SUMMARY
How asymmetric divisions are connected to the terminal differentiation program of neuronal subtypes is poorly understood. In C. elegans, two homeodomain transcription factors, TTX-3 (a LHX2/9 orthologue) and CEH-10 (a CHX10 orthologue), directly activate a large battery of terminal differentiation genes in the cholinergic interneuron AIY. We establish here a transcriptional cascade linking asymmetric division to this differentiation program. A transient lineage-specific input formed by the Zic factor REF-2 and the bHLH factor HLH-2 directly activates ttx-3 expression in the AIY mother. During the terminal division of the AIY mother, an asymmetric Wnt/β-catenin pathway cooperates with TTX-3 to directly restrict ceh-10 expression to only one of the two daughter cells. TTX-3 and CEH-10 automaintain their expression, thereby locking in the differentiation state. Our study establishes how transient lineage and asymmetric division inputs are integrated and suggests that the Wnt/β-catenin pathway is widely used to control the identity of neuronal lineages.
INTRODUCTION
The specific shape, connectivity and function of a neuron is determined by the set of terminal differentiation genes that it expresses, such as genes coding for particular neurotransmitter receptors, ion channels or cell adhesion proteins. These differentiation genes are organized into large batteries that often share similar cis-regulatory element(s) directly activated by a common terminal transcription factor or transcription factor complex (Livesey et al., 2000; Wenick and Hobert, 2004; Zhang et al., 2002). Genes that code for such identity-determining transcription factors directly controlling the expression of terminal differentiation genes have been termed “terminal selector genes” (Hobert, 2008). Several examples of such terminal selector genes have been reported in the nervous system, including the homeodomain protein CRX which directly activates a battery of genes responsible for vertebrate photoreceptor differentiation (Livesey et al., 2000) or the zinc finger transcription factor CHE-1 which directly regulates the expression of a battery of genes that define the ASE gustatory neuron in C. elegans (Etchberger et al., 2007). However, how terminal selector genes are coupled to earlier specification processes, particularly those that involve asymmetric division of neural progenitors, remains poorly understood.
One mechanism to generate cellular diversity in the nervous system is through asymmetric cell divisions, as documented in several organisms including vertebrates, Drosophila and C. elegans (Huttner and Kosodo, 2005; Sulston et al., 1983; Wodarz and Huttner, 2003). During this process, a progenitor cleaves to generate a postmitotic neuron and a new progenitor, or two postmitotic neurons with different identities. In Drosophila, the Notch pathway regulator Numb is asymmetrically distributed between the daughter cells endowing them with different fate (Knoblich, 2008). Numb has also been implicated in this process in vertebrates, however its precise function remains controversial and alternative mechanisms have been proposed involving other molecules, such as β-catenin (Doe, 2008; Knoblich, 2008; Woodhead et al., 2006; Wrobel et al., 2007). Finally no role has been reported for Numb in C. elegans but an unrelated protein, HAM-1, is asymmetrically distributed and regulates some but not all terminal divisions of embryonic neuroblasts (Guenther and Garriga, 1996) leaving open the possibility of additional mechanisms.
In order to better understand the regulation of terminal selector genes that determine neuronal identity and their connection to preceding asymmetric precursor divisions, we focus on the functionally and molecularly well characterized AIY cholinergic interneuron, which is generated by an asymmetric cell division giving rise to AIY and its sister neuron, SMDD. The AIY pair of primary interneurons is located in the head of C. elegans and integrates diverse sensory inputs such as taste, smell and temperature (Tsalik and Hobert, 2003). It is also involved in memory processes such as temperature adaptation (Mori and Ohshima, 1995) and aversive olfactory learning (Zhang et al., 2005). A battery of at least 40 terminal differentiation genes uniquely define the identity of the AIY interneuron (Wenick and Hobert, 2004) including unc-17, the vesicular transporter for its neurotransmitter acetylcholine and mod-1, a serotonin-gated ion channel involved in aversive olfactory learning (Zhang et al., 2005). These genes are directly activated in the postmitotic AIY interneuron by a common cis-regulatory element, called the AIY motif, present in their promoter (Wenick and Hobert, 2004). This motif is a binding site for a complex of two terminal selector genes: TTX-3, a LIM-homeodomain factor orthologous to Apterous/LHX-2/9, and CEH-10, a CVC-homeodomain factor orthologous to CHX-10/VSX-1 (Altun-Gultekin et al., 2001; Forrester et al., 1998; Hobert et al., 1997; Svendsen and McGhee, 1995; Wenick and Hobert, 2004). Besides activating and maintaining the expression of the gene battery, TTX-3 and CEH-10 also maintain their own expression by binding to an AIY motif present in their respective promoters. How ttx-3 and ceh-10 are themselves initially activated is, however, unknown thereby leaving the question of how the AIY neuron acquires its specific identity still unanswered.
In C. elegans, most neurons are produced during embryogenesis from the AB blastomere (anterior daughter of the first cleavage) which also gives rise through a series of asymmetric cell divisions to glial cells, epidermis and some cells of the pharynx and excretory system (Sulston et al., 1983). Each neuron is generated by a unique and stereotyped succession of cleavages oriented most of the time along the antero-posterior axis. For example, the right AIY neuron is “ABprpapaaap”, which indicates for the each of the 9 successive cleavages of the AB blastomeres whether it derives from the anterior (a), posterior (p), right (r) or left (l) daughter of a cell division. During the early cleavages of the AB blastomere the TCF transcription factor POP-1 is asymmetrically localized between the anterior and posterior daughters (Lin et al., 1998). This POP-1 asymmetry is regulated by a particular Wnt pathway recently named the Wnt/β-catenin asymmetry pathway (Mizumoto and Sawa, 2007), which endows the anterior and posterior daughters of early blastomeres with different identities (Kaletta et al., 1997; Lin et al., 1998). Whether this pathway is also involved in the terminal division of embryonic neuroblasts and how it is connected to the terminal differentiation program of postmitotic neurons remains to be established.
To determine how terminal neuronal selector genes are coupled to earlier lineage inputs and to investigate a potential role of the Wnt/β-catenin asymmetry pathway in neuronal fate determination, we took a combination of forward and reverse genetic approaches. Conducting a forward mutant screen and a promoter dissection, we identified a transient lineage-specific input (a combination of Zic and bHLH transcription factors) that directly sets up the expression of terminal selector genes in the mother cell of the AIY interneuron, before it asymmetrically divides to generate AIY and its sister neuron, SMDD. Using temperature sensitive alleles of Wnt pathway components and promoter analysis, we directly implicate POP-1 asymmetry in restricting the activation of the terminal transcription factor complex TTX-3/CEH-10 to the posterior daughter (AIY) of the terminal division. We finally show that the Wnt/β-catenin asymmetry pathway is also implicated in other terminal neuroblast divisions in the embryonic nervous system. Our study provides an overall logic for how specific lineage inputs and asymmetric division cues are integrated to initiate the terminal differentiation program of a neuron. We also discuss two other general implications of our study, namely, a common theme of Zic factor function in neuroblast development and a potentially broad conservation of the role of Wnt/β-catenin signaling in the regulation of neural progenitor asymmetric divisions.
RESULTS
The Zic transcription factor REF-2 affects the specification of the AIY interneuron
The AIY interneuron is specified by a pair of terminal selector transcription factors, TTX-3 and CEH-10, which directly activate a large battery of terminal differentiation genes (Altun-Gultekin et al., 2001; Wenick and Hobert, 2004). In order to determine how this transcription factor complex is regulated we undertook a forward genetic screen and uncovered several mutants with abnormal ttx-3 expression (see experimental procedures). In one of these mutants, ot327, both ttx-3 and ceh-10 expression is lost in the AIY neurons at larval stages (Figures 1A and 1C; ot327 is recessive; the larval lethality of ot327 homozygote mutants prevents the analysis of the expression of ttx-3 and ceh-10 in adults). As expected from the loss of ttx-3 and ceh-10 expression, ot327 also causes a loss of the expression of several terminal differentiation markers of AIY fate, including sra-11 (a putative neurotransmitter receptor) and inx-18 (an innexin) (Table S1). However, in ot327 mutants we still observe by DIC microscopy a neuron at the position normally occupied by AIY and this neuron still expresses the pan-neuronal marker F25B3.3 (Figure 1D). This suggests that while ot327 causes a loss of the specific subtype identity of the AIY interneuron, it may not affect its pan-neuronal features, a phenotype similar to the one observed in ttx-3 and ceh-10 mutants (Altun-Gultekin et al., 2001). ot327 also affects the specification of some other neurons such as the interneuron AVK (Table S1). However the global organization of the nervous system seems normal as examined with a pan-neuronal promoter driving GFP (data not shown) and the analysis of several cell-specific markers indicates that some other neuronal subtypes are correctly specified (Table S1). This suggests that the effects of ot327 in the nervous system are neuron-type specific.
Figure 1. ot327 and gk178 affect the expression of the TTX-3/CEH-10 complex in AIY.

(A) In ot327 and gk178 animals, ttx-3 (ttx-3promB::gfp, otIs173) and ceh-10 (ceh-10promA::gfp, lqIs4) expression is lost in AIY at larval stages (lateral view, anterior is left, dorsal is up, Amp: amphid support cell, scale bar = 20 μm).
(B) Expression of the same reporters is also lost in the AIY lineage in the embryo (ttx-3: epidermal enclosure, ventral view, anterior is left; ceh-10: 1.5-fold, lateral view, anterior is left, dorsal is up; AINm: AIN mother; Ant neu: anterior neurons; Pha: pharyngeal cell, scale bar = 10 μm).
(C) Percentage of animals which have lost reporter expression in the AIY lineage. As ot327 and gk178 are larval lethal, the F1 progeny of heterozygote mothers was analyzed. Around 1/4 of the progeny displays a mutant phenotype, as expected from Mendel’s laws. Embryos were scored between epidermal enclosure and 2-fold for ttx-3, and at 1.5-fold for ceh-10 (*: expression is absent from only one of the two AIY; §: in 3 to 4% of the animals expression is lost in only one of the two AIY).
(D) In wild type larvae AIY is located in a group of three neurons just posterior to the excretory cell (Exc) and expressing the pan-neuronal marker F25B3.3 (F25B3.3::dsRed2, otIs173, n=10). In ot327 homozygotes (recognized by the loss of ttx-3promB::gfp expression) a neuron is still present at the position normally occupied by AIY and still expresses F25B3.3::dsRed2 (n=11) (scale bar = 5 μm).
By genetic mapping, RNAi phenocopy and transformation rescue we find that ot327 affects the ref-2 gene (Figure S1A). This gene encodes the only C. elegans ortholog of the odd paired/Zic family of zinc finger transcription factors (Alper and Kenyon, 2002). Only one mutation in this locus has been reported so far, a dominant mutation, mu218, located 3′ to the coding region of ref-2 (Figure S1A; (Alper and Kenyon, 2002)). This allele causes overexpression of the REF-2 protein in a particular type of post-embryonic blast-cells, the P-cells, and subsequent problems in their fusion pattern (hence the name REF-2 for REgulator of Fusion). Sequencing of the ref-2 coding region from ot327 mutant animals reveals a G to A transition that converts a cysteine of the fourth zinc finger into a tyrosine (Figure S1B), which is expected to result in a loss of DNA binding activity of this zinc finger (Wolfe et al., 2000) and is thus predicted to be a strong loss-of-function allele. We also obtained a deletion allele for ref-2 from the C. elegans Gene Knockout Consortium (gk178, Figure S1A). This deletion physically removes the region coding for the first two zinc fingers, is expected to result in a frame shift eliminating the remaining three zinc fingers and is therefore a likely null allele. gk178, as ot327, is recessive, causes larval lethality and a loss of both ttx-3 and ceh-10 expression (Figure 1A,C), further confirming that ref-2 is required for the specification of AIY. The dominant allele mu218 has no effect on viability or ttx-3 expression suggesting that the presumptive ref-2 regulatory element affected in mu218 is not involved in AIY development (100% of mu218 larvae express ttx-3promB::gfp, otIs173, in AIY, n=50).
REF-2 initiates the expression of the TTX-3/CEH-10 complex
In order to better understand how ref-2 regulates the specification of the AIY interneuron we first analyzed its expression pattern. We inserted the venus yfp reporter into a fosmid containing the ref-2 locus as well as 15 kb of upstream and 10 kb of downstream sequences (covering also three upstream and two downstream predicted genes) (Figure 2A). Venus was inserted in frame just before the stop codon of ref-2. Injection of the ref-2::venus fusion fosmid rescues both the lethality and loss of ttx-3 expression of ref-2(ot327) mutant (3 independent lines) suggesting that all the cis-regulatory elements necessary for the role of ref-2 in AIY specification are present in this construct.
Figure 2. Sequential activation of ref-2, ttx-3, ceh-10 and the AIY motif.

(A) Expression pattern of a ref-2::venus rescuing translational fusion gene, observed with the transgenic array otEx3091. (i-i″) Expression in the SMDD/AIY mother (SMDD/AIYm) at the end of gastrulation (ventral view anterior is left, scale bar = 10 μm). (ii) Expression of REF-2::VENUS in the SMDD/AIY lineage (marked by ttx-3promB::mcherry, otIs181) before (interphase), during (mitosis) and after (post-mitosis) cleavage of the SMDD/AIY mother (scale bar = 2 μm). (iii, iii′) Expression in the excretory pore cell (Exc pore) and excretory gland (Exc gld) at L2 larval stage (lateral view, anterior is left, dorsal is up, scale bar = 10 μm). (iv) expression in the P, Y and B-cells at L1 larval stage (lateral view, anterior is left, dorsal is up, scale bar = 20 μm).
(B) Comparison of the expression of ttx-3 (ttx-3promB::gfp, mgIs18 or ttx-3promB::mcherry, otEx2644), ceh-10 (ceh-10promA::gfp, lqIs4) and the AIY motif (AIY motif::gfp, otEx1597) at the end of gastrulation (End gast., ventral view, anterior is left), 1.5- and 3-fold (lateral view, anterior is left). At 1.5-fold, the ttx-3 and ceh-10 pictures show colocalization of ttx-3promB::mcherry and ceh-10promA::gfp expression in AIY in the same embryo (scale bar = 10 μm).
ref-2::venus is detected in a substantial number of dividing cells in the embryo. At the end of gastrulation the reporter is detected in some neuroblasts on the ventral side of the embryo (Figure 2A-i). These neuroblasts include the left/right symmetric pair of ABpl/rpapaaa neuroblasts, which will give rise to AIY and its sister cell, the SMDD motor neuron through an asymmetric cell division (see experimental procedures for cell identification). During interphase, the REF-2::VENUS protein is detected in the nucleus of the SMDD/AIY mothers (Figure 2A-ii, interphase), then spreads into the cytoplasm just before cleavage (Figure 2A-ii, mitosis) and disappears from the two daughter cells, AIY and SMDD, after cleavage (Figure 2A-ii, post-mitosis). No expression is observed in AIY during larval and adult stages. Thus ref-2 appears to be expressed only transiently in the AIY lineage during embryogenesis suggesting that it may participate in the initiation of the AIY differentiation program rather than in the maintenance of AIY fate. REF-2::VENUS is also expressed in the excretory system (G1, G2, excretory pore and excretory gland, which may explain the larval lethality of ref-2 mutants), in the P-cell lineage and in the Y and B cells (Figure 2A-iii and 2A-iv).
To further investigate a potential role of ref-2 in initiating the expression of the TTX-3/CEH-10 selector complex, we first analyzed when exactly ttx-3 and ceh-10 start to be expressed in the AIY lineage during embryogenesis. ttx-3 expression was monitored with both a transcriptional gfp or mcherry fusion (ttx-3promB::gfp/mcherry) and a translational gfp fusion (ttx-3promA::gfp) in which all coding regions of ttx-3 are included and which rescues the ttx-3 null mutant phenotype ((Altun-Gultekin et al., 2001; Hobert et al., 1997), see Figure 4A and supplemental experimental procedures). To monitor ceh-10 expression we used both a gfp (ceh-10promA::gfp (Wenick and Hobert, 2004)) and a lacZ (ceh-10::lacZ (Svendsen and McGhee, 1995)) transcriptional fusion containing all the upstream 5′ regulatory regions required to rescue AIY developmental defects in a ceh-10 null mutant (see Figure 7 and supplemental experimental procedures). Finally, we also monitored ttx-3 and ceh-10 expression by assaying the activation of their binding site, the AIY motif, placed in front of gfp (Wenick and Hobert, 2004). ttx-3 starts to be expressed at the end of gastrulation in a pair of ventral neuroblasts (Figures 2A and 2B). By lineaging we determined that this corresponds to the left/right symmetric pair of SMDD/AIY mothers (ABpl/rpapaaa) before their last cleavage. At this time neither ceh-10 expression nor the AIY motif have been activated. After cleavage of the SMDD/AIY mother, the ttx-3 transcriptional and translational fusions are detected in both daughter cells AIY and SMDD (Figure 2B and data not shown), and ceh-10 starts to be expressed in AIY but not in SMDD (Figure 2B). Finally, during embryonic elongation, ttx-3 expression disappears from SMDD while the expression of ttx-3 and ceh-10 increases in AIY and the AIY motif starts to be activated in AIY (Figure 2B).
Figure 4. The initiation of ttx-3 expression relies on Zic and bHLH binding sites.

(A) Isolation of the cis-regulatory element responsible for the initiation of ttx-3 expression. For each deletion construct the activity in the AIY lineage is presented to the right (initiation: epidermal enclosure to 2-fold, maintenance: 3-fold to adult, purple box: AIY motif, scale in bp, see Figure S2 for quantification).
(B) Analysis of the initiator element (C4.4). The upper part presents the alignment of the initiator element sequence between C. elegans (C. ele), C. briggsae (C. bri), C. remanei (C. rem) and C. brenneri (C. bre). The graph presents the percentage of embryos showing expression in the SMDD/AIY lineage (SMDD/AIY, red), AIN lineage (AINm, green) or other lineages (mostly pharynx, grey). The F1 progeny of Rol mothers (positive for the coinjection marker rol-6(d)) was analyzed between epidermal enclosure and 2-fold stage. Three independent lines were scored for each construct (n=100, error bars = standard error to the proportion).
(C) Effect of hlh-2 RNAi on the initiation of ttx-3 expression. The table presents the percentage of embryos showing expression of ttx-3promB::gfp (mgIs18) in the SMDD/AIY lineage and AIN lineage during epidermal enclosure after RNAi treatment.
Figure 7. The initiation of ceh-10 expression relies on TTX-3 and POP-1 binding sites.

The upper part depicts the gfp reporter constructs tested relative to the ceh-10 locus (ceh-10 and its cis-regulatory sequences are located in introns of the polymerase gene polq-1 which is oriented in the opposite direction). The graph presents the percentage of animals showing expression in AIY at the 1.5-fold stage (initiation) or early larval stage (maintenance). Transgenic animals were recognized by the presence of reporter gene expression in the CAN lineage, which consistently expresses ceh-10. Three independent lines were scored for each construct (n=50, error bars = standard error to the proportion).
The early expression of ttx-3 in the SMDD/AIY mother before its last cleavage and in the newly born AIY and SMDD just after cleavage is unaffected in ttx-3 and in zygotic ceh-10 null mutants (Figures 3A and 3C), but is affected in ref-2 mutants (Figures 1B and 1C). In contrast, the later expression of ttx-3 in AIY at the end of embryogenesis (3-fold) and at larval stage genetically depends on ttx-3 and ceh-10 (Figures 3A and 3C). ttx-3 expression can therefore be separated into an initiation phase (dependent on ref-2 but independent of ttx-3 and ceh-10) and an automaintenance phase (dependent on ttx-3 and ceh-10). Similarly, the early expression of ceh-10 in AIY at the 1.5-fold stage (right after most cells in the embryo have been generated) is independent of zygotic ceh-10 (initiation phase) while the later expression at the end of embryogenesis (3-fold) and larval stage depends on ceh-10 and ttx-3 (automaintenance phase) (Figures 3B and 3C). Notably, the initiation of ceh-10 expression is strongly reduced in ttx-3 null mutants (Figures 3B and 3C; in contrast, the hypomorphic ttx-3 allele ks5 does not affect ceh-10 expression (Altun-Gultekin et al., 2001)). Therefore, ttx-3, which is expressed in AIY before ceh-10, participates in the initiation of ceh-10 expression.
Figure 3. Initiation and automaintenance phases of ttx-3 and ceh-10.
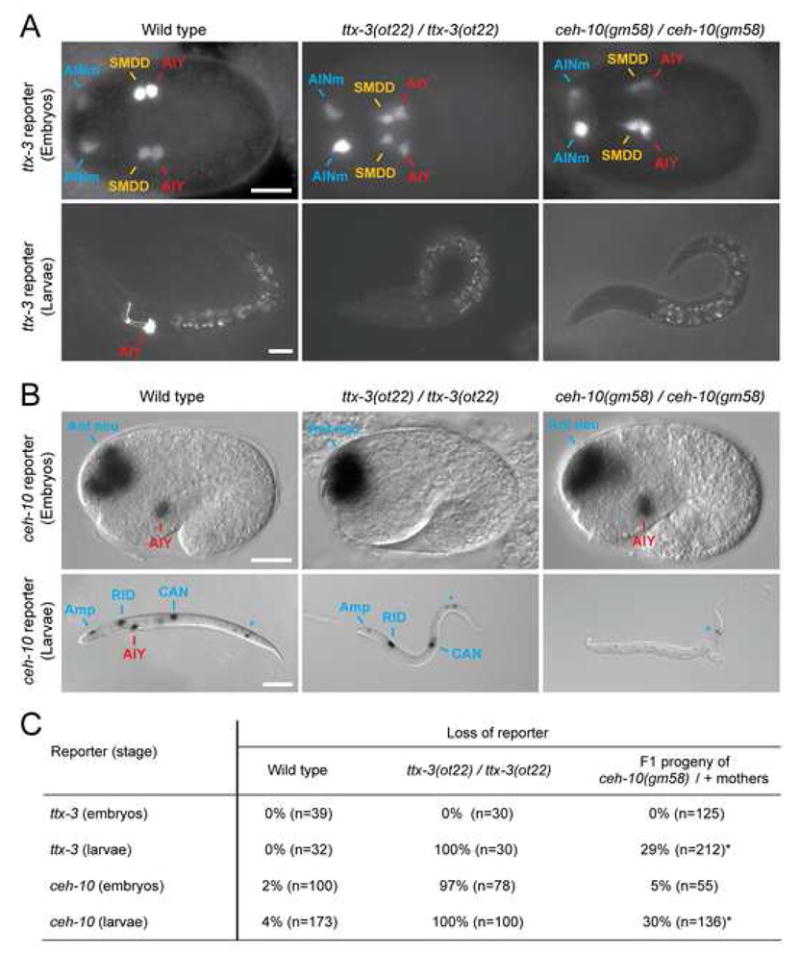
(A) Effect of ttx-3(ot22) and ceh-10(gm58) alleles on ttx-3 expression (ttx-3promB::gfp, mgIs18) at epidermal enclosure (ventral view, anterior to the left, AINm = AIN mother, scale bar = 10 μm) and larval stage (lateral view, anterior to the left, dorsal to the top, scale bar = 20 μm).
(B) Effect of ttx-3(ot22) and ceh-10(gm58) alleles on ceh-10 expression (ceh-10::lacZ, pInt1) at 1.5-fold and larval stage (lateral view, anterior to the left, dorsal to the top, Ant neu: anterior neurons; Amp: amphid support cell; *: variable expression in the posterior intestine, scale bar = 10 μm for 1.5-fold and 50 μm for larva).
(C) Percentage of animals which have lost, in the AIY lineage, expression of ttx-3 (ttx-3promB::gfp, mgIs18; embryos were scored between epidermal enclosure and 2-fold) or ceh-10 (ceh-10::lacZ, pInt1; embryos were scored at 1.5-fold). As ceh-10(gm58) is larval lethal, the F1 progeny of heterozygote mothers was analyzed (*: around 1/4 of the progeny displays a mutant phenotype, as expected from Mendel’s laws). A very dim GFP signal was detected in some ttx-3(ot22); ttx-3promB::gfp larvae and in some ceh-10(gm58); ttx-3promB::gfp larvae which may reflect perdurance of GFP signal from earlier expression.
Predicted REF-2 and HLH-2 binding sites are required for ttx-3 expression in the SMDD/AIY mother
The REF-2 protein is present in the SMDD/AIY mother when ttx-3 starts to be expressed and is required for the initiation of ttx-3 expression, making it conceivable that REF-2 may directly initiate ttx-3 expression by binding to its promoter. To test this notion, we analyzed the regulatory regions of the ttx-3 locus using transgenic reporter assays. By deletion analysis we isolated a 113 bp cis-regulatory element that is sufficient for the initiation of ttx-3 expression in the SMDD/AIY mother (construct C4.4, Figure 4A and S2). Notably, this initiator element does not drive the later automaintenance phase (end of embryogenesis to adulthood), which relies on an intronic cis-regulatory element containing the AIY motif (construct K, Figure 4A, (Wenick and Hobert, 2004)), demonstrating that these two phases of expression can not only be separated genetically (in ref-2 versus ttx-3 or ceh-10 mutant backgrounds) but can also be uncoupled at the cis-regulatory level.
Alignment of the initiator element sequence from four Caenorhabditis species reveals the presence of a block of 12 perfectly conserved nucleotides that displays strong similarity with the in vitro defined binding site for the Zic transcription factor Macho-1 in Ciona intestinalis (Yagi et al., 2004) and an in vivo binding site for Zic1 in mouse (Ebert et al., 2003) (Figure 4B). As REF-2 is the only Zic transcription factor in the C. elegans genome, this site is likely to constitute a direct binding site for REF-2. Altering 3 base pairs in this binding site indeed causes a loss of the activity of the initiator element in the SMDD/AIY lineage (Figure 4B), thereby phenocopying the effect of a loss of function of the REF-2 protein (Figure 1B). Taken together our experiments suggest that REF-2 directly activates ttx-3 expression in the SMDD/AIY mother.
The initiator element also contains three putative bHLH binding sites, one perfectly conserved in all 4 nematode species and two less well conserved (Figure 4B, (Ledent and Vervoort, 2001)). Point mutations in all three binding sites also result in a loss of the activity of the initiator element in the SMDD/AIY lineage (Figure 4B). The bHLH factor HLH-2, an orthologue of E/Daughterless, has been reported to be transiently expressed in most ventral neuroblasts at the time when ttx-3 starts to be expressed in the SMDD/AIY mother (Krause et al., 1997). A knock-down of hlh-2 by RNAi phenocopies the effect of the bHLH binding sites mutations on the initiation of ttx-3 expression (Figure 4C), suggesting that REF-2 and HLH-2 cooperate to directly activate ttx-3 expression in the SMDD/AIY mother.
The Wnt/β-catenin asymmetry pathway cooperates with TTX-3 to initiate ceh-10 expression in postmitotic AIY
Just after the anterior-posterior cleavage of the SMDD/AIY mother ceh-10 expression is activated in AIY but not SMDD (Figure 2B) and ceh-10 not only activates the AIY terminal differentiation program (Wenick and Hobert, 2004) but also represses SMDD fate (Figure S3). Therefore the activation of ceh-10 expression specifically in AIY is a key step in the AIY versus SMDD cell fate choice. REF-2-induced TTX-3 is present in both AIY and SMDD just after the terminal division of the SMDD/AIY mother but activates ceh-10 expression only in AIY. What is the molecular mechanism that ensures the differential activity of TTX-3 in AIY versus SMDD? A Wnt signaling pathway, termed the Wnt/β-catenin asymmetry pathway, is involved in many antero-posterior asymmetric divisions during early embryogenesis in C. elegans as well as during several postembryonic cell divisions (Kaletta et al., 1997; Lin et al., 1998; Mizumoto and Sawa, 2007). Most neurons, including AIY, are born during mid-embryogenesis (between the end of gastrulation and epidermal enclosure) (Sulston et al., 1983) and it is not known whether the Wnt/β-catenin asymmetry pathway is also involved in these terminal neuroblast divisions.
In those cases previously studied, the Wnt/β-catenin asymmetry pathway establishes a reciprocal nuclear asymmetry of the TCF transcription factor POP-1 and its co-activator SYS-1/β-catenin in the two daughters of an asymmetric cell division (Mizumoto and Sawa, 2007) (Figure 5A). This pathway regulates both POP-1 and SYS-1 at a post-translational level. In the posterior daughter, low levels of POP-1 allow most POP-1 to bind to SYS-1/β-catenin and to activate gene expression, while in the anterior daughter a surplus of POP-1 compared to SYS-1 results in POP-1 repressing gene expression (Figure 5A). To test whether this pathway may be involved in determining the difference between AIY and its sister SMDD we first analyzed the expression of POP-1::GFP and SYS-1::VENUS translational fusions during AIY development (Phillips et al., 2007; Siegfried et al., 2004). We find that just after the terminal division of the SMDD/AIY mother, POP-1::GFP is more concentrated in the SMDD nucleus (anterior sister) than in the AIY nucleus (posterior) while SYS-1::VENUS shows an opposite distribution (Figure 5B). These POP-1 and SYS-1 asymmetries disappear rapidly after cell division (the asymmetry being no longer detectable at the 1.5-fold stage) suggesting that any potential POP-1 regulatory input is transient. To test whether this POP-1 asymmetry is indeed responsible for the difference between AIY and its sister, we used temperature-sensitive alleles of the kinase genes mom-4 (an ortholog of TAK1) and lit-1 (an ortholog of NLK) (Takeshita and Sawa, 2005). MOM-4 and LIT-1 are required for the Wnt signaling-dependent export of POP-1 from the posterior sister nucleus (Figure 5A) and a loss of function of mom-4 and lit-1 leads to an accumulation of POP-1 in the posterior sister nucleus to a level similar to the one normally observed in the anterior sister (Mizumoto and Sawa, 2007). We used the temperature-sensitive double mutant mom-4(ne1539); lit-1(t1512) as it disrupts the Wnt/β-catenin asymmetry pathway more efficiently than the single mutants (Takeshita and Sawa, 2005). mom-4(ne1539); lit-1(t1512) embryos were shifted to the restrictive temperature just before the cleavage of the SMDD/AIY mother (see experimental procedures for details). In shifted mutant embryos ttx-3 is expressed in the SMDD/AIY mother and in both SMDD and AIY just after cleavage as in wild-type embryos, but ceh-10 expression fails to be initiated in the newly born AIY and ttx-3 expression fails to be maintained in AIY during elongation and larval stage (Figures 5C and 5D). This suggests that the Wnt/-catenin asymmetry pathway is indeed responsible for restricting ceh-10 expression to AIY therefore establishing the difference between SMDD and AIY during the terminal division.
Figure 5. The Wnt/-catenin pathway is involved in the asymmetric division of the SMDD/AIY mother.

(A) General diagram of the Wnt/β-catenin asymmetry pathway. Only the components used in this study are presented, for a more complete description of the pathway see Mizumoto and Sawa (2007). Upon stimulation of a Wnt receptor Frizzled, the kinases MOM-4/TAK-1 and LIT-1/NLK are activated in the posterior daughter cell. LIT-1 enters the nucleus and phosphorylates POP-1/TCF promoting its nuclear export. As a consequence POP-1 concentration is low in the posterior nucleus allowing most of the POP-1 proteins to be associated with the limiting coactivator SYS-1/β-catenin and activate transcription, while in the anterior nucleus POP-1 concentration is high, most of the POP-1 proteins are not associated with SYS-1 and therefore repress transcription.
(B) Localization of POP-1::GFP (qIs74) or SYS-1::VENUS (qIs95) fusion proteins just after cleavage of the SMDD/AIY mother (epidermal enclosure, ventral view, anterior to the left, SMDD/AIY lineage marked by ttx-3promB::mcherry, otIs181 or otEx2644).
(C) Expression of ttx-3 (ttx-3promB::gfp, mgIs18) in wild type or mom-4(ne1539); lit-1(t1512) temperature-sensitive mutants. Embryos were shifted to the restrictive temperature just before cleavage of the SMDD/AIY mother and analyzed at the 1.5-fold stage or at hatching (lateral view, anterior to the left). The results are summed up at the bottom (red = ttx-3 expression, numbers = number of animals showing expression in the cell depicted/total number of animals).
(D) Expression of ceh-10 (ceh-10promA::gfp, lqIs4) in wild type or mom-4(ne1539); lit-1(t1512) temperature-sensitive mutants. Embryos were shifted to the restrictive temperature just before cleavage of the SMDD/AIY mother and analyzed at the 1.5-fold stage (lateral view, anterior to the left). The results are summed up at the bottom (blue = ceh-10 expression, numbers = number of embryos showing expression in the cell depicted/total number of embryos). Scale bar for all panels = 2 μm.
We then tested whether the Wnt/β-catenin asymmetry pathway could be used in an iterative manner in the AIY lineage by analyzing the effect of manipulating this pathway one cell division earlier, at the stage of formation of the SMDD/AIY mother, in which ttx-3 expression is initially activated (see above). During gastrulation, the ABpl/rpapaa neuroblast divide to give rise to the SMDD/AIY mother anteriorly and the SIAD/SIBV mother posteriorly (Figure 6A). Removal of mom-4 and lit-1 activities just before the cleavage of the ABpl/rpapaa neuroblast leads to the ectopic activation of ttx-3 (which is normally restricted to the anterior SMDD/AIY mother) in the SIAD/SIBV lineage. This suggests that the Wnt/β-catenin asymmetry pathway is also involved in the division that generates the SMDD/AIY mother (Figure 6A).
Figure 6. The Wnt/-catenin pathway is involved in the asymmetric division of other embryonic neuroblasts.

(A) Effect of mom-4(ne1539); lit-1(t1512) temperature-sensitive mutants on the asymmetric division of the ABpl/rpapaa neuroblast. This division generates the SMDD/AIY mother anteriorly and the SIAD/SIBV mother posteriorly. ttx-3 (red) is expressed in the SMDD/AIY mother and the newly born SMDD and AIY but not in the SIAD/SIBV mother or the newly born SIAD and SIBV. Embryos were shifted to the restrictive temperature just before cleavage of the ABpl/rpapaa neuroblast and analyzed at the end of epidermal enclosure (just after the birth of SMDD, AIY, SIAD and SIBV; ventral view, anterior to the left, numbers = number of embryos showing ttx-3promB::gfp, mgIs18 expression in the newly born SMDD and AIY only (left column) or in both the newly born SMDD, AIY and the newly born SIAD, SIBV (right column) over total number of embryos analyzed).
(B) Effect of sys-1/-catenin and mom-5/frizzled overexpression on the asymmetric division of the ABpl/rpapaa neuroblast. The graph presents the percentage of embryos showing expression of ttx-3promB::gfp, mgIs18 in the SMDD/AIY lineage but not the SIAD/SIBV lineage (grey) or no expression in those lineages (black). The F1 progeny of control mothers or Rol mothers (positive for the coinjection marker rol-6(d) that marks the sys-1 and mom-5 overexpression arrays) was analyzed between the end of epidermal enclosure and the 1.5-fold stage. Three independent lines were scored for each construct (n>50, error bars = standard error to the proportion).
(C) Effect of mom-4(ne1539); lit-1(t1512) temperature-sensitive mutants on the asymmetric division of the AIN mother. This division generates a cell death (rounded shape) and the AIN neuron (elongated shape), both marked by ttx-3promB::gfp expression (red). Although this division is left-right oriented, the daughter that will eventually die is located more anteriorly just after cleavage and displays a higher POP-1 nuclear level than its sister cell AIN. Embryos were shifted to the restrictive temperature just before cleavage of the AIN mother and analyzed at the 1.5-fold stage (lateral view, numbers = number of embryos showing a cell death + neuron pair (left column) or a cell death + cell death pair (right column) over total number of embryos analyzed).
(D) Effect of mom-4(ne1539); lit-1(t1512) temperature-sensitive mutants on the asymmetric division of the ASER mother. This division generates the ASER neuron anteriorly (marked by gcy-5::gfp, ntIs1 expression, red) and a cell death posteriorly. Embryos were shifted to the restrictive temperature just before cleavage of the ASER mother and analyzed at hatching (lateral view, anterior to the left, numbers = number of embryos showing only one ASER neuron (left column) or a pair of ASER neurons (right column) over total number of embryos analyzed). Scale bar for all panels = 2 μm.
To confirm this result we performed the reciprocal experiment by analyzing the effect of ectopically activating the Wnt/β-catenin asymmetry pathway in the SMDD/AIY mother, through over-expressing the β-catenin, SYS-1 or the Frizzled receptor, MOM-5, using the ttx-3 initiator element as a driver (Figures 5A and 6B). Forced expression of either protein has been shown to cause the activation of this pathway in other cellular contexts (Kidd et al., 2005; Park et al., 2004). As expected, overexpression of either SYS-1 or MOM-5 in the SMDD/AIY mother leads to loss of the identity of this neuroblast, as assessed by a loss of ttx-3 expression (Figure 6B), a phenotype opposite to the one observed when the pathway is blocked in the posterior cell (Figure 6A). This experiments also indicates that the Wnt/β-catenin asymmetry pathway acts autonomously within the SMDD/AIY lineage.
Predicted POP-1/TCF and TTX-3 binding sites are required for the initiation of ceh-10 expression in postmitotic AIY
The initiation of ceh-10 expression in postmitotic AIY is dependent on both TTX-3 and the Wnt/β-catenin asymmetry pathway. We therefore tested whether these two signals could be directly integrated at the level of the ceh-10 promoter. POP-1 has been shown to bind in vitro and in vivo to sites matching the general TCF consensus sequence A/T A/T CAAA G/A (Arata et al., 2006; Korswagen et al., 2000; Lam et al., 2006; Maduro et al., 2005). A 3.7 kb region upstream of ceh-10 that is sufficient to rescue AIY developmental defects of ceh-10 mutants when fused to ceh-10 coding sequences (see supplemental experimental procedures) contains 27 putative POP-1/TCF binding sites (Figure 7). This is significantly higher than what would have been expected by random (p=0.0028, comparison with a Markov model order 2). Point mutations or deletions of predicted POP-1 binding sites reduce the initiation of ceh-10 expression in the embryo while they do not affect the maintenance of the expression at larval stage (Figure 7), suggesting that POP-1 directly participate to the initiation of ceh-10 expression but has no role in its maintenance. The AIY motif is a low affinity binding site for TTX-3 alone and is bound by the TTX-3/CEH-10 complex with higher affinity (Wenick and Hobert, 2004). Mutating the AIY motif present in the ceh-10 promoter strongly reduces the initiation of ceh-10 expression in the embryo (Figure 7) suggesting that TTX-3 may directly initiate ceh-10 expression by initially binding to the AIY motif on its own. Mutating the AIY motif also completely eliminates the maintenance of ceh-10 expression at larval stage, reflecting its later role as an automaintenance motif by the TTX-3/CEH-10 complex. Taken together these data suggest that the lineage specific transcription factor TTX-3 and the Wnt/β-catenin asymmetry pathway are directly integrated at the level of the ceh-10 promoter.
The Wnt/β-catenin asymmetry pathway is widely used to regulate the terminal division of embryonic neuroblasts
Finally, we tested whether the Wnt/β-catenin asymmetry pathway is also involved in the terminal division of other embryonic neuroblasts by analyzing the effect of a precisely-timed loss of mom-4 and lit-1 activity on two other lineally unrelated neuronal lineages, one giving rise to the AIN interneuron, another to the ASER sensory neuron (Figure 6C and 6D). Removal of mom-4 and lit-1 activities just before the terminal division of the AIN or ASER mothers affects the generation of the AIN and ASER neurons (Figure 6C and 6D). This suggests that the Wnt/β-catenin asymmetry pathway is widely used to regulate the terminal division of embryonic neuroblasts, therefore the regulatory logic that we establish here, connecting asymmetric division to the terminal differentiation program in the case of the AIY interneuron, is likely to be used also by other embryonic neurons.
DISCUSSION
Several examples have by now well illustrated that the differentiation of individual neuron types is governed by terminal selector genes, that encode transcription factors which directly activate large batteries of terminal differentiation genes (Etchberger et al., 2007; Hobert, 2008; Livesey et al., 2000; Wenick and Hobert, 2004; Zhang et al., 2002). However, how these terminal selector genes are regulated by earlier specification processes, in particular asymmetric divisions, remains poorly understood. Here we have uncovered a direct regulatory cascade that links the asymmetric division machinery to the activation of the terminal selector genes ttx-3 and ceh-10 during embryogenesis in C. elegans (Figure 8A). We will first discuss our results in the context of the broad concept of progressive regulatory states, before analyzing two other general implications of our studies, namely, a common theme of Zic gene function in neural precursors and a potentially broadly conserved role of Wnt signaling in neuronal specification.
Figure 8. A model for the initiation of the terminal differentiation program of the AIY interneuron.
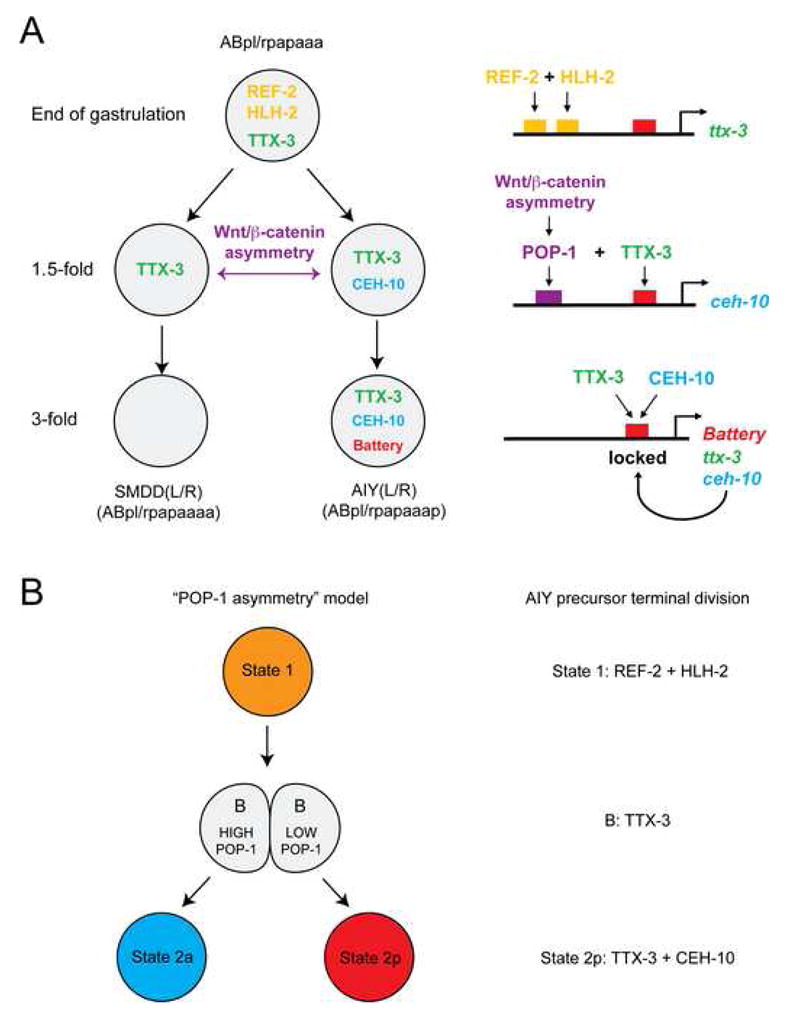
(A) Summary of the regulatory events occurring upon terminal division of the SMDD/AIY mother. Note that arrows between transcription factors and cis-regulatory regions represent likely direct interactions based on the fact that a loss of function of the trans-acting factor is phenocopied by the mutation of its predicted binding site(s) in the respective cis-regulatory region.
(B) The terminal division of AIY can be put into a previously proposed theoretical framework (Lin et al., 1998) describing how the “POP-1 asymmetry” can generate specific regulatory states. Note that the putative transcription factor “B” corresponds to TTX-3 in the case of AIY.
Regulatory states that define the terminal differentiation program of the cholinergic interneuron AIY
The Zic transcription factor REF-2 is transiently expressed in the SMDD/AIY mother where it directly activates the expression of the ttx-3 LIM homeobox gene in cooperation with the bHLH transcription factor HLH-2. Following division of the mother cell, TTX-3 is inherited in both SMDD and AIY and activates ceh-10 expression in AIY but not in SMDD. We have shown here that the difference in ttx-3 activity between AIY and SMDD is due to the Wnt/β-catenin asymmetry pathway. The transcriptional mediators of this pathway, the TCF transcription factor POP-1 and its coactivator the β-catenin SYS-1, are asymmetrically localized after division of the SMDD/AIY mother. In AIY, the POP-1 nuclear concentration is low and SYS-1 concentration is high. This may allow most of the POP-1 proteins to be associated with the coactivator SYS-1 and to activate the transcription of ceh-10 via the predicted POP-1 binding sites present in its promoter. In SMDD, the POP-1 nuclear concentration is high and SYS-1 concentration is low, most of the POP-1 proteins may not be associated with SYS-1 and therefore repress ceh-10 transcription. Finally, once coexpressed in postmitotic AIY, TTX-3 and CEH-10 directly activate a large battery of terminal differentiation genes responsible for AIY differentiation and specific function. TTX-3 and CEH-10 also maintain their own expression so that the system is locked during larval and adult stage (this study, (Wenick and Hobert, 2004)).
It has been proposed that during development a cell progresses through a succession of “regulatory states”, each characterized by a combination of specific gene regulatory factors (Davidson et al., 2003). In the case of the AIY terminal division two regulatory states are observed. The first one (state 1) is characterized by the transient expression of REF-2 and HLH-2 in the SMDD/AIY mother (Figure 8B). The second (state 2p) corresponds to the terminal differentiation state defined by the expression of the terminal complex TTX-3/CEH-10 and the battery of terminal differentiation genes. The transition between those two states is driven by a binary decision system based on the Wnt/β-catenin asymmetry pathway.
Our findings provide explicit support for a theoretical model initially proposed by Priess and co-workers (Lin et al., 1998). In this model a transcription factor “B” is expressed in both daughter cells following the division, cooperates with a high POP-1 level in the anterior cell to specify state 2a and cooperates with a low POP-1 level in the posterior cell to specify state 2p (Figure 8B). In the case of AIY, this lineage specific factor “B” corresponds to the transcription factor TTX-3.
Zic transcription factors and neuroblast development
Before discussing general principles of Wnt/β-catenin signaling in neuronal specification, we would like to briefly discuss one specific member of the regulatory network that we have studied here, ref-2. We have shown that ref-2 is expressed in several neuronal precursors in the embryo; in contrast, there is no detectable expression of ref-2 in postmitotic neurons at larval and adult stages. Similarly, in Hydra and vertebrates, Zic transcription factors are also expressed in several neural progenitors while expression in adult postmitotic neurons is only rarely seen (Aruga, 2004; Merzdorf, 2007). This indicates that Zic transcription factors may have a conserved function in neural precursor development. While in vertebrates Zic transcription factors have been shown to play a role in promoting the proliferation of the progenitors (Aruga, 2004; Merzdorf, 2007), it is conceivable that they also function as transient initiators of the terminal differentiation program of specific neurons, as observed here in the case of AIY. For example an intriguing parallel can be drawn between the development of the AIY interneurons and the cholinergic projection neurons/interneurons of the vertebrate basal forebrain. These vertebrate cholinergic neurons have an important function in memory formation (Oda, 1999), as is the case for the cholinergic interneuron AIY. In vertebrates, these postmitotic neurons and their progenitors express the TTX-3-related LIM-homeodomain transcription factor Lhx7/8, which is required for their differentiation (Asbreuk et al., 2002; Zhao et al., 2003). It has been recently reported that the Zic transcription factors Zic1 and Zic3 are also expressed in these progenitors and that inactivation of both genes reduces the number of cholinergic neurons (Inoue et al., 2007). While these Zic factors seem to regulate primarily the proliferation of the precursors it would be interesting to test whether, in analogy to ttx-3 initiation by REF-2, they also initiate the expression of Lhx7/8 and endow the progenitors with the ability to generate cholinergic neurons.
Wnt signaling and specification of the embryonic nervous system
A particular Wnt pathway, the Wnt/β-catenin asymmetry pathway, is involved in many asymmetric blastomere divisions in the early embryo as well as some asymmetric divisions during larval development in C. elegans (Hawkins et al., 2005; Kaletta et al., 1997; Lin et al., 1998; Mizumoto and Sawa, 2007; Siegfried and Kimble, 2002). Analysis of temperature-sensitive mutants of the upstream kinase gene lit-1(t1512) has shown that this pathway is involved in six successive asymmetric division rounds in the early embryo (Kaletta et al., 1997). However, this pathway has not been shown so far to be implicated in the terminal division of embryonic neuroblasts. We have observed that the three terminal neuroblast divisions that we analyzed (giving rise to AIY, AIN and ASER respectively) are affected by disrupting this Wnt pathway. Moreover, lit-1(t1512); mom-4(ne1539) embryos shifted at restrictive temperature just before most embryonic neuroblasts undergo their last division give rise to larvae showing strongly uncoordinated movements, suggesting additional defects in motor neuron lineages. These observations predict that the Wnt/β-catenin asymmetry pathway is widely used in terminal neuroblast division in the C. elegans embryo. Our findings therefore extend the binary specification model proposed by Kaletta and colleagues (Kaletta et al., 1997) and the POP-1 “coordinate system” proposed by Lin and colleagues (Lin et al., 1998).
While we have shown that the transcriptional mediators of this pathway, POP-1/TCF and SYS-1/β-catenin, are asymmetrically localized after the terminal division of embryonic neuroblasts, how the asymmetry in this pathway is initially established remains obscure. Both POP-1 and SYS-1 are regulated by this pathway at a post-translational level (Mizumoto and Sawa, 2007). In the early embryo POP-1 asymmetry in the AB lineage requires an initial MOM-2/Wnt signal coming from the P1 lineage (Park and Priess, 2003) that may be transmitted among AB blastomeres by a relay mechanism (Bischoff and Schnabel, 2006), but POP-1 asymmetry becomes later independent of MOM-2/Wnt (Huang et al., 2007; Park and Priess, 2003). MOM-5/Frizzled is enriched in the posterior pole of early AB derivatives and in analogy to the planar cell polarity in Drosophila, a Wnt-independent asymmetric Frizzled localization could be responsible for generating asymmetric cell divisions (Park et al., 2004). Additional studies on Wnt-requirement and Frizzled localization are required to assess their mode of function in the context of the terminal division of embryonic neuroblasts.
Neurons are also generated via asymmetric divisions in Drosophila and vertebrates (Huttner and Kosodo, 2005; Wodarz and Huttner, 2003). Recent results suggest a possible role for β-catenin in the asymmetric division of neural progenitors in the mouse brain (Doe, 2008; Knoblich, 2008). For example it has been proposed that β-catenin may regulate the asymmetric division generating intermediate progenitors from radial glial cells during corticogenesis (Woodhead et al., 2006; Wrobel et al., 2007). A Wnt/β-catenin system, similar to the one shown here to operate in terminal neuroblast divisions in C. elegans, may therefore be used in binary cell fate decisions during the development of the nervous system in other organisms.
EXPERIMENTAL PROCEDURES
Genetics
ot327 was isolated from a conventional genetic screen in which we used EMS to mutagenize a wild-type strain that carries a ttx-3::gfp transgene (mgIs18) (V.B. and O.H. unpublished). We retrieved several complementation groups in which ttx-3::gfp expression is defective and focus here on the ref-2 allele ot327. Both ot327 and gk178 (kindly provided by the Vancouver knockout consortium) were backcrossed 5 times before phenotypic characterization.
Expression constructs and transgenic strains
The making of constructs and transgenic strains is described in detail in the supplemental data section.
Xgal staining
The activity of the β-galactosidase reporter in pInt1, ceh-10::lacZ (Svendsen and McGhee, 1995) integrated animals was analyzed following the classic drying (for larvae) or freeze-cracking (for embryos) protocols as previously described (Fire, 1992).
RNAi
RNAi was performed in a sensitized rrf-3 mutant background (Simmer et al., 2003). ttx-3promB::gfp (mgIs18); rrf-3 (pk1426) hermaphrodites at L4 stage were grown at 20°C on bacteria harboring a plasmid to express dsRNA (pKM1196 for hlh-2 (Karp and Greenwald, 2003) or J. Ahringer library for other clones) as described (Fraser et al., 2000) and the F1 progeny was scored.
Temperature shifts
lit-1(t1512); mom-4(ne1539) double mutants and control strains were grown at 11°C. For temperature shifts, 2-cell stage embryos were mounted on slides at 4°C and incubated at 11°C until they reached the desired stage. They were then shifted to 25°C and maintained at 25°C until scoring. Embryos were shifted at 180 min in Sulston timetable (Sulston et al., 1983) for the ABpl/rpapaa neuroblast, 250 min for the SMDD/AIY mother and AIN mother, and 280 min for the ASER mother, corresponding respectively to 600 min, 840 min and 930 min after 2-cell stage at 11°C.
Cell identifications
The cells expressing ttx-3 at epidermal enclosure where identified as the SMDD/AIY mother (followed by its daughters SMDD and AIY after cleavage) and the AIN mother (followed by its daughters AIN and dying sister cell after cleavage) by lineaging both ttx-3promB::gfp (mgIs18) and ttx-3promA::gfp (otEx57) embryos. Embryos were mounted at the 2-cell stage and their development recorded by 4D-DIC-videomicroscopy (Schnabel et al., 1997) until epidermal enclosure when GFP expression was also recorded. The GFP positive cells were then lineaged using the Simi Biocell software (Simi GmbH). Expression of ref-2::venus (otEx3091, otEx3092 and otEx3093), ceh-10promB::gfp (lqIs4), pop-1::gfp (qIs74) and sys-1::venus (qIs95) was subsequently monitored in the AIY and AIN lineages by colabelling with ttx-3promB::mcherry (otIs181 and otEx2644). At larval and adult stages AIY was identified under DIC by its stereotypical location in a group of 3 neurons aligned posteriorly to the excretory cell nucleus (Sulston et al., 1983).
Supplementary Material
Acknowledgments
We thank Q. Chen for expert injection assistance, C. Benard, N. Bravo and J. Recio for help with the genetic screen, H. Bigelow for help with statistical analysis, the Caenorhabditis Genetics Center, the C. elegans Gene Knockout Consortium in Vancouver, S. Alper, J. Kimble and R. Lin for strains and reagents, H. Sawa for suggesting the use of the lit-1(ts); mom-4(ts) double and I. Greenwald, R. Mann, P. Sengupta, M. Chalfie and members of the Hobert lab for comments on the manuscript. This work was funded by the National Institutes of Health (R01NS039996-05; R01NS050266-03), the Howard Hughes Medical Institute and postdoctoral fellowships by the EMBO and HFSPO to V.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alper S, Kenyon C. The zinc finger protein REF-2 functions with the Hox genes to inhibit cell fusion in the ventral epidermis of C. elegans. Development. 2002;129:3335–3348. doi: 10.1242/dev.129.14.3335. [DOI] [PubMed] [Google Scholar]
- Altun-Gultekin Z, Andachi Y, Tsalik EL, Pilgrim D, Kohara Y, Hobert O. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development. 2001;128:1951–1969. doi: 10.1242/dev.128.11.1951. [DOI] [PubMed] [Google Scholar]
- Arata Y, Kouike H, Zhang Y, Herman MA, Okano H, Sawa H. Wnt signaling and a Hox protein cooperatively regulate psa-3/Meis to determine daughter cell fate after asymmetric cell division in C. elegans. Dev Cell. 2006;11:105–115. doi: 10.1016/j.devcel.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Aruga J. The role of Zic genes in neural development. Mol Cell Neurosci. 2004;26:205–221. doi: 10.1016/j.mcn.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Asbreuk CH, van Schaick HS, Cox JJ, Kromkamp M, Smidt MP, Burbach JP. The homeobox genes Lhx7 and Gbx1 are expressed in the basal forebrain cholinergic system. Neuroscience. 2002;109:287–298. doi: 10.1016/s0306-4522(01)00466-3. [DOI] [PubMed] [Google Scholar]
- Bischoff M, Schnabel R. A posterior centre establishes and maintains polarity of the Caenorhabditis elegans embryo by a Wnt-dependent relay mechanism. PLoS Biol. 2006;4:e396. doi: 10.1371/journal.pbio.0040396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EH, McClay DR, Hood L. Regulatory gene networks and the properties of the developmental process. Proc Natl Acad Sci U S A. 2003;100:1475–1480. doi: 10.1073/pnas.0437746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development. 2008;135:1575–1587. doi: 10.1242/dev.014977. [DOI] [PubMed] [Google Scholar]
- Ebert PJ, Timmer JR, Nakada Y, Helms AW, Parab PB, Liu Y, Hunsaker TL, Johnson JE. Zic1 represses Math1 expression via interactions with the Math1 enhancer and modulation of Math1 autoregulation. Development. 2003;130:1949–1959. doi: 10.1242/dev.00419. [DOI] [PubMed] [Google Scholar]
- Etchberger JF, Lorch A, Sleumer MC, Zapf R, Jones SJ, Marra MA, Holt RA, Moerman DG, Hobert O. The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev. 2007;21:1653–1674. doi: 10.1101/gad.1560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A. Histochemical techniques for locating Escherichia coli beta-galactosidase activity in transgenic organisms. Genet Anal Tech Appl. 1992;9:151–158. doi: 10.1016/1050-3862(92)90042-4. [DOI] [PubMed] [Google Scholar]
- Forrester WC, Perens E, Zallen JA, Garriga G. Identification of Caenorhabditis elegans genes required for neuronal differentiation and migration. Genetics. 1998;148:151–165. doi: 10.1093/genetics/148.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Guenther C, Garriga G. Asymmetric distribution of the C. elegans HAM-1 protein in neuroblasts enables daughter cells to adopt distinct fates. Development. 1996;122:3509–3518. doi: 10.1242/dev.122.11.3509. [DOI] [PubMed] [Google Scholar]
- Hawkins NC, Ellis GC, Bowerman B, Garriga G. MOM-5 frizzled regulates the distribution of DSH-2 to control C. elegans asymmetric neuroblast divisions. Dev Biol. 2005;284:246–259. doi: 10.1016/j.ydbio.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Hobert O. Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc Natl Acad Sci U S A. 2008;105:20067–20071. doi: 10.1073/pnas.0806070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O, Mori I, Yamashita Y, Honda H, Ohshima Y, Liu Y, Ruvkun G. Regulation of interneuron function in the C. elegans thermoregulatory pathway by the ttx-3 LIM homeobox gene. Neuron. 1997;19:345–357. doi: 10.1016/s0896-6273(00)80944-7. [DOI] [PubMed] [Google Scholar]
- Huang S, Shetty P, Robertson SM, Lin R. Binary cell fate specification during C. elegans embryogenesis driven by reiterated reciprocal asymmetry of TCF POP-1 and its coactivator beta-catenin SYS-1. Development. 2007;134:2685–2695. doi: 10.1242/dev.008268. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol. 2005;17:648–657. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Inoue T, Ota M, Ogawa M, Mikoshiba K, Aruga J. Zic1 and Zic3 regulate medial forebrain development through expansion of neuronal progenitors. J Neurosci. 2007;27:5461–5473. doi: 10.1523/JNEUROSCI.4046-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletta T, Schnabel H, Schnabel R. Binary specification of the embryonic lineage in Caenorhabditis elegans. Nature. 1997;390:294–298. doi: 10.1038/36869. [DOI] [PubMed] [Google Scholar]
- Karp X, Greenwald I. Post-transcriptional regulation of the E/Daughterless ortholog HLH-2, negative feedback, and birth order bias during the AC/VU decision in C. elegans. Genes Dev. 2003;17:3100–3111. doi: 10.1101/gad.1160803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd AR, 3rd, Miskowski JA, Siegfried KR, Sawa H, Kimble J. A beta-catenin identified by functional rather than sequence criteria and its role in Wnt/MAPK signaling. Cell. 2005;121:761–772. doi: 10.1016/j.cell.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Korswagen HC, Herman MA, Clevers HC. Distinct beta-catenins mediate adhesion and signalling functions in C. elegans. Nature. 2000;406:527–532. doi: 10.1038/35020099. [DOI] [PubMed] [Google Scholar]
- Krause M, Park M, Zhang JM, Yuan J, Harfe B, Xu SQ, Greenwald I, Cole M, Paterson B, Fire A. A C. elegans E/Daughterless bHLH protein marks neuronal but not striated muscle development. Development. 1997;124:2179–2189. doi: 10.1242/dev.124.11.2179. [DOI] [PubMed] [Google Scholar]
- Lam N, Chesney MA, Kimble J. Wnt signaling and CEH-22/tinman/Nkx2.5 specify a stem cell niche in C. elegans. Curr Biol. 2006;16:287–295. doi: 10.1016/j.cub.2005.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent V, Vervoort M. The basic helix-loop-helix protein family: comparative genomics and phylogenetic analysis. Genome Res. 2001;11:754–770. doi: 10.1101/gr.177001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Hill RJ, Priess JR. POP-1 and anterior-posterior fate decisions in C. elegans embryos. Cell. 1998;92:229–239. doi: 10.1016/s0092-8674(00)80917-4. [DOI] [PubMed] [Google Scholar]
- Livesey FJ, Furukawa T, Steffen MA, Church GM, Cepko CL. Microarray analysis of the transcriptional network controlled by the photoreceptor homeobox gene Crx. Curr Biol. 2000;10:301–310. doi: 10.1016/s0960-9822(00)00379-1. [DOI] [PubMed] [Google Scholar]
- Maduro MF, Kasmir JJ, Zhu J, Rothman JH. The Wnt effector POP-1 and the PAL-1/Caudal homeoprotein collaborate with SKN-1 to activate C. elegans endoderm development. Dev Biol. 2005;285:510–523. doi: 10.1016/j.ydbio.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Merzdorf CS. Emerging roles for zic genes in early development. Dev Dyn. 2007;236:922–940. doi: 10.1002/dvdy.21098. [DOI] [PubMed] [Google Scholar]
- Mizumoto K, Sawa H. Two betas or not two betas: regulation of asymmetric division by beta-catenin. Trends Cell Biol. 2007;17:465–473. doi: 10.1016/j.tcb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Mori I, Ohshima Y. Neural regulation of thermotaxis in Caenorhabditis elegans. Nature. 1995;376:344–348. doi: 10.1038/376344a0. [DOI] [PubMed] [Google Scholar]
- Oda Y. Choline acetyltransferase: the structure, distribution and pathologic changes in the central nervous system. Pathol Int. 1999;49:921–937. doi: 10.1046/j.1440-1827.1999.00977.x. [DOI] [PubMed] [Google Scholar]
- Park FD, Priess JR. Establishment of POP-1 asymmetry in early C. elegans embryos. Development. 2003;130:3547–3556. doi: 10.1242/dev.00563. [DOI] [PubMed] [Google Scholar]
- Park FD, Tenlen JR, Priess JR. C. elegans MOM-5/frizzled functions in MOM-2/Wnt-independent cell polarity and is localized asymmetrically prior to cell division. Curr Biol. 2004;14:2252–2258. doi: 10.1016/j.cub.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Phillips BT, Kidd AR, 3rd, King R, Hardin J, Kimble J. Reciprocal asymmetry of SYS-1/beta-catenin and POP-1/TCF controls asymmetric divisions in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:3231–3236. doi: 10.1073/pnas.0611507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel R, Hutter H, Moerman D, Schnabel H. Assessing normal embryogenesis in Caenorhabditis elegans using a 4D microscope: variability of development and regional specification. Dev Biol. 1997;184:234–265. doi: 10.1006/dbio.1997.8509. [DOI] [PubMed] [Google Scholar]
- Siegfried KR, Kidd AR, 3rd, Chesney MA, Kimble J. The sys-1 and sys-3 genes cooperate with Wnt signaling to establish the proximal-distal axis of the Caenorhabditis elegans gonad. Genetics. 2004;166:171–186. doi: 10.1534/genetics.166.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried KR, Kimble J. POP-1 controls axis formation during early gonadogenesis in C. elegans. Development. 2002;129:443–453. doi: 10.1242/dev.129.2.443. [DOI] [PubMed] [Google Scholar]
- Simmer F, Moorman C, Van Der Linden AM, Kuijk E, Van Den Berghe PV, Kamath R, Fraser AG, Ahringer J, Plasterk RH. Genome-Wide RNAi of C. elegans Using the Hypersensitive rrf-3 Strain Reveals Novel Gene Functions. PLoS Biol. 2003;1:E12. doi: 10.1371/journal.pbio.0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Svendsen PC, McGhee JD. The C. elegans neuronally expressed homeobox gene ceh-10 is closely related to genes expressed in the vertebrate eye. Development. 1995;121:1253–1262. doi: 10.1242/dev.121.5.1253. [DOI] [PubMed] [Google Scholar]
- Takeshita H, Sawa H. Asymmetric cortical and nuclear localizations of WRM-1/beta-catenin during asymmetric cell division in C. elegans. Genes Dev. 2005;19:1743–1748. doi: 10.1101/gad.1322805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalik EL, Hobert O. Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J Neurobiol. 2003;56:178–197. doi: 10.1002/neu.10245. [DOI] [PubMed] [Google Scholar]
- Wenick AS, Hobert O. Genomic cis-Regulatory Architecture and trans-Acting Regulators of a Single Interneuron-Specific Gene Battery in C. elegans. Dev Cell. 2004;6:757–770. doi: 10.1016/j.devcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Huttner WB. Asymmetric cell division during neurogenesis in Drosophila and vertebrates. Mech Dev. 2003;120:1297–1309. doi: 10.1016/j.mod.2003.06.003. [DOI] [PubMed] [Google Scholar]
- Wolfe SA, Nekludova L, Pabo CO. DNA recognition by Cys2His2 zinc finger proteins. Annu Rev Biophys Biomol Struct. 2000;29:183–212. doi: 10.1146/annurev.biophys.29.1.183. [DOI] [PubMed] [Google Scholar]
- Woodhead GJ, Mutch CA, Olson EC, Chenn A. Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J Neurosci. 2006;26:12620–12630. doi: 10.1523/JNEUROSCI.3180-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel CN, Mutch CA, Swaminathan S, Taketo MM, Chenn A. Persistent expression of stabilized beta-catenin delays maturation of radial glial cells into intermediate progenitors. Dev Biol. 2007;309:285–297. doi: 10.1016/j.ydbio.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K, Satoh N, Satou Y. Identification of downstream genes of the ascidian muscle determinant gene Ci-macho1. Dev Biol. 2004;274:478–489. doi: 10.1016/j.ydbio.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ma C, Delohery T, Nasipak B, Foat BC, Bounoutas A, Bussemaker HJ, Kim SK, Chalfie M. Identification of genes expressed in C. elegans touch receptor neurons. Nature. 2002;418:331–335. doi: 10.1038/nature00891. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Marin O, Hermesz E, Powell A, Flames N, Palkovits M, Rubenstein JL, Westphal H. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proc Natl Acad Sci U S A. 2003;100:9005–9010. doi: 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


