Abstract
In Caenorhabditis elegans, the central cell-killing process is essentially controlled by the interplay of four apoptotic factors: EGL-1/BH3-only protein, CED-9/Bcl2, CED-4/Apaf1, and CED-3/caspase. In cells destined to die, EGL-1 binds to CED-9 and results in the release of CED-4 from the mitochondrion-tethered CED-9-CED-4 complex to the perinucleus, which facilitates processing of the CED-3 caspase to cause apoptosis. However, whether additional factors exist to regulate the cell-killing process remains largely unknown. We have identified here WAN-1, the C. elegans ortholog of mammalian adenine nucleotide translocator, as an important cell death regulator. Genetic inactivation of wan-1 significantly suppressed both somatic and germ line cell deaths in C. elegans. Consistently, chemical inhibition of WAN-1 activity also caused strong reduction of germ line apoptosis. WAN-1 localizes to mitochondria and can form complex with both CED-4 and CED-9. Importantly, the cell death initiator EGL-1 can disrupt the interaction between CED-9 and WAN-1. In addition, overexpression of WAN-1 induced ectopic cell killing dependently on the core cell death pathway. These findings suggest that WAN-1 is involved in the central cell-killing process and cooperates with the core cell death machinery to promote programmed cell death in C. elegans.
Programmed cell death is an evolutionarily conserved cellular process that is critical for animal development and tissue homeostasis. In the nematode Caenorhabditis elegans, genetic and biochemical studies have identified a regulatory pathway that controls different events of programmed cell death, including the specification of cell death, the activation of cell death, the engulfment of cell corpses, and the degradation of internalized cell debris (17). The process of cell death activation involves four factors: the BH3-only protein EGL-1, the Bcl2 homolog CED-9, the Apaf-1-like protein CED-4, and the caspase CED-3. It has been recognized that the genetic and biochemical interactions among these four factors determine the onset of apoptosis. Several lines of evidence have shown that, in living cells, the antiapoptotic factor CED-9 localizes to mitochondria and binds to CED-4, thus sequestering the proapoptotic activity of CED-4 on mitochondria. In cells that are destined to die, the apoptosis initiator EGL-1 binds to CED-9 with higher binding affinity and induces a conformational change of the latter, which causes translocation of CED-4 from mitochondria to the perinuclear region and triggers apoptosis (6, 27, 39). In vitro, it has been demonstrated that CED-4 binds to CED-9 in a 2:1 ratio. After its release from the CED-9-CED-4 complex, the dimerized CED-4 further forms tetramer to facilitate the processing of the CED-3 procaspase (39). Importantly, the initiation of this protein interaction cascade leading to CED-3 activation requires transcriptional upregulation of EGL-1, which has been proved to be crucial for the onset of apoptosis at least in certain types of cells (8, 22).
The initiation of cell death by protein interactions in C. elegans is quite similar to that in mammals in which BH3-only proteins such as tBid, Bim, Bik, and Bad mediate different cell death stimuli and antagonize antiapoptotic proteins (e.g., Bcl2 and Bcl-xL) to promote the release of several proapoptotic factors from mitochondria, including cytochrome c, Smac/Diablo, AIF, and EndoG. The proapoptotic proteins Apaf-1, cytochrome c, and procaspase-9 form apoptosome in the presence of ATP/dATP, leading to processing of procaspase-9 into an activated form that can further activate effector caspases including caspase-3 and caspase-7 to cause apoptosis (33). The release of proapoptotic factors from mitochondria is controlled by interplays between the pro- and antiapoptotic factors of Bcl2 family proteins (9) and involves the mitochondrion permeability transition pore complex (PTPC). PTPC is believed to be mainly composed of a mitochondrial inner membrane protein, adenine nucleotide translocator (ANT), a mitochondrial outer-membrane protein, voltage-dependent anion channel (VDAC), and a mitochondrial matrix protein, cyclophilin D (10, 16). PTPC can mediate Ca2+- or oxidative stress-induced mitochondrion permeability transition (MPT) and promote the release of proapoptotic factors. Bcl2 family proteins such as Bax, tBid, and Bcl2 interact with PTPC components including ANT and VDAC to regulate mitochondrion membrane permeability (MMP) that is important for the release of proapoptotic factors during apoptosis (20).
In C. elegans, recent studies have shed light on the importance of mitochondrial proteins in programmed cell death. For example, CPS-6 and WAH-1, the C. elegans homologs of Endo G and AIF, respectively, localize to mitochondria and regulate the progression of cell death (26, 36). The BH3-only protein EGL-1 can induce translocation of WAH-1 from mitochondria to cytosol and nucleus. After its translocation, WAH-1 cooperates with CPS-6 to promote apoptotic DNA degradation (26, 36). In the meantime, WAH-1 also synergizes with the phospholipid scramlase SCRM-1/PLSCR to expose phosphatidylserine on the surface of apoptotic cells as an “eat me” signal (34). In addition, ICD-1, a mitochondrial protein homologous to human βNAC, was found to suppress CED-3-independent apoptosis in C. elegans (4). Moreover, it has been reported that mitochondria undergo fragmentation during apoptosis in C. elegans, a process that likely promotes the release of proapoptotic factors such as CPS-6 and WAH-1 (18). Thus, it is becoming evident that mitochondria play critical roles in programmed cell death in C. elegans as in mammals. However, whether other mitochondrial factors function in the cell death activation process in C. elegans is still largely unknown. Particularly, whether the protein interaction cascade leading to apoptosis involves additional mitochondrial regulators remains elusive.
Here we report the identification of wan-1, which encodes the C. elegans ortholog of mammalian ANT, as an important regulator of programmed cell death in C. elegans. Taking biochemical approaches, we have shown that WAN-1 physically interacts with CED-4 both in vivo and in vitro. Furthermore, we have found that WAN-1 also forms complex with CED-9, which can be disrupted by the cell death initiator EGL-1. We demonstrate that wan-1 is important for both somatic and germ line cell deaths in C. elegans by genetic inactivation or chemical inhibition of its activity. In addition, we found that overexpression of WAN-1 caused ectopic cell killing which was dependent on the core cell death pathway. These results establish that WAN-1/ANT, like many other cell death regulators, functions to regulate apoptosis in an evolutionarily conserved manner. Moreover, our findings further underscore that mitochondria play crucial roles in C. elegans programmed cell death.
MATERIALS AND METHODS
C. elegans strains and genetics.
C. elegans strains were provided by C. elegans genetic center (CGC) and worms were cultured and maintained by using standard procedures (5). The Bristol N2 strain was used as wild type. The deletion strains used in the present study are the wan-1(gk172), wan-2(gk294), wan-3(gk300), and wan-4(ok2209) strains. The wan-1(gk172) strain contains a deletion of 604 bp that removes most part of exon 1, the whole exons 2 and 3, and part of exon 4; the wan-2(gk294) strain carries a deletion region of 1,249 bp that removes the whole open reading frame and 324 bp of the promoter region of the wan-2 gene; the wan-3(gk300) strain contains a deletion of 471 bp that removes part of exons 1 and 2 and likely results in an early stop codon in the transcript; and the wan-4(ok2209) strain contains a deletion region of 892 bp including 646 bp of the promoter region and 246 bp of exons 1 and 2. Deletion mutants were outcrossed with N2 strain for at least four times. Double mutants were constructed by using standard protocol (5). Germ line transformation experiments were performed essentially as described previously (25).
Immunoprecipitation and MALDI-TOF analysis.
The yqIs1 transgenic strain expressing Phsp ced-4flag was grown with liquid culture at 20°C. To induce the expression of CED-4Flag protein, worms were heat shocked at 33°C for 1 h and continued to grow at 20°C for another 3 h. Worms were then collected and broken in liquid nitrogen, and proteins were extracted in a lysis buffer (25 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1% Triton X-100, and 10% glycerol) to yield whole-worm lysate. For immunoprecipitation, whole-worm lysate was incubated with agarose beads conjugated with anti-Flag antibody (M2; Sigma) overnight at 4°C. Beads were extensively washed, and bound proteins were resolved on 12% sodium dodecyl sulfate (SDS) polyacrylamide gel and visualized with silver or Coomassie blue staining. Proteins of interest were subjected to matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) analysis. Briefly, gel slices were treated with 100 mM NH4HCO3 to remove Coomassie blue and dehydrated with 50% acetonitrile in 50 mM NH4HCO3. Gel pieces were then sequentially treated with 5 mM dithiothreitol for reduction and 0.5 M iodoacetamide for alkylation. After appropriate washing and dehydration, gel slices were soaked overnight in protein digestion buffer (0.02 μg of trypsin/μl in 25 mM NH4HCO3 [pH 8.0]) at 37°C. Reactions were quenched with 88% formic acid and sonicated to release protein peptides. The supernatant was further cleaned with zip-tip and applied to AutoFlex (Bruker) for mass spectrometric analysis.
RNAi.
The 3′-untranslated region (3′UTR) of wan-1 (125 bp) was in vitro synthesized into double-stranded RNA (dsRNA) and injected into gonads of young adult worms. Surviving progeny that developed normally were scored for embryonic cell corpses 48 h after injection. dsRNA of green fluorescent protein (GFP) was injected as control. Animals developed normally to L4 stage were scored for extra cells in the anterior pharynx. To examine wan-1 RNA interference (RNAi)-caused embryonic lethality, the dsRNA synthesized from the 3′UTR of wan-1 or the cDNA of GFP were injected as described above. At 48 h after injection, eggs were transferred to fresh plates, and hatched animals were counted 12 h later. To examine the wan-1 RNAi effect on germ line apoptosis, two approaches were used. First, a bacterial feeding assay was performed as described previously (36). Briefly, worms synchronized to L3 stage were fed with bacteria expressing either control dsRNA or full-length wan-1 dsRNA, and germ cell corpses were scored at different adult ages of the P0 worms. Second, dsRNAs synthesized from either wan-1 3′UTR or GFP cDNA were injected into the body cavity of L4-stage animals as described by Mello and Fire (24), and germ cell corpses were scored at different time points after injection.
Quantification of cell corpses and extra cells.
Cell corpses and extra cells were scored by using Nomarski optics. For somatic cell corpses, only those in the head region of embryos were scored. Extra cells in the anterior pharyngeal region of L4 animals were scored. To quantify germ cell corpses, cell corpses in the germ line meiotic region of one gonad arm were scored 12, 24, 36, 48, and 60 h after the L4 stage.
Chemical treatment and measurement of ATP level.
Synchronized young adult worms were incubated in Tris buffer (10 mM Tris [pH 7.5]) containing different concentration of Bongkrekic acid (BA; Sigma) or atractyloside potassium (Atr; Sigma) at 20°C for 14 h with supplement of OP50 bacteria. Chemicals were removed by transferring worms onto nematode growth medium plates. Germ cell corpses were scored at indicated time points after the removal of chemicals. To measure ATP levels, 500 animals treated with above chemicals were collected in 100 μl of buffer (100 mM Tris-HCl [pH 7.5], 40 mM EDTA). Worms were flash-frozen in liquid nitrogen and further boiled for 15 min, the resulting supernatant was collected, and the ATP concentration was measured by using an ENLITEN ATP assay kit (Promega). The luminescence was read on the Fluoroskan Ascent FL microplate reader (Thermo), and measurements were performed in triplicate.
Four-dimensional analysis of cell corpse duration.
The recording of both embryonic and germ line cell death events was performed as previously described (38).
Mammalian cell transfection and immunoprecipitation.
HEK293 cells were cultured in Dulbecco modified Eagle medium (HyClone) supplemented with 10% fetal bovine serum (HyClone), and transfections were performed by using the calcium phosphate method or Lipofectamine 2000 (Invitrogen) according to the supplier's instructions. Cells were harvested 36 h posttransfection and lysed in the lysis buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 0.5% sodium deoxycholate, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride). Cell lysate was incubated with the indicated antibodies and protein A/G-Sepharose beads (Roche) for more than 2 h, and the beads were extensively washed. Bound proteins were resolved on SDS-polyacrylamide gel and detected by Western blotting. Immunoprecipitation with purified mitochondria was similarly performed. For immunostaining, transfected cells were fixed with 3.7% formaldehyde and stained with anti-Flag antibody.
Protein expression and GST pull-down.
Recombinant GST-CED-4 and GST-CED-9 proteins were expressed in bacterial BL21(DE3) cells and purified with glutathione-Sepharose beads (Amersham) according to the instructions provided by the supplier. GST pull-down was performed as described previously (36). Briefly, purified GST or GST fusion proteins were immobilized on glutathione-Sepharose beads and incubated with [35S]methionine-labeled proteins at 4°C for more than 2 h. The beads were washed extensively, and bound proteins were separated on an SDS-polyacrylamide gel and viewed by autoradiography.
RESULTS
ANT interacts with CED-4 in C. elegans.
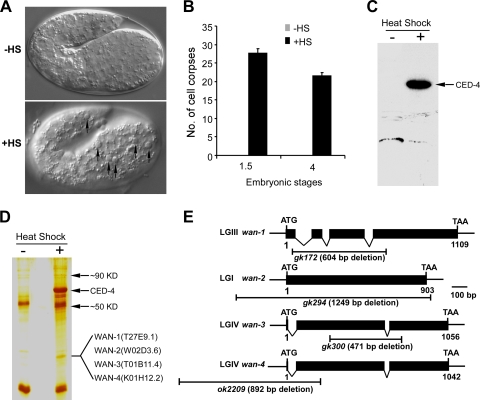
To identify additional factors that are involved in the central cell-killing process in C. elegans, we used a coimmunoprecipitation assay to search for proteins that are associated with the proapoptotic protein CED-4. We first expressed a Flag-tagged CED-4 (CED-4Flag) under the control of a heat shock promoter in ced-1(e1735); ced-4(n1162) double mutants. After heat shock, a large number of cell corpses were observed in transgenic embryos, indicating that the transgenic CED-4Flag was functional to rescue the cell death defects caused by ced-4 loss-of-function mutation (Fig. 1A and B). Western blot analysis further confirmed that CED-4Flag was well expressed in transgenic animals (Fig. 1C). We next prepared whole-worm lysate from the transgenic animals and performed immunoprecipitation of CED-4Flag with anti-Flag antibody. Three proteins with molecular masses of ∼90, ∼50, and ∼33 kDa were found to associate with CED-4 (Fig. 1D). Mass spectrometric analyses revealed that the peptide sequences of the 33-kDa protein corresponded to four proteins belonging to a family of highly homologous proteins, ANTs (Fig. 1D and see Fig. S1A in the supplemental material). Sequence comparison and structure modeling indicated that C. elegans ANTs share high homology with human ANT (see Fig. S1B and C in the supplemental material). We named them wan genes for “worm adenine nucleotide translocators.” wan-1 and wan-2 encode for proteins of 300 amino acids, while wan-3 and wan-4 encode for proteins of 313 amino acids, respectively (see Fig. S1B in the supplemental material).
FIG. 1.
Identification of ANT as CED-4-interacting protein. (A) Integrated transgenic array (yqIs1) expressing Phsp ced-4flag rescued the cell death defect in ced-1(e1735); ced-4(n1162) mutants. The top and bottom panels show the embryos of ced-1(e1735); yqIs1; ced-4(n1162) worms before and after heat shock, respectively. Arrows indicate cell corpses. (B) Quantification of embryonic cell corpses of above animals treated with heat shock. Embryos at early stages were heat-shocked at 33°C for 1 h and continued to grow at 20°C. Embryos that developed to 1.5- and 4-fold stages before and after heat shock were scored for the number of cell corpses. Error bars represent the standard error of the mean (SEM). (C) The expression of CED-4Flag in above animals was detected by Western blot assay. (D) WAN proteins were associated with CED-4. Immunoprecipitation was performed by using anti-Flag antibody from the lysate of above worms and proteins were resolved on 12% SDS polyacrylamide gel and viewed by silver staining. Proteins identified by mass spectrometric analysis are indicated by arrows. (E) Schematic representation of deletion mutations of wan genes. LGI, LGIII, and LGIV indicate linkage group I, III, and IV, respectively. Solid boxes indicate exons and waved lines indicate introns. The fragment below each gene indicates the region and size of deletion of each wan gene.
Human possesses four ANT isoforms that are expressed in tissue- and development-specific manner. These ANTs function in mitochondria for the exchange of mitochondrial ATP with cytosolic ADP (14, 32). Importantly, ANT is a major component of mitochondrial PTPC that promotes the release of apoptotic factors from mitochondria during apoptosis (2, 16, 45). In addition, overexpression of ANT1 and ANT3 dominantly induced apoptosis (1, 44), while the knockdown of ANT2 sensitized cells to chemical-induced apoptosis (21). To determine whether C. elegans wan genes are important for programmed cell death, we analyzed the profiles of developmental cell deaths in wan-1(gk172), wan-2(gk294), wan-3(gk300), and wan-4(ok2209) deletion mutants which likely represent strong loss-of-function or null mutations of individual wan genes (Fig. 1E). Homozygous wan-1(gk172) worms are lethal. However, homozygous wan-2, -3, and -4 deletion mutants are viable and exhibit no obvious growth defects, allowing us to examine the cell death profiles in these mutants. A time course analysis of both embryonic and germ line cell deaths indicated that the wan-2(gk294), wan-3(gk300), and wan-4(ok2209) single mutants did not show pronounced defects in either somatic or germ line apoptosis compared to that of wild-type animals (see Fig. S2A in the supplemental material and data not shown). Furthermore, the wan-2(gk294); wan-3(gk300) and wan-2(gk294); wan-4(ok2209) double mutants, as well as the wan-2(gk294); wan-3(gk300) wan-4(RNAi) triple mutants did not show obvious cell death defects (see Fig. S2B in the supplemental material). In addition, we examined the cell death profiles of these wan gene mutants in the background of ced-1(e1735), which causes the accumulation of cell corpses owing to defects in cell corpse engulfment. In the double mutants between ced-1 and wan-2, -3, and -4, neither embryonic nor germ line cell death profiles are distinct from that of ced-1(e1735) single mutants alone (see Fig. S2C and D in the supplemental material). Taken together, these results indicate that wan-2, -3, and -4 are not essential for programmed cell death in C. elegans.
wan-1 functions in the cell-killing process.
Since wan-1(gk172) deletion mutants are embryonic lethal, we performed RNAi to inactivate wan-1 to determine whether it is involved in programmed cell death. For this purpose, we used the dsRNA derived from the wan-1 3′UTR, which shares no homology with other wan genes. wan-1 RNAi gave rise to an efficient inactivation of wan-1 (see Fig. S3A in the supplemental material) and caused ca. 60% embryonic lethality in wild-type animals, which was less severe than observed in wan-1(gk172) animals (see Table S1 in the supplemental material). Nevertheless, in animals expressing a WAN-1::GFP translation fusion protein under the control of the wan-1 promoter and the 3′UTR of the unc-54 gene (Pwan-1 wan-1::gfp::unc-54 3′UTR), the embryonic lethality caused by wan-1 RNAi was strongly suppressed (see Table S1 in the supplemental material), indicating that the 3′UTR-derived wan-1 RNAi specifically targeted wan-1 for inactivation. However, since fair number of wan-1(RNAi) embryos survived and developed normally to L4 stage, we were able to examine the cell death profiles in these escapers. In wan-1(RNAi) embryos, the number of cell corpses was significantly decreased at all examined embryonic stages compared to that in control RNAi-treated N2 animals (Fig. 2A). In contrast, in embryos expressing Pwan-1 wan-1::gfp::unc-54 3′UTR, wan-1 RNAi failed to cause obvious decrease of cell corpses (Fig. 2A), indicating that the expression of WAN-1::GFP rescued the embryonic cell death defects caused by the 3′UTR-derived wan-1 RNAi. wan-1 RNAi also significantly reduced the number of cell corpses in the triple mutants of wan-2, -3, and -4 (see Fig. S2B in the supplemental material). Moreover, we used four-dimensional microscopy analysis to determine the number of cell death events from the first cell division to 400 min during embryogenesis. We found that significantly fewer cell deaths occurred in wan-1(RNAi) embryos that developed normally than in control RNAi-treated animals (Fig. 2B). In addition, we examined whether wan-1 RNAi could enhance the cell death defects in the ced-3 and ced-4 weak loss-of-function mutants. In wild-type worms, 16 cells in the anterior pharynx normally undergo programmed cell death and barely extra “undead” cells can be observed. In the ced-3(n2438) and ced-4(n2273) weak loss-of-function mutants, however, cell death is partially blocked, and usually one to three extra cells can be seen. Importantly, wan-1 RNAi significantly enhanced the cell survival phenotype in both weak ced-3 and ced-4 mutants (Table 1) . Taken together, these findings suggest that wan-1 RNAi inhibited programmed cell death in C. elegans.
FIG. 2.
wan-1 RNAi affects embryonic cell death. (A) wan-1 RNAi inhibited embryonic cell death in wild type but not in animals expressing WAN-1::GFP. dsRNA was synthesized from the 3′UTR of wan-1 (wan-1 RNAi) or GFP cDNA (control RNAi) and injected into the gonads of the wild type (N2) and worms expressing Pwan-1 wan-1::gfp::unc54 3′UTR (wan-1::gfp). At 48 h after injection, surviving progeny were scored for cell corpses of different embryonic stages. Fifteen animals were scored for each embryonic stage. Error bars represent the SEM. Comparisons were performed between control RNAi and wan-1 RNAi treatment using an unpaired t test. Single asterisks indicate P < 0.05, and double asterisks indicate P < 0.001. (B) The occurrence of embryonic cell death events from the first cell division to 400 min during embryogenesis. Five embryos from control RNAi- and wan-1 RNAi-treated N2 animals were recorded by using four-dimensional microscopy, respectively. The y axis indicates the average number of total cell death events. (C to F) The embryonic cell death profiles of wan-1 RNAi-treated mutants defective in cell corpse engulfment. Results for the ced-1(e1735) (C), ced-2(e1752) (D), ced-7(n1892) (E), and ced-5(n1812) (F) strains are shown. Cell corpses were scored and analyzed as in panel A.
TABLE 1.
wan-1 RNAi enhances the cell death defects in ced-3 and ced-4 weak loss-of-function mutants
| Strain | No. of samples scored | No. of extra cells
|
|
|---|---|---|---|
| Mean ± SEMa | Range | ||
| N2; control RNA: | 27 | 0 | 0 |
| N2; wan-1 RNA: | 27 | 0.26 ± 0.12* | 0-2 |
| ced-3(n2438); control RNA: | 42 | 1.48 ± 0.10 | 0-3 |
| ced-3(n2438); wan-1 RNA: | 30 | 2.70 ± 0.16† | 1-5 |
| ced-4(n2273); control RNA: | 24 | 2.17 ± 0.20 | 1-4 |
| ced-4(n2273); wan-1 RNA: | 20 | 3.80 ± 0.27† | 2-6 |
The numbers of extra cells of control RNAi- and wan-1 RNAi- treated animals were compared by using an unpaired Student t test. *, P < 0.05; †, P < 0.001.
To further prove that wan-1 is important for cell death, we performed wan-1 RNAi in animals defective in cell corpse engulfment. In C. elegans, the engulfment of cell corpses is mainly controlled by two parallel and partially redundant genetic pathways, with the genes ced-1, -6, and -7 and dyn-1 functioning in one pathway and the genes ced-2, -5, -10, and -12 and psr-1 functioning in the other pathway (29, 35, 43). In ced-1(e1735) and ced-7(n1892) animals, wan-1 RNAi significantly decreased the number of cell corpses at all examined embryonic stages (Fig. 2C and E). Similarly, wan-1 RNAi also caused strong reduction of cell corpses in ced-2(e1752) and ced-5(n1812) animals (Fig. 2D and F). The reduction of cell corpses in both engulfment pathways further proved that wan-1 RNAi indeed inhibited cell death, suggesting that wan-1 likely functions in the cell-killing process.
wan-1 is important for germ cell death.
Except for somatic cell death, C. elegans germ cells also undergo apoptosis, which is controlled by the same regulatory pathway as in somatic cells (15). To determine whether wan-1 is also important for germ cell death, we examined wan-1 RNAi-treated wild type as well as mutants defective in cell corpse engulfment. In the wild type, dying cells are swiftly engulfed and degraded so that only a small number of germ cell corpses can be seen at different adult ages (15), but even fewer cell corpses were observed after wan-1 RNAi treatment (Fig. 3A), suggesting that wan-1 RNAi likely inhibited germ cell death as well. Moreover, wan-1 RNAi treatment by either feeding with bacteria expressing dsRNA derived from the cDNA of wan-1 (Fig. 3B and C) or injection of dsRNA corresponding to the 3′UTR of wan-1 (see Fig. S4 in the supplemental material) strongly reduced the number of germ cell corpses to a similar level in all examined mutants defective in cell corpse engulfment. The reduction of germ cell corpses was more profound with the aging of animals (Fig. 3B and C and see Fig. S4 in the supplemental material); this was not likely due to defects in germ line development because the germ lines of ced-1(e1735); wan-1(RNAi) worms were morphologically indistinguishable from those of control RNAi-treated animals (data not shown). In addition, the number of nuclei in the germ line mitotic regions of both control RNAi- and wan-1 RNAi-treated ced-1(e1735) worms showed no significant differences (Fig. 3D). To determine whether the wan-1 RNAi-induced reduction of germ cell corpses had resulted from a suppression of apoptosis or an enhancement of cell corpse engulfment, we used four-dimensional microscopy analysis to measure the duration of germ cell corpse persistence and to monitor the occurrence of germ cell death. Our results indicated that germ cell corpses on average existed longer than 200 min in both control RNAi- and wan-1 RNAi-treated ced-1(e1735) worms, suggesting that wan-1 RNAi did not obviously affect the engulfment of cell corpses. However, wan-1 RNAi significantly reduced the number of cell death events. For example, we observed 5.6 ± 0.7 cells die within 200 min in the gonadal U-turn region in control RNAi-treated ced-1(e1735) animals with an adult age of 36 h post-L4 stage, but we only observed 1.1 ± 0.4 cells die within 200 min in the same gonadal region of wan-1 RNAi-treated ced-1(e1735) animals at the same adult age (Fig. 3E, region 1). In the oocyte region, we also found that wan-1 RNAi significantly reduced the number of cell death events compared to control RNAi treatment (Fig. 3E, region 2). These findings indicated that wan-1 RNAi suppressed germ cell death rather than enhanced cell corpse engulfment. Collectively, our results demonstrated that wan-1 regulates germ line apoptosis in C. elegans.
FIG. 3.
wan-1 RNAi suppresses germ line apoptosis in C. elegans. (A) The germ cell death profile in N2 animals treated with wan-1 RNAi. (B) wan-1 RNAi inhibited germ cell death in ced-1(e1735), ced-6(n2095), and ced-7(n1892) mutants. (C) wan-1 RNAi inhibited germ cell death in ced-2(e1752), ced-5(n1812), and ced-12(tp2) mutants. RNAi was performed by using a bacterial feeding assay, and germ cell corpses in one gonad arm were scored every 12 h after L4 stage for each animal. Fifteen animals were scored for every time point. The y axis indicates the average number of germ cell corpses of one gonad arm. Error bars represent the SEM. Comparisons were performed as described above. (D) wan-1 RNAi did not affect germ line development. The germ lines of control RNAi- and wan-1 RNAi-treated ced-1(e1735) animals were dissected and stained with DAPI (4′,6′-diamidino-2-phenylindole) (top panel), and the nuclei number of the indicated germ line mitotic region were counted (bottom panel). N represents the number of animals scored. (E) wan-1 RNAi significantly reduced the occurrence of germ cell death events. The occurrence of germ cell death in both control RNAi- and wan-1 RNAi-treated ced-1(e1735) animals at 36 h post-L4 stage were monitored with four-dimensional microscopy recording. The top panel shows the schematic representation of the gonadal regions examined. Region 1 indicates the gonadal U-turn region, and region 2 indicates the oocyte region. The bottom panel shows the quantification of cell death events within 200 min in the examined gonadal regions. Error bars represent the SEM. Comparisons were performed as described above. Seven animals were analyzed for each treatment.
The ANT inhibitor BA suppresses apoptosis in C. elegans.
Mammalian ANT has two states, the cytosolic state (c-state) and the matrix state (m-state) (28). The PTP-inhibitory compounds Atr and BA can selectively bind to different regions of ANT (Fig. 4A). The binding of Atr to ANT fixes the latter to the c-state, which induces the swelling of mitochondria and facilitates apoptosis (23). In contrast, BA fixes ANT to the m-state and suppresses Atr- or Bax-induced apoptosis (23). Because WAN-1 highly resembles mammalian ANT (28) (see Fig. S1B and C in the supplemental material), we tested whether BA and Atr could affect programmed cell death in C. elegans by treating ced-1(e1735) animals with BA or Atr at concentrations comparable with those applied to mammalian cells for interfering apoptosis (1, 23). Although both BA and Atr caused significant reduction of ATP level in worms (Fig. 4B), only BA induced a strong suppression of germ line apoptosis in a concentration-dependent manner (Fig. 4C, D, and E). For example, 6 h after removal of BA, only a mean of 5.4 germ cell corpses were observed in ced-1(e1735) animals treated with 100 μM BA, compared to a mean of 31.2 germ cell corpses at the same time point in the control animals (Fig. 4D). In contrast, Atr did not cause obvious change of germ line apoptosis (Fig. 4E), indicating that the suppression of apoptosis by BA was not likely due to a general inhibition of ATP/ADP exchange. The suppression of germ line apoptosis by BA suggests that WAN-1 may function similarly to its mammalian equivalents in regulating apoptosis.
FIG. 4.
BA inhibits germ line apoptosis in C. elegans. (A) Schematic representation of BA and Atr molecules. (B) BA and Atr decreased ATP levels in ced-1(e1735) mutants. Young adult worms of ced-1(e1735) were treated with BA or Atr at indicated concentrations for 14 h, and the ATP levels were determined. (C) BA suppressed germ line apoptosis in ced-1(e1735) animals. Young adult worms of ced-1(e1735) were treated as in panel B, and germ cell death was examined after the removal of chemicals. The pictures show the germ lines of a mock-treated animal and a BA-treated (100 μM) animal 6 h after removal of BA. Arrows indicate germ cell corpses. (D and E) Quantification of germ cell corpses of ced-1(e1735) animals treated with BA and Atr. Germ cell corpses of BA-treated (50 and 100 μM) (D) and Atr-treated (100 and 200 μM) (E) animals were scored at indicated time points after the removal of chemicals. Fifteen animals were scored for each time point. Error bars represent the SEM.
WAN-1 localizes to mitochondria.
To understand how WAN-1 may regulate programmed cell death, we examined the subcellular localization of the translational WAN-1::GFP fusion protein (Pwan-1 wan-1::gfp::unc-54 3′UTR). WAN-1::GFP expression was observed from embryonic to adult stages. In adult worms, WAN-1::GFP was seen in a wide range of tissues including the intestines, hypoderm, vulva, and some neurons (see Fig. S3B in the supplemental material). In embryos, WAN-1::GFP exhibited a punctate, cytoplasmic staining pattern which overlapped with that of the mitochondrion-specific fluorescent dye, Mitotracker Red (Fig. 5A), indicating that WAN-1 is localized to mitochondria. To confirm this, we expressed WAN-1Flag in HEK293 cells and found that it also displayed a punctate staining pattern that completely overlapped with that of Mitotracker Red (Fig. 5B). A further subcellular fractionation analysis revealed that WAN-1Flag existed in the same fraction with that of cytochrome c (see Fig. S3C in the supplemental material). These results indicate that WAN-1 indeed localizes to mitochondria.
FIG. 5.
WAN-1 localizes to mitochondria. (A) WAN-1 localizes to mitochondria in C. elegans. The DIC, GFP, and Mitotracker Red images and the merged image of GFP and Mitotracker Red of an early transgenic embryo expressing the WAN-1::GFP fusion protein are shown. (B) WAN-1 localizes to mitochondria in HEK293 cells. WAN-1Flag was expressed in HEK293 cells and viewed by immunostaining with anti-Flag antibody. Mitochondria were stained with Mitotracker Red, and nucleus was stained with DAPI. (C and D) WAN-1 likely oligomerizes. MycHis- and Flag-tagged WAN-1 proteins were cotransfected in HEK293 cells and purified with Ni-agarose beads (C) or immunoprecipitated with anti-Flag antibody (D) and detected by Western blotting.
Biochemical and structural studies have suggested that mammalian ANT functions as a dimer (28). Since WAN-1 highly resembles mammalian ANT, we tested whether WAN-1 could also dimerize. We coexpressed WAN-1 proteins with different tags in HEK293 cells and performed immunoprecipitation. Our results indicated that WAN-1Myc could nicely pull down WAN-1Flag and vice versa (Fig. 5C and D). Thus, WAN-1 likely dimerizes or oligomerizes to fulfill its normal function in living cells.
WAN-1 interplays with core regulators for cell death activation.
Previously, heterologous expression of C. elegans core cell death regulators in mammalian cultured cells has been used very successfully in dissecting the interaction among these proteins, which suggested a molecular mechanism underlying programmed cell death (12, 13, 37, 41). Since WAN-1 could localize to mitochondria in HEK293 cells as in worms (Fig. 5B), we decided to take advantage of mammalian cells to examine the potential protein interactions between WAN-1 and other core cell death regulators.
We first tested whether CED-4 and WAN-1 could interact in mammalian cells since WAN-1 was initially identified as a CED-4-interacting protein in C. elegans (Fig. 1). When WAN-1 and CED-4 labeled with different tags were coexpressed in HEK293 cells, CED-4 nicely pulled down WAN-1 as revealed by immunoprecipitation (Fig. 6A). In contrast, CED-4 failed to interact with an unrelated protein, the C. elegans p53 homolog CEP-1, when they were similarly coexpressed (Fig. 6A), indicating that CED-4 and WAN-1 interacted specifically in mammalian cells. Next, we examined whether CED-4 and WAN-1 could directly interact using the glutathione S-transferase (GST) fusion protein pull-down assay. Our data indicated that 35S-labeled WAN-1 interacted with GST-CED-4 immobilized on glutathione-Sepharose beads but not with GST (Fig. 6B), further demonstrating that WAN-1 interacted with CED-4 directly and specifically.
FIG. 6.
WAN-1 interacts with core cell death regulators. (A) CED-4 interacted with WAN-1 in HEK293 cells. CED-4Myc was coexpressed with WAN-1Flag or CEP-1Flag in HEK293 cells, immunoprecipitated with anti-Myc antibody, and detected by Western blotting with the indicated antibodies. (B) CED-4 interacted with WAN-1 directly. Purified GST or GST-CED-4 protein (3 μg of each) immobilized on glutathione-Sepharose beads was incubated with 35S-labeled WAN-1 or luciferase (LUC) for more than 2 h and washed extensively. Bound proteins were resolved on 12% SDS-polyacrylamide gel and visualized by autoradiography. Lanes 1 and 4 indicate 10% of input proteins. (C) CED-9 interacted with WAN-1 in HEK293 cells. CED-9MycHis was coexpressed with WAN-1Flag or CEP-1Flag in HEK293 cells, immunoprecipitated with anti-Flag antibody, and detected by Western blotting. (D) GST-CED-9 and 35S-labeled WAN-1 interacted in vitro. The experiment was performed as described in panel B. (E) WAN-1, CED-4, and CED-9 form a complex in HEK293 cells. WAN-1Flag, CED-9MycHis, and CED-4Myc were coexpressed in HEK293 cells, immunoprecipitated with anti-Flag antibody, and detected by Western blotting. (F) EGL-1 disrupts the interaction between CED-9 and WAN-1. WAN-1Flag and CED-9MycHis were coexpressed with or without EGL-1Flag in HEK293 cells. Immunoprecipitation was performed by using anti-Myc antibody and detected with the indicated antibodies. (G) EGL-1 interacted with CED-9 but not with WAN-1. EGL-1Myc and CED-9Flag were coexpressed with or without WAN-1Flag in HEK293 cells. Immunoprecipitation was performed using anti-Myc antibody and detected by Western blotting.
In living cells, CED-9 forms complex with CED-4 to prevent the latter from promoting CED-3 processing. Since WAN-1 directly interacts with CED-4, we tested whether WAN-1 could also interact with CED-9. We found that CED-9 was associated with WAN-1 but not CEP-1 in HEK293 cells (Fig. 6C). In addition, the GST pull-down assay revealed that WAN-1 could directly interact with CED-9 in vitro (Fig. 6D). These results indicated that WAN-1 and CED-9 could also form complex.
As WAN-1, CED-4, and CED-9 directly interacted with one another, we examined whether they could form a tripartite complex by performing immunoprecipitation of WAN-1Flag, CED-4Myc, and CED-9Myc, which were coexpressed in HEK293 cells. As shown in Fig. 6E, both CED-4 and CED-9 were coimmunoprecipitated with WAN-1, indicating that they formed a complex. Next, we investigated whether these interactions occurred on mitochondria. We isolated the mitochondrial fraction from HEK293 cells coexpressing WAN-1 and CED-4 or WAN-1 and CED-9 and found that WAN-1 interacted with both CED-4 and CED-9 in purified mitochondria (see Fig. S3D and E in the supplemental material). Nevertheless, these interactions seemed not to be affected by either BA or Atr (see Fig. S3D and E in the supplemental material), implying that BA may not function by directly interfering with the protein interaction between WAN-1 and these two cell death regulators.
The binding of the cell death initiator EGL-1 to CED-9 to disrupt CED-9-CED-4 complex is the key to the onset of apoptosis in C. elegans (6, 39). Because WAN-1 interacts with CED-9, we tested whether EGL-1 could disrupt the complex of these two proteins. In HEK293 cells, WAN-1 was associated with CED-9 as shown by coimmunoprecipitation (Fig. 6C and E). However, when EGL-1 was coexpressed with WAN-1 and CED-9, the interaction between WAN-1 and CED-9 was strongly reduced (Fig. 6F). Moreover, we found that EGL-1 efficiently pulled down CED-9 but not WAN-1 when these three proteins were coexpressed in HEK293 cells, indicating that EGL-1 and WAN-1 did not interact with each other (Fig. 6G). Thus, the strong reduction of WAN-1-CED-9 interaction was likely caused by an EGL-1-induced conformational change of CED-9 (40). The disruption of CED-9-WAN-1 interaction by EGL-1 may release the proapoptotic activity of WAN-1 which together with CED-4 promotes the activation of cell death in C. elegans.
wan-1 overexpression induces ectopic cell death in C. elegans.
Because WAN-1 interacts with core cell death regulators and plays an essential role in programmed cell death, we further examined whether WAN-1 could induce cell death by ectopically expressing WAN-1 in touch receptor neurons under the control of the promoter of the mec-7 gene (Pmec-7 wan-1) (30). We then scored the percentage of posterior lateral microtubule (PLM) touch receptor neurons that were killed. Our data revealed that overexpression of WAN-1 resulted in >50% death of PLM neurons (51 to 56% PLM death in three independent transgenic lines), which was similar to that caused by CED-4 overexpression (Pmec-7 ced-4) (48 to 56% PLM death) (Table 2). These results indicate that WAN-1 on its own can induce ectopic cell death. To determine whether WAN-1-induced cell death requires the core cell death machinery, we crossed transgenic lines expressing WAN-1 into the ced-3(n717) and ced-4(n1162) strong loss-of-function mutants, as well as into the ced-9(n1950) gain-of-function mutants. We found that PLM killing caused by WAN-1 overexpression was almost completely abolished in these mutants, indicating that WAN-1 induced cell killing through the core cell death pathway (Table 2).
TABLE 2.
Overexpression of wan-1 induces ectopic cell killing in PLM touch receptor neuronsa
| Transgene(s)b | Array | PLM survival (%) |
|---|---|---|
| None | 100 | |
| Pmec-7wan-1 | 1 | 44 |
| 2 | 46 | |
| 3 | 49 | |
| ced-3(n717); Pmec-7wan-1* | 1 | 99 |
| 2 | 97 | |
| ced-4(n1162); Pmec-7wan-1* | 1 | 96 |
| 2 | 95 | |
| ced-9(n1950gf); Pmec-7wan-1* | 1 | 98 |
| 2 | 99 | |
| Pmec-7ced-4 | 1 | 52 |
| 2 | 44 | |
| ced-9(n1950gf); Pmec-7ced-4c | 1 | 54 |
| 2 | 50 | |
| Pmec-7wan-1; Pmec-7ced-4 | 1 | 32 |
| 2 | 44 | |
| ced-9(n1950gf); Pmec-7wan-1; Pmec-7ced-4d | 1 | 55 |
| 2 | 57 |
Pmec-7 wan-1 construct (50 ng/μl) and Pmec-7 ced-4 construct (25 ng/μl) alone or combined were coinjected with the injection marker pRF4 (50 ng/μl) into the C. elegans bzIs8 strain in which the six touch receptor neurons are labeled by GFP. pRF4 causes a roller phenotype. Each numbered array indicates an independent transgenic line in which the existence of green PLM neurons was scored.
*, The first two transgenic lines of Pmec-7wan-1 were crossed into ced-3(n717) and ced-4(n1162) strong loss-of-function mutants, as well as into a ced-9(n1950) gain-of-function mutant.
The two transgenic lines of Pmec-7 ced-4 were crossed into the ced-9(n1950) mutants.
The two transgenic lines of Pmec-7wan-1; Pmec-7 ced-4 were crossed into the ced-9(n1950) mutants. More than 45 transgenic animals with roller phenotype (>90 PLM neurons) were scored for each transgenic line.
To investigate further how WAN-1 may act to induce PLM death, we coexpressed WAN-1 and CED-4 in touch receptor neurons and found that it caused a higher percentage of PLM death (56 to 68%) than that caused by either WAN-1 or CED-4 alone (Table 2). Previously, it was shown that CED-4-induced ectopic cell killing was enhanced by ced-9 loss-of-function mutation (31), which suggests that endogenous CED-9 protects against cell killing by CED-4 overexpression. Thus, we speculated that the increased PLM death observed in animals coexpressing WAN-1 and CED-4 was due to an antagonistic effect of WAN-1 on endogenous CED-9. To test this possibility, we crossed transgenic lines expressing CED-4 alone and lines coexpressing WAN-1 and CED-4 into the ced-9(n1950) gain-of-function mutants. Consistent with previous report (31), the ced-9(n1950) mutation did not obviously inhibit the PLM death induced by CED-4. In contrast, the ectopic PLM death induced by coexpressing WAN-1 and CED-4 was partially suppressed by the ced-9(n1950) gain-of-function mutation (Table 2), which resulted in PLM death similarly to that caused by overexpressed CED-4 in the ced-9(n1950) mutants (Table 2). Altogether, these data suggest that WAN-1 likely promotes cell killing by antagonizing the antiapoptotic role of CED-9.
DISCUSSION
We have shown here that C. elegans ANT, WAN-1, is important for programmed cell death. WAN-1 was identified as a CED-4-interacting protein by coimmunoprecipitation. We further demonstrated that WAN-1 and CED-4 specifically interacted with one another in mammalian cells, and they directly interacted in vitro. Moreover, we found that WAN-1 could also interact with CED-9, the antiapoptotic protein that binds to CED-4 and prevents apoptosis in C. elegans. Our biochemical data indicate that WAN-1, CED-4, and CED-9 likely form a complex on mitochondria. Importantly, we found that the interaction between WAN-1 and CED-9 could be disrupted by EGL-1, the CED-9 antagonist that initiates the cell-killing process. These results suggest that WAN-1/ANT is a novel and important cell death regulator in C. elegans, which is supported by our in vivo observations that wan-1 RNAi inhibited both somatic and germ line cell deaths and promoted cell survival in C. elegans. This conclusion is reinforced by the observation that the ANT-inhibitory compound BA, which fixes ANT to an m-conformation, inhibited germ cell apoptosis in C. elegans. Moreover, WAN-1 on its own can induce ectopic cell death that requires the core cell death pathway. Taken together, these findings suggest that WAN-1 cooperates with core cell death machinery to promote programmed cell death in C. elegans.
Although wan-2, -3, and -4 are highly homologous to wan-1, our genetic data indicate that they are not essential for either cell survival or programmed cell death in C. elegans, although they may be involved in other, yet-unidentified cellular processes. The potential interaction between CED-4 and the nonapoptotic proteins WAN-2, -3, and -4 may be involved in regulating other cellular processes. For instance, CED-4 has been found recently to participate in hypoxic response and cell size control in addition to apoptosis (7, 11). The facts that deletion of wan-1 gene led to embryonic lethality and wan-1(RNAi) animals exhibited significant reduction of both somatic and germ line apoptosis suggest that WAN-1 is important for both ATP/ADP exchange and apoptosis in worm. This dual function of WAN-1 is similar to its mammalian counterpart. In mammals, previous studies have shown that ANT is a bifunctional protein. On one hand, ANT controls the exchange of cytosolic ADP with mitochondrial ATP to provide energy for cell metabolism. On the other hand, the human ANT isoforms ANT1 and ANT3 can dominantly induce apoptosis in a wide variety of cell types independently of their ATP/ADP exchange activity (1, 44), and ANT3 is required for tumor necrosis factor alpha-induced apoptosis in MCF-7 cells (42). Thus, C. elegans WAN-1 and mammalian ANT share evolutionarily conserved functions.
The exact mechanisms for ANT to regulate apoptosis in mammals have been controversial. It is believed that ANT, VDAC and cyclophilin D form nonspecific permeability transition pore (PTP) at the contact site between mitochondrial outer and inner membranes to control MMP that promotes the release of proapoptotic proteins during apoptosis (2, 20). Bcl2 and Bax are found to individually interact with mammalian ANT to modulate its dual function. Bcl2 enhances but Bax suppresses the ATP/ADP exchange through a direct action on ANT (3). Moreover, Bax cooperates with ANT to increase mitochondrion membrane permeability because these two proteins, but not either alone, formed Atr-responsive channels in artificial membranes. Inhibition of ANT activity by BA abrogated Bax-induced nuclear apoptosis (23). Nevertheless, a mouse knockout study showed that mouse hepatocytes deficient in both Ant1 and Ant2 responded to several apoptosis stimuli equally well to wild-type hepatocytes although increased resistance of isolated mitochondria to Ca2+-induced MPT were observed in these Ant knockout mice, opening the debate if ANT is essential for PTP which might be dispensable for MPT-associated apoptosis (19). Intriguingly, overexpression of ANT1 and ANT3 induced apoptosis with a concomitant decrease of mitochondrial inner membrane potential in several types of human cultured cells except hepatocyte, and apoptosis induced by ANT1 and ANT3 was suppressed by the ANT ligand BA or cyclophilin D (1, 44). In addition, MCF-7 cells harboring a hypomorphic mutation of Ant3 were resistant to tumor necrosis factor alpha-induced apoptosis, which was associated with a deficiency in MMP regulation and cytochrome c release (42). Thus, a reasonable interpretation for these seemingly contradictory observations is that ANT might not be an essential component for PTP, but it functions as an important regulator of PTP by interacting with VDAC and cyclophilin D. Alternatively, ANT may regulate apoptosis by interacting with other pro- or antiapoptotic factors. Regardless of these mechanistic arguments on the role of mammalian ANT in regulating apoptosis, our findings that WAN-1/ANT plays an essential role in C. elegans apoptosis strongly suggest that ANT is an important cell death regulator evolutionarily conserved from nematodes to humans. Our results indicate that the protein interactions between WAN-1/ANT and the core cell death regulators likely account for its role in regulating programmed cell death. In living cells, WAN-1, CED-4, and CED-9 likely form a complex and the interaction between WAN-1 and CED-9 possibly facilitates ATP/ADP exchange and promotes survival in C. elegans in a similar manner to that of ANT and Bcl2 in mammalian cells (3). In the meantime, CED-9 inhibits the proapoptotic functions of both CED-4 and WAN-1 by forming a complex with these two proteins. In cells destined to die, by contrast, EGL-1 is upregulated and binds to CED-9, leading to the disruption of the interactions between WAN-1 and CED-9, as well as between CED-4 and CED-9. Thus, the inhibitory effect on WAN-1-CED-4 complex is removed. Although we currently do not know clearly what other cell death events that CED-4 may affect through interacting with WAN-1 before its release from mitochondria, our genetic data support the notion that WAN-1 and CED-4 likely act together to antagonize the antiapoptotic activity of CED-9 to promote the cell-killing process.
Whether C. elegans mitochondria possess mammalian PTPC-like complex still needs to be uncovered. The inhibition of apoptosis by the ANT ligand BA in C. elegans germ line suggests that a specific conformation of WAN-1/ANT is required for its proper function in promoting apoptosis. Since it seems BA does not affect the interactions between WAN-1 and CED-4 or CED-9, the BA-specific conformation of WAN-1/ANT may be important for a PTP-like structure in C. elegans. Alternatively, such conformation of WAN-1/ANT might affect other cellular events on mitochondria that may be initiated by the interaction between WAN-1 and the core cell death regulators.
In conclusion, we have identified WAN-1 as a novel factor that cooperates with core cell death regulators including CED-4, CED-9, and EGL-1 to regulate apoptosis in C. elegans. Our findings demonstrate that ANT is an evolutionarily conserved cell death regulator. Moreover, our discovery that the mitochondrial protein WAN-1/ANT is important for programmed cell death further supports previous findings (18, 26, 34, 36) that mitochondria play critical roles in programmed cell death in C. elegans.
Supplementary Material
Acknowledgments
We thank the C. elegans Genetic Center (CGC) for providing worm deletion strains; Shanting Hao for help with mass spectrometric analysis, Yingfang Liu for structure modeling of WAN-1, and Xiaochen Wang and Xun Huang for helpful suggestions and critical reading of the manuscript.
This research was supported by grants 2007CB947201 and 2006CB504100 from the National Basic Research Program of China, grants 30771059 and 30871266 from the National Natural Science Foundation of China, grant 2006AA02Z147 from the 863 Program of China, and grant KSCX1-YW-R-70 from the Chinese Academy of Sciences. C.Y. is supported by the 100-Talents Program of the Chinese Academy of Sciences.
Footnotes
Published ahead of print on 4 May 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bauer, M. K., A. Schubert, O. Rocks, and S. Grimm. 1999. Adenine nucleotide translocase-1, a component of the permeability transition pore, can dominantly induce apoptosis. J. Cell Biol. 1471493-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belzacq, A. S., H. L. Vieira, G. Kroemer, and C. Brenner. 2002. The adenine nucleotide translocator in apoptosis. Biochimie 84167-176. [DOI] [PubMed] [Google Scholar]
- 3.Belzacq, A. S., H. L. Vieira, F. Verrier, G. Vandecasteele, I. Cohen, M. C. Prevost, E. Larquet, F. Pariselli, P. X. Petit, A. Kahn, R. Rizzuto, C. Brenner, and G. Kroemer. 2003. Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res. 63541-546. [PubMed] [Google Scholar]
- 4.Bloss, T. A., E. S. Witze, and J. H. Rothman. 2003. Suppression of CED-3-independent apoptosis by mitochondrial betaNAC in Caenorhabditis elegans. Nature 4241066-1071. [DOI] [PubMed] [Google Scholar]
- 5.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 7771-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, F., B. M. Hersh, B. Conradt, Z. Zhou, D. Riemer, Y. Gruenbaum, and H. R. Horvitz. 2000. Translocation of Caenorhabditis elegans CED-4 to nuclear membranes during programmed cell death. Science 2871485-1489. [DOI] [PubMed] [Google Scholar]
- 7.Chen, L., T. McCloskey, P. M. Joshi, and J. H. Rothman. 2008. ced-4 and proto-oncogene tfg-1 antagonistically regulate cell size and apoptosis in Caenorhabditis elegans. Curr. Biol. 181025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conradt, B., and H. R. Horvitz. 1998. The Caenorhabditis elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell 93519-529. [DOI] [PubMed] [Google Scholar]
- 9.Cory, S., and J. M. Adams. 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2647-656. [DOI] [PubMed] [Google Scholar]
- 10.Crompton, M., E. Barksby, N. Johnson, and M. Capano. 2002. Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie 84143-152. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta, N., A. M. Patel, B. A. Scott, and C. M. Crowder. 2007. Hypoxic preconditioning requires the apoptosis protein CED-4 in Caenorhabditis elegans. Curr. Biol. 171954-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delivani, P., C. Adrain, R. C. Taylor, P. J. Duriez, and S. J. Martin. 2006. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol. Cell 21761-773. [DOI] [PubMed] [Google Scholar]
- 13.del Peso, L., V. M. Gonzalez, and G. Nunez. 1998. Caenorhabditis elegans EGL-1 disrupts the interaction of CED-9 with CED-4 and promotes CED-3 activation. J. Biol. Chem. 27333495-33500. [DOI] [PubMed] [Google Scholar]
- 14.Dolce, V., P. Scarcia, D. Iacopetta, and F. Palmieri. 2005. A fourth ADP/ATP carrier isoform in man: identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett. 579633-637. [DOI] [PubMed] [Google Scholar]
- 15.Gumienny, T. L., E. Lambie, E. Hartwieg, H. R. Horvitz, and M. O. Hengartner. 1999. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 1261011-1022. [DOI] [PubMed] [Google Scholar]
- 16.Halestrap, A. P., G. P. McStay, and S. J. Clarke. 2002. The permeability transition pore complex: another view. Biochimie 84153-166. [DOI] [PubMed] [Google Scholar]
- 17.Horvitz, H. R. 2003. Nobel lecture: worms, life, and death. Biosci. Rep. 23239-303. [DOI] [PubMed] [Google Scholar]
- 18.Jagasia, R., P. Grote, B. Westermann, and B. Conradt. 2005. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in Caenorhabditis elegans. Nature 433754-760. [DOI] [PubMed] [Google Scholar]
- 19.Kokoszka, J. E., K. G. Waymire, S. E. Levy, J. E. Sligh, J. Cai, D. P. Jones, G. R. MacGregor, and D. C. Wallace. 2004. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 427461-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroemer, G., L. Galluzzi, and C. Brenner. 2007. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 8799-163. [DOI] [PubMed] [Google Scholar]
- 21.Le Bras, M., A. Borgne-Sanchez, Z. Touat, O. S. El Dein, A. Deniaud, E. Maillier, G. Lecellier, D. Rebouillat, C. Lemaire, G. Kroemer, E. Jacotot, and C. Brenner. 2006. Chemosensitization by knockdown of adenine nucleotide translocase-2. Cancer Res. 669143-9152. [DOI] [PubMed] [Google Scholar]
- 22.Liu, H., T. J. Strauss, M. B. Potts, and S. Cameron. 2006. Direct regulation of egl-1 and of programmed cell death by the Hox protein MAB-5 and by CEH-20, a Caenorhabditis elegans homolog of Pbx1. Development 133641-650. [DOI] [PubMed] [Google Scholar]
- 23.Marzo, I., C. Brenner, N. Zamzami, J. M. Jurgensmeier, S. A. Susin, H. L. Vieira, M. C. Prevost, Z. Xie, S. Matsuyama, J. C. Reed, and G. Kroemer. 1998. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 2812027-2031. [DOI] [PubMed] [Google Scholar]
- 24.Mello, C., and A. Fire. 1995. DNA transformation. Methods Cell Biol. 48451-482. [PubMed] [Google Scholar]
- 25.Mello, C. C., J. M. Kramer, D. Stinchcomb, and V. Ambros. 1991. Efficient gene transfer in Caenorhabditis elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 103959-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parrish, J., L. Li, K. Klotz, D. Ledwich, X. Wang, and D. Xue. 2001. Mitochondrial endonuclease G is important for apoptosis in Caenorhabditis elegans. Nature 41290-94. [DOI] [PubMed] [Google Scholar]
- 27.Parrish, J., H. Metters, L. Chen, and D. Xue. 2000. Demonstration of the in vivo interaction of key cell death regulators by structure-based design of second-site suppressors. Proc. Natl. Acad. Sci. USA 9711916-11921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pebay-Peyroula, E., C. Dahout-Gonzalez, R. Kahn, V. Trezeguet, G. J. Lauquin, and G. Brandolin. 2003. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 42639-44. [DOI] [PubMed] [Google Scholar]
- 29.Reddien, P. W., and H. R. Horvitz. 2004. The engulfment process of programmed cell death in Caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 41298-202. [DOI] [PubMed] [Google Scholar]
- 30.Savage, C., M. Hamelin, J. G. Culotti, A. Coulson, D. G. Albertson, and M. Chalfie. 1989. mec-7 is a β-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes Dev. 3870-881. [DOI] [PubMed] [Google Scholar]
- 31.Shaham, S., and H. R. Horvitz. 1996. Developing Caenorhabditis elegans neurons may contain both cell-death protective and killer activities. Genes Dev. 10578-591. [DOI] [PubMed] [Google Scholar]
- 32.Stepien, G., A. Torroni, A. B. Chung, J. A. Hodge, and D. C. Wallace. 1992. Differential expression of adenine nucleotide translocator isoforms in mammalian tissues and during muscle cell differentiation. J. Biol. Chem. 26714592-14597. [PubMed] [Google Scholar]
- 33.Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 152922-2933. [PubMed] [Google Scholar]
- 34.Wang, X., J. Wang, K. Gengyo-Ando, L. Gu, C. L. Sun, C. Yang, Y. Shi, T. Kobayashi, Y. Shi, S. Mitani, X. S. Xie, and D. Xue. 2007. Caenorhabditis elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nat. Cell Biol. 9541-549. [DOI] [PubMed] [Google Scholar]
- 35.Wang, X., Y. C. Wu, V. A. Fadok, M. C. Lee, K. Gengyo-Ando, L. C. Cheng, D. Ledwich, P. K. Hsu, J. Y. Chen, B. K. Chou, P. Henson, S. Mitani, and D. Xue. 2003. Cell corpse engulfment mediated by Caenorhabditis elegans phosphatidylserine receptor through CED-5 and CED-12. Science 3021563-1566. [DOI] [PubMed] [Google Scholar]
- 36.Wang, X., C. Yang, J. Chai, Y. Shi, and D. Xue. 2002. Mechanisms of AIF-mediated apoptotic DNA degradation in Caenorhabditis elegans. Science 2981587-1592. [DOI] [PubMed] [Google Scholar]
- 37.Wu, D., H. D. Wallen, and G. Nunez. 1997. Interaction and regulation of subcellular localization of CED-4 by CED-9. Science 2751126-1129. [DOI] [PubMed] [Google Scholar]
- 38.Xiao, H., D. Chen, Z. Fang, J. Xu, X. Sun, S. Song, J. Liu, and C. Yang. 2009. Lysosome biogenesis mediated by vps-18 affects apoptotic cell degradation in Caenorhabditis elegans. Mol. Biol. Cell 2021-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan, N., J. Chai, E. S. Lee, L. Gu, Q. Liu, J. He, J. W. Wu, D. Kokel, H. Li, Q. Hao, D. Xue, and Y. Shi. 2005. Structure of the CED-4-CED-9 complex provides insights into programmed cell death in Caenorhabditis elegans. Nature 437831-837. [DOI] [PubMed] [Google Scholar]
- 40.Yan, N., L. Gu, D. Kokel, J. Chai, W. Li, A. Han, L. Chen, D. Xue, and Y. Shi. 2004. Structural, biochemical, and functional analyses of CED-9 recognition by the proapoptotic proteins EGL-1 and CED-4. Mol. Cell 15999-1006. [DOI] [PubMed] [Google Scholar]
- 41.Yang, X., H. Y. Chang, and D. Baltimore. 1998. Essential role of CED-4 oligomerization in CED-3 activation and apoptosis. Science 2811355-1357. [DOI] [PubMed] [Google Scholar]
- 42.Yang, Z., W. Cheng, L. Hong, W. Chen, Y. Wang, S. Lin, J. Han, H. Zhou, and J. Gu. 2007. Adenine nucleotide (ADP/ATP) translocase 3 participates in the tumor necrosis factor induced apoptosis of MCF-7 cells. Mol. Biol. Cell 184681-4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu, X., S. Odera, C. H. Chuang, N. Lu, and Z. Zhou. 2006. Caenorhabditis elegans dynamin mediates the signaling of phagocytic receptor CED-1 for the engulfment and degradation of apoptotic cells. Dev. Cell 10743-757. [DOI] [PubMed] [Google Scholar]
- 44.Zamora, M., M. Granell, T. Mampel, and O. Vinas. 2004. Adenine nucleotide translocase 3 (ANT3) overexpression induces apoptosis in cultured cells. FEBS Lett. 563155-160. [DOI] [PubMed] [Google Scholar]
- 45.Zamzami, N., S. A. Susin, P. Marchetti, T. Hirsch, I. Gomez-Monterrey, M. Castedo, and G. Kroemer. 1996. Mitochondrial control of nuclear apoptosis. J. Exp. Med. 1831533-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.