Abstract
The objective of this study was to determine how changes in the network structure and properties of hyaluronic acid (HA) hydrogels, due to variations in the macromer molecular weight (50 to 1100 kDa) and macromer concentration (2 to 20 wt%), affect neocartilage formation by encapsulated auricular chondrocytes. To investigate tissue formation, swine auricular chondrocytes were photoencapsulated in the various networks, implanted subcutaneously in the dorsum of nude mice, and explanted after 6 and 12 weeks for biochemical and histological analysis. After 12 weeks, the various constructs were 81 to 93% water, contained between 0.1 × 106 and 0.6 × 106 chondrocytes per sample, and consisted of 0 to 0.049 μg chondroitin sulfate/μg wet weight (glycosaminoglycan content) and 0.002 to 0.060μg collagen/μg wet weight. Histological staining showed an even distribution of chondrocytes and glycosaminoglycans in addition to minimal type I collagen staining and intense and uniform type II collagen staining in the constructs with greatest neocartilage production. Hydrogels fabricated from 2 wt% of the 50 kDa HA macromer most resembled the properties of native cartilage and show the greatest promise for continued development for cartilage regeneration.
Keywords: cartilage, tissue engineering, hyaluronic acid, photopolymerization, hydrogel
Introduction
Tissue engineering is emerging as a field with potential to develop viable treatment alternatives to allogenic and alloplastic implants to repair damaged cartilage1. Because cartilage has many inherent limitations for regeneration, techniques to regenerate this avascular tissue are especially needed. These approaches typically involve the delivery of cells (e.g., chondrocytes, stem cells) in a resorbable scaffold that is either grown in vitro, potentially in conjunction with bioreactors, or is implanted directly in vivo and gradually replaced with cartilaginous tissue. The design and fabrication of the scaffold is extremely important and a wide range of materials have been investigated for these applications including: collagen2, fibrin3,4, alginates5,6, and poly(glycolic acid)7,8.
Elisseeff and coworkers9,10 first pioneered the use of a photopolymerization process as a method to suspend and deliver chondrocytes in hydrogel networks for the treatment of damaged cartilage. The photopolymerization process allows for the in vivo formation of cell/material constructs which is advantageous for filling irregular defects and providing good contact between the tissue-engineered construct and the surrounding tissue. In general, photoencapsulation is rapid and eliminates the use of potentially toxic solvents. Initial efforts focused on the use of photopolymerizable hydrogels based on poly(ethylene glycol) (PEG). These studies showed that the cell/monomer solution could be injected subcutaneously and polymerized with transdermal light exposure9. To accelerate the degradation of the hydrogels and improve the distribution of large extracellular matrix molecules produced by the encapsulated cells, Bryant et al.11 introduced degradable lactic acid units into the networks. This work illustrates the importance of material design in the development of tissue engineering scaffolds, where factors such as scaffold degradation, mechanics, and cell recognition capabilities are essential.
One molecule of particular interest in cartilage regeneration is hyaluronic acid (HA). HA is a linear polysaccharide comprised of alternating D-glucuronic acid and N-acetyl-D-glucosamine that is found in connective tissues, including cartilage, and degrades primarily by hyaluronidases found throughout the body12. HA plays a role in cellular processes including cell proliferation, morphogenesis, inflammation, and wound repair13 and several cell surface receptors for hyaluronic acid (e.g. CD44, ICAM-1, and RHAMM) have been identified12. In addition to these factors, HA is readily modified through both its carboxy groups with esterification14,15 and hydroxyl groups that are crosslinked with divinyl sulfone16 or via photopolymerization17,18. The potential cell recognition of this polysaccharide, the cell dictated degradation by secreted enzymes, and the ease of processing of HA through chemical modification makes it a potentially suitable matrix for the delivery of chondrocytes in cartilage tissue engineering applications.
We previously showed that photocrosslinkable hyaluronic acid macromers could form networks with a variety of properties (e.g., mechanics, degradation, cell viability) that were dependent on the concentration and molecular weight of the starting macromer19. The overall objective of the current study was to determine how these same changes in the network structure and properties influence the ability of photoencapsulated auricular chondrocytes to produce cartilaginous tissue in vivo. To accomplish this, auricular chondrocytes were harvested from swine, photoencapsulated in the various HA hydrogels, and implanted subcutaneously in nude mice. Biochemical assays and histological analysis were then used to compare cartilage production between the various hydrogels.
Materials and Methods
Monomer Synthesis and Polymerization
Methacrylated hyaluronic acid (MeHA) was synthesized by a previously reported technique18,19. Briefly, methacrylic anhydride (Sigma) was added to a solution of 1 wt% hyaluronic acid (Lifecore, microbial fermentation process, MW = 50 kDa, 350 kDa, 1100 kDa) in deionized water, adjusted to a pH of 8 with 5 N NaOH, and reacted on ice for 24 hours. For purification, the macromer solution was dialyzed (MW cutoff 5–8k) against deionized water for at least 48 hours with repeated changes of water and the final product was obtained by lyophilization. 1H-NMR was used to determine the final functionality and purity of the MeHA. The macromer was stored at −20°C in powder form prior to use. For cell encapsulation, the macromer was sterilized with exposure to the germicidal lamp in a laminar flow hood for 30 minutes and dissolved in a sterile solution of phosphate buffered saline (PBS) containing 0.05 wt% 2-methyl-1-[4-(hydroxyethoxy)phenyl]-2-methyl-1-propanone (Irgacure 2959, I2959). This technique was used for sterilization since the higher concentration MeHA solutions were too viscous to filter sterilize.
Chondrocyte Isolation and Photoencapsulation
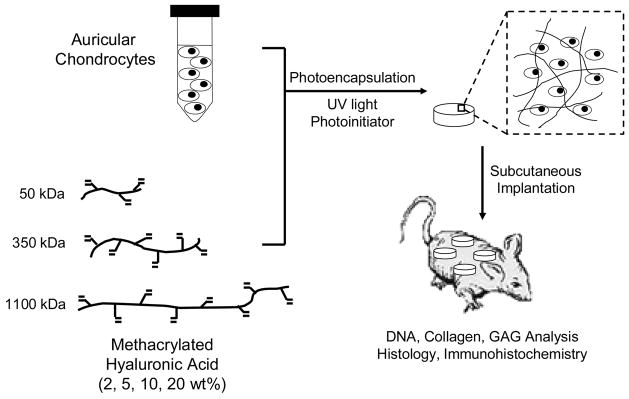
Swine aged 3 to 6 months were euthanized using an overdose of Pentobarbital (100 mg/kg IV) and cartilage tissue was harvested in a sterile fashion from the ears (auricular) and knees (articular) of the swine. The harvested auricular cartilage was cut into ~1mm3 pieces, washed in PBS, and digested for 18 hours at 37°C in a sterile 0.1% collagenase (Worthington) solution in Ham’s F-12 medium. After digestion, the solution was passed through a 100 μm filter to remove undigested cartilage and centrifuged to obtain a chondrocyte pellet. The chondrocytes were washed twice with PBS, counted using a hemacytometer, and determined viable using the trypan blue exclusion dye test prior to encapsulation. Auricular chondrocytes (40 × 106 cells/ml) were photoencapsulated in the various hydrogel networks by suspension in a solution of 2, 5, 10, and 20 wt% macromer (MeHA) containing 0.05 wt% I2959. Solutions were pipetted into sterile molds (50 μl volume) and polymerized with ~4 mW/cm2 ultraviolet light for 10 minutes using a long-wave ultraviolet lamp (Model 100AP, Blak-Ray). These conditions were previously determined to be cytocompatible for the photoencapsulation of chondrocytes20. A schematic of this process is illustrated in Figure 1. Auricular and articular cartilage were harvested from the same source and used as controls for biochemical analyses.
Figure 1.
General schematic of the chondrocyte photoencapsulation process and subsequent analysis.
Implantation in Nude Mice
Nude mice were anesthetized with ketamine (80 mg/kg) and xylazine (12 mg/kg). A 2 cm midline incision was made on the back of each mouse and 4 subcutaneous pockets were made using blunt dissection. A chondrocyte/hydrogel construct was placed in each of these pockets and the wound was closed with sterile stainless steel skin clips. After 6 and 12 weeks, mice were euthanized and constructs were harvested for analysis. NIH guidelines for the care and use of laboratory animals (NIH Publication #85-23 Rev. 1985) were observed.
Biochemical Analysis
For biochemical analysis (n = 4), constructs were weighed initially (wet weight), lyophilized and weighed (dry weight). Next, samples were digested in a papain solution (125 μg/ml papain type III, 10 mM l-cysteine, 100 mM phosphate, and 10 mM EDTA at pH 6.3) for 15 hours at 60oC. Total DNA content was determined using a PicoGreen dsDNA Assay21 with chondrocyte number determined using a conversion factor of 7.7 pg of DNA per chondrocyte 22. Total GAG content was determined using the dimethylmethylene blue dye method 23 with chondroitin sulfate (CS) as a standard. Total collagen content was determined using the hydroxyproline assay 24, with a collagen to hydroxyproline ratio of 7.2525,26. Values reported for GAG and collagen content were normalized to construct wet weight.
Histological Analysis
For histological analysis, constructs were fixed in 10% formalin for 24 hours, embedded in paraffin, and processed using standard histological procedures. The histological sections (7 μm thick) were stained with hematoxylin and eosin to observe the morphology and distribution of encapsulated chondrocytes and Safranin O to visualize glycosaminoglycans (GAG). Type I and type II collagen distributions were detected using a Vectastain Universal Elite ABC Kit (Vector Laboratories) and a DAB Substrate Kit for Peroxidase (Vector Laboratories). Sections were predigested in 0.5 mg/ml hyaluronidase for 30 min at 37°C and incubated in 0.5 N acetic acid for 4 hours at 4°C prior to overnight incubation with primary antibodies at dilutions of 1:200 and 1:3 for type I (mouse monoclonal anti-collagen type 1, Sigma) and type II collagen antibodies (mouse monoclonal anti-collagen type II, Developmental Studies Hybridoma Bank), respectively.
Statistical Analysis
Anova with Tukey’s post-hoc test was used to determine significant difference among groups, with p < 0.05. All values are reported as the mean ± the standard deviation.
Results
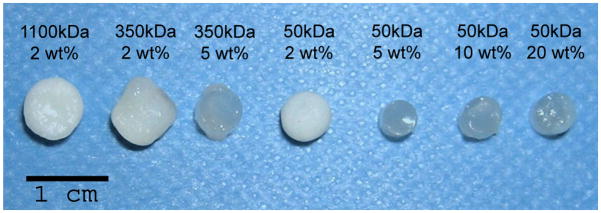
To investigate the influence of the hydrogel structure on neocartilage formation, primary auricular swine chondrocytes were photoencapsulated in HA hydrogels with macromer molecular weights ranges from 50 to 1100 kDa and macromer concentrations ranging from 2 to 20 wt%. These constructs were implanted subcutaneously in nude mice to provide an in vivo environment for tissue formation. After culture in the dorsum of nude mice for 6 or 12 weeks, the constructs were harvested and analyzed. A schematic of this process is shown in Figure 1. After 12 weeks, the macroscopic appearance (Figure 2) of the constructs varied dramatically depending on the prepolymer solution (e.g., macromer molecular weight and concentration) used for encapsulation. Specifically, constructs fabricated from 2 wt% of the macromers were noticeably more opaque than those fabricated with higher macromer concentrations. The 2 wt% hydrogels formed from both the 1100 kDa and 50 kDa macromers most resembled native cartilage and exhibited a white, shiny appearance. In addition, the 2 wt% 350 and 1100 kDa hydrogels exhibited a noticeable increase in size after the 12 weeks of subcutaneous culture in nude mice, whereas the 2 wt% 50 kDa hydrogel more closely maintained initial construct dimensions (i.e., 5 mm diameter).
Figure 2.
Explanted HA constructs 12 weeks after subcutaneous implantation of HA/auricular chondrocyte constructs in nude mice. The 2 wt% constructs resemble native cartilage tissue, whereas other HA constructs remained relatively translucent with little change in their macroscopic appearance since implantation.
Biochemical Analysis
The water content of the explanted constructs was determined from wet and dry weights (Figure 3). In general, constructs exhibited a decrease in water content from 6 weeks to 12 weeks, where a significant decrease was noted for 2 wt% 1100 kDa, 2 to 5 wt% 350 kDa, and 2 wt% 50 kDa constructs. Of the 12 week explants, the 2 wt% 50 kDa construct (81 ± 2.7% water) was most comparable to auricular (74 ± 2.4% water) and articular (79 ± 5.1% water) cartilage, and no statistically significant differences were found between the values.
Figure 3.
Water content of HA constructs after 6 (black) and 12 (white) weeks of subcutaneous culture in nude mice (n=4). A significant decrease (p<0.05) in water content from 6 to 12 weeks was found for all of the 2 wt% constructs and for the 5 wt% 350 kDa construct, with the 2 wt% 50 kDa construct exhibiting the greatest decrease in water content.
The total DNA content was determined for all harvested constructs and converted into numbers of chondrocytes per sample (Figure 4). The majority of the constructs exhibited an increase in chondrocyte number from 6 to 12 weeks, indicating cellular proliferation with culture time. Constructs fabricated with the lowest macromer concentration (2 wt%) appeared to support the greatest number of chondrocytes regardless of molecular weight. The 2 wt% 50 kDa constructs had the greatest number of chondrocytes at ~600,000 cells per sample after 12 weeks. Values were also normalized to sample wet weight for comparison to control auricular and articular cartilage (not shown). The 2 wt% 50 kDa constructs exhibited the greatest amount of DNA per wet weight, which was approximately 80 and 87% of that found for native auricular and articular cartilage, respectively. No significant differences were detected for the 2 wt% 1100 kDa and 2 wt% 50 kDa hydrogels when compared to auricular and articular cartilage for the normalized DNA values. Hydrogels (2 wt% 50 kDa) without encapsulated cells served as a control and exhibited minimal fluorescence, comprising less than 3.5% of any sample.
Figure 4.
Number of chondrocytes per sample for HA constructs after 6 (black) and 12 (white) weeks of subcutaneous culture in nude mice (n=4). Values, reported in millions, were determined using the PicoGreen dsDNA assay with a 7.7 pg DNA/chondrocyte conversion. For the 12 week explants, the 2 wt% constructs showed a statistical difference (p<0.05) from constructs with higher macromer concentrations.
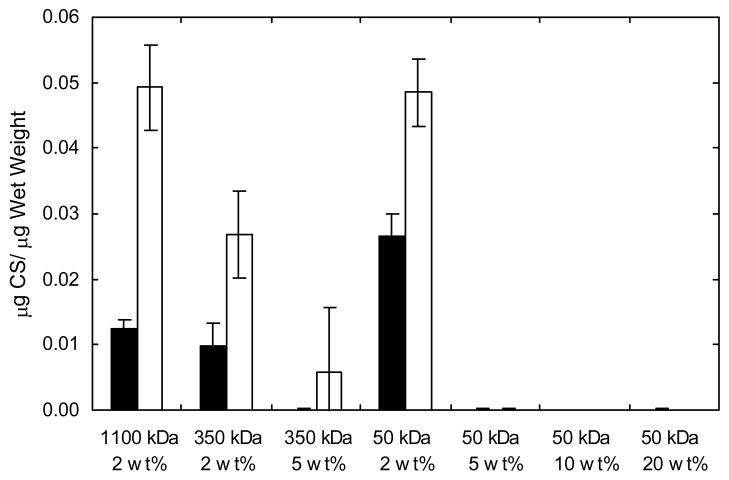
GAG content was measured in the explanted constructs and is reported as the quantity of chondroitin sulfate normalized to sample wet weight (Figure 5). The GAG content in constructs increased from 6 to 12 weeks, where the 2 wt% 1100 and 50 kDa constructs showed the greatest amount of GAGs (0.049 ± 0.007 and 0.049 ± 0.005 μg CS/μg wet weight, respectively) after 12 weeks of culture. This value is approximately 80% and 53% of the GAG content found in native auricular and articular cartilage, respectively. There was no significant difference between GAG production by chondrocytes encapsulated in the 2 wt% networks formed from both the 1100 and 50 kDa macromers compared to native auricular cartilage. Virtually no measurable GAGs were found in the hydrogels fabricated from the higher macromer concentrations (5 to 20 wt%). HA hydrogel controls showed little GAG detection and were similar to control hydrogels formed from a non-polysaccharide PEG hydrogel 19.
Figure 5.
Glycosaminoglycan content of HA constructs normalized to construct wet weight after 6 (black) and 12 (white) weeks of subcutaneous culture in nude mice (n=4). The 2 wt% constructs are statistically different (p<0.05) from those with higher macromer concentration after both 6 and 12 weeks.
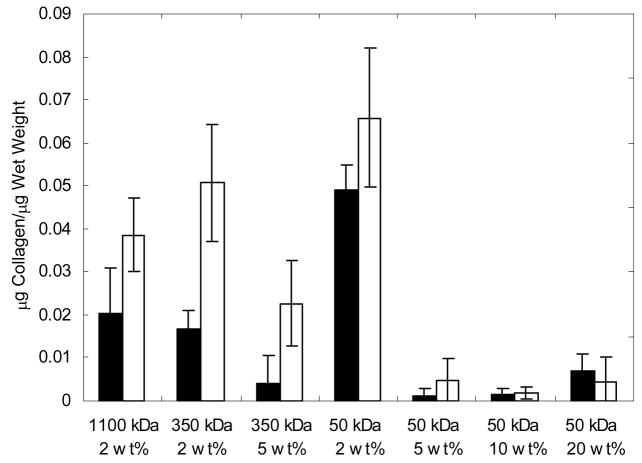
As a final quantification of biochemical content, the amount of collagen in the explanted constructs was determined by the hydroxyproline content and is normalized to sample wet weight (Figure 6). Similar to the GAG content, the majority of the constructs exhibited an increase in collagen content from 6 to 12 weeks. The 2 wt% 50 kDa construct is statistically different (p<0.05) from all of the samples after 6 weeks and showed the greatest amount collagen content (0.0658 ± 0.0161 μg collagen/μg wet weight) after 12 weeks. This value represents ~65% and ~74% of the total collagen that was found in control samples of auricular and articular cartilage, respectively. Again, only small amounts of collagen were detected in the hydrogels fabricated from the higher macromer concentrations (5 to 20 wt%). The values for both GAG and collagen content were also normalized to DNA content (results not shown) and showed similar trends to values normalized to sample wet weight.
Figure 6.
Total collagen content of HA constructs normalized to construct wet weight after 6 (black) and 12 (white) weeks of subcutaneous culture in nude mice (n=4). The 2 wt% 50 kDa construct is statistically different (p<0.05) from all of the samples after 6 weeks and has the greatest amount collagen content after 12 weeks.
Histological Analysis
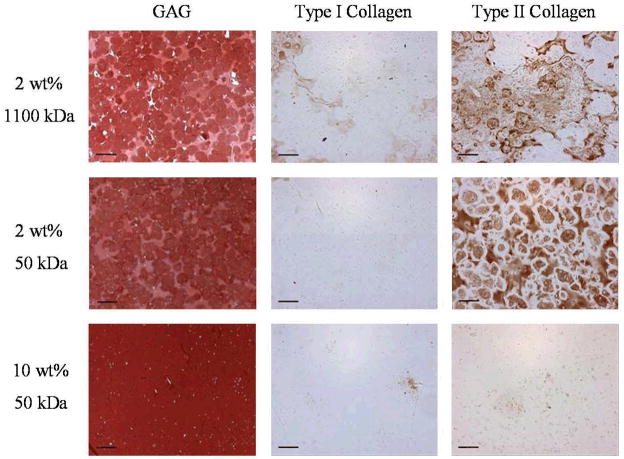
Representative stains for GAG, type I collagen, and type II collagen are shown in Figure 7 for several constructs harvested 12 weeks after implantation. Specifically, the histology is shown for the two compositions that looked the most promising both macroscopically and biochemically (i.e., 2 wt% of 1100 and 50 kDa macromers) and, for contrast, a sample that showed little production of extracellular matrix (10 wt% 50 kDa macromer). Unfortunately, the hydrogels exhibit background staining for HA when using the Safranin O stain, but the images still capture the large production of GAG after 12 weeks in vivo and illustrate the morphology and distribution of the cells. In constructs fabricated with a 2 wt% macromer solution, chondrocytes are evenly distributed and appear viable throughout the gels. In contrast, morphology indicative of cell death is evident in constructs fabricated with higher macromer concentrations (5 to 20 wt%) as illustrated by the 10 wt% 50 kDa construct. GAG staining is found abundantly throughout the histological sections, including the pericellular regions surrounding the cells in the 2 wt% hydrogels. The representative stains for type I and type II collagen support results observed in the biochemical analysis, where more intense staining is observed in the 2 wt% 1100 and 50 kDa constructs in contrast to the other samples. Though light staining for type I collagen is observed sporadically throughout the 1100 kDa sample, type II collagen appears to dominate in these constructs. For the 2 wt% 50 kDa sample, there was no type I collagen observed, but an even distribution of type II collagen is found throughout the networks. Again, no staining is observed in the 5 to 20 wt% 50kDa samples for either type I or type II collagen.
Figure 7.
Representative histological sections of 2 wt% 1100 kDa, 2 wt% 50 kDa, and 10 wt% 50 kDa constructs stained for glycosaminoglycans, type I collagen, and type II collagen after 12 weeks of subcutaneous culture in nude mice. Scale bar = 100 μm.
Discussion
Photocrosslinkable hyaluronic acid hydrogels were investigated in this study as carriers for auricular chondrocytes for cartilage regeneration. Hyaluronic acid has many beneficial properties for these applications. Specifically, HA is a molecule that is recognized by specific cell-surface receptors and degraded by enzymes produced by the encapsulated chondrocytes, potentially leading to a cell-dictated degradation mechanism. HA is readily modified with photoreactive groups to allow for cell encapsulation when a solution of the monomer, cells, and photoinitiator are exposed to the correct wavelength of light. In the fabrication of these hydrogels, several factors including the molecular weight of the HA, the acrylation percentage, and the concentration of the HA in the prepolymer solution can influence the properties of the resulting construct.
Our previous work 19 investigated the fabrication of these networks and illustrated the wide range of hydrogel properties that can be obtained. Alterations in HA molecular weight and macromer concentration affected precursor solution viscosities and hydrogel properties such as volumetric swelling ratio, compressive modulus, degradation, and cell viability, which are summarized in Table 1. Specifically, a decrease in the volumetric swelling ratio and an increase in both the compressive modulus and time for complete degradation of the networks were found as the macromer concentration (e.g., crosslinking density) was increased. However, only minor changes in these properties were observed with different macromer molecular weights. It should be noted that the hyaluronidase concentration used in this study (i.e., 100 U/ml) is not indicative of in vivo levels, but was used only to monitor relative degradation differences between the various hydrogels. When cells were encapsulated in the networks in vitro, a decrease in cell viability was found as the macromer concentration was increased. This in vitro decrease in cell viability was attributed to an increase in the radical concentration during encapsulation and a decrease in the diffusion of nutrients and wastes as the crosslinking density increased.
Table 1.
Summary of various properties of HA hydrogels19.
| MW of Macromer (kDa) | Methacrylation (%)a | Macromer in precursor solution (wt%) | Volumetric Swelling Ratio | Compressive Modulus (kPa) | Degradation Time (Days)b | Cell Viability (Absorbance @ 560 nm)c |
|---|---|---|---|---|---|---|
| 1100 | 6 | 2 | 36.5 ± 6.4 | 2.1 ± 0.6 | 1.0 ± 0.0 | 0.96 ± 0.06 |
| 350 | 7 | 2 | 42.6 ± 0.6 | 2.5 ± 0.6 | 1.0 ± 0.0 | 0.82 ± 0.06 |
| 5 | 25.9 ± 2.9 | 26.1 ± 1.1 | 2.0 ± 0.0 | 0.68 ± 0.08 | ||
| 50 | 12 | 2 | 41.7 ± 4.1 | 11.3 ± 4.0 | 1.0 ± 0.0 | 0.92 ± 0.04 |
| 5 | 26.5 ± 0.7 | 35.7 ± 3.8 | 8.3 ± 1.2 | 0.76 ± 0.06 | ||
| 10 | 17.4 ± 1.0 | 65.6 ± 9.3 | 19.0 ± 0.0 | 0.37 ± 0.07 | ||
| 20 | 8.2 ± 0.3 | 100.5 ± 11.0 | 37.7 ± 0.6 | 0.19 ± 0.04 |
Determined by 1H-NMR
Time for complete degradation of hydrogel disks in 100U hyaluronidase per ml PBS
Normalized viability of encapsulated fibroblasts after 1 week of in vitro culture
To further explore the potential of these photopolymerizable networks, the current work investigated the formation of neocartilage from various HA hydrogels in vivo. Auricular chondrocytes were used in this study to investigate neocartilage formation in the hydrogels based on hydrogel chemistry. This has direct implications for plastic surgery applications, but is also a consideration for articular cartilage regeneration. Auricular cartilage is easily harvested with little donor-site morbity and auricular chondroctyes can be obtained at yields twice as high as articular chondrocytes27. Additionally, auricular chondrocytes proliferate approximately four times faster than articular chondrocytes in monolayer culture28 and express high levels of type II collagen and glycosaminoglycans when implanted in vivo on 3-dimensional scaffolds27.
Macroscopic observations indicated that the hydrogels with the 2 wt% macromer concentration exhibited the greatest neocartilage formation and produced cartilage that most closely resembled native cartilage tissue. Higher macromer concentrations resulted in relatively translucent constructs, where restrictions in nutrient transport through the construct and high radical concentration during polymerization potentially compromised cell viability, growth, and extracellular matrix production. This reflects cell viability results from our previous in vitro study. With culture time, the decrease in water content is likely due to increased tissue formation (e.g., cell proliferation and extracellular matrix deposition) within the construct. After 12 weeks of culture, the water content for the 2 wt% 50 kDa construct was closest to that of native auricular and articular cartilage.
Constructs derived from macromer concentrations greater than 2 wt% showed little cell growth, as they contained approximately 2 × 105 chondrocytes per sample when implanted. Although the cells show some proliferation between the 6 and 12 week time points, the lack of type I collagen staining and abundant type II collagen staining in the 2 wt% hydrogels indicate that the cells are maintaining their chondrogenic phenotype. Again, this is potentially related to compromised cell viability during encapsulation, but could also be due to higher crosslinking densities that degrade slower than the more loosely crosslinked one, limiting 3-dimensional cell proliferation. In addition, results from GAG measurements exhibit a similar trend, where 2 wt% 1100 kDa and 2 wt% 50 kDa networks contained the greatest quantity of GAGs while constructs with macromer concentrations greater that 2 wt% exhibited extremely low GAG levels. Additionally, measured values for both 2 wt% 1100 kDa and 50 kDa constructs were comparable to the GAG content of native auricular cartilage. Collagen content followed a similar trend, with highly crosslinked networks showing very little collagen production by encapsulated cells.
Histological analysis was used to detect the distribution of GAGs, collagen type I, and collagen type II within the constructs. GAGs appeared to be evenly distributed throughout constructs with neocartilage growth, reflecting an even distribution of chondrocytes within the scaffold. On the other hand, cell death was evident in constructs without growth (5 to 20 wt% 50 kDa). Though some type I collagen staining was observed for 2 wt% 1100 kDa and 2wt% 50 kDa constructs, more intense and more uniform staining was found for type II collagen. The lack of type II collagen staining in certain regions of the 2 wt% 1100 kDa construct is potentially due to cell clustering, as the highly viscous precursor solution may have prevented an even cell distribution. On the other hand, type II collagen appears to be evenly distributed in the 2 wt% 50 kDa samples where viscosity did not play a hindering role. In general, histological observations are consistent with the results from the biochemical analysis and indicate that the cells are maintaining their phenotype even after long culture periods.
Overall, this analysis shows that the HA hydrogel structure is an important design parameter in cartilage tissue engineering and plays an important role in both the quality and distribution of tissue produced by encapsulated cells. After screening several factors, our results indicate that hydrogels formed from 2 wt% of the 50 kDa macromer supported the greatest amount of biochemical components and the best distribution of extracellular matrix components. We believe that enhanced neocartilage production in the 2 wt% hydrogels is due to: (i) a lower radical concentration during the polymerization of the 2 wt% hydrogels than for higher macromer concentrations, which may increase cell viability, (ii) an increase in nutrient and waste transport with a more loosely crosslinked hydrogel, and (iii) more rapid hydrogel degradation with a larger hydrogel mesh size, which may increase the distribution of extracellular matrix components. Additionally, the low viscosity of the 2 wt% solution makes it more amenable not only in the distribution of encapsulated chondrocytes, but also clinical application of these gel solutions through arthroscopic techniques. These results have shown very promising results for the production of cartilaginous tissue by encapsulated auricular chondrocytes with engineered tissue resembling native tissue both biochemically and histologically.
Acknowledgments
Support for this research was provided through an NIH grant (K22 DE-015761). The authors would like to thank Dr. Steven Nicoll for use of his laboratory for sample analysis.
References
- 1.Laurencin CT, Ambrosio AM, Borden MD, Cooper JA., Jr Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Kimura T, Yasui N, Ohsawa S, Ono K. Chondrocytes embedded in collagen gels maintain cartilage phenotype during long-term cultures. Clin Orthop Relat Res. 1984;(186):231–9. [PubMed] [Google Scholar]
- 3.Ting V, Sims CD, Brecht LE, McCarthy JG, Kasabian AK, Connelly PR, Elisseeff J, Gittes GK, Longaker MT. In vitro prefabrication of human cartilage shapes using fibrin glue and human chondrocytes. Ann Plast Surg. 1998;40(4):413–20. doi: 10.1097/00000637-199804000-00016. discussion 420–1. [DOI] [PubMed] [Google Scholar]
- 4.Sims CD, Butler PE, Cao YL, Casanova R, Randolph MA, Black A, Vacanti CA, Yaremchuk MJ. Tissue engineered neocartilage using plasma derived polymer substrates and chondrocytes. Plast Reconstr Surg. 1998;101(6):1580–5. doi: 10.1097/00006534-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Paige KT, Cima LG, Yaremchuk MJ, Vacanti JP, Vacanti CA. Injectable cartilage. Plast Reconstr Surg. 1995;96(6):1390–8. doi: 10.1097/00006534-199511000-00024. discussion 1399–400. [DOI] [PubMed] [Google Scholar]
- 6.Fragonas E, Valente M, Pozzi-Mucelli M, Toffanin R, Rizzo R, Silvestri F, Vittur F. Articular cartilage repair in rabbits by using suspensions of allogenic chondrocytes in alginate. Biomaterials. 2000;21(8):795–801. doi: 10.1016/s0142-9612(99)00241-0. [DOI] [PubMed] [Google Scholar]
- 7.Sittinger M, Reitzel D, Dauner M, Hierlemann H, Hammer C, Kastenbauer E, Planck H, Burmester GR, Bujia J. Resorbable polyesters in cartilage engineering: affinity and biocompatibility of polymer fiber structures to chondrocytes. J Biomed Mater Res. 1996;33(2):57–63. doi: 10.1002/(SICI)1097-4636(199622)33:2<57::AID-JBM1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Freed LE, Vunjak-Novakovic G, Biron RJ, Eagles DB, Lesnoy DC, Barlow SK, Langer R. Biodegradable polymer scaffolds for tissue engineering. Biotechnology (N Y) 1994;12(7):689–93. doi: 10.1038/nbt0794-689. [DOI] [PubMed] [Google Scholar]
- 9.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Langer R. Transdermal photopolymerization for minimally invasive implantation. Proc Natl Acad Sci U S A. 1999;96(6):3104–7. doi: 10.1073/pnas.96.6.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51(2):164–71. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Bryant SJ, Bender RJ, Durand KL, Anseth KS. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol Bioeng. 2004;86(7):747–55. doi: 10.1002/bit.20160. [DOI] [PubMed] [Google Scholar]
- 12.Menzel EJ, Farr C. Hyaluronidase and its substrate hyaluronan: biochemistry, biological activities and therapeutic uses. Cancer Lett. 1998;131(1):3–11. doi: 10.1016/s0304-3835(98)00195-5. [DOI] [PubMed] [Google Scholar]
- 13.Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7(2):79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 14.Grigolo B, Roseti L, Fiorini M, Fini M, Giavaresi G, Aldini NN, Giardino R, Facchini A. Transplantation of chondrocytes seeded on a hyaluronan derivative (hyaff-11) into cartilage defects in rabbits. Biomaterials. 2001;22(17):2417–24. doi: 10.1016/s0142-9612(00)00429-4. [DOI] [PubMed] [Google Scholar]
- 15.Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, Kon E, Pederzini L, Rosa D, Sacchetti GL, et al. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;(435):96–105. doi: 10.1097/01.blo.0000165737.87628.5b. [DOI] [PubMed] [Google Scholar]
- 16.Ramamurthi A, Vesely I. Ultraviolet light-induced modification of crosslinked hyaluronan gels. J Biomed Mater Res A. 2003;66(2):317–29. doi: 10.1002/jbm.a.10588. [DOI] [PubMed] [Google Scholar]
- 17.Nettles DL, Vail TP, Morgan MT, Grinstaff MW, Setton LA. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann Biomed Eng. 2004;32(3):391–7. doi: 10.1023/b:abme.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- 18.Smeds KA, Pfister-Serres A, Miki D, Dastgheib K, Inoue M, Hatchell DL, Grinstaff MW. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2001;54(1):115–21. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Burdick JA, Chung C, Jia X, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6(1):386–91. doi: 10.1021/bm049508a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryant SJ, Nuttelman CR, Anseth KS. Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed. 2000;11(5):439–57. doi: 10.1163/156856200743805. [DOI] [PubMed] [Google Scholar]
- 21.Singer VL, Jones LJ, Yue ST, Haugland RP. Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Anal Biochem. 1997;249(2):228–38. doi: 10.1006/abio.1997.2177. [DOI] [PubMed] [Google Scholar]
- 22.Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174(1):168–76. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 23.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–8. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 24.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18(2):267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 25.Herbage D, Bouillet J, Bernengo JC. Biochemical and physiochemical characterization of pepsin-solubilized type-II collagen from bovine articular cartilage. Biochem J. 1977;161(2):303–12. doi: 10.1042/bj1610303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson AK, Chen AC, Sah RL. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res. 2001;19(6):1113–21. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- 27.van Osch GJVM, Mandl EW, Jahr H, Koevoet W, Nolst-Trenite G, Verhaar JAN. Considerations on the use of ear chondrocytes as donor chondrocytes for cartilage tissue engineering. Biorheology. 2004;41(34):411–21. [PubMed] [Google Scholar]
- 28.Kafienah W, Jakob M, Demarteau O, Frazer A, Barker MD, Martin I, Hollander AP. Three-dimensional tissue engineering of hyaline cartilage: comparison of adult nasal and articular chondrocytes. Tissue Eng. 2002;8(5):817–26. doi: 10.1089/10763270260424178. [DOI] [PubMed] [Google Scholar]