ischemia
Hypoxia-driven promoters are also of great benefit against other diseases, most notably ischemia. Myocardial ischemia can be a chronic illness with repeated bouts of severe hypoxia and can eventually result in myocardial fibrosis and death. By placing hypoxia-responsive elements within the promoter MLC2v, researchers have developed a tissue-specific hypoxia-responsive promoter expressed predominantly in heart tissue that can induce reporter gene expression by up to 400-fold when expressed by adeno-associated viral vectors [132]. Similar vectors expressing VEGF have been demonstrated to increase angiogenesis, improve cardiac function, and reduce myocardial infarcts in mouse model of ischemia [133].
Diabetes
Diabetes is currently treated by daily injections of recombinant insulin to compensate for a deficiency in either production or function of insulin. Although successful for treating the disease, it can be expensive and can also result in a reduction of quality of life due to daily injections and imposed dietary restrictions. Inducible insulin expression by gene therapeutic vectors would circumnavigate many of the disadvantages of conventional insulin therapy. Researchers have investigated the ability to engineer hepatocytes to express and secrete a functional, modified insulin protein under the control of a rapamycin-responsive promoter. Using adenoviral-associated vectors to infect hepatocytes, gene expression was found to be negligible in the absence of rapamycin, but was potently and reversibly expressed in a mouse model of the disease when treated with rapamycin [134]. In a related study, glucose-responsive elements were used to drive insulin expression in hepatocytes in vitro and in vivo. The authors found that fasting blood glucose levels were returned to normal in a rat model of diabetes [135].
Arthritis
Arthritis affects 1% of the population and is primarily a disease of the elderly. It is characterized by chronic and painful inflammation at the joints of individuals. This chronic inflammatory response has been used by researchers attempting to treat symptoms of arthritis with inducible gene therapeutic vectors. Although cytokine-responsive promoters were successfully used to drive the expression of reporter genes in a model of arthritis [136], most current research is now focused on the robust Tet-ON regulatory system for treating this disease [137].
Tetracycline-Dependent Regulatable Gene Expression Systems
The Tet-ON system offers many advantages over other regulatable gene expression systems. The inducer has been used as an antibiotic for decades and it has been well characterized in a clinical setting. It is nontoxic at doses required for gene activation in preclinical and clinical studies, and the margin of safety is high. Tetracycline, (and its analogue doxycycline), is rapidly metabolized and cleared from the body, making it an ideal drug for the rapid increase in expression, long-term expression, and rapid decrease in expression of the desired transgene. The components of the Tet-ON system recognize unique sequences of DNA, and doxycycline does not interfere with native proteins, reducing the potential of serious side effects when using this system. However, because these proteins were derived from bacteria, they may be immunogenic. Further study is required to determine whether the immune system can recognize components of the Tet-ON system over the long time periods required for the treatment of such chronic illnesses as Parkinson disease and multiple sclerosis.
Dynamics of the Tet-OFF and Tet-ON Regulatory Systems
The first studies of Tet-regulatory transcriptional expression systems emerged from experimental studies on the prokaryotic bacterial strain Escherichia coli [138]. Since then, the utilization of Tet-inducible molecular switches to regulate the transcription of genes has become an effective and popular tool to control mammalian gene expression. The Tet-OFF system operates via a coordinated interaction of two important components (Fig. 2A): the Tet repressor protein (TetR) and the tetracycline-response element (TRE). The 23.6-kDa [139,140] protein TetR inhibits the transcription of genes in the tetracycline-resistance operon on the Tn10 transposon. In bacteria, the TetR hinders transcription of these genes by docking to the Tet operator sequences (tetO) in the absence of tetracycline. Fusion of the TetR to a viral protein (VP) domain, VP16, a eukaryotic transactivator derived from herpes simplex virus type 1, converts the TetR from a transcriptional repressor to an activator termed the transactivator (tTA). The tTA is operational under its own promoter and polyadenylation sequences and is autonomous of the TRE-driven transgene encoding cassette. A framework of seven tandem tetO sequences is positioned upstream of the immediate transgene initiation codon sequence and of the minimal cytomegalovirus (CMV) promoter. When the inducer binds to the transactivator, the complex activates the minimal human CMV promoter driving transgene expression. The tetO sequences and the minimal CMV promoter together constitute the TRE. Independent promoters, TRE and CMV or other cell-type-specific promoters, control the transgene and the transactivator. Gene regulation is achieved by the synthesis of transactivators by the tTA-encoded regulatory switch and their further interaction with the tetracycline inducer and TRE. The Tet-OFF system works by switching gene expression on and off in the absence or presence of the inducer, respectively. In the off situation, i.e., upon delivery or presence of the tetracycline derivative antibiotic, the administered inducer binds to the tTA, thereby hindering the capacity of the tTA to become docked to its tetO sequences within the TRE, thus impeding subsequent transcription from the TRE. In the on situation, i.e., upon removal or absence of inducer, tTA binds to its tetO sequences within the TRE, thus activating TRE and inducing gene expression (Fig. 2A).
FIG. 2.
Tetracycline-dependent regulatory systems. (A) Tet-OFF regulatable switch. Tetracycline inducers such as doxycycline (Dox) bind to the transactivator (tTA), resulting in the prevention of its binding to the tetO elements, thereby blocking promoter activation and subsequent gene expression. In the absence of Dox, the binding of the synthesized tTA to the tetO sequences induces promoter activation and turns on gene expression. (B) Tet-ON regulatable switch. The absence of Dox results in the inability of the synthesized tTA to bind to the tetO elements, subsequently blocking promoter activation and subsequent gene expression. The presence of Dox results in its binding to the tTA, and then the tTA/Dox complex binds to the tetO sequences, allowing promoter activation and gene expression.
Randomly induced mutations of the Tet-OFF-based tTA led to the subsequent development of the Tet-ON system [141] (Fig. 2B). Gossen and colleagues induced mutations in the original TetR that is bound to the VP16 domain, which resulted in four amino acid exchanges, yielding a protein that exhibits opposite functions. This mutant, termed rtTA, triggers transcription by activating the TRE only in the presence of the inducer, thereby activating the silent minimal CMV promoter. Transgene expression is switched off in the absence of the inducer. The rtTA reverse transactivator has been utilized successfully by achieving the desired regulation of gene expression in a number of applications, but has some limitations with respect to the precision of gene regulation. Despite the complete withdrawal or absence of the inducer, Tet-ON-based rtTA was found to exhibit some degree of affinity for the Tet operator sequences within the TRE, thus activating the minimal promoter and inducing low levels of gene transcription in the off state. This interaction between the response element and the transactivator, which produces basal activity in the off mode, is the main setback in attaining tight gene regulation using this system.
Since tetracycline-dependent and steroid hormone receptor regulatory systems are the most widely used regulatory systems, studies have attempted to compare both. Adenoviral vectors expressing the chloramphenicol acetyl transferase (CAT) reporter gene under the regulation of either the Tet-ON- or a mifepristone-regulated system were generated and compared. Both systems permit tight control of CAT reporter gene expression in vitro, with induction levels of approximately 1800- (Tet-ON system) and 600-fold (RU486-regulated system) [142]. Though in vitro reporter protein expression with the mifepristone regulatory system was more sensitive to the levels of the inducer, this was not demonstrated in vivo. The ecdysteroid regulatory system was compared with both Tet-ON and Tet-OFF regulatory systems in transient transfection studies. The ecdysteroid muristerone A was reported to boost reporter activity by nearly 1000-fold. In contrast, the Tet-OFF system displayed a 59-fold change in gene expression in the absence of doxycycline and the Tet-ON system showed only a 2.5-fold induction in the presence of doxycycline. The maximum activity of the Tet-ON system was higher than that of all other regulatory systems analyzed but high basal activity of the reporter gene was present even in the absence of doxycycline [121]. Tetracycline is commonly fed to cattle and contaminates fetal bovine serum from sources in the United States and many other countries. The high levels of expression in the absence of doxycycline in the Tet-ON system suggest that medium components were not screened for tetracycline contamination in this study. A number of reports have demonstrated effective silencing of gene expression in the Tet-ON system in the absence of doxycycline [139,143-147]. More recently, novel tetracycline-regulated systems that display high inducibility and very low to negligible basal activity have been developed [148-150].
To improve Tet-ON regulatory systems further, Hillen, Bujard, and colleagues induced random and directed mutagenesis, searching for novel rtTA forms that could enhance tight regulation and minimize background expression in the absence of the inducer [139]. Using Saccharomyces cerevisiae for screening assays, their experiments uncovered five rtTA mutants, from which one mutant transactivator generated virtually negligible basal expression in the absence of the inducer and required a 10-fold lower doxycycline concentration for TRE activation. Quantitative luciferase assay data on the novel mutant transactivator, rtTA2S-M2, revealed tight regulation of Tet-regulatory switch-dependent β-galactosidase gene expression in transfected HeLa X1/6 cells in the presence and absence of the inducer [139].
Another strategy to reduce unwanted gene expression from the Tet-regulatable system in the off state was also pursued successfully by Bujard and colleagues. A chimeric protein incorporating a modified TetR fused to transcriptional silencing domains of the protein Kid was generated. When this protein (tTSKid) was coexpressed with rtTA proteins it was unable to heterodimerize. This was due to modifications on the dimerizing surfaces of the TetR component. In vitro studies of the silencing efficiency demonstrated that aberrant reporter transgene expression was reduced by as much as 1000-fold by tTSKid in the absence of Dox. Furthermore, tTSKid did not significantly affect maximal expression of the reporter transgene [151].
A multicomponent regulatory switch composed of a tetracycline response promoter coengineered with rtTA2S-M2 transactivator and a tTSKid repressor element, separated by an internal ribosome entry site (IRES), has been shown to be very effective in achieving tight regulation of transgene expression in mice and nonhuman primates [148,149]. Lamartina and co-workers engineered and examined ex vivo a bicistronic vector containing a single regulatory cassette, encompassing the rtTA2S-M2 and tTSKid elements. In the absence of doxycycline, the rtTA2S-M2/tTSKid-encoded Tet-regulatory switch produced negligible basal activity. In the presence of doxycycline, there was a 1000-fold inducibility of serum alkaline phosphatase gene expression and elevated sensitivity to doxycycline [148]. Such novel transcriptional regulatory switches are advantageous for tight regulation of transgene expression to facilitate further their application for gene therapies [150].
Effects of Different Ratios of TRE to Transactivators on Regulation, Kinetics, and Levels of Transgene Expression Using the Tet-Regulatable System
The VP16 transactivator domain is an important unit of the regulatory switch that interacts with a diverse pool of transcriptional regulatory proteins, exhibiting tight regulation of a gene of interest [152]. The use of different versions of the transactivator elements revealed important data on the ratios of the TRE promoter and tTA transactivator elements required to produce the desired transgene induction levels, while simultaneously minimizing basal expression of the transgene in the uninduced state. Studies on the rtTA and tTA transactivator ratios versus levels of TRE appear to produce varying results as to what proportion of each element is required for optimal induction levels and negligible basal expression [153,154].
While some authors report that a 1:1 tTA:TRE ratio is required for optimal induction and negligible background expression [155], others report the need for excess tTA vs TRE [153,156,157]. This highlights the dependency of the system on the promoters used for driving the expression of the transactivator, cell lines, or in vivo target organ; the delivery system used; and the sensitivity of the method used to evaluate levels of transgene expression. It is therefore important to set up the optimal conditions for each application or target organ.
Characteristics of an Ideal Tet-ON Regulatory System for Clinical Gene Therapy Applications
It has become apparent that tight regulation of therapeutic genes is of critical importance in clinical gene therapy. Tet-ON-based regulatable gene-expression systems are preferred for use in clinical gene therapeutics principally because of the constant administration of antibiotics needed to silence gene expression with the Tet-OFF system. The Tet-OFF switch system would allow for transgene expression only in the absence of antibiotics, and a continuous administration of tetracycline would be required to switch off the system. The constant high levels of antibiotics in the general circulation could lead to other complications such as patients’ increased tolerance to the tetracycline derivative, cytotoxicity, or adverse side effects. Therefore, novel Tet-ON transactivator systems are more attractive in a clinical setting as they do not require constant administration of the inducer and can be switched on only when needed. Nevertheless, choosing the optimal Tet-dependent regulatory system will rely on the pathogenicity and etiology of the disease.
The optimal Tet-ON gene regulatory system should exhibit (a) no basal activity of the tTA/TRE unit in the absence of the tetracycline inducer, (b) good regulation and induction kinetics, (c) quick response to the administration or removal of the inducer, (d) stringent transgene regulation, and (e) negligible cytotoxic or inflammatory responses associated with the regulatory elements within the switch system. For a regulatory system to be used in clinical gene therapy, stringent gene regulation must be achieved such that transgene induction and protein synthesis do not occur in the absence of the inducer. When dealing with therapeutic transgenes, the basal expression of the transgene in the absence of a tetracycline-derived inducer, known as “leakiness,” has been the major obstacle in achieving stringent regulation kinetics. Newly emerging transactivators, like the rtTA2S-M2, provide superior control of gene expression in vivo, allowing tightly regulated gene expression [148,149].
The regulatory switch engineered with a strong promoter, i.e., mouse CMV [158], to drive transactivator gene expression, will exhibit higher levels of transgene expression upon induction with a given dose of the inducer [150]. The higher levels of therapeutic transgene expression attained in the on state will allow the use of lower titers of the vector to express and regulate the therapeutic gene during treatment (Fig. 3). This will minimize the chance of induced toxicity and inflammation due to high vector doses [158,159]. High sensitivity of the transactivator to the tetracycline inducer is a desirable feature in this kind of system since enhanced sensitivity means that lower antibiotic concentrations will be required to trigger transgene expression. The quantity, concentration, and frequency of antibiotic administration needed to attain the desirable levels of therapeutic product are crucial factors that require careful evaluation when considering antibiotic-based gene control systems for clinical use (Table 2). The well-characterized tetracycline derivative doxycycline is a broadly employed antibiotic in medical treatment largely due to its nontoxic effects. A recent study by Chtarto et al. [146] revealed an additional tetracycline derivative that was shown to be effective and less cytotoxic than doxycycline in vivo. Minocycline, an antibiotic that is considered to possess antiapoptotic and anti-inflammatory properties, exhibits reduced cytotoxicity and more rapid elimination from the body than doxycycline. Although improved doxycycline-based Tet-ON regulatory systems show promising results, additional studies are needed to advance the effectiveness and minimize the side effects of antibiotics used in conjunction with the Tet-ON system.
FIG. 3.
An ideal gutless vector for regulatable gene therapeutic strategies. A gutless vector engineered with combined regulatory and therapeutic cassettes to express genes efficiently and regulate expression tightly. The Tet-ON regulatory switch turns transgene expression on and off in the presence and absence, respectively, of the tetracycline derivative. In addition to a TRE obligatory for tTA binding to the tetO sequences and promoter induction, cell-type-specific promoters will be essential, should localized transgene expression be needed. An ideal regulatory switch should be under the control of a strong promoter such as the murine cytomegalovirus promoter, which can trigger high transactivator activation and subsequently generate high induction levels of the transgene. The switch should contain transcriptional silencers such as the tTSKid repressor to avoid significant TRE/tTA interaction and consequently achieve virtually negligible background expression in the off state. In addition to utilizing effective promoters and transcriptional silencers, engineered transgene cassettes with promoters driving the transgene and transactivator spaced apart maximally can minimize promoter cross talk in the off state, thus generating tighter regulatable gene expression.
TABLE 2.
Considerations for successful clinical gene therapy employing antibiotic-based transcriptional regulatory systems
| 1. Evaluate the safest and most effective antibiotic concentrations required for short- and long-term therapeutic gene induction |
| Determine duration and levels of therapeutic induction needed, which in turn rely on the type of disease and relapsing symptoms |
| Establish this by means of sufficient preclinical testing in vitro and in vivo in animal models |
| Evaluation of therapeutic outcomes and levels and duration of transgene expression from preclinical studies and phase I human clinical trials |
| 2. Determine the possible adverse side effects of repeated antibiotic administration over time |
| Good understanding of pharmacokinetics upon periodic re- administration and withdrawal of the inducer |
| Analyze possible side effects from tolerance to the inducer during long-term treatment of antibiotic-based gene regulation |
| Studies involving various periods of antibiotic administration and withdrawal as needed should the disease symptoms recur over time |
| 3. Evaluate possible molecular and cellular mechanisms of putative inflammatory and cytotoxic side effects caused by overexpression of transactivators |
Regulatable Tetracycline-Dependent Gene Expression Systems Used in Preclinical Gene Therapy Applications
Tetracycline-inducible promoter elements have been encoded within several gene delivery systems and used in preclinical animal models. We will discuss the main vector systems that have been used to transfer genes under regulation of the tetracycline-inducible promoters, since this is the most widely characterized system available to date.
Regulating Transgene Expression from First-Generation Adenoviral Vectors
A number of studies utilizing first-generation adenoviral vectors with Tet-regulatory systems targeted at specific cell types produced successful transgene regulation within the brain in vivo [156,160-162]. Smith-Arica and co-workers [156] have established effective doxycycline-dependent transcriptional regulation and cell-type-specific transcriptional targeting, producing successful regulatable transgenesis in cell lines, primary brain cultures, and localized regions within the rat brain. A first-generation adenoviral vector encoding the glial fibrillary acidic protein promoter driving the expression of β-galactosidase under the control of the Tet-OFF regulatory switch produced localized doxycycline-dependent β-galactosidase expression within glial cells in vitro and in vivo in a dose-dependent manner [156]. The neuron-specific enolase (NSE) promoter was used to induce expression of β-galactosidase in the absence of doxycycline within neurons. Although the NSE promoter did not exhibit neuronal-restricted transgene expression in vitro, it attained successful neuronal specificity in vivo [156]. These results indicate that the choice of a specific promoter designed for expressing a therapeutic or cytotoxic gene does not always result in the expected localized transgene expression when tested in vitro; therefore it is imperative to test these systems in vivo. Another study that employed the prolactin promoter, specific to anterior pituitary lactotrophic cells, encoded within a first-generation adenoviral vector driving the Tet-OFF regulatory cassette elicited inducible β-galactosidase expression within lactotrophs, both in vitro and in vivo [160].
Using the Tet-OFF system, successful regulation of tyrosine hydroxylase (TH) expression elicited effective regression of estrogen-induced pituitary prolactinomas in vivo [163]. TH is the rate-limiting enzyme in the dopamine biosynthetic pathway. Dopamine, in addition to its principal role in keeping the motor circuitry of the basal ganglia intact, is recognized as having inhibitory effects on adenohypophysial prolactin-producing cells of the pituitary gland. An adenoviral vector engineered with a TH transgene cassette and a Tet-OFF regulatory switch produced regulated TH expression in anterior pituitary (AP) cell lines [163]. Also, rats with estrogen-induced hyperprolactonemia and pituitary lactotroph hyperplasia showed regression of pituitary mass and normalization of serum prolactin levels when injected with the Ad engineered vector into the AP gland, in the absence of doxycycline, resulting in normal levels of circulating prolactin [163]. A first-generation Ad was used in mammary cancer cell lines for the expression of human H2 preprorelaxin (hH2), a precursor of the protein relaxin whose role is critical in tumor cell migration, attachment, and invasion. These in vitro experiments demonstrated that the addition of inducer led to hH2 expression, tumor cell migration, and proliferation [164]; this represents a new tool for the study of mammalian tumor physiology. Tietge and co-workers [168] generated a RAd encoding the human group IIA secretary phospholipase A2 (sPLA2), a protein that has been shown to have the ability to degrade plasma membrane phospholipids [165,166] and possibly to play a role in removal of metabolically compromised and injured cells [167]. Unregulated expression of sPLA2 might be toxic to 293 cells, thereby preventing RAd generation and scale up. When a RAd encoding a group IIA secretary phospholipase A2 under the Tet-OFF regulatable system (Adtet-OFF.hsPLA (2)) was utilized, the authors describe sPLA2 expression in vitro but they could not detect plasmatic sPLA2 protein in vivo. Providing additional tTA protein either by stable transfection or by co-infection with a tTA-expressing Ad resulted in increased levels of sPLA2 and β-galactosidase expression. Plasmatic sPLA2 was detectable in a tTA dose-dependent pattern that lasted for 7 days, and β-gal expression was observed in the lung and liver exhibiting a tTA dose-dependent pattern [168]. This system represents an interesting tool for the generation of RAds encoding potentially toxic genes, allowing vector multiplication in the absence of transgene expression. Gene delivery using viral vectors can result in long-term sustained transgene expression in the subretinal space, the area that separates the photoreceptor outer segments from the retinal pigment epithelium (RPE), resulting in vector transduction of RPE [169,170]. Due to the unique immune-privileged status of this site, systemic immune responses are minimized [171], making it possible to deliver genes to the ocular cells and control them much like any other conventional drug, administered as needed for therapeutic purposes. Dejneka et al. [147] described that when a RAd encoding the hGH under the Tet-ON system was administered to the subretinal space in mice, 100% of RPE cells were transduced within 48 h of administration. The hGH produced in the retina was secreted into the bloodstream in a doxycycline dose-dependent manner [147]. The delivery route and the gene regulation described in this paper offer a unique way to evaluate gene function in the eye and represent a novel method for introducing therapeutic proteins into the retina. One example of a Tet-OFF-based regulatory system using a bidirectional tetracycline-responsive promoter driving both the transgene and the regulatory switch cassettes is the regulation of single-chain interleukin 12 (IL-12) gene expression using first-generation adenoviral vectors [172,173]. Block and co-workers [172] described the adenoviral-mediated expression of murine IL-12 under Tet regulation, encoded within the same Ad vector. IL-12 is chosen for cancer gene therapy because it can stimulate antitumoral immune responses and it exerts proliferation of T lymphocytes and NK cells. Thus, the cytolytic activity of these cells is enhanced by IL-12-mediated production of interferon-γ. Furthermore, IL-12-secreting tumor cells were shown to induce a strong antitumoral response in different models [173,174]. The authors demonstrate that IL-12 expression was suppressed up to 6000-fold in the presence of doxycycline, resulting in a considerable decrease in interferon-γ secretion by activated splenocytes and, consequently, a marked reduction in immune response [175]. Considering that the systemic administration of recombinant human IL-12 has been associated with severe side effects like hemorrhagic colitis, leukopenia, and elevated liver enzymes; the development of a RAd providing regulatable single-chain IL-12 expression for the transduction of tumor cells would significantly contribute to the safety of these innovative approaches in cytokine gene therapy. For applications in neurological diseases, direct injections into the striatum and the substantia nigra of RAds encoding the neurotropic factor glial-derived neurotropic factor (GDNF) have been shown to induce growth and protection of viable dopaminergic neurons [176-181]. These results using Ad-vector-mediated GDNF gene transfer may substantially slow dopaminergic neuronal cell loss in patients with Parkinson disease [180]. Kojima and co-workers [182] described the Tet-regulated expression of human GDNF preventing dopamine depletion in brain striatum, in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced injury to nigrostriatal neurons [182]. They found human GDNF to be expressed at high levels in vitro in primary cultures of rat nigral neurons. By injecting 2 × 106 PFUs into MPTP-treated mice in vivo, they found a decreased loss of dopamine content in the injected side of the striatum (to 16%), whereas administration of the control virus expressing β-gal did not have this effect (9%). Although the administration of a higher dose was supposed to provide a more beneficial effect, the delivery of 5 × 107 PFUs showed a lower protection against MPTP-induced dopaminergic cell loss, probably due to vector-induced neurotoxicity [183].
Regulating Transgene Expression from High-Capacity Adenoviral Vectors
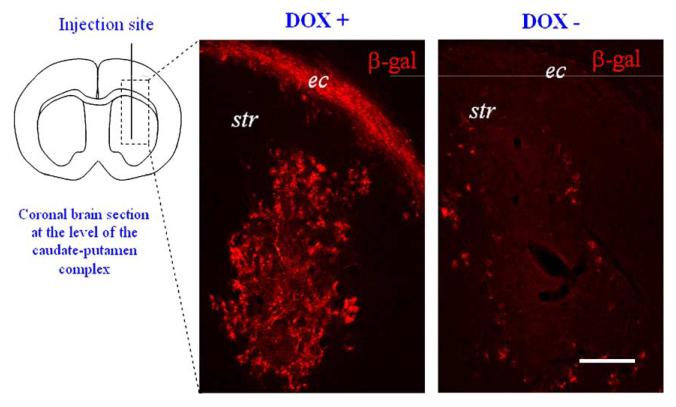
The first in vivo gene transfer and expression study employing a combined Tet-ON transactivator and repressor system using HC-Ad vectors produced tight regulation kinetics with maximal induction in the on state, while minimizing leakiness of gene expression in the off state [143]. Engineered with the Tet-ON transactivator rtTA2S-M2 and the transcriptional repressor tTSKid, doxycycline-dependent secreted alkaline phosphatase reporter gene expression was tightly regulated upon intramuscular injection of the engineered HC-Ad vector in C57/B6 and Balb/C nude mice [143]. In this study, the IRES addition to ensure translation of the tTSKid silencer downstream of the CMV promoter-driven transactivator produced an almost 100,000-fold increase in transgene expression in stable HeLa cell lines. Tight regulation of reporter gene expression was achieved using HC-Ad vector constructs with head-to-head configuration of the promoters driving the transgene and regulatable switch cassettes [143]. These results are also consistent with another report of adeno-associated viral vector-based transcriptional gene regulation using the Tet-ON system. In this study, the two promoters driving the expression of the transgene and transactivator were maximally spaced from each other in a head-to-head configuration, producing tight regulation kinetics of transgene expression while sustaining minimal levels of background activity in the uninduced state [184]. When analyzed together, these data suggest that the distance between the individual promoters and the orientation of the transgene- and transactivator-encoding cassettes could play an important role in achieving good induction kinetics. Also, the promoters positioned closer together might induce possible cross talk between them, thus increasing the likelihood of transactivator activation, thereby inducing transgene expression in the absence of the inducer. The first studies of intrastriatal injections of the Tet regulatory switch encoded within first-generation vectors, i.e., RAd hGDNF, demonstrated tight regulation of the transgene expression in both neuronal and glial cells [182]. Our own results using HC-Ad indicate that these vectors engineered with the rtTA2S-M2/IRES/tTSKid regulatory switch produced strong β-galactosidase expression within the striatum in the induced state and negligible basal activity in the uninduced state (Fig. 4) [150,185]. Immunohistochemistry and fluorescence confocal microscopy analysis revealed colocalization of transduced β-galactosidase expression within neurons and astrocytes in the striatum (Fig. 5) [150]. The strong murine CMV promoter driving the transactivator produced high transgene expression levels with negligible basal activity in the uninduced state compared to the Ad vectors encoding β-galactosidase driven by the constitutive mCMV or hCMV promoter [8,186].
FIG. 4.
Rat striatum sections showing doxycycline (DOX)-dependent β-galactosidase (β-gal) expression in cells infected with a high-capacity, gutless adenovirus vector, i.e., pSTK120m[TRE-βGal-pA]-[mCMV-rtTA2SM2-IRES-tTSkid-pA]. Lewis rats were injected with 1 × 107 infectious units of the HC-Ad vector into the striatum. Twenty-four hours before surgery rats were given drinking water containing 2 mg/ml DOX. Rats were perfused, fixed, and processed for β-galactosidase immunogenicity and confocal microscopy 4 days after HC-Ad delivery. Note the expression of β-gal-immunoreactive cells in the striatum (str) and external capsule (ec) in rats treated with DOX (+) compared with negligible background expression levels in animals not treated with DOX (-). Scale bar, 500 μm.
FIG. 5.

Striatal sections of rats injected with HC-Ad vector pSTK120m[TRE-βGal-pA]-[mCMV-rtTA2SM2-IRES-tTSkid-pA] showing β-gal expression in (A) neurons and (B) astrocytes. Neurons were detected by double immunostaining for β-galactosidase and microtubule-associated protein-2 (MAP-2) (A). Astrocytes were detected by double immunostaining for β-galactosidase and glial fibrillary acidic protein (GFAP) (B). Scale bar, 50 μm.
Thus utilizing these HC-Ad vectors, engineered to encode specific neurotropic factors under the control of a regulatory switch containing the rtTA2S-M2 transactivator, in conjunction with transcriptional silencers, tight regulation of neurotropic factor gene expression as would be needed for gene therapy of Parkinson disease should be achievable. Another useful application of tetracycline-controlled transactivators emerged from studies conducted on the molecular basis of long-term potentiation (LTP) to understand further the mechanisms of learning and memory. Synaptic plasticity, a key player in the LTP phenomenon, has been thought to be under the control of protein kinases and protein phosphatases that are dependent on the enzyme calcium/calmodulin-dependent protein kinase IIα (CaMKIIα) [187]. Using the original Tet-ON rtTA system, Hedou and Mansuy [188] demonstrated that protein activity of phosphatase CN, a protein opposing CaMKIIα, is important for determining synaptic strength and subsequently LTP. The application of upgraded tetracycline-dependent molecular switches may be utilized to examine further and establish the role of these important kinases in the LTP pathway.
Since HC-Ad are much safer than their predecessor first-generation adenoviral vectors, regulatable switches engineered within these viral vectors will be critical to acquiring safe levels and regulation of transgene expression in vivo. The current issues that require further research are to examine the extent of HC-Ad vector-induced immune responses, establishing viral vector stability and spread once injected into the targeted tissue or organ, and to determine safe viral vector titers needed for effective reversion of an affected phenotype in a clinical setting (Table 2).
Regulating Transgene Expression from Retroviral Vectors
Engineered retroviral vectors produced successful transgene regulation using the Tet-OFF-based tetracycline-dependent system. One of the first Tet-regulatory systems to be used in a retroviral vector involved a modified Tet-OFF/tTA that produced successful and stable transgene expression of the VSV-G toxin gene in mammalian cell lines in the absence of the doxycycline inducer [189]. In this study, a modified version of the tetracycline-controlled inducible system was produced by the addition of a ligand-binding domain from the estrogen receptor (ER) to the carboxy terminus of the tTA, yielding tTA-ER. A single retroviral vector, encoding the VSV-G gene regulated by the tTA-ER element, exhibited a 30-fold induction in the absence of the inducer, 17β-estradiol, in transfected mammalian cell lines, producing stable expression of the tTA-ER. Moreover, the addition of the ER ligand-binding domain to the tTA was shown to reduce tTA toxicity in the induced state in the absence of 17β-estradiol [189]. In another study using the Tet-OFF tetracycline-responsive expression system, a single retroviral vector containing TRE and the HSV-1-TK cytotoxic gene under the control of the tetO synthetic promoter produced high levels of TK gene expression in transduced murine NIH 3T3 cells in the absence of the inducer tetracycline [190]. They observed a 417-fold increase in HSV-1-TK enzyme activity in NIH 3T3 cells compared to enzyme activity in control cells. These in vitro studies demonstrate good efficiency in attaining high levels of HSV-1-TK expression to improve gene therapy strategies for brain tumors. Studies by Kenny and co-workers [191] established successful Tet-OFF-based regulation from retroviral vectors and demonstrated the effectiveness of the TRE promoter in achieving stringent regulation of gene expression [191]. Upon evaluation of different promoters to drive tTA expression, such as CMV, elongation factor 1α, and phosphoglycerate kinase-1, in combination with the TRE they observed that only the CMV promoter in combination with the TRE promoter produced successful regulatable β-galactosidase expression when controlled by the Tet-OFF regulatory switch in HC11 mouse mammary epithelial cell lines [191].
Although stable and efficient regulatable transgenesis can be attainable with retroviral vectors, their restricted cloning size of 8 kb constrains their wide use in gene therapy. Hofmann et al. [192] describe a single autoregulatory cassette, containing tandem Tet operator sequences and the cytomegalovirus minimal promoter driving the expression of a bicistronic mRNA, leading to transcription of the gene of interest (β-gal) and the internal ribosome entry site-controlled transactivator (Tetrepressor—VP16 fusion protein). This system allowed reversible induction of transgene expression in response to Tet; in the absence of Tet there was a progressive increase in transactivator by means of an autoregulatory loop, whereas in the presence of Tet, gene expression was prevented [192]. The use of this retroviral vector resulted in rapid gene delivery of inducible genes into cell types, i.e., primary mouse myoblasts, in which direct transfection has low efficiency [192].
Regulating Transgene Expression from Lentiviral Vectors
Lentiviral vectors have been demonstrated to transduce successfully and drive gene expression in a number of mammalian cells and animal models [42,193] and have been engineered using the Tet-ON- and Tet-OFF-based tetracycline-dependent systems to achieve successful regulation of gene expression in vitro into 293 cells [194], ex vivo in cells of the central nervous system [195], and in vivo in a rat model for Huntington disease [196]. Kafri et al. [194] demonstrated that after administration of a green fluorescent protein (GFP)-expressing lentiviral vector to rats, in a variety of tissues including differentiated neurons, myotubes, hepatocytes, and hematopoietic stem cells, under the regulation of the Tet system, GFP expression was tightly regulated in vivo following addition or withdrawal of Dox in the drinking water. They described a 500-fold increase in cellular GFP levels upon withdrawal of the inducer from the culture medium in transfected mammalian cell lines. Successful regulatable transgenesis was also achieved in vivo following transduction of rat brain; doxycycline-regulated GFP expression was observed in terminally differentiated neurons and was regulated by adding or withdrawing doxycycline from the rats’ drinking water [194]. In addition to single-gene-engineered lentiviral vectors, doxycycline-mediated regulation was also successfully attained from bicistronic lentiviral vectors, as demonstrated by Reiser and co-workers [144]. A dual HIV-1-based vector system, one vector encompassing the rtTA-based Tet-ON construct and the other encoding the TRE and an enhanced (E) GFP reporter gene, generated effective regulatable EGFP expression in primary human skin fibroblasts (HSFs) in the presence of doxycycline [144]. Fluorescence microscopy of HSFs transduced with the NL-rtTA/TRE-EGFP bicistronic vector demonstrated a 1.7-fold difference in fluorescence intensities in the presence and absence of doxycycline. CD81 is a transmembrane protein that is upregulated in the mesolimbic dopaminergic pathway after acute administration of high doses of cocaine [197]. Its expression contributes to some of the behavioral changes associated with cocaine sensitization [198]. A lentiviral vector encoding CD81 under the control of the Tet-ON system was stereotaxically delivered into the ventral tegmental area (VTA) of two groups of rats that were given water alone (CD81 expression) or water with doxycycline solution (down-regulates CD81 expression). A 3.2-fold augmented locomotor activity was observed in those animals expressing CD81 in the VTA vs animals placed on doxycycline, which downregulated CD81 expression. Additionally, when the administration of the virus was to the nucleus accumbens, it resulted in higher effects, yielding a 4.2-fold increase in locomotor activity [198].
Regulating Transgene Expression from Adeno-associated Vectors
One of the first successful applications of achieving regulatable gene expression within the rAAV vectors was the efficient Tet-ON-based regulation of the green fluorescent protein. Two rAAV vectors, one engineered with a GFP-encoding cassette and the other with the silencer-flanked rtTA Tet-ON transactivator tTSKid-rtTA, were constructed [199]. Subretinal injection of these engineered rAAV vector particles into rats produced tightly regulated GFP expression. In vitro studies performed by the same group also demonstrated that a higher ratio of silencer-encoded AAV vector to transactivator-encoded AAV vector produced higher levels of gene expression in the on state compared with an equal ratio of the two vectors. The data also showed that an 8:1 ratio of the silencer-encoded vector to the transactivator-encoded vector produced an almost 12-fold increase in gene expression. However, compared to an equal ratio of the two vectors, basal activity in the absence of the inducer was not further suppressed when using the 8:1 ratio [199]. Studies by Rendahl et al. [145] also reported Tet-ON-based stringent gene regulation in vivo by utilizing two rAAV vectors, one with a regulatory switch-encoding vector and the other with an erythropoietin (Epo) transgene-encoding vector. The tTSKid repressor and rtTA-engineered rAAV vector produced both tight and prolonged levels of Epo expression upon intramuscular injection of the engineered vector in mice in the presence of doxycycline [145]. Periodic administration of the inducer over a 32-week period produced constant Epo expression, achieving successful long-term transgene regulation [145]. It has been described that leptin, the product of the Ob gene, acts on satiety centers in the hypothalamus to both decrease food intake and increase energy expenditure. Virus-mediated leptin gene delivery has been shown to cause a rapid and complete disappearance of white adipose tissue in genetically normal animals [200]. In a study by Wilsey et al. [201] the use of two rAAV vector systems yielded concise Tet-ON-dependent regulation of the expression of leptin in young rats injected with the viral vector into the hypothalamus, producing weight gain in the induced state [201]. The transgene-engineered rAAV vector contained the inducible TetR promoter, driving a bicistronic construct for the expression of Ob and GFP with the addition in the construct of the woodchuck hepatitis virus posttranscriptional regulatory element (WPRE) downstream of the transgene to augment expression. In the presence of doxycycline, the silencer-encoded rtTA induced Ob gene expression, producing an 8-fold increase in hypothalamic leptin [201].
In addition to successful tetracycline-inducible transgene expression using dual AAV vectors, the utilization of a single AAV vector encoding an autoregulatory cassette that encompasses a bidirectional tetracycline-responsive promoter using the Tet-dependent regulatory system was also effective [146]. Tet-OFF systems with bidirectional tetracycline-responsive promoter-encoded cassettes designed into AAV vectors produced successful transgene expression in the uninduced state [202,203]. Folliot et al. [204] used a single rAAV vector engineered with the Tet-OFF regulatory switch devoid of the silencer, which elicited efficient and prolonged transgene regulation of GFP reporter gene expression. Intravitreal injections of a single rAAV vector coengineered with a destabilized GFP gene and Tet-OFF tTA regulatory cassette produced only 5% residual expression of GFP 48 h after doxycycline administration, sustaining GFP transduction shutdown for as long as 1 week in ganglion cells.
In an another study by Chtarto and co-workers [146], an autoregulatory rAAV vector was engineered with the Tet-ON rtTA and the EGFP reporter gene, both driven by a bidirectional Tet-responsive promoter and the complete cassette flanked with bidirectional SV40 polyadenylation sites at both ITRs. The autoregulatory cassette produced around 50-fold induction of EGFP expression in the presence of doxycycline in human tumor cell lines, and in vivo data revealed an 80-fold increase in the induced state [146]. Slow regulation kinetics was observed after doxycycline administration but rapid termination of the gene expression was observed in the uninduced state. However, the use of new Tet-ON-based transactivators encoded with silencers and post-transcriptional elements will be difficult using rAAV vectors due to the constrained cloning space within the core rAAV vector. One feasible approach of regulating transgene expression using rAAV vectors is through a bidirectional autoregulatory cassette, in which more cloning capacity is available for including silencer elements and other short transgenes. In addition to the type of Tet-ON transactivator employed to attain optimal gene regulation, the configuration of the transgene and regulatory switch cassettes was found to be an important factor in achieving maximum gene expression and virtually negligible expression in the on and off state, respectively. In a recent report by Chenuaud et al. [184], an optimal design of a single recombinant AAV using two independent promoters was established with the use of the Tet-ON transactivator rtTA2S-M2. Promoters driving the transactivator and the erythropoietin DNA reporter gene-encoding cassettes situated distant from each other, but oriented in the same direction, produced maximal induction and minimal background activity in the off state when injected intramuscularly into mice [184].
Recombinant AAV vector-based studies suggest advantages to using these vectors to regulate gene expression, but there are also possible drawbacks with respect to their application in human gene therapy. Due to the predetermined cloning capacity of 5 kb of the AAV vectors, most of the studies described to date utilize two vector systems to deliver both the transgene and the regulatory cassettes, limiting the choice of transgenes to clone. Second, in the clinical perspective, the administration of a higher ratio of silencer- and transactivator-encoding rAAV vector to therapeutic gene-encoding rAAV vector to achieve higher transgene expression levels can result in delivery of a combined higher vector dose, thus creating a greater potential for adverse immune responses. In a Tet-OFF rAAV vector, gene expression has been quantified by very precise techniques [205]; Tet-regulated transgene expression has been studied in rat retinal ganglion cells [204], the regulated expression of erythropoietin from a AAV vector has been proven to improve anemia in a mouse model of β-thalassemia [206], and Tet-inducible IL-10 expression has been applied to the experimental arthritis model [207]. The interaction between the Tet-ON/OFF regulatory systems and the mammalian immune system has yet to be fully characterized. The degree of immune response to a viral vector can be a consequence of one or more factors that include the method and route of vector delivery at the target site [208], cell-type-specific gene expression [209], and immunogenicity of the vector capsid and the transactivator elements making up the regulatory switch [210].
Regulating Transgene Expression from Herpes Simplex Vectors
A number of studies generated successful and efficient regulatable transgenesis from HSV-1-derived amplicon vectors engineered with the Tet-OFF tetracycline-responsive system. In one study, localized and high levels of β-galactosidase reporter gene expression were observed in the dendrate gyrus of the mouse brain with over 10-fold suppression in gene expression and controlled cytotoxicity in the presence of doxycycline [211]. In another study, effective inducible reporter gene expression was effectively regulated in vitro and in vivo with a Tet-OFF system-based HSV-based amplicon vector [212]. Hippocampal cultures infected with the engineered amplicon vectors produced over 50-fold repression of firefly luciferase reporter gene expression in the presence of tetracycline, with maximal gene expression levels achieved within 10–12 h after the withdrawal of tetracycline [212]. Adding tetracycline to cultures without prior tetracycline exposure resulted in quick silencing of gene expression, by which luciferase reporter gene activity was reduced to less than 8% within 24 h. Maximum gene expression levels from the amplicon vectors occurred within 2–3 days postinfection and gradually declined subsequently [212]. Combinations of emerging amplicon vectors and superior Tet-ON- and Tet-OFF-based tetracycline-regulatable system technologies have been shown to generate better and effective HSV-1-derived ampliconmediated therapeutic gene transfer. One example of successful implementation of adapted Tet-regulatable systems can be shown from work by Schmeisser and co-workers [213]. In an attempt to find suitable tetracycline-inducible HSV vector-based systems that exhibit enhanced induction characteristics and flexibility, several combinations of TRE regulatory/promoter elements were constructed to regulate β-galactosidase reporter gene expression in cells [213]. These promoters contained a TRE linked to a minimal CMV immediate early promoter, a minimal HSV ICP0 promoter, or a truncated HSV ICP0 promoter. The ICP0 promoter constructs expressed the highest and most sustained levels of β-gal. Vectors were also constructed that coexpressed an inducible Tet transactivator in addition to the inducible β-gal marker gene. This resulted in tetracycline-inducible gene expression that was not restricted to specific cell lines, and this vector was capable of inducible expression in irreversibly differentiated NT2 cells (NT neurons) for several days [213]. In another study, Herrlinger et al. demonstrated tetracycline-mediated regulation of the luciferase reporter gene under the control of either the Tet-OFF or the Tet-ON system from HSV-1 mutants in infected Vero cells [214]. The study revealed the role and importance of selective transactivating factors encoded by immediate early genes and their effect on the TRE complex and transgene regulation. The presence of specific transactivating factor proteins and viral proteins has been shown to induce the tetO/minimal TRE complex in the uninduced state, resulting in their interference in generating effective tetracycline-regulated transgene expression [214]. Transient transfection into Vero cells of a mutant HSV-1 expressing ICP4 and VP16 transactivating factors failed to minimize or eliminate basal levels of transgene expression in the uninduced state with the Tet-OFF and Tet-ON systems. In contrast, mutant HSV-1 vectors devoid of ICP4 and VP16 abolished this basal activity in the uninduced state, suggesting the likely role of specific viral proteins in generating an effective tetracycline-based regulatory system [214]. This study demonstrates that in the HSV-1 genome, especially the transactivating immediate early gene products (ICP4, ICP27, and ICP0) and the VP16 tegument protein can activate the tetO/minimal CMV promoter and thereby interfere with the integrity of tetracycline-regulated transgene expression [214].
Regulatable Expression Systems as Applied to Clinical Trials: Importance and Key Challenges for Their Implementation in Human Patients
The goal of gene therapy is to provide treatment for diseases that currently have limited or no therapeutic options. Below, we will review the importance and challenges of the future implementation of regulatable gene expression systems in gene therapy clinical trials. To date, phase I, II, and III clinical trials have utilized retroviral or adenoviral vectors in treating malignant glioma; meanwhile, the first trial utilizing an adeno-associated virus for Parkinson disease treatment has recently been approved [215]. Several gene therapeutic strategies have been used to treat glioblastoma. In two such trials, although some degree of immune response was elicited, a combination of herpes simplex-thymidine kinase/ganciclovir after surgical resection of recurrent glioblastoma was observed to be relatively safe and showed statistically significant efficacy [216,217]. In this scenario, the use of an inducible gene expression system would allow shutting down of expression of HSV-1 TK once tumor regression occurs as assessed by MRI or other imaging systems. Another gene therapy trial for glioblastoma involved intratumoral injection of an adenoviral vector carrying wild-type p53 [218]. A replication-disabled Semliki Forest viral vector carrying the human interleukin 12 gene, which has been shown to be safe and effective in treating breast and prostate cancer in prior animal studies [219], was also used in glioblastoma treatment. Again it would be clinically advantageous to be able to turn therapeutic gene expression off once the therapy is no longer needed. Clinical trials that use viral vectors to treat other neurological diseases such as Parkinson disease are also in progress. One such trial is being proposed for the use of subthalamic, glutamic acid decarboxylase gene transfer via an adeno-associated viral vector [220]. If successfully transduced, the transgene will increase subthalamic nucleus levels of GABA, an inhibitory neurotransmitter involved in the pathological pathway. Although none of the vectors in current use contain regulatable systems within their viral vector constructs, utilization of such regulatable systems will provide greater safety, thereby increasing the potential for clinical use of viral and nonviral vectors for in vivo gene delivery.
Systems in which the transgene must be repressed by the constant presence of a substance such as antibiotics may pose potential clinical problems, such as side effects, making them undesirable. However, the use of regulatable systems in which high-level, specific, and transient expression of a therapeutic gene can be induced, i.e., the Tet-ON regulatable gene expression system, may prove to be a therapeutic powerful tool. Instances in which regulatable switches would have a powerful impact might include turning on a transgene used to eliminate a tumor and subsequently turning it off to prevent aberrant destruction of other tissues or inflammatory reactions. Also when expressing a factor to stimulate growth of specific cells, i.e., GDNF for Parkinson disease, it would be crucial to be able to turn gene expression off or be able to regulate levels of expression tightly to prevent adverse side effects due to abnormal cell growth, aberrant connections between cells, or abnormal neurotransmitter release.
In solid tumors, radiation-inducible promoters have proven very successful in preclinical models of the disease. One such vector, called TNFerade, has been tested in phase I clinical trials on patients with solid tumors and soft-tissue sarcoma’s [221,222]. This replication-deficient adenoviral vector contains the TNF-α gene and expression is regulated by the Egr1 promoter. Although patient numbers were small in these phase I clinical trials, objective responses were high even in patients with refractory tumors. This therapy utilizes the ability to focus ionizing radiation at the tumor to limit damage to normal tissues and side effects were minor in the majority of individuals. Consequently, this novel therapy appears to warrant further investigation in larger, randomized trials.
Myocardial ischemia is also a system in which inducible gene therapy vectors have proven very successful in preclinical models of the disease. Although no clinical trials are yet under way, the vector system currently in development utilizes a hypoxia-sensitive promoter to regulate gene expression [223] and affords us a glimpse of an exciting future in gene therapy in which inducible vectors may lie dormant for months or even years before therapeutic gene expression is switched on in response to danger signals such as hypoxia. Clinical trials in humans with diabetes are unfortunately farther into the future and many technical challenges must still be overcome, including correct processing of the insulin transgene and stable expression of an inducible system that must be switched on and off many thousands of times. However, early research in liver cells has validated the use of gene therapy to replace insulin levels and perhaps an approach unifying stem cell research and gene therapy can go some way to surmounting these obstacles in the future.
Combining regulatable switches with other genetic elements, i.e., the WPRE and regions of the HSV-1-TK gene, known to increase mRNA stability and levels of transgene expression will further enhance their usefulness by promoting long-term and widespread distribution of transgene expression [224]. Alternatively, the development of strategies to promote predetermined therapeutic gene insertion within the host genome will also enhance the utility and safety of gene transfer vectors. Utilization of regulatable switches driven by strong promoters will allow high levels of transgene expression, thus requiring less viral vector dose to be utilized. This in turn will minimize possible toxicity and inflammation, making these viral vectors safer for clinical use. Development of tools that allow for greater control of gene expression will reduce untoward side effects, creating wider and safer clinical settings in which gene therapies could be implemented.
ACKNOWLEDGMENTS
Work described in this review is funded by the National Institute of Neurological Disorders & Stroke (NINDS), Grant 1 R01 NS44556.01; by NIDDK Grant 1 RO3 TW006273-01 to M.G.C.; by NINDS Grants 1 RO1 NS42893, U54 4 NS04-5309, and R21 NS47298 to P.R.L.; by The Linda Tallen & David Paul Kane Annual Fellowship to M.G.C. and P.R.L.; and by the Board of Governors at Cedars—Sinai Medical Center. We also thank the unparalleled support and academic leadership of Dr. Shlomo Melmed. We are grateful to Mr. Richard Katzman and Dr. David Meyer for their superb administrative support.
REFERENCES
- 1.Bergelson JM, et al. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 2.Nemerow GR, Stewart PL. Role of alpha(v) integrins in adenovirus cell entry and gene delivery. Microbiol. Mol. Biol. Rev. 1999;63:725–734. doi: 10.1128/mmbr.63.3.725-734.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leopold PL, et al. Fluorescent virions: dynamic tracking of the pathway of adenoviral gene transfer vectors in living cells. Hum. Gene Ther. 1998;9:367–378. doi: 10.1089/hum.1998.9.3-367. [DOI] [PubMed] [Google Scholar]
- 4.Morral N, et al. Administration of helper-dependent adenoviral vectors and sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc. Natl. Acad. Sci. USA. 1999;96:12816–12821. doi: 10.1073/pnas.96.22.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowenstein PR, Castro MG. Inflammation and adaptive immune responses to adenoviral vectors injected into the brain: peculiarities, mechanisms, and consequences. Gene Ther. 2003;10:946–954. doi: 10.1038/sj.gt.3302048. [DOI] [PubMed] [Google Scholar]
- 6.Lowenstein PR, Castro MG. Recent advances in the pharmacology of neurological gene therapy. Curr. Opin. Pharmacol. 2004;4:91–97. doi: 10.1016/j.coph.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christ M, et al. Modulation of the inflammatory properties and hepatotoxicity of recombinant adenovirus vectors by the viral E4 gene products. Hum. Gene Ther. 2000;11:415–427. doi: 10.1089/10430340050015888. [DOI] [PubMed] [Google Scholar]
- 8.Thomas CE, et al. Preexisting antiadenoviral immunity is not a barrier to efficient and stable transduction of the brain, mediated by novel high-capacity adenovirus vectors. Hum. Gene Ther. 2001;12:839–846. doi: 10.1089/104303401750148829. [DOI] [PubMed] [Google Scholar]
- 9.Grave L, et al. Differential influence of the E4 adenoviral genes on viral and cellular promoters. J. Gene Med. 2000;2:433–443. doi: 10.1002/1521-2254(200011/12)2:6<433::AID-JGM143>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Hu H, Serra D, Amalfitano A. Persistence of an [E1—, polymerase—] adenovirus vector despite transduction of a neoantigen into immune-competent mice. Hum. Gene Ther. 1999;10:355–364. doi: 10.1089/10430349950018805. [DOI] [PubMed] [Google Scholar]
- 11.Hodges BL, et al. Adenovirus vectors with the 100K gene deleted and their potential for multiple gene therapy applications. J. Virol. 2001;75:5913–5920. doi: 10.1128/JVI.75.13.5913-5920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kochanek S, et al. A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc. Natl. Acad. Sci. USA. 1996;93:5731–5736. doi: 10.1073/pnas.93.12.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parks RJ, et al. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA. 1996;93:13565–13570. doi: 10.1073/pnas.93.24.13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng P, et al. Development of a FLP/frt system for generating helper-dependent adenoviral vectors. Mol. Ther. 2001;3(5 Pt 1):809–815. doi: 10.1006/mthe.2001.0323. [DOI] [PubMed] [Google Scholar]
- 15.Umana P, et al. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat. Biotechnol. 2001;19:582–585. doi: 10.1038/89349. [DOI] [PubMed] [Google Scholar]
- 16.Lowenstein PR, et al. High-capacity, helper-dependent, “gutless” adenoviral vectors for gene transfer into the brain. Methods Enzymol. 2002;346:292–311. doi: 10.1016/s0076-6879(02)46062-4. [DOI] [PubMed] [Google Scholar]
- 17.Grable M, Hearing P. cis and trans requirements for the selective packaging of adenovirus type 5 DNA. J. Virol. 1992;66:723–731. doi: 10.1128/jvi.66.2.723-731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiedner G, et al. Genomic DNA transfer with a high-capacity adenovirus vector results in improved in vivo gene expression and decreased toxicity. Nat. Genet. 1998;18:180–183. doi: 10.1038/ng0298-180. [DOI] [PubMed] [Google Scholar]
- 19.Morsy MA, et al. An adenoviral vector deleted for all viral coding sequences results in enhanced safety and extended expression of a leptin transgene. Proc. Natl. Acad. Sci. USA. 1998;95:7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas CE, et al. Peripheral infection with adenovirus causes unexpected long-term brain inflammation in animals injected intracranially with first-generation, but not with high-capacity, adenovirus vectors: toward realistic long-term neurological gene therapy for chronic diseases. Proc. Natl. Acad. Sci. USA. 2000;97:7482–7487. doi: 10.1073/pnas.120474397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Neal WK, et al. Toxicity associated with repeated administration of first-generation adenovirus vectors does not occur with a helper-dependent vector. Mol. Med. 2000;6:179–195. [PMC free article] [PubMed] [Google Scholar]
- 22.Maione D, et al. An improved helper-dependent adenoviral vector allows persistent gene expression after intramuscular delivery and overcomes preexisting immunity to adenovirus. Proc. Natl. Acad. Sci. USA. 2001;98:5986–5991. doi: 10.1073/pnas.101122498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toietta G, et al. Reduced inflammation and improved airway expression using helper-dependent adenoviral vectors with a K18 promoter. Mol. Ther. 2003;7(5 Pt 1):649–658. doi: 10.1016/s1525-0016(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 24.Hermonat PL, Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc. Natl. Acad. Sci. USA. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samulski RJ, Chang LS, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J. Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakai H, Storm TA, Kay MA. Increasing the size of rAAV-mediated expression cassettes in vivo by intermolecular joining of two complementary vectors. Nat. Biotechnol. 2000;18:527–532. doi: 10.1038/75390. [DOI] [PubMed] [Google Scholar]
- 28.Yan Z, et al. Trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc. Natl. Acad. Sci. USA. 2000;97:6716–6721. doi: 10.1073/pnas.97.12.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma HI, et al. Intratumoral gene therapy of malignant brain tumor in a rat model with angiostatin delivered by adeno-associated viral (AAV) vector. Gene Ther. 2002;9:2–11. doi: 10.1038/sj.gt.3301616. [DOI] [PubMed] [Google Scholar]
- 30.Bordignon C, et al. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA-immunodeficient patients. Science. 1995;270:470–475. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- 31.Pannell D, Ellis J. Silencing of gene expression: implications for design of retrovirus vectors. Rev. Med. Virol. 2001;11:205–217. doi: 10.1002/rmv.316. [DOI] [PubMed] [Google Scholar]
- 32.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, et al. Murine leukemia induced by retroviral gene marking. Science. 2002;296:497. doi: 10.1126/science.1068893. [DOI] [PubMed] [Google Scholar]
- 34.Baum BJ, et al. Advances in vector-mediated gene transfer. Immunol. Lett. 2003;90:145–149. doi: 10.1016/j.imlet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Jakobsson J, et al. Targeted transgene expression in rat brain using lentiviral vectors. J. Neurosci. Res. 2003;73:876–885. doi: 10.1002/jnr.10719. [DOI] [PubMed] [Google Scholar]
- 36.Trobridge G, Russell DW. Cell cycle requirements for transduction by foamy virus vectors compared to those of oncovirus and lentivirus vectors. J. Virol. 2004;78:2327–2335. doi: 10.1128/JVI.78.5.2327-2335.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blesch A. Lentiviral and MLV based retroviral vectors for ex vivo and in vivo gene transfer. Methods. 2004;33:164–172. doi: 10.1016/j.ymeth.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Carlotti F, et al. Lentiviral vectors efficiently transduce quiescent mature 3T3-L1 adipocytes. Mol. Ther. 2004;9:209–217. doi: 10.1016/j.ymthe.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 39.Vogel R, et al. A single lentivirus vector mediates doxycycline-regulated expression of transgenes in the brain. Hum. Gene Ther. 2004;15:157–165. doi: 10.1089/104303404772679968. [DOI] [PubMed] [Google Scholar]
- 40.Zufferey R, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dull T, et al. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naldini L, et al. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Humeau LM, et al. Efficient lentiviral vector-mediated control of HIV-1 replication in CD4 lymphocytes from diverse HIV+ infected patients grouped according to CD4 count and viral load. Mol. Ther. 2004;9:902–913. doi: 10.1016/j.ymthe.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Kearns-Jonker M, et al. Use of lentiviral vectors to induce long-term tolerance to gal(+) heart grafts. Transplantation. 2004;77:1748–1754. doi: 10.1097/01.tp.0000131174.52424.4a. [DOI] [PubMed] [Google Scholar]
- 45.Whitley R. Herpes simplex viruses. In: Fields B, Knipe D, Howley PM, editors. Fields Virology. Lippincott-Raven; New York: 1996. pp. 2297–2342. [Google Scholar]
- 46.Glorioso JC, DeLuca NA, Fink DJ. Development and application of herpes simplex virus vectors for human gene therapy. Annu. Rev. Microbiol. 1995;49:675–710. doi: 10.1146/annurev.mi.49.100195.003331. [DOI] [PubMed] [Google Scholar]
- 47.Ace CI, et al. Construction and characterization of a herpes simplex virus type 1 mutant unable to transinduce immediate-early gene expression. J. Virol. 1989;63:2260–2269. doi: 10.1128/jvi.63.5.2260-2269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeLuca NA, McCarthy AM, Schaffer PA. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J. Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho DY, et al. Herpes simplex virus vector system: analysis of its in vivo and in vitro cytopathic effects. J. Neurosci. Methods. 1995;57:205–215. doi: 10.1016/0165-0270(94)00150-f. [DOI] [PubMed] [Google Scholar]
- 50.Wu N, et al. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J. Virol. 1996;70:6358–6369. doi: 10.1128/jvi.70.9.6358-6369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward PL, Roizman B. Herpes simplex genes: the blueprint of a successful human pathogen. Trends Genet. 1994;10:267–274. doi: 10.1016/0168-9525(90)90009-u. [DOI] [PubMed] [Google Scholar]
- 52.Ho DY, Mocarski ES, Sapolsky RM. Altering central nervous system physiology with a defective herpes simplex virus vector expressing the glucose transporter gene. Proc. Natl. Acad. Sci. USA. 1993;90:3655–3659. doi: 10.1073/pnas.90.8.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.During MJ, et al. Long-term behavioral recovery in parkinsonian rats by an HSV vector expressing tyrosine hydroxylase. Science. 1994;266:1399–1403. doi: 10.1126/science.266.5189.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaplitt MG, et al. Preproenkephalin promoter yields region-specific and long-term expression in adult brain after direct in vivo gene transfer via a defective herpes simplex viral vector. Proc. Natl. Acad. Sci. USA. 1994;91:8979–8983. doi: 10.1073/pnas.91.19.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith RL, et al. Long-term expression in sensory neurons in tissue culture from herpes simplex virus type 1 (HSV-1) promoters in an HSV-1-derived vector. J. Virol. 1995;69:4593–4599. doi: 10.1128/jvi.69.8.4593-4599.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wood MJ, et al. Inflammatory effects of gene transfer into the CNS with defective HSV-1 vectors. Gene Ther. 1994;1:283–291. [PubMed] [Google Scholar]
- 57.Palmer JA, et al. Development and optimization of herpes simplex virus vectors for multiple long-term gene delivery to the peripheral nervous system. J. Virol. 2000;74:5604–5618. doi: 10.1128/jvi.74.12.5604-5618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dobson AT, et al. A latent, nonpathogenic HSV-1-derived vector stably expresses beta-galactosidase in mouse neurons. Neuron. 1990;5:353–360. doi: 10.1016/0896-6273(90)90171-b. [DOI] [PubMed] [Google Scholar]
- 59.Fink DJ, et al. In vivo expression of beta-galactosidase in hippocampal neurons by HSV-mediated gene transfer. Hum. Gene Ther. 1992;3:11–19. doi: 10.1089/hum.1992.3.1-11. [DOI] [PubMed] [Google Scholar]
- 60.Breakefield XO, et al. Gene transfer into the nervous system using recombinant herpes virus vectors. In: Gage F, Christen Y, editors. Gene Transfer and Therapy in the Nervous System. Springer-Verlag; Berlin: 1992. pp. 118–132. [Google Scholar]
- 61.Glorioso JC, et al. Development of herpes simplex virus as a gene transfer vector for central nervous system. In: Gage F, Christen Y, editors. Gene Transfer and Therapy in the Nervous System. Springer-Verlag; Berlin: 1992. pp. 133–145. [Google Scholar]
- 62.Spaete RR, Frenkel N. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell. 1982;30:295–304. doi: 10.1016/0092-8674(82)90035-6. [DOI] [PubMed] [Google Scholar]
- 63.Geller AI, Breakefield XO. A defective HSV-1 vector expresses Escherichia coli beta-galactosidase in cultured peripheral neurons. Science. 1988;241:1667–1669. doi: 10.1126/science.241.4873.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burton EA, Fink DJ, Glorioso JC. Gene delivery using herpes simplex virus vectors. DNA Cell Biol. 2002;21:915–936. doi: 10.1089/104454902762053864. [DOI] [PubMed] [Google Scholar]
- 65.Wood MJ, et al. Immunological consequences of HSV-1-mediated gene transfer into the CNS. Gene Ther. 1994;1(Suppl 1):S82. [PubMed] [Google Scholar]
- 66.Laquerre S, et al. Recombinant herpes simplex virus type 1 engineered for targeted binding to erythropoietin receptor-bearing cells. J. Virol. 1998;72:9683–9697. doi: 10.1128/jvi.72.12.9683-9697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lilley CE, Branston RH, Coffin RS. Herpes simplex virus vectors for the nervous system. Curr. Gene Ther. 2001;1:339–358. doi: 10.2174/1566523013348346. [DOI] [PubMed] [Google Scholar]
- 68.Wakimoto H, et al. Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells. Gene Ther. 2003;10:983–990. doi: 10.1038/sj.gt.3302038. [DOI] [PubMed] [Google Scholar]
- 69.Herrlinger U, et al. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases, but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 1998;5:809–819. doi: 10.1038/sj.gt.3300643. [DOI] [PubMed] [Google Scholar]
- 70.Gillis JM. Utrophin, a way to cure Duchenne muscle dystrophy. Med. Sci. (Paris) 2004;20:442–447. doi: 10.1051/medsci/2004204442. [DOI] [PubMed] [Google Scholar]
- 71.da Cruz MT, Simoes S, de Lima MC. Improving lipoplex-mediated gene transfer into C6 glioma cells and primary neurons. Exp. Neurol. 2004;187:65–75. doi: 10.1016/j.expneurol.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 72.Estruch EJ, et al. Non-viral, integrin-mediated gene transfer into fibroblasts from patients with lysosomal storage diseases. J. Gene Med. 2001;3:488–497. doi: 10.1002/jgm.214. [DOI] [PubMed] [Google Scholar]
- 73.Wang R, et al. Gene transfer of vascular endothelial growth factor plasmid/liposome complexes in glioma cells in vitro: the implication for the treatment of cerebral ischemic diseases. Clin. Hemorheol. Microcirc. 2000;23:303–306. [PubMed] [Google Scholar]
- 74.Hsiao M, et al. Intracavitary liposome-mediated p53 gene transfer into glioblastoma with endogenous wild-type p53 in vivo results in tumor suppression and long-term survival. Biochem. Biophys. Res. Commun. 1997;233:359–364. doi: 10.1006/bbrc.1997.6459. [DOI] [PubMed] [Google Scholar]
- 75.Wolff JA. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 76.Houk BE, Hochhaus G, Hughes JA. Kinetic modeling of plasmid DNA degradation in rat plasma. AAPS PharmSci. 1999;1:E9. doi: 10.1208/ps010309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niven R, et al. Biodistribution of radiolabeled lipid-DNA complexes and DNA in mice. J. Pharm. Sci. 1998;87:1292–1299. doi: 10.1021/js980087a. [DOI] [PubMed] [Google Scholar]
- 78.Golan R, et al. DNA toroids: stages in condensation. Biochemistry. 1999;38:14069–14076. doi: 10.1021/bi990901o. [DOI] [PubMed] [Google Scholar]
- 79.Dunlap DD, et al. Nanoscopic structure of DNA condensed for gene delivery. Nucleic Acids Res. 1997;25:3095–3101. doi: 10.1093/nar/25.15.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schatzlein AG. Non-viral vectors in cancer gene therapy: principles and progress. Anticancer Drugs. 2001;12:275–304. doi: 10.1097/00001813-200104000-00001. [DOI] [PubMed] [Google Scholar]
- 81.Wolfert MA, et al. Characterization of vectors for gene therapy formed by self-assembly of DNA with synthetic block co-polymers. Hum. Gene Ther. 1996;7:2123–2133. doi: 10.1089/hum.1996.7.17-2123. [DOI] [PubMed] [Google Scholar]
- 82.Katayose S, Kataoka K. Water-soluble polyion complex associates of DNA and poly(ethylene glycol)-poly (L-lysine) block copolymer. Bioconjugate Chem. 1997;8:702–707. doi: 10.1021/bc9701306. [DOI] [PubMed] [Google Scholar]
- 83.Katayose S, Kataoka K. Remarkable increase in nuclease resistance of plasmid DNA through supramolecular assembly with poly(ethylene glycol)-poly(L-lysine) block copolymer. J. Pharm. Sci. 1998;87:160–163. doi: 10.1021/js970304s. [DOI] [PubMed] [Google Scholar]
- 84.Toncheva V, et al. Novel vectors for gene delivery formed by self-assembly of DNA with poly(L-lysine) grafted with hydrophilic polymers. Biochim. Biophys. Acta. 1998;1380:354–368. doi: 10.1016/s0304-4165(98)00004-x. [DOI] [PubMed] [Google Scholar]
- 85.Choi YH, et al. Polyethylene glycol-grafted poly-L-lysine as polymeric gene carrier. J. Controlled Release. 1998;54:39–48. doi: 10.1016/s0168-3659(97)00174-0. [DOI] [PubMed] [Google Scholar]
- 86.Kim JS, et al. A new non-viral DNA delivery vector: the terplex system. J. Controlled Release. 1998;53:175–182. doi: 10.1016/s0168-3659(97)00251-4. [DOI] [PubMed] [Google Scholar]
- 87.Wu GY, Wu CH. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J. Biol. Chem. 1987;262:4429–4432. [PubMed] [Google Scholar]
- 88.Wu CH, Wilson JM, Wu GY. Targeting genes: delivery and persistent expression of a foreign gene driven by mammalian regulatory elements in vivo. J. Biol. Chem. 1989;264:16985–16987. [PubMed] [Google Scholar]
- 89.Cotten M, et al. Transferrin-polycation-mediated introduction of DNA into human leukemic cells: stimulation by agents that affect the survival of transfected DNA or modulate transferrin receptor levels. Proc. Natl. Acad. Sci. USA. 1990;87:4033–4037. doi: 10.1073/pnas.87.11.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coll JL, et al. In vitro targeting and specific transfection of human neuroblastoma cells by chCE7 antibody-mediated gene transfer. Gene Ther. 1997;4:156–161. doi: 10.1038/sj.gt.3300375. [DOI] [PubMed] [Google Scholar]
- 91.Mahato RI, et al. Physicochemical and disposition characteristics of antisense oligonucleotides complexed with glycosylated poly(L-lysine) Biochem. Pharmacol. 1997;53:887–895. doi: 10.1016/s0006-2952(96)00880-5. [DOI] [PubMed] [Google Scholar]
- 92.Fajac I, et al. Sugar-mediated uptake of glycosylated polylysines and gene transfer into normal and cystic fibrosis airway epithelial cells. Hum. Gene Ther. 1999;10:395–406. doi: 10.1089/10430349950018841. [DOI] [PubMed] [Google Scholar]
- 93.Ziady AG, et al. Ligand substitution of receptor targeted DNA complexes affects gene transfer into hepatoma cells. Gene Ther. 1998;5:1685–1697. doi: 10.1038/sj.gt.3300777. [DOI] [PubMed] [Google Scholar]
- 94.Schaffer DV, Lauffenburger DA. Optimization of cell surface binding enhances efficiency and specificity of molecular conjugate gene delivery. J. Biol. Chem. 1998;273:28004–28009. doi: 10.1074/jbc.273.43.28004. [DOI] [PubMed] [Google Scholar]
- 95.Xu B, et al. The contribution of poly-L-lysine, epidermal growth factor and streptavidin to EGF/PLL/DNA polyplex formation. Gene Ther. 1998;5:1235–1243. doi: 10.1038/sj.gt.3300719. [DOI] [PubMed] [Google Scholar]
- 96.Li S, et al. Targeted gene delivery to pulmonary endothelium by anti-PECAM antibody. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;278:L504–L511. doi: 10.1152/ajplung.2000.278.3.L504. [DOI] [PubMed] [Google Scholar]
- 97.Zanta MA, et al. In vitro gene delivery to hepatocytes with galactosylated polyethylenimine. Bioconjugate Chem. 1997;8:839–844. doi: 10.1021/bc970098f. [DOI] [PubMed] [Google Scholar]
- 98.Bettinger T, Remy JS, Erbacher P. Size reduction of galactosylated PEI/DNA complexes improves lectin-mediated gene transfer into hepatocytes. Bioconjugate Chem. 1999;10:558–561. doi: 10.1021/bc990006h. [DOI] [PubMed] [Google Scholar]
- 99.Diebold SS, et al. Efficient gene delivery into human dendritic cells by adenovirus polyethylenimine and mannose polyethylenimine transfection. Hum. Gene Ther. 1999;10:775–786. doi: 10.1089/10430349950018535. [DOI] [PubMed] [Google Scholar]
- 100.Kircheis R, et al. Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. Gene Ther. 1997;4:409–418. doi: 10.1038/sj.gt.3300418. [DOI] [PubMed] [Google Scholar]
- 101.Bielinska AU, et al. Application of membrane-based dendrimer/DNA complexes for solid phase transfection in vitro and in vivo. Biomaterials. 2000;21:877–887. doi: 10.1016/s0142-9612(99)00229-x. [DOI] [PubMed] [Google Scholar]
- 102.Mumper RJ, et al. Polyvinyl derivatives as novel interactive polymers for controlled gene delivery to muscle. Pharm. Res. 1996;13:701–709. doi: 10.1023/a:1016039330870. [DOI] [PubMed] [Google Scholar]
- 103.Leong KW, et al. DNA-polycation nanospheres as non-viral gene delivery vehicles. J. Controlled Release. 1998;53:183–193. doi: 10.1016/s0168-3659(97)00252-6. [DOI] [PubMed] [Google Scholar]
- 104.Isobe H, et al. Atomic force microscope studies on condensation of plasmid DNA with functionalized fullerenes. Angew. Chem. Int. Ed. Engl. 2001;40:3364–3367. doi: 10.1002/1521-3773(20010917)40:18<3364::aid-anie3364>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 105.Benns JM, et al. pH-sensitive cationic polymer gene delivery vehicle: N-Acpoly(L-histidine)-graft-poly(L-lysine) comb shaped polymer. Bioconjugate Chem. 2000;11:637–645. doi: 10.1021/bc0000177. [DOI] [PubMed] [Google Scholar]
- 106.Liu W. Guang, De Yao K. Chitosan and its derivatives—a promising non-viral vector for gene transfection. J. Controlled Release. 2002;83:1–11. doi: 10.1016/s0168-3659(02)00144-x. [DOI] [PubMed] [Google Scholar]
- 107.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wurm FM, Gwinn KA, Kingston RE. Inducible overproduction of the mouse c-myc protein in mammalian cells. Proc. Natl. Acad. Sci. USA. 1986;83:5414–5418. doi: 10.1073/pnas.83.15.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mayo KE, Warren R, Palmiter RD. The mouse metallothionein-I gene is transcriptionally regulated by cadmium following transfection into human or mouse cells. Cell. 1982;29:99–108. doi: 10.1016/0092-8674(82)90094-0. [DOI] [PubMed] [Google Scholar]
- 110.Ryals J, et al. A 46-nucleotide promoter segment from an IFN-alpha gene renders an unrelated promoter inducible by virus. Cell. 1985;41:497–507. doi: 10.1016/s0092-8674(85)80023-4. [DOI] [PubMed] [Google Scholar]
- 111.Hynes NE, Groner B. Mammary tumor formation and hormonal control of mouse mammary tumor virus expression. Curr. Top. Microbiol. Immunol. 1982;101:51–74. doi: 10.1007/978-3-642-68654-2_3. [DOI] [PubMed] [Google Scholar]
- 112.Hu MC, Davidson N. The inducible lac operator-repressor system is functional in mammalian cells. Cell. 1987;48:555–566. doi: 10.1016/0092-8674(87)90234-0. [DOI] [PubMed] [Google Scholar]
- 113.Rivera VM, et al. A humanized system for pharmacologic control of gene expression. Nat. Med. 1996;2:1028–1032. doi: 10.1038/nm0996-1028. [DOI] [PubMed] [Google Scholar]
- 114.Di Croce L, et al. Steroid and nuclear receptors. EMBO J. 1999;18:6201–6210. doi: 10.1093/emboj/18.22.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burcin MM, O’Malley BW, Tsai SY. A regulatory system for target gene expression. Front. Biosci. 1998;3:c1–c7. doi: 10.2741/a258. [DOI] [PubMed] [Google Scholar]
- 116.Ngan ES, et al. The mifepristone-inducible gene regulatory system in mouse models of disease and gene therapy. Semin. Cell Dev. Biol. 2002;13:143–149. doi: 10.1016/s1084-9521(02)00020-4. [DOI] [PubMed] [Google Scholar]
- 117.Baulieu EE. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science. 1989;245:1351–1357. doi: 10.1126/science.2781282. [DOI] [PubMed] [Google Scholar]
- 118.Wang Y, et al. A regulatory system for use in gene transfer. Proc. Natl. Acad. Sci. USA. 1994;91:8180–8184. doi: 10.1073/pnas.91.17.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burcin MM, et al. Adenovirus-mediated regulable target gene expression in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:355–360. doi: 10.1073/pnas.96.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yao TP, et al. Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature. 1993;366:476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]
- 121.No D, Yao TP, Evans RM. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. USA. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karns LR, et al. Manipulation of gene expression by an ecdysone-inducible gene switch in tumor xenografts. BMC Biotechnol. 2001;1:11. doi: 10.1186/1472-6750-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johns DC, et al. Inducible genetic suppression of neuronal excitability. J. Neurosci. 1999;19:1691–1697. doi: 10.1523/JNEUROSCI.19-05-01691.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kumar MB, et al. A single point mutation in ecdysone receptor leads to increased ligand specificity: implications for gene switch applications. Proc. Natl. Acad. Sci. USA. 2002;99:14710–14715. doi: 10.1073/pnas.222278999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hallahan DE, et al. Spatial and temporal control of gene therapy using ionizing radiation. Nat. Med. 1995;1:786–791. doi: 10.1038/nm0895-786. [DOI] [PubMed] [Google Scholar]
- 126.Kaliberov SA, Kaliberova LN, Buchsbaum DJ. Combined ionizing radiation and sKDR gene delivery for treatment of prostate carcinomas. Gene Ther. 2004;126:407–417. doi: 10.1038/sj.gt.3302432. [DOI] [PubMed] [Google Scholar]
- 127.Nuyts S, et al. Radio-responsive recA promoter significantly increases TNFalpha production in recombinant clostridia after 2 Gy irradiation. Gene Ther. 2001;8:1197–1201. doi: 10.1038/sj.gt.3301499. [DOI] [PubMed] [Google Scholar]
- 128.Worthington J, et al. Modification of vascular tone using iNOS under the control of a radiation-inducible promoter. Gene Ther. 2000;7:1126–1131. doi: 10.1038/sj.gt.3301224. [DOI] [PubMed] [Google Scholar]
- 129.Chung P, et al. Overexpression of the human inducible nitric oxide synthase gene enhances radiation-induced apoptosis in colorectal cancer cells via a caspase-dependent mechanism. Nitric Oxide. 2003;8:119–126. doi: 10.1016/s1089-8603(02)00147-7. [DOI] [PubMed] [Google Scholar]
- 130.Gazit G, et al. Use of the glucose starvation-inducible glucose-regulated protein 78 promoter in suicide gene therapy of murine fibrosarcoma. Cancer Res. 1999;59:3100–3106. [PubMed] [Google Scholar]
- 131.Ido A, et al. Gene therapy targeting for hepatocellular carcinoma: selective and enhanced suicide gene expression regulated by a hypoxia-inducible enhancer linked to a human alpha-fetoprotein promoter. Cancer Res. 2001;61:3016–3021. [PubMed] [Google Scholar]
- 132.Phillips MI, et al. Vigilant vector: heart-specific promoter in an adeno-associated virus vector for cardioprotection. Hypertension. 2002;39(2 Pt 2):651–655. doi: 10.1161/hy0202.103472. [DOI] [PubMed] [Google Scholar]
- 133.Su H, et al. Adeno-associated viral vector delivers cardiac-specific and hypoxia-inducible VEGF expression in ischemic mouse hearts. Proc. Natl. Acad. Sci. USA. 2004;101:16280–16285. doi: 10.1073/pnas.0407449101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Auricchio A, et al. Constitutive and regulated expression of processed insulin following in vivo hepatic gene transfer. Gene Ther. 2002;9:963–971. doi: 10.1038/sj.gt.3301746. [DOI] [PubMed] [Google Scholar]
- 135.Alam T, Sollinger HW. Glucose-regulated insulin production in hepatocytes. Transplantation. 2002;74:1781–1787. doi: 10.1097/00007890-200212270-00024. [DOI] [PubMed] [Google Scholar]
- 136.Pan RY, et al. Disease-inducible transgene expression from a recombinant adeno-associated virus vector in a rat arthritis model. J. Virol. 1999;73:3410–3417. doi: 10.1128/jvi.73.4.3410-3417.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Perez N, et al. Tetracycline transcriptional silencer tightly controls transgene expression after in vivo intramuscular electrotransfer: application to interleukin 10 therapy in experimental arthritis. Hum. Gene Ther. 2002;13:2161–2172. doi: 10.1089/104303402320987851. [DOI] [PubMed] [Google Scholar]
- 138.Hillen W, Berens C. Mechanisms underlying expression of Tn10 encoded tetracycline resistance. Annu. Rev. Microbiol. 1994;48:345–369. doi: 10.1146/annurev.mi.48.100194.002021. [DOI] [PubMed] [Google Scholar]
- 139.Urlinger S, et al. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. USA. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kim HJ, et al. Tetracycline repressor-regulated gene repression in recombinant human cytomegalovirus. J. Virol. 1995;69:2565–2573. doi: 10.1128/jvi.69.4.2565-2573.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gossen M, Bujard H. Efficacy of tetracycline-controlled gene expression is influenced by cell type. Biotechniques. 1995;19:213–216. [PubMed] [Google Scholar]
- 142.Molin M, et al. Two novel adenovirus vector systems permitting regulated protein expression in gene transfer experiments. J. Virol. 1998;72:8358–8361. doi: 10.1128/jvi.72.10.8358-8361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Salucci V, et al. Tight control of gene expression by a helper-dependent adenovirus vector carrying the rtTA2(s)-M2 tetracycline transactivator and repressor system. Gene Ther. 2002;9:1415–1421. doi: 10.1038/sj.gt.3301813. [DOI] [PubMed] [Google Scholar]
- 144.Reiser J, et al. Development of multigene and regulated lentivirus vectors. J. Virol. 2000;74:10589–10599. doi: 10.1128/jvi.74.22.10589-10599.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rendahl KG, et al. Tightly regulated long-term erythropoietin expression in vivo using tet-inducible recombinant adeno-associated viral vectors. Hum. Gene Ther. 2002;13:335–342. doi: 10.1089/10430340252769842. [DOI] [PubMed] [Google Scholar]
- 146.Chtarto A, et al. Tetracycline-inducible transgene expression mediated by a single AAV vector. Gene Ther. 2003;10:84–94. doi: 10.1038/sj.gt.3301838. [DOI] [PubMed] [Google Scholar]
- 147.Dejneka NS, et al. Pharmacologically regulated gene expression in the retina following transduction with viral vectors. Gene Ther. 2001;8:442–446. doi: 10.1038/sj.gt.3301413. [DOI] [PubMed] [Google Scholar]
- 148.Lamartina S, et al. Construction of an rtTA2(s)-m2/tts(kid)-based transcription regulatory switch that displays no basal activity, good inducibility, and high responsiveness to doxycycline in mice and nonhuman primates. Mol. Ther. 2003;7:271–280. doi: 10.1016/s1525-0016(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 149.Lamartina S, et al. Stringent control of gene expression in vivo by using novel doxycycline-dependent trans-activators. Hum. Gene Ther. 2002;13:199–210. doi: 10.1089/10430340252769734. [DOI] [PubMed] [Google Scholar]
- 150.Goverdhana S. Regulatable transgene expression from gutless adenoviral vectors. Molecular Therapy Abstracts; The American Society of Gene Therapy 7th Annual Meeting; Minneapolis. 2004. [Google Scholar]
- 151.Freundlieb S, Schirra-Muller C, Bujard H. A tetracycline controlled activation/repression system with increased potential for gene transfer into mammalian cells. J. Gene Med. 1999;1:4–12. doi: 10.1002/(SICI)1521-2254(199901/02)1:1<4::AID-JGM4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 152.Flint J, Shenk T. Viral transactivating proteins. Annu. Rev. Genet. 1997;31:177–212. doi: 10.1146/annurev.genet.31.1.177. [DOI] [PubMed] [Google Scholar]
- 153.Harding TC, et al. Switching transgene expression in the brain using an adenoviral tetracycline-regulatable system. Nat. Biotechnol. 1998;16:553–555. doi: 10.1038/nbt0698-553. [DOI] [PubMed] [Google Scholar]
- 154.Corti O, et al. A single adenovirus vector mediates doxycycline-controlled expression of tyrosine hydroxylase in brain grafts of human neural progenitors. Nat. Biotechnol. 1999;17:349–354. doi: 10.1038/7901. [DOI] [PubMed] [Google Scholar]
- 155.Corti O, et al. Long-term doxycycline-controlled expression of human tyrosine hydroxylase after direct adenovirus-mediated gene transfer to a rat model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 1999;96:12120–12125. doi: 10.1073/pnas.96.21.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Smith-Arica JR, et al. Cell-type-specific and regulatable transgenesis in the adult brain: adenovirus-encoded combined transcriptional targeting and inducible transgene expression. Mol. Ther. 2000;2:579–587. doi: 10.1006/mthe.2000.0215. [DOI] [PubMed] [Google Scholar]
- 157.Harding TC, et al. Tetracycline-regulated transgene expression in hippocampal neurones following transfection with adenoviral vectors. J. Neurochem. 1997;69:2620–2623. doi: 10.1046/j.1471-4159.1997.69062620.x. [DOI] [PubMed] [Google Scholar]
- 158.Gerdes CA, Castro MG, Lowenstein PR. Strong promoters are the key to highly efficient, noninflammatory and noncytotoxic adenoviral-mediated transgene delivery into the brain in vivo. Mol. Ther. 2000;2:330–338. doi: 10.1006/mthe.2000.0140. [DOI] [PubMed] [Google Scholar]
- 159.Dewey RA, et al. Chronic brain inflammation and persistent herpes simplex virus 1 thymidine kinase expression in survivors of syngeneic glioma treated by adenovirus-mediated gene therapy: implications for clinical trials. Nat. Med. 1999;5:1256–1263. doi: 10.1038/15207. [DOI] [PubMed] [Google Scholar]
- 160.Smith-Arica JR, et al. Switching on and off transgene expression within lactotrophic cells in the anterior pituitary gland in vivo. Endocrinology. 2001;142:2521–2532. doi: 10.1210/endo.142.6.8183. [DOI] [PubMed] [Google Scholar]
- 161.Southgate TD, et al. Transcriptional targeting to anterior pituitary lactotrophic cells using recombinant adenovirus vectors in vitro and in vivo in normal and estrogen/sulpiride-induced hyperplastic anterior pituitaries. Endocrinology. 2000;141:3493–3505. doi: 10.1210/endo.141.9.7639. [DOI] [PubMed] [Google Scholar]
- 162.Castro MG, et al. Cell-type specific expression in the pituitary: physiology and gene therapy. Biochem. Soc. Trans. 1999;27:858–863. doi: 10.1042/bst0270858. [DOI] [PubMed] [Google Scholar]
- 163.Williams JC, et al. Regulated, adenovirus-mediated delivery of tyrosine hydroxylase suppresses growth of estrogen-induced pituitary prolactinomas. Mol. Ther. 2001;4:593–602. doi: 10.1006/mthe.2001.0499. [DOI] [PubMed] [Google Scholar]
- 164.Silvertown JD, Geddes BJ, Summerlee AJ. Adenovirus-mediated expression of human prorelaxin promotes the invasive potential of canine mammary cancer cells. Endocrinology. 2003;144:3683–3691. doi: 10.1210/en.2003-0248. [DOI] [PubMed] [Google Scholar]
- 165.Balsinde J, Balboa MA, Dennis EA. Functional coupling between secretory phospholipase A2 and cyclooxygenase-2 and its regulation by cytosolic group IV phospholipase A2. Proc. Natl. Acad. Sci. USA. 1998;95:7951–7956. doi: 10.1073/pnas.95.14.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Murakami M, et al. Functional coupling between various phospholipase A2s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J. Biol. Chem. 1999;274:3103–3115. doi: 10.1074/jbc.274.5.3103. [DOI] [PubMed] [Google Scholar]
- 167.Hack CE, et al. A role for secretory phospholipase A2 and C-reactive protein in the removal of injured cells. Immunol. Today. 1997;18:111–115. doi: 10.1016/s0167-5699(97)01002-5. [DOI] [PubMed] [Google Scholar]
- 168.Tietge UJ, et al. A tetracycline-regulated adenoviral expression system for in vivo delivery of transgenes to lung and liver. J. Gene Med. 2003;5:567–575. doi: 10.1002/jgm.384. [DOI] [PubMed] [Google Scholar]
- 169.Bennett J, et al. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest. Ophthalmol. Visual Sci. 1994;35:2535–2542. [PubMed] [Google Scholar]
- 170.Li T, et al. In vivo transfer of a reporter gene to the retina mediated by an adenoviral vector. Invest. Ophthalmol. Visual Sci. 1994;35:2543–2549. [PubMed] [Google Scholar]
- 171.Wenkel H, Streilein JW. Analysis of immune deviation elicited by antigens injected into the subretinal space. Invest. Ophthalmol. Visual Sci. 1998;39:1823–1834. [PubMed] [Google Scholar]
- 172.Block A, et al. Amplified Muc1-specific gene expression in colon cancer cells utilizing a binary system in adenoviral vectors. Anticancer Res. 2002;22:3285–3292. [PubMed] [Google Scholar]
- 173.Tahara H, et al. IL-12 gene therapy using direct injection of tumors with genetically engineered autologous fibroblasts. Hum. Gene Ther. 1995;6:1607–1624. doi: 10.1089/hum.1995.6.12-1607. [DOI] [PubMed] [Google Scholar]
- 174.Caruso M, et al. Adenovirus-mediated interleukin-12 gene therapy for metastatic colon carcinoma. Proc. Natl. Acad. Sci. USA. 1996;93:11302–11306. doi: 10.1073/pnas.93.21.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Block A, et al. Highly suppressible expression of single-chain interleukin-12 by doxycycline following adenoviral infection with a single-vector Tet-regulatory system. J. Gene Med. 2003;5:190–200. doi: 10.1002/jgm.334. [DOI] [PubMed] [Google Scholar]
- 176.Hurtado-Lorenzo A, et al. Use of recombinant adenovirus for gene transfer into the rat brain: evaluation of gene transfer efficiency, toxicity, and inflammatory and immune reactions. Methods Mol. Med. 2003;76:113–133. doi: 10.1385/1-59259-304-6:113. [DOI] [PubMed] [Google Scholar]
- 177.Lowenstein PR, et al. Nonneurotropic adenovirus: a vector for gene transfer to the brain and gene therapy of neurological disorders. Int. Rev. Neurobiol. 2003;55:3–64. doi: 10.1016/s0074-7742(03)01001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Do Thi NA. Delivery of GDNF by an E1,E3/E4 deleted adenoviral vector and driven by a GFAP promoter prevents dopaminergic neuron degeneration in a rat model of Parkinson’s disease. Gene Ther. 2004;11:746–756. doi: 10.1038/sj.gt.3302222. [DOI] [PubMed] [Google Scholar]
- 179.Kozlowski DA, et al. Quantitative analysis of transgene protein, mRNA, and vector DNA following injection of an adenoviral vector harboring glial cell line-derived neurotrophic factor into the primate caudate nucleus. Mol. Ther. 2001;3:256–261. doi: 10.1006/mthe.2000.0256. [DOI] [PubMed] [Google Scholar]
- 180.Choi-Lundberg DL, et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- 181.Choi-Lundberg DL, et al. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Exp. Neurol. 1998;154:261–275. doi: 10.1006/exnr.1998.6887. [DOI] [PubMed] [Google Scholar]
- 182.Kojima H, et al. Adenovirus-mediated transduction with human glial cell line-derived neurotrophic factor gene prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopamine depletion in striatum of mouse brain. Biochem. Biophys. Res. Commun. 1997;238:569–573. doi: 10.1006/bbrc.1997.7183. [DOI] [PubMed] [Google Scholar]
- 183.Thomas CE, et al. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther. 2001;3:36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- 184.Chenuaud P, et al. Optimal design of a single recombinant adeno-associated virus derived from serotypes 1 and 2 to achieve more tightly regulated transgene expression from nonhuman primate muscle. Mol. Ther. 2004;9:410–418. doi: 10.1016/j.ymthe.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 185.Xiong W, et al. Tightly regulated helper-dependent adenoviral-mediated transgene expression in vitro and in vivo. Society for Neuroscience, 34th Annual Meeting; San Diego, CA. October 23–27, 2004.2004. [Google Scholar]
- 186.Thomas CE, et al. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther. 2001;3:36–46. doi: 10.1006/mthe.2000.0224. [DOI] [PubMed] [Google Scholar]
- 187.Lisman JE. Proc. Natl. Acad. Sci. USA. 1985;82:3055–3057. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hedou G, Mansuy IM. Inducible molecular switches for the study of long-term potentiation. Philos. Trans. R. Soc. London B Biol. Sci. 2003;358:797–804. doi: 10.1098/rstb.2002.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Iida A, et al. Inducible gene expression by retrovirus-mediated transfer of a modified tetracycline-regulated system. J. Virol. 1996;70:6054–6059. doi: 10.1128/jvi.70.9.6054-6059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hwang JJ, Scuric Z, Anderson WF. Novel retroviral vector transferring a suicide gene and a selectable marker gene with enhanced gene expression by using a tetracycline-responsive expression system. J. Virol. 1996;70:8138–8141. doi: 10.1128/jvi.70.11.8138-8141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kenny PA, Enver T, Ashworth A. Retroviral vectors for establishing tetracycline-regulated gene expression in an otherwise recalcitrant cell line. BMC Mol. Biol. 2002;3:13. doi: 10.1186/1471-2199-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hofmann A, Nolan GP, Blau HM. Rapid retroviral delivery of tetracycline-inducible genes in a single autoregulatory cassette. Proc. Natl. Acad. Sci. USA. 1996;93:5185–5190. doi: 10.1073/pnas.93.11.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 194.Kafri T, et al. Lentiviral vectors: regulated gene expression. Mol. Ther. 2000;1:516–521. doi: 10.1006/mthe.2000.0083. [DOI] [PubMed] [Google Scholar]
- 195.Johansen J, et al. Evaluation of Tet-on system to avoid transgene down-regulation in ex vivo gene transfer to the CNS. Gene Ther. 2002;9:1291–1301. doi: 10.1038/sj.gt.3301778. [DOI] [PubMed] [Google Scholar]
- 196.Regulier E, et al. Dose-dependent neuroprotective effect of ciliary neurotrophic factor delivered via tetracycline-regulated lentiviral vectors in the quinolinic acid rat model of Huntington’s disease. Hum. Gene Ther. 2002;13:1981–1990. doi: 10.1089/10430340260355383. [DOI] [PubMed] [Google Scholar]
- 197.Verca M. S. Brenz, et al. Cocaine-induced expression of the tetraspanin CD81 and its relation to hypothalamic function. Mol. Cell. Neurosci. 2001;17:303–316. doi: 10.1006/mcne.2000.0942. [DOI] [PubMed] [Google Scholar]
- 198.Bahi A, et al. CD81-induced behavioural changes during chronic cocaine administration: in vivo gene delivery with regulatable lentivirus. Eur. J. Neurosci. 2004;19:1621–1633. doi: 10.1111/j.1460-9568.2004.03260.x. [DOI] [PubMed] [Google Scholar]
- 199.Sanftner L. H. McGee, et al. Recombinant AAV-mediated delivery of a tet-inducible reporter gene to the rat retina. Mol. Ther. 2001;3(5 Pt 1):688–696. doi: 10.1006/mthe.2001.0308. [DOI] [PubMed] [Google Scholar]
- 200.Chen G, et al. Disappearance of body fat in normal rats induced by adenovirus-mediated leptin gene therapy. Proc. Natl. Acad. Sci. USA. 1996;93:14795–14799. doi: 10.1073/pnas.93.25.14795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wilsey J, et al. Hypothalamic delivery of doxycycline-inducible leptin gene allows for reversible transgene expression and physiological responses. Gene Ther. 2002;9:1492–1499. doi: 10.1038/sj.gt.3301835. [DOI] [PubMed] [Google Scholar]
- 202.Gallia GL, Khalili K. Evaluation of an autoregulatory tetracycline regulated system. Oncogene. 1998;16:1879–1884. doi: 10.1038/sj.onc.1201706. [DOI] [PubMed] [Google Scholar]
- 203.Strathdee CA, McLeod MR, Hall JR. Efficient control of tetracycline-responsive gene expression from an autoregulated bi-directional expression vector. Gene. 1999;229:21–29. doi: 10.1016/s0378-1119(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 204.Folliot S, et al. Sustained tetracycline-regulated transgene expression in vivo in rat retinal ganglion cells using a single type 2 adeno-associated viral vector. J. Gene Med. 2003;5:493–501. doi: 10.1002/jgm.367. [DOI] [PubMed] [Google Scholar]
- 205.Jiang L, et al. Tight regulation from a single tet-off rAAV vector as demonstrated by flow cytometry and quantitative, real-time PCR. Gene Ther. 2004;11:1057–1067. doi: 10.1038/sj.gt.3302245. [DOI] [PubMed] [Google Scholar]
- 206.Johnston J, et al. Regulated expression of erythropoietin from an AAV vector safely improves the anemia of β-thalassemia in a mouse model. Mol. Ther. 2003;7:493–497. doi: 10.1016/s1525-0016(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 207.Apparailly F, et al. Tetracycline-inducible interleukin-10 gene transfer mediated by an adeno-associated virus: application to experimental arthritis. Hum. Gene Ther. 2002;13:1179–1188. doi: 10.1089/104303402320138961. [DOI] [PubMed] [Google Scholar]
- 208.Brockstedt DG, et al. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin. Immunol. 1999;92:67–75. doi: 10.1006/clim.1999.4724. [DOI] [PubMed] [Google Scholar]
- 209.Cordier L, et al. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum. Gene Ther. 2001;12:205–215. doi: 10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- 210.Favre D, et al. Lack of an immune response against the tetracycline-dependent transactivator correlates with long-term doxycycline-regulated transgene expression in nonhuman primates after intramuscular injection of recombinant adeno-associated virus. J. Virol. 2002;76:11605–11611. doi: 10.1128/JVI.76.22.11605-11611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fotaki ME, Pink JR, Mous J. Tetracycline-responsive gene expression in mouse brain after amplicon-mediated gene transfer. Gene Ther. 1997;4:901–908. doi: 10.1038/sj.gt.3300487. [DOI] [PubMed] [Google Scholar]
- 212.Ho DY, McLaughlin JR, Sapolsky RM. Inducible gene expression from defective herpes simplex virus vectors using the tetracycline-responsive promoter system. Brain Res. Mol. Brain Res. 1996;41:200–209. doi: 10.1016/0169-328x(96)00097-6. [DOI] [PubMed] [Google Scholar]
- 213.Schmeisser F, Donohue M, Weir JP. Tetracycline-regulated gene expression in replication-incompetent herpes simplex virus vectors. Hum. Gene Ther. 2002;13:2113–2124. doi: 10.1089/104303402320987815. [DOI] [PubMed] [Google Scholar]
- 214.Herrlinger U, et al. HSV-1 infected cell proteins influence tetracycline-regulated transgene expression. J. Gene Med. 2000;2:379–389. doi: 10.1002/1521-2254(200009/10)2:5<379::AID-JGM126>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 215.Staff B. First clinical trial for Parkinson’s gene therapy gets approval. 2002.
- 216.Germano IM, et al. Adenovirus/herpes simplex-thymidine kinase/ganciclo-vir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J. Neurooncol. 2003;65:279–289. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- 217.Prados MD, et al. Treatment of progressive or recurrent glioblastoma multiforme in adults with herpes simplex virus thymidine kinase gene vector-producer cells followed by intravenous ganciclovir administration: a phase I/II multi-institutional trial. J. Neurooncol. 2003;65:269–278. doi: 10.1023/b:neon.0000003588.18644.9c. [DOI] [PubMed] [Google Scholar]
- 218.Lang FF, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J. Clin. Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 219.Ren H, et al. Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki Forest virus vector carrying the human interleukin-12 gene—a phase I/II clinical protocol. J. Neurooncol. 2003;64:147–154. doi: 10.1007/BF02700029. [DOI] [PubMed] [Google Scholar]
- 220.During MJ, et al. Subthalamic GAD gene transfer in Parkinson disease patients who are candidates for deep brain stimulation. Hum. Gene Ther. 2001;12:1589–1591. [PubMed] [Google Scholar]
- 221.Mundt AJ, et al. A Phase I trial of TNFerade biologic in patients with soft tissue sarcoma in the extremities. Clin. Cancer Res. 2004;10:5747–5753. doi: 10.1158/1078-0432.CCR-04-0296. [DOI] [PubMed] [Google Scholar]
- 222.Senzer N, et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: a phase I study in patients with solid tumors. J. Clin. Oncol. 2004;22:592–601. doi: 10.1200/JCO.2004.01.227. [DOI] [PubMed] [Google Scholar]
- 223.Tang Y, et al. Vigilant vectors: adeno-associated virus with a biosensor to switch on amplified therapeutic genes in specific tissues in life-threatening diseases. Methods. 2002;28:259–266. doi: 10.1016/s1046-2023(02)00231-1. [DOI] [PubMed] [Google Scholar]
- 224.Donello JE, Loeb JE, Hope TJ. Woodchuck hepatitis virus contains a tripartite posttranscriptional regulatory element. J. Virol. 1998;72:5085–5092. doi: 10.1128/jvi.72.6.5085-5092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]