Abstract
Increased hydrostatic pressure can damage neurons, although the mechanisms linking pressure to neurochemical imbalance or cell injury are not fully established. Throughout the body, mechanical perturbations such as shear stress, cell stretching, or changes in pressure can lead to excessive release of ATP. It is thus possible that increased pressure across neural tissues triggers an elevated release of ATP into extracellular space. As stimulation of the P2X7 receptor for ATP on retinal ganglion cells leads to elevation of intracellular calcium and excitotoxic death, we asked whether increased levels of extracellular ATP accompanied an elevation in pressure across the retina. The hydrostatic pressure surrounding bovine retinal eyecups was increased and the ATP content of the vitreal compartment adjacent to the retina was determined. A step increase of only 20 mmHg induced a three-fold increase in the vitreal ATP concentration. The ATP levels correlated closely with the degree of pressure increase over 20–100 mmHg range. The increase was transient at lower pressures but sustained at higher pressures. The rise in vitreal ATP was the same regardless of whether nitrogen or air was used to increase pressure, implying changes in oxygen partial pressure did not contribute. Lactate dehydrogenase activity was not affected by pressure, ruling out a substantial contribution from cell lysis. The ATP increase was largely inhibited by either 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) or carbenoxolone (CBX). While this is consistent with physiological release of ATP through pannexins hemichannels, a contribution from anion channels, vesicular release or other mechanisms cannot be ruled out. In conclusion, a step elevation in pressure leads to a physiologic increase in the levels of extracellular ATP bathing retinal neurons. This excess extracellular ATP may link increased pressure to the death of ganglion cells in acute glaucoma, and suggests a role for ATP in the neuronal damage accompanying increased intracranial pressure.
Keywords: Pannexin hemichannel, pressure, retinal ganglion cells excitotoxicity, stroke, purinergic signaling, neuroprotection
The purines ATP and adenosine are now recognized for their essential role in extracellular signaling. ATP can be released from both neural and non-neural cells into extracellular spaces, where it can act at a variety of ionotropic P2X or G-protein linked P2Y receptors (Burnstock and Williams, 2000). The precise trigger for ATP release varies with cell type. Depolarization can initiate a classic vesicular release from neurons (Fiedler et al., 1992). Transmitters such as thrombin (Pearson and Gordon, 1979) acetylcholine (Yang et al., 1994) and glutamate (Reigada et al., 2006a) can initiate release from endothelial or epithelial cells. In contrast, mechanical perturbation is one of the most reliable ways to initiate release from non-neuronal cells (Wang et al., 1996; Feranchak et al., 2000). Moreover, extracellular levels of ATP following cell swelling can be considerably greater than those associated with transmitter stimulation (Mitchell, 2001, Reigada et al., 2006a). In addition to swelling, ATP is released from cells after stretching (Patel et al., 2005), direct cell surface mechanical stimulation (Guthrie et al., 1999), shear stress (Milner et al., 1992, Bodin and Burnstock, 2001), medium disturbance (Grygorczyk and Hanrahan, 1997) or increased hydrostatic pressure (Ferguson et al., 1997, Cockayne et al., 2000, Knight and Burnstock, 2001). It follows that mechanical distention is one of the most effective triggers for ATP release (Burnstock, 1999).
Excess extracellular ATP may have particular relevance to neuropathologies where inflammation in a closed space or restriction of flow elevates pressure. Intracranial pressure can increase in response to a variety of disorders including blockade of the drainage of cerebral spinal fluid, increased venous pressure, or cerebral edema following infarction (Bardutzky and Schwab, 2007). Acute subdural hematomas can raise pressure and trigger neuronal apoptosis (Alessandri et al., 2006). A rise in intracranial pressure is closely correlated with cell death following traumatic brain injury (Yang et al., 2005), and increased hydrostatic pressure induces apoptotic death in neuronal cell lines (Agar et al., 2000). Within the eye, the increased intraocular pressure associated with glaucoma can lead to the death of ganglion cells (Quigley, 1999, Gordon et al., 2002), yet the link between increased pressure and neuronal death is not fully understood. It is possible that excessive levels of extracellular ATP can link elevated intraocular pressure and ganglion cell death. Levels of ATP are elevated in the anterior chamber of patients with primary acute angle closure glaucoma, and the concentration of ATP in this extracellular space is correlated with the magnitude of pressure increase (Zhang et al., 2007).
The pathological potential of excess extracellular ATP to retinal ganglion cells was recently illustrated (Resta et al., 2007). The damage to retinal ganglion cells following rapid elevations in ocular pressure was attenuated by dephosphorylating ATP with apyrase, or by blocking purinergic receptors (Resta et al., 2007). In addition, levels of extracellular ATP were elevated by increased ocular pressure. While these observations nicely emphasized the possible contribution ATP made to pressure-induced damage in the retina, the relationship between pressure and ATP levels over time, in addition to the mechanisms of release, remain to be determined. Invasive sampling from the small spaces surrounding retinal ganglion cells would involve passing probes through cells, and as the intracellular concentration of ATP is several orders of magnitude higher than extracellular levels this would interfere with the extracellular signal. However, ganglion cells lie in the inner retina, in close proximity to the to the vitreal chamber, and it is possible that excess ATP release from retinal cells diffuses into the vitreous humor where sampling would not itself be damaging. Experiments were thus performed ex vivo in a bovine eyecup preparation that allowed undisturbed access to the vitreal cavity surrounding the retina. This preparation also enabled manipulation of the composition and thus provided an insight into the mechanisms involved in this release.
Experimental procedures
Solutions and drugs
All the chemicals were from Sigma-Aldrich Corp. (St. Louis, MO) unless otherwise noted. An isotonic solution was used to rinse the eyecup, obtain samples and dilute drugs and the luminescent assay. It consisted of (in mM): 105 NaCl, 5 KCl, 6 HEPES acid, 4 Na-HEPES, 5 NaHCO3, 60 Mannitol, 5 glucose, 0.5 MgCl2 and 1.3 CaCl2, at pH 7.4 with NaOH. The general anion channel blocker 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) was stored as a 10 mM stock solution in dimethyl sulfoxide (DMSO) and diluted to working strength of 30 µM. Mefloquine was stored as a 10 mM stock solution in DMSO and diluted to 200 µM. Carbenoxolone (CBX) was stored at as a 10 mM stock in H20 and diluted to 10 µM.
Retinal eyecup preparation
Bovine eyes were obtained from an abattoir, transported to the laboratory and maintained on ice in the dark until use. Eyes were used with approval of the University of Pennsylvania Institutional Animal Care and Use Committee. Experiments were typically performed 4–8 hrs post mortem. Eyes were bisected through the ora serrata, with the anterior portion and vitreous gel discarded carefully to avoid detachment of the retina. The posterior eyecup was placed in a pressure chamber and rinsed 3 times with 5 ml of isotonic solution. Immediately before the onset of the experiment, 1 ml of isotonic solution was placed in the eyecup and the chamber was closed. This volume ensured sufficient space to collect the sample without the risk of touching the retina with the pipette tip, as mechanical prodding of the inner retinal membrane can trigger ATP release (Newman, 2001) and as sampling itself can lead to ATP release if performed carelessly (Grygorczyk and Hanrahan, 1997). With 1 ml solution in the eyecup, the surface was also sufficiently below the cut edge of the eyecup to ensure ATP from the interior of cells at this cut edge did not readily seep into the solution. In some eyes, 30 µM NPPB, 10 µM carbenoxolone or 200 µM mefloquine dissolved in isotonic solution was placed in the eyecup for 15 minutes. After this preincubation, 1 ml of fresh drug solution was reintroduced and the eyecup subjected to an increase in pressure.
Pressure chamber
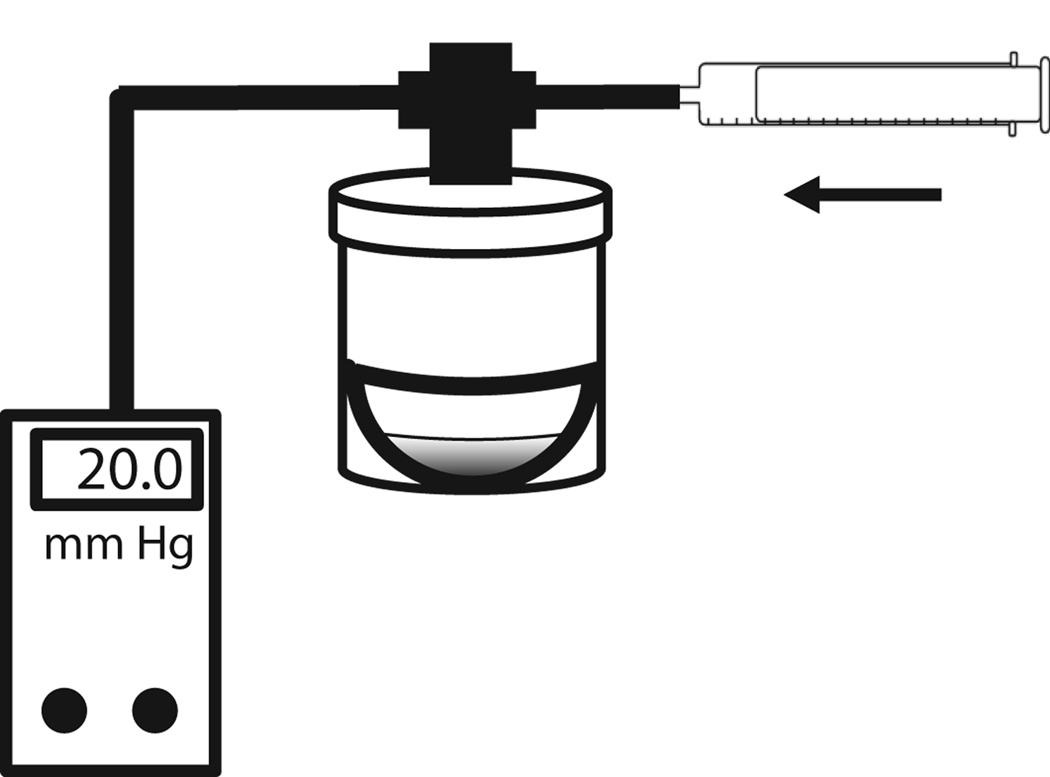
A simplified pressure chamber was designed from a 60 ml plastic jar with a screw cap, as modeled in Figure 1. A 3-way valve was attached to a hole drilled in the lid to connect to a 20 ml syringe with Tygon tubing. The pressure inside the chamber was constantly monitored with a PM015D digital pressure transducer (World Precision Instruments Inc., Sarasota, FL). The screw cap and the 3-way valve joints were sealed with Teflon tape and silicone grease to prevent air leak as the internal pressure was increased. The pressure in the chamber was increased by introducing air or nitrogen through the syringe. While relatively simple, this device allowed control over the internal pressure for the relatively short duration of these experiments (up to 60 min). Appropriate controls are described throughout the paper to validate the experimental system.
Figure 1. Model of the pressure chamber.
Retinal eyecups were placed in the chamber, and washed with control solution. One ml solution was added to the bottom of each eyecup and the lid was sealed. Air or nitrogen was injected from the syringe until the pressure reading given by the digital manometer reached the appropriate level. The pressure was then locked by adjusting the 3-way valve. The pressure was monitored continuously for the indicated time period. When appropriate precautions were taken, pressure levels remained constant throughout the experiment.
After maintaining the chamber at the set pressure for the indicated time, the pressure inside the chamber was carefully decreased, the lid was opened and a 100 µl sample was obtained from the eyecup. Control eyecup preparations were placed in identical chambers and the lid closed as done for pressurized eyes, but the chambers were maintained at atmospheric pressure. All samples were taken at the same position midway between the retina and the solution surface; this eliminated the possibility of contact between the pipette tip and retina surface while ensuring the diffusional distance was constant. Samples were rapidly frozen at −80°C until ATP measurement. In experiments with multiple time points, the chamber was resealed after sample collection and the internal pressure restored to experimental levels.
Measurement of ATP
The ATP concentration in samples was determined with the chemoluminescent luciferin-luciferase reaction, as previously described (Reigada and Mitchell, 2005). In brief, a stock solution was prepared by diluting one vial of ATP assay kit (FLAA, Sigma Chemical Corp.) in 450 µl of isotonic solution plus 50 µl distilled water. The luciferin-luciferase working solution was prepared diluting 80 µl of the stock solution in 1 ml isotonic solution. To determine ATP levels, 45 µl of the sample obtained from the pressure experiment was added to a well in a 96-well white assay plate (Corning Inc,, Corning, NY) and placed in a microplate luminometer (Luminoskan Ascent, ThermoFisher Scientific, Waltham, MA). The luciferin-luciferase working solution (10 µl) was injected in each well using the internal injector system. The light emitted was recorded with an integration time of 100 ms/measurement every minute for 10 min. ATP levels were calculated by integrating the area under the luminescence curve. The luminescence was converted to ATP concentration using an ATP standard curve prepared daily. Neither 30 µM NPPB not 10 µM carbenoxolone affected the luciferase assay. While mefloquine led to a reduction in assay sensitivity, the lack of a reduction of experimental ATP levels implies this did not alter the conclusions.
Lactate dehydrogenase measurements
The activity of lactate dehydrogenase (LDH) was determined, based upon the principal that the enzyme is not present in the extracellular solution unless cells have ruptured. Samples, reagents and standard LDH concentrations were diluted in a working solution composed by tris[Hydorxymethyl]amino-methane (trizma base) and NaCl, pH 7.2. 75 µl of sample was added to a 1.5 ml semimicro cuvete containing 925 µl of 0.3 mg/ml reduced β-nicotinamide adenine dinucleotide (β-NADH) and 0.2 mg/ml pyruvic acid diluted in working solution. Once the reaction reached equilibrium, (30 sec after addition) the absorbance at 340 nm was measured (Spectronic 1201, Milton Roy Company, Ivyland, PA). Levels were converted to enzyme units using a standard curve.
Data analysis
All the data are expressed as mean ± SE. Significance was tested with an unpaired Student’s t test for two variables and an ANOVA with appropriate post-hoc test for more than two variables using SigmaStat software (Systat Software, Inc. San Jose, CA). On occasions when data was not normally distributed, ANOVA’s were performed on ranks. Significance was defined as p<0.05. Linear regression was calculated using Sigmaplot software based on a least squares analysis (Systat Software, Inc.) “n” indicates the number of retinal eyecups per experiment. While ATP levels were consistently elevated in eyes exposed to increased pressure, there was variation in the absolute levels on different experimental days. As a maximum of three control and three experimental eyes could be processed on a given day, ATP levels were sometimes normalized to the mean control for a given day’s experiments.
Results
Elevated pressure leads to rise in extracellular ATP
The amount of ATP present in the vitreal chamber increased as pressure across the eyecup preparation was raised (Fig. 2A). Levels of ATP in eyecups not exposed to a change in pressure were low, at 6.2 ± 0.7 nM. Elevating the pressure by only 20 mmHg above atmospheric for 10 min increased ATP to 13.0 ± 1.9 nM (p<0.005 vs. unpressurized control). While the increase in the ATP levels of the vitreous chamber in response to brief, moderate increases in pressure suggested ATP could transduce the pressure rise into a neurochemical signal, additional experiments were necessary to confirm the finding and probe the effect of different parameters.
Figure 2. Increased pressure leads to an increase in vitreal ATP.
A. A significant increase in the ATP concentration was detected in vitreous of eyecups held at 20 mmHg above atmospheric pressure for 10 min (black bar), as compared to the non-pressured controls (white bar; n=12 for each, * p<0.005). In this panel and throughout, bars and circles represent the mean ± standard error of the mean; “n” indicates the number of retinal eyecups per experiment.
B. The kinetics of the ATP response were dependent on the size of the pressure step. While ATP levels remained elevated over 30 min in eyecups exposed to a step change of 70 mmHg (black squares), ATP levels returned to baseline in preparations exposed to only 20 mmHg (white triangles). ATP remained elevated for 60 min with 70 mmHg (not shown). Lines represent a spline curve fit to the data. All concentrations were normalized to the mean control value (black circles) for a given day’s experiments (n=7–18, * p<0.05).
C. The ATP concentration increased in relationship to the magnitude of the pressure step in the samples collected from the vitreous chamber after 20 minutes of pressure elevation. The line represents a linear regression fit to the data with y=1.089 +8.662x; r2=0.947. * p<0.05 vs 0 mmHg control; n= 20, 8, 3, 7, and 4 for 0 mmHg, 20 mmHg, 50 mmHg, 70 mmHg and 100 mmHg respectively.
The elevation in ATP in response to a step increase in pressure was transient at lower magnitudes. When pressure was elevated by 20 mmHg, ATP levels fell back to baseline within 30 min (Fig. 2B). In contrast, the rise in extracellular ATP levels was sustained at higher pressures. With a pressure increase of 70 mmHg, ATP concentration remained elevated for the duration of the 30 min experiment.
The retina contains many ecto-ATPases (Iandiev et al., 2007), and sustained extracellular concentrations of ATP reflect a balance between continuous release and degradation. While this complex temporal relationship makes broad comparisons somewhat convoluted, it was clear that increased pressured generally led to higher levels of ATP in the vitreous chamber. For example, there was a linear relationship between the concentration of ATP and the size of the pressure change when examined after 20 min of pressure elevation (Fig. 1C). This supports the conclusion that the increase in vitreal ATP was causally related to the elevated pressure.
ATP rise not due to change in partial pressure or cell lysis
Initial experiments were performed by injecting room air into the chamber to increase the pressure. As the relative partial pressures of gasses can theoretically change in response to altered pressure, it was possible that alterations in the partial pressure of oxygen influenced ATP release. To determine whether changes in the partial pressure of different gasses contributed to the increase in ATP, the experiments were repeated with nitrogen instead of air. The pressure chambers were flushed through with 3–5 volumes of nitrogen to remove room air, then the chambers were closed and pressure was increased with a syringe of nitrogen. As demonstrated in Figure 3A, the increase in the ATP concentration of the vitreous compartment was the same regardless of whether pressure was elevated using air or nitrogen. This suggested that small alterations in the relative partial pressures of oxygen or other gasses were not responsible for the change in ATP.
Figure 3. Increased ATP not due to differential partial pressure or cell lysis.
A. ATP levels in the vitreal compartment increased similarly regardless of whether air or nitrogen was injected into the chamber. Raising pressure by 100 mmHg with either air (n=4) or nitrogen (N2; n=5) significantly raised ATP levels compared to eyecups kept at atmospheric pressure (n=9), although there was no significant difference between them. * p<0.05 vs non-pressurized control, NS = not significantly different.
B. LDH levels were measured in the vitreal samples to test for cell lysis. Extracellular LDH levels were not significantly different in samples from retina eyecups challenged with increases of 20 mmHg (n=4) or 50 mmHg (n=4) for 10 minutes versus the control levels (n=7). NS = not significantly different.
The intracellular content of ATP is 4–5 orders of magnitude higher than baseline external levels, and concentrations that maximally activate receptors are typically far below cytoplasmic levels. While elevations in extracellular ATP can result from physiological release mechanisms, ATP can also leak from damaged cells (Davalos et al., 2005). To determine whether the rise of ATP in the vitreous space was due to pressure-dependent cell lysis, the activity of lactose dehydrogenase (LDH) in the vitreal samples was measured. The cytoplasmic enzyme is only detected extracellularly after cell rupture, and as such is commonly used to detect cell death. Elevating pressure did not alter levels of LDH (Fig. 3B), implying that the increased ATP present in vitreous solution did not originate from ruptured cells.
Conduit for ATP release
Many different cell types release ATP, and various routes for the exit of ATP exist. Recently, pannexin hemichannels have been implicated in the physiologic release of ATP (Huang et al., 2007). The potential contribution of pannexins to the pressure-dependent release of ATP was examined using the same approach we have applied to study pannexin channels in the ciliary epithelium (Li, 2008). Eyecups were preincubated with inhibitors for 15 min before pressure was increased to allow them to permeate the retina. Pressure was increased by 20 mmHg for 10 min after which samples were obtained.
Carbenoxolone (10 µM) reduced the pressure-dependent rise in vitreal ATP levels by 87% (Fig. 4A), while NPPB (30 µM) reduced the rise in vitreal ATP levels by 89% (Fig. 4B). While this concentration of carbenoxelone has been recognized to block pannexin hemichannels for several years (Bruzzone et al., 2005), NPPB was recently shown to inhibit pannexins (Silverman et al., 2008). NPPB is a well known to inhibit anion channels (Buyse et al., 1997), but the ability of both drugs to produce a near-complete block suggests they are both acting at a common site. The gap junction/hemichannel blocker mefloquine did not alter ATP release, even at 200 µM (Fig. 4C).
Figure 4. Pharmacology of ATP release.
A. Carbenoxolone (CBX, 10 µM) blocked the release of ATP in eyecups exposed to pressure (grey bar, n=5) compared to cells exposed to pressure alone (black bar, n=5). The ATP levels in the presence of carbenoxolone and pressure were not significantly different from non-pressurized controls (while bar, n=5). The absolute increase in pressure was relatively high in this set of experiments, further emphasizing the magnitude of the block by carbenoxolone * p<0.05 vs control; **p <0.05 vs. pressure alone.
B. NPPB prevented the release of ATP in response to pressure. Eyecups were incubated with 30 µM NPPB solution for 15 minutes after which pressure was raised by 20 mmHg and samples were collected 10 min later. NPPB prevented the rise in ATP in eyecups exposed to pressure (grey bar, n=4) as compared to cells exposed to pressure alone (black bar, n=5). The ATP levels were normalized to the non-pressure and NPPB-free eyecup control levels (white bar, n=6). * p<0.05 vs control; **p <0.05 vs pressure alone.
C. No decrease in ATP levels was produced by the gap/hemichannel blocker melfoquine (Mefl), even at 200 µM. Pressure was increased by 20 mmHg and vitreal samples retrieved after 10 min. * p<0.05 vs control, NS = not significantly different, n=9 for each.
Discussion
The results above establish an association between elevated hydrostatic pressure and the release of ATP from the retina. The data indicate there is an increase in the extracellular levels of ATP when pressure is raised above atmospheric. Both the kinetics and the magnitude of ATP elevation in the vitreous space were influenced by the extent of the pressure increase, supporting this link. Controls imply this rise in ATP is not due to cell rupture, while the pharmacology suggests pannexin hemichannels contribute to at least some of the ATP release. The presence of a pressure-dependent rise in extracellular ATP in the inner retinal provides further evidence that release of ATP can transduce mechanical perturbations into neurochemical signals, and may have implications for pathological pressure increases across neural tissue in general.
Trigger for ATP release is mechanical
Mechanical stress is one of the most common triggers inducing ATP release (Ferguson et al., 1997, Cockayne et al., 2000, Vlaskovska et al., 2001). While the system used in the current study is relatively simple, key controls substantiate the conclusion that mechanical changes were responsible for the pressure-dependent release of ATP. Although the relative partial pressures of different gasses can alter with absolute pressure, the vitreal concentrations of ATP were not affected by changing the pressurized gas in the chamber from air to nitrogen. This is consistent with previous reports suggesting there is typically little change in response to moderate hydrostatic pressure elevation (Yang et al., 2004), and implies changes in relative partial pressure were not responsible for the excess ATP release. Hypoxia/ischemia can trigger a release of ATP from many cell types (Buttigieg and Nurse, 2004, Gourine et al., 2005) including the RPE cells in the outer retina (Mitchell and Reigada, 2008), and the closed chambers into which the eyecups were placed may have restricted access to oxygen. However, both control and pressurized eyecups were maintained in closed chambers for the same duration, implying hypoxia was unlikely to explain the increased ATP. The similarity of the response from eyecups maintained in pure nitrogen and air also argues against hypoxia as a trigger for ATP release.
While these arguments imply that the mechanical effects of elevated pressure contribute to the release of ATP, the type of stress is unclear. Mechanical perturbations are a common trigger for ATP release (Milner et al., 1992; Grygorczyk and Hanrahan, 1997; Guthrie et al., 1999; Bodin and Burnstock, 2001), but the response to elevated pressure in the retinal eyecup most closely resembles that in the bladder, where the extracellular ATP concentration rose in proportion to the magnitude of pressure applied across the tissue (Ferguson et al., 1997; Knight et al., 2002). It is not clear whether these relatively small rises in hydrostatic pressure are themselves responsible for the response, at least in the retina, as the resulting forces are thought to be too small to induce changes on a molecular level (Ethier, 2006). Within the posterior eye, it has been suggested that the primary effect of elevated intraocular pressure is mediated by differential compression (Sigal et al., 2007) or membrane stresses (Tan et al., 2006). However, hydrostatic pressure can also influence ocular cells by modifying their cytoskelletal organization and extracellular matrices (Wax et al., 2000), while the differential compression of the lipid bilayer between the fluid-filled intra- and extracellular spaces could produce additional forces that trigger ATP release. The presence of a many-layered retina, a spongy choroid and a more rigid sclera in the eye provide an opportunity for differential compressibility and stretch in response to the pressure applied across the eyecup.
Mechanism of ATP release
The rise in vitreal ATP following an increase in pressure likely represents a physiologic response. Increased pressure did not raise the concentration of lactose dehydrogenase in the vitreal chamber, implying that ATP did not emanate from ruptured cells. While the identity of the ATP conduit release remains to be determined, pharmacological analysis suggests pannexins could contribute. Pannexins exist as stable hemichannels in the membrane, and can open in the presence of physiological levels of extracellular calcium (Dahl and Locovei, 2006; Spray et al., 2006). Pannexins are prime candidates for a role in pressure-dependent release of ATP from the inner retina. They gate open in response to increased pressure, and are permeable to ATP (Bao et al., 2004). The release of ATP through pannexins is involved in the calcium signaling within the taste buds (Huang et al., 2007). Pannexins co-localize with PSD-96 in the post-synaptic region of hippocampal cells (Zoidl et al., 2007), and pharmacology suggests they may be involved in the activation of a 530 pS hemichannel in ischemic hippocampal neurons (Thompson et al., 2006). Pannexins are particularly sensitive to carbenoxolone, with an IC50 of 5 µM, but are relatively insensitive to other connexin blockers (Bruzzone et al., 2005). In contrast, connexin hemichannels are relatively insensitive to carbenoxolone, with 10 µM inducing less than a 5% block of current.
The block of pressure-triggered ATP release into the bovine vitreal chamber by 10 µM carbenoxolone suggests that ATP could pass through pannexin channels. As both carbenoxolone and NPPB inhibited the pressure-dependent ATP release by >85%, it is unlikely that the drugs acted in parallel at different targets. The recent demonstration that NPPB inhibits currents through pannexin1 expressed on Xenopus oocytes (Silverman et al., 2008) suggests that both drugs are acting at pannexins, although definitive identification awaits more specific tools.
In addition to pannexins, ATP could be released through several other mechanisms including anion channels and vesicular pathways. NPPB can block a variety of Cl− channels including volume sensitive channels (Furukawa et al., 1998), voltage-dependent anion channels (VDAC; Sabirov et al., 2001), and the cystic fibrosis transmembrane conductance regulator (CFTR; Cuthbert, 2001), all of which have been associated with ATP release in some capacity (Wang et al., 1996, Braunstein et al., 2001; Hisadome et al., 2002; Dutta et al., 2004; Sabirov and Okada, 2005). Vesicular release of ATP occurs in both neural and non-neural cells (Maroto and Hamill, 2001; Reigada et al., 2003), with release from astrocytes of potential relevance here (Bowser and Khakh, 2007; Pangrsic et al., 2007). Connexin hemichannels have also been implicated in release (Cotrina et al., 1998). As mefloquine blocks both gap junctions and hemichannels composed of connexins 46 or connexin 50 (Srinivas et al., 2005), the lack of response to mefloquine suggests these connexins at least are not involved in the release. It shout be noted that several different mechanisms could contribute to the pressure-dependent release of ATP from the retina, as distinct release pathways can co-exist even within the same cell (Joseph et al., 2003).
While the cellular source of the pressure-dependent ATP release from the retina remains to be determined, several candidates should be considered. Glial cells are being increasingly recognized for their role in retinal communication (Newman, 2006). Müller cells release ATP in proximity to the ganglion cells following mechanical prodding of the inner retinal membrane, making this a likely source for pressure-dependent release (Newman, 2001; Newman, 2003). Whether ATP would be released in levels high enough to overcome rapid conversion to adenosine is not known. The endfeet of Müller cells face the vitreal chamber and would be well suited for delivery of the ATP detected in this study, with ATP stimulating P2 receptors on the endfeet (Uckermann et al., 2002). RPE cells bordering the outer retina release ATP into the retina in response to various stimuli including mechanical swelling (Mitchell, 2001; Mitchell and Reigada, 2008), and the ATP released from RPE hemichannels may contribute (Pearson et al., 2005). Whether the high activity of ecto-nucleotidases in subretinal space dephosphorylates the ATP before it can diffuse to the vitreous chamber is not known (Reigada et al., 2006b). Finally, the ability of neurons to release transmitter should not be forgotten. Release of ATP from retinal neurons has been reported (Neal and Cunningham, 1994). In this regard, the high levels of pannexin expression on retinal ganglion cells may be of relevance (Dvoriantchikova et al., 2006).
Physiological implications
It is tempting to hypothesize that the increased pressure of acute glaucoma leads to an elevation in extracellular ATP levels that damages the ganglion cells. This theory is strongly supported by ability of the soluble ATPase apyrase and of P2X7 receptor antagonists to reduce the ganglion cell damage induced by acute elevations in ocular pressure (Resta et al., 2007). The demonstration that stimulation of the P2X7 receptor for ATP on retinal ganglion cells elevates intracellular calcium and kills retinal ganglion cells in vitro is also consistent with this model (Zhang et al., 2005; Zhang et al., 2006b), although attempts to examine ganglion cell death in the bovine model were confounded by structural complexities.
While many steps in the putative connection between elevated pressure, excess ATP and ganglion cell death require validation, two concerns in particular merit discussion here. Firstly, the absolute levels of ATP measured in the vitreous were low, and below that capable of activating P2 receptors. However, the volume of extracellular space in the retina is miniscule compared to the 1 ml solution placed in the eyecup, so the levels of ATP we measured were diluted many fold below that surrounding retinal cells. The presence of the aforementioned ectoATPases in the retina, combined with diffusional forces, would further reduce ATP concentration of the vitreal sample. In this regard, membrane-attached luciferase has demonstrated that levels of ATP are considerable higher on the membrane than in bulk solution (Joseph et al., 2003), suggesting the ATP concentrations surrounding retinal cells will be substantially higher. Regardless, the ability of P2X7 receptor antagonists to reduce ganglion cell damage accompanying elevated ocular pressure by implies that endogenous levels of ATP are sufficient to stimulate the receptor and damage ganglion cells in vivo (Resta et al., 2007).
Secondly, the transient elevation at low pressure steps may alter the physiological signaling. At this point it is not clear whether this transient elevation at 20 mmHg reflects adaptation of the tissue or the release mechanisms. It does imply any responses attributed to ATP will be more sustained at higher pressures. The magnitude of diurnal pressure oscillations are larger than normal for many patients with glaucoma, suggesting changes in pressure may be relevant even at modest absolute elevations in pressure (Sehi et al., 2005). The presence of elevated ATP levels in the aqueous humor of patients with acute angle closure glaucoma concentration implies that ATP remains elevated over several days of increased pressure (Zhang et al., 2007), although examination of glaucoma models is clearly necessary before these findings can be extrapolated to the retina.
Finally, this findings suggest the proposed link between elevated pressure, ATP release and neuronal damage in the brain also warrants further investigation. Pharmacologic treatments to limit pressure-dependent damage would be particularly useful in cases where current shunting therapy is insufficient, such as during extended periods of hydrocephalus (Del Bigio, 2001) or in the developing world where surgery can be more problematic (Warf, 2005).
Acknowledgements
The authors would like to thank Alan M. Laties and Mortimer M. Civan for useful discussions on glaucoma and pannexins, respectively. We are also grateful for initial work on carbenoxolone performed by Li Ang. This work was supported by grants EY015537, EY013434 and a Vision Research Core Grant EY001583 all from the NIH, and by the Jody Sack Fund.
Preliminary portions of this work have been previously presented in abstract form (Zhang et al., 2006a).
Abbreviations
- ATP
adenosine triphosphate
- CBX
carbenoxolone
- CFTR
cystic fibrosis transmembrane conductance regulator
- DMSO
dimethyl sulfoxide
- LDH
lactate dehydrogenase
- NPPB
5-nitro-2-(3-phenylpropylamino) benzoic acid
- RPE
retinal pigment epithelium
- VDAC
voltage-dependent anion channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agar A, Yip SS, Hill MA, Coroneo MT. Pressure related apoptosis in neuronal cell lines. J Neurosci Res. 2000;60:495–503. doi: 10.1002/(SICI)1097-4547(20000515)60:4<495::AID-JNR8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Alessandri B, Nishioka T, Heimann A, Bullock RM, Kempski O. Caspase-dependent cell death involved in brain damage after acute subdural hematoma in rats. Br Res. 2006;1111:196–202. doi: 10.1016/j.brainres.2006.06.105. [DOI] [PubMed] [Google Scholar]
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Letts. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Bardutzky J, Schwab S. Antiedema therapy in ischemic stroke. Stroke. 2007;38:3084–3094. doi: 10.1161/STROKEAHA.107.490193. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Bowser DN, Khakh BS. Two forms of single-vesicle astrocyte exocytosis imaged with total internal reflection fluorescence microscopy. ProcNat Acad Sci USA. 2007;104:4212–4217. doi: 10.1073/pnas.0607625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein GM, Roman RM, Clancy JP, Kudlow BA, Taylor AL, Shylonsky VG, Jovov B, Peter K, Jilling T, Ismailov II, Benos DJ, Schwiebert LM, Fitz JG, Schwiebert EM. Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J Biol Chem. 2001;276:6621–6630. doi: 10.1074/jbc.M005893200. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Therapeut. 2000;295:862–869. [PubMed] [Google Scholar]
- Buttigieg J, Nurse CA. Detection of hypoxia-evoked ATP release from chemoreceptor cells of the rat carotid body. Biochem Biophys Res Comm. 2004;322:82–87. doi: 10.1016/j.bbrc.2004.07.081. [DOI] [PubMed] [Google Scholar]
- Buyse G, Voets T, Tytgat J, De Greef C, Droogmans G, Nilius B, Eggermont J. Expression of human pICln and ClC-6 in Xenopus oocytes induces an identical endogenous chloride conductance. J Biol Chem. 1997;272:3615–3621. doi: 10.1074/jbc.272.6.3615. [DOI] [PubMed] [Google Scholar]
- Cockayne D, Hamilton S, Zhu Q, Dunn P, Zhong Y, Novakovic S, Malmberg A, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit W, Burnstock G, McMahon S, Ford A. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci. 1998;18:8794–8804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert AW. Assessment of CFTR chloride channel openers in intact normal and cystic fibrosis murine epithelia. Br J Pharmacol. 2001;132:659–668. doi: 10.1038/sj.bjp.0703859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G, Locovei S. Pannexin: To gap or not to gap, is that a question? IUBMB Life. 2006;58:409–419. doi: 10.1080/15216540600794526. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim J, Zuo Y, Jung S, Littman D, Dustin M, Gan W. ATP mediates rapid microglial response to local brain injury in vivo. Nature Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Del Bigio MR. Pathophysiologic consequences of hydrocephalus. Neurosurg Clin North Am. 2001;12:639–649. [PubMed] [Google Scholar]
- Dutta AK, Sabirov RZ, Uramoto H, Okada Y. Role of ATP-conductive anion channel in ATP release from neonatal rat cardiomyocytes in ischaemic or hypoxic conditions. J Physiol. 2004;559:799–812. doi: 10.1113/jphysiol.2004.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvoriantchikova G, Ivanov D, Panchin Y, Shestopalov VI. Expression of pannexin family of proteins in the retina. FEBS Letts. 2006;580:2178–2182. doi: 10.1016/j.febslet.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Ethier CR. Hydrostatic pressure Is not a surrogate for IOP in glaucoma. Invest Ophthalmol Vis Sci. 2006 E-letter: http://www.iovs.org/cgi/eletters/46/48/2829. [Google Scholar]
- Ferguson D, Kennedy I, Burton T. ATP is released from rabbit urinarybladder epithelial cells by hydrostatic pressure changes--a possible sensory mechanism? J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler JL, Pollard HB, Rojas E. Quantitative analysis of depolarization-induced ATP release from mouse brain synaptosomes: external calcium dependent and independent processes. J Membr Biol. 1992;127:21–33. doi: 10.1007/BF00232755. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Ogura T, Katayama Y, Hiraoka M. Characteristics of rabbit ClC-2 current expressed in Xenopus oocytes and its contribution to volume regulation. Am J Physiol. 1998;274:C500–C512. doi: 10.1152/ajpcell.1998.274.2.C500. [DOI] [PubMed] [Google Scholar]
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory response. J Neurosci. 2005;25:1211–1218. doi: 10.1523/JNEUROSCI.3763-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygorczyk R, Hanrahan J. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol. 1997;272:C1058–C1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- Guthrie P, Knappenberger J, Segal M, Bennett M, Charles A, Kater S. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisadome K, Koyama T, Kimura C, Droogmans G, Ito Y, Oike M. Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J Gen Physiol. 2002;119:511–520. doi: 10.1085/jgp.20028540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Nat Acad Sci USA. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem. 2003;278:23331–23342. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- Knight G, Burnstock G. Identification of P1 and P2 purinoceptors in the aorta of the lizard (Agama sp.) Comp Biochem Physiol Toxicol & Pharmacol. 2001;128:413–423. doi: 10.1016/s1532-0456(00)00214-3. [DOI] [PubMed] [Google Scholar]
- Knight GE, Bodin P, De Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- Li A, Lu W, Leung GC-T, Peterson-Yantorno K, Mitchell CH, Civan MM. Potential role of pannexin hemichannels in ATP release from native bovine ciliary epithelial cells. Association for Research in Vision and Ophthalmology (ARVO) 2008 E-Abstract #3161. [Google Scholar]
- Maroto R, Hamill OP. Brefeldin A block of integrin-dependent mechanosensitive ATP release from xenopus oocytes reveals a novel mechanism of mechanotransduction. J Biol Chem. 2001;276:23867–23872. doi: 10.1074/jbc.M101500200. [DOI] [PubMed] [Google Scholar]
- Milner P, Bodin P, Loesch A, Burnstock G. Increased shear stress leads to differential release of endothelin and ATP from isolated endothelial cells from 4- and 12-month-old male rabbit aorta. J Vasc Res. 1992;29:420–425. doi: 10.1159/000158960. [DOI] [PubMed] [Google Scholar]
- Mitchell CH. Release of ATP by a human retinal pigment epithelial cell line: potential for autocrine stimulation through subretinal space. J Physiol. 2001;534:193–202. doi: 10.1111/j.1469-7793.2001.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell CH, Reigada D. Purinergic signalling in the subretinal space: a role in the communication between the retina and the RPE. Purinergic Signall. 2008;4:101–107. doi: 10.1007/s11302-007-9054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M, Cunningham J. Modulation by endogenous ATP of the light-evoked release of ACh from retinal cholinergic neurones. Br J Pharmacol. 1994;113:1085–1087. doi: 10.1111/j.1476-5381.1994.tb17106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial cell inhibition of neurons by release of ATP. J Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. A purinergic dialogue between glia and neurons in the retina. Novartis Found Symp. 2006;276:193–202. [PubMed] [Google Scholar]
- Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- Patel A, Reigada D, Mitchell C, Bates S, Margulies S, Koval M. Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am J Physiol Lung. 2005;289:L489–L496. doi: 10.1152/ajplung.00074.2005. [DOI] [PubMed] [Google Scholar]
- Pearson J, Gordon J. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979;281:382–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron. 2005;46:731–744. doi: 10.1016/j.neuron.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Quigley HA. Neuronal death in glaucoma. Prog Ret Eye Res. 1999;18:39–57. doi: 10.1016/s1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- Reigada D, Diez-Perez I, Gorostiza P, Verdaguer A, Gomez de Aranda I, Pineda O, Vilarrasa J, Marsal J, Blasi J, Aleu J, Solsona C. Control of neurotransmitter release by an internal gel matrix in synaptic vesicles. Proc Nat Acad Sci USA. 2003;100:3485–3490. doi: 10.1073/pnas.0336914100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigada D, Lu W, Mitchell CH. Glutamate acts at NMDA receptors on fresh bovine and on cultured human retinal pigment epithelial cells to trigger release of ATP. J Physiol. 2006a;575:707–720. doi: 10.1113/jphysiol.2006.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reigada D, Mitchell C. Release of ATP from retinal pigment epithelial cells involves both CFTR and vesicular transport. Am J Physiol Cell Physiol. 2005;288:C132–C140. doi: 10.1152/ajpcell.00201.2004. [DOI] [PubMed] [Google Scholar]
- Reigada D, Zhang X, Crespo A, Nguyen J, Liu J, Pendrak K, Stone RA, Laties AM, M CH. Stimulation of an 1-adrenergic receptor downregulates ecto-5' nucleotidase activity on the apical membrane of RPE cells. Purinergic Signalling. 2006b;2:499–507. doi: 10.1007/s11302-005-3980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov RZ, Dutta AK, Okada Y. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J Gen Physiol. 2001;118:251–266. doi: 10.1085/jgp.118.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov RZ, Okada Y. ATP release via anion channels. Purinergic Signalling. 2005;1:311–328. doi: 10.1007/s11302-005-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehi M, Flanagan JG, Zeng L, Cook RJ, Trope GE. Relative change in diurnal mean ocular perfusion pressure: a risk factor for the diagnosis of primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2005;46:561–567. doi: 10.1167/iovs.04-1033. [DOI] [PubMed] [Google Scholar]
- Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Predicted extension, compression and shearing of optic nerve head tissues. Exp Eye Res. 2007;85:312–322. doi: 10.1016/j.exer.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Silverman W, Locovei S, Dahl GP. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008 doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray DC, Ye ZC, Ransom BR. Functional connexin "hemichannels": a critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verselis VK. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys J. 2005;88:1725–1739. doi: 10.1529/biophysj.104.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JC, Kalapesi FB, Coroneo MT. Mechanosensitivity and the eye: cells coping with the pressure. Br J Ophthalmol. 2006;90:383–388. doi: 10.1136/bjo.2005.079905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- Uckermann O, Grosche J, Reichenbach A, Bringmann A. ATP-evoked calcium responses of radial glial (Müller) cells in the postnatal rabbit retina. J Neurosci Res. 2002;70:209–218. doi: 10.1002/jnr.10406. [DOI] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne D, Ford A, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Roman R, Lidofsky SD, Fitz JG. Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. ProcNat Acad Sci USA. 1996;93:12020–12025. doi: 10.1073/pnas.93.21.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax MB, Tezel G, Kobayashi S, Hernandez MR. Responses of different cell lines from ocular tissues to elevated hydrostatic pressure. Br J Ophthalmol. 2000;84:423–428. doi: 10.1136/bjo.84.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Agapova O, Parker A, Shannon W, Pecen P, Duncan J, Salvador-Silva M, Hernandez MR. DNA microarray analysis of gene expression in human optic nerve head astrocytes in response to hydrostatic pressure. Physiol Genom. 2004;17:157–169. doi: 10.1152/physiolgenomics.00182.2003. [DOI] [PubMed] [Google Scholar]
- Yang S, Cheek D, Westfall D, Buxton I. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res. 1994;74:401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- Yang XF, Liu WG, Shen H, Gong JB, Yu J, Hu WW, Lu ST, Zheng XJ, Fu WM. Correlation of cell apoptosis with brain edema and elevated intracranial pressure in traumatic brain injury. Ch J Traumatol. 2005;8:96–100. [PubMed] [Google Scholar]
- Zhang X, Li A, Ge J, Reigada D, Laties A, Mitchell C. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp Eye Res. 2007;85:637–643. doi: 10.1016/j.exer.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Zhang X, Reigada D, Zhang M, Laties AM, Mitchell CH. Increased ocular pressure increases vitreal levels of ATP. Association for Research in Vision and Ophthalmology (ARVO) 2006a E-Abstract #426. [Google Scholar]
- Zhang X, Zhang M, Laties AM, Mitchell CH. Stimulation of P2X7 receptors elevates Ca2+ and kills retinal ganglion cells. Invest Ophthmol Vis Sci. 2005;46:2183–2191. doi: 10.1167/iovs.05-0052. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang M, Laties AM, Mitchell CH. Balance of purines may determine life or death as A3 adenosine receptors prevent loss of retinal ganglion cells following P2X7 receptor stimulation. J Neurochem. 2006b;98:566–575. doi: 10.1111/j.1471-4159.2006.03900.x. [DOI] [PubMed] [Google Scholar]
- Zoidl G, Petrasch-Parwez E, Ray A, Meier C, Bunse S, Habbes HW, Dahl G, Dermietzel R. Localization of the pannexin1 protein at postsynaptic sites in the cerebral cortex and hippocampus. Neurosci. 2007;146:9–16. doi: 10.1016/j.neuroscience.2007.01.061. [DOI] [PubMed] [Google Scholar]