Abstract
Loss of function after neurological injury frequently occurs through the interruption of axonal connectivity, rather than through cell loss. Functional deficits persist because a multitude of inhibitory factors in degenerating myelin and astroglial scar prevent axonal growth in the adult brain and spinal cord. Given the high clinical significance of achieving functional recovery through axonal growth, substantial research effort has been, and will be, devoted toward this desirable goal. Unfortunately, the labels commonly used in the literature to categorize post-injury axonal anatomy might hinder advancement. In this article, we present an argument for the importance of developing precise terms that describe axonal growth in terms of the inciting event, the distance of axonal extension and the timing of axonal growth. The phenotypes produced by molecular interventions that overcome astroglial scar or myelin-associated inhibitors are reframed and discussed in this context.
Introduction
Neurological damage to the adult central nervous system of mammals commonly produces persistent deficits with limited recovery of function. The restricted capacity of the adult brain and spinal cord to support the re-extension and rearrangement of axonal connections is a primary determinant of failed recovery. A century of research into the sequelae of brain and spinal injury has identified several cell-autonomous and environmental factors that contribute to axon growth failure. Here we focus on the two broad classes of environmental inhibitors: proteins associated with degenerating myelin (myelin-associated inhibitors; MAIs) [1] and with glial scarring (chondroitin sulfate proteoglycans; CSPGs) [1]. These insights have enabled the development of therapeutic interventions designed to enhance axonal growth after experimental CNS lesion [2]. Several methods have advanced to Phase I clinical trials [3].
The nature, location, extent and maturity of a neurological insult influence the degree and specificity of axonal growth necessary to restore function. Optimally, our therapeutic arsenal will include methods capable of stimulating a spectrum of controlled growth responses from damaged and intact axons. To this end, much progress has been made in understanding the intrinsic mechanisms that influence the growth repertoire of the developing and immature nervous system (for reviews, see Refs [4,5]). However, in the spinal cord injury (SCI) literature, the terms regeneration, sprouting and plasticity have been employed to characterize axonal growth responses after various interventions [2]. The implication exists that these forms of axonal growth are inherently distinct, serving separate functions and being regulated by unique molecular pathways. In this essay, we explore three variables to characterize injury-responsive axonal growth. Further, we relate these variables to the three commonly used descriptive terms. We also examine the extent to which molecular perturbations separate, or fail to segregate, axonal growth as regeneration, sprouting and plasticity.
Characterizing axonal response to injury
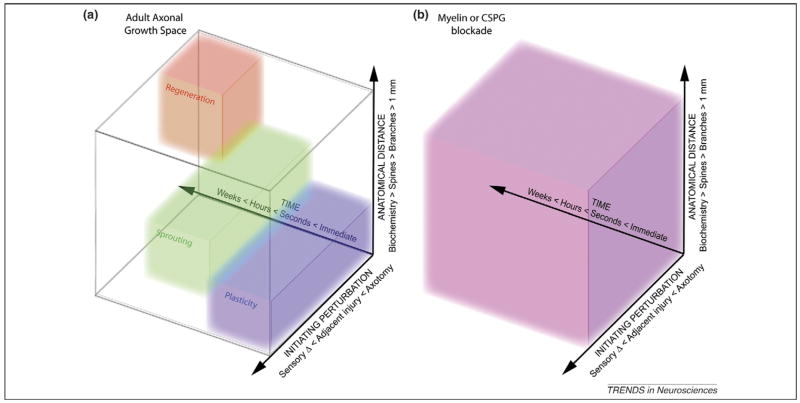
In an attempt to capture the multitude of growth responses that occur after CNS injury, three variables seem most relevant (Figure 1a). The first is the nature of the inciting perturbation. Axonal growth can be stimulated by various therapies after a wide range of events. The most simple growth response would be an axon that has been severed by a disease process might itself extend from the severed end. Another manifestation of growth is the creation of a collateral from the proximal segment of an injured axon. Alternatively, axonal growth can also be stimulated from fiber systems that are not themselves damaged but are positioned adjacent or parallel to a cut pathway. Finally, growth is sometimes stimulated not by an injury per se, but rather by altered sensory inputs from nonneuronal peripheral damage or from altered experience.
Figure 1.
Axonal growth space. The pattern of axonal growth observed after CNS injury has been disparately described as regeneration, sprouting or plasticity. In an attempt to qualify these terms, we have depicted them as occupying overlapping domains in three-dimensional space. This space is defined by the nature of the inciting perturbation, the anatomical distance of the axonal response and the time after the perturbation. (a) Although these terms appear to have substantive meaning, their relevance is diminished when assessing their functional significance, as therapeutic blockade of myelin-associated inhibitors and CSPGs result in functional axonal growth patterns that occupy the entire continuum (b).
The second key variable is the anatomical extent of any axonal growth. Without intervention, axonal growth of greater than 1 mm rarely (if ever) occurs in the intact or the damaged adult mammalian central nervous system. When such growth is stimulated by a therapeutic intervention, it can follow pre-existing or novel pathways [6]. Anatomical changes can also be observed at more localized levels, altering branch morphology in terminal fields or rearranging axonal varicosities and dendritic spines. Although anatomically more confined, such changes might have substantial functional relevance. Many forms of plasticity are known to involve biochemical changes in synaptic efficacy without anatomical reorganization.
Finally, the response to neurological insult also has a critical temporal pattern. The changes induced acutely and those occurring chronically can be widely disparate, and in some cases later changes might require earlier adaptations.
How do the commonly used terms regeneration, sprouting and plasticity relate to the space defined by the variables of time, distance and perturbation? In a relative sense, regeneration refers to growth over longer distances and greater time periods from transected axons. In the most extreme definition, it might refer to the growth of a severed fiber over centimeters along its previous trajectory to its original synaptic partner. Plasticity is commonly used to refer to changes of lesser anatomical extent from uninjured fibers. Plasticity has a specialized use in cases where experience-dependent stimuli are altered without frank neuronal injury. The label sprouting is applied to the growth from either cut or intact fibers over moderate distances. There is no fixed length at which growth transitions from sprouting to regeneration. Although some forms of injury-induced growth fit well with one or the other term, many (or most) combinations of perturbation, distance and time describe axon growth that does not fit with one of the common terms. For example, consider a case in which a spinal cord injury disconnects a percentage of fibers in one descending tract, and then some intervention stimulates new growth from cut and uncut fibers in this tract rostral to the injury and from uncut fibers caudal to the injury. Clearly a mixture of terms is required to describe the response.
Despite the fact that all three of the terms relate to axon growth, a heightened degree of importance surrounds the label applied [7–9]. Classifying a potential therapy as regenerative or plastic or sprouting can obscure its full utility and stunt investigation of its efficacy. These considerations raise the issue of whether separating such forms of growth has a molecular basis. Therefore, we review whether blockade of axonal growth inhibitors produces a discrete form of growth or ignores the perceived boundaries between these labels.
Myelin-associated inhibitors and axonal growth
First, we consider the myelin-associated inhibitors NogoA, MAG (myelin-associated glycoprotein) and OMgp (oligodendrocyte myelin glycoprotein) and ask whether anti-MAI strategies result in a consistent and therefore predictable axon growth response after CNS injury. Schwab and colleagues characterized the axon growth-inhibiting activity in CNS myelin more than 20 years ago [10]. These early studies illustrated that a 35 kDa and a 250 kDa membrane protein fraction from CNS myelin were inhibitory to neurite outgrowth in vitro [11]. This inhibitory protein was later cloned by three independent groups [12–14] and is now termed NogoA. The Nogo-66 receptor (NgR1) was subsequently identified [15] and mediates the inhibitory activity of MAG and OMgp in addition to NogoA [16–18] in a complex that requires one or more co-receptors, including p75/Lingo-1/Taj [19–22]. Ligand binding to NgR1 results in RhoA-mediated stimulation of Rho-associated kinase (ROCK) and actinomyosin contractility, which ultimately results in inhibition of neurite outgrowth and growth cone collapse [23]. NgR1 might be most critical for acute inhibitory effect [24]. The potent effects of these proteins and NgR on growth cone collapse in vitro led to the design of in vivo experiments focused on interfering with myriad points along this ligand–receptor axis.
The rodent spinal cord has proven to be the most tractable model to assess the potential efficacy of SCI therapies. Most lesion models transect a specific portion of the spinal cord to produce a stereotypical compromise of certain behaviors. Commonly, the dorsal half of the spinal cord is cut, severing the entire dorsal and the dorsolateral columns and locally destroying the dorsal horns. The desired target of dorsal hemisection lesions is the corticospinal tract (CST), the bulk of which resides in the ventral aspect of the dorsal columns (in the rodent), with the remainder in the lateral columns (<5% resides in the ventral columns). In the absence of any treatment, the CST degenerates a few millimeters rostral to the lesion and completely caudal to the lesion. Interfering with the Nogo–NgR axis by antagonizing the inhibitory effects of NogoA pharmacologically using the monoclonal antibody IN-1 [25–29] or in nogo-a knockout mice [30–32] has shown significant long-distance growth of damaged CST axons after dorsal hemisection with concomitant restoration of locomotor function. Similar results were observed with therapies that antagonized the activation of NgR1, using either the Nogo receptor antagonist peptide NEP1–40 or a soluble version of NgR1 called NgR(310)ecto-Fc [33–37]. These studies directly correlate the growth of damaged CST axons into the caudal spinal cord with recovery of locomotion. It would appear from these studies that long-distance axon growth (regeneration?) is crucial to restore function after CNS injury.
However, closer anatomical inspection of spinal cords from these studies reveals that most of the treated and mutant animals that exhibited significant growth of damaged fibers caudal to the lesion site also showed significant local growth (sprouting?) of lesioned fibers rostral to the lesion in comparison to controls. The function of these fibers is not fully understood, and it would seem unreasonable to rule out a possible role for them in restoring function after hemisection. Indeed, a recent study by Bareyre et al., showed that local growth (sprouting?) of damaged CST axons resulted in new synapse formation between the damaged CST and propriospinal neurons, and furthermore that these new intrinsic spinal circuits enhanced recovery of locomotion after experimental SCI [38]. This study would argue that restoration of function is possible in the absence of long-distance axon growth (regeneration?) of damaged axons, and that localized spinal circuit rearrangements of damaged and intact systems can have a profound functional impact. Indeed, localized axonal growth (sprouting, plasticity?) of damaged and intact fiber systems has been observed in the spinal cord and brain in rodents that have been treated with anti-MAI therapies. Growth of intact CST fibers into the denervated brainstem and cervical spinal cord after unilateral corticospinal tract lesion (pyramidotomy) and restitution of fine motor skill were observed in adult rats treated with IN-1 [39–42] and in mice null mutant for nogo-a and ngr1 [43]. Perhaps the most convincing data illustrating a functional role for de novo growth of intact fiber systems come from stroke studies that have shown the potent effects of both IN-1 [44,45] and NgR(310)ecto-Fc [46] on functional restoration after middle cerebral artery occlusion. Common to all these studies is the efficacy of treatment in restoring function post-lesion. Different between them is the type of axon growth most likely to mediate functional restoration. Targeting inhibitory proteins in CNS myelin can restore function by stimulating axon growth over short, medium and long distances, and cannot be described as mediating solely axonal regeneration, sprouting or plasticity.
Astroglial scars and CSPGs
Chondroitin sulfate proteoglycans are also potent inhibitors of neurite outgrowth in vitro [47] and growth of damaged axons in vivo [48,49]. CSPGs together with hyaluronan and tenascins make up the extracellular matrix of the CNS. CSPGs consist of a core protein to which a variable number of unbranched sugar (glucosaminoglycan; GAG) side chains are covalently attached. Hydrolyzing GAG side chains with the bacterial enzyme chondroitinase ABC (ChABC) has been shown to make extracellular matrix-rich substrata more permissive to neurite outgrowth [49]; however, stubs (carbohydrate linker regions) remain after hydrolysis which retain some inhibitory activity [50], and furthermore proteogylcan core proteins might also be inhibitory irrespective of their GAG status [51]. Inhibitory CSPGs are robustly expressed in the adult CNS [48] and their expression is greatly elevated at sites of CNS lesion and are the major inhibitory species associated with the glial scar. Together with reactive astrocytes, meningeal cells and fibroblasts [52], CSPGs in the glial scar present a physical and chemical barrier to axonal growth after lesion. All physical trauma to the spinal cord results in the formation of a glial scar and therefore the scar is a prime target for therapeutic intervention.
Fewer studies have assessed the impact of degrading CSPGs on axonal growth than nullifying CNS myelin inhibitors. The diverse structure and function of CSPGs [53] render them complicated targets for therapeutic intervention. Furthermore, as yet, no general CSPG ‘receptor’ has been identified that mediates their function. However, activation of the small GTPase RhoA appears to be a necessary step in mediating CSPG-associated inhibition in vitro. Several studies have reported disinhibition of neurite outgrowth on CSPG-rich substrates in the presence of ROCK inhibitors [54,55]. Therefore, blockade of the Rho/ROCK axis holds the potential to block signaling by myelin and CSPGs. One recent study has implicated PKC activation as critical in transducing CSPG-mediated RhoA activation and hence growth cone collapse. Intrathecal delivery of the PKC inhibitor Go6976 resulted in modest regeneration of ascending dorsal column collaterals after dorsal hemisection [56]; however, this study has yet to be reproduced. More recently, epidermal growth factor receptor (EGFR) activation [57] has emerged as another intermediate in transducing the action of both CSPG and myelin inhibitory function. These studies showed that inhibition of EGFR kinase function was capable of ablating the neurite outgrowth inhibitory effects of CSPGs. In the absence of detailed knowledge regarding the signaling consequences of CSPG binding in neurons, our ability to target glial scar-associated axonal growth inhibition is limited to two strategies that target the presence or the addition of GAG side chains. Inhibiting the addition of GAG side chains to injury upregulated proteoglycans using a DNA enzyme that targets xylosyltransferase 1 [58] has been shown to render the damaged CNS more permissive to axon growth at the lesion site. ChABC has been widely used to liberate GAGs from intrinsic and injury upregulated CSPGs in vivo (for a review, see Ref. [49]).
Several studies have now shown that ChABC infusion in vivo enhances functional recovery after brain [59] and spinal cord injury [60–64]. It is less clear whether the functional restoration observed in these studies was a result of localized or long-distance axonal growth. The growth of damaged CST axons caudal to a lesion has been observed after intrathecal infusion of ChABC [60]. The growth of these axons was correlated with a restoration of behavioral and electrophysiological function. These anatomical data support the hypothesis that long-distance axonal growth (regeneration?) is the primary result of ChABC application. By contrast, little CST growth or recovery was detected in a dorsal hemisection lesion of adult mice that transgenically express ChABC in reactive astrocytes and digest CSPGs locally [61]. These two studies suggest a more sophisticated role for ChABC in mediating axonal growth. Widespread CSPG digestion by intrathecal administration might be critical, and distant axonal growth in multiple pathways might mediate recovery. Our transgenic ChABC study did demonstrate the growth of damaged sensory systems [61]. Specifically, primary afferents grew through the dorsal root entry zone after multiple cervical dorsal rhizotomies and supported restoration of thermal and mechanical cutaneous sensation in ChABC-transgenic mice [61]. Thus, the specific fiber system and injury type also influence axonal growth responses to CSPG digestion.
It is clear that ChABC also stimulates the growth of uninjured systems within the CNS. A single application of ChABC stimulates transient growth of Purkinje cells in the cerebellum in the absence of injury [65], and encourages the growth of intact cuneate primary afferent collaterals into partially denervated gracile nuclei [66]. Intact nociceptive primary afferent and raphespinal terminals have also been shown to rearrange locally (sprout?) in response to dorsal hemisection and ChABC application [67] without functional consequences. These data clearly demonstrate the potent effects that ChABC can exert over both damaged and intact fiber systems to yield short- and long-range growth.
Experience-dependent responses: role of MAIs and CSPGs
Interestingly, MAIs signaling through NgR1 and CSPGs also inhibit physiologic plasticity in the intact CNS. In the mammalian visual system, these factors restrict a classic form of experience-dependent plasticity, ocular dominance plasticity, to a developmental critical period. Brief monocular deprivation shifts ocular dominance by decreasing the relative responsiveness of neurons in the visual cortex to input from the deprived eye, resulting in a loss of visual acuity. Identical perturbations to visual experience in adults do not affect ocular dominance or visual acuity [68].
The distribution of myelinated fibers and the deposition of CSPGs both increase during development and plateau concomitantly with the close of the critical period [69–71]. Mice lacking a function gene for NogoA or NgR1 exhibit ocular dominance plasticity as adults, well beyond the end of the critical period [72]. Similarly, removal of CSPG GAGs by repeated injection of ChABC proximal to the visual cortex partially reactivates ocular dominance plasticity [73]. In addition, ChABC treatment in combination with reverse occlusion, a standard treatment for improving visual acuity during the critical period, increases visual acuity in adult rats deprived of vision earlier in life [74]. Thus, a physiological function of these inhibitors might be to consolidate activity-dependent refinements to neural circuitry achieved during development by limiting further plasticity. How interfering with the functions of NgR1 or CSPGs promotes this plasticity is not yet clear, but understanding how these factors regulate anatomical modification and synaptic function in the visual system might reveal how these same mechanisms can be engaged to enhance both axonal growth and efficiency of rehabilitation following injury.
Conclusions
It is clear from a battery of studies that blocking the action of MAIs and CSPGs releases a diverse pattern of axonal growth responses in the CNS. Depending on the lesion model, the site of application and the timing of treatment, different patterns of axonal growth from cut and uncut systems occur. These patterns are best characterized across the matrix of time, anatomy and perturbation (Figure 1b). Axonal growth that fits each of the terms regeneration, sprouting and plasticity occurs simultaneously with specific molecular interventions. The use of the terms regeneration, sprouting and plasticity obscure neurobiological responses rather than clarifying them. Neither MAI blockade or CSPG interference induces a complete recapitulation or restoration of the injured system, but both create some combination of multiple forms of axonal growth. Recognizing the diversity of responses for axonal growth therapies promises the greatest likelihood of realizing their potential clinical benefits.
Acknowledgments
This work is supported by research grants from the NIH, the Falk Medical Research Trust and the Wings for Life Foundation to S.M.S., and by the Burroughs Wellcome Fund to A.W.M. The authors declare no conflict of interest.
References
- 1.Liu BP, et al. Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond B Biol Sci. 2006;361:1593–1610. doi: 10.1098/rstb.2006.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- 3.Baptiste DC, Fehlings MG. Update on the treatment of spinal cord injury. Prog Brain Res. 2007;161:217–233. doi: 10.1016/S0079-6123(06)61015-7. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz LI, Yin Y. Combinatorial treatments for promoting axon regeneration in the CNS: strategies for overcoming inhibitory signals and activating neurons’ intrinsic growth state. Dev Neurobiol. 2007;67:1148–1165. doi: 10.1002/dneu.20515. [DOI] [PubMed] [Google Scholar]
- 5.Rossi F, et al. Regulation of intrinsic neuronal properties for axon growth and regeneration. Prog Neurobiol. 2007;81:1–28. doi: 10.1016/j.pneurobio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci. 2006;7:603–616. doi: 10.1038/nrn1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cafferty WB, et al. Response to correspondence: Kim et al. ‘Axon regeneration in young adult mice lacking Nogo-A/B.’. NeuronNeuron. 2007;3854:187–199. 195–199. doi: 10.1016/j.neuron.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steward O, et al. Response to: Kim et al. ‘Axon regeneration in young adult mice lacking Nogo-A/B.’. Neuron. 2007;38:187–199. doi: 10.1016/j.neuron.2007.04.004. [DOI] [PubMed] [Google Scholar]; Neuron. 54:191–195. [Google Scholar]
- 9.Steward O, et al. False resurrections: distinguishing regenerated from spared axons in the injured central nervous system. J Comp Neurol. 2003;459:1–8. doi: 10.1002/cne.10593. [DOI] [PubMed] [Google Scholar]
- 10.Caroni P, Schwab ME. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol. 1988;106:1281–1288. doi: 10.1083/jcb.106.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spillmann AA, et al. Identification and characterization of a bovine neurite growth inhibitor (bNI-220) J Biol Chem. 1998;273:19283–19293. doi: 10.1074/jbc.273.30.19283. [DOI] [PubMed] [Google Scholar]
- 12.Chen MS, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 13.GrandPre T, et al. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- 14.Prinjha R, et al. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 15.Fournier AE, et al. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 16.Domeniconi M, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 17.Liu BP, et al. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 18.Wang KC, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 19.Mi S, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 20.Park JB, et al. A TNF receptor family member, TROY, is a coreceptor with Nogo receptor in mediating the inhibitory activity of myelin inhibitors. Neuron. 2005;45:345–351. doi: 10.1016/j.neuron.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 21.Shao Z, et al. TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron. 2005;45:353–359. doi: 10.1016/j.neuron.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 22.Wang KC, et al. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- 23.Fournier AE, et al. Rho GTPases and axonal growth cone collapse. Methods Enzymol. 2000;325:473–482. doi: 10.1016/s0076-6879(00)25467-0. [DOI] [PubMed] [Google Scholar]
- 24.Venkatesh K, et al. Molecular dissection of the myelin-associated glycoprotein receptor complex reveals cell type-specific mechanisms for neurite outgrowth inhibition. J Cell Biol. 2007;177:393–399. doi: 10.1083/jcb.200702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bregman BS, et al. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 26.Brosamle C, et al. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. J Neurosci. 2000;20:8061–8068. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liebscher T, et al. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- 28.Schnell L, Schwab ME. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 1990;343:269–272. doi: 10.1038/343269a0. [DOI] [PubMed] [Google Scholar]
- 29.Schnell L, Schwab ME. Sprouting and regeneration of lesioned corticospinal tract fibres in the adult rat spinal cord. Eur J Neurosci. 1993;5:1156–1171. doi: 10.1111/j.1460-9568.1993.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 30.Dimou L, et al. Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J Neurosci. 2006;26:5591–5603. doi: 10.1523/JNEUROSCI.1103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JE, et al. Axon regeneration in young adult mice lacking Nogo-A/B. Neuron. 2003;38:187–199. doi: 10.1016/s0896-6273(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 32.Simonen M, et al. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 33.GrandPre T, et al. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 34.Li S, et al. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol Cell Neurosci. 2005;29:26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci. 2004;24:10511–10520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Strittmatter SM. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, et al. Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol. 2006;60:540–549. doi: 10.1002/ana.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bareyre FM, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 39.Raineteau O, et al. Functional switch between motor tracts in the presence of the mAb IN-1 in the adult rat. Proc Natl Acad Sci U S A. 2001;98:6929–6934. doi: 10.1073/pnas.111165498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raineteau O, et al. Sprouting and regeneration after pyramidotomy and blockade of the myelin-associated neurite growth inhibitors NI 35/250 in adult rats. Eur J Neurosci. 1999;11:1486–1490. doi: 10.1046/j.1460-9568.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 41.Thallmair M, et al. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat Neurosci. 1998;1:124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- 42.Z’Graggen WJ, et al. Compensatory sprouting and impulse rerouting after unilateral pyramidal tract lesion in neonatal rats. J Neurosci. 2000;20:6561–6569. doi: 10.1523/JNEUROSCI.20-17-06561.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci. 2006;26:12242–12250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papadopoulos CM, et al. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol. 2002;51:433–441. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- 45.Seymour AB, et al. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J Cereb Blood Flow Metab. 2005;25:1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- 46.Lee JK, et al. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Crespo D, et al. How does chondroitinase promote functional recovery in the damaged CNS? Exp Neurol. 2007;206:159–171. doi: 10.1016/j.expneurol.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Lemons ML, et al. Intact aggrecan and chondroitin sulfate-depleted aggrecan core glycoprotein inhibit axon growth in the adult rat spinal cord. Exp Neurol. 2003;184:981–990. doi: 10.1016/S0014-4886(03)00383-2. [DOI] [PubMed] [Google Scholar]
- 51.Tan AM, et al. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci. 2006;26:4729–4739. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faulkner JR, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- 54.Borisoff JF, et al. Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci. 2003;22:405–416. doi: 10.1016/s1044-7431(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 55.Monnier PP, et al. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci. 2003;22:319–330. doi: 10.1016/s1044-7431(02)00035-0. [DOI] [PubMed] [Google Scholar]
- 56.Sivasankaran R, et al. PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci. 2004;7:261–268. doi: 10.1038/nn1193. [DOI] [PubMed] [Google Scholar]
- 57.Koprivica V, et al. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- 58.Grimpe B, Silver J. A novel DNA enzyme reduces glycosaminoglycan chains in the glial scar and allows microtransplanted dorsal root ganglia axons to regenerate beyond lesions in the spinal cord. J Neurosci. 2004;24:1393–1397. doi: 10.1523/JNEUROSCI.4986-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moon LD, et al. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci. 2001;4:465–466. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- 60.Bradbury EJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 61.Cafferty WB, et al. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27:2176–2185. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caggiano AO, et al. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma. 2005;22:226–239. doi: 10.1089/neu.2005.22.226. [DOI] [PubMed] [Google Scholar]
- 63.Yick LW, et al. Axonal regeneration of Clarke’s neurons beyond the spinal cord injury scar after treatment with chondroitinase ABC. Exp Neurol. 2003;182:160–168. doi: 10.1016/s0014-4886(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 64.Yick LW, et al. Chondroitinase ABC promotes axonal regeneration of Clarke’s neurons after spinal cord injury. Neuroreport. 2000;11:1063–1067. doi: 10.1097/00001756-200004070-00032. [DOI] [PubMed] [Google Scholar]
- 65.Corvetti L, Rossi F. Degradation of chondroitin sulfate proteoglycans induces sprouting of intact Purkinje axons in the cerebellum of the adult rat. J Neurosci. 2005;25:7150–7158. doi: 10.1523/JNEUROSCI.0683-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Massey JM, et al. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci. 2006;26:4406–4414. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barritt AW, et al. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci. 2006;26:10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hooks BM, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron. 2007;56:312–326. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 69.Hockfield S, et al. Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harb Symp Quant Biol. 1990;55:505–514. doi: 10.1101/sqb.1990.055.01.049. [DOI] [PubMed] [Google Scholar]
- 70.Muller CM, et al. Role of myelin-associated neurite growth inhibitorN!35/250 in the determination of critial period plasticity in kitten visual cortex. Soc Neurosci Abstr. 1994;20:1471. [Google Scholar]
- 71.Sur M, et al. Expression of a surface-associated antigen on Y-cells in the cat lateral geniculate nucleus is regulated by visual experience. J Neurosci. 1988;8:874–882. doi: 10.1523/JNEUROSCI.08-03-00874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGee AW, et al. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pizzorusso T, et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 74.Pizzorusso T, et al. Structural and functional recovery from early monocular deprivation in adult rats. Proc Natl Acad Sci U S A. 2006;103:8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]