Abstract
Lipoxygenases (LOX) and cyclooxygenases (COX) react an achiral polyunsaturated fatty acid with oxygen to form a chiral peroxide product of high regio- and stereochemical purity. Both enzymes employ free radical chemistry reminiscent of hydrocarbon autoxidation but execute efficient control during catalysis to form a specific product over the multitude of isomers found in the non-enzymatic reaction. Exactly how both dioxygenases achieve this positional and stereo control is far from clear. We present four mechanistic models, not mutually exclusive, that could account for the specific reactions of molecular oxygen with a fatty acid in the LOX or COX active site.
Introduction
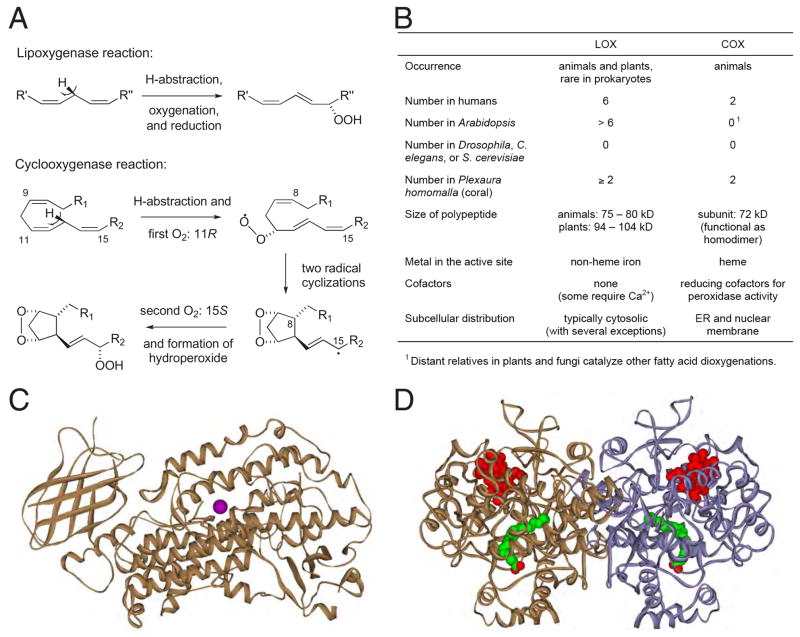
The oxygenation of polyunsaturated fatty acids by lipoxygenase (LOX) and cyclooxygenase (COX) enzymes is the initial step in the formation of a diverse array of lipid mediators [1–4]. LOX and COX products in animals commonly act by signaling through G-protein coupled receptors, and different eicosanoids appear to be involved in cellular homeostasis, proliferation and differentiation, and also in pathophysiological processes (inflammation and cancer). Generally the plant LOX pathway products are involved in the resistance to environmental stress and the defense against microbe and herbivore attack; some of the volatile products, like jasmonic acid and short-chain aldehydes, have a function in plant-plant communication. Arachidonic acid (20.4ω6) is the prototypical substrate in animals, linoleic or linolenic acids (18.2ω6, 18.3ω3) in plants. The initial products are fatty acid peroxides that give rise to local hormones classified collectively as “eicosanoids” (20-carbon lipids), or by the more general term “oxylipins” [5]. Although LOX and COX catalyze a similar dioxygenation of their fatty acid substrate (Figure 1A), the two enzymes are completely unrelated in sequence and three-dimensional structure as well as in their tissue expression and cellular localization (Figure 1B).
Figure 1. The lipoxygenase (LOX) and cyclooxygenase (COX) proteins.
(A) LOX and COX catalysis, forming fatty acid hydroperoxide and PGH2, respectively.
(B) Biochemical characteristics of LOX and COX enzymes.
(C) P. homomalla 8R-LOX with N-terminal β-barrel domain (left) and catalytic domain carrying the non-heme iron (magenta) [12].
(D) COX is a homodimer. The heme group (red) indicates the peroxidase active site, and arachidonic acid (green) is bound in the oxygenase active site.
LOX are non-heme iron enzymes consisting of a single polypeptide chain that is folded into two domains, the mostly α-helical catalytic domain and the N-terminal β-barrel domain that is involved in membrane binding (Figure 1C) [6–12]. There are five LOX enzymes in humans that are active on arachidonic acid, and these catalyze four distinct reactions (5S, 12R, 12S, or 15S oxygenation), the products being individual fatty acid hydroperoxides [1]. When one considers other species, another five distinct reactions are added (5R, 8R, 8S, 9S, 11R) [13–15]. An additional level of complexity is added by the numerous plant LOX enzymes, nearly all of which have evolved to utilize linoleic or linolenic acid as substrate, and many of which are quite specific for these fatty acids [5, 16]. As a consequence, only the catalytic iron and three out of the five amino acids that serve as iron ligands are absolutely conserved within the LOX gene family. There is considerable variation in all the other amino acids that could impinge into the LOX active site, perhaps not surprisingly in view of the spectrum of specific oxygenations that can be catalyzed. Even so, each specific LOX-catalyzed oxygenation involves a similar pair of double bonds on the fatty acid substrate and its coupling with O2, and therefore it seems almost certain that there is a common mechanism underlying the different LOX reaction specificities.
COX is a hemoprotein active as a homodimer that is inserted into one leaflet of the endoplasmic reticulum or nuclear membrane through four amphipathic α-helices (Figure 1D) [17–20]. Each monomer of the COX protein contains a separate peroxidase and oxygenase active site: the heme confers the peroxidase activity and also serves to oxidize a key tyrosine residue (Tyr385) that initiates substrate activation in the cyclooxygenase site [4]. The COX enzymes catalyze two oxygenations in the conversion of arachidonic acid to the prostaglandin endoperoxide product [4, 21, 22] (Figure 1A). Within the same oxygenase active site, the first oxygenation occurs in the 11R configuration and, following two cyclization reactions, the second oxygenation is 15S (Figure 1A). Thus, two specific reactions are accommodated sequentially within the same active site. The two mammalian cyclooxygenase isozymes, COX-1 and COX-2, each catalyze the identical reaction in converting arachidonic acid to PGH2. As a consequence, not only are key amino acids involved in catalysis conserved, but with a very few notable exceptions there is almost perfect conservation of all residues that line the oxygenase active site [23, 24].
Particularly from the point of view of what happens to the substrate, LOX and COX dioxygenases use similar chemistry in the oxygenation of the fatty acids [25, 26]. This chemistry is comparable to the reactions of polyunsaturated fatty acids during autoxidation [27–29]. For this reason we consider the mechanisms of regio- and stereocontrol in both enzymes in parallel. Part of the fascination related to this issue is that molecular oxygen is not covalently bound in the enzyme prior to reaction with substrate. And since the starting fatty acid substrates are achiral, the question arises how the LOX and COX enzymes oxygenate the substrate with such exquisite regio- and stereoselectivity? When trying to describe potential mechanisms for the control of oxygenation in the enzymatic reaction, we could discern four distinct concepts that may be applied by the LOX and COX dioxygenases. These may not be mutually exclusive. In the first part of this review we will briefly highlight the similarities between the oxygenation reactions catalyzed by LOX and COX enzymes (Part I). A short section will then cover reactions of peroxyl radicals with relevance to the discussion of the enzymatic reactions (Part II). We will then present four potential mechanisms which may contribute to the control of oxygenation by LOX and COX (Part III). Finally we will review specific experimental evidence as it relates to the different concepts of control that may be adopted by the structurally unrelated active sites of LOX and COX enzymes (Part IV).
Part I: Substrate activation and oxygenation
Similarities in fatty acid oxygenation in LOX and COX catalysis
The LOX and COX enzymes employ different strategies to activate the fatty acid substrate and thus facilitate reaction with O2. The proteins themselves require oxidation from the resting state to the catalytically active form. In both cases enzyme activation is accomplished by trace amounts of fatty acid hydroperoxides [30–33]. In LOX enzymes, a Fe2+ to Fe3+ oxidation generates a ferric iron-(Fe3+)-hydroxy species [34]. In COX enzymes, oxidation at the peroxidase active site is conducted through the protein to generate a tyrosyl radical (Tyr385) in the oxygenase active site [35]. The ensuing reactions on their fatty acid substrate are remarkably similar:
Both enzymes initiate a stereoselective hydrogen abstraction from the substrate, which is thus oxidized to a free radical capable of reaction with unactivated molecular oxygen.
The hydrogen is removed from the methylene (CH2) carbon of a selected 1,4-cis,cis pentadiene unit on the fatty acid substrate.
-
In both enzymes, hydrogen abstraction is followed by regioselective oxygen addition on the opposite face of the substrate (an antarafacial relationship between H-removal and oxygenation) to form a fatty acid peroxyl radical as the primary oxygenation product.
Steps in between then differ, with a more complex reaction pathway in COX catalysis.
Both enzymes complete the catalytic cycle and restore the catalytically competent enzyme by reduction of the peroxyl radical to form a hydroperoxide product (in LOX catalysis, a fatty acid hydroperoxide; in COX catalysis, the more complex endoperoxide-hydroperoxide, PGG2).
Once the catalytic cycles are started, both enzymes operate independent of external cofactors. Nonetheless, the catalytic cycles do not continue indefinitely, since to varying extents, the cyclooxygenases and lipoxygenases require periodic re-activation as the radical is occasionally lost from the active site, and activity may also diminish due to irreversible suicide inactivation.
Having noted that the LOX and COX reactions are so similar from the point of view of the substrate chemistry, we should also give emphasis to contrasts in the mechanisms of hydrogen abstraction from the point of view of the enzymes. Whereas the COX enzyme develops a tyrosyl radical to effect a direct radical hydrogen abstraction, current thinking on the LOX reaction mechanism implicates not a direct hydrogen atom transfer to the iron, but a proton coupled electron transfer (PCET) [36–39]; this invokes tunneling of an electron to the ferric iron while the proton simultaneously transfers to the hydroxide ligand, effectively completing a “H-atom” transfer with reduction of Fe3+-OH to Fe2+-OH2.
Oxygen handling by LOX and COX enzymes – contrast to monooxygenases such as cytochrome P450
There is no experimental evidence to support the possibility that LOX and COX enzymes actively bind molecular oxygen in order to control substrate oxygenation. In cyclooxygenases there are no appropriate sites for covalent oxygen binding in the oxygenase active site. In lipoxygenases, the non-heme iron participates directly in catalysis, but there is no evidence of Fe-O2 or Fe-OOH species, and it can be concluded from kinetic isotope effects that molecular oxygen is not bound to the iron, at least up to and including the initial hydrogen abstraction from the substrate [40]. These inferences on lack of oxygen binding make sense in terms of the free radical chemistry involved. O2 binding and/or activation is not necessary once the substrate fatty acid is oxidized to a free radical. Based on autoxidation chemistry there is essentially no energy barrier for formation of a peroxyl radical by addition of molecular oxygen to a fatty acid pentadienyl radical [28]. But this conclusion leaves open the major issue of how specific oxygenation is achieved.
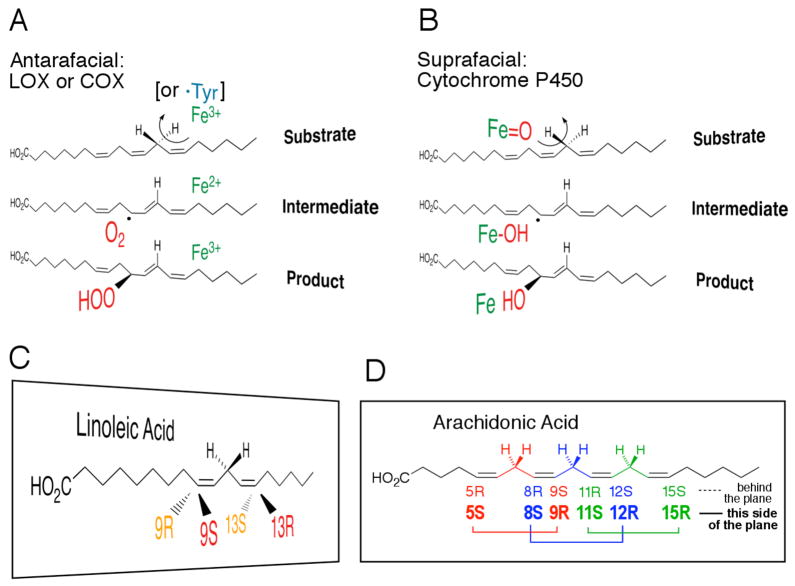
With regard to the question of stereocontrol, differences in oxygen handling are seen at their most extreme in comparing dioxygenases such as LOX and COX with monooxygenases such as the cytochrome P450s (Figure 2). P450 generates a high valent iron-oxo (FeV=O) species and uses this to abstract a hydrogen and catalyze hydroxylation through oxygen rebound predominantly on the same face of the substrate (suprafacial oxygenation) [41–43]. COX and LOX, having activated the substrate by a stereoselective hydrogen abstraction, insert O2 on the opposite face of the substrate relative to the hydrogen abstracted. A specific LOX reaction is known for each of the four available positions around the cis,cis pentadiene of linoleic acid (Figure 2C) and each of ten out of the available twelve positions around the four double bonds of arachidonic acid (Figure 2D). The antarafacial oxygenation has been demonstrated experimentally for different COX and LOX reactions by use of fatty acid substrates carrying a single stereospecific tritium or deuterium label on the CH2 between the two cis double bonds (e.g. refs [25, 26, 44–46]). Quite why the antarafacial “rule” is so stringently held to is not altogether clear, but it might relate to the “steric shielding” to be discussed in Part III; it could be that the substrate is pressed against, or melded up against the enzyme structure involved in H-abstraction, while the opposite, antarafacial, side is left open to interaction with O2.
Figure 2. Hydrogen abstraction and oxygenation.
(A) Antarafacial hydrogen abstraction and addition of oxygen in LOX and COX.
(B) Suprafacial reaction in cytochrome P450s.
(C) The relationship of R and S chirality of oxygenations in linoleic acid.
(D) The twelve possible positions of oxygenation in arachidonic acid: R and S configuration products are paired on either face of the fatty acid.
Part II: Free radical chemistry: some relevant reactions of peroxyl radicals
The initial hydrogen abstraction from the fatty acid generates a carbon radical that reacts freely with O2, giving a peroxyl radical. Based on autoxidation chemistry, peroxyl radicals have several possibilities for reaction. In trying to understand how the enzymes select only a single outcome, it is instructive to consider the potential options. In typical LOX reactions the peroxyl is quickly and efficiently reduced to a hydroperoxide. In COX, the initial peroxyl at C-11 instead forms the 9,11-endoperoxide, whereas the second peroxyl is reduced to form the 15-hydroperoxide product PGG2.
1. β-Fragmentation - the “off” reaction of O2
It is inherent to the chemistry of peroxyl radicals that, in the absence of other options, O2 will flip on and off an activated fatty acid carbon chain (Figure 3A). Whereas hydrogen abstraction from the fatty acid is essentially irreversible and fully commits the substrate to a reaction pathway, the “on” reaction of molecular oxygen, giving the peroxyl radical, is a reversible step. This reverse reaction with oxygen is termed β-fragmentation because the bond that breaks is one removed (in the β position) to the radical center. The off rate can be very fast and depends on which carbon the oxygen couples to on the activated substrate. The peroxyls formed at the end positions of the activated pentadiene (C-9 or C-13 in linoleic acid) and having a conjugated diene are relatively stable, exhibiting a half-life of milliseconds to seconds, although this is very dependent on the environment [47, 48]. By contrast, although peroxyls are readily formed on the middle carbon of an activated fatty acid pentadiene (the bis-allylic position and the site of hydrogen abstraction), the off rate is very fast (>2 × 106 s−1, at least 4000 times faster than for the conjugated peroxyls), making it difficult to trap the peroxyl as a hydroperoxide end-product [49–51]. This exemplifies a competition that is seen in all the following scenarios. The final pathway is determined by which reaction out competes the others.
Figure 3. Reactions of fatty acid peroxyl radicals.
(A)β-Fragmentation.
(B) cis to trans double bond isomerization.
(C) Formation of a cyclic peroxide (endoperoxide).
(D) Reduction of the peroxyl radical to a hydroperoxide end product.
2. cis to trans double bond isomerization
This is commonly observed in uncontrolled autoxidations, giving rise to hydroperoxides with a trans-trans conjugated diene [52] (Figure 3B), yet is rarely evident in enzymatic reactions. The exceptions are LOX-catalyzed transformations in which it could be inferred that stereocontrol is sacrificed through mobility of the reacting substrate in the active site, or indeed, escape of the peroxyl radical from the active site [53, 54]. A cis-to-trans double bond isomerization can occur during the on-off reactions of O2, i.e. β-fragmentation of the peroxyl radical. Only when the oxygen is attached can the carbon chain that was originally cis isomerize to the trans conformation (Figure 3B). The fact that this is an unusual occurrence in LOX and COX indicates that the enzymes direct the reaction pathway to another outcome that kinetically out-competes β- fragmentation.
3. Cyclization to an endoperoxide
A facile reaction of peroxyls, if a β,γ-double bond is available relative to the peroxyl-bearing carbon, is formation of a cyclic peroxide (endoperoxide) and an adjacent carbon radical [28] (Figure 3C). Similar to reduction to a hydroperoxide, this is an essentially irreversible step that promotes an ongoing reaction with the possibilities for a second oxygenation - the very basis of prostaglandin endoperoxide biosynthesis by the COX enzymes. Since this cyclization requires a β,γ-double bond in the molecule it is restricted to fatty acids with at least 3 double bonds (e.g. linolenic and arachidonic acids). Significantly, in LOX catalysis the cyclization to an endoperoxide is never observed. This suggests that the enzyme avoids this outcome by physically holding the fatty acid carbon chain with a potentially reactive β,γ-double bond in an unproductive conformation and by very efficiently trapping the peroxyl radical.
4. Reduction to a hydroperoxide
The peroxyl radical can be reduced by a hydrogen atom donor (e.g. the catalytic tyrosine as in cyclooxygenase) (Figure 3D), or by it accepting an electron rather than an H atom. In such a proton-coupled electron transfer, a lower valence metal will reduce the peroxyl and be oxidized in the process, a likely mechanism for forming the hydroperoxide in LOX catalysis. O2 has the potential for on-off reactions, and therefore the availability and correct positioning of the hydrogen/electron donor could have a major influence on the specificity of oxygenation catalyzed by the enzyme. Notably, hydroperoxide reduction does not occur in the initial 11R oxygenation in COX, but is vital to closing both LOX and COX transformations.
Part III: Four potential mechanisms of oxygenation control
Four concepts for the control of oxygenation of arachidonic acid that may be employed by LOX and COX enzymes are illustrated in Figure 4. Although each concept is presented in a way to distinguish it from the others, we want to emphasize that a combination of two or more of these mechanisms may actually operate.
Figure 4. Illustration of four hypotheses for oxygenation control in COX and LOX catalysis.
(A) Steric shielding: the enzyme blocks all but the desired reactive center of the pentadiene radical.
(B) Oxygen channeling: oxygen is directed from the outside to the desired site of reaction of the pentadiene radical.
(C) Selective peroxyl radical trapping: oxygen is free to react and β-fragment at all sites of the pentadiene. A strategically placed hydrogen donor traps only the desired product by reduction to the hydroperoxide.
(D) Radical localization: enzyme-induced rotation around a carbon bond traps the radical and leaves but one carbon reactive with oxygen.
1. Steric shielding
Steric shielding requires that the fatty acid substrate is bound in a way that only one of the reactive carbon centers of the pentadienyl radical is accessible to molecular oxygen (Figure 4A). Oxygen might have free access to some parts of the active site, but be restricted from others by the protein structure. There may also be a recess or pocket within the active site where O2 can reside due to favorable solubility properties and available space such that it reacts with the fatty acid radical intermediate in the intended positional and stereochemical configuration. It follows that the concept of an oxygen pocket goes part and parcel with steric shielding. In considering the steric shielding mechanism we recognize a major overlap with the function of the protein in binding and holding the substrate in the correct position for hydrogen abstraction and in allowing all subsequent steps of the catalytic cycle. This is almost certain to mask parts of the reactive substrate. It is evident that steric shielding is involved in controlling oxygenation, and possibly it is the sole relevant mechanism.
2. Oxygen channeling
In principle, a channel through the protein may direct molecular oxygen to the desired site of oxygenation on the activated fatty acid substrate, thus ensuring positional and stereospecificity (Figure 4B). Oxygen does have its particular solubility properties, favoring its high concentration in a hydrophobic environment, and this may influence the selection of a route and accumulation in membranes or within an area or compartment of the protein.
3. Selective peroxyl radical trapping
This concept entails that the oxygenation specificity of the enzyme depends not on the step of oxygen addition but on trapping of the peroxyl radical that leads to the intended product (Figure 4C). The key line of thinking here is that, although oxygen can react with the activated fatty acid at many positions, all these reactions are readily reversible (β-fragmentation). Formation of a distinct product depends on preventing further on-off reactions either by selective reduction of the intended peroxyl radical to a hydroperoxide or, in the case of the first oxygenation in COX catalysis, by promoting its cyclization to an endoperoxide. Clearly, this mechanism requires favorable positioning of a reductant (or a fatty acid double bond, in the case of the endoperoxide pathway). The resultant kinetic competition favors an enzyme-mediated trapping of one peroxyl radical over the trapping of all other peroxyls.
4. Radical localization
During fatty acid autoxidation the key pentadienyl radical intermediate adopts the lowest energy conformation, that of a planar structure with the unpaired electron spin (radical) delocalized over all 5 carbons. This permits reaction with oxygen in multiple positions and in R or S chirality. However, if force is applied and the pentadienyl radical is twisted out of plane, the unpaired electron (radical) becomes localized, giving way to selective reaction with O2 at that position (Figure 4D). This phenomenon is well precedented in free radical chemistry, in which the addition of bulky substituents has been shown to distort an otherwise planar system and prevent radical delocalization. Furthermore, localized radicals possess inherent chirality, and therefore in principle this mechanism could account for both the regio- and stereoselectivity of oxygenation.
Part IV: Experimental evidence for the four mechanisms of oxygenation control
The evidence in support of steric shielding, oxygen channeling and selective radical trapping are considered here individually for LOX and COX; the localized radical hypothesis will be reviewed thereafter.
Fatty acid binding in LOX, two directions of oxygen attack
The LOX enzyme family can synthesize an array of products and this is accommodated within closely related protein structures by changes in substrate binding. It is clear that the depth of fatty acid entry into the LOX active site is an important determinant of which set of cis,cis double bonds on the fatty acid chain are targeted for reaction [55, 56], and the head-to-tail orientation of the fatty acid in the active site is a further determinant of reaction specificity [57–59]. Superimposed on these substrate binding modes is one more distinct parameter: all oxygenations with S stereochemistry occur deep in the active site, relative to the point of substrate entry and the catalytic iron, while all oxygenations with R stereochemistry occur on the proximal or near side of the iron. The evidence for this remarkable conclusion rests partly on deduction based on conserved structure and mechanism [60], and partly on mutagenesis studies of a single active site residue conserved as Ala in S-LOX and Gly in R-LOX and shown to be a determinant of S or R stereocontrol [61]. Swapping the Ala to Gly in different S-LOX and Gly to Ala in R-LOX is found to largely switch the position and stereochemistry of oxygenation across one face of the reacting pentadiene (e.g. from 13S to 9R on linoleate, from 8R to 12S or 15S to 11R on arachidonate (cf. Figure 2C and 2D) [61, 62].
Steric shielding and oxygen channeling in LOX enzymes
It is impossible to separate a role for the enzyme in holding the fatty acid substrate in place from a potential role in shielding parts of the activated substrate from reaction with O2. The methylene (CH2) hydrogen targeted for removal has to be directed towards the catalytic iron as the site of hydrogen abstraction, and in the process, that face of the substrate may be shielded by protein, allowing reaction on the opposite face with molecular oxygen (antarafacial oxygenation). And part of that opposite face may be confined within the protein structure, leaving too little room for O2 to penetrate. If so, shielding could account for control of oxygenation. But the residues implicated in shielding are also intimately involved in “pressing” the substrate into a favorable position relative to the catalytic iron. Therefore, when “deshielding” is induced by substitution with a less space-filling residue, this is likely to permit a substrate mobility which is detrimental to catalysis. This issue is nicely illustrated by some mutations conducted as an ancillary part of a study on a potential oxygen channel in soybean LOX-1 [40, 63]. Linoleic acid was modeled into the active site, and two residues residing next to the face of the reacting pentadiene, Leu-546 and Leu-754, were postulated to confer a shielding function. While the individual mutations of Leu to Ala disturbed the normal product profile, the most prominent effect was a hundred-fold (Leu546Ala) or thousand-fold (Leu754Ala) drop in catalytic efficiency of the enzyme. This concurs with our observations that mutation of amino acid residues making more space in the active site often is associated with a drastic reduction in enzyme catalytic activity [61, 62]. Such results might imply that the normal cohesive fit of the naturally evolved enzyme structure with substrate is seriously disrupted by the appearance of more space per se, as well as by ensuing realignments of the protein that have permitted accommodation of the new amino acid within the overall structure. This type of effect can complicate interpretation of results within the idealized concepts of steric shielding or oxygen channeling.
It is implicit in the steric shielding concept that one position on the substrate is accessible to O2. This could be simply an appropriately placed pocket in the active site in which oxygen resides until substrate activation allows formation of the peroxyl radical. Alternatively, directed access of O2 could be facilitated by a channel transferring oxygen from outside the protein onto the desired position on the reacting substrate. The first X-ray crystal structure of soybean LOX-1 indicated a potential access route for oxygen (being too small for the fatty acid substrate), but this lacked a clear relationship to substrate binding or other experimental support [6]. Subsequently, Axelrod and colleagues presented more detailed proposals on the basis of a higher resolution structure, indicating that a different channel from the bulk solvent to the active site was more likely for oxygen transport [7]. Starting from these ideas, Klinman and co-workers recently showed that when Ile-553, one of the residues whose side chain lies next to the proposed oxygen channel, was mutated to Phe, the rate of oxygenation chemistry (kcat/KM(O2)) dropped by 20-fold, while kcat dropped only 2-fold [63]. This was interpreted as the larger Phe residue impeding access of O2 to the intermediate pentadienyl radical. Ile-553, however, is not only part of the proposed oxygen channel, this residue also forms part of the active site with potential contact to the fatty acid substrate. Experimental proof for a putative oxygen channel, therefore, would be the introduction of a block further up the oxygen channel where an interaction of the mutated residue with the fatty acid substrate can be excluded.
If oxygen channeling is a basis for stereocontrol in LOX enzymes, then it has to occur throughout the LOX protein family. The X-ray structure of the mammalian reticulocyte enzyme, a 15-LOX similar to soybean LOX-1, did not reveal a separate channel for oxygen delivery [8], and moreover, Kühn and coworkers report that mutagenesis of the residue equivalent to the putative channel-determinant of the soybean LOX-1 had no significant impact on catalysis [64]. Through molecular dynamics modeling of oxygen movement, however, a different channel for oxygen was identified, and this appeared to deliver O2 to the appropriate place for reaction in the 15S position of arachidonic acid [64]. It remains to be determined whether this is a functional oxygen channel during catalysis and whether it is conserved in other LOX enzymes.
One of the challenges to the oxygen channel concept, and perhaps to other models as well, is finding a suitable explanation for the ready switch in oxygenation induced by the Ala-Gly mutation referred to earlier [61]. How can directed oxygen delivery be of practical import when simply removing or adding a methyl group can switch specific oxygenation from one end of the reacting pentadiene to the other? The Ala-to-Gly mutation should make more space available, not block a channel. Indeed, the extra space near the Gly is associated with R-oxygenation nearby, but it is unclear how this also depresses reaction at the deep end of the active site where the normal S oxygenation occurs. Some insight into the complex ramifications of changing the Ala to Gly comes from the observation that the EPR spectrum of the Ala542Gly mutant of soybean LOX-1 is markedly altered from wild-type, suggesting a change in the ligand environment of the catalytic iron, presumably induced by conformational adjustments within the protein [62]. Also the catalytic activities were generally reduced, by up to 90% of wild-type [61, 62], indicating another facet of the wide-ranging effects of this mutation within the LOX active site.
Another confounding observation is that fatty acid isomers containing an extra double bond in conjugation with the reacting pentadiene are stereospecifically oxygenated two carbons away from the usual position. For example, when the 13trans double bond analogue of arachidonic acid (5,8,11,13–20.4ω7) is used as a substrate for 12S-LOX, 14S-HPETE is produced as opposed to the usual 12S-HPETE product from arachidonic acid [65]. Similarly, the 5Z,8Z,11Z,14Z,16E-analogue of eicosapentaenoic acid is preferentially oxygenated by soybean LOX-1 at the 17S position, rather than at 15S [66]. These results might argue against the concept that oxygen is channeled toward one specific position on the carbon chain. Certainly, whatever mechanism controls oxygenation, there is considerable flexibility in the system.
Radical trapping and LOX reaction specificity
The control of oxygenation could be provided, in principle, by selective trapping of the desired peroxyl radical as the hydroperoxide. Taking the extreme case, if this were the sole basis of stereocontrol, oxygen would have to go on and off the wrong carbons until being trapped at the correct position. It follows, therefore, that the off rates of peroxyl oxygens on the fatty acid be considerably faster than the overall turnover rate of the enzyme. For some LOX enzymes, this criterion presents an almost insurmountable challenge. The soybean L-1 enzyme and the coral 8R-LOX exhibit turnover numbers of ~300/sec [67, 68], meaning that an average catalytic cycle requires only ~3 msec for completion. Although peroxyl radicals on the middle bis-allylic carbon are exceedingly unstable, with off rates of 106/sec, the greater stability of the conjugated peroxyls is hardly compatible with multiple on-off cycles being accrued within 3 msec. Admittedly, many LOX enzymes exhibit hundred-fold lower turnover numbers than the fastest enzymes, in which case selective radical trapping is a more viable mechanism of stereocontrol, but this would require that the slow and fast enzymes have fundamentally different mechanisms, which is improbable in view of all the other reaction characteristics they share in common.
Although by the preceding argument we would dismiss the involvement of radical trapping as a primary means of stereocontrol, it must be involved as a crucial step in trapping the previously selected peroxyl radical as the final hydroperoxide product. Its importance in maintaining high LOX reaction specificity is illustrated by contrast with an exceptional case in which radical trapping is amiss: the type-2 plant LOX produce a mixture of hydroperoxides with cis,trans or trans,trans conjugated dienes [54], and as outlined earlier the trans,trans products are generated through the on/off reactions of peroxyl radicals. The implications are that either the fatty acid radicals have considerable mobility and a long lifetime within the active site, or that they escape and undergo rearrangements outside the enzyme. (The fidelity of the hydrogen abstraction step has not been reported for these type-2 plant LOX enzymes, but presumably this approach could help distinguish between these two possibilities.) Either way, the results affirm the importance of peroxyl radical trapping as part of the strict stereocontrol mechanisms characteristic of the majority of LOX enzymes.
Steric shielding in the cyclooxygenase reaction
In addition to providing direct insight into the control of oxygenation, the X-ray structures of COX containing bound fatty acid substrate have helped direct mutagenesis studies and served as a platform for molecular dynamics simulations. In a COX enzyme containing a catalytically inert cobalt heme group, arachidonic acid is bound in the oxygenase active site with the critical pro-S hydrogen at C-13 suitably positioned for hydrogen abstraction by an incipient Tyr-385 radical (Figure 6A) [20]. During catalysis, this hydrogen removal is followed by 11R oxygenation, and all the experimental evidence from product analyses suggests that the stereocontrol of this step is very tight. Despite repeated attempts, no mutant COX enzyme has been described that catalyzes the inverted 11S oxygenation to a significant extent (> 2%) even though many of the mutants synthesized more 11R-HETE at the expense of the prostaglandin product [69–71] – clear evidence of an effect on the later steps in catalysis. Only in the reaction with linoleic acid has a significant production of the equivalent 9S-HPODE been observed by wild-type COX enzymes: the ratio of 9R-HPODE to 9S-HPODE was 4:1 for COX-1 and 5:1 for COX-2 [72, 73]. Inspection of the structure of linoleic acid bound in the COX-1 active site and comparison with arachidonic acid reveals that their carboxylate groups are in very similar positions at the entrance to the oxygenase active site, as are the linoleate 9,12 double bonds with the equivalent 11,14 double bonds of arachidonate [74]. As the intervening chain of linoleate is two carbons shorter, it is stretched out while the arachidonate chain has more options for flexibility and can be more convoluted. Perhaps this allows arachidonate to better shield its C-11 position against the hydrophobic face of the oxygenase channel.
Figure 6. Stereocontrol of the initial 11R oxygenation in COX catalysis: key amino acids and molecular dynamic computations of available space and oxygen mobility.
(A) Reaction is initiated by conduction of a radical from the heme group through Tyr-385 to C-13 of arachidonic acid (red).
(B) The arachidonate C11-C15 pentadienyl radical (cyan) in the two monomers of COX-1 showing the computed free space above (blue bars) and below (red bars) showing the most free space under the 11R position [76].
(C) Representative traces of O2 mobility during a timeframe of 0 to 500 ps (blue to red) [76]).
Molecular dynamics simulations support steric shielding as an important element of control in the 11R oxygenation [75, 76]. The 11S face of the pentadienyl radical (the hydrogen abstraction side) is well shielded from access of molecular oxygen; the distance between carbons 11, 13, and 15 of a planar arachidonate-derived pentadienyl radical and the nearest protein atom is less than 1 Å on average (Figure 6B) [76]. In contrast, the simulations indicate between 2 and 3 Å of open space on the pro-R side of C-11, providing sufficient room for the access of oxygen at this position. Based on the x-ray crystal structure of arachidonic acid-bound COX-1, Malkowski and co-workers suggested that oxygen resides in an oxygen pocket on the pro-R side of C-11 [20]. Mutation of residues forming this putative oxygen pocket does not, however, provide a clear picture. The mutations still allow prostaglandin synthesis to occur with a tendency to change the proportions of the 11R-HETE by-product [70, 77]. The MD calculations, however, provide support for the existence of this putative oxygen pocket. Not only do the calculations reveal the favored space under the 11R face of the arachidonyl radical (Fig. 6B), they also show that this space remains occupied by individual oxygen molecules. In the MD simulations, an oxygen molecule was placed below the 11R position and its mobility monitored during a timeframe of 500 ps [76]. The two most commonly observed outcomes were that the O2 exited the active site through the substrate entry channel, or remained in place, the latter strongly suggesting the existence of a shielded O2 pocket (Fig. 6C). Interestingly, the MD calculations reveal different outcomes for each monomer of the COX dimer, and more recent experimental work suggest that the two monomers display half-site reactivity, i.e. only one is functional during catalysis [78]. Half-of-sites reactivity is a well-recognized phenomenon in oligomeric enzymes composed of identical subunits, a negative cooperativity that is usually attributed to the initial binding of ligand inducing a conformational change that affects the whole molecule [79, 80]. Here, the apparent concordance of calculation and experiment should be viewed with some caution, because the X-ray data on which the MD calculations are modeled provided data for only one monomer, and this was duplicated in silico to give a dimer with an arachidonate molecule in each half – a condition that may not occur in actual COX catalysis. The issue of half-site reactivity could be further explored with MD calculations were data available for a COX dimer containing a single molecule of arachidonic acid.
Trapping the 11-peroxyl radical
In the next catalytic step, the task of the enzyme is to facilitate formation of the 9,11-endoperoxide by holding the reacting fatty acid in a kinked arrangement with the 11R-peroxyl close to the pro-R face of C-9. The 5-exo cyclization has to out-compete hydrogen atom transfer (reduction to the hydroperoxide) and β-fragmentation. Based on solution phase measurements alone this would be problematic. Approximate lower bounds for the relevant rate constants are >800 s−1 for 5-exo cyclization, versus >690 s−1 for β-fragmentation and a potential value of 1.4 × 104 M−1s−1 for reduction, the latter based on the reaction of peroxyl radicals with p-methylphenol in benzene at 37°C [81 ]. If this was all there was to it, 5-exo cyclization would not be able to compete, being too slow by a factor of ~10. Yet 9,11-endoperoxide formation wins out over reduction to 11R-HPETE by margins of 20-fold, possibly for several reasons. First, the enzyme ensures a suitable fatty acid conformation, and thus keeps the reaction geometry favorable. Secondly, the enzyme may not permit the fatty acid mobility required for reduction of the 11R-peroxyl by Tyr-385. And thirdly, the rate measurements for model compounds in benzene may be an overestimate because reaction of peroxyl radicals with phenols drops markedly in hydrogen bond-accepting solvents [82], and in the enzyme Tyr-385 is hydrogen-bonded to Tyr-348 [83]. Added together, 5-exo-cyclization wins out handsomely over reduction, especially in the wild-type COX enzymes. That the reduction to 11R-HPETE is a potentially facile reaction is evidenced by the increased abundance of this side-product in many COX mutants in which the normal spatial arrangements are upset [69, 70, 77].
Oxygenation control by radical localization
When it comes to the final 15S-oxygenation, it is important to consider that the reacting fatty acid intermediate is structurally quite unlike arachidonic acid. The newly formed cyclopentyl ring with two fixed side-chain substituents and harboring the 9,11-endoperoxide is a foreshortened molecule that must be restrained in the active site quite differently from the linear arachidonic acid. One reflection of this relates to contacts with the active site residue Val-349. X-ray crystallography shows that Val-349 has contacts with the C-3 and C-4 carbons of the arachidonic acid chain, far removed from the oxygenation action at the 11,14-double bonds [20]. Yet Val-349 is a critical determinant of the oxygenation specificity at C-15 [84, 85]. One can imagine that the reacting fatty acid must undergo substantial movement to bring Val-349 into play. This brings up an important distinction between the first and second oxygenations of COX enzymes: in marked contrast to the initial 11R oxygenation, several examples in COX catalysis show variable specificity of the second oxygenation with the normal 15S specificity swapping to 15R [24, 84]. Before addressing the basis for this stereocontrol we want to consider the pros and cons of localized radicals in COX and LOX catalysis. If radical distortion plays a role in any of these reactions, it is in the C-15 oxygenation of prostaglandin synthesis.
EPR studies on model compounds set the precedent for the existence of localized radicals, Figure 7A [86, 87]. Although simple benzyl radicals are planar and therefore delocalized, when the two benzylic hydrogens are replaced by bulky tertiary-butyl substituents, steric interactions force the substituents out of the plane of the aromatic ring, delocalization is no longer possible, and the radical is localized on the benzylic carbon [86]. The unpaired electron is localized because its 2pz orbitals are perpendicular relative to the 2pz orbitals of the aromatic ring therefore preventing overlapping and radical delocalization. A similar enforced lack of planarity will localize an allyl radical on a single carbon [87]. In a pentadienyl radical, two different localized radicals can be formed, depending on which carbon-carbon bond is twisted out of plane, (Figure 7B). Rotation around the 3,4 carbon bond results in an allyl radical delocalized from C-1 to C-3 and a 4Z-double bond; rotation around the 1,2 carbon bond leaves a conjugated 2E,4Z-diene and a localized radical at C-1. Whereas delocalized radicals are planar and have no stereochemical preference for reaction, a localized carbon radical is pyramidal and potentially chiral (Figure 7C). The direction of rotation could dictate the stereochemistry through the different steric interactions of the three substituents (H, CH, and CH2, Figure 7C), illustrated for counter-clockwise or clockwise rotation at C-1 of a fatty acid pentadiene in Figure 7D.
Figure 7. Delocalized and localized radicals.
(A) Planar radicals are delocalized; tertiary-butyl substituents twist the carbons out of plane, producing a localized radical.
(B) Rotation of a pentadienyl radical out of plane produces a localized or allyl radical.
(C) Localized radicals are pyramidal and flip between two conformations (stereoisomers).
(D) Depending on the direction of rotation of the 1,2-bond the localized radical adopts a pro-S or pro-R configuration.
On applying these principles to the first oxygenation in COX catalysis (equivalent also to a typical LOX-catalyzed oxygenation), a 90° rotation around the 11,12 bond would generate a complete bias for stereospecific oxygenation at C-11 [76, 88]. This sounds attractive, except that this would require either a large extra energy expenditure and/or very large movements of the protein during catalysis. If the double bonds were suitably positioned (out of plane) prior to hydrogen abstraction, then the H-removal is estimated to require an extra energy input of ~22 kcal/mol (equivalent to the difference between the C-H bond dissociation energies in propane, 98 kcal/mol, and 1,4-pentadiene, 76 kcal/mol). On the other hand, if the pentadiene remained planar to facilitate hydrogen abstraction, subsequently the protein would be required to twist the pentadiene radical to enforce its localization at C-11. Admittedly, by this route the energy expenditures would be sequential rather than additive as in the first scenario. But is there any precedent for such a twist being effected during enzymatic catalysis? Although it has been argued eloquently that enzymes are mechanical devices – large structures that utilize more than simply the active site structure in catalysis [89] – the evidence points to the occurrence of relatively small motions within the protein during a catalytic cycle [90–94]. Could such small motions result in a twisted pentadienyl radical? In favor of the argument, it is not necessary to rotate the bond by 90° to achieve a sufficiently strong bias for radical localization. For example, a rotation of about 55° (10 kcal/mol energy barrier) will result in a 50% higher spin density at C-1 compared to C-3 and C-5 of a pentadiene radical [75]. Furthermore, regiocontrol of the oxygenation in COX or LOX catalysis is not absolute since a small percentage of oxygenation at the other end of the pentadiene is always observed.
Notably, the arguments against the localized radical hypothesis are less formidable in one special instance – in the second oxygenation of COX catalysis. Here, the reactant carbon radical is already formed, so the extra energy input is limited to the modest requirement for torsion. Furthermore, and in complete contrast to the initial oxygenation at C-11, the C-15 oxygenation is readily switched from 15S to 15R or vice versa by Val349Ile or Ser530Thr mutations in the COX active site [84, 85]. Nominally, the reacting intermediate is an allyl radical delocalized through carbons C13,14,15. Since an allylic radical is about 12 kcal/mol less stable than a pentadienyl radical, the barrier for rotation of the 13,14 bond by 90° can be estimated at ~10 kcal/mol. In fact, the required energy could be far less than this, because observations from autoxidative formation of prostaglandin analogues indicate there is already a bias that strongly favors C-15 oxygenation over reaction at C-13. Only the 15-hydroxy prostaglandin isomers have been identified among the major autoxidation products [95–97], most likely due to steric hindrance from the cyclopentane ring [98]. If the omega carbon chain of the reacting endoperoxide were twisted one way or the other, this could be the mechanism underlying the 15R/15S oxygenation specificity. By the steric shielding hypothesis, it is hard to understand how a 95% switch in stereocontrol could be induced by a simple Ser530Thr substitution, whereas it is less of a stretch to imagine this could alter the position of the reacting fatty acid omega chain and produce a localized radical. The concept that the physical conformation of the reactant is a determinant of 15R/15S specificity musters support from observations with two mutant forms of COX-2 (Leu384Phe and Gly526Ser) [70]. These enzymes each form PGG2 with the usual 15S-hydroperoxide together with another product, a diepoxide that is oxygenated to the 15R-hydroperoxide in this final step. So, these COX enzymes form purely 15S-hydroperoxy-PGG2 or purely 15R-hydroperoxy-diepoxide in the same active site. We infer from this that the structure of the active site is not set up or designed for synthesis of a particular stereochemistry. Rather, formation of a 15R- or 15S-hydroperoxy product may depend on the induced conformation of the omega side chain of the reacting intermediate. One interpretation is that the resulting radical localization changes with the physical positioning of the reacting intermediate. Alternatively, the physical positioning of the reacting intermediate might affect which face of the radical is shielded. In COX, for example, if a different conformation was assumed about the 12–13 single bond in the allyl radical (Figure 1A), the configuration at C-15 could be reversed. In this mechanism, the shielded and open regions of the protein are unchanged, but the face of the radical exposed to the open face depends on the conformation about one C-C single bond, and this conformation could be affected by the interaction of the omega side chain with the mutant residues. This argument does not depend on the localized radical hypothesis. We note that similar issues arise in the remarkable case of acetylation of Ser-530 by aspirin in COX-2, a modification that blocks PG endoperoxide synthesis and instead induces formation solely of 15R-HETE [99, 100]. Incidentally, note that this switch in stereochemistry at C-15 differs from the stereochemical switch induced in LOX enzymes by the Ala-Gly mutations, which redirect oxygenation from one end of the pentadiene to the other (e.g. 15S to 11R).
Just as EPR was used to establish the chemical precedent for the existence of localized radicals, so the methodology can be applied to the detection and characterization of fatty acid radicals in LOX and COX enzymes. One of the fundamental issues is the relatively slow time-scale involved in recording the EPR spectrum. Given the turnover numbers of the enzymes (~ 300/sec for soybean LOX-1, about 60/sec for COX), it is practically impossible to initiate the enzymic reaction under an O2 atmosphere and then freeze-trap the fatty acid radical to liquid nitrogen temperatures or below. Anaerobic conditions can prevent multiple turnovers and the use of selective deuterium labels on the fatty acid helps pinpoint the location of the radical on the carbon chain. Nonetheless, although EPR has been used successfully to detect radicals in “purple” soybean LOX (a ferric iron-peroxide complex) [101], and amongst derivatives of the oxygenated products [102], there is no direct evidence related to the structure of the initial fatty acid radical in lipoxygenases. By contrast, EPR techniques applied to COX catalysis have approached closer to the issue. Tsai and co-workers found no indication for radical localization in the initial reaction of native COX-2 implying a planar delocalized pentadienyl radical as the intermediate that reacts with oxygen. Since these studies were carried out in the absence of oxygen, only the radical obtained from the initial hydrogen abstraction could be analyzed, equivalent to the formation of the 11R-peroxyl radical in native COX. Thus, it was not possible to study the second oxygenation at C-15 which uses the bicyclic C13-C15 allyl radical as the substrate. When the formation of 15R-HETE by aspirin-acetylated COX-2 was analyzed using various deuterated arachidonic acids some of the EPR spectra were different from native COX-2 and resembled spectra found earlier for COX-1 [88, 103]. The data were most fittingly explained by assuming a twisted allyl radical which was delocalized either from C-11 to C-13 or from C-13 to C-15. The possible occurrence of a different radical (allylic versus pentadiene) was explained by the more constrained active site of COX-1 and acetylated COX-2 in comparison to COX-2. The authors also inferred from the similarity of the EPR spectra with different deuterated arachidonic acid substrates that the divergence of catalysis into different products (PGG2, 11-HPETE, 15-HPETE) occurs at a point after formation of the pentadienyl radical [88]. This hypothesis remains compatible with the findings from kinetic analyses which propose three different arrangements of the arachidonate molecule in the active site, each giving rise to one of the three products [104]. Overall, the balance of evidence suggests that a localized radical is an unlikely explanation for the control of the initial oxygenation in COX catalysis, or in LOX, although the evidence there is less direct. If there is a role, then the second oxygenation of COX catalysis seems the most likely candidate for involvement of a localized radical in stereocontrol.
Concluding remarks
The major puzzling issue in understanding how the LOX and COX enzymes control the regio-and stereochemistry of their catalytic reactions is that the second substrate, oxygen, has untethered if not uncontrolled access to the reactive intermediate. If sufficient computing power could be brought to bear on the issue, one approach to addressing the reaction mechanisms would be to extend the type of molecular dynamics study already reported for the first oxygenation of COX catalysis [75, 76]. The MD simulations of oxygen mobility in Figure 6 as well as the more extensive results presented in the original article give an excellent impression of the power of this methodology. Several of the reaction trajectories (the computed movements of individual O2 molecules) provide quite compelling evidence for the existence of a shielded oxygen pocket. In principle there is no limitation to extending the MD studies to the second oxygenation. A virtual bicylic endoperoxide with C13-15 allyl radical would be “observed” in the oxygenase active site. Mutation of Ser530 to Thr almost completely switches the C-15 hydroxyl stereochemistry of the prostaglandin products [84], providing a discrete focus for interpreting the credibility of the computations. Similar diagnostic mutations could be applied in evaluating the MD computations in LOX catalysis.
To sum up: Steric shielding of the reactive intermediate is an integral part of the control of regio-and stereochemistry in LOX and COX catalysis – and this is true whether or not any other mechanisms come into play. Direction of O2 via an oxygen channel onto the correct part of the substrate free radical has been advocated for very select instances and with evidence in support. But it is not obvious that oxygen channeling is conserved as a general mechanism, and given the identical mechanistic issues that are successfully tackled within the entire LOX superfamily (and the similarity of fatty acid oxygenation in COX catalysis), this leads us to question whether oxygen channeling could be a significant controlling factor. Although, in principle, selective radical trapping in combination with multiple on-off cycles of O2 onto the reacting carbon radical could account for the correct regio- and stereoselectivity of oxygenation, it appears that the rate is too slow for such a mechanism to be viable. On the other hand, selective radical trapping is important in completing both the LOX and COX catalytic cycles and it also constitutes an integral step in the cyclooxygenase reaction in driving formation of the 9,11-endoperoxide ring. Finally, radical localization via distortion of planar fatty acid carbon radicals has somewhat formidable energy barriers, but current evidence cannot discount the possibility of its involvement in the second oxygenation of COX catalysis.
Figure 5. Structures of R- and S-lipoxygenases and modeling of arachidonic acid in the 8R-LOX active site.
(A) Superposition of the X-ray structures of coral 8R-LOX (blue) [12] and mammalian 15S-LOX-1 (gold) [8]. Arachidonic acid (gray, with red carboxyl group) and molecular oxygen (green) are modeled in the active site in suitable positions for 8R-LOX catalysis.
(B) Close-up of the 8R-LOX active site in the same orientation. C-10 of arachidonic acid is located at the open position of the iron coordination sphere in a suitable position for hydrogen abstraction, and O2 is depicted in the Gly-428 pocket [12].
Acknowledgments
This work was supported by NIH grants GM-53638, GM-15431 and GM-076592.
Abbreviations
- AA
arachidonic acid
- LOX
lipoxygenase
- COX
cyclooxygenase
- HETE
hydroxyeicosatetraenoic acid
- HPETE
hydroperoxyeicosatetraenoic acid
- HPODE
hydroperoxyoctadecadieneoic acid
- PG
prostaglandin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brash AR. Lipoxygenases: Occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- 2.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004;39:1125–1132. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- 4.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: Structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 5.Liavonchanka A, Feussner I. Lipoxygenases: occurrence, functions and catalysis. J Plant Physiol. 2006;163:348–357. doi: 10.1016/j.jplph.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Boyington JC, Gaffney BJ, Amzel LM. The three-dimensional structure of an arachidonic acid 15-lipoxygenase. Science. 1993;260:1482–1486. doi: 10.1126/science.8502991. [DOI] [PubMed] [Google Scholar]
- 7.Minor W, Steczko J, Stec B, Otwinowski Z, Bolin JT, Walter R, Axelrod B. Crystal structure of the soybean lipoxygenase L-1 at 1.4Å resolution. Biochemistry. 1996;35:10687–10701. doi: 10.1021/bi960576u. [DOI] [PubMed] [Google Scholar]
- 8.Gillmor SA, Villaseñor A, Fletterick R, Sigal E, Browner MF. The structure of mammalian 15-lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity. Nature Struct Biol. 1997;4:1003–1009. doi: 10.1038/nsb1297-1003. [DOI] [PubMed] [Google Scholar]
- 9.Skrzypczak-Jankun E, Bross RA, Carroll RT, Dunham WR, Funk MO., Jr Three-dimensional structure of a purple lipoxygenase. J Am Chem Soc. 2001;123:10814–10820. doi: 10.1021/ja011759t. [DOI] [PubMed] [Google Scholar]
- 10.Dainese E, Sabatucci A, van Zadelhoff G, Angelucci CB, Vachette P, Veldink GA, Agro AF, Maccarrone M. Structural stability of soybean lipoxygenase-1 in solution as probed by small angle X-ray scattering. J Mol Biol. 2005;349:143–152. doi: 10.1016/j.jmb.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Hammel M, Walther M, Prassl R, Kuhn H. Structural flexibility of the N-terminal beta-barrel domain of 15-lipoxygenase-1 probed by small angle X-ray scattering. Functional consequences for activity regulation and membrane binding. J Mol Biol. 2004;343:917–929. doi: 10.1016/j.jmb.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 12.Oldham ML, Brash AR, Newcomer ME. Insights from the X-ray crystal structure of coral 8R-lipoxygenase: Calcium activation via a C2-like domain and a structural basis of product chirality. J Biol Chem. 2005;39:39545–39552. doi: 10.1074/jbc.M506675200. [DOI] [PubMed] [Google Scholar]
- 13.Krieg P, Marks F, Fürstenberger G. A gene cluster encoding human epidermis-type lipoxygenases at chromosome 17p13.1: cloning, physical mapping, and expression. Genomics. 2001;73:323–300. doi: 10.1006/geno.2001.6519. [DOI] [PubMed] [Google Scholar]
- 14.Funk CD, Chen XS, Johnson EN, Zhao L. Lipoxygenase genes and their targeted disruption. Prostaglandins Other Lipid Mediat. 2002;68–69:303–312. doi: 10.1016/s0090-6980(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 15.Schneider C, Brash AR. Lipoxygenase-catalyzed formation of R-configuration hydroperoxides. Prostaglandins Other Lipid Mediat. 2002;68–69:291–301. doi: 10.1016/s0090-6980(02)00041-2. [DOI] [PubMed] [Google Scholar]
- 16.Feussner I, Wasternack C. The lipoxygenase pathway. Annu Rev Plant Biol. 2002;53:275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- 17.Picot D, Loll PJ, Garavito RM. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature. 1994;367:243–249. doi: 10.1038/367243a0. [DOI] [PubMed] [Google Scholar]
- 18.Luong C, Miller A, Barnett J, Chow J, Ramesha C, Browner MF. Flexibility of the NSAID binding site in the structure of human cyclooxygenase-2. Nature Struct Biol. 1996;3:927–933. doi: 10.1038/nsb1196-927. [DOI] [PubMed] [Google Scholar]
- 19.Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY, Gildehaus JM, Miyashiro JM, Penning TD, Seibert K, Isakson PC, Stallings WC. Structural basis for the selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384:644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 20.Malkowski MG, Ginell SL, Smith WL, Garavito RM. The productive conformation of arachidonic acid bound to prostaglandin synthase. Science. 2000;289:1933–1937. doi: 10.1126/science.289.5486.1933. [DOI] [PubMed] [Google Scholar]
- 21.van der Donk WA, Tsai AL, Kulmacz RJ. The cyclooxygenase reaction mechanism. Biochemistry. 2002;41:15451–15458. doi: 10.1021/bi026938h. [DOI] [PubMed] [Google Scholar]
- 22.Rouzer CA, Marnett LJ. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem Rev. 2003;103:2239–2304. doi: 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- 23.Gierse JK, McDonald JJ, Hauser SD, Rangwala SH, Koboldt CM, Seibert K. A single amino acid difference between cyclooxygenase-1 (COX-1) and -2 (COX-2) reverses the selectivity of COX-2 specific inhibitors. J Biol Chem. 1996;271:15810–15814. doi: 10.1074/jbc.271.26.15810. [DOI] [PubMed] [Google Scholar]
- 24.Valmsen K, Järving I, Boeglin WE, Varvas K, Koljak R, Pehk T, Brash AR, Samel N. The origin of 15R-prostaglandins in the Caribbean coral Plexaura homomalla: Molecular cloning and expression of a novel cyclooxygenase. Proc Natl Acad Sci USA. 2001;98:7700–7705. doi: 10.1073/pnas.131022398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamberg M, Samuelsson B. On the specificity of the oxygenation of unsaturated fatty acids catalyzed by soybean lipoxidase. J Biol Chem. 1967;242:5329–5335. [PubMed] [Google Scholar]
- 26.Hamberg M, Samuelsson B. Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J Biol Chem. 1967;242:5344–5354. [PubMed] [Google Scholar]
- 27.Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radical Biol Med. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- 28.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 29.Yin H, Porter NA. New insights regarding the autoxidation of polyunsaturated fatty acids. Antioxid Redox Signal. 2005;7:170–184. doi: 10.1089/ars.2005.7.170. [DOI] [PubMed] [Google Scholar]
- 30.Smith WL, Lands WE. Oxygenation of unsaturated fatty acids by soybean lipoxygenase. J Biol Chem. 1972;247:1038–1047. [PubMed] [Google Scholar]
- 31.Schilstra MJ, Veldink GA, Verhagen J, Vliegenthart JFG. Effect of lipid hydroperoxide on lipoxygenase kinetics. Biochemistry. 1992;31:7692–7799. doi: 10.1021/bi00148a033. [DOI] [PubMed] [Google Scholar]
- 32.Smith WL, Lands WE. Oxygenation of polyunsaturated fatty acids during prostaglandin biosynthesis by sheep vesicular gland. Biochemistry. 1972;11:3276–3285. doi: 10.1021/bi00767a024. [DOI] [PubMed] [Google Scholar]
- 33.Kulmacz RJ, Ren Y, Tsai AL, Palmer G. Prostaglandin H synthase: spectroscopic studies of the interaction with hydroperoxides and with indomethacin. Biochemistry. 1990;29:8760–8771. doi: 10.1021/bi00489a037. [DOI] [PubMed] [Google Scholar]
- 34.Scarrow RC, Trimitsis MG, Buck CP, Grove GN, Cowling RA, Nelson MJ. X-ray spectroscopy of the iron site in soybean lipoxygenase-1: changes in coordination upon oxidation or addition of methanol. Biochemistry. 1994;33:15023–15035. doi: 10.1021/bi00254a011. [DOI] [PubMed] [Google Scholar]
- 35.Dietz R, Nastainczyk W, Ruf HH. Higher oxidation states of prostaglandin H synthase. Rapid electronic spectroscopy detected two spectral intermediates during the peroxidase reaction with prostaglandin G2. Eur J Biochem. 1988;171:321–328. doi: 10.1111/j.1432-1033.1988.tb13793.x. [DOI] [PubMed] [Google Scholar]
- 36.Jonsson T, Glickman MH, Sun SJ, Klinman JP. Experimental evidence for extensive tunneling of hydrogen in the lipoxygenase reaction: Implications for enzyme catalysis. J Am Chem Soc. 1996;118:10319–10320. [Google Scholar]
- 37.Lehnert N, Solomon EI. Density-functional investigation on the mechanism of H-atom abstraction by lipoxygenase. J Biol Inorg Chem. 2003;8:294–305. doi: 10.1007/s00775-002-0415-6. [DOI] [PubMed] [Google Scholar]
- 38.Hatcher E, Soudackov AV, Hammes-Schiffer S. Proton-coupled electron transfer in soybean lipoxygenase. J Am Chem Soc. 2004;126:5763–5775. doi: 10.1021/ja039606o. [DOI] [PubMed] [Google Scholar]
- 39.Fukuzumi S. Proton-coupled electron transfer of unsaturated fatty acids and mechanistic insight into lipoxygenase. Helv Chim Acta. 2006;89:2425–2440. [Google Scholar]
- 40.Knapp MJ, Klinman JP. Kinetic studies of oxygen reactivity in soybean lipoxygenase-1. Biochemistry. 2003;42:11466–11475. doi: 10.1021/bi0300884. [DOI] [PubMed] [Google Scholar]
- 41.Groves JT, McClusky GA, White RE, Coon MJ. Aliphatic hydroxylation by highly purified liver microsomal cytochrome P-450. Evidence for a carbon radical intermediate. Biochem Biophys Res Commun. 1978;81:154–160. doi: 10.1016/0006-291x(78)91643-1. [DOI] [PubMed] [Google Scholar]
- 42.White RE, Miller JP, Favreau LV, Bhattacharyya A. Stereochemical dynamics of aliphatic hydroxylation by cytochrome P450. J Am Chem Soc. 1986;108:6024–6031. doi: 10.1021/ja00279a059. [DOI] [PubMed] [Google Scholar]
- 43.Oliw EH, Brodowsky D, Hörnsten L, Hamberg M. bis-Allylic hydroxylation of polyunsaturated fatty acids by hepatic monooxygenases and its relation to the enzymatic and nonenzymatic formation of conjugated hydroxy fatty acids. Arch Biochem Biophys. 1993;300:434–439. doi: 10.1006/abbi.1993.1059. [DOI] [PubMed] [Google Scholar]
- 44.Egmond MR, Vliegenthart JFG, Boldingh J. Stereospecificity of the hydrogen abstraction at carbon atom n-8 in the oxygenation of linoleic acid by lipoxygenases from corn germs and soya beans. Biochem Biophys Res Commun. 1972;48:1055–1060. doi: 10.1016/0006-291x(72)90815-7. [DOI] [PubMed] [Google Scholar]
- 45.Corey EJ, Lansbury PT., Jr Stereochemical course of 5-lipoxygenation of arachidonate by rat basophil leukemic cell (RBL-1) and potato enzymes. J Am Chem Soc. 1983;105:4093–4094. [Google Scholar]
- 46.Hughes MA, Brash AR. Investigation of the mechanism of biosynthesis of 8-hydroxyeicosatetraenoic acid in mouse skin. Biochim Biophys Acta. 1991;1081:347–354. doi: 10.1016/0005-2760(91)90292-p. [DOI] [PubMed] [Google Scholar]
- 47.Marnett LJ. Peroxyl free radicals: potential mediators of tumor initiation and promotion. Carcinogenesis. 1987;8:1365–1373. doi: 10.1093/carcin/8.10.1365. [DOI] [PubMed] [Google Scholar]
- 48.Roschek B, Jr, Tallman KA, Rector CL, Gillmore JG, Pratt DA, Punta C, Porter NA. Peroxyl radical clocks. J Org Chem. 2006;71:3527–3532. doi: 10.1021/jo0601462. [DOI] [PubMed] [Google Scholar]
- 49.Brash AR. Autoxidation of methyl linoleate: identification of the bis-allylic 11-hydroperoxide. Lipids. 2000;35:947–952. doi: 10.1007/s11745-000-0604-0. [DOI] [PubMed] [Google Scholar]
- 50.Tallman KA, Pratt DA, Porter NA. Kinetic products of linoleate peroxidation: rapid β-fragmentation of nonconjugated peroxyls. J Am Chem Soc. 2001;123:11827–11828. doi: 10.1021/ja0169724. [DOI] [PubMed] [Google Scholar]
- 51.Kitaguchi H, Ohkubo K, Ogo S, Fukuzumi S. Direct ESR detection of pentadienyl radicals and peroxyl radicals in lipid peroxidation: mechanistic insight into regioselective oxygenation in lipoxygenases. J Am Chem Soc. 2005;127:6605–6609. doi: 10.1021/ja044345j. [DOI] [PubMed] [Google Scholar]
- 52.Porter NA, Weber BA, Weenen H, Khan JA. Autoxidation of polyunsaturated lipids. Factors controlling the stereochemistry of product hydroperoxides. J Am Chem Soc. 1980;102:5597–5601. [Google Scholar]
- 53.Kühn H, Belkner J, Wiesner R, Brash AR. Oxygenation of biological membranes by the pure reticulocyte lipoxygenase. J Biol Chem. 1990;265:18351–18361. [PubMed] [Google Scholar]
- 54.Fukushige H, Wang C, Simpson TD, Gardner HW, Hildebrand DF. Purification and identification of linoleic acid hydroperoxides generated by soybean seed lipoxygenases 2 and 3. J Agric Food Chem. 2005;53:5691–5694. doi: 10.1021/jf047958o. [DOI] [PubMed] [Google Scholar]
- 55.Kühn H, Sprecher H, Brash AR. On the singular or dual specificity of lipoxygenases. The number of chiral products varies with alignment of methylene groups at the active site of the enzyme. J Biol Chem. 1990;265:16300–16305. [PubMed] [Google Scholar]
- 56.Sloane DL, Leung R, Craik CS, Sigal E. A primary determinant for lipoxygenase positional specificity. Nature. 1991;354:149–152. doi: 10.1038/354149a0. [DOI] [PubMed] [Google Scholar]
- 57.Gardner HW. Soybean lipoxygenase-1 enzymically forms both (9S)- and (13S)-hydroperoxides from linoleic acid by a pH-dependent mechanism. Biochim Biophys Acta. 1989;1001:274–281. doi: 10.1016/0005-2760(89)90111-2. [DOI] [PubMed] [Google Scholar]
- 58.Hornung E, Walther M, Kühn H, Feussner I. Conversion of cucumber linoleate 13-lipoxygenase to a 9-lipoxygenating species by site directed mutagenesis. Proc Natl Acad Sci USA. 1999;96:4192–4197. doi: 10.1073/pnas.96.7.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jisaka M, Kim RB, Boeglin WE, Brash AR. Identification of amino acid determinants of the positional specificity of mouse 8S-lipoxygenase and human 15S-lipoxygenase-2. J Biol Chem. 2000;275:1287–1293. doi: 10.1074/jbc.275.2.1287. [DOI] [PubMed] [Google Scholar]
- 60.Brash AR, Boeglin WE, Chang MS, Shieh BH. Purification and molecular cloning of an 8R-lipoxygenase from the coral Plexaura homomalla reveal the related primary structures of R- and S-lipoxygenases. J Biol Chem. 1996;271:20949–20957. doi: 10.1074/jbc.271.34.20949. [DOI] [PubMed] [Google Scholar]
- 61.Coffa G, Brash AR. A single active site residue directs oxygenation stereospecificity in lipoxygenases: stereocontrol is linked to the position of oxygenation. Proc Natl Acad Sci USA. 2004;101:15579–15584. doi: 10.1073/pnas.0406727101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coffa G, Imber AN, Maguire BC, Laxmikanthan G, Schneider C, Gaffney BJ, Brash AR. On the relationships of substrate orientation, hydrogen abstraction and product stereochemistry in single and double dioxygenations by soybean lipoxygenase-1 and its Ala542Gly mutant. J Biol Chem. 2005;280:38756–38766. doi: 10.1074/jbc.M504870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knapp MJ, Seebeck FP, Klinman JP. Steric control of oxygenation regiochemistry in soybean lipoxygenase-1. J Am Chem Soc. 2001;123:2931–2932. doi: 10.1021/ja003855k. [DOI] [PubMed] [Google Scholar]
- 64.Kuhn H, Saam J, Eibach S, Holzhutter HG, Ivanov I, Walther M. Structural biology of mammalian lipoxygenases: enzymatic consequences of targeted alterations of the protein structure. Biochem Biophys Res Commun. 2005;338:93–101. doi: 10.1016/j.bbrc.2005.08.238. [DOI] [PubMed] [Google Scholar]
- 65.Labelle M, Falgueyret JP, Riendeau D, Rokach J. Synthesis of 2 analogs of arachidonic acid and their reaction with 12-lipoxygenase. Tetrahedron. 1990;46:6301–6310. [Google Scholar]
- 66.Corey EJ, Nagata R. Synthesis of three new dehydroarachidonic acid derivatives and their oxidation by soybean lipoxygenase. Tetrahed Lett. 1987;45:5391–5394. [Google Scholar]
- 67.Vliegenthart JFG, Veldink GA. Lipoxygenases. Free Rad Biol. 1982;5:29–64. [Google Scholar]
- 68.Boutaud O, Brash AR. Purification and catalytic activities of the two domains of the allene oxide synthase-lipoxygenase fusion protein of the coral Plexaura homomalla. J Biol Chem. 1999;274:33764–33770. doi: 10.1074/jbc.274.47.33764. [DOI] [PubMed] [Google Scholar]
- 69.Rowlinson SW, Crews BC, Goodwin DC, Schneider C, Gierse JK, Marnett LJ. Spatial requirements for 15-(R)-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid synthesis within the cyclooxygenase active site of murine COX-2. Why acetylated COX-1 does not synthesize 15-(R)-HETE. J Biol Chem. 2000;275:6586–6591. doi: 10.1074/jbc.275.9.6586. [DOI] [PubMed] [Google Scholar]
- 70.Schneider C, Boeglin WE, Brash AR. Identification of two cyclooxygenase active site residues, leucine-384 and glycine-526, that control carbon ring cyclization in prostaglandin biosynthesis. J Biol Chem. 2004;279:4404–4414. doi: 10.1074/jbc.M307431200. [DOI] [PubMed] [Google Scholar]
- 71.Harman CA, Rieke CJ, Garavito RM, Smith WL. Crystal structure of arachidonic acid bound to a mutant of prostaglandin endoperoxide H synthase-1 that forms predominantly 11-hydroperoxyeicosatetraenoic acid. J Biol Chem. 2004;279:42929–42935. doi: 10.1074/jbc.M403013200. [DOI] [PubMed] [Google Scholar]
- 72.Hamberg M. Stereochemistry of oxygenation of linoleic acid catalyzed by prostaglandin-endoperoxide H synthase-2. Arch Biochem Biophys. 1998;349:376–380. doi: 10.1006/abbi.1997.0443. [DOI] [PubMed] [Google Scholar]
- 73.Hamberg M, Samuelsson B. Stereochemistry in the formation of 9-hydroxy-10,12-octadecadienoic acid and 13-hydroxy-9,11-octadecadienoic acid from linoleic acid by fatty acid cyclooxygenase. Biochim Biophys Acta. 1980;617:545–547. doi: 10.1016/0005-2760(80)90022-3. [DOI] [PubMed] [Google Scholar]
- 74.Malkowski MG, Thuresson ED, Lakkides KM, Rieke CJ, Micielli R, Smith WL, Garavito RM. Structure of eicosapentaenoic and linoleic acids in the cyclooxygenase site of prostaglandin endoperoxide H synthase-1. J Biol Chem. 2001;276:37547–37555. doi: 10.1074/jbc.M105982200. [DOI] [PubMed] [Google Scholar]
- 75.Furse KE, Pratt DA, Porter NA, Lybrand TP. Molecular dynamics simulations of arachidonic acid complexes with COX-1 and COX-2: insights into equilibrium behavior. Biochemistry. 2006;45:3189–3205. doi: 10.1021/bi052337p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Furse KE, Pratt DA, Schneider C, Brash AR, Porter NA, Lybrand TP. Molecular dynamics simulations of arachidonic acid-derived pentadienyl radical intermediate complexes with COX-1 and COX-2: insights into oxygenation regio- and stereoselectivity. Biochemistry. 2006;45:3206–3218. doi: 10.1021/bi052338h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thuresson ED, Lakkides KM, Rieke JC, Sun Y, Wingerd BA, Micielli R, Mulichak AM, Malkowski MG, Garavito RM, Smith WL. Prostaglandin endoperoxide H synthase-1. The functions of cyclooxygenase active site residues in the binding, positioning, and oxygenation of arachidonic acid. J Biol Chem. 2001;276:10347–10357. doi: 10.1074/jbc.M009377200. [DOI] [PubMed] [Google Scholar]
- 78.Yuan C, Rieke CJ, Rimon G, Wingerd BA, Smith WL. Partnering between monomers of cyclooxygenase-2 homodimers. Proc Natl Acad Sci USA. 2006;103:6142–6147. doi: 10.1073/pnas.0601805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bernhard SA, MacQuarrie RA. Half-site reactivity and the “induced-fit” hypothesis. J Mol Biol. 1973;74:73–78. doi: 10.1016/0022-2836(73)90356-2. [DOI] [PubMed] [Google Scholar]
- 80.Levitzki A, Stallcup WB, Koshland DE., Jr Half-of-the-sites reactivity and the conformational states of cytidine triphosphate synthetase. Biochemistry. 1971;10:3371–3378. doi: 10.1021/bi00794a009. [DOI] [PubMed] [Google Scholar]
- 81.Chenier JHB, Furimsky E, Howard JA. Arrhenius parameters for reaction of tert-butylperoxy and 2-ethyl-2-propylperoxy radicals with some nonhindered phenols, aromatic-amines, and thiophenols. Can J Chem. 1974;52:3682–3688. [Google Scholar]
- 82.Snelgrove DW, Lusztyk J, Banks JT, Mulder P, Ingold KU. Kinetic Solvent Effects on Hydrogen-Atom Abstractions: Reliable, Quantitative Predictions via a Single Empirical Equation. J Am Chem Soc. 2001;123:469–477. [Google Scholar]
- 83.Hochgesang GP, Rowlinson SW, Marnett LJ. Tyrosine-385 is critical for acetylation of cyclooxygenase-2 by aspirin. J Am Chem Soc. 2000;122:6514–6515. [Google Scholar]
- 84.Schneider C, Boeglin WE, Prusakiewicz JJ, Rowlinson SW, Marnett LJ, Samel N, Brash AR. Control of prostaglandin stereochemistry at the 15-carbon by cyclooxygenases-1 and 2. A critical role for serine 530 and valine 349. J Biol Chem. 2002;277:478–485. doi: 10.1074/jbc.M107471200. [DOI] [PubMed] [Google Scholar]
- 85.Valmsen K, Boeglin WE, Järving J, Schneider C, Varvas K, Brash AR, Samel N. Structural and functional comparison of 15S- and 15R-specific cyclooxygenases from the coral Plexaura homomalla. Eur J Biochem. 2004;271:3533–3538. doi: 10.1111/j.0014-2956.2004.04289.x. [DOI] [PubMed] [Google Scholar]
- 86.Schreiner K, Berndt A. ESR-Spectrum of a perpendicular benzyl radical. Angew Chem Int Edit. 1974;13:144–145. [Google Scholar]
- 87.Regenstein H, Berndt A. Homohyperconjugation in a perpendicular allyl radical. Angew Chem Int Edit. 1974;13:145–146. [Google Scholar]
- 88.Tsai A-l, Palmer G, Wu G, Peng S, Okeley NM, van der Donk WA, Kulmacz RJ. Structural characterization of arachidonyl radicals formed by aspirin-treated prostaglandin H synthase-2. J Biol Chem. 2002;277:38311–38321. doi: 10.1074/jbc.M206961200. [DOI] [PubMed] [Google Scholar]
- 89.Williams RJP. Are enzymes mechanical devices? Trends Biochem Sci. 1993;18:115–117. doi: 10.1016/0968-0004(93)90015-f. [DOI] [PubMed] [Google Scholar]
- 90.Agarwal PK, Billeter SR, Rajagopalan PT, Benkovic SJ, Hammes-Schiffer S. Network of coupled promoting motions in enzyme catalysis. Proc Natl Acad Sci USA. 2002;99:2794–2799. doi: 10.1073/pnas.052005999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Daniel RM, Dunn RV, Finney JL, Smith JC. The role of dynamics in enzyme activity. Annu Rev Biophys Biomol Struct. 2003;32:69–92. doi: 10.1146/annurev.biophys.32.110601.142445. [DOI] [PubMed] [Google Scholar]
- 92.Tousignant A, Pelletier JN. Protein motions promote catalysis. Chem Biol. 2004;11:1037–1042. doi: 10.1016/j.chembiol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 93.Schramm VL. Enzymatic transition states and transition state analogues. Curr Opin Struct Biol. 2005;15:604–613. doi: 10.1016/j.sbi.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 94.Hammes-Schiffer S, Benkovic SJ. Relating protein motion to catalysis. Annu Rev Biochem. 2006;75:519–541. doi: 10.1146/annurev.biochem.75.103004.142800. [DOI] [PubMed] [Google Scholar]
- 95.O’Connor DE, Mihelich ED, Coleman MC. Stereochemical course of the autoxidative cyclization of lipid hydroperoxides to prostaglandin-like bicyclo endoperoxides. J Am Chem Soc. 1984;106:3577–3584. [Google Scholar]
- 96.Yin H, Havrilla CM, Morrow JD, Porter NA. Formation of isoprostane bicyclic endoperoxides from the autoxidation of cholesteryl arachidonate. J Am Chem Soc. 2002;124:7745–7754. doi: 10.1021/ja0201092. [DOI] [PubMed] [Google Scholar]
- 97.Thoma I, Krischke M, Loeffler C, Mueller MJ. The isoprostanoid pathway in plants. Chem Phys Lipids. 2004;128:135–148. doi: 10.1016/j.chemphyslip.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 98.Tallman KA, Porter NA. Factors influencing the autoxidation of non-conjugated dienes. Abstracts of Papers, 227th ACS National Meeting; Anaheim, CA, United States. March 28–April 1, 2004.2004. [Google Scholar]
- 99.Lecomte M, Laneuville O, Ji C, DeWitt DL, Smith WL. Acetylation of human prostaglandin endoperoxide synthase-2 (cyclooxygenase-2) by aspirin. J Biol Chem. 1994;269:13207–13215. [PubMed] [Google Scholar]
- 100.Schneider C, Brash AR. Stereospecificity of hydrogen abstraction in the conversion of arachidonic acid to 15R-HETE by aspirin-treated cyclooxygenase-2. J Biol Chem. 2000;275:4743–4746. doi: 10.1074/jbc.275.7.4743. [DOI] [PubMed] [Google Scholar]
- 101.Nelson MJ, Cowling RA, Seitz SP. Structural characterization of alkyl and peroxyl radicals in solutions of purple lipoxygenase. Biochemistry. 1994;33:4966–4973. doi: 10.1021/bi00182a027. [DOI] [PubMed] [Google Scholar]
- 102.Qian SY, Yue GH, Tomer KB, Mason RP. Identification of all classes of spin-trapped carbon-centered radicals in soybean lipoxygenase-dependent lipid peroxidations of omega-6 polyunsaturated fatty acids via LC/ESR, LC/MS, and tandem MS. Free Radic Biol Med. 2003;34:1017–1028. doi: 10.1016/s0891-5849(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 103.Tsai A, Kulmacz RJ, Palmer G. Spectroscopic evidence for reaction of prostaglandin H synthase-1 tyrosyl radical with arachidonic acid. J Biol Chem. 1995;270:10503–10508. doi: 10.1074/jbc.270.18.10503. [DOI] [PubMed] [Google Scholar]
- 104.Thuresson ED, Lakkides KM, Smith WL. Different catalytically competent arrangements of arachidonic acid within the cyclooxygenase active site of prostaglandin endoperoxide H synthase-1 lead to the formation of different oxygenated products. J Biol Chem. 2000;275:8501–8507. doi: 10.1074/jbc.275.12.8501. [DOI] [PubMed] [Google Scholar]