Abstract
Colonic carcinogenesis involves the progressive dysregulation of homeostatic mechanisms that control growth. The epidermal growth factor (EGF) receptor (EGFR) regulates colonocyte growth and differentiation and is overexpressed in many human colon cancers. A requirement for EGFR in colonic premalignancy, however, has not been shown. In the current study, we used a specific EGFR antagonist, gefitinib, to investigate this role of the receptor in azoxymethane colonic premalignancy. The azoxymethane model shares many clinical, histologic, and molecular features of human colon cancer. Mice received azoxymethane i.p. (5 mg/kg/wk) or saline for 6 weeks. Animals were also gavaged with gefitinib (10 mg/kg body weight) or vehicle (DMSO) thrice weekly for 18 weeks, a dose schedule that inhibited normal receptor activation by exogenous EGF. Compared with control colonocytes [bromodeoxyuridine (BrdUrd), 2.2 ± 1.2%], azoxymethane significantly increased proliferation (BrdUrd, 12.6 ± 2.8%), whereas gefitinib inhibited this hyperproliferation (BrdUrd, 6.2 ± 4.0%; <0.005). Azoxymethane significantly induced pro-transforming growth factor-α (6.4 ± 1.3–fold) and increased phospho-(active) EGFR (5.9 ± 1.1–fold), phospho-(active) ErbB2 (2.3 ± 0.2–fold), and phospho-(active) extracellular signal-regulated kinase (3.3 ± 0.4–fold) in premalignant colonocytes. Gefitinib inhibited activations of these kinases by >75% (P < 0.05). Gefitinib also significantly reduced the number of large aberrant crypt foci and decreased the incidence of colonic microadenomas from 75% to 33% (P < 0.05). Gefitinib concomitantly decreased cell cycle–regulating cyclin D1 and prostanoid biosynthetic enzyme cyclooxygenase-2 in microadenomas, suggesting that these regulators are key targets of EGFR in colonic carcinogenesis. These results show for the first time that EGFR signaling is required for early stages of colonic carcinogenesis. Our findings suggest, moreover, that inhibitors of EGFR might be useful in chemopreventive strategies in individuals at increased risk for colonic malignancies.
Introduction
Colonic carcinogenesis involves a stepwise accumulation of mutations in tumor suppressor genes and proto-oncogenes (1). These mutations in turn dysregulate mechanisms controlling crypt cell proliferation, maturation, and apoptosis that are normally controlled by multiple homeostatic mechanisms, including signals from the epidermal growth factor (EGF) receptor (EGFR; ref. 2). EGFR belongs to the ErbB family of receptor tyrosine kinases (RTK) that also includes ErbB2, ErbB3, and ErbB4. Normal colonocytes express EGFR, ErbB2, and ErbB3 (3–5). ErbB receptors have an extracellular domain that binds ErbB ligands with high affinity, a single transmembrane domain, and an intracellular domain. The intracellular domain contains a tyrosine kinase and multiple effector domains. With ligand binding, the ErbB receptors form homodimeric or heterodimeric complexes. This dimerization activates the intrinsic RTK that in turn phosphorylates in trans multiple tyrosine residues in the cytoplasmic receptor tail. These autophosphorylation sites, and adjacent residues, create domains that recruit adapter and effector proteins that propagate downstream signals (2). Downstream pathways include Ras/extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase/AKT (2). Targets of these signaling pathways include cyclin D1 and cyclooxygenase-2 (COX-2; refs. 6–8). Cyclin D1 is an important EGFR target that controls G1-S cell cycle progression (9). COX-2 is the key inducible and rate-limiting enzyme required for prostaglandin biosynthesis (10).
Multiple ligands for ErbB receptors have been described. Some ligands bind to unique ErbB receptors, whereas others can bind to multiple ErbB receptors. The diverse combinations of ErbB ligands and receptors afford multiple levels of control by these potent growth-regulating signals (2, 11). In human colon cancers, alterations in ErbB receptors, including up-regulation of EGFR and ErbB2, and increases in EGFR ligands, including transforming growth factor-α (TGF-α), have been described (4, 12, 13). Increases in EGFR or ErbB2 expression portend greater invasiveness of these tumors and a worse prognosis (14, 15). Cyclin D1 and COX-2 are also up-regulated in human and experimental colon cancers (8, 16, 17).
Although studies indicate that several ErbB receptors are overexpressed in many advanced human colon cancers, inhibitors or antibodies to these receptors alone possess limited clinical efficacy (18). Presumably, many tumors have acquired activating mutations downstream of EGFR or exploited other growth-promoting signals, rendering EGFR signaling redundant. In contrast to the relatively small number of established colon cancers responsive to EGFR inhibitors, colonic epithelial cells early in malignant transformation are more likely to require EGFR signaling for proliferation and survival and hence to remain susceptible to cell cycle arrest or death by EGFR blockade (19, 20). For this reason, determining EGFR requirements early in colonic premalignancy is especially important.
We have used the azoxymethane model of colon cancer to elucidate EGFR requirements in colonic premalignancy. Azoxymethane is a selective colonic procarcinogen that induces G to A transitions and leads to activating mutations in K-ras and β-catenin in colonocytes (21). The azoxymethane model recapitulates many of the clinical, histologic, and molecular features of sporadic human colon cancers, with tumors arising within 24 weeks of azoxymethane treatment (21). We showed previously that ErbB2 can be up-regulated in azoxymethane-induced tumors, along with activation of wild-type (WT) Ras and ERK and increases in COX-2, downstream effectors of EGFR (8). In this model, there is a progression from aberrant crypt foci (ACF) to adenomas and finally invasive cancers (22). ACF are the earliest identifiable mucosal abnormalities in carcinogen-treated rodents and are also frequently present in humans harboring colonic neoplastic lesions (22, 23). Large ACF with dysplastic features are often hyperproliferative and believed to be precursors of colon cancer (24, 25). In a recent study, we showed that EGFR signaling was up-regulated in >40% of human hyperproliferative ACF with chromoendoscopic features of dysplasia (26).
In this study, we examined the effects of gefitinib to assess the causal role of EGFR in colonic carcinogenesis. Gefitinib, a synthetic analinoquinazoline, is an orally bioavailable specific EGFR inhibitor that is active in the submicromolar concentration range (IC50, 0.03 µmol/L). This inhibitor possesses a 100-fold greater selectivity for EGFR over other ErbB members. Gefitinib is even more selective when inhibitory concentrations for EGFR are compared with those required to inhibit other tyrosine and serine/threonine kinases (27). In preclinical studies, gefitinib potently inhibited EGFR-dependent cell proliferation in vitro and retarded neoplastic growth in vivo in tumor xenograft models (27). As a single agent, however, gefitinib has limited clinical efficacy against established colon cancers that are likely driven by multiple signaling pathways (18). It was of interest, therefore, to examine the effect of this agent in early stages of colonic carcinogenesis.
Because there is no curative therapy for advanced tumors, efforts are increasingly focused on chemopreventive strategies. Progress in this area will depend on improved understanding of signaling pathways required for tumorigenesis. This study was undertaken to test the hypothesis that EGFR is required for colonic tumorigenesis at the premalignant phase. We first established a dose and treatment schedule with gefitinib that inhibited EGFR signaling in normal mouse colon. We then assessed the effects of azoxymethane induction and gefitinib treatment on early stages of colonic carcinogenesis in premalignant colonocytes. We established for the first time that azoxymethane-induced crypt cell hyperproliferation, ACF growth, and microadenoma appearance were accompanied by increased EGFR signaling in this model. Furthermore, gefitinib inhibited EGFR signaling and concomitantly reduced the incidence of microadenomas. These studies indicate that EGFR is required for colonic premalignant progression. In addition, EGFR inhibition offers a potentially important strategy for chemoprevention of colon cancer in high-risk individuals.
Materials and Methods
Materials
Male A/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). AIN-76A rodent chow diet was purchased from Harlan Teklad Laboratories (Madison, WI). Azoxymethane was obtained from the National Cancer Institute Chemical Carcinogen Reference Standard Repository, Midwest Research (Kansas City, MO). Gefitinib was generously provided by AstraZeneca (Cheshire, England). EGF was purchased from Calbiochem (San Diego, CA). DNA extractions were done using the Qiagen DNeasy kit (Qiagen, Valencia, CA). Polyclonal antibodies to phospho-(active) EGFR (pEGFR), phospho-(active) ErbB2 (pErbB2), phospho-(active) ERK (pERK)-1 and pERK-2, TGF-α, and cyclin D1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal phospho-(active) AKT antibodies were obtained from Cell Signaling Technology (Beverly, MA). Monoclonal antibodies against Ki-67 (clone SP1) were obtained from Neomarkers (Fremont, CA). Polyclonal pan-EGFR antibodies were obtained from Upstate Biotechnology (Waltham, MA). COX-2 rabbit polyclonal antibodies were purchased from Cayman Chemicals (Ann Arbor, MI). Monoclonal β-actin antibodies and the bromodeoxyuridine (BrdUrd) immunostaining kit were obtained from Zymed Laboratories, Inc. (South San Francisco, CA). Electrophoretic grade acrylamide, bisacrylamide, Tris, SDS, and prestained molecular weight markers were from Bio-Rad Laboratories (Richmond, CA). Kodak (Rochester, NY) supplied the X-OMAT AR film. Polyvinylidene difluoride (PVDF) membranes (Immobilon-P) were purchased from Millipore, Inc. (Bedford, MA). All other reagents were of the highest quality available and were obtained from Sigma-Aldrich Corp. (St. Louis, MO), unless otherwise noted.
Methods
Experimental animal protocols and tissue harvest
EGFR signaling in normal colon
Male A/J mice (25 g) were maintained on AIN-76A rodent chow diet in approved specific pathogen-free facilities with 12-h light and 12-h darkness in a temperature range of 22°C to 25°C and humidity of 25% to 35%. All animal procedures were reviewed and approved by the University of Chicago Institutional Animal Care and Use Committee and followed NIH guidelines. For EGFR signaling in normal mouse colon, animals were gavaged thrice weekly with 50 µL gefitinib (10 mg/kg body weight) or DMSO (vehicle). Two hours after the third gavage, mice were anesthetized with ketamine (70 mg i.p./kg body weight) and xylazine (7 mg i.p./kg body weight). The abdominal viscera were exposed by a laparotomy. Mice were given the indicated doses of EGF ( final volume of 25 µL) or vehicle (PBS) by intracardiac injection. Colons were harvested and flash frozen in liquid nitrogen. In preliminary time course studies, maximal receptor activation occurred 5 min after EGF injection. For some experiments, a segment of left colon was fixed in 10% buffered formalin.
Azoxymethane studies
For carcinogen studies, male A/J mice, initially weighing 20 to 25 g, were treated with 5 mg azoxymethane i.p./kg body weight or vehicle (saline) weekly for 6 weeks. Carcinogen-treated (40 mice) and saline-treated (20 mice) groups were divided equally to receive gefitinib (10 mg/kg body weight) or vehicle (DMSO) by gavage thrice weekly beginning in week 1. Animals were weighed weekly and sacrificed 18 weeks after the first azoxymethane injection (Fig. 1). Two hours before sacrifice, animals received 50 mg BrdUrd i.p./kg body weight to label S-phase colonocytes. A circular segment of distal left colon was fixed in 10% buffered formalin for BrdUrd incorporation. The remaining colons were opened longitudinally and fixed flat in 10% buffered formalin for immunostaining or flash frozen for Western blotting. In colons fixed flat in formalin, ACF were visualized by methylene blue staining with a ×20 dissecting microscope by an investigator unaware of the treatment conditions. ACF were identified as crypts with increased methylene blue staining and expanded pericryptal spaces. ACF were predominantly localized to the left colon. Total ACF numbers and size (number of component crypts) were counted and expressed as mean ± SE for each group. Large ACF were operationally defined as ACF with six or more component crypts. For K-ras mutation analysis, large ACF were isolated by dermal punch and trimmed to remove adjacent normal-appearing crypts. Colons were then divided longitudinally and paraffin embedded as Swiss rolls as described (28). H&E-stained sections were examined for colonic microadenomas identified by an expert gastrointestinal pathologist (J.H.) following recently published consensus criteria (29).
Figure 1.
Experimental treatment protocol and growth curve. A, treatment protocol. Mice received azoxymethane (AOM; 5 mg/kg) or saline (azoxymethane vehicle) weekly by i.p. injection for six weeks (arrows). Mice were also treated with gefitinib (10 mg/kg) or DMSO (vehicle) by gavage thrice weekly throughout the study. Animals were sacrificed in week 18. B, growth curves. Weights for the AIN-76A alone and AIN-76A + gefitinib group did not differ from each other and values were combined and presented as control. The azoxymethane-treated and azoxymethane + gefitinib–treated animals showed delayed growth during azoxymethane treatment but resumed normal growth rates afterwards.
Immunostaining
For immunostaining, 5-micron sections of formalin-fixed paraffin-embedded colons, prepared as Swiss rolls or as transverse circular colonic segments (BrdUrd), were cut and mounted on Vectabond-coated Superfrost Plus slides. The slides were heated to 60°C for 1 h, deparaffinized by 5-min washes for three times in xylene, hydrated by a graded series of ethanol washes, and rinsed with distilled water. BrdUrd, pERK, and COX-2 antigens were retrieved by microwave heating for 15 min in 0.01 mol/Lcitra te buffer (pH 7.0) or Ki-67 (pH 9) as described (30). For cyclin D1, epitope retrieval was achieved by heating slides in a steamer with Tris-EDTA buffer (pH 9). Slides were then washed in TBS Tween 20 (TBST) thrice for 2 min. The endogenous peroxidase activity was quenched with methanol/H2O2 solution (1.0%) in the dark for 15 min. Sections were washed thrice with TBST for 2 min and nonspecific binding was blocked using Protein Block for 20 min. Sections were incubated at 4° C with primary antibodies (overnight with anti-BrdUrd, 1:50; anti-pERK, 1:450; anti-COX-2, 1:200 or for 1 h with anti-Ki-67, 1:300, or anti-cyclin D1, 1:50) followed by incubation with biotinylated secondary antibodies (1:200) at room temperature. Antigen-antibody complexes were detected by DAKO EnVision Plus System, Peroxidase (DAKO EnVision Plus System, horseradish peroxidase), using 3,3′-diaminobenzidine as substrate. After washing with distilled water, the slides were counterstained with Gill’s III hematoxylin, rinsed with water, dehydrated with ethanol, and cleared with xylene. For negative controls, sections were incubated with isotype-matched irrelevant antibodies. For BrdUrd labeling, only complete crypts, extending from the muscularis mucosa to the colonic lumen, were counted. Colonic sections contained on average >20 complete crypts. Proliferation was assessed by BrdUrd incorporation and expressed as the percentage of crypt cells labeled with BrdUrd.
Western Blotting
Frozen left colons were mechanically pulverized in liquid nitrogen with a mortar and pestle chilled in a liquid nitrogen bath. Tissues were sonicated and then homogenized with a Polytron (power setting of 4 × 30 s) in 1.5 mL nondenaturing lysis buffer containing 25 mmol/L HEPE S (pH 7.5), 125 mmol/L NaC l, 1% IGEPAL, 10 mmol/L MgCl2, 1 mmol/L EDTA, and 2% glycerol. Colonic proteins were extracted in SDS-containing Laemmli buffer and measured by Bio-Rad protein assay. Proteins were subjected to quantitative Western blotting analysis as described (8). Briefly, proteins were separated by SDS-PAGE on 4% to 20% resolving polyacrylamide gradient gels and electroblotted to PVDF membranes. Blots were incubated overnight at room temperature with specific primary antibodies followed by 2 h of incubation with appropriate peroxidase-coupled secondary antibodies and subsequent detection on X-OMAT film by enhanced chemiluminescence. Xerograms were digitized with an Epson scanner (San Jose, CA), and band intensity was quantified using IP Lab Gel (Scanalytics, Rockville, MD). Western blotting band intensities were expressed in arbitrary units and normalized to fold increases compared with control colons from mice treated with saline (azoxymethane vehicle) and receiving DMSO (gefitinib vehicle) by gavage.
K-ras Mutation Analysis
DNA was purified from isolated ACF using the Qiagen DNeasy kit following the recommendation of the manufacturer. Primer-mediated RFLP Colonic EGFR Signaling in Azoxymethane Tumorigenesis (PM-RFLP) was used to detect mutations in K-ras codon 12 in isolated ACF. Briefly, mismatched 5′ primers created restriction sites for BstN1 in WT K-ras that were abolished by G to A mutations in codon 12. The primers, PCR conditions, and restriction digests were described previously (31).
Statistical Methods
Data were expressed as mean ± SD or SE as indicated. Statistical comparisons of differences in total and large ACF and in microadenoma multiplicities were determined by Kruskal-Wallis nonparametric analyses. Western blotting protein expression levels and BrdUrd labeling were compared by unpaired Student’s t test. Incidences of microadenomas and K-ras mutations were compared by Fisher exact test. Values of P < 0.05 were considered statistically significant.
Results
Effects of gefitinib on normal EGFR signaling and mouse growth
For these studies, we chose A/J mice as they are susceptible to tumor induction by azoxymethane (32). Because our major objective was to examine the effect of EGFR inhibition in colonic carcinogenesis, before conducting the carcinogen study, we assessed the ability of gefitinib to block signaling induced by exogenous EGF in normal mouse colon. Using the dose schedule described in Materials and Methods, gefitinib significantly inhibited EGF-induced activations of EGFR and ErbB2 and downstream effectors, ERK and AKT (Supplementary Fig. S1A–C). We confirmed by immunostaining that ERK activation was predominantly confined to the colonic epithelial cells and blocked by gefitinib (Supplementary Fig. S1D). Thus, in normal mouse colon, gefitinib inhibited EGFR signals when given by gavage at this dose and frequency.
For carcinogenesis studies, mice were treated with azoxymethane (5 mg i.p./kg body weight) or saline weekly for 6 weeks. The animals concomitantly received gefitinib (10 mg/kg body weight) or DMSO (vehicle) by gavage thrice weekly as summarized in the protocol in Fig. 1A. For mice not receiving azoxymethane, there were no differences in weight gain between the control group on AIN-76A alone and the group supplemented with gefitinib. Therefore, for the growth analysis, these two groups were combined and shown as control in Fig. 1B. While not affecting body weight, gefitinib caused the development of a wavy hair coat and curly whiskers. These results are an indirect confirmation of active drug effects as this hair pattern is characteristic of loss of EGFR signaling in the epidermis of mice deficient in TGF-α (33). During carcinogen treatment, the azoxymethane alone and azoxymethane plus gefitinib groups lagged behind the control groups with respect to body weight but then resumed comparable growth rates for the remainder of the study. The carcinogen-treated groups were ~10% lower in final body weights compared with controls (Fig. 1B). Importantly, there were no differences in growth curves or mean body weights between the azoxymethane alone group and the azoxymethane plus gefitinib group (Fig. 1B).
Gefitinib inhibits azoxymethane-induced hyperproliferation and ACF growth
Numerous studies, including our own investigations in the azoxymethane model, have indicated that colonic neoplastic transformation is accompanied by crypt cell hyperproliferation and ACF development (25, 30). In agreement with prior reports, azoxymethane increased proliferation in the colonic mucosa as assessed by BrdUrd incorporation (Fig. 2). Crypt cell proliferation in the control- and gefitinib-treated groups were low, with BrdUrd incorporation of 2.2 ± 1.2% and 1.8 ± 0.9%, respectively. Azoxymethane treatment increased BrdUrd incorporation significantly to 12.6 ± 2.8% (P < 0.05). In contrast, gefitinib significantly inhibited azoxymethane-induced hyperproliferation to 6.2 ± 4.0, a 50% reduction in BrdUrd incorporation (Fig. 2; Table 1; P < 0.05). These changes in proliferation were mirrored by similar changes in ACF growth. ACF were found predominantly in the left colon in agreement with prior studies (24). Compared with azoxymethane alone, gefitinib caused a 24.4% reduction in the mean number of total ACF/mouse, from 41 ± 6 to 32 ± 8 (Table 1). Importantly, gefitinib caused a significant reduction in the mean number of large ACF/mouse (ACF containing six or more component crypts) from 12 ± 2 in the azoxymethane alone group to 6 ± 1 (50% reduction) in the azoxymethane plus gefitinib group (Table 1; P < 0.05). Because large ACF are associated with an increased risk for malignant progression, inhibition of ACF growth is likely of biological relevance (24).
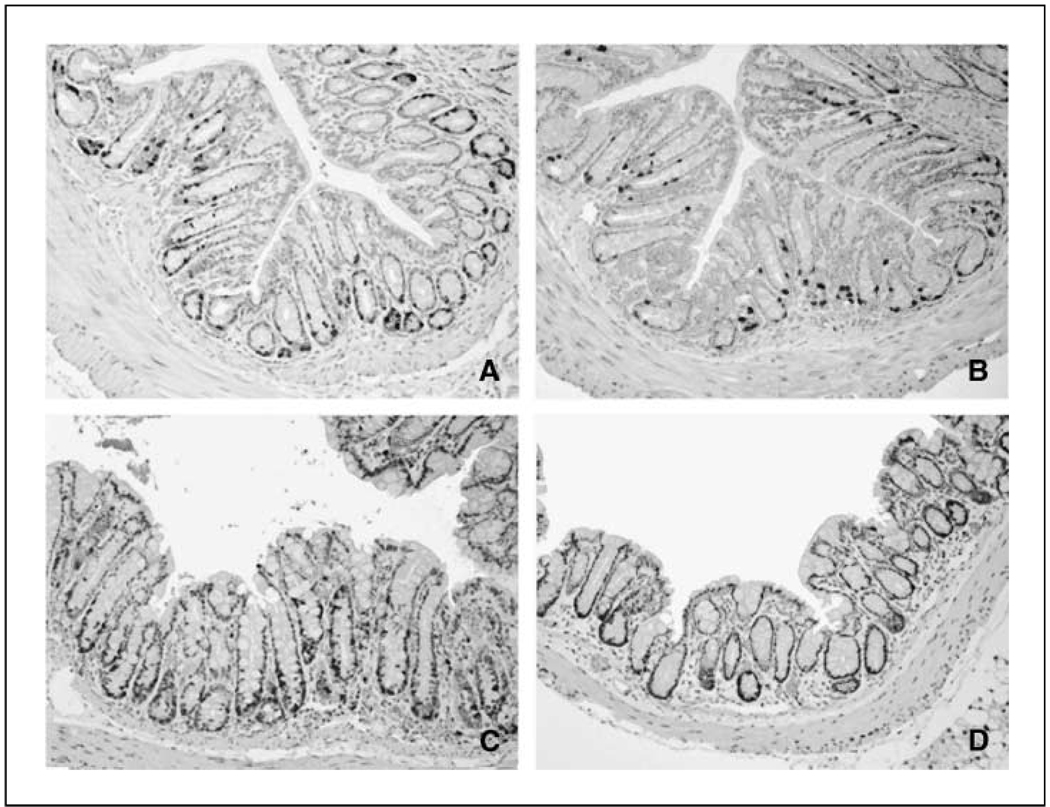
Figure 2.
Gefitinib inhibits azoxymethane-induced hyperproliferation in mouse premalignant colon. A/J mice were treated as described in Materials and Methods. Two hours before sacrifice, mice received BrdUrd (50 mg i.p./kg body weight). Circular left colon segments were fixed in 10% buffered formalin and BrdUrd incorporation detected by immunostaining as described in Materials and Methods. Representative sections from each group. A, control animals injected with saline (azoxymethane vehicle) and gavaged with DMSO (gefitinib vehicle). B, gefitinib alone. C, azoxymethane alone. D, azoxymethane + gefitinib. Note the increased BrdUrd labeling (black staining) in the azoxymethane alone group compared with animals in the control group. Compared with azoxymethane alone, gefitinib decreased the carcinogen-induced BrdUrd labeling (compare D with C).
Table 1.
Gefitinib inhibits hyperproliferation, ACF growth, and microadenoma development
| Group | Cell proliferation (% BrdUrd labeled) | ACF | Microadenomas | ||||
|---|---|---|---|---|---|---|---|
| ACF | Large ACF | Ras mutations (%) | Microadenomas | Tumor incidence | Tumor multiplicity | ||
| AOM | 12.6 ± 2.8 | 41 ± 6 | 12 ± 2 | 5/26 (19) | 17 | 0.75 | 1.9 |
| AOM + gefitinib | 6.2 ± 4.0* | 32 ± 8 | 6 ± 1* | 0/15 (0) | 5 | 0.33* | 1.2* |
NOTE: Animals were treated as described in Fig. 1. Proliferation was measured by BrdUrd incorporation. Control colons showed 2.2 ± 1.2% BrdUrd labeling. ACF in formalin-fixed colons were identified as crypts with increased methylene blue staining and expanded pericryptal spaces (n = 15). The total number of ACF and number of large ACF (≥6 component crypts/focus) were expressed as mean ± SE per mouse. Ras mutations in large ACF were determined by PM-RFLP. For microadenoma analyses, colons were prepared as Swiss rolls (n = 12). Three 5-micron sections (50 microns apart) from each Swiss roll were stained with H&E and examined for microadenomas. Note that there were no tumors in the saline (azoxymethane vehicle)–treated group or the gefitinib alone group. Rates of BrdUrd labeling were compared by Student’s t test. Total and large ACF and microadenoma multiplicities were compared by Kruskal-Wallis test, and tumor incidences were compared by Fisher exact test.
Abbreviation: AOM, azoxymethane.
P < 0.05, compared with AOM alone.
Gefitinib inhibits azoxymethane-induced microadenoma formation
There were very few visible colonic lesions when animals were sacrificed at 18 weeks. Typically, azoxymethane tumor studies are carried out beyond 24 weeks (34). To detect intraepithelial neoplastic lesions, we prepared Swiss rolls from full-length colons in the azoxymethane alone group and the azoxymethane plus gefitinib group. Three sections, each separated by 50 microns, were stained with H&E and examined for microadenomas. Intervening unstained sections were used for immunostaining. We identified 17 microadenomas from 9 of 12 animals in the azoxymethane alone group. In contrast, we found five microadenomas from 4 of 12 animals in the azoxymethane plus gefitinib group (Table 1). Thus, gefitinib significantly reduced the incidence of microadenomas from 75% to 33% (P < 0.05). Gefitinib also significantly reduced tumor multiplicity from 1.9 to 1.2 microadenomas per tumor-bearing animal (Table 1; P = 0.02) Inhibition of microadenoma formation confirms the biological relevance of EGFR blockade with gefitinib in the azoxymethane model. In the gefitinib-treated group, adenomas also appeared to have decreased pERK immunostaining (Fig. 3, compare A and B) and lower proliferation, as assessed by Ki-67 staining (Fig. 3, compare C and D). These local reductions in proliferation and ERK signaling within the microadenomas were in agreement with the generalized decreased crypt cell proliferation and inhibited EGFR signaling that were found as field effects in the colonic mucosa (see Fig. 4 below).
Figure 3.
Gefitinib inhibits microadenoma formation and pERK and Ki-67 staining. A/J mice were treated as described in Materials and Methods. Mice were sacrificed and colons were harvested after 18 weeks. Colons were fixed flat in formalin and divided longitudinally. Swiss rolls were prepared and embedded in paraffin and sections stained. Representative microadenomas from carcinogen-treated groups. A, pERK in azoxymethane group. Magnification, ×200. B, pERK in azoxymethane + gefitinib group. Magnification, ×200. C, Ki-67 in azoxymethane group. Magnification, ×200. D, Ki-67 in azoxymethane + gefitinib group. Magnification, ×200. Note the decreased proliferation and reduced pERK staining in microadenomas from gefitinib-treated mice. Sections are representative of 11 microadenomas from 7 mice in the azoxymethane alone group and 4 microadenomas from 3 mice in the azoxymethane + gefitinib group.
Figure 4.
Gefitinib inhibits azoxymethane-induced EGFR signaling in premalignant mouse colon. A/J mice were treated as described in Materials and Methods. Eighteen weeks after the first azoxymethane injection, mice were sacrificed and colons were flash frozen. Whole-colon lysates were prepared as described in Materials and Methods. A, Western blots of pro-TGF-α, pEGFR, pan-EGFR, pErbB2, pERK, and β-actin expression. A+G, azoxymethane + gefitinib group. B, quantitative densitometry. Fold changes in pro-TGF-α and phospho-active proteins were assessed by densitometry. Four to six animals were analyzed in each group. Values are expressed as fold of control. Columns, mean; bars, SD. ‡ and *, P < 0.05 compared with control; †, P < 0.05 compared with azoxymethane alone.
Gefitinib inhibits azoxymethane-induced EGFR signaling
To identify EGFR signaling correlates of azoxymethane-induced hyperproliferation and microadenoma formation, we examined activation of EGFR and ErbB2 and downstream effectors in azoxymethane premalignancy. As shown in Fig. 4, azoxymethane significantly induced the activation of EGFR (5.9 ± 1.2–fold; P < 0.05), ErbB2 (2.3 ± 0.2–fold; P < 0.05), and ERK (3.3 ± 0.4–fold; P < 0.05) in mouse colon, as assessed by increased levels of phospho-active kinases. Moreover, gefitinib significantly inhibited these azoxymethane-induced activations in premalignant mucosa (Fig. 4). We also found that the proform of TGF-α (Mr = 26 kDa) was up-regulated 6-fold in the azoxymethane group (Fig. 4). Presumably, increased TGF-α and possibly other EGFR ligands trigger EGFR signaling in azoxymethane colonic carcinogenesis. Interestingly, gefitinib alone increased TGF-α, perhaps as a feedback response to EGFR blockade.
Gefitinib inhibits azoxymethane-induced cyclin D1 and COX-2 but not β-catenin up-regulation
We examined microadenomas for β-catenin expression, as many studies have shown adenomatous polyposis coli (APC)/β-catenin signals play critical roles in colonic carcinogenesis (35). We found by immunostaining that β-catenin was up-regulated in nuclear and cytoplasmic distributions in all microadenomas, irrespective of treatment group (data not shown). Although cyclin D1 is a well-recognized target of β-catenin, it is also a downstream target of EGFR (6, 36). In other tumor cell types, EGFR blockade has been shown to inhibit cyclin D1 expression (37). We therefore examined the effects of gefitinib on cyclin D1 as potentially contributing to the chemo-preventive effects of gefitinib. As shown in Fig. 5A, cyclin D1 was strongly expressed in a diffuse and nuclear pattern in azoxymethane-induced microadenomas. In contrast, azoxymethane-induced cyclin D1 overexpression was reduced in microadenomas from gefitinib-treated animals (Fig. 5B). Cyclin D1 was increased in 7 of 10 azoxymethane-induced microadenomas, whereas, in the gefitinib-treated group, expression levels were comparable with adjacent mucosa in all five microadenomas examined. Thus, up-regulated β-catenin in the presence of EGFR blockade is not sufficient to drive increased cyclin D1 expression in azoxymethane microadenomas. Furthermore, the effects of gefitinib on cyclin D1 (Fig. 5B) paralleled the proliferation changes we observed in microadenomas as assessed by Ki-67 staining (Fig. 3D).
Figure 5.
Gefitinib inhibits cyclin D1 and COX-2 overexpression in microadenomas. A/J mice were treated as described in Materials and Methods. Mice were sacrificed after 18 weeks and colonic Swiss rolls were prepared. Representative sections from carcinogen-treated groups. A, cyclin D1 expression in azoxymethane alone group. B, cyclin D1 expression in azoxymethane + gefitinib group. Cyclin D1 was markedly increased in 7 of 10 tumors from azoxymethane-treated animals, whereas, in the azoxymethane + gefitinib group, staining was comparable with adjacent uninvolved crypts in five of five tumors. C, COX-2 expression in azoxymethane group. D, COX-2 expression in azoxymethane + gefitinib group. COX-2 was increased in 8 of 10 microadenomas from the azoxymethane group compared with one of five microadenomas from the azoxymethane + gefitinib–treated group.
COX-2 also plays a critical role in colonic carcinogenesis and is another target of APC/β-catenin signaling (10, 38). Like cyclin D1, COX-2 is also a downstream target of EGFR (39). We showed previously that COX-2 was increased in azoxymethane tumors expressing WT Ras activated by EGFR-related ErbB2 (8). In agreement with these studies, COX-2 was up-regulated in azoxymethane microadenomas (Fig. 5C). In contrast, gefitinib inhibited COX-2 expression in these microadenomas (Fig. 5D). Thus, gefitinib inhibited up-regulations of cyclin D1 and COX-2 that we speculate are EGFR targets required for microadenoma development.
Effects of azoxymethane and gefitinib on K-ras mutations
K-ras mutations occur in the azoxymethane model and could be responsible for EGFR-independent activation of downstream effectors and targets, including ERK and COX-2 (8, 31). In other cell types, K-ras mutations have been associated with resistance to gefitinib (40). We therefore examined ACF for G to A K-ras mutations in codon 12, the predominant site of mutations in this model. We detected mutations in 5 of 26 (19%) ACF in the azoxymethane alone group and in 0 of 15 (0%) ACF from the azoxymethane plus gefitinib group (Table 1). Although these numerical differences suggested that gefitinib might inhibit the development of tumors with K-ras mutations, they did not reach statistical significance (P = 0.09). Moreover, this frequency of K-ras mutations was in agreement with prior reports (41). Because Ras was WT (and therefore regulated by EGFR) in ACF from gefitinib-treated animals, we infer that gefitinib would inhibit EGFR downstream effectors, such as ERK, an effector of Ras, in these ACF. Interestingly, we have shown that ursodeoxycholic acid, another chemopreventive agent in the azoxymethane model, suppressed the development of tumors with K-ras mutations or activated WT Ras (34). In future longer-term studies with overt tumors, it will be of interest to assess whether gefitinib can in fact inhibit the development of tumors with mutant Ras similar to other chemopreventive agents in this model (34, 42).
Discussion
In this study, we have shown for the first time that EGFR signaling is up-regulated by the microadenoma stage in the azoxymethane model of colon cancer. We have also shown that gefitinib inhibits EGFR signaling and concomitantly limits neoplastic progression in this premalignant phase. These studies are important because they have elucidated an important growth-promoting requirement for EGFR in colonic premalignancy and identified this growth factor signaling pathway as a target for new chemoprevention strategies.
To confirm that EGFR activation was important in malignant transformation, we used a pharmacologic approach using the EGFR-specific tyrosine kinase inhibitor gefitinib. Gefitinib is a substituted analinoquinazoline that interacts with the ATP binding site of EGFR to block kinase activity (27). Quinazolines have been shown to cause receptor dimerization and internalization while blocking receptor autophosphorylation and signal propagation (43). The specificity of the inhibitor was important because genistein, a broad-spectrum tyrosine kinase inhibitor, had been shown previously to paradoxically increase tumor development in the azoxymethane model (44). Before embarking on premalignant studies, we examined the ability of gefitinib to inhibit EGF-induced signaling in normal colon. Using the dose schedule described in Fig. 1, we showed that gefitinib significantly inhibited EGF-induced EGFR and ErbB2 tyrosine phosphorylations and decreased ERK and AKT activations in normal mouse colonocytes (Supplementary Fig. S1).
We showed previously in rat azoxymethane tumors that ErbB2 up-regulation can activate WT Ras and increase ERK signaling and COX-2 expression (8). In this study, we examined EGFR signaling in the premalignant phase of azoxymethane-treated mice. EGFR and ErbB2 were activated and downstream signaling up-regulated in a generalized manner in the colon, reflecting a “field effect” at the microadenoma stage before the emergence of visible tumors. The proform of TGF-α was increased 18 weeks after azoxymethane treatment and presumably drives this increased EGFR signaling. Gefitinib decreased this growth signaling of the receptor and limited azoxymethane-induced hyperproliferation, ACF growth, and microadenoma appearance. Our results are in broad agreement with studies in the Min mouse, a genetic model of intestinal tumorigenesis. Min mice carry a germ-line mutation of the APC gene and consequently possess increased β-catenin signaling. Pharmacologic or genetic blockade of EGFR inhibited the growth of Min adenomas in the small intestine (45). Thus, in addition to β-catenin, it would seem that EGFR signaling is also required for azoxymethane tumorigenesis.
Cyclin D1 dysregulation contributes to aberrant proliferation in many types of tumors, including colon cancer, and is overexpressed in azoxymethane and human colonic carcinogenesis as early as the ACF stage (16, 26, 30, 46). Cyclin D1 is also an important mitogenic target of TGF-α and EGFR signaling (6, 47). In this study, we showed that gefitinib inhibited the up-regulation of cyclin D1 in microadenomas. Recent studies showing EGFR in the nucleus suggest an intriguing role for this receptor as a transcriptional regulator (6). In this regard, EGFR has been shown to bind to an AT-rich region in the cyclin D1 promoter and to activate this promoter (6).We have also shown recently that cyclin D1 is overexpressed in a subset of large hyperproliferative human ACF with up-regulated EGFR signaling (26). Thus, cyclin D1 seems to be an important EGFR target in colonic carcinogenesis that is required for development of microadenomas.
COX-2 is also up-regulated in azoxymethane and human colonic tumors (8, 10, 34). This overexpression has been reported as early as the ACF stage (48, 49). Enzymatic blockade of COX-2 significantly reduced tumorigenesis in the azoxymethane model, showing the critical role this protein plays in neoplastic transformation (50). We showed previously that EGFR-related ErbB2 increased COX-2 in azoxymethane tumors (8). In agreement with those findings, other authors have shown that COX-2 is a target of EGFR signaling in colon cancer cells (39). In the current study, EGFR activation was accompanied by up-regulation of pERK and its downstream effector COX-2 in microadenomas. The ability of gefitinib to suppress ERK activation and COX-2 up-regulation in microadenomas likely contributes to the chemopreventive effects of this agent in this model. Thus, although APC/β-catenin signals regulate cyclin D1 and COX-2 in colonic carcinogenesis (36, 38), we have shown that their up-regulations also require EGFR signaling in the azoxymethane model. Our studies, moreover, have extended the Min mouse findings by identifying cyclin D1 and COX-2 as EGFR targets of the chemopreventive effects of gefitinib.
Our findings have important clinical implications for receptor blockade as a chemopreventive strategy in individuals at increased risk for this malignancy (51). Prior studies have shown that gefitinib can inhibit both EGFR- and ErbB2-dependent colonic and noncolonic cancer cell growth in vitro and reduce neoplastic growth in tumor xenograft models (27, 52). In a chemopreventive setting, transforming colonic epithelial cells are likely to remain susceptible to cell cycle arrest or death by EGFR blockade (19, 20). Gefitinib, when used in combination with EGFR-independent inhibitors, has also been shown to synergize for growth inhibition of tumor xenografts (53). Combination chemopreventive therapies might allow lower doses of individual agents that are better tolerated and associated with less drug resistance in transforming cells. Theoretically, more than one mutation might be required to block the inhibitory effects of drugs that act by independent mechanisms.
In summary, this is the first study to our knowledge to show that EGFR is activated and downstream effector signaling increased at the microadenoma stage of colonic tumorigenesis. Furthermore, gefitinib inhibited microadenoma formation and concomitantly blocked up-regulation of EGFR targets, cyclin D1 and COX-2, in these lesions. Because cyclin D1 and COX-2 play critical roles in colonic tumorigenesis, gefitinib inhibition of these EGFR signals and blockade of neoplastic progression strongly support our hypothesis that early colonic malignant transformation requires EGFR signaling. Furthermore, our results suggest that EGFR inhibitors could provide new chemopreventive strategies in patients at increased risk for colon cancer.
Acknowledgments
Grant support: Grants P30DK42086 (Digestive Diseases Research Core Center), CA036745 (M. Bissonnette), and CA097540 (S. Khare); Samuel Freedman Research Laboratories for Gastrointestinal Cancer Research; and Department of Pathology Research Core Facilities of the University of Chicago.
We thank Drs. Yan Chun Li and Ezra Cohen for the careful reading and helpful suggestions.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Vogelstein B, Kinzler K. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 2.Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. The epidermal growth factor receptor family as a central element for cellular signal transduction and diversification. Endocr Relat Cancer. 2001;8:11–31. doi: 10.1677/erc.0.0080011. [DOI] [PubMed] [Google Scholar]
- 3.Playford RJ, Hanby AM, Gschmeissner S, Peiffer LP, Wright NA, McGarrity T. The epidermal growth factor receptor (EGF-R) is present on the basolateral, but not the apical, surface of enterocytes in the human gastrointestinal tract. Gut. 1996;39:262–266. doi: 10.1136/gut.39.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Half E, Broaddus R, Danenberg KD, Danenberg PV, Ayers GD, Sinicrope FA. HER-2 receptor expression, localization, and activation in colorectal cancer cell lines and human tumors. Int J Cancer. 2004;108:540–548. doi: 10.1002/ijc.11599. [DOI] [PubMed] [Google Scholar]
- 5.Maurer CA, Friess H, Kretschmann B, et al. Increased expression of erbB3 in colorectal cancer is associated with concomitant increase in the level of erbB2. Hum Pathol. 1998;29:771–777. doi: 10.1016/s0046-8177(98)90444-0. [DOI] [PubMed] [Google Scholar]
- 6.Lin SY, Makino K, Xia W, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]
- 7.Muise-Helmericks RC, Grimes HL, Bellacosa A, Malstrom SE, Tsichlis PN, Rosen N. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem. 1998;273:29864–29872. doi: 10.1074/jbc.273.45.29864. [DOI] [PubMed] [Google Scholar]
- 8.Bissonnette M, Khare S, von Lintig FC, et al. Mutational and nonmutational activation of p21ras in rat colonic azoxymethane-induced tumors: effects on mitogen-activated protein kinase, cyclooxygenase-2, and cyclin D1. Cancer Res. 2000;60:4602–4609. [PubMed] [Google Scholar]
- 9.Sherr CJ. D-type cyclins. Trends Biochem Sci. 1995;20:187–190. doi: 10.1016/s0968-0004(00)89005-2. [DOI] [PubMed] [Google Scholar]
- 10.Mann JR, DuBois RN. Cyclooxygenase-2 and gastrointestinal cancer. Cancer J. 2004;10:145–152. doi: 10.1097/00130404-200405000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasui W, Sumiyoshi H, Hata J, et al. Expression of epidermal growth factor receptor in human gastric and colonic carcinomas. Cancer Res. 1988;48:137–141. [PubMed] [Google Scholar]
- 13.Ciardiello F, Kim N, Saeki T, et al. Differential expression of epidermal growth factor-related proteins in human colorectal tumors. Proc Natl Acad Sci U S A. 1991;88:7792–7796. doi: 10.1073/pnas.88.17.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radinsky R, Risin S, Fan D, et al. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res. 1995;1:19–31. [PubMed] [Google Scholar]
- 15.Kapitanovic S, Radosevic S, Kapitanovic M, et al. The expression of p185(HER-2/neu) correlates with the stage of disease and survival in colorectal cancer. Gastroenterology. 1997;112:1103–1113. doi: 10.1016/s0016-5085(97)70120-3. [DOI] [PubMed] [Google Scholar]
- 16.Arber N, Hibshoosh H, Moss SF, et al. Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology. 1996;110:669–674. doi: 10.1053/gast.1996.v110.pm8608874. [DOI] [PubMed] [Google Scholar]
- 17.DuBois RN, Radhika A, Reddy BS, Entingh AJ. Increased cyclooxygenase-2 levels in carcinogeninduced rat colonic tumors. Gastroenterology. 1996;110:1259–1262. doi: 10.1053/gast.1996.v110.pm8613017. [DOI] [PubMed] [Google Scholar]
- 18.Kindler HL, Friberg G, Skoog L, Wade-Oliver K, Vokes EE. Phase I/II trial of gefitinib and oxaliplatin in patients with advanced colorectal cancer. Am J Clin Oncol. 2005;28:340–344. doi: 10.1097/01.coc.0000159558.19631.d5. [DOI] [PubMed] [Google Scholar]
- 19.Markowitz SD, Molkentin K, Gerbic C, Jackson J, Stellato T, Willson JK. Growth stimulation by coexpression of transforming growth factor-a and epidermal growth factor-receptor in normal and adenomatous human colon epithelium. J Clin Invest. 1990;86:356–362. doi: 10.1172/JCI114709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paraskeva C, Buckle BG, Sheer D, Wigley CB. The isolation and characterization of colorectal epithelial cell lines at different stages in malignant transformation from familial polyposis coli patients. Int J Cancer. 1984;34:49–56. doi: 10.1002/ijc.2910340109. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M, Wakabayashi K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004;95:475–480. doi: 10.1111/j.1349-7006.2004.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995;93:55–71. doi: 10.1016/0304-3835(95)03788-X. [DOI] [PubMed] [Google Scholar]
- 23.Takayama T, Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med. 1998;339:1277–1284. doi: 10.1056/NEJM199810293391803. [DOI] [PubMed] [Google Scholar]
- 24.McLellan EA, Medline A, Bird RP. Sequential analyses of the growth and morphological characteristics of aberrant crypt foci: putative preneoplastic lesions. Cancer Res. 1991;51:5270–5274. [PubMed] [Google Scholar]
- 25.Otori K, Sugiyama K, Hasebe T, Fukushima S, Esumi H. Emergence of adenomatous aberrant crypt foci (ACF) from hyperplastic ACF with concomitant increase in cell proliferation. Cancer Res. 1995;55:4743–4746. [PubMed] [Google Scholar]
- 26.Cohen G, Mustafi R, Chumsangsri A, et al. Epidermal growth factor receptor signaling Is up-regulated in human colonic aberrant crypt foci. Cancer Res. 2006;66:5656–5664. doi: 10.1158/0008-5472.CAN-05-0308. [DOI] [PubMed] [Google Scholar]
- 27.Wakeling AE, Guy SP, Woodburn JR, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–5754. [PubMed] [Google Scholar]
- 28.Moolenbeek C, Ruitenberg EJ. The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab Anim. 1981;15:57–59. doi: 10.1258/002367781780958577. [DOI] [PubMed] [Google Scholar]
- 29.Boivin GP, Washington K, Yang K, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 30.Wali R, Khare S, Tretiakova M, et al. Ursodeoxycholic acid and F6-3 inhibit aberrant crypt proliferation in the rat AOM model of colon cancer: roles of cyclin D1 and E-cadherin. Cancer Epidemiol Biomarkers Prev. 2002;11:1653–1662. [PubMed] [Google Scholar]
- 31.Zaidi NH, Pretlow TP, O’Riordan MA, Dumenco LL, Allay E, Gerson SL. Transgenic expression of human MGMT protects against azoxymethane-induced aberrant crypt foci and G to A mutations in the K-ras oncogene of mouse colon. Carcinogenesis. 1995;16:451–456. doi: 10.1093/carcin/16.3.451. [DOI] [PubMed] [Google Scholar]
- 32.Nambiar PR, Girnun G, Lillo NA, Guda K, Whiteley HE, Rosenberg DW. Preliminary analysis of azoxymethane induced colon tumors in inbred mice commonly used as transgenic/knockout progenitors. Int J Oncol. 2003;22:145–150. [PubMed] [Google Scholar]
- 33.Luetteke NC, Qiu TH, Peiffer RL, Oliver P, Smithies O, Lee DC. TGFa deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993;73:263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- 34.Khare S, Cerda S, Wali RK, et al. Ursodeoxycholic acid inhibits ras mutations, wild-type ras activation, and cyclooxygenase-2 expression in colon cancer. Cancer Res. 2003;63:3517–3523. [PubMed] [Google Scholar]
- 35.Morin PJ, Sparks AB, Korinek V, et al. Activation of bcatenin-Tcf signaling in colon cancer by mutations in bcatenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 36.Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the b-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petty WJ, Dragnev KH, Memoli VA, et al. Epidermal growth factor receptor tyrosine kinase inhibition represses cyclin D1 in aerodigestive tract cancers. Clin Cancer Res. 2004;10:7547–7554. doi: 10.1158/1078-0432.CCR-04-1169. [DOI] [PubMed] [Google Scholar]
- 38.Eisinger AL, Nadauld LD, Shelton DN, et al. The adenomatous polyposis coli tumor suppressor gene regulates expression of cyclooxygenase-2 by a mechanism that involves retinoic acid. J Biol Chem. 2006;281:20474–20482. doi: 10.1074/jbc.M602859200. [DOI] [PubMed] [Google Scholar]
- 39.Coffey RJ, Hawkey CJ, Damstrup L, et al. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci U S A. 1997;94:657–662. doi: 10.1073/pnas.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janmaat ML, Rodriguez JA, Gallegos-Ruiz M, Kruyt FA, Giaccone G. Enhanced cytotoxicity induced by gefitinib and specific inhibitors of the Ras or phosphatidyl inositol-3 kinase pathways in non-small cell lung cancer cells. Int J Cancer. 2006;118:209–214. doi: 10.1002/ijc.21290. [DOI] [PubMed] [Google Scholar]
- 41.Guda K, Upender MB, Belinsky G, et al. Carcinogeninduced colon tumors in mice are chromosomally stable and are characterized by low-level microsatellite instability. Oncogene. 2004;23:3813–3821. doi: 10.1038/sj.onc.1207489. [DOI] [PubMed] [Google Scholar]
- 42.Singh J, Kulkarni N, Kelloff G, Reddy BS. Modulation of azoxymethane-induced mutational activation of ras protooncogenes by chemopreventive agents in colon carcinogenesis. Carcinogenesis. 1994;15:1317–1323. doi: 10.1093/carcin/15.7.1317. [DOI] [PubMed] [Google Scholar]
- 43.Arteaga CL, Ramsey TT, Shawver LK, Guyer CA. Unliganded epidermal growth factor receptor dimerization induced by direct interaction of quinazolines with the ATP binding site. J Biol Chem. 1997;272:23247–23254. doi: 10.1074/jbc.272.37.23247. [DOI] [PubMed] [Google Scholar]
- 44.Rao CV, Wang CX, Simi B, et al. Enhancement of experimental colon cancer by genistein. Cancer Res. 1997;57:3717–3722. [PubMed] [Google Scholar]
- 45.Roberts RB, Min L, Washington MK, et al. Importance of epidermal growth factor receptor signaling in establishment of adenomas and maintenance of carcinomas during intestinal tumorigenesis. Proc Natl Acad Sci U S A. 2002;99:1521–1526. doi: 10.1073/pnas.032678499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang QS, Papanikolaou A, Sabourin CL, Rosenberg DW. Altered expression of cyclin D1 and cyclindependent kinase 4 in azoxymethane-induced mouse colon tumorigenesis. Carcinogenesis. 1998;19:2001–2006. doi: 10.1093/carcin/19.11.2001. [DOI] [PubMed] [Google Scholar]
- 47.Yan YX, Nakagawa H, Lee MH, Rustgi AK. Transforming growth factor-a enhances cyclin D1 transcription through the binding of early growth response protein to a cis-regulatory element in the cyclin D1 promoter. J Biol Chem. 1997;272:33181–33190. doi: 10.1074/jbc.272.52.33181. [DOI] [PubMed] [Google Scholar]
- 48.Dong M, Johnson M, Rezaie A, et al. Cytoplasmic phospholipase A2 levels correlate with apoptosis in human colon tumorigenesis. Clin Cancer Res. 2005;11:2265–2271. doi: 10.1158/1078-0432.CCR-04-1079. [DOI] [PubMed] [Google Scholar]
- 49.Dong M, Guda K, Nambiar PR, et al. Inverse association between phospholipase A2 and COX-2 expression during mouse colon tumorigenesis. Carcinogenesis. 2003;24:307–315. doi: 10.1093/carcin/24.2.307. [DOI] [PubMed] [Google Scholar]
- 50.Reddy BS, Hirose Y, Lubet R, et al. Chemoprevention of colon cancer by specific cyclooxygenase-2 inhibitor, celecoxib, administered during different stages of carcinogenesis. Cancer Res. 2000;60:293–297. [PubMed] [Google Scholar]
- 51.Kirschbaum MH, Yarden Y. The ErbB/HER family of receptor tyrosine kinases: a potential target for chemoprevention of epithelial neoplasms. J Cell Biochem Suppl. 2000;34:52–60. [PubMed] [Google Scholar]
- 52.Moasser MM, Basso A, Averbuch SD, Rosen N. The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001;61:7184–7188. [PubMed] [Google Scholar]
- 53.Ciardiello F, Caputo R, Bianco R, et al. Antitumor effect and potentiation of cytotoxic drugs activity in human cancer cells by ZD-1839 (Iressa), an epidermal growth factor receptor-selective tyrosine kinase inhibitor. Clin Cancer Res. 2000;6:2053–2063. [PubMed] [Google Scholar]