Abstract
The eukaryotic translation initiation factor 4E (eIF4E) controls gene expression through its effects on mRNA export and cap-dependent translation, both of which contribute to its oncogenic potential. In contrast to its translation function, the mRNA export function of eIF4E is poorly understood. Using an RNP isolation/mass spectrometry approach, we identified candidate cofactors of eIF4E mRNA export including LRPPRC. This protein associates with mRNAs containing the eIF4E-sensitivity element (4E-SE), and its overexpression alters the nuclear export of several eIF4E-sensitive mRNAs. LRPPRC-mediated alteration of eIF4E's mRNA export function requires the integrity of its eIF4E-binding site and it coincides with the subcellular re-distribution of eIF4E. The eIF4E export RNP is distinct in composition from the bulk mRNA export pathway, in that eIF4E- and eIF4E-sensitive mRNAs do not associate with general mRNA export factors such as TAP/NXF1 or REF/Aly. Our data indicate that mRNA export pathways have evolved for specific mRNAs enabling the differential regulation of biochemical pathways by modulating the expression of groups of genes at the level of their export.
Keywords: eIF4E, LRPPRC, nuclear mRNA export, PML
Introduction
Control of gene expression is critical to ensure normal cellular proliferation and survival. The RNA regulon model has been proposed to explain how gene expression can be combinatorially and coordinately modulated post-transcriptionally (Keene and Lager, 2005; Keene, 2007). Only with coordinated production of key factors can required biochemical reactions occur in the cell. In this model, mRNAs containing similar elements in their untranslated regions (UTRs) sensitize RNAs to regulation at distinct levels, for example, stability, mRNA export or translation. These elements, referred to as USER codes, enable the transcript to recruit the correct factors to facilitate the given process (Keene and Lager, 2005; Keene, 2007). In fact, RNAs regulated in a coordinated manner have been observed to contain the same set of USER codes (Keene, 2007). Thus, the expression of these genes can be coordinated post-transcriptionally.
The eukaryotic translation initiation factor 4E (eIF4E) is a central node in a RNA regulon that modulates the expression of genes involved in proliferation and survival (Culjkovic et al, 2007). For instance, eIF4E coordinately modulates the expression of critical genes involved in specific pathways, for example, genes that function upstream and downstream of Akt (Culjkovic et al, 2008). Biochemically, eIF4E functions at two levels: cap-dependent translation and nuclear mRNA export, both of which require its m7G cap-binding activity (Culjkovic et al, 2007). Specifically, eIF4E enhances the mRNA export of genes that contain an ∼50-nt element in the 3′ UTR known as an eIF4E-sensitivity element (4E-SE) (Culjkovic et al, 2005, 2006). In the cytoplasm, eIF4E enhances the translational efficiency of specific genes and this is based on the presence of complex 5′ UTRs (Koromilas et al, 1992). Thus, eIF4E enhances the expression of a given transcript by increasing its cytoplasmic levels and/or by increasing the efficiency of its association with polysomes. These activities are distinct, each relying on the presence of the appropriate USER code. Some transcripts are controlled at both levels (e.g., ODC), whereas others are controlled only at the level of export (e.g., cyclin D1) or translation (e.g., VEGF) (Culjkovic et al, 2007). Given its ability to coordinately modulate gene expression, eIF4E is positioned to profoundly impact the cellular proteome.
Previous studies indicate that eIF4E overexpression leads to oncogenic transformation in tissue culture (Sonenberg and Gingras, 1998; Mamane et al, 2004). In animal models, eIF4E overexpression leads to increased tumours and metastases (Graff and Zimmer, 2003; Montanaro and Pandolfi, 2004), and its overexpression is a hallmark of poor prognosis in many human cancers (Graff and Zimmer, 2003; De Benedetti and Graff, 2004). Thus, there is an ongoing effort to develop therapeutic strategies targeting eIF4E. Importantly, cells transformed by elevated eIF4E develop an oncogene dependency, or addiction to, eIF4E (Kentsis et al, 2004; Graff et al, 2008). New therapies have been introduced targeting eIF4E in the clinic, including the use of a physical mimic of the m7G cap ribavirin in refractory acute myeloid leukaemias (AMLs) in a phase I/II clinical trial (www.ribatrial.com).
To develop other therapies based on eIF4E, one requires a detailed understanding of how eIF4E transforms the cell. The traditional view is that the translation function of eIF4E underlies its oncogenic properties. However, several lines of evidence implicate its role in mRNA export as contributing substantially to its transforming potential (Culjkovic et al, 2007). One key finding indicates that a mutant form of eIF4E, W73A, can, when overexpressed, transform cells to the same extent as wild-type eIF4E and rescue these cells from serum deprivation-induced apoptosis to the same extent (for example, Cohen et al, 2001; Culjkovic et al, 2008). The W73A mutant is fully functional in mRNA export but cannot promote translation as it cannot bind eIF4G and thus cannot recruit the remaining translation machinery (Cohen et al, 2001; Kentsis et al, 2001; Topisirovic et al, 2003a; Culjkovic et al, 2008). Importantly, this mutant has a structure indistinguishable from wild-type eIF4E and binds the m7G cap with similar affinity (Kentsis et al, 2001). A second line of evidence arises from studies focusing on the 3′ UTR element, the 4E-SE (Culjkovic et al, 2005). It was found that, in the eIF4E-overexpressing cells, cyclin D1 constructs containing only the 4E-SE transformed cells as readily as cyclin D1 constructs with full-length 3′ UT. In contrast, cells expressing cyclin D1 constructs lacking the 50-nt 4E-SE resulted in transformation levels similar to vector controls (Culjkovic et al, 2005). Previous studies indicated that cyclin D1 is controlled at the level of mRNA export, not of translation (Rousseau et al, 1996). Further in primary human AML specimens, eIF4E levels are not only highly elevated, but the majority of eIF4E is found in the nucleus and eIF4E-dependent mRNA export of cyclin D1 is also substantially upregulated (Topisirovic et al, 2003b). Taken together, these data suggest that the nuclear mRNA export activity of eIF4E has a key function in this oncogenic process.
Given its clear implications in the transforming potential of eIF4E, we set out to understand the mechanism(s) by which eIF4E modulates mRNA export. Our previous studies indicated that eIF4E-dependent mRNA export is fundamentally different from bulk mRNA export (Culjkovic et al, 2005, 2006). For instance, knockdown of the nuclear receptor NXF1/TAP does not affect eIF4E-dependent mRNA export, whereas bulk export is profoundly impacted (Culjkovic et al, 2006). In contrast, eIF4E-dependent mRNA export is leptomycin B (LMB) sensitive, indicating that it is CRM1 mediated, unlike bulk mRNA export (Culjkovic et al, 2006). Finally, eIF4E-dependent mRNA export requires the 50-nt 4E-SE element in the 3′ UTR, which is not required for bulk mRNA export (Culjkovic et al, 2006). To understand the molecular underpinnings of this mRNA export pathway, we identified proteins that specifically associated with the 4E-SE element and additionally, we characterized the interaction of key cellular mRNA export factors and their role in this eIF4E specific process.
Results
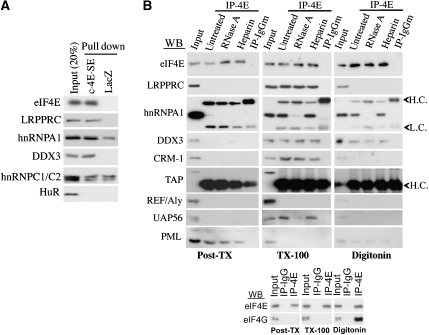
We initiated our studies into eIF4E-dependent mRNA export by exploiting the requirement for the 4E-SE element. Our previous studies indicated that this element is structurally conserved, although substantial sequence variation exists among 4E-SEs from different transcripts (Culjkovic et al, 2006). For instance, the 4E-SEs from both cyclin D1 and Pim-1 contain the paired stem-loop structure but differ substantially at the sequence level (Culjkovic et al, 2006). To identify proteins associated with the 4E-SE, we carried out RNP isolations using biotinylated transcripts containing the 4E-SE from either cyclin D1 (c-4E-SE) or from Pim-1 (p-4E-SE), reasoning that proteins that associated with both chimaeras were likely to recognize the secondary structure element, and not sequence-specific features, of either 4E-SE. The biotinylated transcripts were incubated with U2OS cell lysates, washed and bound proteins were separated by SDS–PAGE (Supplementary Figure 1A). Pulled-down proteins were analysed by mass spectrometry and their identity was verified by western blot analysis (Figure 1A). Transcripts used in these in vitro studies were not capped or polyadenylated. Importantly, we observed that eIF4E selectively associates with these mRNAs, indicating that this association is through interactions with proteins that recognize the 4E-SE (Figure 1A). Interestingly, the leucine-rich protein LRPPRC was identified as a factor that bound only to transcripts that contained the 4E-SE as observed both by mass spectrometry and confirmed by western blot analysis (Figure 1A). Little is known about LRPPRC but it has been implicated in nuclear mRNA metabolism by its association with nuclear mRNPs (Mili et al, 2001). We also identified the DEAD box helicase DDX3 implicated in the export of cellular mRNAs and HIV Rev RNA (Figure 1A) (Yedavalli et al, 2004; Lai et al, 2008). A full list of associated proteins is given in Supplementary Table I. As a negative control, we investigated the association of the ARE-binding protein HuR with the RNA baits. As expected, we observed no interaction with LacZ or c-4E-SE (Figure 1A). We also isolated proteins involved in general mRNA metabolism, such as hnRNPA1 and hnRNPC1/C2, which bound to both c-4E-SE and LacZ baits (Figure 1A).
Figure 1.
Association of eIF4E with 4E-SE mRNPs and RNA processing factors depends on its subcellular localization. (A) RNA pull-down assay using the 4E-SE from cyclin D1 (c-4E-SE) as a bait was analysed by western blot. Note that eIF4E was specifically pulled down with uncapped RNA as a bait. Hence, under the conditions used, eIF4E is recruited efficiently to the bait RNA through 4E-SE-binding factors. (B) Immunoprecipitations (IPs) were carried out in the indicated fraction of U2OS cells with an anti-eIF4E antibody (IP-4E) or the appropriate IgG control (IP-IgGm). IPs were treated with either heparin or RNase A and analysed by western blot using indicated antibodies. HC and LC, heavy and light immunoglobulin chains, respectively.
Interactions with the eIF4E RNP
Next, we determined whether endogenous LRPPRC and other, established, mRNA export factors were associated with endogenous eIF4E. To better understand the maturation of eIF4E-containing RNP, we monitored interactions as a function of subcellular distribution that likely reflects RNPs at different stages of maturation. The following fractions were isolated: digitonin (cytosolic fraction containing soluble cytoplasmic RNP), Triton X-100 (TX-100; nucleoplasmic fraction enriched in export competent, or mature nuclear RNPs) and post-Triton (post-TX; nuclear ‘matrix' fraction containing heterogeneous nuclear RNPs) (Mili et al, 2001). Note that TX-100 also contains insoluble cytoplasmic structures. eIF4E was immunoprecipitated from each fraction and its interacting proteins were monitored by western blot analysis. Fractionation controls are given in Supplementary Figure 1B.
The composition of the eIF4E RNP varied across fractions (Figure 1B). For instance, in the post-TX fraction, eIF4E interacted with the promyelocytic leukaemia (PML) protein, which is an established negative regulator of eIF4E-dependent mRNA export (Cohen et al, 2001; Topisirovic et al, 2002; Culjkovic et al, 2006). eIF4E interacted only with LRPPRC in the TX-100 fraction (Figure 1B). Also, in this fraction enriched in export-competent RNPs, eIF4E associated with the export receptor CRM1, hnRNP A1, the dead box helicase UAP56 and DDX3, a factor that functions in CRM1-dependent export of HIV Rev RNA (Yedavalli et al, 2004). RNAse digestion experiments indicated that eIF4E interacted with LRPPRC, PML, CRM1 and DDX3 in an RNA-independent manner, whereas its interaction with UAP56 was RNA dependent. Addition of heparin to the immunoprecipitations (IPs) did not affect the composition of the complexes, indicating interactions were specific (Figure 1B). Finally, we examined the association of eIF4E with eIF4G, which we observed only in the digitonin fraction, as expected (Figure 1B). Importantly, eIF4E did not associate with bulk mRNA export components TAP/NXF1 or REF/Aly in any fraction (Figure 1B). Further, eIF4E did not associate with the non-shuttling hnRNP C1/C2 (Supplementary Figure 1C).
Traditionally, the major nuclear cap-binding factor is the cap-binding complex (CBC) comprised of two cap-binding proteins (CBP80 and CBP20). The CBC is a key part of the bulk mRNA export machinery, where CBC associates with bulk mRNA until it exits the nuclear pore (Moore, 2005). We carried out IPs for CBP80 (Figure 2) and compared the associated proteins with those found for eIF4E (Figure 1B). Importantly, CBP80 did not associate with eIF4E or LRPPRC in any fraction (Figure 2). As expected, CBP80 did associate with hnRNPC1/C2 in the post-TX fraction and with hnRNPA1 in the post-TX and TX-100 fractions. CBP80 associated with TAP/NXF1 and REF/Aly as expected given its role in bulk mRNA export (Cheng et al, 2006). Further, it also associated with DDX3 (Figure 2). Finally, CBP80 interacts with CRM-1 in the mRNA export-competent fraction, which is expected given the role of CBP80 in UsnRNA export (Figure 2) (Ohno et al, 2000). Thus, there are factors specific to eIF4E-dependent mRNA export such as eIF4E and LRPPRC and factors common to both export pathways such as hnRNPA1 and DDX3. Note that we observed a similar pattern of associations in HEK293 cells, indicating that our results are not restricted to U2OS cells (data not shown).
Figure 2.
mRNPs containing CBC and eIF4E are distinct. Material from the indicated fractions of U2OS cells was immunoprecipitated using an anti-CBP80 antibody (IP-CBP80) or the isotype-matched rabbit IgG control (IP-IgGr). IP reactions were analysed by western blot as indicated.
The composition of 4E-SE-containing RNP parallels the composition of the eIF4E export RNP
We extended our studies to establish whether the LacZ-4E-SE mRNA associated with a similar complement of proteins as eIF4E. RNA was isolated by IP of RNPs from U2OS cells stably transfected with LacZ or LacZ-4E-SE and detected by RT–qPCR or sqRT–PCR (Figure 3). Comparable IP efficiency was observed in each fraction for all antibodies (data not shown). In the post-TX fraction, both LacZ and LacZ-4E-SE transcripts associated with hnRNPA1 and CBP80 (Figure 3). This is consistent with what is known about mRNA maturation, where CBC and hnRNPs associate with transcripts during transcription in the post-TX fraction (Mili et al, 2001; Moore, 2005). Thus, the early steps of maturation of eIF4E-sensitive mRNAs appear similar to other mRNAs. Importantly, differences in associated proteins are observed between LacZ and LacZ-4E-SE in the TX-100 fraction. Here, transcripts containing the 4E-SE associate preferentially with eIF4E and LRPPRC with 50-fold enrichment over LacZ or versus the IgG controls (Figure 3). By contrast, hnRNPA1 associated with both mRNAs to a similar extent. Importantly, LacZ RNA associated with CBP80 with 15-fold enrichment relative to LacZ-4E-SE or to IgG controls (Figure 3). This is consistent with LacZ being exported by the bulk mRNA export machinery and LacZ-4E-SE being transported using distinct factors, including eIF4E and LRPPRC.
Figure 3.
4E-SE is required for mRNA binding to eIF4E and LRPPRC in the TX-100 fraction. RIP reactions were carried out in the indicated fractions of U2OS cells transfected with LacZ or LacZ-4E-SE using the indicated antibodies. Control RIPs were isotype- and species-matched IgGs (Ip-IgGm and IP-IgGr). The quantity of LacZ or LacZ-4E-SE mRNA was determined by RT–qPCR. Fold enrichment is calculated relative to the appropriate IgG control±standard deviations (s.d.) (n=3).
We extended our studies to determine whether endogenous mRNAs acted similarly to our chimaeric model transcripts. We monitored the association of endogenous cyclin D1 and c-myc mRNAs, which are eIF4E sensitive, and of endogenous GAPDH, which is not sensitive to eIF4E at the mRNA export level (Figure 4). In addition, we monitored both spliced and unspliced mRNAs (using exon- and intron-specific primers) as a function of their association with either eIF4E or CBP80 in each fraction. Note that pre-mRNA is detected only in the post-TX fraction, consistent with unspliced RNAs remaining in this fraction (Mili et al, 2001). In the post-TX fraction, CBP80 and hnRNPA1 but not eIF4E or LPRPRC associated with cyclin D1 and GAPDH pre-mRNA. In the TX-100 fraction, both CBP80 and hnRNPA1 associated with GAPDH mRNA, whereas eIF4E and LRPPRC did not. Cyclin D1 and c-myc mRNAs associated with eIF4E and LRPPRC, as well as with CBP80 and hnRNPA1 (Figure 4). This suggests that eIF4E and LRPPRC associate only with eIF4E-sensitive endogenous mRNAs. Note that eIF4E and CBP80 did not co-IP in these cells, indicating that they are not in the same complex (or that they coexist in the same RNP only very transiently) (Figure 2). Further, it suggests that there are (at least) two RNP populations containing cyclin D1 or c-myc mRNAs: those with eIF4E and LRPPRC and those with CBP80. The fact that cyclin D1 and c-myc mRNAs, but not LacZ-4E-SE mRNA, associate with CBP80 likely arises because, in the context of the full-length RNA, there are other elements in the UTR that compete for which mRNA pathway cyclin D1 and c-myc will be exported by i.e., the bulk pathway or the eIF4E-sensitive pathway. In this way, the elements, or USER codes, in cyclin D1 and c-myc competitively determine the export pathway. Further, the endogenous RNAs were spliced, and the machinery important for association with CBC in the TX-100 fraction could be deposited during this process. In contrast, for LacZ-4E-SE, the only signal is the 4E-SE and thus, only the eIF4E mRNA export pathway is used. Alternatively (but not mutually exclusive), the two RNPs may represent temporal units, where cyclin D1 and c-myc mRNAs exit from the post-TX fraction associated with CBP80 and the RNP is remodelled in the TX-100 fraction so that eIF4E now binds the cap in place of CBP80, and LRPPRC is also recruited to the remodelled RNP. Importantly, GAPDH, which is not sensitive to eIF4E, does not associate with eIF4E or LRPPRC in the post-TX or TX-100 fraction (Figure 4).
Figure 4.
LRPPRC selectively associates with model eIF4E export-sensitive mRNAs, cyclin D1 and c-myc, in the TX-100 fraction. U2OS cells were fractionated and RIPs were carried out in each fraction using indicated antibodies. Quantity of the RNA in the inputs (20%) and the immunoprecipitated material was determined using sqRT–PCR. Intron- and exon-specific primers (cyclin D1 and c-myc) and primers spanning across two different exons (GAPDH) were used to distinguish pre-mRNA and mRNA. PCR products were analysed on a 2.5% agarose gel.
In the cytosolic fraction, we observed that eIF4E associates with both LacZ and LacZ-4E-SE mRNAs consistent with its role in cap-dependent translation (Figure 4). Importantly, LRPPRC does not associate with eIF4E- or LacZ-4E-SE-containing mRNA in this fraction (Figure 4). Thus, it is unlikely that LRPPRC is directly modulating cap-dependent translation.
LRPPRC modulates the expression of eIF4E-sensitive genes
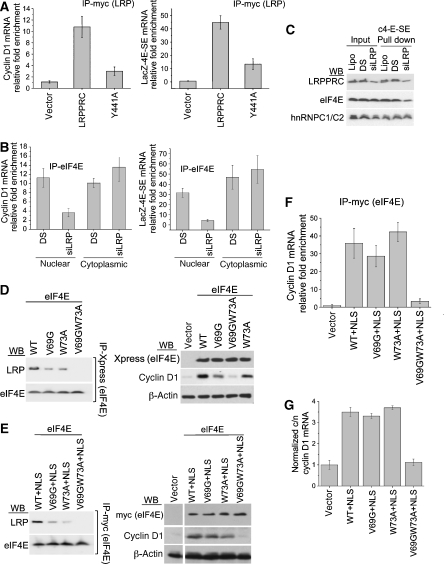
Inspection of the LRPPRC sequence revealed three putative eIF4E-binding sites as defined by YXXXXLΦ motif, where X is any amino acid and Φ is any hydrophobic residue (Supplementary Figure 2) (Koromilas et al, 1992; Sonenberg and Gingras, 1998). To assess whether these sites were eIF4E-binding sites, we transfected U2OS cells with myc-tagged wild-type or mutant LRPPRC and monitored eIF4E-LRPPRC association by IP. eIF4E associates with LRPPRC but mutation of either of the first two binding sites (Y to A) significantly reduces this association (Figure 5A). Mutation of the third binding site has no effect, indicating that this is not an eIF4E-binding site. Controls show that eIF4E itself was immunoprecipitated equally in all cases (Figure 5A). Thus, mutation of either of the first two binding sites abrogates eIF4E association.
Figure 5.
LRPPRC selectively affects eIF4E-dependent mRNA export. (A) IP reactions were carried in U2OS cells overexpressing indicated constructs. Y441A and Y583A mutants diminish eIF4E binding. (B) Protein levels of eIF4E targets were determined as a function of LRPPRC mutation by western blot. (C) U2OS cells were treated with lipofectamine (lipo), double scrambled controls (DS) or LRP siRNA (siLRP). (D) U2OS cells were fractionated into cytoplasmic and nuclear fractions and the amount of indicated mRNA in each fraction was determined using RT–qPCR. For each transcript, values are represented as cytoplasmic to nuclear ratios (c/n)±s.d. (n=3) and were normalized to 18S rRNA. In (D), export was monitored in U2OS cells transfected with LRP constructs as indicated (left panel) or treated with LRPPRC siRNA (siLRP) or the negative control (DS) (right panel). (E) Western blot analyses of U2OS/LacZ and U2OS/LacZ-4E-SE cells transfected with an empty vector or LRP constructs as indicated (left panel) or treated with LRPPRC siRNA (siLRP) or the negative control (DS) (right panel). (B, C, E) α-Tubulin and β-actin served as loading controls. (F) Same set of experiments as (D) but using U2OS/LacZ or U2OS/LacZ-4E-SE cells.
We investigated whether the association of LRPPRC with eIF4E-sensitive transcripts was a functional one. Previous studies showed that eIF4E overexpression promotes mRNA export of cyclin D1, Pim 1 and c-myc and thereby, increases their protein levels (Culjkovic et al, 2006). Thus, we monitored the levels of these proteins as a function of wild-type or mutant LRPPRC overexpression (Figure 5B). Additionally, we monitored the expression of these proteins as a function of RNAi-mediated knockdown of LRPPRC (Figure 5C). LRPPRC overexpression leads to increased levels of cyclin D1, Pim-1 and c-myc proteins. Importantly, LRPPRC does not alter the levels of eIF4E, α-tubulin or β-actin proteins, which are eIF4E insensitive. Overexpression of the LRPPRC Y663A mutant results in the same phenotype as wild type, consistent with its ability to bind eIF4E. However, the mutants that cannot associate with eIF4E (Y441A and Y583A) do not affect the expression of cyclin D1, Pim1 or c-myc (Figure 5B). Conversely, LRPPRC knockdown suppresses cyclin D1, Pim1 and c-myc protein levels, again not altering eIF4E or α-tubulin protein levels (Figure 5C). Previous studies showed this same pattern of effects for the knockdown of eIF4E (Culjkovic et al, 2008). In summary, LRPPRC has parallel effects to those of eIF4E on the expression of endogenous genes. Further, these activities are linked to the ability of LRPPRC to bind eIF4E.
LRPPRC modulates the export of eIF4E-sensitive mRNAs
To determine the stage in gene expression at which LRPPRC ultimately affects protein levels, we monitored the ability of LRPPRC to modulate mRNA export in U2OS cells overexpressing LRPPRC or the LRPPRC mutant that no longer binds eIF4E (Y441A). Cells were fractionated into nuclear and cytoplasmic fractions and mRNA levels were monitored by RT–qPCR. Values are normalized to 18S mRNA, which is not sensitive to eIF4E-dependent mRNA export (Culjkovic et al, 2006). Fractionation controls are shown in Supplementary Figure 3C. LRPPRC overexpression increased (by 5- to 6-fold) the ratio of cytoplasmic to nuclear cyclin D1 and Pim 1 mRNAs versus vector controls (Figure 5D). Importantly, the Y441A mutant does not alter export relative to vector controls. Conversely, RNAi knockdown of LRPPRC reduces the export of these mRNAs by 5- to 10-fold (Figure 5D). In no case were levels of total mRNAs affected (Supplementary Figure 3A and B).
Interestingly, LRPPRC overexpression moderately suppresses the export of α-tubulin and β-actin mRNA (about two-fold) (Figure 5D). Further, this is independent of eIF4E as the Y441A mutant suppresses export to the same extent as wild-type LRPPRC. Conversely, knockdown of LRPPRC leads to a two-fold increase in α-tubulin mRNA export (Figure 5D) We observed no change in the protein levels or total mRNA levels of β-actin or α-tubulin on modulation of LRPPRC levels (Figure 5B and C; Supplementary Figure 3A and B). The absence of changes in protein levels likely arises because of the moderate effects of LRPPRC on export of these transcripts (about two-fold) coupled to the fact that these proteins have long half-lives making it difficult to detect changes in their levels. These findings suggest that LRPPRC sequesters key export components away from the bulk mRNA export pathway to enhance eIF4E-dependent mRNA export and are consistent with previous observations that LRPPRC overexpression led to the nuclear accumulation of poly(A) mRNA (Tsuchiya et al, 2004).
We investigated whether LRPPRC modulates LacZ-4E-SE export. Here, LRPPRC overexpression increased LacZ protein levels in LacZ-4E-SE-overexpressing cells but not in the cells transfected with LacZ (Figure 5E). Further, the Y441A mutant, which is deficient in eIF4E binding, did not have this effect. LRPPRC knockdown led to reduced LacZ protein levels in cells expressing LacZ-4E-SE but not for cells expressing LacZ alone (Figure 5E). Consistently, LacZ-4E-SE export was increased by about seven-fold on LRPPRC overexpression relative to vector controls or the Y441A mutant, whereas LacZ mRNA export was only modestly reduced (by <2-fold; Figure 5F). Further, siRNA knockdown led to a reduction of LacZ-4E-SE mRNA export but did not affect LacZ mRNA export relative to double scrambled controls (Figure 5F). Further, no change in total transcript levels was observed (Supplementary Figure 3D and E). Fractionation controls are shown in Supplementary Figure 3F. To ensure that there were no nonspecific effects of the siRNAs used, we knocked down LRPPRC targeting non-coding regions and added-back exogenous LRPPRC. We see that LRPPRC addition compensates for the knockdown, where there is more cyclin D1 or LacZ-4E-SE produced in the add-back cells than in the controls (Supplementary Figure 4A). Importantly, adding the Y441A mutant back does not have this compensatory effect (Supplementary Figure 4A). In summary, LacZ-4E-SE mRNA export is sensitive to LRPPRC, whereas LacZ alone is not. Thus, the 50-nt 4E-SE is sufficient to recruit LRPPRC (Figure 1A) and sensitize the transcript to this mode of export (Figure 5F). The promotion of export by LRPPRC of LacZ-4E-SE is most likely dependent on its ability to bind to eIF4E, as indicated by the failure of LRPPRC constructs with the Y441A mutation to enhance mRNA export.
Intriguingly, RNAi-mediated knockdown of LRPPRC did not suppress the export of LacZ mRNA (Figure 5F), whereas it did suppress the export of α-tubulin and β-actin mRNAs by nearly two-fold (albeit in an eIF4E-independent manner) (Figure 5D). This suggests that there are elements in the UTRs of α-tubulin and β-actin mRNAs that are absent from LacZ mRNA and that negatively sensitize these transcripts to LRPPRC. Thus, it seems likely that LRPPRC sequesters an important cofactor(s) away from these transcripts, moderately reducing their export.
LRPPRC and eIF4E mutually promote association with 4E-SE mRNAs
We investigated the possibility that eIF4E promotes the interaction of LRPPRC with 4E-SE-containing mRNAs. Thus, we examined whether the Y441A mutation altered the association of LRPPRC with 4E-SE-containing mRNAs. U2OS cells transfected with myc-tagged LRPPRC, or the Y441A mutant, were immunoprecipitated using the myc tag and levels of either endogenous cyclin D1 or LacZ-4E-SE mRNA were quantified using qPCR. In both cases, the mRNA was substantially enriched in the wild-type-expressing cells relative to the mutant or vector controls (Figure 6A). However, we do observe that some 4E-SE-containing mRNAs associated with the mutant (Figure 6A). Thus, LRPPRC interacts, albeit at much lower levels, with 4E-SE-containing mRNAs in the absence of eIF4E association. This is consistent with LRPPRC containing a novel RNA-binding motif.
Figure 6.
LRPPRC requires interaction with eIF4E for its mRNA export function. (A) U2OS (left) or U2OS/LacZ-4E-SE cells (right) were transfected with an empty vector or indicated myc-tagged LRPPRC constructs and immunoprecipitated with myc antibody. (B) RIPs were carried out in the nuclear and cytoplasmic extracts of U2OS or U2OS/LacZ-4E-SE cells treated with negative control (DS) or siLRP. (A, B) The quantity of cyclin D1 or LacZ-4E-SE mRNA was determined by RT–qPCR. Fold enrichment is calculated relative to the appropriate IgG control±standard deviations (s.d.) (n=3). (C) RNA pull downs using LacZ-4E-SE (c-4E-SE) as a bait were carried out in nuclear extracts of U2OS cells treated with lipofectamine (lipo), negative control (DS) or siLRP. Pulled-down material was analysed by western blot using indicated antibodies. (D, left) The association of endogenous LRPPRC with overexpressed eIF4E wild-type or mutant proteins was assessed by immunoprecipitation (IP) in cells transfected with indicated Xpress-tagged eIF4E constructs. (Right) Western blot analysis of eIF4E target proteins as a function of eIF4E mutation. β-Actin was used as a loading control. (E) Cells were transfected with the myc-tagged eIF4E constructs with a heterologous NLS. Experiments were carried out same as in (D). (F) Double mutation of the dorsal surface abrogates the ability of eIF4E to associate with endogenous cyclin D1 mRNA. Cells were immunoprecipitated with the anti-myc antibody and RNA levels were quantified by RT–qPCR. Fold enrichment is relative to the IgG control±s.d. (n=3). (G) mRNA export assay examining the ability of eIF4E mutants to promote cyclin D1 mRNA export. For each transcript, values were normalized to 18S rRNA and presented as cytoplasmic to nuclear ratios (c/n)±s.d. (n=3).
In parallel, we assessed whether the knockdown of LRPPRC affected the affinity of eIF4E for 4E-SE-containing mRNAs. U2OS cells were treated with LRPPRC siRNA, nuclear and cytoplasmic fractions were isolated and immunoprecipitated with an anti-eIF4E antibody. Clearly, knockdown of LRPPRC reduced interactions of eIF4E with either endogenous cyclin D1 or LacZ-4E-SE mRNAs in the nuclear compartment relative to DS controls (Figure 6B). LRPPRC did not affect the association of eIF4E with these RNAs in the cytoplasmic fraction (Figure 6B). In parallel, RNA pull-down experiments using LacZ and c-4E-SE as baits were carried out in the nuclear fraction of U2OS cells. Here, the association of eIF4E with c-4E-SE was impaired by the knockdown of LRPPRC related to DS controls (Figure 6C). Importantly, the association of LacZ-4E-SE with hnRNPC1/C2 was not effected by reduction in LRPPRC protein levels (Figure 6C). In both IP and pull-down experiments where LRPPRC levels were reduced, some RNAs were still associated with eIF4E. This is likely due to incomplete knockdown of LRPPRC levels.
Effects of eIF4E mutations on LRPPRC mRNA export function
Proteins using consensus eIF4E-binding sites interact with the dorsal surface of eIF4E, which includes residues W73 and V69 (Koromilas et al, 1992; Sonenberg and Gingras, 1998). Given that mutants on this surface (W73A and V69G) still function in mRNA export (see below and Topisirovic et al, 2002), we examined the effects of these mutations on the LRPPRC interaction. LRPPRC immunoprecipitated with wild-type eIF4E and with either mutant, albeit with reduced affinity (Figure 6D). However, a double mutant, V69GW73A, substantially reduced the association of eIF4E with LRPPRC and failed to elevate cyclin D1 protein levels (Figure 6D). Circular dichroism studies showed that the V69GW73A mutant had an indistinguishable secondary structure from wild-type eIF4E (data not shown). Although V69G and W73A mutations did not affect the subcellular distribution of eIF4E, the double mutant was almost exclusively cytoplasmic (Supplementary Figure 4B). Thus, to investigate whether the double mutant did not bind LRPPRC as it did not enter the nucleus, we engineered wild-type and mutant constructs with a heterologous NLS. By fractionation methods, we observed strong nuclear localization of both wild-type and mutant proteins (Supplementary Figure 4C). Even in the presence of the NLS, the double mutant did not bind LRPPRC, whereas the single mutants and wild-type eIF4E did (Figure 6E).
We examined the functional outcome of these mutations on eIF4E-dependent mRNA export in the context of the heterologous NLS. By monitoring mRNA export by subcellular fractionation, we observed that both single mutants associated with, and promoted the export of, endogenous cyclin D1 mRNA as well as wild-type eIF4E (Figure 6F and G). However, the double mutant failed to associate with or promote the export of cyclin D1 mRNA (Figure 6F and G). Cyclin D1 protein levels were elevated in cells expressing wild-type or single mutants relative to the double mutant or vector controls (Figure 6D and E).
Thus, mutations that abrogate binding of LRPPRC to eIF4E also impair eIF4E-dependent mRNA export. It seems likely that the binding site for LRPPRC and eIF4G do not completely overlap on eIF4E. Consistent with our findings, mutations on the dorsal surface of eIF4E differentially affect its binding to the 4E-BPs (Ptushkina et al, 1999).
LRPPRC modulates the subcellular distribution of eIF4E
We investigated how LRPPRC potentiates the mRNA export activity of eIF4E. LRPPRC did not affect eIF4E protein levels and the ability of LRPPRC to modulate eIF4E activity appeared to depend on a direct association with LRPPRC. Thus, we assessed whether LRPPRC modulated the subcellular distribution of eIF4E. The nuclear fraction of eIF4E forms bodies, a subset of which associate with mRNAs such as cyclin D1 and LacZ-4E-SE, but not with LacZ and GAPDH transcripts (Culjkovic et al, 2005, 2006). The remaining eIF4E bodies colocalize with PML nuclear bodies. PML represses eIF4E-dependent mRNA export by directly binding eIF4E and reducing its affinity for the m7G cap by ∼100-fold (Cohen et al, 2001; Kentsis et al, 2001). This subset of eIF4E nuclear bodies appears ‘off' and bodies that contain mRNA may be sites for remodelling the eIF4E export RNPs. Thus, we examined the effects of wild-type or mutant LRPPRC overexpression on the subcellular distribution of eIF4E in U2OS cells. We also examined the effects of knockdown of LRPPRC. The subcellular distributions of LRPPRC and eIF4E were monitored both by confocal microscopy and by subcellular fractionation and western blotting.
Endogenous LRPPRC has a weak nuclear localization and a more substantial cytoplasmic staining where LRPPRC is found in mitochondria (Supplementary Figure 5A) (Mili and Pinol-Roma, 2003). Interestingly, treatment of cells with LMB leads to the nuclear accumulation of both endogenous LRPPRC and eIF4E (Supplementary Figure 5A and B). This is interesting given that mRNA export of eIF4E-sensitive mRNAs such as cyclin D1 and LacZ-4E-SE is LMB sensitive, whereas bulk mRNA export is not (Culjkovic et al, 2006). Further, these findings indicate that a substantial fraction of LRPPRC traffics through the nucleus and support its role in eIF4E-dependent mRNA export.
There are three fractions of eIF4E in the nucleus: nucleoplasmic eIF4E, eIF4E nuclear bodies that colocalize with mRNA and eIF4E nuclear bodies that colocalize with PML and are devoid of mRNA (Culjkovic et al, 2005). Typically, eIF4E nuclear bodies are very round (Cohen et al, 2001). LRPPRC overexpression results in a more irregular eIF4E body morphology that is distinct from vector controls or Y441A LRPPRC-expressing cells (Figure 7A; Supplementary Figure 6A). We also examined the effects of LRPPRC on the distribution of eIF4E relative to PML. Markedly, LRPPRC overexpression leads to a loss of PML-eIF4E nuclear body colocalization (Figure 7A). Loss of the colocalization of eIF4E with PML nuclear bodies on LRPPRC overexpression coincides with eIF4E shifting from the post-TX to the ‘export-competent' TX-100 fraction (Figure 7C). This could be achieved by effectively competing with the inhibitor of eIF4E mRNA export function, PML, for binding to the dorsal surface of eIF4E. Consistently, we observe PML and eIF4E colocalization in bodies indistinguishable from vector controls in cells overexpressing the Y441A mutant (Figure 7A). Accordingly, Y441A mutant overexpressing cells did not accumulate eIF4E in the TX-100 fraction (Figure 7C). As a control, we show that both wild type and Y441A LRPPRC do not affect the subcellular distribution of CBP80 (Figure 7C).
Figure 7.
LRPPRC alters the subcellular distribution of eIF4E. (A) Confocal micrographs of U2OS cells transfected as indicated. eIF4E and PML staining is shown in green and red, respectively. Nuclei were counterstained with DAPI (blue) in (A, B). Overlay of all three channels is shown on the right. On the far right, overlays were zoomed × 2.5. In the control, vector transfected cells and cells overexpressing Y441A LRPPRC mutant, most of PML (red arrowheads) and eIF4E (green arrowheads) nuclear bodies are round and frequently colocalize (yellow arrowheads indicate typical PML/eIF4E body colocalization). In contrast, eIF4E nuclear bodies in cells transfected with the wild-type LRPPRC are irregularly shaped and do not colocalize with PML. (B) Confocal micrographs of U2OS cells treated with either LRPPRC siRNA (siLRP) or negative control (DS). eIF4E and Dcp1 are shown in green and red, respectively, with colocalization in yellow. Far right insets represent overlays that were zoomed × 2. In contrast to controls, cells treated with LRPPRC siRNA show increased numbers of Dcp1 cytoplasmic bodies (P-bodies; red arrowheads) and increased colocalization of eIF4E with these structures (yellow arrowhead). Green arrowheads indicate eIF4E nuclear bodies. (C) Cells transfected with empty vector, wild-type LRPPRC and Y441A LRPPRC mutant (Y441A) were fractionated into post-TX, TX-100 and digitonin fractions and the relative distribution of CBP80 and eIF4E proteins in each fraction was monitored using western blot (WB).
Knock down of LRPPRC resulted in depletion of the nuclear eIF4E, (Supplementary Figure 6B), thereby providing an explanation for how LRPPRC knockdown reduces eIF4E-dependent mRNA export. Interestingly, depletion of LRPPRC levels coincided with the accumulation of eIF4E in cytoplasmic P-bodies (Figure 7B) as illustrated by its increased colocalization with the P-body marker, Dcp1.
Discussion
eIF4E-dependent mRNA export contributes to the transformation potential of eIF4E. Although the biochemistry of eIF4E's role in cytoplasmic cap-dependent translation is well understood (Sonenberg and Gingras, 1998; Gingras et al, 1999), much less is known about its nuclear function. Thus, we set out to elucidate the molecular underpinnings of eIF4E's function in nuclear mRNA export. Here, we identified a novel factor in this process, LRPPRC. We showed that LRPPRC and eIF4E associate with eIF4E-sensitive mRNAs within export-competent RNPs. LRPPRC positively modulates mRNA export of 4E-SE-containing mRNAs in an eIF4E-dependent manner. Further, the eIF4E-dependent mRNA export pathway is distinct from bulk mRNA export. The eIF4E pathway contains specific factors such as eIF4E and LRPPRC and excludes factors involved in bulk mRNA export, for example, the CBC and TAP/NXF1. However, some factors, such as UAP56, hnRNPA1 and DDX3 are common to both pathways (this study and Moore, 2005). Further, our studies suggest that initial maturation of 4E-SE-containing mRNAs is similar to that for bulk mRNA (Figure 8).
Figure 8.
Model depicting the differences in metabolism of 4E-SE-containing transcripts and bulk mRNA. 4E-SE mRNAs are processed in the same way as bulk mRNA but are exported through 4E-SE export-competent mRNP, containing eIF4E, CRM-1, an LRPPRC (shown in green). Export-competent mRNP comprising bulk mRNA, CBC and TAP is shown in blue. See text for further details.
Our data suggest that LRPPRC binds the 4E-SE and promotes the association of eIF4E with the 4E-SE-containing RNA. In support of this notion, LRPPRC binds RNA directly (Mili et al, 2001) and contains several copies of the PPR motif, which is thought to be a signature for a family of RNA-binding proteins (Small and Peeters, 2000). We show here that LRPPRC requires association with eIF4E to modulate mRNA export and that LRPPRC potentiates the interactions between eIF4E- and 4E-SE-containing mRNAs. Alternatively, LRPPRC may bridge the association through additional cofactors. In any case, LRPPRC and eIF4E mutually potentiate the formation of the 4E-SE export-competent mRNP (Figure 8).
Further, LRPPRC leads to re-distribution of eIF4E within the nucleus, where it no longer associates with PML nuclear bodies. This strongly suggests that LRPPRC promotes mRNA export by recruiting eIF4E away from its potent inhibitor, PML (Cohen et al, 2001; Culjkovic et al, 2008). Both PML and LRPPRC bind the dorsal surface of eIF4E, thus LRPPRC is well positioned to compete with PML for eIF4E. Loss of LRPPRC reduces nuclear eIF4E levels, consistent with the requirement for eIF4E to be in the nucleus to function in mRNA export.
These findings indicate that LRPPRC is a member of an emerging class of factors that control eIF4E activity through modulation of eIF4E location. Other such factors include the eIF4E transporter protein 4E-T, the eIF4E-binding protein 4E-BP1 and the homeodomain protein PRH/Hex (Topisirovic et al, 2003a; Ferraiuolo et al, 2005; Rong et al, 2008). Small molecules such as LMB, m7G and ribavirin also modulate eIF4E localization (Dostie et al, 2000; Kentsis et al, 2004). LMB treatment leads to nuclear accumulation of eIF4E and ribavirin or m7G treatment leads to its cytoplasmic accumulation. Given that the nuclear functions of eIF4E contribute to its oncogenic activities even in humans, understanding the mechanisms by which this is controlled is important for understanding the molecular underpinning of eIF4E's transformation capacity.
The association of LRPPRC with eIF4E and its ability to modulate eIF4E function suggest a potential mechanism for the communication between post-transcriptional gene expression and energy production in the cell. This is because LRPPRC is also found in mitochondria where it has a critical function in mRNA expression (Mili and Pinol-Roma, 2003; Cooper et al, 2006). Importantly, wild-type LRPPRC and the Y441A mutant bind to an eIF4E-insensitive mitochondrial mRNA (ATP synthase 6) equally well, indicating that this mutation does not directly affect the RNA-binding ability of LRPPRC and suggests that its nuclear and mitochondrial functions are distinct (data not shown). Furthermore, LRPPRC knockdown induced P-body formation, increasing the accumulation of eIF4E within these structures. Thus, LRPPRC may function as a regulator of eIF4E activity as a function of the energy status of the cell. Here, reduced levels of LRPPRC would impair nuclear export of eIF4E-sensitive transcripts and sequester cytoplasmic transcripts within P-bodies. It is important to note that we do not observe any mitochondrial localization of eIF4E or interaction between LRPPRC and eIF4E in the cytoplasm. Importantly, LMB treatment leads to the nuclear accumulation of LRPPRC. Although LRPPRC is mainly cytoplasmic, a significant fraction transits through the nucleus. Thus, the extent of the nuclear versus mitochondrial localization of LRPPRPC may have an important function in coupling gene expression with energy production.
Finally, our studies suggest that specific mRNA export pathways have evolved to promote, in concert with other processes, the activity of distinct biochemical pathways. For example, eIF4E impacts on multiple targets in the Akt pathway by stimulating the mRNA export of both an upstream activator of Akt, NBS1, as well as downstream effectors such as mdm2, cyclin D1 and c-myc (Culjkovic et al, 2008). In this way, the eIF4E RNA regulon can modulate biochemical pathways. Factors, such as LRPPRC, that are required for the nuclear export arm of the regulon may function as energy sensors in the cell, coupling proliferation and survival to energy status.
Materials and methods
Cell culture, plasmids, RNAi and transfections
U2OS and HEK293 cells (from the ATCC) were maintained in DMEM supplemented with 10% FBS and penicillin/streptomycin (all obtained from Invitrogen). U2OS stably transfected with LacZ and LacZ-4E-SE and pcDNA3.1MycLRPPRC wild-type construct are described by Mili and Pinol-Roma (2003) and Culjkovic et al (2005, 2006), respectively. Information about constructs and transfections are provided in the Supplementary data.
Synthesis of biotinylated transcripts, RNA pull down and mass spectrometry
In vitro transcribed, biotinylated transcripts were generated by PCR using pcDNA3.1His/LacZ (Invitrogen) and pcDNA3.1His/LacZ-4E-SE constructs as described in the Supplementary data. RNA pull-down assays were carried out as in Takagi et al (2005). Pulled-down protein was eluted in Laemmli buffer, loaded onto 10–15% SDS–PAGE gel, visualized with Sypro Ruby (Bio-Rad) and analysed using western blot and mass spectrometry as described in the Supplementary data.
Western blot, IP and RNA IP (RIP)
Western blot analyses were carried out as in Topisirovic et al (2002). Antibodies used for western blot are provided in the Supplementary data. To minimize the possibility of mRNP reassortment in cell extracts (Mili and Steitz, 2004), initial IPs and RIPs were carried out using two different protocols (see Supplementary data). Notably, both protocols gave similar results, suggesting that the observed protein–RNA and protein–protein interactions were not due to the reassortment of mRNPs in the lysate.
Subcellular fractionation, sqRT–PCR and RT–qPCR
Subcellular fractionation and RNA isolation were performed as described in the Supplementary data and in Mili et al (2001) and Topisirovic et al (2002). sqRT–PCR and RT–qPCR were performed using One-step RT–PCR kit (Qiagene) and SYBR Green PCR Master Mix (Applied Biosystems), respectively.
LMB, immunofluorescence and laser-scanning confocal microscopy
For LMB treatment, U2OS cells were grown on coverslips and incubated with LMB (50 nM; Sigma) or vehicle (ethanol) for 4 h at 37°C. Immunofluorescence was carried out as described in the Supplementary data using a laser-scanning confocal microscope (LSM510; Carl Zeiss).
Supplementary Material
Supplementary Information
Supplementary Table 1
Supplementary Materials and Methods
Supplementary Raw MS Data
Acknowledgments
We are grateful to J Keene, E Izzauralde and M Green for providing antibodies to HuR, CBP20&80 and UAP56, respectively. We thank L Volpon, C Charbonneau and B Gibbs for help with protein purification, microscopy and MS, respectively. This study was supported by funding from NIH (RO1 80728) to KLBB and R01 GM067894 to SP-R. IRIC receives infrastructure support funds from the FRSQ and from a CIHR multi-resource grant. KLBB and PT hold Canada Research Chairs in Molecular Biology of the Cell Nucleus and in proteomics and bioanalytical mass spectrometry, respectively. IT is a special fellow of the Leukemia and Lymphoma Society, USA and CB is supported by a UNCF-Merck Science Initiative Graduate Fellowship.
References
- Cheng H, Dufu K, Lee CS, Hsu JL, Dias A, Reed R (2006) Human mRNA export machinery recruited to the 5′ end of mRNA. Cell 127: 1389–1400 [DOI] [PubMed] [Google Scholar]
- Cohen N, Sharma M, Kentsis A, Perez JM, Strudwick S, Borden KL (2001) PML RING suppresses oncogenic transformation by reducing the affinity of eIF4E for mRNA. EMBO J 20: 4547–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MP, Qu L, Rohas LM, Lin J, Yang W, Erdjument-Bromage H, Tempst P, Spiegelman BM (2006) Defects in energy homeostasis in Leigh syndrome French Canadian variant through PGC-1alpha/LRP130 complex. Genes Dev 20: 2996–3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culjkovic B, Tan K, Orolicki S, Amri A, Meloche S, Borden KL (2008) The eIF4E RNA regulon promotes the Akt signaling pathway. J Cell Biol 181: 51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culjkovic B, Topisirovic I, Borden KL (2007) Controlling gene expression through RNA regulons: the role of the eukaryotic translation initiation factor eIF4E. Cell Cycle 6: 65–69 [DOI] [PubMed] [Google Scholar]
- Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL (2005) eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3′UTR. J Cell Biol 169: 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KL (2006) eIF4E is a central node of an RNA regulon that governs cellular proliferation. J Cell Biol 175: 415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A, Graff JR (2004) eIF-4E expression and its role in malignancies and metastases. Oncogene 23: 3189–3199 [DOI] [PubMed] [Google Scholar]
- Dostie J, Ferraiuolo M, Pause A, Adam SA, Sonenberg N (2000) A novel shuttling protein, 4E-T, mediates the nuclear import of the mRNA 5′ cap-binding protein, eIF4E. EMBO J 19: 3142–3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraiuolo MA, Basak S, Dostie J, Murray EL, Schoenberg DR, Sonenberg N (2005) A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay. J Cell Biol 170: 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N (1999) eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem 68: 913–963 [DOI] [PubMed] [Google Scholar]
- Graff JR, Konicek BW, Carter JH, Marcusson EG (2008) Targeting the eukaryotic translation initiation factor 4E for cancer therapy. Cancer Res 68: 631–634 [DOI] [PubMed] [Google Scholar]
- Graff JR, Zimmer SG (2003) Translational control and metastatic progression: enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clin Exp Metastasis 20: 265–273 [DOI] [PubMed] [Google Scholar]
- Keene JD (2007) RNA regulons: coordination of post-transcriptional events. Nat Rev Genet 8: 533–543 [DOI] [PubMed] [Google Scholar]
- Keene JD, Lager PJ (2005) Post-transcriptional operons and regulons co-ordinating gene expression. Chromosome Res 13: 327–337 [DOI] [PubMed] [Google Scholar]
- Kentsis A, Dwyer EC, Perez JM, Sharma M, Chen A, Pan ZQ, Borden KL (2001) The RING domains of the promyelocytic leukemia protein PML and the arenaviral protein Z repress translation by directly inhibiting translation initiation factor eIF4E. J Mol Biol 312: 609–623 [DOI] [PubMed] [Google Scholar]
- Kentsis A, Topisirovic I, Culjkovic B, Shao L, Borden KL (2004) Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci USA 101: 18105–18110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koromilas AE, Lazaris-Karatzas A, Sonenberg N (1992) mRNAs containing extensive secondary structure in their 5′ non-coding region translate efficiently in cells overexpressing initiation factor eIF-4E. EMBO J 11: 4153–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Lee YH, Tarn WY (2008) The DEAD-box RNA helicase DDX3 associates with export messenger ribonucleoproteins as well as tip-associated protein and participates in translational control. Mol Biol Cell 19: 3847–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N (2004) eIF4E––from translation to transformation. Oncogene 23: 3172–3179 [DOI] [PubMed] [Google Scholar]
- Mili S, Pinol-Roma S (2003) LRP130, a pentatricopeptide motif protein with a noncanonical RNA-binding domain, is bound in vivo to mitochondrial and nuclear RNAs. Mol Cell Biol 23: 4972–4982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mili S, Shu HJ, Zhao Y, Pinol-Roma S (2001) Distinct RNP complexes of shuttling hnRNP proteins with pre-mRNA and mRNA: candidate intermediates in formation and export of mRNA. Mol Cell Biol 21: 7307–7319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mili S, Steitz JA (2004) Evidence for reassociation of RNA-binding proteins after cell lysis: implications for the interpretation of immunoprecipitation analyses. RNA 10: 1692–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanaro L, Pandolfi PP (2004) Initiation of mRNA translation in oncogenesis: the role of eIF4E. Cell Cycle 3: 1387–1389 [DOI] [PubMed] [Google Scholar]
- Moore MJ (2005) From birth to death: the complex lives of eukaryotic mRNAs. Science 309: 1514–1518 [DOI] [PubMed] [Google Scholar]
- Ohno M, Segref A, Bachi A, Wilm M, Mattaj IW (2000) PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell 101: 187–198 [DOI] [PubMed] [Google Scholar]
- Ptushkina M, von der Haar T, Karim MM, Hughes JM, McCarthy JE (1999) Repressor binding to a dorsal regulatory site traps human eIF4E in a high cap-affinity state. EMBO J 18: 4068–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong L, Livingstone M, Sukarieh R, Petroulakis E, Gingras AC, Crosby K, Smith B, Polakiewicz RD, Pelletier J, Ferraiuolo MA, Sonenberg N (2008) Control of eIF4E cellular localization by eIF4E-binding proteins, 4E-BPs. RNA 14: 1318–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N (1996) Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci USA 93: 1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small ID, Peeters N (2000) The PPR motif––a TPR-related motif prevalent in plant organellar proteins. Trends Biochem Sci 25: 46–47 [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Gingras AC (1998) The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr Opin Cell Biol 10: 268–275 [DOI] [PubMed] [Google Scholar]
- Takagi M, Absalon MJ, McLure KG, Kastan MB (2005) Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell 123: 49–63 [DOI] [PubMed] [Google Scholar]
- Topisirovic I, Capili AD, Borden KL (2002) Gamma interferon and cadmium treatments modulate eukaryotic initiation factor 4E-dependent mRNA transport of cyclin D1 in a PML-dependent manner. Mol Cell Biol 22: 6183–6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topisirovic I, Culjkovic B, Cohen N, Perez JM, Skrabanek L, Borden KL (2003a) The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth. EMBO J 22: 689–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topisirovic I, Guzman ML, McConnell MJ, Licht JD, Culjkovic B, Neering SJ, Jordan CT, Borden KL (2003b) Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis. Mol Cell Biol 23: 8992–9002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya N, Fukuda H, Nakashima K, Nagao M, Sugimura T, Nakagama H (2004) LRP130, a single-stranded DNA/RNA-binding protein, localizes at the outer nuclear and endoplasmic reticulum membrane, and interacts with mRNA in vivo. Biochem Biophys Res Commun 317: 736–743 [DOI] [PubMed] [Google Scholar]
- Yedavalli VS, Neuveut C, Chi YH, Kleiman L, Jeang KT (2004) Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 119: 381–392 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Table 1
Supplementary Materials and Methods
Supplementary Raw MS Data