Abstract
Down syndrome (DS) individuals develop several neuropathological hallmarks seen in Alzheimer's disease, including cognitive decline and the early loss of cholinergic markers in the basal forebrain. These deficits are replicated in the Ts65Dn mouse, which contains a partial trisomy of murine chromosome 16, the orthologous genetic segment to human chromosome 21. Oxidative stress levels are elevated early in DS, and may contribute to the neurodegeneration seen in these individuals. We evaluated oxidative stress in Ts65Dn mice, and assessed the efficacy of long-term antioxidant supplementation on memory and basal forebrain pathology. We report that oxidative stress was elevated in the adult Ts65Dn brain, and that supplementation with the antioxidant vitamin E effectively reduced these markers. Also, Ts65Dn mice receiving vitamin E exhibited improved performance on a spatial working memory task and showed an attenuation of cholinergic neuron pathology in the basal forebrain. This study provides evidence that vitamin E delays onset of cognitive and morphological abnormalities in a mouse model of DS, and may represent a safe and effective treatment early in the progression of DS neuropathology.
Keywords: Ts65Dn, Basal forebrain cholinergic neuron, Working memory, Alzheimer's disease, Neurodegeneration, Calbindin D-28k, Alpha-tocopherol
Introduction
Down syndrome (DS) is characterized as a complete or partial trisomy of chromosome 21 leading to variable degrees of learning disabilities. In addition, individuals with DS develop pathologies reminiscent of Alzheimer's disease (AD) during middle-age, including amyloid plaques and progressive degeneration of basal forebrain cholinergic neurons (BFCN) (Casanova et al., 1985; Mufson et al., 2003), and many individuals develop further cognitive deterioration consistent with Alzheimer's-type dementia (Wisniewski, et al., 1985). BFCNs provide the major cholinergic innervation to the hippocampus and cortex (Mesulam et al., 1983), and thereby influence the processing of information necessary for attention and cognition in both animals and humans (Perry et al., 1977; Whitehouse et al., 1982). The phenotypic loss of cholinergic neurons is a hallmark of AD, and correlates strongly with memory loss (Ginsberg et al., 2006). An animal model for DS, the Ts65Dn mouse, contains a segmental trisomy of murine chromosome 16 that is orthologous to the genetic segment of human chromosome 21 most commonly implicated in DS (Davisson et al., 1990; Reeves et al., 1995). Ts65Dn mice exhibit several deficits seen in DS individuals, including progressive cognitive decline (Hunter et al., 2003a,b; Reeves et al., 1995) and adult-onset degeneration of BFCNs (Cooper et al., 2001;Granholm et al., 2000; Holtzman et al.,1996). The amyloid precursor protein (App) gene is triplicated in the Ts65Dn mouse, and this gene triplication is required for early endosomal abnormalities (Cataldo et al., 2003), diminished nerve growth factor transport, and BFCN loss in the Ts65Dnmouse (Salehi et al., 2006). These pathologies occur despite the absence of amyloid plaques in the Ts65Dn mouse (Reeves et al., 1995), though elevations in both APP (Seo and Isacson,2005) and β-amyloid (Hunter et al., 2004a,b) occur with aging in the hippocampus of this model, indicating that a build up of APP metabolites may contribute to this pathology. Spatial memory deficits have been well characterized in this model using hippocampal-dependent tasks such as the radial arm (Bimonte-Nelson et al., 2003; Hunter et al., 2003a,b) and Morris water mazes (Escorihuela et al.,1998; Reeves et al.,1995), and together with altered synaptic transmission and morphology (Cataldo et al., 2003; Insausti et al.,1998; Siarey et al.,1997) suggest impaired hippocampal function. These shared features suggest that Ts65Dn mice serve to model the progression of neuropathology in DS and provide the opportunity to study potential therapeutic interventions for DS and all Alzheimer's-like diseases.
In addition to BFCN pathology, another hallmark now recognized in the progression of Alzheimer's disease is oxidative stress. Brain regions affected in AD show elevated levels of lipid peroxidation (Lovell et al., 1995). Neurofibrillary tangles and amyloid plaques, both hallmarks of AD, colocalize with markers of oxidative stress (Sayre et al., 1997). Elevated levels of β-amyloid can induce oxidative damage in vitro (Behl et al., 1992), and mice transgenic for amyloid accumulation exhibit elevations in markers for oxidative stress (Smith et al., 1998). As in AD, DS individuals have elevated levels of DNA damage and lipid peroxidation (Jovanovic et al.,1998), and a pro-oxidant state is present early in the lifespan of DS (Pallardo et al., 2006). DS cortical neurons in vitro show enhanced reactive oxygen species (ROS) production resulting in increased neuronal degeneration and apoptosis (Busciglio and Yankner, 1995). Studies in Trisomy 16 neurons show that defects in mitochondrial complex I contribute to these elevations in ROS (Bambrick et al., 2003). Neuronal survival, which is greatly reduced as a result of oxidative stress levels, can be restored in vitro using antioxidants such as vitamin E (Behar and Colton, 2003; Schuchmann and Heinemann, 2000). These studies add to growing evidence that the increase in oxidative stress which occurs with aging and neurodegenerative diseases plays an important role in the development of cognitive decline (Lovell and Markesbery, 2007), and suggests that antioxidant therapy may serve to improve abnormalities in learning and memory in DS.
Normally found in high quantities in the brain, decreased levels of endogenous antioxidants such as α-tocopherol are reduced in individuals with AD (Bourdel-Marchasson et al., 2001; Jimenez-Jimenez et al., 1997) indicating either an enhanced consumption or reduced production of endogenous antioxidants. The primary component of vitamin E, α-tocopherol, is a powerful lipophilic chain-breaking antioxidant that acts as an inhibitor of lipid peroxidation (Azzi et al., 2003). There have been many clinical trials attempting to establish whether antioxidant treatment is efficacious in neurodegenerative disease, including two large-scale interventional trials in AD (Petersen et al., 2005; Sano et al., 1997). However, to our knowledge, no studies have evaluated the efficacy of antioxidant administration as a means to prevent Alzheimer's-like symptoms that occur in DS adults, and it is not known if oxidative stress is a major cause of degenerative changes observed in the Ts65Dn model.
The aims of the present study were therefore to assess levels of oxidative stress in the brain of the Ts65Dnmouse, and determine if long-term supplementation with vitamin E could prevent the age-associated cognitive decline and morphological alterations seen in this model. To meet these aims, we tested the efficacy of vitamin E in Ts65Dn using a working and reference memory version of the water-escape radial arm maze (RA maze). This maze avoids the potential confounds of food motivation and thigmotaxic behavior often associated with the land RA maze and Morris maze, respectively, and has been used previously to characterize deficits in spatial memory function in the Ts65Dn mouse (Bimonte-Nelson et al., 2003; Hunter et al., 2003a,b). We also assessed whether vitamin E treatment was sufficient to prevent morphological changes that occur with age in the Ts65Dn mouse. In addition to assessing BFCN degeneration, we also examined the effects of vitamin E on APP expression, which is increased in Ts65Dn mice, and the expression levels of Calbindin D-28k (CB), a calcium-binding protein which is reduced in the brain of both aging humans (Geula et al., 2003) and Ts65Dn mice (Hunter et al., 2004a,b), and whose loss may sensitize neurons to oxidative damage (Guo et al., 1998).
Materials and methods
Subjects
Mice with partial trisomy for a segment of murine chromosome 16 just proximal to the gene for App and extending to the gene for myxovirus resistance (Mx) were developed by Davisson et al. at Jackson Laboratories (Davisson et al., 1990). Controls for this experiment were normosomic littermates to the Ts65Dn mice with the same genetic background (B6C3HF1). As the C3H mouse strain carries the retinal degeneration allele (rd), the Ts65Dn and normosomic mice were screened and found free of retinal degeneration at Jackson Laboratories before behavioral testing. The trisomy is maintained by mating female carriers (the males are sterile) to C57Bl/6Jeicher × C3H/HeSnJ F1 males on a segregated genetic back-ground (Davisson et al., 1990).
Subjects consisted of male Ts65Dn mice and normosomic littermates that were acquired from Jackson Laboratories (Bar Harbor, ME) and housed in our animal facility for 1 to 2 months before testing. Due to the difficulty in breeding these mice and therefore obtaining large cohorts, this study was performed in two waves. All mice were single housed, received food and water ad libitum, and were maintained on a 12-hour light/dark cycle. Because the phenotype of Ts65Dn mice is subtle, behavioral testing was conducted blind to the genotype of the animals.
Long-term vitamin E supplementation
All diets and protocols were approved by the institutional animal care and use committee (IACUC) of the Medical University of South Carolina. At 4 months of age, male Ts65Dn mice and littermate controls were randomly selected to be placed on a NIH-31 base diet supplemented with 400 mg dl- β-tocopheryl acetate per kg diet (Harlan, Indianapolis, IN), a ten-fold increase over concentrations in the base diet. Food consumption was monitored throughout treatment, and daily intake of vitamin E was measured at 50 ± 5 mg/kg mouse. This dosage would correspond to 3000 IU in a 60 kg adult, or slightly higher than previous clinical AD trials (Sano et al.,1997). Long-term animal studies have shown that this dosage reduces levels of oxidative stress in the brain and prevents cognitive deficits in aged rats (Joseph et al., 1998). Controls were maintained on a standard NIH-31 diet. Control and Vitamin E diets were iso-caloric and similar in taste and color. Food intake was monitored throughout testing, and there were no differences in consumption between treatments.
Visible platform procedure
Mice were tested on a simple visible platform task, described previously (Bimonte-Nelson et al., 2003; Hunter et al., 2003a,b), 3 days before maze testing began. Briefly, the subject was placed in a black, plastic round tub 75 cm in diameter and partially filled with water (24 °C). The platform was painted black, had a flag with a bold “S” shaped character embossed on it, and was placed about 1 in. above the water level in the tub. Each mouse was given 120 s to find the platform. If it did not find the platform, the subject was guided to it using large forceps. Once on the platform, the mouse remained on it for 20 s. Following each trial, the subject was returned to its heated home cage. After all mice had completed their first trial, the platform was moved to a different previously assigned location. All mice in the group were then given their subsequent trial, until all mice received five trials. Data from the cued visible platform task was analyzed with a 1-Between (Group) 1-Within (Trial) repeated measures analysis of variance (ANOVA).
Water-escape radial-arm maze testing procedure
RA maze testing in the first cohort began 15 weeks after the initiation of treatment, when mice (8 normosomic and 8 Ts65Dn) were approximately 7.5 months of age. The second cohort of mice (7 normosomic and 10 Ts65Dn) was maintained on the diet 2 months longer, until 9.5 months of age, before testing (Fig. 1A). In order to maintain as much consistency as possible, all testing was performed using an identical protocol in the same room, by the same experimenter. As Ts65Dn mice are susceptible to swim-induced hypothermia at lower water temperatures (Stasko and Costa, 2004), water and ambient temperatures were maintained at 24 °C, and mice were towel dried and placed under heat lamps between trials. Diet regimens continued throughout testing. The testing procedure using the RA maze in mice has been reported in detail previously (Bimonte et al., 2000; Hunter et al., 2004a,b; Hunter et al., 2003a,b). In brief, an eight-arm maze containing hidden escape platforms in the ends of four of the eight arms was located in a room with salient extra-maze cues that remained constant throughout testing. Each subject was released from the start arm and had 2 min to locate a platform. Once a platform was found, the mouse remained on it for 15 s and was then returned to its heated home cage for 30 s until its next trial. During the interval, the previously chosen platform was removed from the maze before the procedure was repeated. All arm entries were recorded and analyzed for errors. Errors were quantified for each daily session using the orthogonal measures of working and reference memory errors of Jarrard et al. (1984) as done previously in studies using the RA maze (Bimonte-Nelson et al., 2003; Bimonte et al., 2000; Hunter et al., 2004a,b; Hunter et al., 2003a,b). Working Memory Correct (WMC) errors were the number of first and repeat entries into any arm from which a platform had been removed during that session. Reference Memory (RM) errors were the number of first entries into any arm that never contained a platform. Working Memory Incorrect (WMI) errors were the number of repeat entries into an arm that never contained a platform in the past (thus, repeat entries into a reference memory arm). To determine when during a session errors were made, the number of WMC and WMI errors committed during each trial within each session was determined. This allowed evaluation of differences in performance of the three treatment groups as trials progressed and working memory load increased. Each subject was given one session a day for 15 consecutive days. Day 1 was considered a training session, and days 2 to 15 were testing sessions. The data from days 2 through 15 were grouped into 2-day blocks for graphical analysis, and separated into an acquisition phase (Days 2–9, Blocks 1–4) and asymptotic phase (Days 10–15, Blocks 5–7), as described previously (Hunter et al., 2003a,b).
Fig. 1.
Treatment strategy. (A) Ts65Dn mice begin to show deficits in spatial and working memory tests between 4 and 6 months, while degeneration of basal forebrain cholinergic neurons is first evident at 6 months with decreased cell size, followed by a reduced cell number by 8–10 months. This study initiated vitamin E dietary supplementation at 4 months of age, prior to cognitive and morphological alterations in Ts65Dn mice, and mice were maintained on control or vitamin E-enriched diets through 2 weeks of behavioral testing in the radial arm maze. Mice were sacrificed between 8 and 10 months of age, immediately following behavioral testing. (B) Latency to find the platform (seconds) during the first and final (fifth) trials on the visible platform task. There were no significant differences in performance on the simple visible platform test.
Tissue preparation
All animals used for histochemical analysis were anesthetized with halothane and sacrificed by decapitation. First, a sagittal cut to the brain was made to separate the prefrontal cortex, which was frozen on dry ice and kept at −80 °C until Western blot analysis. Another sagittal cut was made to isolate the remaining rostral third of the cerebrum, which included the basal forebrain. This tissue was fixed in 4% paraformaldehyde. The remaining brain was divided into hemispheres, and the right hemisphere was fixed in 4% paraformaldehyde. The left hemisphere was immediately dissected, and the cerebral cortex fraction were frozen on dry ice and kept at −80 °C, and later utilized for the analysis of ROS formation (see below). The forebrain and right hemisphere fractions remained in paraformaldehyde for 48 h and were then transferred to 30% sucrose in 0.1 M phosphate buffer for at least 24 h before sectioning. Serial sections of the forebrain area, containing the medial septal nucleus, and of the right hemisphere, containing the hippocampus, were generated at a thickness of 45 µm on a cryostat.
Immunohistochemistry
Immunohistochemistry for the high-affinity NGF receptor TrkA in the basal forebrain was conducted as described previously (Granholm et al., 2000). TrkA was chosen as a marker for BFCNs instead of choline acetyl transferase (ChAT) as it shares a >99% colocalization with ChAT (Sobreviela et al.,1994), and has been shown to deteriorate early in the degenerative process in BFCN of AD and DS subjects (Ginsberg et al., 2006; Mufson et al., 2006). Briefly, free-floating sections were rinsed with 0.01 M TBS, incubated in a solution containing 0.3% hydrogen peroxide and 20% methanol in TBS to inhibit residual endogenous peroxidase, and blocked for 1 h in 10% normal goat serum (NGS) and 0.3% Triton X-100. Sections were incubated for 48 h at 4 °C with antibody directed against the high-affinity nerve growth factor receptor TrkA (1:10,000, generously provided by Dr. L. Reichardt). Next, sections were incubated for 1 h with biotinylated anti-rabbit IgG (1:200; Vector Laboratories, Burlingame, CA) followed by incubation in an avidin–biotin complex (ABC Elite; Vector).
Calbindin D-28k histology in the hippocampus was conducted as previously reported utilizing the peroxidase–antiperoxidase method (Hunter et al., 2004a,b). In brief, using a 0.5 M Tris-buffered saline and 1.5% NaCl buffer (1.5 T), sections were incubated with 0.3% hydrogen peroxide, blocked for 1 h in 10% NGS (as above), and incubated for 48 h at 4 °C with a primary antibody solution (rabbit anti-calbindin D-28k, 1:1,000, Chemicon, Temecula, CA). Sections were incubated with goat anti-rabbit solution (1:200; Sternberger Monoclonals, Lutherville, MD) followed by a rabbit peroxidase–antiperoxidase solution (1:200; Sternberger Monoclonals). All sections were rinsed in 0.01 M imidazole buffer and developed in a nickel ammonium-enhanced diaminobenzidine reaction. Sections were mounted on subbed slides, dehydrated with increasing gradients of ethanol, cleared with two incubations of xylene, and coverslipped with Permount solution.
To control for antibody specificity, we incubated one section without primary antibody and one section without secondary antibody for each different type of staining. No immunostaining was observed in any of these sections. Specificity of TrkA antibody (Sobreviela et al., 1994) and calbindin D-28k (Porter et al., 2001) have previously been reported. All sections for each different antibody used were processed together under the same conditions to avoid batch-to-batch differences.
Stereology and image analysis
Quantitative estimates of the total number of TrkA-positive neurons in the medial septal nucleus (MSN) were performed using the optical fractionator method as described previously (Granholm et al., 2002; Hunter et al., 2004a,b;West et al., 1991). This is an unbiased, stereological cell counting method that is not affected by the volume of reference (MSN) or size of the counted elements (TrkA-positive neurons). After randomly selecting the initial section, every 4th subsequent section was stained for TrkA, providing for a systematic random design. Using StereoInvestigator software (Micro Bright field, Colchester, VT), the MSN region was outlined using the following landmarks: the rostral border consisted of the medial orbital cortex at the level of the midline fusion of the corpus callosum, the caudal border consisted of the midline fusion of the anterior commissure, and the lateral borders consisted of the shell of the accumbens nucleus. Counting began on the first section following the fusion of the corpus callosum, and roughly 8 sections were counted per brain. The outlined region was then measured using optical fractionation, which maintains systematic random design by utilizing disector counting frames (100 × 100 µm), and neurons were counted using a 60× objective lens (1.4 numerical aperture). Cell area and volume measurements for TrkA-positive neurons were performed on the same serial sections using the nucleator probe within StereoyInvestigator (Gundersen et al., 1988). The nucleator uses a series of six rays that extend out from a point marked at the nucleus. Each intersection of the rays with the cell boundary is located and marked, and together they provide an estimation of cell area. Neurons were randomly selected via the optical fractionator program, with at least 10 neurons sampled per section and at least 50 neurons sampled per brain by an independent and blinded investigator.
Staining intensity of calbindin D-28k immunoreactivity in the cornu ammonis 1 and 2 regions of the hippocampus (CA1/CA2)was performed as described previously (Hunter et al., 2004a,b). As we were interested in quantifying the relative strength of calbindin-immunoreactivity in CA1 neurons rather than the number of neurons that were calbindin-positive, densitometric measurements were utilized rather than stereologic cell counting. While relative immunoreactivity may also be measured via immunoblot, the size of the region of interest precluded an accurate microdissection. In brief, staining intensity was determined using NIH Image software that measures a gray scale value within the range of 0 to 256, where 0 represents white and 256 black. A template was utilized and applied at the same coordinates for all brains and images were captured with a Nikon Eclipse E-600 microscope, an Olympus-750 video camera system, and a Dell Pentium III computer. Staining density was obtained when background staining was subtracted from mean staining intensities. Measurements were performed blinded on every 12th section through the hippocampus using the same systemic random design described above for cell counts.
Determination of oxidative stress
The production of ROS was assessed on cortical tissues using a f1uorometric assay, as described previously (Joseph et al., 1998; Murray and Lynch, 1998). An oxidative reaction converting the stable, non-f1uorescent 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) to the highly fluorescent 2′,7′-dichlorofluorescein (DCF) occurs in the presence of ROS, such as hydrogen peroxide. Pre-weighed frozen cortical homogenates were sonicated in 1 ml 40 mM Tris–HCl buffer (pH 7.4) on ice. Homogenates were incubated with H2DCFDA (5 µM final concentration; Molecular Probes, Eugene, OR) at 37 °C for 15 min. Fluorescence was monitored on a Spectra Max M5 set for 488 nm excitation and 525 nm emission at intervals of 15 min. Quantification of ROS was assessed from a standard curve of DCF (Sigma, St. Louis, MO) in methanol (range 1 µM–0.01 µM). Values were normalized by protein weight, and results were expressed as µM DCF produced per mg protein.
Western blot analysis
Prefrontal cortex tissue was homogenized in 10 volumes 10 mM HEPES,pH7.4 containing 1mMEDTA, 0.25Msucrose,1 × protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and 5 mM PNT (1,10-Phenanthro-line) using a Pro homogenizer with a 5 mm generator (PRO Scientific, Oxford, CT) and sonicated twice for 25 s. The homogenate was spun at 2500 rpm for 10 min to remove the nuclear pellet and the supernatant obtained was centrifuged further at 100,000 ×g for 60 min in order to collect the total membrane pellet for analysis of full length APP (APP-FL) and APP C-terminal fragments α and β (CTFα and CTFβ) and supernatants for analysis of secreted APP (sAPP). Western blotting was carried out as described previously (Pinnix et al., 2001). Briefly, using 15 µg protein from each sample was fractionated on 4–12% Bis–Tris gradient gel (Biorad, Hercules, CA) and transferred onto nitrocellulose filters using the Criterion wet transfer system. The blot was air dried and wetted with boiling PBS (8.1 mM disodium hydrogen phosphate, 1.5 mM potassium dihydrogen phosphate, 137 mM NaCL and 2.7 mM KCL) and blocked in 10% newborn calf serum (Atlanta Biologicals, Atlanta, GA) in TBS (50 mM Tris pH 8.0, 150 mM NaCl, 10 mM EDTA) for 1 h before probing with the primary antibodies – 22C11 (1:1000 dilution, residues 67–81 of APP-FL, Chemicon, Temecula, CA) and O443 (1:10,000 dilution, 20 residues on C-terminus of APP) (Pinnix et al., 2001) – for a 24 h incubation at 4 °C. The blots were then washed in TBS containing 0.1% Tween-20 (TBST) and incubated with appropriate horse-radish peroxidase-coupled secondary antibodies (Jackson Immunoresearch, West Grove, PA) for 1 h at room temperature and washed again with TBST and developed with chemiluminescent reagents from Millipore corporation (Bedford, MA). The chemiluminescent signal was detected using a Fluorchem HD imager (Alpha Innotech, San Leandro, CA), and quantified using the Alpha Ease software that comes with the instrument. APP-FL and sAPP levels were normalized to total protein levels visualized using a prominently stained band from Ponceau S. The density of metabolites of APP-FL were normalized directly to the APP-FL densities.
Statistics
Unless otherwise specified, all data were analyzed by analysis of variance (ANOVA) for genotype, treatment, and genotype by treatment interactions. To study effects of genotype and treatment on the rate of learning across days, a Poisson regression model was fitted for each of WMI, WMC, and RM, respectively. The data for phase and trial analyses of WMC and WMI were analyzed using a 1-Between (group)×2-Within (days and trials) repeated measures ANOVA (Statview Version 4.0), while RM data was analyzed using a 1-Between (group)×1-Within (days) repeated measures ANOVA to evaluate two-group comparisons of interest. Graphs are presented as errors±standard error of the mean (SEM). As it is not possible to make a WMC error on trial 1, trial 1 was omitted from analysis of WMC errors. Correlations within each group were performed using a correlationZ test. Software Splus 7.0 for Windows was used in the computation. We set significance level at 0.05, and all reported p-values were from 2-tailed tests unless otherwise noted.
Results
Subjects
This experiment was performed in order to determine whether long-term supplementation with vitamin E, a lipophilic molecule with antioxidant potential, would alleviate the cholinergic degeneration and cognitive decline seen in Ts65Dn mice. We administered a diet enriched with the antioxidant vitamin E (Ts65Dn vitamin E, n=10, Normosomic n=5, 50 mg/kg mouse) or a control diet (Normosomic, n=9; Ts65Dn control, n=7) in two cohorts. In the first cohort, a subset of the age-matched, littermate controls were given vitamin E (n=5, 50 mg/kg mouse). Treatment began when the mice were 4 months of age (Fig. 1A), as the onset of both BFCN degeneration and deficits in spatial memory occur during this time frame in Ts65Dn animals (Granholm et al., 2000; Hunter et al., 2003a, b). Weight was monitored throughout the study to determine whether significant weight alterations would result from vitamin E supplementation (Normosomic control=42.1±1.9 g; Normosomic Vit E=40.9±2.5 g Ts65Dn Control=31.8±1.6 g Ts65Dn Vit E=35.1±2.0 g). There were significant differences with regard to body weight [F3,28=6.87, p=0.0013], as, consistent with previous studies, the Ts65Dn mice maintained 20% lower body weights than their normosomic littermates [genotype effect: F1,30=15.56; p=0.0004]. There were no significant treatment effects on animal weight [treatment effect: F1,30=0.11], and vitamin E did not alter weight in Ts65Dn mice (p=0.25). There were no changes to general behavior (grooming, eating, drinking, open field testing) observed between treatments.
Prior to testing in the RA maze, mice were tested in a cued, visible platform task. This task investigated potential genotype differences in swimming and visual ability and introduced the mice to the demands of a water-escape task, such as swimming and searching for the platform. Fig. 1B shows the latency to find the visible platform on the first and last trials of testing. While there was a significant Trials effect [F4,15=4.73, p=0.003], there were no differences among the groups (p=0.18). This indicates that all groups improved their performance across trials and were able to perform the task, and suggests that visual and swimming skills were consistent between groups.
Vitamin E improved Ts65Dn cognitive performance in the radial arm maze
Memory function in the Ts65Dnwas tested using the RA Maze. The RA Maze is a sensitive behavioral task that is able to measure working memory and long-term spatial reference memory simultaneously, and has been used frequently for Ts65Dn mice (Bimonte-Nelson et al., 2003; Hunter et al., 2003a,b).
The first cohort included an additional normosomic group that received vitamin E to determine whether treatment would affect normosomic mice. A 2-by-2 repeated measures ANOVA of the first cohort assessing the interaction between genotype (Ts65Dn and normosomic) and treatment (vitamin E and control) showed a significant interaction in WMC errors [F1,1=6.94; p=0.022] and a marginal interaction in WMI errors [F1,1=3.48; p=0.087]. These statistics indicate that vitamin E had differential effects on genotype, as it improved behavioral performance in Ts65Dn mice and provided no cognitive benefit to normosomic animals. Furthermore, normosomic littermates receiving vitamin E showed no differences with normosomic mice receiving the control diet on any measure, behavioral or morphological. Thus we omitted the normosomic vitamin E group from the 2nd cohort.
Performance in the RA Maze is displayed in Fig. 2, which shows working memory correct (WMC, 2A), reference memory (RM, 2B), and working memory incorrect (WMI, 2C) errors (mean number±SEM) by normosomic control (black squares), Ts65Dn control (grey circles), and Ts65Dn vitamin E (open circles) groups across days of testing. The data are presented in 2-day blocks, while the dotted vertical line demarcates the early learning, or acquisition, phase (Blocks 1–4) and the later maintenance, or asymptotic, phase (Blocks 5–7).
Fig. 2.
Vitamin E-treated Ts65Dn mice improve performance on RA maze. WMC (A), RM (B), and WMI (C) errors±SEM collapsed across 2-day trial blocks. Testing blocks were separated into an acquisition, or learning, phase (days 2–9) and an asymptotic, or maintenance, phase (days 10–15), as shown by the vertical dotted line. A 3D graph of WMI errors plotted across both days and trials showing normosomic (D), Ts65Dn control (E) and Ts65Dn Vitamin E (F). Normosomic and Ts65Dn Vitamin E groups exhibited learning on WMC, RM, and WMI, while the Ts65Dn control group only showed a modest error reduction on WMI. Each block was analyzed separately using a simple ANOVA, and group differences were represented by * (p<0.05). The Ts65Dn control group performed more WMC errors than normosomics and more WMI errors than normosomic and Ts65Dn vitamin E mice. There were no statistical differences between the normosomic and Ts65Dn Vit E groups in the asymptotic phase of testing (normosomic control, n=9; Ts65Dn control, n=7; Ts65Dn vitamin E, n=10).
Learning
In order to examine task learning within each group, a Poisson regression model was fitted to error levels across days. A significant decrease in error rate across days in the RA maze represents learning. The normosomic group showed significant error reductions in WMC (Fig. 2A, p<0.0001), RM (Fig. 2B, p<0.0001), and WMI (Fig. 2C, p<0.0001). As in previous studies, the Ts65Dn control group showed significant learning impairments, as they were unable to reduce errors in WMC (p=0.31) and RM (p=0.07), only showing improvement in WMI (P=0.0003). The inability of Ts65Dn mice to reduce error levels across testing blocks in both working and reference memory tasks may be indicative of a failure in hippocampal-dependent spatial learning. In contrast, Ts65Dn mice receiving vitamin E supplementation exhibited learning across phases in all measures [WMC (p<0.0001); RM (p<0.0001) and WMI (p<0.0001)]. In addition, the Ts65Dn Vitamin E group reduced their errors across days at a faster rate than the Ts65Dn control group in both working memory measures [WMC (p=0.004) and WMI (p<0.0001)]. To further illustrate the disparity on WMI performance between groups, Figs. 2D–F represent three-dimensional graphs of mean errors plotted against both days and trials in each group. While the Ts65Dn control group continued to maintain a high rate of errors throughout testing, Ts65Dn Vitamin E mice reduced their errors in all the trials. The greater error reduction seen in the Ts65Dn Vitamin E group suggests that vitamin E supplementation was sufficient to facilitate spatial learning.
Acquisition vs. asymptotic phases of testing
Analysis of the acquisition phase of testing showed no significant differences between groups in WMC, RM, or WMI measures [F2,23=1.67, 2.28, and 2.93, respectively]. In contrast, differences in WMC [F2,23=3.57; p =0.045] and WMI [F2,23=7.77; p =0.0026] emerged during the asymptotic phase of testing. The Ts65Dn control group committed significantly more WMC errors than their normosomic littermates (Fig. 2A, p=0.014) during the asymptotic phase, while Ts65Dn mice supplemented with vitamin E showed no increase in errors from the normosomic group, and a marginal decrease in errors over the Ts65Dn control group (p=0.089). Vitamin E exerted an even stronger effect on WMI errors in Ts65Dn mice, as Ts65Dn mice receiving vitamin E committed significantly fewer WMI errors than Ts65Dn controls (Fig. 2C, p=0.0021). Taken together, these data indicate that the vitamin E supplementation was able to improve spatial learning and memory in Ts65Dn mice.
Working memory load
In addition to showing differences in error rates across days, Figs. 2D–F also depict changes in error rates across trials between the groups, an effect noted especially during the asymptotic testing phase. Fig. 3 illustrates the number of WMC(Fig. 3A) and WMI (Fig. 3B) errors made by each group for each trial collapsed across the asymptotic portion of the RA maze. As each subsequent trial requires memory of the platform locations found on previous trials, analysis of error rate across trials represents performance as working memory load increases. There was an overall trial × group interaction in WMI errors [F3,6=5.73; p<0.0001] during the asymptotic phase of testing. This interaction points to the highly significant increase in WMI trial 4 errors in the Ts65Dn control group over the other groups. To confirm this trial 4 effect, a separate analysis of the final trial showed significant differences in WMI [Fig. 3B; F2,23=6.82; p=0.0047], with the Ts65Dn Vitamin E group performing better than Ts65Dn control mice (p=0.0062) and nearly as well as their normosomic littermates. There were marginal differences in WMC errors in trial 4 [Fig. 3A; F2,23=2.78; p=0.083], with Ts65Dn control mice performing worse than normosomic mice (p=0.028), while vitamin E supplemented Ts65Dn mice were not statistically different. Collectively, these findings suggest that Ts65Dn vitamin E mice were able to maintain a higher working memory load than Ts65Dn mice receiving control diet.
Fig. 3.
Working memory load. Trial by group interactions for the asymptotic phase (days 10–15) of testing are shown for WMC (A) and WMI (B), with the y-axis depicting mean errors per trial±SEM, averaged over the last three blocks of testing. Trial progression escalates the amount of information required for task completion, and therefore serves to model increases in memory load. Ts65Dn control mice committed more errors on trial 4, when working memory load was highest. This impairment in Ts65Dn memory load appears to be improved with vitamin E supplementation (*p<0.05, **p<0.01).
Vitamin E lowered oxidative stress levels in the Ts65Dn brain
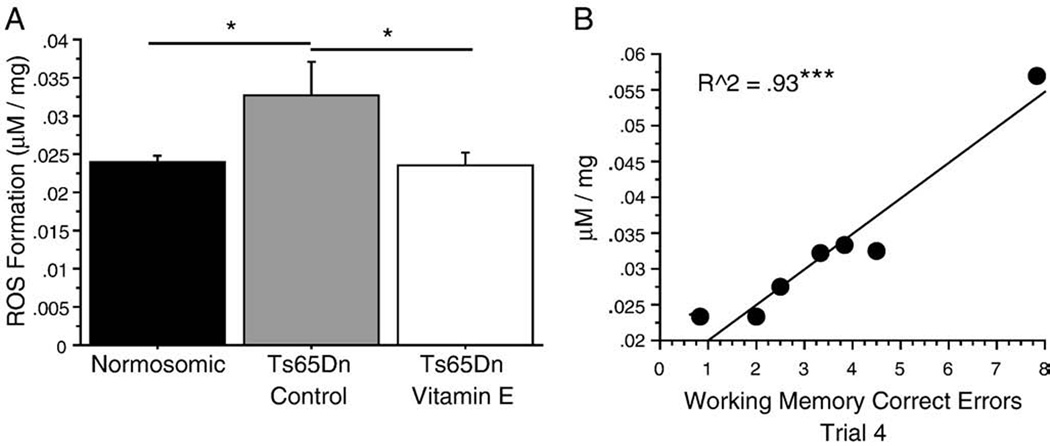
In order to evaluate the effects of vitamin E on the levels of oxidative stress in the Ts65Dn mouse, overall ROS activity was determined using cortical whole cell homogenates (Fig. 4A). Oxidative potential in Ts65Dn control mice was increased [F2,23=4.45; p = 0.023] by nearly 40% over normosomic mice (p=0.015), while vitamin E-treated Ts65Dn mice exhibited ROS activity levels that were lower than Ts65Dn controls (p=0.013) and comparable with normosomics. Not only were cortical ROS levels in Ts65Dn controls elevated, these levels correlated strongly with behavior, as Ts65Dn control mice that exhibited higher ROS levels tended to commit more errors in the fourth and final trial of testing during the asymptotic phase in both WMC (r2=0.93, p<0.0001; Fig. 4B) and WMI (r2=0.83, p<0.0018; data not shown) measures. Due to the low variability, or clustering, of ROS values seen in the other groups, this correlation could only be performed in the Ts65Dn control group. The strong correlation between oxidative stress and working memory measures further indicates a potential involvement of oxidative stress with the decline of memory function in the Ts65Dn mouse.
Fig. 4.
Vitamin E reverses elevations in oxidative stress in Ts65Dn mice. (A) H2DCFDA assay of cortical tissue homogenates demonstrated a significant increase in cortical ROS levels in Ts65Dn controls over both normosomic and Ts65Dn vitamin E mice. Values are expressed as mean±SEM in µM DCF fluorescence per milligram protein. (B) Scattergram showing the significant correlation between cortical ROS levels and WMC errors in Trial 4 of the Asymptotic phase of testing for Ts65Dn control animals. Ts65Dn mice that made more WMC errors on the final trial of testing tended to have higher levels of ROS in the cortex (Ts65Dn control, n=7; ***p<0.001).
Vitamin E reduced pathology of BFCNs in Ts65Dn mice
In order to determine whether vitamin E reduced the BFCN degeneration in Ts65Dn mice, we performed immunohistochemical analysis for TrkA, the high affinity nerve growth factor receptor and a reliable marker for BFCNs. Representative images of the MSN (Figs. 5A–C) depict that reductions, often asymmetric, occurred in TrkA-positive neurons in Ts65Dn mice. These losses in BFCNs were not present in Ts65Dn mice treated with vitamin E. Stereologic cell counting of TrkA-positive neurons in the MSN revealed a significant difference in the number of cholinergic neurons between groups [Fig. 5D; F2,22=3.13; p=0.032] using a one-tailed test, as we have previously shown decreases in TrkA-positive neurons (Granholm et al., 2000), with Ts65Dn controls exhibiting the lowest neuron counts. Post-hoc analysis showed a significant difference in neuron number between the normosomic and Ts65Dn control groups (p=0.029), and near significance between Ts65Dn control and Ts65Dn vitamin E groups (p=0.053). There were no differences between normosomic and Ts65Dn vitamin E groups (p=0.66). On closer analysis (Fig. 5F), there appeared to be greater neuron loss on the second cohort of mice that reached 10 months of age. An independent analysis of the 10-month cohort revealed even stronger differences in cell number [F2,12=5.51, p=0.02]. Not only did Ts65Dn mice show a significant drop in the number of BFCN at the older age (against normosomic mice, p=0.015), but Ts65Dn vitamin E-treated mice exhibited more BFCNs than Ts65Dn control and no cell loss compared to the normosomic group.
Fig. 5.
Vitamin E prevented cholinergic degeneration in the medial septal nucleus (MSN) of Ts65Dn mice. Cholinergic neurons were identified by the high affinity NGF receptor TrkA immunostaining in 8–10-month mice. Normosomic animals demonstrated a strong TrkA immunoreactivity in the MSN (A), while Ts65Dn controls exhibited weaker staining as well as regional asymmetry of TrkA-positive neuron distribution in the MSN (B). These abnormalities were not seen in Ts65Dn vitamin E mice (C). Stereological cell counting showed that Ts65Dn mice exhibited neuronal loss (*p<0.05, +p=0.053) in the medial septal nucleus (D), while separate analysis of cohorts demonstrated the greatest cell loss in the 10-month-old cohort (F). Vitamin E-treated mice did not exhibit of BFCN compared with normosomic littermates. Cell area measurements also showed a significant reduction in TrkA-positive cell area in Ts65Dn controls (*p<0.05, ***p<0.001) compared to normosomic and Ts65Dn vitamin E mice (E), while cohort analysis demonstrated that vitamin E had the greatest effect on the 8-month-old cohort (G). Values are mean cell size±SEM (normosomic control, n=9; Ts65Dn control, n=7; Ts65Dn vitamin E, n=10). Scale bar=100 µm.
Cell size is another reliable indicator of BFCN viability, and cholinergic neuron atrophy is a common pathological hallmark for AD, DS, and their animal models, and correlates well with memory loss (Koh et al., 1989). Image analysis of sections stained with TrkA antibody using the nucleator cell size measurement method (Fig. 5E) revealed that the Ts65Dn control group exhibited lower neuronal areas in TrkA-positive neurons [overall effect: F2,23=7.826; p=0.0026], consistent with neuronal atrophy. Not only were Ts65Dn control cholinergic neurons nearly 20% smaller than in normosomic controls (p=0.0006), they were also significantly smaller than Ts65Dn vitamin E neurons (p=0.0183), further indicating that vitamin E was able to attenuate the amount of BFCN atrophy seen in the Ts65Dn mice. A similar analysis of cohorts showed that vitamin E had a greater effect on neuron volume in Ts65Dn mice in the 8-month-old group (Fig. 5G), while in the 10-month-old cohort Ts65Dn mice receiving vitamin E had decreased neuronal area with respect to normosomic mice. Since BFCN atrophy precedes neuronal loss in Ts65Dn mice (Granholm et al., 2000), the data suggests that vitamin E was effective at delaying the progression of BFCN loss, but was not sufficient to completely prevent cholinergic decline at the later stage of development.
APP processing is affected in Ts65Dn mice following vitamin E administration
The degeneration of cholinergic neurons in the Ts65Dn mouse has been shown to result in part from the additional gene load of APP (Salehi et al., 2006). Therefore we assessed whether vitamin E's protective effects may be mediated through altering APP dynamics. As murine β-amyloid measurements require large amounts of tissue we chose to assess APP processing by analyzing APP metabolites instead. Representative Western blot data depicting the levels of APP-FL and its metabolites, CTF-α and CTF-β, in prefrontal cortex lysates are shown in Fig. 6A. In control Ts65Dn and Ts65Dn treated with vitamin E, APP-FL (Fig. 6B), CTFα (Fig. 6C), and sAPP (Fig. 6E) levels were elevated significantly compared to normosomic mice. Yet while APP-FL and sAPP levels appeared consistent between Ts65Dn and Ts65Dn Vitamin E groups and reflect the 50% increase in gene load of APP, densitometry revealed an increase in CTFα in the Ts65Dn Vitamin E group beyond these levels [F2,9=15.85; Ts65Dn vs. Ts65Dn Vitamin E: p=0.03; n=4 per group]. Normalizing CTFα levels to APP-FL band intensity as an internal control, a method used to assess the activity of the alpha-cleavage pathway (Obregon et al., 2006, Zhou et al., 2008), further indicates that CTFα accumulates in Ts65Dn Vitamin E mice compared to both normosomic and Ts65Dn mice [Fig. 6E, F2,9=6.871, p=0.003 and p=0.06, respectively], while no significant difference emerged between normosomic and Ts65Dn mice. As expected, for wild-type APP, CTFβ is present at very low levels, and thus we could not accurately quantify it, though visual examination of dark exposures suggest that CTFβ levels may also increase following vitamin E. While vitamin E did not appear to affect APP levels and therefore its synthesis, elevated CTFs suggest either an increase in α-secretase activity or a decrease in CTF breakdown, which is regulated primarily through the γ-secretase complex.
Fig. 6.
APP metabolism is altered following vitamin E supplementation in Ts65Dn mice. Western blot depicting the levels of full length APP and its CTFs using O443 and secreted APP using 22C11 (A). Densitometric analysis of bands corresponding to APP-FL (B) and APP-CTFα (C) reveals that APP expression is elevated in both treated and untreated Ts65Dn mice (APP-FL: Normosomic=4.657±0.203; Ts65Dn=5.787±0.159; Ts65Dn Vit E=5.981±0.135), while CTFα levels in Ts65Dn Vitamin E mice are statistically increased over their untreated counterparts (APP-CTF: Normosomic=0.899±0.070; Ts65Dn=1.315±0.102; Ts65Dn Vit E=1.656±0.110). Secreted APP levels are increased in Ts65Dn and Ts65Dn Vitamin E mice, though the treated mice show a non-significant increase over Ts65Dn controls (D, sAPP: Normosomic=2.124±0.131; Ts65Dn=3.056±0.095; Ts65Dn Vit E=3.245±0.027). Ratio of APP-CTFα to APP-FL as a measurement for the relative metabolism of APP in prefrontal cortex tissues (E). Guinea pig brain (GPB) lysate (A) is a positive control for APP-FL. Data are mean±SEM, normalized by the total protein stain Ponceau S (n=4 per group).
Vitamin E attenuated altered hippocampal morphology in Ts65Dn mice
Previous studies in our laboratory have demonstrated that Ts65Dn mice exhibit a decline in CB-positive staining in the cell bodies and apical dendrites of hippocampal pyramidal cells (Hunter et al., 2004). CB is found in a subpopulation of hippocampal neurons, and similar decreases measured in Alzheimer's individuals have been implicated in contributing to a rise in excitotoxicity and oxidative stress (Sutherland et al., 1993). In order to determine whether vitamin E influenced CB expression in Ts65Dn mice, immunohistochemical analysis of CB-positive neurons was performed. Ts65Dn control mice exhibited a reduction in CB-immunoreactivity in the CA1/CA2 regions of the hippocampus (Fig. 7B) relative to normosomic controls (Fig. 7A), while the Ts65Dn vitamin E group showed an attenuated loss (Fig. 7C). This decrease in staining was most evident in the apical dendrites of pyramidal neurons within the molecular layer of CA1. Semiquantitative densitometry confirmed this loss [Fig. 7D; F2,23=6.95; p=.004] and revealed a substantial decrease in CB-immunoreactivity in the CA1/CA2 region of Ts65Dn control animals (p=0.001). Vitamin E-treated Ts65Dn mice showed a marginal improvement over the Ts65Dn control group (p=0.07). A cohort analysis (Fig. 7E) revealed that CB-immunoreactivity was lower in the 10-month-old cohort of vitamin E-treated Ts65Dn mice, and was not statistically different compared to normosomic expression density in the 8-month group. This suggests that vitamin E was able to slow the loss of CB expression that occurs in Ts65Dn mice.
Fig. 7.
Vitamin E attenuated the loss of calbindin D-28k expression in the hippocampus of Ts65Dn mice. Immunohistochemical analysis of hippocampal neurons demonstrate robust Calbindin immunoreactivity in CA1 pyramidal neurons of normosomic animals (A). Ts65Dn control animals exhibit reduced staining at the cell body and apical dendrite (B), while vitamin E treatment partially recovers this loss (C). Densitometry revealed that Ts65Dn control mice had reduced CB-immunoreactivity in the CA1/CA2 region compared to normosomic controls (D, ***p<0.001). Ts65Dn vitamin E mice show a marginal increase in CB over Ts65Dn control (D, +p=0.07) mice, while a cohort analysis showed that this increase was most prominent at 8 months of age (E). Values are mean±SEM staining intensity normalized to background. Scale bar=100 µm.
Discussion
The current findings indicate that long-term supplementation with vitamin E delays impairment in cognitive performance, preserves markers of cholinergic cell survival and sustains hippocampal morphology in Ts65Dn mice. Oxidative stress levels were normalized in Ts65Dn mice treated with vitamin E and APP metabolism appeared altered, indicating a potential mechanism for its action. The efficacy with which vitamin E was able to improve neuronal and oxidative stress markers in Ts65Dn mice suggests that dietary intake of vitamin E may be sufficient to counteract the increased oxidative stress that was observed in this particular mouse model, and may be generalized to neurodegenerative diseases such as DS and AD in humans.
Here we found that untreated Ts65Dn mice were unable to reduce their error rates across days of testing. The lack of sustained behavioral improvement in this study was consistent with previous work in our laboratory and others which found deficits in spatial tests in adult Ts65Dn mice, indicating learning and memory impairment (Granholm et al., 2000; Hunter et al., 2003a,b; Reeves et al., 1995). In contrast to Ts65Dn controls, Ts65Dn mice receiving vitamin E supplementation were able to reduce their rate of errors on all indices across days of testing in the RA Maze (Fig. 2), suggesting learning capability. Vitamin E-treated Ts65Dn mice showed a slower rate of error reduction than the normosomic controls, and did not differentiate themselves from Ts65Dn controls until the last three blocks of testing. The Ts65Dn vitamin E group performed as well as the normosomic group in both WMC and WMI measures by the final block of testing (Figs. 2A and C). These learning curves suggest that Ts65Dn mice treated with vitamin E acquired the task more slowly than control animals, yet once the task was learned they exhibited proficient memory function.
Trial by group analysis revealed that vitamin E treatment provided an even greater effect on Ts65Dn performance during trials with higher memory loads. A disproportionate number of errors committed by the Ts65Dn controls occurred during the later trials, when working memory load was highest. This sensitivity to memory load has been recognized in previous studies (Bimonte-Nelson et al., 2003; Hunter et al., 2004a,b; Hunter et al., 2003a,b). Ts65Dn mice treated with vitamin E did not exhibit increased errors with the higher memory load, and performed at or near normal levels on the final trial during the asymptotic phase of testing. The indices in the studies presented here, especially working memory load measures, may have direct or indirect implications for learning impairments such as the declines in word and digit span which develop in AD. These deficits in working memory load are among the earliest indicators of memory dysfunction in dementia (Devenny et al., 2000), and can be found in DS adults prior to cognitive deterioration (Oliver et al., 2005). Therefore, early vitamin E supplementation in DS individuals may aid in delaying the cognitive decline seen as they enter middle-age. This will be an interesting target group for future clinical studies.
As vitamin E was effective in delaying memory deficits in the RA Maze, this study sought to determine if vitamin E exerted these effects through the protection of BFCNs. Reduced cholinergic signaling to cortical systems correlates strongly with the decline in memory function in both human and animal systems (Mufson et al., 2006), and our laboratory has previously shown a temporal connection between the onset of spatial memory deficits and the atrophy and subsequent loss of BFCNs in the Ts65Dn mouse (Granholm et al., 2000). Through unbiased stereological cell counting and cell area measurements we found that vitamin E delayed the onset of BFCN pathology in Ts65Dn mice by reducing neuronal atrophy in the 8-month-old cohort, and preventing the loss of TrkA-positive cells in the 10-month-old cohort. Our findings in the Ts65Dn controls are in line with previous work, which found reduced cholinergic neuron size in Ts65Dn mice by 6 months of age (Granholm et al., 2000), followed by progressive neuronal loss starting at 10 months of age (Cooper et al., 2001; Hunter et al., 2004a,b; Seo and Isacson, 2005) as evidenced by others as well (Cooper et al., 2001; Holtzman et al., 1996). Cholinergic atrophy is an age-associated event in humans, non-human primates, and rodents (Holtzman et al.,1993; Smith et al.,1999; Whitehouse et al.,1982), and the reversal of this atrophy has been linked to improvement in memory function (Martinez-Serrano et al., 1996). Therefore; it seems likely that prevention of cholinergic atrophy in Ts65Dn mice treated with vitamin E contributed to the improved memory function found in this study.
Vitamin E has proved efficacious in reducing markers of oxidative stress (Hong et al., 2004, Pratico et al., 1998), restoring endogenous antioxidant enzyme functions to normal levels (Zaidi and Banu, 2004), and improving performance in hippocampal-dependent tasks such as the radial arm and Morris water mazes in rodent models of oxidative stress (Fukui et al., 2002; Veinbergs et al., 2000).We have demonstrated that the Ts65Dn mice examined here have higher levels of ROS activity in cortical regions. This finding has not been reported in Ts65Dn mice before, though it is consistent with elevated oxidative stress markers in DS individuals (Jovanovic et al., 1998), and impaired mitochondrial function in another DS model, the Ts1Cje mouse (Shukkur et al., 2006). Oxidative stress further defines the Ts65Dn mouse as an appropriate model for the neuropathology seen in DS. Vitamin E supplementation was able to reduce these markers of oxidative stress to the levels of their normosomic counterparts, indicating that antioxidants may be effective in normalizing stress levels. Also noteworthy is the significant correlation between cortical ROS levels in Ts65Dn and impaired behavior in working memory components of the RA maze. Previous rodent studies have shown that vitamin E is able to recover impairments to long-term potentiation (LTP) seen with elevated levels of oxidative stress (Murray and Lynch,1998; Xie and Sastry,1993). Vitamin E may be acting to facilitate learning and memory by a similar mechanism here, as the Ts65Dn mouse has been found to exhibit significant LTP impairments (Siarey et al., 1997).
Despite the absence of amyloid plaques in the Ts65Dn mouse, APP appears to play a crucial role in the onset of memory loss and cholinergic neurons (Salehi et al., 2006). There is substantial evidence that monomers and oligomers of β-amyloid, derived from β-secretase activity, are toxic in the brain, disrupting cell signaling, causing synapse degeneration, and in some cases leading to neuronal death (Bayer and Wirths, 2008). One possible mechanism through which vitamin E may exert its influence is through the modification of APP metabolism, therefore reducing toxic species. Vitamin E did not appear to influence the production of APP-FL, and in fact showed modestly higher levels of sAPP (Fig. 6D). These data are consistent with previous work from our laboratory and others (Salehi et al., 2006) showing that APP-FL elevated levels according to gene load, while sAPP accumulates with age (Hunter et al., 2003a,b). This accumulation is consistent with the idea that intracellularly processed APP (Sambamurti et al., 1992) accumulates in vesicles known to build up in this model (Cataldo et al., 2003). Interestingly, CTFα levels were higher in Ts65Dn mice treated with vitamin E (Fig. 6C), and normalization of the ratio of this fragment to APP-FL (Fig. 6D) indicates that this increase in CTFα did not result from enhanced APP synthesis, but rather suggests a shift towards an enhancement of APP turnover via α-secretase cleavage elevated in the prefrontal cortex tissue in Ts65Dn Vitamin E. Secreted APPα, derived from the alternate cleavage pattern via α-secretases, has been shown to have neuroprotective effects in the brain (Goodman and Mattson, 1994), and may facilitate learning and memory (Mattson and Pedersen, 1998). The homeostasis between the α- and β-secretase cleavage pathways may be modified by oxidative stress, as BACE-1, a β-secretase enzyme, is upregulated following lipid peroxidation (Tamagno et al., 2005), and oxidative stress can facilitate β-amyloid formation (Coma et al., 2008). The antioxidant potential of vitamin E may be acting to modify APP cleavage patterns in the Ts65Dn mouse in a similar fashion, as suggested by our Western blot data described above. Another possible cause of this accumulation of CTFα would be a reduction in its breakdown via γ-secretase. The γ-secretase complex is most notably involved in the breakdown of CTFβ fragment that unleashes β-amyloid, thus any reduction in its activity may reduce intracellular β-amyloid accumulation. Future studies should elucidate whether an alteration in APP processing is responsible for the preservation of BFCNs and memory function in this model.
Although cholinergic degeneration is recognized as an important biomarker for dysfunctional limbic system pathways, the hippocampus exhibits early morphologic and electrophysiologic pathology in the Ts65Dn mouse (Belichenko et al., 2004). Here we show that vitamin E may also have a neuroprotective function in the hippocampus. Our laboratory has previously documented a reduction in CB-immunoreactivity in the CA1 region of the hippocampus (Hunter et al., 2004a,b). In the present study, Ts65Dn mice receiving vitamin E supplementation exhibited higher CB levels than untreated Ts65Dn mice. CB levels are reduced in the hippocampus of AD patients (Sutherland et al., 1993), and upregulation of CB expression reduces mitochondrial dysfunction and oxidative stress triggered by β-amyloid in vitro (Guo et al., 1998). It may therefore be relevant that an increase in CB levels is associated with decreases in oxidative stress, and it is possible that vitamin E may exert some of its neuroprotective properties via an increase in CB. Further studies must determine whether the increase in CB expression observed with vitamin E here is a direct response of vitamin E treatment in Ts65Dn mice, or if it merely represents preserved neuronal phenotype of the hippocampal pyramidal cells. Nonetheless, these neurons represent an interesting target for neuroprotection, since they are preferentially innervated by BFCNs (Ludkiewicz et al., 2002), thus providing another physiological link between cholinergic and hippocampal neurons in degenerative disease.
The lifespan of DS individuals has increased dramatically in recent years, emphasizing the need to develop sustainable treatments for dementia in these individuals. The progression of pathology that occurs in DS is poorly understood, and it is not currently known whether the developmental delays and mental retardation seen in DS children are caused by the same genes that induce this cognitive deterioration in DS adults. There have been clinical trials testing antioxidant supplements in young children with DS, and these have shown little or no efficacy in improving cognitive development (Bennett et al., 1983; Ellis et al., 2008). These results may indicate that there are differing etiologies between the early pathology seen in children and the dementia in DS adulthood, or they may indicate the limitations of vitamin E as therapy. While randomized clinical trials assessing treatment show far less efficacy (Petersen et al., 2005; Sano et al., 1997), longitudinal studies have shown vitamin E to reduce cognitive loss and dementia (Grodstein et al., 2003; Masaki et al., 2000). While DS shares with AD this early rise in oxidative stress levels, there may be divergent etiologies of this stress. DS in particular seems to contain a genetic predisposition to increased levels of oxidative species (Busciglio and Yankner, 1995), and an antioxidant intervention may show more promise with this group than with the population at large. Also, vitamin E may require long-term supplementation to provide bene.t as a protective mechanism (Yaffe, 2007), and short interventions may not be sufficient to reverse the neuronal changes already occurring in children with DS. There is an on-going phase III clinical trial assessing the efficacy of vitamin E in aging persons with DS (Dalton, 2006). This important trial will provide definitive evidence as to whether vitamin E can restore language and motor function in an aging population. Our study, which begins dietary supplementation prior to memory decline, indicates that vitamin E supplementation may show benefit in younger individuals as preventive therapy. Utilizing a mouse model for the cholinergic degeneration and cognitive decline seen in adult DS individuals, this study showed that vitamin E supplementation, when initiated early in adulthood, was effective in attenuating cognitive changes and emphasizes the use of vitamin E prior to the outward manifestation of cognitive decline. We believe that these results advocate clinical trials in DS individuals prior to advanced memory impairment. As vitamin E is a common, accessible supplement that is normally well tolerated in adults, this would be a low risk, potentially efficacious target substance to further study in a condition that inevitably leads to AD-like pathology in DS.
Acknowledgments
This work was supported by United States Public Health Service (AG12122) and the Anna and John J. Sie Foundation for Down Syndrome. We thank Muriel T. Davisson for the gift of breeding stocks and Dr. Louis Reichardt for the generous gift of TrkA antibodies. In addition, we thank Dr. Lauren Willis and Dr. Heather Boger for valuable scientific discussions and Selena Sumner, Alfred Moore, and Claudia Umphlet for outstanding technical assistance.
References
- Azzi A, Gysin R, Kempna P, Ricciarelli R, Villacorta L, Visarius T, Zingg JM. The role of alpha-tocopherol in preventing disease: from epidemiology to molecular events. Mol. Aspects Med. 2003;24:325–336. doi: 10.1016/s0098-2997(03)00028-1. [DOI] [PubMed] [Google Scholar]
- Bambrick LL, Yarowsky PJ, Krueger BK. Altered astrocyte calcium homeostasis and proliferation in theTs65Dn mouse, a model of Down syndrome. J. Neurosci. Res. 2003;73:89–94. doi: 10.1002/jnr.10630. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Wirths O. Review on the APP/PS1KI mouse model: intraneuronal Abeta accumulation triggers axonopathy, neuron loss and working memory impairment. Genes Brain Behav. 2008;7(Suppl 1):6–11. doi: 10.1111/j.1601-183X.2007.00372.x. [DOI] [PubMed] [Google Scholar]
- Behar TN, Colton CA. Redox regulation of neuronal migration in a Down Syndrome model. Free Radic. Biol. Med. 2003;35:566–575. doi: 10.1016/s0891-5849(03)00329-0. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis J, Cole GM, Schubert D. Vitamin E protects nerve cells from amyloid beta protein toxicity. Biochem. Biophys. Res. Commun. 1992;186:944–950. doi: 10.1016/0006-291x(92)90837-b. [DOI] [PubMed] [Google Scholar]
- Belichenko PV, Masliah E, Kleschevnikov AM, Villar AJ, Epstein CJ, Salehi A, Mobley WC. Synaptic structural abnormalities in the Ts65Dn mouse model of Down Syndrome. J. Comp. Neurol. 2004;480:281–298. doi: 10.1002/cne.20337. [DOI] [PubMed] [Google Scholar]
- Bennett FC, McClelland S, Kriegsmann EA, Andrus LB, Sells CJ. Vitamin and mineral supplementation in Down's syndrome. Pediatrics. 1983;72:707–713. [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav. Brain Res. 2003;139:47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol. Behav. 2000;70:311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Bourdel-Marchasson I, Delmas-Beauvieux MC, Peuchant E, Richard-Harston S, Decamps A, Reignier B, Emeriau JP, Rainfray M. Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing. 2001;30:235–241. doi: 10.1093/ageing/30.3.235. [DOI] [PubMed] [Google Scholar]
- Busciglio J, Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature. 1995;378:776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Walker LC, Whitehouse PJ, Price DL. Abnormalities of the nucleus basalis in Down's syndrome. Ann. Neurol. 1985;18:310–313. doi: 10.1002/ana.410180306. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Petanceska S, Peterhoff CM, Terio NB, Epstein CJ, Villar A, Carlson EJ, Staufenbiel M, Nixon RA. App gene dosage modulates endosomal abnormalities of Alzheimer's disease in a segmental trisomy 16 mouse model of down syndrome. J. Neurosci. 2003;23:6788–6792. doi: 10.1523/JNEUROSCI.23-17-06788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coma M, Guix FX, Ill-Raga G, Uribesalgo I, Alameda F, Valverde MA, Munoz FJ. Oxidative stress triggers the amyloidogenic pathway in human vascular smooth muscle cells. Neurobiol. Aging. 2008;29:969–980. doi: 10.1016/j.neurobiolaging.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Cooper JD, Salehi A, Delcroix JD, Howe CL, Belichenko PV, Chua-Couzens J, Kilbridge JF, Carlson EJ, Epstein CJ, Mobley WC. Failed retrograde transport of NGF in a mouse model of Down's syndrome: reversal of cholinergic neurodegenerative phenotypes following NGF infusion. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10439–10444. doi: 10.1073/pnas.181219298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton A. Vitamin E in aging persons with Down syndrome. [Accessed March 20, 2007];National Institute of Health Website. 2006 http://www.clinicaltrials.gov/ct/gui/show/NCT00056329.
- Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog. Clin. Biol. Res. 1990;360:263–280. [PubMed] [Google Scholar]
- Devenny DA, Krinsky-McHale SJ, Sersen G, Silverman WP. Sequence of cognitive decline in dementia in adults with Down's syndrome. J. Intellect. Disabil. Res. 2000;44(Pt 6):654–665. doi: 10.1046/j.1365-2788.2000.00305.x. [DOI] [PubMed] [Google Scholar]
- Ellis JM, Tan HK, Gilbert RE, Muller DP, Henley W, Moy R, Pumphrey R, Ani C, Davies S, Edwards V, Green H, Salt A, Logan S. Supplementation with antioxidants and folinic acid for children with Down's syndrome: randomised controlled trial. Bmj. 2008;336:594–597. doi: 10.1136/bmj.39465.544028.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escorihuela RM, Vallina IF, Martinez-Cue C, Baamonde C, Dierssen M, Tobena A, Florez J, Fernandez-Teruel A. Impaired short- and long-term memory in Ts65Dn mice, a model for Down syndrome. Neurosci. Lett. 1998;247:171–174. doi: 10.1016/s0304-3940(98)00317-6. [DOI] [PubMed] [Google Scholar]
- Fukui K, Omoi NO, Hayasaka T, Shinnkai T, Suzuki S, Abe K, Urano S. Cognitive impairment of rats caused by oxidative stress and aging, and its prevention by vitamin E. Ann. N. Y. Acad. Sci. 2002;959:275–284. doi: 10.1111/j.1749-6632.2002.tb02099.x. [DOI] [PubMed] [Google Scholar]
- Geula C, Bu J, Nagykery N, Scinto LF, Chan J, Joseph J, Parker R, Wu CK. Loss of calbindin-D28k from aging human cholinergic basal forebrain: relation to neuronal loss. J. Comp. Neurol. 2003;455:249–259. doi: 10.1002/cne.10475. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Che S, Wuu J, Counts SE, Mufson EJ. Down regulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer's disease. J. Neurochem. 2006;97:475–487. doi: 10.1111/j.1471-4159.2006.03764.x. [DOI] [PubMed] [Google Scholar]
- Goodman Y, Mattson MP. Secreted forms of beta-amyloid precursor protein protect hippocampal neurons against amyloid beta-peptide-induced oxidative injury. Exp. Neurol. 1994;128:1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Ford KA, Hyde LA, Bimonte HA, Hunter CL, Nelson M, Albeck D, Sanders LA, Mufson EJ, Crnic LS. Estrogen restores cognition and cholinergic phenotype in an animal model of Down syndrome. Physiol. Behav. 2002;77:371–385. doi: 10.1016/s0031-9384(02)00884-3. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Sanders LA, Crnic LS. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down's syndrome. Exp. Neurol. 2000;161:647–663. doi: 10.1006/exnr.1999.7289. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Chen J, Willett WC. High-dose antioxidant supplements and cognitive function in community-dwelling elderly women. Am. J. Clin. Nutr. 2003;77:975–984. doi: 10.1093/ajcn/77.4.975. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, et al. The new stereological tools: dissector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Apmis. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Guo Q, Christakos S, Robinson N, Mattson MP. Calbindin D28k blocks the proapoptotic actions of mutant presenilin 1: reduced oxidative stress and preserved mitochondrial function. Proc. Natl. Acad. Sci. U. S. A. 1998;95:3227–3232. doi: 10.1073/pnas.95.6.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Li Y, Chen K, Gage FH, Epstein CJ, Mobley WC. Nerve growth factor reverses neuronal atrophy in a Down syndrome model of age-related neurodegeneration. Neurology. 1993;43:2668–2673. doi: 10.1212/wnl.43.12.2668. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Santucci D, Kilbridge J, Chua-Couzens J, Fontana DJ, Daniels SE, Johnson RM, Chen K, Sun Y, Carlson E, Alleva E, Epstein CJ, Mobley WC. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc. Natl. Acad. Sci. U. S. A. 1996;93:13333–13338. doi: 10.1073/pnas.93.23.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Kim MJ, Park MR, Kwag OG, Lee IS, Byun BH, Lee SC, Lee KB, Rhee SJ. Effects of vitamin E on oxidative stress and membrane fluidity in brain of streptozotocin-induced diabetic rats. Clin. Chim. Acta. 2004;340:107–115. doi: 10.1016/j.cccn.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Bimonte HA, Granholm AC. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav. Brain Res. 2003a;138:121–131. doi: 10.1016/s0166-4328(02)00275-9. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Isacson O, Nelson M, Bimonte-Nelson H, Seo H, Lin L, Ford K, Kindy MS, Granholm AC. Regional alterations in amyloid precursor protein and nerve growth factor across age in a mouse model of Down's syndrome. Neurosci. Res. 2003b;45:437–445. doi: 10.1016/s0168-0102(03)00005-1. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Bachman D, Granholm AC. Minocycline prevents cholinergic loss in a mouse model of Down's syndrome. Ann. Neurol. 2004a;56:675–688. doi: 10.1002/ana.20250. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Bimonte-Nelson HA, Nelson M, Eckman CB, Granholm AC. Behavioral and neurobiological markers of Alzheimer's disease in Ts65Dn mice: effects of estrogen. Neurobiol. Aging. 2004b;25:873–884. doi: 10.1016/j.neurobiolaging.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Insausti AM, Megias M, Crespo D, Cruz-Orive LM, Dierssen M, Vallina IF, Insausti R, Florez J. Hippocampal volume and neuronal number in Ts65Dn mice: a murine model of Down syndrome. Neurosci. Lett. 1998;253:175–178. doi: 10.1016/s0304-3940(98)00641-7. [DOI] [PubMed] [Google Scholar]
- Jarrard LE, Okaichi H, Steward O, Goldschmidt RB. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav. Neurosci. 1984;98:946–954. doi: 10.1037//0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- Jimenez-Jimenez FJ, de Bustos F, Molina JA, Benito-Leon J, Tallon-Barranco A, Gasalla T, Orti-Pareja M, Guillamon F, Rubio JC, Arenas J, Enriquez-de-Salamanca R. Cerebrospinal fluid levels of alpha-tocopherol (vitamin E) in Alzheimer's disease. J. Neural. Transm. 1997;104:703–710. doi: 10.1007/BF01291887. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Prior RL, Cao G, Martin A, Taglialatela G, Bickford PC. Long-term dietary strawberry, spinach, or vitamin E supplementation retards the onset of age-related neuronal signal-transduction and cognitive behavioral deficits. J. Neurosci. 1998;18:8047–8055. doi: 10.1523/JNEUROSCI.18-19-08047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic SV, Clements D, MacLeod K. Biomarkers of oxidative stress are significantly elevated in Down syndrome. Free Radic. Biol. Med. 1998;25:1044–1048. doi: 10.1016/s0891-5849(98)00137-3. [DOI] [PubMed] [Google Scholar]
- Koh S, Chang P, Collier TJ, Loy R. Loss of NGF receptor immunoreactivity in basal forebrain neurons of aged rats: correlation with spatial memory impairment. Brain Res. 1989;498:397–404. doi: 10.1016/0006-8993(89)91125-6. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Ehmann WD, Butler SM, Markesbery WR. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer's disease. Neurology. 1995;45:1594–1601. doi: 10.1212/wnl.45.8.1594. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer's disease. Nucleic Acids Res. 2007;35:7497–7504. doi: 10.1093/nar/gkm821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludkiewicz B, Wojcik S, Spodnik E, Domaradzka-Pytel B, Klejbor I, Morys J. Cholinergic innervation of parvalbumin- and calbindin-containing neurones in the hippocampus during postnatal development of the rat brain. Folia Morphol. (Warsz) 2002;61:89–96. [PubMed] [Google Scholar]
- Martinez-Serrano A, Fischer W, Soderstrom S, Ebendal T, Bjorklund A. Long-term functional recovery from age-induced spatial memory impairments by nerve growth factor gene transfer to the rat basal forebrain. Proc. Natl. Acad. Sci. U. S. A. 1996;93:6355–6360. doi: 10.1073/pnas.93.13.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki KH, Losonczy KG, Izmirlian G, Foley DJ, Ross GW, Petrovitch H, Havlik R, White LR. Association of vitamin E and C supplement use with cognitive function and dementia in elderly men. Neurology. 2000;54:1265–1272. doi: 10.1212/wnl.54.6.1265. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Pedersen WA. Effects of amyloid precursor protein derivatives and oxidative stress on basal forebrain cholinergic systems in Alzheimer's disease. Int. J. Dev. Neurosci. 1998;16:737–753. doi: 10.1016/s0736-5748(98)00082-3. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J. Comp. Neurol. 1983;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Counts SE, Che S, Ginsberg SD. Neuronal gene expression profiling: uncovering the molecular biology of neurodegenerative disease. Prog. Brain Res. 2006;158:197–222. doi: 10.1016/S0079-6123(06)58010-0. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Ginsberg SD, Ikonomovic MD, DeKosky ST. Human cholinergic basal forebrain: chemoanatomy and neurologic dysfunction. J. Chem. Neuroanat. 2003;26:233–242. doi: 10.1016/s0891-0618(03)00068-1. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Dietary supplementation with vitamin E reverses the age-related deficit in long term potentiation in dentate gyrus. J. Biol. Chem. 1998;273:12161–12168. doi: 10.1074/jbc.273.20.12161. [DOI] [PubMed] [Google Scholar]
- Obregon DF, Rezai-Zadeh K, Bai Y, Sun N, Hou H, Ehrhart J, Zeng J, Mori T, Arendash GW, Shytle D, Town T, Tan J. ADAM10 activation is required for green tea (−)-epigallocatechin-3-gallate-induced alpha-secretase cleavage of amyloid precursor protein. J. Biol. Chem. 2006;281:16419–16427. doi: 10.1074/jbc.M600617200. [DOI] [PubMed] [Google Scholar]
- Oliver C, Holland T, Hall S, Crayton L. Effects of increasing task load on memory impairment in adults with Down syndrome. Am. J. Ment. Retard. 2005;110:339–345. doi: 10.1352/0895-8017(2005)110[339:EOITLO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pallardo FV, Degan P, d'Ischia M, Kelly FJ, Zatterale A, Calzone R, Castello G, Fernandez-Delgado R, Dunster C, Lloret A, Manini P, Pisanti MA, Vuttariello E, Pagano G. Multiple evidence for an early age pro-oxidant state in Down Syndrome patients. Biogerontology. 2006;7:211–220. doi: 10.1007/s10522-006-9002-5. [DOI] [PubMed] [Google Scholar]
- Perry EK, Perry RH, Blessed G, Tomlinson BE. Necropsy evidence of central cholinergic deficits in senile dementia. Lancet. 1977;1:189. doi: 10.1016/s0140-6736(77)91780-9. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Pinnix I, Council JE, Roseberry B, Onstead L, Mallender W, Sucic J, Sambamurti K. Convertases other than furin cleave beta-secretase to its mature form. Faseb. J. 2001;15:1810–1812. doi: 10.1096/fj.00-0891fje. [DOI] [PubMed] [Google Scholar]
- Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feed forward inhibition in the mouse barrel cortex. J. Neurosci. 2001;21:2699–2710. doi: 10.1523/JNEUROSCI.21-08-02699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratico D, Tangirala RK, Rader DJ, Rokach J, FitzGerald GA. Vitamin E suppresses isoprostane generation in vivo and reduces atherosclerosis in ApoE-deficient mice. Nat. Med. 1998;4:1189–1192. doi: 10.1038/2685. [DOI] [PubMed] [Google Scholar]
- Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat. Genet. 1995;11:177–184. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, Xia W, Villar A, Campbell WA, Kulnane LS, Nixon RA, Lamb BT, Epstein CJ, Stokin GB, Goldstein LS, Mobley WC. Increased App expression in a mouse model of Down's syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Shioi J, Anderson JP, Pappolla MA, Robakis NK. Evidence for intracellular cleavage of the Alzheimer's amyloid precursor in PC12 cells. J. Neurosci. Res. 1992;33:319–329. doi: 10.1002/jnr.490330216. [DOI] [PubMed] [Google Scholar]
- Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N. Engl. J. Med. 1997;336:1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J. Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- Schuchmann S, Heinemann U. Diminished glutathione levels cause spontaneous and mitochondria-mediated cell death in neurons from trisomy 16 mice: a model of Down's syndrome. J. Neurochem. 2000;74:1205–1214. doi: 10.1046/j.1471-4159.2000.741205.x. [DOI] [PubMed] [Google Scholar]
- Seo H, Isacson O. Abnormal APP, cholinergic and cognitive function in Ts65Dn Down's model mice. Exp. Neurol. 2005;193:469–480. doi: 10.1016/j.expneurol.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Shukkur EA, Shimohata A, Akagi T, Yu W, Yamaguchi M, Murayama M, Chui D, Takeuchi T, Amano K, Subramhanya KH, Hashikawa T, Sago H, Epstein CJ, Takashima A, Yamakawa K. Mitochondrial dysfunction and tau hyperpho-sphorylation in Ts1Cje, a mouse model for Down syndrome. Hum. Mol. Genet. 2006;15:2752–2762. doi: 10.1093/hmg/ddl211. [DOI] [PubMed] [Google Scholar]
- Siarey RJ, Stoll J, Rapoport SI, Galdzicki Z. Altered long-term potentiation in the young and old Ts65Dn mouse, a model for Down Syndrome. Neuropharmacology. 1997;36:1549–1554. doi: 10.1016/s0028-3908(97)00157-3. [DOI] [PubMed] [Google Scholar]
- Smith DE, Roberts J, Gage FH, Tuszynski MH. Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proc. Natl. Acad. Sci. U. S. A. 1999;96:10893–10898. doi: 10.1073/pnas.96.19.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Hirai K, Hsiao K, Pappolla MA, Harris PL, Siedlak SL, Tabaton M, Perry G. Amyloid-beta deposition in Alzheimer transgenic mice is associated with oxidative stress. J. Neurochem. 1998;70:2212–2215. doi: 10.1046/j.1471-4159.1998.70052212.x. [DOI] [PubMed] [Google Scholar]
- Sobreviela T, Clary DO, Reichardt LF, Brandabur MM, Kordower JH, Mufson EJ. TrkA-immunoreactive profiles in the central nervous system: colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. J. Comp. Neurol. 1994;350:587–611. doi: 10.1002/cne.903500407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasko MR, Costa AC. Experimental parameters affecting the Morris water maze performance of a mouse model of Down syndrome. Behav. Brain Res. 2004;154:1–17. doi: 10.1016/j.bbr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Sutherland MK, Wong L, Somerville MJ, Yoong LK, Bergeron C, Parmentier M, McLachlan DR. Reduction of calbindin-28k mRNA levels in Alzheimer as compared to Huntington hippocampus. Brain Res. Mol. Brain Res. 1993;18:32–42. doi: 10.1016/0169-328x(93)90171-k. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A, Danni O, Smith MA, Perry G, Tabaton M. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J. Neurochem. 2005;92:628–636. doi: 10.1111/j.1471-4159.2004.02895.x. [DOI] [PubMed] [Google Scholar]
- Veinbergs I, Mallory M, Sagara Y, Masliah E. Vitamin E supplementation prevents spatial learning deficits and dendritic alterations in aged apolipoprotein E-deficient mice. Eur. J. Neurosci. 2000;12:4541–4546. [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann. Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- Xie Z, Sastry BR. Induction of hippocampal long-term potentiation by alpha-tocopherol. Brain Res. 1993;604:173–179. doi: 10.1016/0006-8993(93)90365-t. [DOI] [PubMed] [Google Scholar]
- Yaffe K. Antioxidants and prevention of cognitive decline: does duration of use matter? Arch. Intern. Med. 2007;167:2167–2168. doi: 10.1001/archinte.167.20.2167. [DOI] [PubMed] [Google Scholar]
- Zaidi SM, Banu N. Antioxidant potential of vitamins A, E and C in modulating oxidative stress in rat brain. Clin. Chim. Acta. 2004;340:229–233. doi: 10.1016/j.cccn.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Suram A, Venugopal C, Prakasam A, Lin S, Su Y, Li B, Paul SM, Sambamurti K. Geranylgeranyl pyrophosphate stimulates gamma-secretase to increase the generation of Abeta and APP-CTFgamma. Faseb. J. 2008;22:47–54. doi: 10.1096/fj.07-8175com. [DOI] [PMC free article] [PubMed] [Google Scholar]