Abstract
Fragile X syndrome is caused by a lack of fragile X mental retardation protein (FMRP) due to silencing of the FMR1 gene. The metabotropic glutamate receptors (mGluRs) in the central nervous system contribute to higher brain functions including learning/memory, persistent pain, and mental disorders. Our recent study has shown that activation of Group I mGluR up-regulated FMRP in anterior cingulate cortex (ACC), a key region for brain cognitive and executive functions; Ca2+ signaling pathways could be involved in the regulation of FMRP by Group I mGluRs. In this study we demonstrate that stimulating Group I mGluRs activates Ca2+/calmodulin-dependent protein kinase IV (CaMKIV) in ACC neurons. In ACC neurons of adult mice overexpressing CaMKIV, the up-regulation of FMRP by stimulating Group I mGluR is enhanced. The enhancement occurs at the transcriptional level as the Fmr1 mRNA level was further elevated compared with wild-type mice. Using pharmacological approaches, we found that inhibition of CaMKIV could attenuate the up-regulation of FMRP by Group I mGluRs. CaMKIV contribute to the regulation of FMRP by Group I mGluRs probably through cyclic AMP-responsive element binding protein (CREB) activation, as manipulation of CaMKIV could simultaneously cause the change of CREB phosphorylation induced by Group I mGluR activation. Our study has provided strong evidence for CaMKIV as a molecular link between Group I mGluRs and FMRP in ACC neurons and may help us to elucidate the pathogenesis of fragile X syndrome.
Fragile X syndrome, the most common inherited form of human mental retardation, is caused by mutations of the FMR1 gene that encodes the fragile X mental retardation protein (FMRP)4 (1–5). FMRP, an mRNA-binding protein, is involved in activity-dependent synaptic plasticity through regulation of local protein synthesis at synapses (6–12). The function of Group I metabotropic glutamate receptor (mGluR) activation require the translation of pre-existing mRNA near active synapses. The abnormal functions of Group I mGluR-dependent synaptic plasticity have been observed in hippocampus of Fmr1 knock-out (KO) mice (8, 13–15). Because FMRP normally functions as a repressor of translation of specific mRNAs (6, 7, 14, 16), it is supposed that the protein synthesis-dependent functions of Group I mGluRs are exaggerated because of the lack of FMRP in fragile X syndrome (13, 14, 17).
The anterior cingulate cortex (ACC) plays an important role in cognitive learning, fear memory, and persistent pain (18–24). Previous studies have shown that trace fear memory is impaired in Fmr1 KO mice accompanied by alterations in synaptic plasticity in ACC (25, 26). These findings suggest that the dysfunction of ACC due to lack of FMRP may be responsible for certain types of mental disorders in fragile X syndrome. Electrophysiological and behavioral studies in animals found that the mGluRs in ACC may contribute to activity-dependent synaptic plasticity and behavioral fear memory (27, 28). The regulation of FMRP by mGluRs has been mostly studied in hippocampal neurons (9, 13, 14, 29, 30) (see Table 1). Our recent study has found that activation of Group I mGluRs regulates the expression of FMRP in ACC neurons; Ca2+-dependent signaling is involved in the regulation of FMRP by Group I mGluRs (31). These findings indicate a possible signaling pathway linking mGluRs to FMRP in ACC. Loss of this signaling pathway may contribute to the pathogenesis of fragile X syndrome.
TABLE 1.
Studies on regulation of FMRP by Group I mGluRs in central nervous system
| Neural cultures or tissues | Manipulations | Effects | References |
|---|---|---|---|
| Cortical synaptoneurosomes | Group I mGluR agonist DHPG | FMRP expression was increased | Weiler et al. (30) |
| Cultured cortical neuron | Group I mGluR agonist DHPG | FMRP is rapidly up-regulated | Todd et al. (56) |
| Hippocampal neuron culture | Group I mGluR agonist DHPG, mGluR, mGluR1, and mGluR5 antagonists | Group I mGluRs regulate trafficking of FMRP and Fmr1 mRNA into dendrites and at synapses | Antar et al. (73) |
| Hippocampal slices | Group I mGluR agonist DHPG | Translation-dependent increase in FMRP expression | Hou et al. (13) |
| Hippocampal neuron culture | Group I mGluR agonist DHPG | Group I mGluR activity -dependent changes in FMRP phosphorylation | Narayanan et al. (57, 58) |
| Anterior cingulate cotex slices | Group I mGluR agonist DHPG | Transcription-dependent up-regulation of FMRP | Wang et al. (31) and this study |
In the present study we have demonstrated that Ca2+/calmodulin-dependent protein kinase IV (CaMKIV), a key neuronal Ca2+ signaling molecule in synaptic plasticity (32–36), is critical for regulation of FMRP by Group I mGluRs in ACC neurons. Stimulating Group I mGluRs with DHPG, an agonist of Group I mGluRs, activates CaMKIV in cingulate cortex. Using transgenic mice overexpressing CaMKIV (35), we found that CaMKIV contributes to the up-regulation of FMRP by Group I mGluRs. By measuring Fmr1 mRNA, we found that overexpression of CaMKIV enhanced the transcription of Fmr1 gene. In addition, we provided evidence that CaMKIV is involved in regulation of FMRP by Group I mGluRs through activation of cyclic AMP-responsive element binding protein (CREB). These findings were further confirmed by pharmacological inhibition of CaMKIV. We propose that CaMKIV is a key molecule in regulation of FMRP Group I mGluRs through the Ca2+-stimulated CREB signaling pathway. CaMKIV may play an isoform-specific role in regulation of FMRP by Group I mGluRs in ACC neurons, as the CaMK inhibitor did not show effect in CaMKIV KO mice.
EXPERIMENTAL PROCEDURES
Animals
Adult male C57Bl/6 mice were used in most of experiments. The transgenic mice overexpressing CaMKIV and CaMKIV KO mice were generated and maintained as reported previously (32, 33, 35). All mice were housed under a 12:12 light cycle with food and water provided ad libitum. All mouse protocols are in accordance with National Institutes of Health guidelines and approved by the Animal Care and Use Committee of University of Toronto.
Drugs and Antibodies
(R,S)-3,5-Dihydroxyphenylglycine (DHPG), (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate, and (1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid, MPMQ, 3-amino-6-chloro-5-dimethylamino-N-2-pyridinylpyrazine carboxamide hydrochloride (ACDPP), and dl-AP3 were purchased from Tocris Bioscience (Ellisville, MO). Cyclopiazonic acid, nifedipine, calcium ionophore A23187, KN93, KN62, protease inhibitor mixture, and phosphatase inhibitor mixture 1 and 2 were purchased from Sigma-Aldrich. The anti-FMRP antibody, horseradish peroxidase-linked goat anti-mouse IgG, and goat anti-rabbit IgG for Western blot were purchased from Chemicon International (Temecula, CA). The anti-phosphothreonine antibody, anti-CREB antibody, and anti-phospho CREB antibody were purchased from Cell Signaling Technology (Danvers, MA). The anti-actin antibody was from Sigma-Aldrich. The anti-CaMKIV antibody was from BD Biosciences.
Brain Slice Preparations
Mice were anesthetized with 2% halothane, and brain slices (300 μm) containing ACC were cut at 4 °C using a Vibratome, in oxygenated artificial cerebrospinal fluid containing 124 mm NaCl, 4 mm KCl, 26 mm NaHCO3, 2.0 mm CaCl2, 1.0 mm MgSO4, 1.0 mm NaH2PO4, 10 mm d-glucose, pH 7.4]. The slices were slowly brought to final temperature of 30 °C in artificial cerebrospinal fluid gassed with 95% O2, 5% CO2 and incubated for at least 1 h before experiments. Slices then were exposed to different compounds of interest for the indicated times and snap-frozen over dry ice. For biochemical experiments, the ACC regions were microdissected and sonicated in ice-cold homogenization buffer containing phosphatase and protease inhibitors.
Immunoprecipitation
For detection of CaMKIV phosphorylation, the solubilized protein samples were prepared with lysis buffer and precipitated with 50 μl of protein G-agarose (Sigma-Aldrich) precoupled with anti-CaMKIV antibody for 4 h at 4 °C. The reaction mixtures were then washed three times, eluted by boiling in loading buffer, and subjected to Western blot using anti-phosphothreonine antibody.
Western Blot Analysis
Western blot was conducted as previously described (21, 37). The brain tissues were dissected and homogenized in lysis buffer containing 10 mm Tris-HCl, pH 7.4, 2 mm EDTA, 1% SDS, 1× protease inhibitor mixture, and 1× phosphatase inhibitor mixture 1 and 2. Protein concentration was measured by Bradford protein assay (Bio-Rad). Electrophoresis of equal amounts of total protein was performed on NuPAGE 4–12% Bis-Tris gels (Invitrogen). Separated proteins were transferred to polyvinylidene fluoride membranes (Pall Corp., East Hills, NY) at 4 °C for analysis. Membranes were probed with a 1:3000 dilution of anti-FMRP, or a 1:1000 dilution of anti-phospho-CREB (Ser-133) and anti-CREB, or a 1:2000 dilution of anti-CaMKIV antibodies and anti-phosphothreonine antibody. The membranes were incubated in the appropriate horseradish peroxidase-coupled secondary antibody diluted 1:3000 for 2 h followed by enhanced chemiluminescence (ECL) detection of the proteins with Western Lightning Plus-ECL (PerkinElmer Life Sciences) according to the manufacturer's instructions. To verify equal loading, membranes were also probed with a 1:3000 dilution of anti-actin antibody. The density of immunoblots was measured using the NIH ImageJ program.
RT-PCR
The total RNA from the ACC was isolated using RNAspin Mini kit (GE Healthcare). RT-PCR was carried out using Qiagen® One-Step RT-PCR kit (Qiagen, Hilden, Germany). A 25-μl PCR reaction contained 0.5 μg of total RNA, 5 μl of 5× RT-PCR buffer, 400 μm dNTP, and 0.5 μm concentrations of each primer and 1 μl of RT-PCR enzyme. PCR conditions were adjusted to be in a linear range of amplification. The PCR cycles consisted of initial incubation at 94 °C for 1 min, denaturation at 94 °C for 45 s, annealing at 56 °C for 45 s, and extension at 72 °C for 1 min, for 30 cycles, and a final extension at 72 °C for 10 min. The primers for Fmr1 used in this experiment were as follows: sense, 5′-CCGAACAGATAATCGTCCACG-3′; antisense, 5′-ACGCTGTCTGGCTTTTCCTTC-3′. Glyceraldehyde-3-phosphate dehydrogenase was amplified as an internal control by using the primer sets: sense, 5-AACGACCCCTTCATTGAC-3′; antisense, 5′-TCCACGACATACTCAGCAC-3′. RT-PCR products were electrophoresed on 1.5% agarose gels and visualized under UV light by ethidium bromide staining. The relative density of bands was analyzed by the NIH ImageJ program.
Data Analysis
All data were presented as the mean ± S.E. Statistical comparisons were made using the paired t test. In all cases p < 0.05 is considered statistically significant.
RESULTS
Activation of CaMKIV by Group I mGluRs in the ACC Neurons
Activation of Group I mGluRs cause the increase of intracellular Ca2+ and initiates the Ca2+ signaling pathways (31, 38–43). CaMKIV, a key Ca2+ signaling molecule in synaptic plasticity, can be activated by the increase of intracellular Ca2+ (31, 33, 34, 44, 45). The phosphorylation of CaMKIV at threonine residues is known to be critical for its kinase activity (44, 46–48). Can CaMKIV be activated by stimulating Group I mGluRs in ACC neurons? To address this we investigated the effects of mGluR activation on the phosphorylation of CaMKIV at threonine residues in ACC slices from adult mice. We found that application of Group I mGluR agonist DHPG (100 μm) to ACC slices for 15 min caused an increase in the phosphorylation of CaMKIV at threonine site in ACC slices (218 ± 14% of the control levels, p < 0.01, compared with control, n = 6, Fig. 1B). By contrast, neither application of Group II mGluR agonist (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (10 μm) nor Group III mGluR agonist (1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid (100 μm) for 15 min affected the phosphorylation of CaMKIV in ACC slices (p > 0.05, compared with control, n = 4, Fig. 1C). DHPG (100 μm) increased the phosphorylation of CaMKIV in a time-dependent manner, the increase could be observed at 5 min, and the highest level was reached at 15 min (p < 0.01 or p < 0.05, compared with control, n = 4, Fig. 1D). However, the presence of DHPG (100 μm) did not affect the expression of CaMKIV in ACC slices (p > 0.05, compared with control, n = 4, Fig. 1E). These data indicate that stimulating Group I mGluRs activates CaMKIV in the ACC neurons. To our knowledge this is the first time that it has been shown that CaMKIV can be activated by stimulating Group I mGluRs. The activation of CaMKIV suggests that CaMKIV might be a downstream effector for Group I mGluRs in cingulate cortex.
FIGURE 1.
Activation of CaMKIV by stimulating Group I mGluRs in ACC. A, a model for preparation of ACC slices and slice incubation. ACSF, artificial cerebrospinal fluid. B, application of Group I mGluR agonist DHPG (100 μm) for 15 min increased the phosphorylation of CaMKIV at threonine residues in ACC, as measured by Western blot. C, application of Group II mGluR agonist (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate (APDC; 10 μm) or Group III mGluR agonist (1S,3R,4S)-1-aminocyclopentane-1,3,4-tricarboxylic acid (ACPT-I, 100 μm) for 15 min did not affect the phosphorylation of CaMKIV at threonine residues in ACC slices. D, phosphorylation of CaMKIV was increased by DHPG (100 μm) in a time-dependent manner; the increase was observed at 5 min, and the highest increase was reached at 15 min. E, DHPG (100 μm) did not affect the expression of CaMKIV in ACC slices. Representative Western blot (top) and quantification data (bottom) are shown for corresponding treatments from B–E. Data were normalized by the control values. p < 0.05 (*) and p < 0.01 (**) compared with control. n = 6 mice for each group in B; n = 4 mice for each group in C–E.
There are two subtypes, including mGluR1 and mGluR5, in Group I mGluRs (13, 49–51). To identify which Group I mGluR subtype(s) was responsible for the activation of CaMKIV caused by DHPG, we applied DHPG (100 μm) to ACC slices in the presence of either a selective mGluR1 antagonist MPMQ (10 μm) or a specific mGluR5 antagonist of ACDPP (10 μm). We found that the phosphorylation of CaMKIV caused by DHPG was only partially blocked by MPMQ or ACDPP (p < 0.05, compared with DHPG treatment, n = 6, Fig. 2, A and B). The presence of Group I mGluR antagonist dl-AP3 (100 μm) completely blocked the phosphorylation of CaMKIV caused by DHPG in ACC slices (221 ± 13 and 102 ± 12% of the control levels for DHPG or dl-AP3 treatment, respectively, p < 0.01, compared with DHPG treatment, n = 6, Fig. 2C). However, the application of MPMQ, ACDPP, or dl-AP3 alone did not affect the basal phosphorylation levels of CaMKIV in ACC slices (p > 0.05, compared with control, n = 4, Fig. 2D). These data further confirm that stimulating Group I mGluRs can activate CaMKIV, and both mGluR1 and mGluR5 are involved in the phosphorylation of CaMKIV caused by DHPG in ACC neurons.
FIGURE 2.
Both mGluR1 and mGluR5 contribute to activation of CaMKIV by Group I mGluRs. A, the phosphorylation of CaMKIV caused by DHPG was partially blocked by a selective mGluR1 antagonist MPMQ (10 μm). MPMQ was applied to slices 15 min before and during the DHPG treatment (100 μm, 15 min). B, the phosphorylation of CaMKIV caused by DHPG was partially blocked by a specific mGluR5 antagonist of ACDPP (10 μm). ACDPP was applied to slices 15 min before and during the DHPG treatment. C, the presence of Group I mGluR antagonist, dl-AP3 (100 μm), completely blocked the increase of FMRP caused by DHPG in ACC slices. dl-AP3 was applied to slices 15 min before and during the DHPG treatment. D, application of MPMQ (10 μm), ACDPP (10 μm), or dl-AP3 (100 μm) for 30 min did not affect the basal phosphorylation levels of CaMKIV at threonine residues in ACC slices. Representative Western blot (top) and quantification data (bottom) are shown for the corresponding treatments. Data were normalized by the control values. p < 0.05 (*) and p < 0.01 (**) compared with control; p < 0.05 (#) and p < 0.01 (##) compared with DHPG treatment. n = 6 mice for each group in A–C; n = 4 mice for each group in D.
Calcium Is Critical for the Activation of CaMKIV by Group I mGluRs
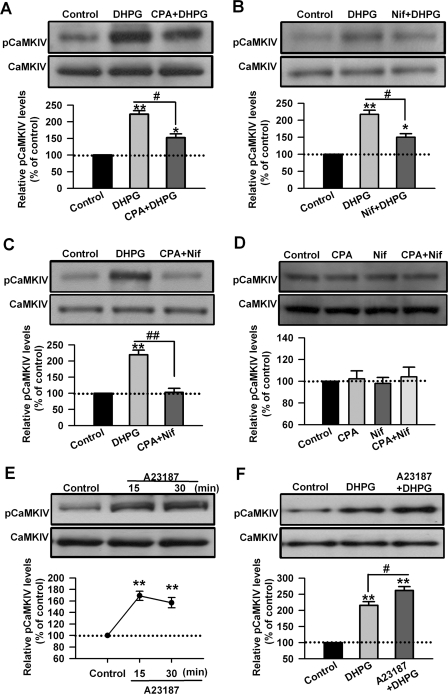
The activation of Group I mGluRs can induce Ca2+ release from trisphosphate-sensitive intracellular stores (41, 49, 52, 53). The sarco/endoplasmic reticulum Ca2+/ATPase (SERCA) pump inhibitor cyclopiazonic acid depletes intracellular stores of Ca2+ by blocking Ca2+ re-uptake into the stores (41, 53). To examine the role of Ca2+ release from intracellular stores in the phosphorylation of CaMKIV induced by DHPG, cyclopiazonic acid (CPA, 30 μm) was applied to ACC slices 20 min before and during the DHPG (100 μm, 15 min) treatment. We found that the phosphorylation of CaMKIV induced by DHPG was partially blocked by cyclopiazonic acid (223 ± 10 and 152 ± 12% that of the control levels for DHPG and CPA treatment, respectively, p < 0.05, compared with DHPG treatment, n = 6, Fig. 3A).
FIGURE 3.
Calcium mediates activation of CaMKIV by Group I mGluRs in ACC neurons. A, the sarco/endoplasmic reticulum Ca2+/ATPase pump inhibitor CPA (50 μm) partially blocked the phosphorylation of CaMKIV at threonine residues by DHPG (100 μm, 15 min). Cyclopiazonic acid was applied to slices 20 min before and during DHPG treatment. B, L-type Ca2+ channels blocker nifedipine (Nif, 25 μm) partially blocked the phosphorylation of CaMKIV at threonine residues caused by DHPG treatment. Nifedipine was applied to slices 20 min before and during DHPG treatment. C, coapplication of cyclopiazonic acid with nifedipine almost completely blocked the phosphorylation of CaMKIV at threonine residues by DHPG treatment. CPA (50 μm) and nifedipine (25 μm) were applied to slices 20 min before and during DHPG treatment. D, application of cyclopiazonic acid (50 μm), nifedipine (25 μm), or coapplication of cyclopiazonic acid with nifedipine for 15 min did not affect the basal phosphorylation levels of CaMKIV at threonine residues in ACC slices. E, application of calcium ionophore A23187 (5 μm, 15 and 30 min) could induce the phosphorylation of CaMKIV in ACC slices. F, coapplication of calcium ionophore A23187 (5 μm, 15 min) and DHPG (100 μm, 15 min) enhanced the phosphorylation of CaMKIV caused by DHPG. Representative Western blot (top) and quantification data (bottom) are shown for the corresponding treatments. Data were normalized by the control values. p < 0.05 (*) and p < 0.01 (**) compared with control; p < 0.05 (#) and p < 0.01 (##) compared with DHPG treatment. n = 6 mice for each group in A–C; n = 4 mice for each group in D–F.
Stimulating Group I mGluRs can facilitate L-type voltage-dependent Ca2+ channels (L-VDCCs) in different cell types (39–42). DHPG treatment may induce Ca2+ influx through L-VDCCs in striatal neurons (40). In this study we found that application of L-VDCC blocker nifedipine (Nif, 25 μm) 20 min before and during the DHPG treatment also partially blocked the phosphorylation of CaMKIV caused by DHPG (217 ± 13 and 150 ± 10% of the control levels for DHPG and nifedipine treatment, respectively, p < 0.05, compared with DHPG treatment, n = 6, Fig. 3B). In addition, coapplication of cyclopiazonic acid with nifedipine almost completely blocked the phosphorylation of CaMKIV induced by DHPG treatment (220 ± 14 and 103 ± 13% that of the control levels for DHPG and CPA plus nifedipine treatment, respectively, p < 0.01, compared with DHPG treatment, n = 6, Fig. 3C). By contrast, cyclopiazonic acid, nifedipine, or coapplication of cyclopiazonic acid and nifedipine did not affect the basal phosphorylation levels of CaMKIV in ACC slices (p > 0.05, compared with control, n = 4, Fig. 3D). These data indicate that both Ca2+ release from intracellular stores and Ca2+ influx through L-VDCCs are involved in the activation of CaMKIV by Group I mGluRs in ACC neurons.
To further confirm the role of calcium in the activation of CaMKIV by Group I mGluRs, we then tested the effect of raising intracellular Ca2+ by application of calcium ionophore A23187 (54, 55)in ACC slices. We found that application of calcium ionophore A23187 (5 μm, 15 min, and 30 min) could induce the phosphorylation of CaMKIV in ACC slices (169 ± 8 and 157 ± 9% of the control levels for A23187 at 15 and 30 min, respectively; p < 0.01, compared with control, n = 4, Fig. 3E). Coapplication of calcium ionophore (5 μm, 15 min) and DHPG (100 μm, 15 min) enhanced the phosphorylation of CaMKIV caused by DHPG (216 ± 11 and 262 ± 12% that of the control levels for DHPG or A23187 treatment, respectively, p < 0.05, compared with DHPG treatment, n = 4, Fig. 3F). These data provide additional evidence for the role of calcium in the activation of CaMKIV by Group I mGluRs in ACC neurons.
Raising Intracellular Calcium or CaMKIV Enhances Regulation of FMRP by Group I mGluRs in the ACC Neurons
Previous studies of the regulation of FMRP by mGluRs were mainly carried out in cultured neurons and hippocampal slices (9, 13, 30, 56–58) (see Table 1). Our recent study has found that the activation of Group I mGluRs by DHPG increased the expression of FMRP in ACC slices from adult mice, and calcium signaling is critical for the regulation of FMRP by Group I mGluRs (31).
Because raising intracellular Ca2+ by application of calcium ionophore A23187 could activate CaMKIV and enhance the phosphorylation of CaMKIV by stimulating Group I mGluRs, we then tested the effect of calcium ionophore A23187 on FMRP in ACC neurons. We found that application of calcium ionophore A23187 (5 μm, 15 and 30 min) did not affect the expression FMRP in ACC slices (p > 0.05, compared with control, n = 5, Fig. 4A). These data indicate that simply raising intracellular calcium cannot up-regulate FMRP, although it can activate CaMKIV. However, coapplication of calcium ionophore A23187 (5 μm, 30 min) and DHPG (100 μm, 30 min) enhanced the up-regulation of FMRP caused by DHPG (197 ± 10 and 246 ± 13% that of the control levels for DHPG or A23187 treatment, respectively, p < 0.05, compared with DHPG treatment, n = 5, Fig. 4B). Taken together these results suggest that calcium signaling may specifically contribute to the regulation of FMRP by Group I mGluRs.
FIGURE 4.
Up-regulation of FMRP by Group I mGluRs was enhanced in ACC from mice overexpressing CaMKIV. A, application of calcium ionophore A23187 (5 μm, 15 and 30 min) did not affect the expression of FMRP in ACC slices. B, coapplication of calcium ionophore A23187 (5 μm, 30 min) and DHPG (100 μm, 30 min) enhanced the up-regulation of FMRP caused by DHPG. C, the expression of CaMKIV was up-regulated in ACC slices from mice overexpressing CaMKIV, as compared with WT mice. D, overexpression of CaMKIV did not affect the basal levels of FMRP in ACC slices. E, the increase of FMRP after treatment with DHPG (100 μm) for 30 min was enhanced in ACC slices from mice overexpressing CaMKIV as compared with WT mice. A representative Western blot (top) and quantification data (bottom) of FMRP are shown for the corresponding treatments. Data were normalized by control or WT control values. **, p < 0.01, compared with control or WT mice; #, p < 0.05 compared with WT or compared with DHPG treatment. n = 5 mice for each group.
To explore the role of CaMKIV in the up-regulation of FMRP by stimulating Group I mGluRs, we have taken the advantage of another line of transgenic mice for CaMKIV. In these transgenic mice, the expression of CaMKIV was selectively up-regulated in the forebrain. The transgenic mice could develop normally and did not exhibit any abnormalities in brain structures. However, overexpression of CaMKIV enhanced the animal ability to form long term memory and rescued memory loss with aging (35).
By Western blot we found that the expression of CaMKIV was up-regulated in ACC slices from these transgenic mice (162 ± 8% of the WT levels, p < 0.01, compared with WT mice, n = 5, Fig. 4C). We next tested the effect of DHPG (100 μm, 30 min) treatment in ACC slices from mice overexpressing CaMKIV. There was no difference in the basal levels of FMRP in ACC slices between WT and CaMKIV overexpression mice (p > 0.05, compared with WT mice, n = 5, Fig. 3D). DHPG treatment could increase expression of FMRP in ACC slice; the increase of FMRP was further enhanced in ACC slices from mice overexpressing CaMKIV compared with WT mice (192 ± 9 and 252 ± 11% that of the WT control levels for WT and CaMKIV overexpression mice, respectively; p < 0.05, compared with WT mice, n = 5, Fig. 4E). The data indicate that overexpression of CaMKIV can enhance the up-regulation of FMRP by Group I mGluRs in ACC neurons.
Pharmacological Inhibition of CaMKIV in ACC Neurons
To rule out the possibility that the findings from transgenic mice might be caused by developmental changes due to genetic manipulation, we next tested the effect of CaMK inhibitors KN93 and KN62 (59, 60). We found that application of KN93 (10 μm) or KN62 (10 μm) for 30 min did not affect the basal levels of FMRP in ACC slices (p > 0.05, compared with control, n = 4, Fig. 5A). However, application of KN93 (10 μm) or KN62(10 μm) 20 min before and during the DHPG treatment could partially block the increase of FMRP caused by DHPG (198 ± 12, 149 ± 8, and 146 ± 10% that of the control levels for DHPG, KN93, and KN62 treatment, respectively, p < 0.05, compared with DHPG treatment, n = 6, Fig. 5B). The data indicate that CaMKs are involved in the regulation of FMRP by Group I mGluRs in ACC neurons.
FIGURE 5.
Pharmacological inhibition of CaMKIV partially blocked up-regulation of FMRP by Group I mGluRs in ACC neurons. A, application of CaMK inhibitor KN93 (10 μm) or KN62 (10 μm) for 30 min did not affect the basal levels of FMRP in ACC slices. B, the CaMK inhibitor KN93 (10 μm) or KN62 (10 μm) partially blocked the increase of FMRP caused by DHPG treatment. KN93 or KN62 was applied to slices 20 min before and during DHPG treatment. C, the CaMK inhibitor KN93 (10 μm) could not further reduce the increase of FMRP caused by DHPG treatment in ACC slices from CaMKIV KO mice. KN93 was applied to slices 20 min before and during DHPG treatment. Representative Western blot (top) and quantification data (bottom) of FMRP are shown for the corresponding treatments. Data were normalized by the control values. p < 0.05 (*) and p < 0.01 (**) compared with control; p < 0.05 (#) and p < 0.01 (##) compared with DHPG treatment. n = 4 mice for each group in A and C; n = 6 mice for each group in B.
To address the isoform-specific roles of CaMKIV, we then tested the effect of CaMK inhibitor in ACC slices from CaMKIV KO mice. We found that the increase of FMRP caused by DHPG was partially blocked in ACC slices from CaMKIV KO mice compared with that of WT mice (p < 0.05, n = 4, Fig. 5C). However, application of KN93 (10 μm) 20 min before and during the DHPG treatment did not cause further reduction in FMRP levels in ACC slices from CaMKIV KO mice (p > 0.05, n = 4, Fig. 5C). These data suggested that CaMKIV plays an isoform-specific role in the regulation of FMRP Group I mGluRs in ACC neurons; other isoforms of CaMKs, such as CaMKII, may not be involved in this process.
CaMKIV and Transcriptional Expression of Fmr1 Gene by Group I mGluRs
One previous study has shown that stimulation of Group I mGluRs with DHPG induces the increase of FMRP in a protein synthesis-dependent manner in hippocampus CA1 area (13). However, we found that the regulation of FMRP by Group I mGluRs occurs at the transcriptional level in ACC (31).
To further address the role of CaMKIV in the transcriptional expression of Fmr1 gene by Group I mGluRs in cingulate cortex, we measured the levels of Fmr1 mRNA by RT-PCR. We found that there was no difference in the basal levels of Fmr1 mRNA in ACC between WT and CaMKIV overexpression mice (p > 0.05, n = 4, Fig. 6A). However, the increase of Fmr1 mRNA caused by DHPG treatment was enhanced in ACC slices from mice overexpressing CaMKIV compared with WT mice (205 ± 10 and 259 ± 9% that of the WT control levels for WT and CaMKIV overexpression mice, respectively; p < 0.05, compared with WT mice, n = 4, Fig. 6B). We next tested the effect of CaMKIV inhibitor on transcriptional expression of Fmr1 gene by Group I mGluRs. We found that application of CaMKIV inhibitor KN93 (10 μm) or KN62 (10 μm) for 20 min did not affect the Fmr1 mRNA in ACC slices (p > 0.05, n = 4, Fig. 6C). By contrast, application of KN93 (10 μm) 20 min before and during the DHPG treatment partially blocked the increase of Fmr1 mRNA caused by DHPG (203 ± 12 and 152 ± 9% that of the control levels for DHPG and KN93 treatment, respectively, p < 0.05, compared with DHPG treatment, n = 4, Fig. 6D). These data indicate that CaMKIV is required for the transcriptional regulation of FMRP by Group I mGluRs in ACC neurons.
FIGURE 6.
CaMKIV contributes to up-regulation of FMRP by Group I mGluRs at the transcriptional level. A, overexpression of CaMKIV did not affect the basal levels of Fmr1 mRNA in ACC slices. B, DHPG (100 μm, 15 min) treatment increased the levels of Fmr1 mRNA in ACC slices, as shown by RT-PCR. The increase of Fmr1 mRNA was enhanced in ACC slices from mice overexpressing CaMKIV, compared with WT mice, as shown by RT-PCR. C, application of CaMK inhibitor KN93 (10 μm) or KN62 (10 μm) for 20 min did not affect the basal levels of Fmr1 mRNA in ACC slices. D, the CaMK inhibitor KN93 (10 μm) attenuated the increase of Fmr1 mRNA by DHPG (100 μm, 15 min) in ACC slices. KN93 was applied to slices 20 min before and during DHPG treatment. The size of PCR products is 141 and 191 bp for Fmr1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), respectively. Representative gels (top) and quantification data (bottom) of FMRP or Fmr1 mRNA are shown for the corresponding treatments. Data were normalized by WT (A), WT control (B), or control (C and D) values. p < 0.05 (*) and p < 0.01 (**) compared with control. #, p < 0.05, compared with WT in B; #, p < 0.05 compared with DHPG treatment in D. n = 4 mice for each group in A–D.
CaMKIV and CREB Activation by Stimulating Group I mGluRs
Phosphorylated CREB (pCREB) binds to the cAMP response element (CRE) site in the gene promoter and activates gene transcription (61–63). It has been reported that the FMR1 gene promoter contains the CRE site (64, 65). Our recent study found that DHPG treatment could increase the pCREB levels in ACC slices and suggests that the regulation of FMRP by Group I mGluRs in ACC neurons likely occurs through CREB activation (31).
CaMKIV activates the transcription factor CREB by phosphorylating CREB at the regulatory Ser-133 residue (34, 48, 62). Up-regulation of CaMKIV enhanced learning-induced CREB activity in the CA1 region of hippocampus (35). To further investigate whether CaMKIV is involved in the phosphorylation of CREB caused by stimulating Group I mGluRs, we then tested the phosphorylation of CREB induced by DHPG (100 μm, 15 min) in ACC slices from mice overexpressing CaMKIV. We found that the basal levels of pCREB were not changed in ACC slices from mice overexpressing CaMKIV (p > 0.05, compared with WT, n = 4, Fig. 7A). However, the phosphorylation of CREB induced by DHPG treatment was enhanced in ACC slices from mice overexpressing CaMKIV as compared with WT mice (221 ± 12 and 293 ± 14% that of the WT control levels for WT and CaMKIV overexpression mice, respectively; p < 0.05, compared with WT mice, n = 4, Fig. 7B). These results indicate that CaMKIV contributes to the phosphorylation of CREB induced by stimulating Group I mGluRs in ACC neurons. Consistently, application of CaMKIV inhibitor KN93 (10 μm) or KN62 (10 μm) for 20 min before and during DHPG (100 μm, 15 min) treatment partially blocked the increase of pCREB caused by DHPG in ACC slices (223 ± 11, 155 ± 9, and 156 ± 13% that of the control levels for DHPG, KN93, and KN62 treatment, respectively, p < 0.05, compared with DHPG treatment, n = 6, Fig. 7D). By contrast, application of KN93 (10 μm) or KN62 (10 μm) for 20 min did not affect the basal levels of pCREB in ACC slices (p > 0.05, compared with control, n = 4, Fig. 7C). These results indicate that CaMKIV is the key molecule that phosphorylates CREB during Group I mGluR activation in ACC neurons.
FIGURE 7.
CaMKIV and the phosphorylation of CREB by Group I mGluR activation. A, overexpression of CaMKIV did not affect the basal phosphorylation levels of CREB in ACC slices. The phosphorylation of CREB at Ser-133 residue was tested by Western blot. B, activation of Group I mGluRs by DHPG induced the phosphorylation of CREB in ACC slices. ACC slices were treated by DHPG (100 μm) for 15 min. The phosphorylation of CREB induced by DHPG (100 μm, 15 min) treatment was enhanced in ACC slices from mice overexpressing CaMKIV as compared with WT mice. C, application of CaMKIV inhibitor KN93 (10 μm) or KN62 (10 μm) for 15 min did not affect the basal phosphorylation levels of CREB in ACC slices. D, CaMKIV inhibitors KN93 (10 μm) or KN62 (10 μm) attenuated the phosphorylation of CREB by DHPG treatment. KN93 or KN62 was applied to slices 20 min before and during DHPG treatment. A representative Western blot (top) and quantification data (bottom) of pCREB levels are shown for corresponding treatments. Data were normalized by WT control (A and B) or control (C and D) values. p < 0.05 (*) and p < 0.01 (**) compared with control; #, p < 0.05, compared with WT in B; #, p < 0.05, compared with DHPG treatment in D. n = 4 mice for each group in A–C; n = 6 mice for each group in D.
DISCUSSION
Our previous studies suggest that FMRP is required for the physiological function of ACC (21, 25), and the mGluRs in ACC may contribute to the activity-dependent synaptic plasticity and fear memory (27, 28). Recently, we provided the direct biochemical evidence that activation of Group I mGluRs up-regulates FMRP in the ACC neurons of adult mice. The up-regulation of FMRP by Group I mGluRs occurs at the transcriptional level. Activation of Group I mGluRs induced the phosphorylation of CREB in ACC neurons (31). In this study we provided novel evidence that stimulating Group I mGluRs activates CaMKIV in ACC neurons. CaMKIV contributes to the up-regulation of FMRP and the phosphorylation of CREB induced by stimulating Group I mGluRs. Our results indicate that CaMKIV acts as a key intracellular messenger for signaling pathways between Group I mGluRs and FMRP in cingulate cortex.
Group I mGluRs, Ca2+, and CaMKIV Activation
The Ca2+ signaling pathways are believed to play a pivotal role in synaptic plasticity (45, 62, 63, 66). CaMKIV, a key Ca2+ signaling molecule in Ca2+ signaling pathways, can be activated by the increase of intracellular Ca2+ (32–34, 45, 67). Stimulation of Group I mGluRs can cause the increase of intracellular Ca2+ and initiates the Ca2+ signaling pathways (31, 40, 42). In this study, we found that stimulating Group I mGluRs could induce the phosphorylation of CaMKIV at threonine residues, which is known to be critical for its kinase activity (44, 46, 47, 68). Both mGluR1 and mGluR5 are involved in the activation of CaMKIV by Group I mGluRs. These data provide novel evidence that CaMKIV can be activated by stimulating Group I mGluRs.
Ca2+ is released from trisphosphate-sensitive intracellular stores upon Group I mGluR activation (41, 53, 69). We found that application of sarco/endoplasmic reticulum Ca2+/ATPase pump inhibitor cyclopiazonic acid before and during Group I mGluR activation partially blocked the phosphorylation of CaMKIV due to Group I mGluR activation. Another possible source for intracellular Ca2+ is Ca2+ influx through L-VDCCs. Previous studies have shown that membrane depolarization by DHPG treatment can trigger the opening of L-VDCCs (38, 40, 42). Here, we found that application of l-VDCC blocker nifedipine also partially blocked the phosphorylation of CaMKIV caused by Group I mGluR activation. When the sarco/endoplasmic reticulum Ca2+/ATPase pump inhibitor cyclopiazonic acid was co-applied with nifedipine to ACC slices, the phosphorylation of CaMKIV was almost completely blocked. It indicates that both Ca2+ release from intracellular stores and Ca2+ influx from L-VDCCs are required for the activation of CaMKIV by Group I mGluRs in ACC neurons.
CaMKIV and the Regulation of FMRP by Group I mGluRs
It is well known that CaMKIV transduces Ca2+ signaling and functions as a transcriptional activator in synaptic plasticity (33, 34, 48, 62). It is likely that CaMKIV might be involved in the signaling pathway downstream of Group I mGluRs. By using mice overexpressing CaMKIV, we confirmed that CaMKIV acts downstream of Group I mGluRs and contributes to the regulation of FMRP by Group I mGluRs in ACC neurons.
In addition, genetic deletion of CaMKIV or inhibiting CaMKs could partially block the increase of FMRP induced by stimulating Group I mGluRs in ACC slices. This result further support the conclusion that CaMKIV is required for the up-regulation of FMRP by Group I mGluRs in ACC neurons. Pharmacological inhibition of CaMKs did not affect regulation of FMRP by Group I mGluRs in ACC slices from CaMKIV KO mice. It suggests that CaMKIV may play an isoform-specific role in regulation of FMRP by Group I mGluRs in cingulate cortex.
In this study we also found that raising intracellular calcium by application of calcium ionophore A23187 did not affect the expression of FMRP, although it could activate CaMKIV and enhance the phosphorylation of CaMKIV and the up-regulation of FMRP by stimulating Group I mGluRs in ACC slices. These results suggest that intracellular calcium and CaMKIV, which can be shared by many different signaling pathways, may specifically contribute to the regulation of FMRP by Group I mGluRs.
CaMKIV and CREB Activation by Group I mGluRs
The CREB is a transcriptional factor that plays an important role in synaptic plasticity (62, 70, 71). The activity of CREB is regulated by its phosphorylation; pCREB binds to the CRE site within the gene and activates the gene transcription (62, 63, 71). Previous studies have shown that there is the CRE site in FMR1 promoter and implicated CREB in the regulation of the FMR1 gene transcription in neural cells (65, 72). Our recent study found that the Group I mGluR-dependent regulation of FMRP in ACC neurons occurs at the transcriptional level, as new Fmr1 mRNA is transcribed, as shown by RT-PCR; the up-regulation of FMRP is accompanied by the phosphorylation of CREB (Ser-133) (31). These results supported that CREB acts as a transcriptional factor for Group I mGluR-dependent regulation of FMRP in the neurons.
There are several signaling pathways that lead to the activation of CREB. Ca2+ and CaMKIV is implicated in various aspects of the neuronal Ca2+ signaling pathways, inducing the phosphorylation of CREB and the gene expression in response to excitatory neurotransmission (32, 34, 35, 62). The phosphorylation of CREB induced by the Group I mGluR agonist DHPG was partially blocked in ACC slices from CaMKIV KO mice, suggesting CaMKIV could be involved in the activation of CREB induced by stimulating Group I mGluRs (31). In this study we have shown that the phosphorylation of CREB induced by DHPG was enhanced in ACC slices from mice overexpressing CaMKIV. In addition, pharmacological inhibition of CaMKIV also partially blocked phosphorylation of CREB induced by DHPG. These novel findings further confirm that CaMKIV is critical for phosphorylation of CREB induced by stimulating Group I mGluRs, and CaMKIV contributes to regulation of FMRP by Group I mGluRs probably through CREB activation.
In summary, we have demonstrated that the CaMKIV is critical for regulation of FMRP by Group I mGluRs in ACC neurons by using genetic and pharmacological approaches. CaMKIV is activated by stimulating Group I mGluRs and contributes to the up-regulation of FMRP probably through the activation of CREB. Our study has provided strong evidence for CaMKIV as the key signaling molecule for regulation of FMRP by Group I mGluRs in cingulate cortex and may help to further elucidate the cellular mechanisms underlying fragile X syndrome.
This work was supported by grants from the EJLB-Canadian Institutes of Health Research (CIHR) Michael Smith Chair in Neurosciences and Mental Health, Canada Research Chair, NeuroCanada, and CIHR Operating Grants CIHR81086 and CIHR84256 (to M. Z.).
- FMRP
- fragile X mental retardation protein
- mGluR
- metabotropic glutamate receptor
- CaMKIV
- Ca2+/calmodulin-dependent protein kinase IV
- KO
- knock out
- ACC
- anterior cingulate cortex
- DHPG
- (R,S)-3,5-dihydroxyphenylglycine
- ACDPP
- 3-amino-6-chloro-5-dimethylamino-N-2-pyridinylpyrazine carboxamide hydrochloride
- CPA
- cyclopiazonic acid
- L-VDCCs
- L-type voltage-dependent Ca2+ channel
- CREB
- cyclic AMP-responsive element (CRE)-binding protein
- pCREB
- phosphorylated CREB
- Bis-Tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- RT
- reverse transcription
- WT
- wild type.
REFERENCES
- 1.Belmonte M. K., Bourgeron T. (2006) Nat. Neurosci. 9, 1221–1225 [DOI] [PubMed] [Google Scholar]
- 2.Feng Y., Zhang F., Lokey L. K., Chastain J. L., Lakkis L., Eberhart D., Warren S. T. (1995) Science 268, 731–734 [DOI] [PubMed] [Google Scholar]
- 3.Huber K. (2007) Am. J. Psychiatry 164, 556. [DOI] [PubMed] [Google Scholar]
- 4.Jin P., Warren S. T. (2003) Trends Biochem. Sci. 28, 152–158 [DOI] [PubMed] [Google Scholar]
- 5.Garber K. B., Visootsak J., Warren S. T. (2008) Eur. J. Hum. Genet. 16, 666–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bagni C., Greenough W. T. (2005) Nat. Rev. Neurosci. 6, 376–387 [DOI] [PubMed] [Google Scholar]
- 7.Greenough W. T., Klintsova A. Y., Irwin S. A., Galvez R., Bates K. E., Weiler I. J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 7101–7106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber K. M., Gallagher S. M., Warren S. T., Bear M. F. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 7746–7750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassell G. J., Warren S. T. (2008) Neuron 60, 201–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa-Mattioli M., Sossin W. S., Klann E., Sonenberg N. (2009) Neuron 61, 10–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown V., Small K., Lakkis L., Feng Y., Gunter C., Wilkinson K. D., Warren S. T. (1998) J. Biol. Chem. 273, 15521–15527 [DOI] [PubMed] [Google Scholar]
- 12.Fähling M., Mrowka R., Steege A., Kirschner K. M., Benko E., Förstera B., Persson P. B., Thiele B. J., Meier J. C., Scholz H. (2009) J. Biol. Chem. 284, 4255–4266 [DOI] [PubMed] [Google Scholar]
- 13.Hou L., Antion M. D., Hu D., Spencer C. M., Paylor R., Klann E. (2006) Neuron 51, 441–454 [DOI] [PubMed] [Google Scholar]
- 14.Bear M. F., Huber K. M., Warren S. T. (2004) Trends Neurosci. 27, 370–377 [DOI] [PubMed] [Google Scholar]
- 15.Nakamoto M., Nalavadi V., Epstein M. P., Narayanan U., Bassell G. J., Warren S. T. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 15537–15542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossman A. W., Aldridge G. M., Weiler I. J., Greenough W. T. (2006) J. Neurosci. 26, 7151–7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richter J. D., Klann E. (2009) Genes Dev. 23, 1–11 [DOI] [PubMed] [Google Scholar]
- 18.Frankland P. W., Bontempi B., Talton L. E., Kaczmarek L., Silva A. J. (2004) Science 304, 881–883 [DOI] [PubMed] [Google Scholar]
- 19.Frankland P. W., O'Brien C., Ohno M., Kirkwood A., Silva A. J. (2001) Nature 411, 309–313 [DOI] [PubMed] [Google Scholar]
- 20.Han C. J., O'Tuathaigh C. M., van Trigt L., Quinn J. J., Fanselow M. S., Mongeau R., Koch C., Anderson D. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 13087–13092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H., Wu L. J., Kim S. S., Lee F. J., Gong B., Toyoda H., Ren M., Shang Y. Z., Xu H., Liu F., Zhao M. G., Zhuo M. (2008) Neuron 59, 634–647 [DOI] [PubMed] [Google Scholar]
- 22.Zhao M. G., Toyoda H., Lee Y. S., Wu L. J., Ko S. W., Zhang X. H., Jia Y., Shum F., Xu H., Li B. M., Kaang B. K., Zhuo M. (2005) Neuron 47, 859–872 [DOI] [PubMed] [Google Scholar]
- 23.Zhuo M. (2006) J. Neurosci. Res. 84, 927–933 [DOI] [PubMed] [Google Scholar]
- 24.Zhuo M. (2008) Trends Neurosci. 31, 199–207 [DOI] [PubMed] [Google Scholar]
- 25.Zhao M. G., Toyoda H., Ko S. W., Ding H. K., Wu L. J., Zhuo M. (2005) J. Neurosci. 25, 7385–7392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashi M. L., Rao B. S., Seo J. S., Choi H. S., Dolan B. M., Choi S. Y., Chattarji S., Tonegawa S. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11489–11494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang J., Ko S., Ding H. K., Qiu C. S., Calejesan A. A., Zhuo M. (2005) Mol. Pain 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei F., Li P., Zhuo M. (1999) J. Neurosci. 19, 9346–9354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nosyreva E. D., Huber K. M. (2006) J. Neurophysiol. 95, 3291–3295 [DOI] [PubMed] [Google Scholar]
- 30.Weiler I. J., Irwin S. A., Klintsova A. Y., Spencer C. M., Brazelton A. D., Miyashiro K., Comery T. A., Patel B., Eberwine J., Greenough W. T. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 5395–5400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Wu L. J., Zhang F., Zhuo M. (2008) J. Neurosci. 28, 4385–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho N., Liauw J. A., Blaeser F., Wei F., Hanissian S., Muglia L. M., Wozniak D. F., Nardi A., Arvin K. L., Holtzman D. M., Linden D. J., Zhuo M., Muglia L. J., Chatila T. A. (2000) J. Neurosci. 20, 6459–6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei F., Qiu C. S., Liauw J., Robinson D. A., Ho N., Chatila T., Zhuo M. (2002) Nat. Neurosci. 5, 573–579 [DOI] [PubMed] [Google Scholar]
- 34.Wayman G. A., Lee Y. S., Tokumitsu H., Silva A., Soderling T. R. (2008) Neuron 59, 914–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukushima H., Maeda R., Suzuki R., Suzuki A., Nomoto M., Toyoda H., Wu L. J., Xu H., Zhao M. G., Ueda K., Kitamoto A., Mamiya N., Yoshida T., Homma S., Masushige S., Zhuo M., Kida S. (2008) J. Neurosci. 28, 9910–9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tremper-Wells B., Vallano M. L. (2005) J. Biol. Chem. 280, 2165–2175 [DOI] [PubMed] [Google Scholar]
- 37.Wang H., Gong B., Vadakkan K. I., Toyoda H., Kaang B. K., Zhuo M. (2007) J. Biol. Chem. 282, 1507–1517 [DOI] [PubMed] [Google Scholar]
- 38.Bianchi R., Young S. R., Wong R. K. (1999) J. Neurophysiol. 81, 2903–2913 [DOI] [PubMed] [Google Scholar]
- 39.Chavis P., Fagni L., Bockaert J., Lansman J. B. (1995) Neuropharmacology 34, 929–937 [DOI] [PubMed] [Google Scholar]
- 40.Mao L., Wang J. Q. (2003) Eur. J. Neurosci. 17, 741–750 [DOI] [PubMed] [Google Scholar]
- 41.Heinke B., Sandkühler J. (2007) Neuropharmacology 52, 1015–1023 [DOI] [PubMed] [Google Scholar]
- 42.Heinke B., Sandkühler J. (2005) Pain 118, 145–154 [DOI] [PubMed] [Google Scholar]
- 43.Hu H. J., Bhave G., Gereau R. W., 4th (2002) J. Neurosci. 22, 7444–7452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chow F. A., Anderson K. A., Noeldner P. K., Means A. R. (2005) J. Biol. Chem. 280, 20530–20538 [DOI] [PubMed] [Google Scholar]
- 45.Colomer J., Means A. R. (2007) Subcell. Biochem. 45, 169–214 [DOI] [PubMed] [Google Scholar]
- 46.Selbert M. A., Anderson K. A., Huang Q. H., Goldstein E. G., Means A. R., Edelman A. M. (1995) J. Biol. Chem. 270, 17616–17621 [DOI] [PubMed] [Google Scholar]
- 47.Chatila T., Anderson K. A., Ho N., Means A. R. (1996) J. Biol. Chem. 271, 21542–21548 [DOI] [PubMed] [Google Scholar]
- 48.Hook S. S., Means A. R. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 471–505 [DOI] [PubMed] [Google Scholar]
- 49.Coutinho V., Knöpfel T. (2002) Neuroscientist 8, 551–561 [DOI] [PubMed] [Google Scholar]
- 50.Moult P. R., Gladding C. M., Sanderson T. M., Fitzjohn S. M., Bashir Z. I., Molnar E., Collingridge G. L. (2006) J. Neurosci. 26, 2544–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thuault S. J., Davies C. H., Randall A. D., Collingridge G. L. (2002) Neuropharmacology 43, 141–146 [DOI] [PubMed] [Google Scholar]
- 52.Fitzjohn S. M., Palmer M. J., May J. E., Neeson A., Morris S. A., Collingridge G. L. (2001) J. Physiol. 537, 421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rae M. G., Martin D. J., Collingridge G. L., Irving A. J. (2000) J. Neurosci. 20, 8628–8636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kushnareva Y. E., Wiley S. E., Ward M. W., Andreyev A. Y., Murphy A. N. (2005) J. Biol. Chem. 280, 28894–28902 [DOI] [PubMed] [Google Scholar]
- 55.Sharma A. K., Rohrer B. (2004) J. Biol. Chem. 279, 35564–35572 [DOI] [PubMed] [Google Scholar]
- 56.Todd P. K., Mack K. J., Malter J. S. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14374–14378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Narayanan U., Nalavadi V., Nakamoto M., Pallas D. C., Ceman S., Bassell G. J., Warren S. T. (2007) J. Neurosci. 27, 14349–14357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Narayanan U., Nalavadi V., Nakamoto M., Thomas G., Ceman S., Bassell G. J., Warren S. T. (2008) J. Biol. Chem. 283, 18478–18482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fog J. U., Khoshbouei H., Holy M., Owens W. A., Vaegter C. B., Sen N., Nikandrova Y., Bowton E., McMahon D. G., Colbran R. J., Daws L. C., Sitte H. H., Javitch J. A., Galli A., Gether U. (2006) Neuron 51, 417–429 [DOI] [PubMed] [Google Scholar]
- 60.He X., Yang F., Xie Z., Lu B. (2000) J. Cell Biol. 149, 783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lu Y. F., Kandel E. R., Hawkins R. D. (1999) J. Neurosci. 19, 10250–10261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bito H., Deisseroth K., Tsien R. W. (1997) Curr. Opin. Neurobiol. 7, 419–429 [DOI] [PubMed] [Google Scholar]
- 63.Shaywitz A. J., Greenberg M. E. (1999) Annu. Rev. Biochem. 68, 821–861 [DOI] [PubMed] [Google Scholar]
- 64.Garber K., Smith K. T., Reines D., Warren S. T. (2006) Curr. Opin. Genet. Dev. 16, 270–275 [DOI] [PubMed] [Google Scholar]
- 65.Hwu W. L., Wang T. R., Lee Y. M. (1997) DNA Cell Biol. 16, 449–453 [DOI] [PubMed] [Google Scholar]
- 66.West A. E., Chen W. G., Dalva M. B., Dolmetsch R. E., Kornhauser J. M., Shaywitz A. J., Takasu M. A., Tao X., Greenberg M. E. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 11024–11031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bito H., Deisseroth K., Tsien R. W. (1996) Cell 87, 1203–1214 [DOI] [PubMed] [Google Scholar]
- 68.Kasahara J., Fukunaga K., Miyamoto E. (2001) J. Biol. Chem. 276, 24044–24050 [DOI] [PubMed] [Google Scholar]
- 69.Rae M. G., Irving A. J. (2004) Neuropharmacology 46, 1057–1069 [DOI] [PubMed] [Google Scholar]
- 70.Gong B., Wang H., Gu S., Heximer S. P., Zhuo M. (2007) Eur. J. Neurosci. 26, 275–288 [DOI] [PubMed] [Google Scholar]
- 71.Kornhauser J. M., Cowan C. W., Shaywitz A. J., Dolmetsch R. E., Griffith E. C., Hu L. S., Haddad C., Xia Z., Greenberg M. E. (2002) Neuron 34, 221–233 [DOI] [PubMed] [Google Scholar]
- 72.Smith K. T., Nicholls R. D., Reines D. (2006) Nucleic Acids Res. 34, 1205–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Antar L. N., Afroz R., Dictenberg J. B., Carroll R. C., Bassell G. J. (2004) J. Neurosci. 24, 2648–2655 [DOI] [PMC free article] [PubMed] [Google Scholar]