Abstract
Progressive multifocal leukoencephalopathy (PML) is a rare but frequently fatal disease caused by the uncontrolled replication of JC virus (JCV), a polyomavirus, in the brains of some immunocompromised individuals. Currently, no effective antiviral treatment for this disease has been identified. As a first step in the identification of such therapy, we screened the Spectrum collection of 2,000 approved drugs and biologically active molecules for their anti-JCV activities in an in vitro infection assay. We identified a number of different drugs and compounds that had significant anti-JCV activities at micromolar concentrations and lacked cellular toxicity. Of the compounds with anti-JCV activities, only mefloquine, an antimalarial agent, has been reported to show sufficiently high penetration into the central nervous system such that it would be predicted to achieve efficacious concentrations in the brain. Additional in vitro experiments demonstrated that mefloquine inhibits the viral infection rates of three different JCV isolates, JCV(Mad1), JCV(Mad4), and JCV(M1/SVEΔ), and does so in three different cell types, transformed human glial (SVG-A) cells, primary human fetal glial cells, and primary human astrocytes. Using quantitative PCR to quantify the number of viral copies in cultured cells, we have also shown that mefloquine inhibits viral DNA replication. Finally, we demonstrated that mefloquine does not block viral cell entry; rather, it inhibits viral replication in cells after viral entry. Although no suitable animal model of PML or JCV infection is available for the testing of mefloquine in vivo, our in vitro results, combined with biodistribution data published in the literature, suggest that mefloquine could be an effective therapy for PML.
Progressive multifocal leukoencephalopathy (PML) is a progressive, usually fatal, demyelinating disease caused by JC virus (JCV) infection and the destruction of oligodendrocytes in multiple brain foci of susceptible individuals. JCV is a double-stranded DNA polyomavirus that is believed to cause asymptomatic infections in 65 to 90% of the human population, as judged by the presence of virus-specific antibodies (35). There is persistent viral shedding in the urine of 20 to 40% of individuals (35), which, together with the observed presence of the virus in kidney tubular epithelial cells (32, 49), indicates that JCV establishes a persistent and chronic infection in a large fraction of the human population. Despite this high infection rate and viral prevalence, PML is a rare disease that almost exclusively afflicts individuals who are immunocompromised due to genetic factors, human immunodeficiency virus (HIV) infection, hematological malignancies, or immunosuppressive therapies (8, 14).
Currently there are no approved or proven therapies for PML. Although a number of preclinical reports and case studies have suggested the potential anti-PML effects of antiviral and antineoplastic drugs such as cytarabine, cidofovir, and topotecan, larger case-controlled studies failed to establish the efficacies of these drugs (1, 21, 27, 28, 31, 39). To date, the most effective intervention for the treatment of PML is reconstitution of the patient's immune system. Thus, the introduction of highly active antiretroviral therapy was the single most significant development, reducing the rate of mortality from PML in HIV-positive individuals from 90% to 50% (8, 10, 16-18). Similarly, a reduction in the drug regimen of PML patients undergoing immunosuppressive therapy may halt the worsening of clinical symptoms (20, 54). However, an immune reconstitution approach is not possible or successful in all patients. Therefore, it is imperative that the search for therapeutics targeting JCV directly be continued.
To identify drugs with anti-JCV activity, we screened a commercially available collection of approved drugs and bioactive compounds in an in vitro JCV infection assay. As a primary screen, we monitored inhibition of the viral infection rate of transformed human glial (SVG-A) cells (38) exposed to JCV(M1/SVEΔ), a modified form of JCV (58). The infection rate was measured as the percentage of cells expressing the viral capsid protein VP1. Of the 2,000 compounds in the Spectrum collection, 14 were found to reduce the number of infected cells by ≥50% at concentrations of ≤20 μM (50% effective concentration [EC50], ≤20 μM). Since PML is a result of uncontrolled viral replication in the central nervous system (CNS), it is critical that potential therapeutic agents cross the blood-brain barrier (BBB) at a concentration sufficient to be effective. On the basis of the published literature on the 14 drug candidates with in vitro anti-JCV activity that have been identified, only mefloquine, an antimalarial agent, appears to exhibit a level of CNS penetration that could be expected to achieve in vitro-derived efficacious concentrations in humans (33, 47). Our experiments characterizing the anti-JCV activity of mefloquine, together with the available published data, suggest that the efficacy of mefloquine treatment should be examined in patients with PML.
MATERIALS AND METHODS
Source and identity of potential therapeutic agents.
The Spectrum collection (MicroSource Discovery Inc., Groton, CT) consists of ∼1,000 bioactive compounds and natural products plus ∼1,000 Food and Drug Administration-approved drugs that are defined according to the name designations set forth in the USP Dictionary of USAN and International Drug Names (57). An alphabetical listing of the compounds is available at http://www.msdiscovery.com/spectrum.html. The compounds are supplied as 10 mM solutions in dimethyl sulfoxide (DMSO). In our follow-up experiments, mefloquine and all other drugs were purchased from Sigma-Aldrich (St. Louis, MO), and 100 mM stock solutions were prepared in DMSO. Two mefloquine enantiomers were separated from a commercial mefloquine sample by Chiral Technologies (West Chester, PA) by chiral high-pressure liquid chromatography on a Chiralpak IA column to a purity of >98.6% for each enantiomer. Mefloquine analogs were purchased from BioBlocks, Inc. (San Diego, CA.). Cidofovir (Vistide) was obtained from Gilead as a 0.238 M aqueous stock solution.
Propagation and identity of cultured cells.
SVG-A cells (a gift from Walter Atwood, Brown University), established by transforming human fetal glial cells with an origin-defective simian virus 40 (SV40) mutant (38), were cultured in 1× Eagle minimal essential medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 4 mM l-glutamine (Mediatech, Inc.). Viral infection was performed in the same medium supplemented with 2% heat-inactivated FBS.
Human astrocytes were isolated from a fetal cerebral cortex (ScienCell Research Laboratories, Carlsbad, CA) and cultured in proprietary astrocyte growth medium (ScienCell Research Laboratories). Viral infection of the astrocytes was performed in this medium.
Cultures of primary human fetal glial (PHFG) cells were prepared from fetal brain tissue by using a modification of an earlier protocol (44). Briefly, tissue was received in glial cell medium (Dulbecco modified Eagle's minimal essential medium [DMEM] supplemented with 3% FBS, 7% bovine calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μg/ml gentamicin, and 2.5 μg/ml amphotericin B [Fungizone]). After removal of the meninges and blood vessels, the tissue was washed twice in 10 to 30 ml glial cell medium without gentamicin containing 200 μg/ml amikacin (Sigma) and was then pelleted in a clinical centrifuge (3 min, 1,120 rpm). The tissue was transferred to a sterile petri dish containing 10 to 20 ml medium, and tissue fragments were reduced in size by expressing them through a 10-ml syringe (without a needle) and a 40-mesh screen in a tissue dissociation device (Sigma). Cells derived by this procedure were seeded on 100-ml tissue culture plates in glial cell medium by using a volume of 0.5 ml of cells per plate. After 1 week, if the astrocyte layer was confluent, the cultures were placed in maintenance medium (DMEM supplemented with 3% FBS, penicillin, and streptomycin). If the astrocyte layer was not confluent, the cultures were placed in glial cell medium without amikacin. At 2 to 3 weeks after they were seeded, the cells were prepared for freezing. Briefly, cell cultures were incubated overnight in DMEM containing 10% FBS. On the next day, the heterogeneous population of cells containing astrocytes and small round cells clustered on top of the astrocyte layer was washed with saline A (trypsin buffer, 0.8% NaCl, 0.4% KCl, 0.1% glucose, 0.035% NaHCO3, 0.2% EDTA, pH 7.0). After aspiration, saline A was added again to each plate, and the cells were incubated for 3 min at 37°C. Trypsin (in saline A) was added, and cells were incubated for 4 min at 37°C. DMEM containing 10% FBS was added, and the cells were gently collected in 50-ml conical tubes. The cell suspension was centrifuged, the pellet was washed once with DMEM containing 10% FBS, and the cells were then pelleted again. The cells were suspended in freezing medium (DMEM, 10% FBS, 10% DMSO) and were stored in liquid nitrogen until they were used for infectivity assays with JCV(Mad1).
JCV isolates.
The hybrid virus JCV(M1/SVEΔ) (a gift from Walter Atwood) was constructed by first inserting SV40 regulatory sequences into the JCV(Mad1) transcriptional control region to create the hybrid genome, JCV(M1/SVE) (58). Transfection of SVG-A cells with this DNA yielded JCV(M1/SVEΔ), which comprised JCV-SV40 enhancer signals linked to the JCV(Mad1) genome. To produce purified preparations of virus, SVG-A cells were plated at 50% confluence and were infected with a 1:50 dilution of JCV(M1/SVEΔ) for 1 h at 37°C (25, 36). The cells were cultured for 3 weeks and were then scraped from the flasks, pooled (along with cells that had detached during prior medium changes), and pelleted. The cell pellet was resuspended in 20 ml of supernatant and disrupted in a microfluidizer (Microfluidics, Inc.). Deoxycholate was added to the cell lysates at a final concentration of 0.25%, and the mixture was incubated at 37°C for 30 min. The virus-containing supernatant was centrifuged at 10,000 rpm for 30 min in an SA600 rotor and aliquoted and stored at −80°C. The JCV(Mad4) isolate (46) was obtained from the American Type Tissue Collection (Manassas, VA), and the prototype JCV(Mad1) isolate (24, 45) was a gift from Duard Walker (Madison, WI).
Detection of antibodies.
PAb597 (a gift from Walter Atwood [19]), a mouse monoclonal antibody directed against SV40 major capsid protein VP1, cross-reacts with JCV VP1 (4) and was used with an Alexa-Fluor 488-labeled goat anti-mouse secondary antibody (Invitrogen, Carlsbad, CA) to visualize JCV-infected cells by indirect immunofluorescence. Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen). Neutralizing anti-JCV rabbit antiserum was a gift from Walter Atwood (3).
JCV infectivity assay.
SVG-A cells were seeded at 2,000 cells/well/0.075 ml of culture medium in flat-bottom 96-well plates (Corning). The compounds to be tested for antiviral activity were prepared the next day in assay medium (1× Eagle minimal essential medium with 2% heat-inactivated FBS and 4 mM l-glutamine). A master viral plate was prepared by mixing equal volumes of 2× compound and virus diluted two times to yield the final working concentrations of compound and virus. The plate containing cells was gently inverted and shaken to remove the medium. From the master plate, 0.035 ml of compound plus virus was added to designated wells. The cells were incubated with the compound-virus mixture for 60 min in a humidified, 37°C incubator containing 5% CO2. Medium containing the final concentration of the drug was added to designated wells to bring the final volume to 0.1 ml/well. After incubation of the plates for 3 days, the cells were washed once with 1× phosphate-buffered saline (PBS) and fixed in 2% paraformaldehyde-1× PBS for 30 min at room temperature. The fixative was removed, and the cells were permeabilized with 0.5% Triton X-100 in PBS for 30 min. Cells infected with JCV(M1/SVEΔ) were visualized by staining with 0.05 ml of PAb597 (2 μg/ml in 1× PBS) for 60 min at 37°C. Following a wash step with 1× PBS, an Alexa-Fluor 488-labeled goat anti-mouse secondary antibody (1:100 dilution in 1× PBS) and DAPI (1 μg/ml, 0.05 ml/well) were added to the cells for 30 min at 37°C. The cells were washed with 1× PBS, and field images of each well were acquired and analyzed with a Cellomics ArrayScan imager (Thermo Scientific Inc.) and Target Activation software.
The infectivity assay was also performed with human astrocytes or PHFG cells but with the following modifications. Human astrocytes were seeded at 4,000 cells/well/0.075 ml of culture medium in flat-bottom 96-well plates. The culture medium was used as the virus diluent, and the duration of the infection was 6 to 10 days. PHFG cells (∼1.2 × 105) were seeded onto 35-mm plates containing 2.0 ml DMEM supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine. The cells were exposed 48 h later to 5 hemagglutinating units of JCV(Mad1) diluted in DMEM; 0.5% heat-inactivated FBS; and 2.5 μM, 5 μM, 7.5 μM, or 10 μM of mefloquine in 0.01% DMSO. Three independent sets of experiments were performed, each of which was performed with two samples at each time point. Cells not exposed to mefloquine were infected with 1 or 5 hemagglutinating units of JCV(Mad1) and served as controls to determine mefloquine's inhibitory activity and to confirm that the infected cell numbers increased proportionally with a fivefold increase in the virus inoculum. Virus was allowed to adsorb to the cells for 3 h at 37°C, and then DMEM supplemented with 3% heat-inactivated FBS and the appropriate amount of mefloquine was added. The medium was replaced 5 days postinfection, and DNA was extracted from the cells at days 7 and 10 postinfection.
DNA extraction and sample preparation.
DNA was extracted from cells with a QIAamp 96 blood kit (Qiagen, Inc.) and were treated with RNase A, which is optional. The DNA was quantitated with a Quant-iT double-stranded DNA high-sensitivity assay, according to the manufacturer's recommendations (Molecular Probes Inc., Eugene, OR). The purified DNA was stored at −20°C until use.
Real-time quantitative PCR (qPCR) assay.
TaqMan forward and reverse primers and MGB probes were designed to recognize conserved JCV early coding sequences and were designed with Primer Express (version 3.0) software (Applied Biosystems, Foster City, CA). The sequence of the forward primer was AGGCAGCAAGCAATGAATCC, that of the reverse primer was ATGGCAATGCTGTTTTAGAGCAA, and that of the 6-carboxyfluorescein-labeled probe was CCACCCCAGCCATAT. To create a copy number standard curve for absolute quantification, we linearized plasmid pUC19 containing the JCV genome with SmaI. Quadruplicate PCRs were run in a 384-well optical plate (Applied Biosystems). Real-time reactions were performed in a 7900HT thermal cycler (Applied Biosystems) under the following conditions: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 60 s with 900 nM forward and reverse primers, 200 nM TaqMan probe, and 1× Universal master mix (Applied Biosystems). The JCV copy number was determined for each experimental sample by comparison to the JCV plasmid standard curve by using Sequence Detection software (Applied Biosystems) and normalization to the total amount of DNA extracted from a sample. Zero copies of JCV DNA were detected in a noninfected negative control. P values were calculated by a Student t test.
Determination of rates of viral inhibition.
We determined the percentage of cells infected following exposure to JCV by dividing the number of immunofluorescent cells by the total number of nucleated cells and then multiplying that value by 100, that is, percent JCV-positive cells = (total number of VP1-positive cells)/(total number of DAPI-positive cells)·100. The percent viral inhibition by a compound was calculated on the basis of the percentage of JCV-positive cells rather than the number of VP1-positive cells: percent JCV inhibition = [1 − (percent JCV-positive cells with a compound − percent JCV-positive cells in the negative control)/(percent JCV-positive cells in the positive control − percent JCV-positive cells in the negative control)]·100, where the positive control represents cells infected with virus in the absence of any compound and the negative control denotes cells not infected with virus. The number of JCV-positive cells in the negative control samples quantitated with the Cellomics ArrayScan imager was always <1% of the number of JCV-positive cells in the positive control. The percentage of JCV DNA replication inhibition was calculated as [1 − (JCV DNA copy number in cells treated with a compound/JCV DNA copy number in the positive control)]·100. Zero copies of the JCV genome were detected in negative control samples. For high-throughput screening of the compounds, we calculated the Z factor, which was equal to 1 − [3·(σp + σn)/|μp − μn|], where μ is the mean, σ is the standard deviation, p is the positive control, and n is the negative control (61). The intraplate intragroup coefficient of variance was always below 20%, and Z′ was always >0.5. EC50s were calculated with Prism software (GraphPad Software Inc., La Jolla, CA). The EC50 was calculated by fitting of a sigmoid dose-response curve, Y = bottom + {(top − bottom)/[1 + 10^(X − log EC50)]}, where X is the logarithm of the inhibitor concentration, Y is the response (i.e., percent inhibition), top is the highest observed value, and bottom is the lowest observed value.
Molecular modeling and in silico screening.
The ROCS program (version 2.3.1; Openeye Scientific Software, Santa Fe, NM) was used to compare the three-dimensional shapes of diverse compound classes and to perform virtual screening against clinical compounds extracted from the MDL Drug Data Report database (Symyx Technologies, Inc.). The program OMEGA2 (version 2.3.0; Openeye Scientific Software) was used to generate multiple low-energy conformations of the compounds. The three-dimensional enumerated conformers were compared on the basis of the implicit Mills-Dean atom chemical force field scheme in conjunction with the shape-base matching algorithm of the ROCS program (version 2.3.1; Openeye Scientific Software). The overlays with the best scores were visualized in the PyMOL program (version 1.0.0b15; DeLano Scientific LLC, Palo Alto, CA).
RESULTS
Primary screen: JCV infectivity assay.
To identify drugs with anti-JCV activity, we screened a commercially available collection of approximately 2,000 approved drugs and bioactive compounds, called the Spectrum collection, for their anti-JCV activities in an in vitro viral infectivity assay (48). As a primary screen, we monitored inhibition of the viral infection rate in a human fetal astroglial cell line (SVG-A) infected with JCV(M1/SVEΔ), a modified form of JCV. SVG-A cells (38) were chosen for use in the primary screening assay, as they represent one of the few available cell lines permissive for JCV replication, and JCV(M1/SVEΔ) was chosen because its infection of SVG-A cells results in an accelerated rate of viral replication that permits the earlier detection of infected cells relative to that observed with other cell types and JCV isolates (3 versus 6 to 10 days postinfection). The JCV(M1/SVEΔ) genome includes the coding sequences of prototype strain JCV(Mad1) isolated from a patient with PML (24, 45) linked to hybrid JCV-SV40 noncoding regulatory sequences (58). This rearrangement of transcriptional signals extends the species and cell type host range of the virus.
To facilitate screening of the Spectrum collection, we adapted the JCV infectivity assay (48) to a 96-well format and employed a Cellomix ArrayScan high-content imager to measure JCV replication. In this system, infected cells can be detected by immunofluorescent staining with antibodies that recognize the JCV capsid protein, VP1. The total numbers of cells in the culture were visualized by staining with DAPI, which stains DNA (Fig. 1A). Use of the Cellomics ArrayScan imager allowed us to simultaneously identify and count each DAPI- and VP1-positive event in the assay well and routinely counted 300 to 800 VP1-positive and 8,000 to 16,000 DAPI-positive events per well, thus minimizing variability due to a nonuniform cell growth pattern and/or intrawell viral spread. By using this format, 4 to 7% of all cells were found to express JCV VP1 72 h postinfection, and the number of infected cells (i.e., VP1-positive cells) at the end of the culture period was proportional to the number of infectious viral particles used to infect the cell culture (Fig. 1B). In each experiment, the highest viral dilution that yielded maximum infectivity was used. Using neutralizing rabbit anti-JCV serum as a positive control for the inhibition of infectivity, we showed that the assay responds to viral inhibition in a predictive fashion (Fig. 1C).
FIG. 1.
Detection and measurement of JCV infection. (A) SVG-A cells infected with JCV(M1/SVEΔ) were fixed and stained 3 days postinfection with murine monoclonal antibodies specific for VP1 protein (green staining). All cells present in the culture were visualized with DAPI DNA nuclear staining (blue). The picture was taken with a Cellomics ArrayScan camera. Magnification, ×200. (B) The number of infected cells (i.e., VP1-positive cells) per group is plotted against the dilution factor of the viral stock used to infect the cells (mean ± standard deviation [n = 2]; blue line). The total numbers of cells (yellow bars) were similar for all groups. Cells were infected in the presence of various dilutions of JCV-neutralizing antiserum (C) or cidofovir (D). Cells were fixed and stained at 3 days postinfection, and the total numbers of VP1-positive and DAPI-positive events per treatment group were determined with a Cellomics ArrayScan imager. Data are presented as percent inhibition relative to that for the no-drug control of the number of JCV-positive (JCV+) cells, the total number of cells, or the number of JCV-positive cells normalized by total cell number (percent JCV-positive cells). Data from a representative experiment of at least three performed are shown.
We initially tested the compounds for their antiviral activities at a single dose (10 μM) and noticed that some of the compounds that inhibited the number of virus-infected cells (i.e., VP1-positive cells) to a great extent also dramatically reduced the total number of cells (i.e., DAPI-positive events), suggesting the occurrence of cytotoxic or cytostatic effects. To determine whether a particular compound had decreased the number of virus-infected cells because of its antiviral effect, we calculated the percent viral inhibition using the infection rate (i.e., the percentage of JCV-positive cells) rather than the total number of JCV-infected cells (i.e., the number of VP1-positive cells). With this system, we observed that treatment with cidofovir, a drug that has been tested clinically for its efficacy in patients with PML (21, 27, 39), inhibited the number of infected cells and the total number of cells in culture to the same degree, indicating that the percent inhibition of the infection rate (i.e., the percentage of JCV-positive cells) was not significant (Fig. 1D). Similar effects were noted for other drugs reported to have cytotoxic effects, e.g., mitomycin C and cytarabine (data not shown). Therefore, we determined the antiviral activity of each compound by calculating viral inhibition on the basis of the percentage of JCV-positive cells rather than the absolute number of JCV-positive cells per group.
Drug screening and selection.
A number of drugs and compounds that inhibited the JCV infection rate by ≥20% without causing significant cell toxicity (<20% total cell number inhibition) were identified (Fig. 2). We chose 20% as a cutoff for the first-pass screening because the coefficient of variance value of our assay was consistently <20%; 67 compounds fulfilled this criterion (see the table in the supplemental material). These compounds were subsequently tested in the same assay by using several different concentrations to further evaluate their therapeutic potential. On the basis of the dose-response data, 14 drugs proved to be effective and demonstrated ≥50% inhibition of virus-infected cells (EC50, <20 μM) without inducing significant cell toxicity (<20% total cell number inhibition) (Table 1). The compounds that had reduced the total cell number by ≥80% were retested at lower concentrations, but none that demonstrated a clear anti-JCV effect without a concomitant cytotoxic or cytostatic effect were identified (Fig. 2). We chose to proceed with testing only with the drugs that did not reduce the total cell numbers to diminish the chance of confounding an antiviral effect with a cytotoxic or cytostatic effect.
FIG. 2.

Flowchart describing the steps used to screen the compounds in the Spectrum collection. Primary screening employed the SVG-A cell line and the virus JCV(M1/SVEΔ), and the assay was performed as described in the legend to Fig. 1. TC50, drug concentration for inhibition of total cell numbers by 50%.
TABLE 1.
JCV inhibitors identified from screening of Spectrum collectiona
| Inhibitor name | Therapeutic use | Statusb | TC50c (μM) | EC50 (μM) | Concn (μM)d
|
Reference(s) for pharmacokinetic data | |
|---|---|---|---|---|---|---|---|
| Brain | Plasma | ||||||
| Isotretinon | Antineoplastic | USP, INN, BAN | >40 | 4.4 | ∼7.3e | 24 | 22, 34 |
| Mefloquine | Antimalarial | USAN, INN, BAN | 16.1 | 4.0 | 30-50 | 6.0 | 33, 47 |
| Diclofenac sodium | Antiinflammatory | USP, JAN | 30.5 | 8.3 | 2.7e | 8.0 | 26, 51 |
| Diltiazem hydrochloride | Ca channel blocker | USP, INN, BAN, JAN | >40 | 8.5 | ∼1.1e | 0.5 | 9, 12 |
| Fusidic acid | Antibacterial | USAN, INN, BAN | >40 | 8.6 | 1.9 | 19-171 | 40, 56 |
| Miconazole nitrate | Antifungal | USP, JAN | 22.9 | 8.6 | NAf | 0.024 | 55 |
| Mefenamic acid | Anti-inflammatory | USP, INN, BAN, JAN | >40 | 10.9 | 2.4e | 40-80 | 26, 29 |
| Flunixin meglumine | Anti-inflammatory | USP, veterinary | >40 | 16.6 | |||
| Propanil | NA | >40 | 7.8 | ||||
| Dehydroabietamide | NA | >40 | 13.0 | ||||
| Diffractic acid | NA | >40 | 14.4 | ||||
| Harmane | NA | >40 | 14.4 | ||||
| Xanthone | NA | >40 | 16.8 | ||||
| Methoxyvone | NA | >40 | 17.2 | ||||
The compounds selected had anti-JCV activity (EC50) at ≤20 μM and a therapeutic index (TC50/EC50) of >2.
USP, United States Pharmacopeia; INN, International Nonproprietary Name; BAN, British Approved Name; USAN, U.S. Approved Name; JAN, Japanese Approved Name.
TC50, inhibition of total cell numbers by 50%.
The highest concentration achieved in the brain or plasma/serum, as reported in the literature.
No data for humans are available; the data are based on the data reported from animal studies.
NA, not applicable.
JCV actively replicates and destroys oligodendrocytes in the CNS of PML patients. Therefore, it is crucial to the success of any potential PML therapy that the candidate drug be capable of achieving an efficacious concentration in the brain. Unfortunately, many drugs are incapable of penetrating the BBB. On the basis of a review of the literature (Table 1), only 1 of the 14 compounds with anti-JCV activity, mefloquine, has been shown to accumulate in the brains of treated patients at the concentration at which it is efficacious in vitro (EC50, 3.9 ± 2.1 μM) (Fig. 3A). A postmortem brain analysis of people taking mefloquine prior to their deaths measured 35 to 50 nmol of drug per gram of brain tissue, or approximately 35 to 50 μM (33, 47). Since potentially efficacious doses of mefloquine could be achieved in the brains of patients receiving approved doses of the drug, subsequent experiments focused upon characterization of the anti-JCV activity of mefloquine.
FIG. 3.
Characterization of mefloquine anti-JCV effect. (A and B) To further characterize the anti-JCV effect of mefloquine in different cell types and against different JCV isolates, viral infections were performed over the range of mefloquine concentrations in SVG-A cells with JCV(M1/SVEΔ) (n = 12) (A), in primary human fetal astrocytes with JCV(M1/SVEΔ) (B), or in SVG-A cells with JCV(Mad4) (n = 5) (C). (D and E) Characterization of the effect of mefloquine on the inhibition of JCV DNA replication. (D) Viral T-antigen DNA was quantified in the presence of various drug concentrations by qPCR in SVG-A cells infected with JCV(M1/SVEΔ); the inhibition of JCV DNA copy number and the inhibition of JCV-positive (JCV+) cells were measured in a replicate plates, the results of one representative experiment of two performed are shown. (E) By using the same qPCR assay, mefloquine's ability to inhibit JCV(Mad1) DNA replication in PFHG cells was measured over a range of drug concentrations at days 7 and 10 postinfection. The graph represents the average percent JCV DNA inhibition for three independent experiments with duplicate samples per time point. (F) The effect of the delay of mefloquine addition was measured in cultures of primary human fetal astrocytes infected with JCV(M1/SVEΔ). Cells were exposed to various concentrations of mefloquine at the same time (T) as virus addition or at 3 h or 24 h after virus addition. Ten days after infection with virus, cells were fixed and stained and the number of virus-infected cells was determined. The results of a representative experiment of five experiments conducted with either primary astrocytes or SVG-A cells is shown. The method used for the calculation of percent JCV inhibition is described in Materials and Methods. Inhibition of total cell numbers (i.e., DAPI-positive events) was less than 20% for all drug concentrations plotted. Unless otherwise noted, only one representative graph is shown, but the EC50 is calculated as an average of all experiments.
Characterization of mefloquine activity in primary cell culture.
To further characterize the effect of mefloquine on the activity of JCV, experiments were performed to evaluate whether the JCV-inhibitory effect of mefloquine was dependent on the cell line used in the primary screen. The SVG-A cell line has been propagated in vitro for many generations and was transformed by an SV40 large T antigen that can enhance JCV replication. To evaluate whether mefloquine is capable of inhibiting viral replication in cells more closely resembling the JCV target cell in the human brain, experiments were performed with primary glial cells. In vitro infection of homogeneous cultures of primary oligodendrocytes, a primary JCV target in the brains of patients with PML, has not been established; therefore, we employed human fetal astrocytes to test the ability of mefloquine to inhibit viral infection in a primary cell culture. In these cells, mefloquine inhibits JCV infection with essentially the same efficacy as it inhibits viral infection in SVG-A cells (Fig. 3B). These data suggest that the anti-JCV effect of mefloquine occurs in a more relevant cellular background.
Characterization of mefloquine effects on natural JCV isolates.
Experiments were performed to demonstrate that the inhibitory effect of mefloquine is not limited to the JCV(M1/SVEΔ) construct used in the primary screening assay. JCV(M1/SVEΔ) was used because of its faster growth kinetics and ease of preparation relative to those of in vivo isolates of JCV; however, the transcription of its JCV genes is not regulated by an authentic JCV enhancer element. To ensure that mefloquine's antiviral activity is not limited to this modified virus, we tested mefloquine's ability to inhibit JCV(Mad4), an in vivo isolate from a PML patient (46). On the basis of the results of five independent experiments, mefloquine inhibited JCV(Mad4) infection of SVG-A cells with the same efficacy as it did JCV(M1/SVEΔ) infection (Fig. 3C), demonstrating that mefloquine has an effect against a known pathogenic form of JCV.
Characterization of mefloquine's effect on JCV DNA replication.
To better understand mefloquine's mechanism of action and to address whether this drug inhibits viral DNA replication as opposed to a later step in the viral life cycle, a qPCR assay was employed to measure viral DNA levels in treated and untreated cells. The results presented in Fig. 3D indicate that the percentage of JCV DNA replication inhibited by mefloquine closely paralleled the percent inhibition of the infection rate, suggesting that mefloquine inhibits the infection rate at one of the steps involved in viral DNA replication and not VP1 protein expression. We sought to extend this observation to a JCV(Mad1) infection of the naïve cells most commonly used to propagate the virus in culture, PHFG cells. At days 7 and 10 postinfection, mefloquine inhibited JCV(Mad1) DNA replication in these cells with nearly the same efficacy (EC50, ∼5 μM) that it inhibited JCV(M1/SVEΔ) DNA replication in SVG-A cells (Fig. 3E).
Effect of mefloquine on established JCV infection.
Our experiments indicated that mefloquine inhibited JCV infection when it was added to cells at the same time that the virus was added, but it was not clear from the results of those experiments whether mefloquine inhibited viral entry of the cells or a later step in the viral life cycle. Once PML is diagnosed, many cells have already been infected, and a preferred drug candidate for PML treatment should demonstrate an ability to inhibit an ongoing viral replication cycle. To investigate this possibility, mefloquine was added to cells at the time of infection or 3 or 24 h postinfection and the antiviral activity was measured. Mefloquine effectively inhibited JCV infection under all three conditions (Fig. 3F). Since most of the virus enters the cells within 1 h and all of the virus entered the cells by 24 h postinfection in our assay (the addition of JCV-neutralizing antiserum 24 h after virus addition was completely ineffective at blocking viral infection), our results suggest that mefloquine would effectively inhibit viral replication in cells already infected with JCV.
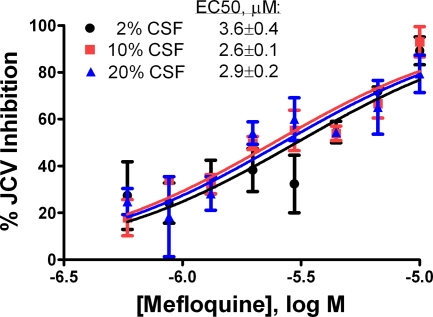
Lack of inhibitory effect of CSF on anti-JCV effect of mefloquine.
While 98% of the mefloquine in plasma is reported to be protein bound, high ratios of the concentration in tissue to the concentration in plasma have been described (41). It is not clear how mefloquine's high level of protein binding might affect its anti-JCV activity; therefore, we tested the drug's efficacy in the presence of increasing concentrations of human cerebrospinal fluid (CSF) to mimic the conditions encountered by the drug in the brain. To this end, we added CSF at a final concentration of 2%, 10%, or 20% over a range of mefloquine concentrations and calculated the EC50 for viral inhibition. As shown in Fig. 4, the EC50s were consistent for all levels of CSF added to the culture (up to 20%).
FIG. 4.
Human CSF does not interfere with mefloquine's anti-JCV activity. SVG-A cells were infected with JCV(M1/SVEΔ) over a range of mefloquine concentrations in the presence of 2%, 10%, or 20% human CSF. Three days later the cells were fixed and stained and the total numbers of VP1-positive cells and DAPI-positive events per treatment group were determined with a Cellomics ArrayScan imager. The results from one representative experiment (of a total of two independent experiments) are shown. The method used for the calculation of the percent JCV inhibition is described in Materials and Methods.
Characterization of antiviral effects of mefloquine enantiomers and analogs.
Mefloquine is a racemic mixture of (11R,12S) and (11S,12R) enantiomers of (2,8-bis-trifluoromethyl-quinolin-4-yl)-piperidin-2-yl-methanol hydrochloride (Fig. 5A and B). While there is a minimal difference between the activities of the enantiomers against malaria (5, 13), the (R,S) enantiomer has a much more potent (∼1,000-fold) antagonistic activity against the A2a adenosine receptor (59). The two enantiomers also display different pharmacokinetics and brain penetration properties (6, 11). To better understand the anti-JCV effects of the two components of the marketed mefloquine racemate, each enantiomer was separated from the racemate by chiral chromatography and tested in the JCV inhibition assay. The two enantiomers were found to have similar efficacies in inhibiting JCV. Although the difference in activity was small, it was statistically significant, with (R,S)-mefloquine being less than twofold more active (2.7 versus 4.5 μM; P = 0.02) than (S,R)-mefloquine or a racemate (2.7 versus 4.0 μM; P = 0.036) (Fig. 5A and B). On the basis of this result and on the reported brain concentrations for mefloquine enantiomers (6), we conclude that an efficacious concentration of mefloquine would be achieved in the brains of PML patients taking the drug. Furthermore, the very small difference in the anti-JCV activities between the enantiomers suggests that this effect is not mediated by the A2a adenosine receptor.
FIG. 5.
Anti-JCV activities of various forms of mefloquine. The (R,S)-mefloquine (A) and (S,R)-mefloquine (B) enantiomers were separated from a mefloquine drug racemate by chiral chromatography; the racemate of (S,S)- and (R,R) enantiomers of mefloquine (C) or (2,8-bis-trifluoromethyl-quinolin-4-yl)-pyridin-2-yl-methanol (D) were added to SVG-A cells simultaneously with JCV(M1/SVEΔ). The cells were fixed and stained at 3 days postinfection, and the total numbers of VP1-positive cells and DAPI-positive events per treatment group were determined with a Cellomics ArrayScan imager. The results of one representative experiment of a total of six (A and B) or two (C and D) performed are shown. EC50s are the means of all experiments performed. Ten micromolar was the highest concentration tested; TI, therapeutic index (TC50/EC50).
To further explore the structure-activity relationship for mefloquine, we acquired and tested other mefloquine analogs. Specifically, we tested a racemic mixture of (11S,12S) and (11R,12R) enantiomers of mefloquine (Fig. 5C) and of the pyridine analog of mefloquine, (2,8-bis-trifluoromethyl-quinolin-4-yl)-pyridin-2-yl-methanol (Fig. 5D). The activity of threo-(R*,R*)-mefloquine (Fig. 5C) was almost the same as the activity of erythro-(R*,S*)-mefloquine (4.6 versus 4.5 μM), indicating that some degree of flexibility exists in the chiral centers at positions 11 and 12 of the mefloquine molecule required for the inhibition of JCV replication. On the other hand, when the piperidine moiety was replaced with the pyridine, as in (2,8-bis-trifluoromethyl-quinolin-4-yl)-pyridin-2-yl-methanol (Fig. 5D), the anti-JCV activity was dramatically reduced. These results indicate that the presence of a saturated heterocycle at this position is crucial for target inhibition.
Modeling studies suggest shape similarities among chemically diverse JCV inhibitors.
To gain insights into the potential mechanism of action or molecular target of mefloquine in the inhibition of JCV replication, we analyzed the chemical classes of drugs with inhibitory activity (see the table in the supplemental material) and found that arylanthranilic and arylalkanoic acid nonsteroidal anti-inflammatory drugs (NSAIDs) (Table 1; Fig. 6) represented the most frequent class of inhibitors. This observation suggests that a common structural motif is shared by at least some JCV inhibitors. To explore the structural relationships among chemically diverse JCV inhibitors, we compared their three-dimensional shapes while assuming that molecules have similar shapes if their volumes overlay well. Conversely, any volume mismatch would represent a measure of dissimilarity. Such shape- and chemical feature-based comparisons (52) indicate that mefloquine, mefenamic acid, and indomethacin can occupy the same conformational space (Fig. 7). We then extended this analysis to compounds in clinical testing reported in the MDL Drug Data Report database and discovered that mefloquine overlaid well with several nucleoside analogues, such as 8-chloroadenosine 3′,5′-monophosphate (Fig. 7) and 3-deazaadenosine (data not shown). The last two compounds inhibited the rate of infection of JCV(M1/SVEΔ) in SVG-A cells with efficacies similar to the efficacy observed with mefloquine (Fig. 7 and data not shown). Taken together, these data suggest that chemically diverse JCV inhibitors may have a common mechanism of viral inhibition and act, in part, as mimetics of nucleoside analogues.
FIG. 6.

Structure-activity relationship of the arylanthranilic and arylalkanoic acid JCV inhibitors. Viral inhibition was measured by an infectivity assay with SVG-A cells and JCV(M1/SVEΔ). The EC50 data represent averages calculated from two or more experiments, and the therapeutic index (TC50/EC50) was >3 for all compounds shown.
FIG. 7.
Shape similarity among chemically diverse JCV inhibitors. The shapes and chemical features of mefloquine (magenta), mefenamic acid (yellow), indomethacin (gray), and 8-chloroadenosine 3′,5′-monophosphate (green) are compared. The overlays were achieved with the ROCS program and were visualized by the use of PyMOL software.
DISCUSSION
PML is a devastating neurodegenerative viral disease that affects some immunosuppressed individuals, including 4 to 5% of HIV-positive patients with AIDS and those undergoing immunosuppressive therapies (for a review, see reference 8). Reconstitution of a patient's immune system either by highly active antiretroviral therapy for HIV-positive individuals or by moderating the immunosuppressive therapies, whenever possible, for others is the only treatment option available today for management of this disease. Although different drugs have been tested as potential treatments for PML, all have failed to demonstrate clinical efficacy, thus keeping the search for drugs with anti-JCV activity a high priority. We report here on the identification of a number of drugs with anti-JCV activity determined by in vitro screening of a commercially available collection of approved drugs and bioactive compounds.
Cytarabine, cidofovir, and alpha interferon have all been reported to exert anti-JCV activities in vitro; but for PML patients they do not offer protection or an improvement in condition over that observed with placebo (for a review, see reference 7). It is possible that the specific pharmacological properties of these drugs in vitro and in vivo are responsible for discrepancies between the results obtained in in vitro infectivity assays and those obtained in in vivo clinical trials. Cytarabine has a short half-life, and although it may reach a concentration of ∼4 μM in the CSF (37), studies with animals suggest that it does not cross the BBB to accumulate in the brain parenchyma in an amount required to inhibit JCV replication (30). The well-known toxicities of cytarabine (i.e., bone marrow suppression and nephrotoxicity) do not allow it to be administered frequently or for an extended period of time to compensate for its unfavorable pharmacokinetic properties. Cidofovir was chosen as a treatment for PML on the basis of its activity against murine polyomavirus and SV40 polyomavirus (2). However, cidofovir displays little (31) or no (Fig. 1D) anti-JCV activity in vitro at the doses achievable in the plasma of treated patients (10 μM); brain biodistribution data do not appear to be available for this drug. Data addressing the ability of alpha interferon to cross the BBB are also lacking, but on the basis of the size of the interferon molecule and its short half-life in blood, one may predict that it would not accumulate in the brain in significant amounts (53). Because JCV infects and replicates in cells throughout the entire white matter of the brain, an effective drug candidate for the treatment of PML must be able to cross the BBB and accumulate throughout the entire brain parenchyma at a dose sufficient to suppress JCV proliferation.
Our initial screening identified several drugs with anti-JCV activities (Table 1), but only mefloquine was shown to accumulate to therapeutically relevant levels in the brain tissue of people receiving clinically approved doses. This drug crosses the BBB and accumulates in the brain at concentrations that, when the drug is tested in cultured cells, lead to the inhibition of JCV infection (33, 47). The drug is likely to accumulate in the brain parenchyma at a concentration much higher than that in the plasma because of its long plasma half-life, lipophilicity (log P = 2.47), and inhibition of MDR-1, a multidrug resistance protein responsible for the efflux of drugs out of the brain (47, 50).
It was recently suggested that 5HT2A receptor blockers might be potential drug candidates for the treatment of PML on the basis of their ability to obstruct the binding of the JCV capsid to its purported cellular receptor (23, 43). This observation remains controversial, as a second group failed to detect the anti-JCV activity of 5HT2A blockers (15) and we failed to uncover such activity for more than 20 drugs in the Spectrum collection belonging to this class of inhibitors (data not shown). Even in those studies reporting that 5HT2A blockers had antiviral activity, the inhibitory mechanism involves a block to JCV cell entry and not a block to viral proliferation in cells already infected with JCV (43). Since a great many glial cells are already infected once a diagnosis of PML is made, the best strategy for the prevention of further brain damage and for treatment would be to inhibit viral replication that has already been established. We report here that mefloquine reduces JCV replication by acting at a step subsequent to viral entry into the cell.
We have employed qPCR to quantify the number of viral genome copies in infected PHFG and SVG-A cell cultures, and we have used this approach to demonstrate that mefloquine inhibits JCV DNA replication. Although mefloquine was discovered more than 30 years ago, its molecular target(s) in patients with malaria has not been identified. Overall, very few molecular targets of mefloquine have been identified, so it is not surprising that the precise molecular mechanism by which mefloquine interferes with JCV DNA replication remains unknown. (11S,12R)-Mefloquine is a specific and high-affinity inhibitor of the adenosine A2a receptor (59). Still, the adenosine A2a receptor does not appear to be a relevant target for JCV inhibition because while (11R,12S)-mefloquine is ∼1,000-fold more active than the (11S,12R) enantiomer against adenosine A2aR, it is only 2-fold more active than the other enantiomer as a JCV inhibitor. Furthermore, we tested a number of specific adenosine receptor inhibitors, and none of them were found to effectively inhibit JCV infection (data not shown). In our search for a common motif among compounds in the Spectrum collection with anti-JCV activities, we noted that N-arylanthranilic and arylalkanoic acid NSAIDs are disproportionately represented as a class. Although many of these molecules inhibit COX-1 and COX-2 activities as well as prostaglandin synthesis, such mechanisms do not seem relevant to their anti-JCV activity, as other NSAIDS in the library from a different molecular class, e.g., arylpropionic acid ibuprofen or flurbiprofen, do not display anti-JCV activity, despite their COX-inhibitory activity.
It is intriguing that the three-dimensional confirmation of mefloquine fits into the shapes of arylanthranilic and arylalkanoic acid NSAIDs (Fig. 7), suggesting that while these compounds belong to different chemical classes, all may share a common molecular target. A search for other molecules with three-dimensional structures similar to those of mefloquine and mefenamic acid revealed several adenosine analogs (e.g., 3-deazaadenosine and 8-chloroadenosine 3′,5′-monophosphate) with anti-JCV activities. One might speculate that these inhibitors bind to an ATP- or nucleotide-binding pocket of a molecule crucial for viral replication and disrupt its function. Since these drugs do not show cytotoxic effects at doses that exhibit anti-JCV effects, it is possible that they directly inhibit T antigen, the JCV-encoded replication protein, rather than the cellular DNA replication machinery required for viral replication. The conserved T antigen of polyomaviruses is a hexameric helicase that hydrolyzes ATP and forms an ATP-dependent replication complex at the AT-rich viral origin of replication (42, 60). Direct biochemical experiments will be required to investigate whether mefloquine targets this multifunctional viral protein.
In summary, mefloquine inhibits the replication of three different JCV isolates in three different cell types. Furthermore, mefloquine inhibits viral replication in cells previously infected with JCV. Finally, mefloquine accumulates in brain tissue at levels more than sixfold its EC50. Although no appropriate animal model is available to test the ability of mefloquine to inhibit JCV in vivo, our in vitro data coupled with published biodistribution data for this drug suggest that mefloquine could represent an effective therapeutic agent for the treatment of PML. Currently, a controlled randomized clinical trial is under way to determine if mefloquine provides clinical efficacy for viral inhibition and protection from neurological damage in PML patients.
Supplementary Material
Acknowledgments
We are grateful to Walter Atwood and Duard Walker for their generosity in providing valuable reagents and to Walter Atwood and Brigitte Bollag (Penn State University) for assistance in establishing the JCV infectivity assay and the protocol for propagating PHFG cells, respectively. We thank Ted Lin and Kevin Guckian (both from Biogen IDEC Inc.) for their help with compound verification. We appreciate editorial help and discussions from Susan Goelz, Petra Duda, and Debra Kinch.
Footnotes
Published ahead of print on 2 March 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Aksamit, A. J. 2001. Treatment of non-AIDS progressive multifocal leukoencephalopathy with cytosine arabinoside. J. Neurovirol. 7:386-390. [DOI] [PubMed] [Google Scholar]
- 2.Andrei, G., R. Snoeck, M. Vandeputte, and E. De Clercq. 1997. Activities of various compounds against murine and primate polyomaviruses. Antimicrob. Agents Chemother. 41:587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atwood, W. J. 2001. A combination of low-dose chlorpromazine and neutralizing antibodies inhibits the spread of JC virus (JCV) in a tissue culture model: implications for prophylactic and therapeutic treatment of progressive multifocal leukencephalopathy. J. Neurovirol. 7:307-310. [DOI] [PubMed] [Google Scholar]
- 4.Atwood, W. J., L. Wang, L. C. Durham, K. Amemiya, R. G. Traub, and E. O. Major. 1995. Evaluation of the role of cytokine activation in the multiplication of JC virus (JCV) in human fetal glial cells. J. Neurovirol. 1:40-49. [DOI] [PubMed] [Google Scholar]
- 5.Basco, L. K., C. Gillotin, F. Gimenez, R. Farinotti, and J. Le Bras. 1992. In vitro activity of the enantiomers of mefloquine, halofantrine and enpiroline against Plasmodium falciparum. Br. J. Clin. Pharmacol. 33:517-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baudry, S., Y. T. Pham, B. Baune, S. Vidrequin, C. Crevoisier, F. Gimenez, and R. Farinotti. 1997. Stereoselective passage of mefloquine through the blood-brain barrier in the rat. J. Pharm. Pharmacol. 49:1086-1090. [DOI] [PubMed] [Google Scholar]
- 7.Berger, J. R. 2000. Progressive multifocal leukoencephalopathy. Curr. Treat. Options Neurol. 2:361-368. [DOI] [PubMed] [Google Scholar]
- 8.Berger, J. R., and E. O. Major. 1999. Progressive multifocal leukoencephalopathy. Semin. Neurol. 19:193-200. [DOI] [PubMed] [Google Scholar]
- 9.Bianchetti, G., S. Billy, V. Ascalone, S. Saivin, G. Houin, and P. Rosenzweig. 1996. Multicenter studies on the pharmacokinetic profile of sustained-release oral diltiazem (300 mg) after once a day repeated administration: influence of age. Int. J. Clin. Pharmacol. Ther. 34:195-201. [PubMed] [Google Scholar]
- 10.Bossolasco, S., G. Calori, F. Moretti, A. Boschini, D. Bertelli, M. Mena, S. Gerevini, A. Bestetti, R. Pedale, S. Sala, S. Sala, A. Lazzarin, and P. Cinque. 2005. Prognostic significance of JC virus DNA levels in cerebrospinal fluid of patients with HIV-associated progressive multifocal leukoencephalopathy. Clin. Infect. Dis. 40:738-744. [DOI] [PubMed] [Google Scholar]
- 11.Bourahla, A., C. Martin, F. Gimenez, V. Singhasivanon, P. Attanath, A. Sabchearon, T. Chongsuphajaisiddhi, and R. Farinotti. 1996. Stereoselective pharmacokinetics of mefloquine in young children. Eur. J. Clin. Pharmacol. 50:241-244. [DOI] [PubMed] [Google Scholar]
- 12.Bregante, M. A., J. J. Aramayona, L. J. Fraile, M. A. Garcia, and C. Solans. 2000. Diltiazem blood pharmacokinetics in the pregnant and non-pregnant rabbit: maternal and foetal tissue levels. Xenobiotica 30:831-841. [DOI] [PubMed] [Google Scholar]
- 13.Brocks, D. R., and R. Mehvar. 2003. Stereoselectivity in the pharmacodynamics and pharmacokinetics of the chiral antimalarial drugs. Clin. Pharmacokinet. 42:1359-1382. [DOI] [PubMed] [Google Scholar]
- 14.Brooks, B. R., and D. L. Walker. 1984. Progressive multifocal leukoencephalopathy. Neurol. Clin. 2:299-313. [PubMed] [Google Scholar]
- 15.Chapagain, M. L., S. Verma, F. Mercier, R. Yanagihara, and V. R. Nerurkar. 2007. Polyomavirus JC infects human brain microvascular endothelial cells independent of serotonin receptor 2A. Virology 364:55-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cinque, P., S. Bossolasco, A. M. Brambilla, A. Boschini, C. Mussini, C. Pierotti, A. Campi, S. Casari, D. Bertelli, M. Mena, and A. Lazzarin. 2003. The effect of highly active antiretroviral therapy-induced immune reconstitution on development and outcome of progressive multifocal leukoencephalopathy: study of 43 cases with review of the literature. J. Neurovirol. 9(Suppl. 1):73-80. [DOI] [PubMed] [Google Scholar]
- 17.Cinque, P., I. J. Koralnik, and D. B. Clifford. 2003. The evolving face of human immunodeficiency virus-related progressive multifocal leukoencephalopathy: defining a consensus terminology. J. Neurovirol. 9(Suppl. 1):88-92. [DOI] [PubMed] [Google Scholar]
- 18.Clifford, D. B., C. Yiannoutsos, M. Glicksman, D. M. Simpson, E. J. Singer, P. J. Piliero, C. M. Marra, G. S. Francis, J. C. McArthur, K. L. Tyler, A. C. Tselis, and N. E. Hyslop. 1999. HAART improves prognosis in HIV-associated progressive multifocal leukoencephalopathy. Neurology 52:623-625. [DOI] [PubMed] [Google Scholar]
- 19.Crawford, L., and E. Harlow. 1982. Uniform nomenclature for monoclonal antibodies directed against virus-coded proteins of simian virus 40 and polyoma virus. J. Virol. 41:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crowder, C. D., K. A. Gyure, C. B. Drachenberg, J. Werner, R. E. Morales, H. H. Hirsch, and E. Ramos. 2005. Successful outcome of progressive multifocal leukoencephalopathy in a renal transplant patient. Am. J. Transplant. 5:1151-1158. [DOI] [PubMed] [Google Scholar]
- 21.De Luca, A., A. Ammassari, P. Pezzotti, P. Cinque, J. Gasnault, J. Berenguer, S. Di Giambenedetto, A. Cingolani, Y. Taoufik, P. Miralles, C. M. Marra, and A. Antinori. 2008. Cidofovir in addition to antiretroviral treatment is not effective for AIDS-associated progressive multifocal leukoencephalopathy: a multicohort analysis. AIDS 22:1759-1767. [DOI] [PubMed] [Google Scholar]
- 22.Eckhoff, C., and C. C. Willhite. 1997. Embryonic delivered dose of isotretinoin (13-cis-retinoic acid) and its metabolites in hamsters. Toxicol. Appl. Pharmacol. 146:79-87. [DOI] [PubMed] [Google Scholar]
- 23.Elphick, G. F., W. Querbes, J. A. Jordan, G. V. Gee, S. Eash, K. Manley, A. Dugan, M. Stanifer, A. Bhatnagar, W. K. Kroeze, B. L. Roth, and W. J. Atwood. 2004. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 306:1380-1383. [DOI] [PubMed] [Google Scholar]
- 24.Frisque, R. J., G. L. Bream, and M. T. Cannella. 1984. Human polyomavirus JC virus genome. J. Virol. 51:458-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frye, S., C. Trebst, U. Dittmer, H. Petry, M. Bodemer, G. Hunsmann, T. Weber, and W. Luke. 1997. Efficient production of JC virus in SVG cells and the use of purified viral antigens for analysis of specific humoral and cellular immune response. J. Virol. Methods 63:81-92. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda, M., K. Kitaichi, F. Abe, Y. Fujimoto, K. Takagi, K. Takagi, T. Morishima, and T. Hasegawa. 2005. Altered brain penetration of diclofenac and mefenamic acid, but not acetaminophen, in Shiga-like toxin II-treated mice. J. Pharmacol. Sci. 97:525-532. [DOI] [PubMed] [Google Scholar]
- 27.Gasnault, J., P. Kousignian, M. Kahraman, J. Rahoiljaon, S. Matheron, J. F. Delfraissy, and Y. Taoufik. 2001. Cidofovir in AIDS-associated progressive multifocal leukoencephalopathy: a monocenter observational study with clinical and JC virus load monitoring. J. Neurovirol. 7:375-381. [DOI] [PubMed] [Google Scholar]
- 28.Gasnault, J., Y. Taoufik, C. Goujard, P. Kousignian, K. Abbed, F. Boue, E. Dussaix, and J. F. Delfraissy. 1999. Prolonged survival without neurological improvement in patients with AIDS-related progressive multifocal leukoencephalopathy on potent combined antiretroviral therapy. J. Neurovirol. 5:421-429. [DOI] [PubMed] [Google Scholar]
- 29.Glazko, A. J. 1966. Experimental observations on flufenamic, mefenamic and meclofenamic acids. 3. Metabolic disposition. Ann. Phys. Med. (Suppl.) :23-36. [PubMed]
- 30.Groothuis, D. R., H. Benalcazar, C. V. Allen, R. M. Wise, C. Dills, C. Dobrescu, V. Rothholtz, and R. M. Levy. 2000. Comparison of cytosine arabinoside delivery to rat brain by intravenous, intrathecal, intraventricular and intraparenchymal routes of administration. Brain Res. 856:281-290. [DOI] [PubMed] [Google Scholar]
- 31.Hall, C. D., U. Dafni, D. Simpson, D. Clifford, P. E. Wetherill, B. Cohen, J. McArthur, H. Hollander, C. Yainnoutsos, E. Major, L. Millar, J. Timpone, et al.. 1998. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. N. Engl. J. Med. 338:1345-1351. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch, H. H., C. B. Drachenberg, J. Steiger, and E. Ramos. 2006. Polyomavirus-associated nephropathy in renal transplantation: critical issues of screening and management. Adv. Exp. Med. Biol. 577:160-173. [DOI] [PubMed] [Google Scholar]
- 33.Jones, R., G. Kunsman, B. Levine, M. Smith, and C. Stahl. 1994. Mefloquine distribution in postmortem cases. Forensic Sci. Int. 68:29-32. [DOI] [PubMed] [Google Scholar]
- 34.Khan, A. A., J. G. Villablanca, C. P. Reynolds, and V. I. Avramis. 1996. Pharmacokinetic studies of 13-cis-retinoic acid in pediatric patients with neuroblastoma following bone marrow transplantation. Cancer Chemother. Pharmacol. 39:34-41. [DOI] [PubMed] [Google Scholar]
- 35.Knowles, W. A. 2006. Discovery and epidemiology of the human polyomaviruses BK virus (BKV) and JC virus (JCV). Adv. Exp. Med. Biol. 577:19-45. [DOI] [PubMed] [Google Scholar]
- 36.Liu, C. K., A. P. Hope, and W. J. Atwood. 1998. The human polyomavirus, JCV, does not share receptor specificity with SV40 on human glial cells. J. Neurovirol. 4:49-58. [DOI] [PubMed] [Google Scholar]
- 37.Lopez, J. A., E. Nassif, P. Vannicola, J. G. Krikorian, and R. P. Agarwal. 1985. Central nervous system pharmacokinetics of high-dose cytosine arabinoside. J. Neurooncol. 3:119-124. [DOI] [PubMed] [Google Scholar]
- 38.Major, E. O., A. E. Miller, P. Mourrain, R. G. Traub, E. de Widt, and J. Sever. 1985. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc. Natl. Acad. Sci. USA 82:1257-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marra, C. M., N. Rajicic, D. E. Barker, B. A. Cohen, D. Clifford, M. J. Donovan Post, A. Ruiz, B. C. Bowen, M. L. Huang, J. Queen-Baker, J. Andersen, S. Kelly, and S. Shriver. 2002. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS 16:1791-1797. [DOI] [PubMed] [Google Scholar]
- 40.Mindermann, T., W. Zimmerli, Z. Rajacic, and O. Gratzl. 1993. Penetration of fusidic acid into human brain tissue and cerebrospinal fluid. Acta Neurochir. (Wien) 121:12-14. [DOI] [PubMed] [Google Scholar]
- 41.Mu, J. Y., Z. H. Israili, and P. G. Dayton. 1975. Studies of the disposition and metabolism of mefloquine HCl (WR 142,490), a quinolinemethanol antimalarial, in the rat. Limited studies with an analog, WR 30,090. Drug Metab. Dispos. 3:198-210. [PubMed] [Google Scholar]
- 42.Murakami, Y., and J. Hurwitz. 1993. DNA polymerase alpha stimulates the ATP-dependent binding of simian virus tumor T antigen to the SV40 origin of replication. J. Biol. Chem. 268:11018-11027. [PubMed] [Google Scholar]
- 43.O'Hara, B. A., and W. J. Atwood. 2008. Interferon beta1-a and selective anti-5HT(2a) receptor antagonists inhibit infection of human glial cells by JC virus. Virus Res. 132:97-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padgett, B. L., C. M. Rogers, and D. L. Walker. 1977. JC virus, a human polyomavirus associated with progressive multifocal leukoencephalopathy: additional biological characteristics and antigenic relationships. Infect. Immun. 15:656-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Padgett, B. L., D. L. Walker, G. M. ZuRhein, R. J. Eckroade, and B. H. Dessel. 1971. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet i:1257-1260. [DOI] [PubMed] [Google Scholar]
- 46.Padgett, B. L., D. L. Walker, G. M. ZuRhein, A. E. Hodach, and S. M. Chou. 1976. JC papovavirus in progressive multifocal leukoencephalopathy. J. Infect. Dis. 133:686-690. [DOI] [PubMed] [Google Scholar]
- 47.Pham, Y. T., F. Nosten, R. Farinotti, N. J. White, and F. Gimenez. 1999. Cerebral uptake of mefloquine enantiomers in fatal cerebral malaria. Int. J. Clin. Pharmacol. Ther. 37:58-61. [PubMed] [Google Scholar]
- 48.Pho, M. T., A. Ashok, and W. J. Atwood. 2000. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 74:2288-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Randhawa, P., F. Baksh, N. Aoki, D. Tschirhart, and S. Finkelstein. 2001. JC virus infection in allograft kidneys: analysis by polymerase chain reaction and immunohistochemistry. Transplantation 71:1300-1303. [DOI] [PubMed] [Google Scholar]
- 50.Riffkin, C. D., R. Chung, D. M. Wall, J. R. Zalcberg, A. F. Cowman, M. Foley, and L. Tilley. 1996. Modulation of the function of human MDR1 P-glycoprotein by the antimalarial drug mefloquine. Biochem. Pharmacol. 52:1545-1552. [DOI] [PubMed] [Google Scholar]
- 51.Romsing, J., D. Ostergaard, T. Senderovitz, D. Drozdziewicz, J. Sonne, and G. Ravn. 2001. Pharmacokinetics of oral diclofenac and acetaminophen in children after surgery. Paediatr. Anaesth. 11:205-213. [DOI] [PubMed] [Google Scholar]
- 52.Rush, T. S., III, J. A. Grant, L. Mosyak, and A. Nicholls. 2005. A shape-based 3-D scaffold hopping method and its application to a bacterial protein-protein interaction. J. Med. Chem. 48:1489-1495. [DOI] [PubMed] [Google Scholar]
- 53.Seelig, A. 2007. The role of size and charge for blood-brain barrier permeation of drugs and fatty acids. J. Mol. Neurosci. 33:32-41. [DOI] [PubMed] [Google Scholar]
- 54.Shitrit, D., L. Nirit, S. I. Shiran, G. Izbicki, D. Sofer, M. Eldad, and M. R. Kramer. 2003. Progressive multifocal leukoencephalopathy in a lung transplant recipient. J. Heart Lung Transplant. 22:946-950. [DOI] [PubMed] [Google Scholar]
- 55.Stevens, R. E., J. Konsil, S. S. Verrill, P. Roy, P. B. Desai, D. H. Upmalis, and F. L. Cone. 2002. Bioavailability study of a 1200 mg miconazole nitrate vaginal ovule in healthy female adults. J. Clin. Pharmacol. 42:52-60. [DOI] [PubMed] [Google Scholar]
- 56.Turnidge, J. 1999. Fusidic acid pharmacology, pharmacokinetics and pharmacodynamics. Int. J. Antimicrob. Agents 12(Suppl. 2):S23-S34. [DOI] [PubMed] [Google Scholar]
- 57.U.S. Pharmacopeia. 2005. USP dictionary of USAN and international drug names. U.S. Pharmacopeia, Rockville, MD.
- 58.Vacante, D. A., R. Traub, and E. O. Major. 1989. Extension of JC virus host range to monkey cells by insertion of a simian virus 40 enhancer into the JC virus regulatory region. Virology 170:353-361. [DOI] [PubMed] [Google Scholar]
- 59.Weiss, S. M., K. Benwell, I. A. Cliffe, R. J. Gillespie, A. R. Knight, J. Lerpiniere, A. Misra, R. M. Pratt, D. Revell, R. Upton, and C. T. Dourish. 2003. Discovery of nonxanthine adenosine A2A receptor antagonists for the treatment of Parkinson's disease. Neurology 61:S101-S106. [DOI] [PubMed] [Google Scholar]
- 60.Wessel, R., J. Schweizer, and H. Stahl. 1992. Simian virus 40 T-antigen DNA helicase is a hexamer which forms a binary complex during bidirectional unwinding from the viral origin of DNA replication. J. Virol. 66:804-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, J. H., T. D. Chung, and K. R. Oldenburg. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 4:67-73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.