Abstract
Integrins are a family of αβ heterodimeric receptors that mediate cell–cell and cell–substratum interactions. Integrin binding to extracellular ligands regulates cell adhesion, shape, motility, intracellular signalling and gene expression1-3. Mechanisms that regulate integrin function are, therefore, central to the participation of integrins in a diverse set of cellular events. Here we report the identification of TASC, a monoclonal antibody to a novel epitope on the integrin β1 subunit, which inhibits cell adhesion to vitronectin but promotes adhesion to laminin and collagen types I and IV. We show that developing retinal neurons that have lost responsiveness to laminin4 regain the ability to bind laminin in the presence of TASC. Thus, β1-class integrins are likely to occupy multiple affinity states that can be modulated at the cell surface.
On developing neurons, β1-class integrins promote adhesion and growth cone motility in response to glycoprotein components of basal laminae and are, therefore, likely to play a prominent part in axon extension and pathfinding in vivo5. Retinal neurons from embryonic day six (E6) chicks express β1-class integrins that mediate adhesion and neurite outgrowth in vitro on purified laminin, collagen IV and fibronectin4. Embryonic day seven (E7) retinal neurons also attach to vitronectin (Fig. 1a). Attachment to vitronectin was abolished by an Arg-Gly-Asp-containing peptide indicative of integrin function6. The function-blocking monoclonal antibody CSAT to the integrin β1 subunit7 inhibited attachment by 32%, implicating β1-class integrins in neuronal attachment to vitronectin (Fig. 1a). The TASC monoclonal antibody was isolated in a screen which used this attachment assay to select clones of interest. TASC IgG inhibited retinal neuron attachment to vitronectin by 37%, and Fab fragments of TASC IgG were also effective (Fig. 1a). The combined effects of TASC plus CSAT were stronger but did not completely block attachment to vitronectin, suggesting that additional vitronectin-binding integrins are expressed by these neurons.
FIG. 1.

TASC inhibits E7 retinal neuron attachment to vitronectin and promotes attachment to laminin and collagens. a, Attachment to vitronectin is inhibited by l00 μg m−1 GRGDSP and 200 μg ml−1 TASC, 50 μg ml−1 TASC Fab, and 50 μg ml−1 CSAT. b, Histograms of retinal neuron attachment to collagen IV (10 min), laminin (20 min) and fibrinogen (1 h). All antibodies are used at 50 μg ml−1. c, Time-course of E7 retinal neuron attachment to collagen I in the presence of no antibody, TASC IgG, TASC Fab and TASC IgG plus CSAT. d, Attachment of E7 retinal neurons to a collagen-I concentration curve at 40 min in the presence of no antibody or TASC at 25 μg ml−1. All values are expressed as a percentage of the attachment observed on poly-d-lysine which is considered to represent a signal of 100%.
METHODS. Vitronectin was purified from fetal bovine serum by chromatography on Heparin Sepharose Cl-6b (Pharmacia)22. Collagen I (Collaborative Research) was diluted to 10 μg ml−1 in 0.1 N acetic acid. Collagen IV (Collaborative Research) was diluted to 10 μg ml−1 in CMF-PBS. Laminin (diluted to 20 (μg ml−1 in CMF-PBS) was purified from murine EHS tumour by I. de Curtis and M. J. Ignatius according to published procedures23. Fibrinogen was the gift of Z. Ruggeri (Research Institute of the Scripps Clinic, La Jolla, California) and was used at 20 μg ml−1 in Ca2+/Mg2+ containing PBS. Poly-d-lysine was from Sigma. IgGs were purified from ascites fluid on protein A/Sepharose Cl-4b (Pharmacia), and GRGDSP was purchased from Collaborative Research. TASC Fab fragments were generated from purified IgG by papain (Sigma) digestion followed by ion-exchange chromatography on DE-52 (What-man). Cell attachment was quantitated as previously described4, except that adherent cells were stained with crystal violet and adsorbtion at 540 nm was subsequently measured24. In each experiment, attachment to BSA-coated wells was subtracted, and all determinations were done in triplicate. To isolate TASC, integrin-rich chick brain glial cells were use d as the immunogen for monoclonal antibody production in mice25. Clonal supernatants were screened for the ability to inhibit retinal neuron attachment to vitronectin.
TASC was subsequently found to promote attachment to other β1-class integrin ligands, including laminin, collagen I and collagen IV (Fig. 1b-d). E7 retinal neuron attachment to laminin was increased about fivefold and to collagen IV about threefold by both bivalent and monovalent TASC in short-term assays (Fig. 1b). The ability of TASC to promote attachment to collagen I was examined in detail. Both TASC IgG and Fab fragments accelerated E7 retinal neuron attachment to collagen I (Fig. 1c): whereas about 70% of the neurons adhered to collagen I in 1 h in the absence of TASC IgG, the same level of adhesion was achieved in the presence of TASC in 5 min. Because monovalent TASC increased attachment to all three ligands (Fig. 1b and c), its effects were not due to bivalent antibody-induced crosslinking of the TASC antigen. All of TASC’s effects were β1-class integrin-specific, as the enhanced binding to laminin, collagen I and collagen IV was abolished by the function-blocking CSAT antibody (Fig. 1b and c). TASC also lowered the dose-dependence of attachment to collagen I by about fourfold (Fig. 1d). Note that TASC did not promote attachment to very low concentrations of ligand or to the BSA-blocked substrate, excluding the possibility that TASC itself had been adsorbed to the substrate. Furthermore, E7 retinal neurons did not attach to fibrinogen in the absence or presence of TASC (Fig 1b) even though β1-dependent attachment to fibrinogen has been detected in a chicken myeloblast cell line and is promoted by TASC (K.M.N. and L.F.R., manuscript in preparation). Thus, TASC does not seem to promote attachment by transforming the ligand specificity of β1-class integrin heterodimers expressed by a particular cell. It is also unlikely that TASC promoted the expression of new receptors on the cell surface, because its effects were detectable within minutes. Rather, TASC seems to increase the efficacy of ligand-specific binding by integrins that are already expressed.
Biochemical characterization of the TASC antigen revealed that TASC recognizes a novel epitope on the integrin β1 subunit. In immunoblots of retinal cell extracts, TASC bound a band of relative molecular mass 105,000–110,000 (Mr 105–110K) (Fig. 2). From 35S-labelled extracts of E7 retinal neurons, TASC IgG precipitated the same set of proteins as those precipitated by the anti-β1 antibody CSAT (Fig. 2, lanes 2 and 8). These included proteins comigrating with the mature (110K) and immature (100K) forms of the β1 subunit1 in addition to the noncovalently associated α subunits (Mr 145 and 135K) previously characterized on retinal neurons4. As immunodepletion of the extract with TASC removed the CSAT antigen and vice versa (Fig. 2), both antibodies seem to recognize the same set of β1-class integrin heterodimers.
FIG. 2.

TASC recognizes the integrin β1 subunit. TASC binds a 105-110K band in an immunoblot of E7 retinal extracts (lane 1). Both TASC and CSAT immunoprecipitate the integrin β1 subunit of 100K and 110K and associated α subunits at 135K and 145K. Immunodepletion analysis of the TASC antigen on E7 retinal neurons reveals that sequential precipitation of labelled extract with CSAT (lanes 2–5) removes the TASC antigen (lane 6). Overexposure of the autoradiogram did not reveal the presence of even minor amounts of either antigen following depletion (not shown). Antigens were unlikely to have been degraded during the course of the experiment, because another cell-surface glycoprotein, the integrin αv subunit, was readily detected by immunoprecipitation from an aliquot of the same depleted sample (lane 7). Depletion with TASC (lanes 8–11) removes the CSAT antigen (lane 12).
METHODS. E7 retinas were extracted for 15 min in an equal volume of ice-cold Ca2+/Mg2+-containing PBS plus 1% Triton X-100 and 2 mM PMSF. Supernatant (200 μg) was run under nonreducing conditions on a 6% gel and transferred to nitrocellulose. The blots were incubated with 20 μg ml−1 TASC overnight at 4 °C, followed by alkaline phosphatase-conjugated second antibody (Promega), used and developed according to manufacturer’s instructions. For immunoprecipitation, E7 retinal neurons were metabolically labelled with 100 μCi ml−1 [35S] methionine/cysteine (NEN) for 16 h. Cells were collected and extracted as above. The supernatant was divided into two 200 μl aliquots, and 20 μg TASC or CSAT was added to each. Samples were rocked overnight at 4 °C and then precleared twice with 50 μl of Sepharose Cl-4b (Pharmacia). Antibody-antigen complexes were precipitated with protein A/Sepharose CI-4B and washed extensively with 0.05M Tris buffer pH 8.0, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 0.5% Triton X-100. To deplete the extract of antigen, supernatants were repeatedly precipitated with 20 μg of each antibody. An additional protein A/Sepharose adsorption step was performed before adding the second antibody to ensure that all of the depleting antibody had been removed. The resulting supernatant was divided in half and precipitated with 20 μg of the second antibody. The monoclonal antibody to αv was isolated in this laboratory25. Pellets were extracted in sample buffer and separated by nonreducing SDS-PAGE on 6% gels. Gels were fixed, stained, treated with En3Hance (NEN), dried, and exposed to Kodak X Omat R film. Mr standards were obtained from Biorad.
Because TASC and CSAT had different effects on cell adhesion, it seemed likely that the two antibodies recognize distinct epitopes on the β1 subunit. To test this possibility, retinal neuron attachment to antibody-coated substrates was measured in the absence and presence of competing antibodies. Although CSAT competed for attachment to CSAT and TASC competed for attachment to TASC, neither antibody inhibited attachment to the other (Table 1). The monoclonal antibody JG22 binds an epitope on β1 which overlaps with CSAT’s8. Antibody JG22 competed for cell binding to CSAT and vice versa, but did not compete for binding to TASC (Table 1). Thus, CSAT and TASC recognize nonoverlapping epitopes on the integrin β1 subunit. The observations that these two anti-β1 antibodies have complementary effects on attachment to laminin and collagens and that TASC inhibits attachment to vitronectin suggest that two distinct sites on the β1 subunit are involved in forming ligand-binding domains. Consistent with this structural prediction, evidence for the presence of two distinct ligand-binding domains on αIIbβ3 and α4β1 heterodimers has been reported9-11.
TABLE 1.
Antibody competition of adhesion to antibody substrates
| Control attachment (%) Competing antibody | |||
|---|---|---|---|
| Substrate antibody | CSAT | JG22 | TASC |
| CSAT | 7 ± 2 | 7 ± 2 | 120 ± 18 |
| JG22 | 5 ± 2 | 10 ± 2 | 131 ± 13 |
| TASC | 96 ± 11 | 90 ± 11 | 5 ± 1 |
E7 retinal neuron attachment to substrates coated with 5 μg ml−1 CSAT, TASC, and JG22 antibodies (see Fig. 1 for methods) was measured. Cells were incubated with the competing antibody for 2 min at room temperature, centrifuged onto the antibody substrates, incubated for 5 min and washed. The results (mean ± s.e.m.) are normalized to the attachment observed to each antibody in the absence of competing antibody. The s.e.m. for control attachment to CSAT was 14%, to JG22 8%, and to TASC 11%.
Purified laminin is a potent promoter of neurite outgrowth by early embryonic chicken retinal neurons and both attachment and neurite outgrowth depend on β1-class integrins4. These responses diminish with increasing developmental age, such that E11 retinal neurons do not attach or extend neurites on purified laminin. But β1-integrins are detectable on the surfaces of these neurons4. In addition, CSAT inhibits E11 retinal neurite outgrowth on astrocyte monolayers where the cell adhesion molecules NCAM and N-cadherin are also active12. These observations suggest that E11 neurons express β1-class integrins that function in situations where multiple neurite-promoting receptors are engaged. Consistent with this, the number of 125I-labelled laminin binding sites on the majority of retinal neurons remains relatively constant between E6 and E11, although retinal ganglion neurons (~10% of all retinal neurons) exhibit a decrease in the number of laminin receptors13. To test the possibility that these unresponsive cells continue to express functional integrin laminin receptors, their attachment to laminin was measured in the presence of TASC. Within 10 min, E11 retinal neurons had begun to attach to laminin in the presence of TASC, and 80% of the cells were attached in 40 min (Fig. 3). Thus, E11 retinal neurons express integrin receptors for laminin and the observed developmental changes must be due, at least in part, to mechanisms which lead to the reversible inactivation of receptor populations on the cell surface. This finding raises the possibility that laminin receptors continue to function on E11 neurons in vivo and that their function may be dynamically regulated by physiological events, such as cell contact and synapse formation.
FIG. 3.
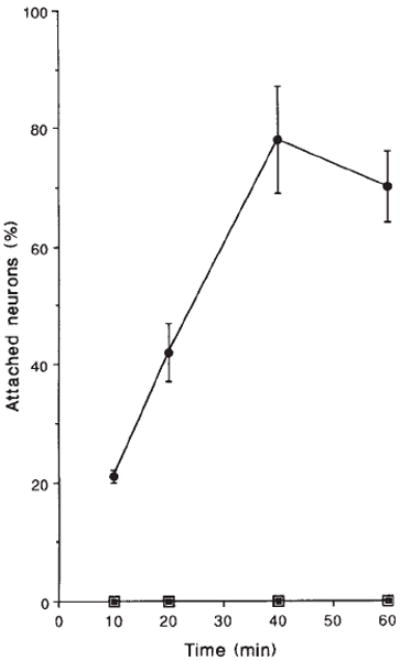
E11 retinal neurons do not adhere to laminin in the absence of TASC at any time point up to 60 min (open squares). In the presence of 100 μg ml−1 TASC (●), attachment to laminin is detectable within 10 min and peaks at 40 min with ~80 % of the neurons attached compared to poly-d-lysine. TASC-induced attachment is completely blocked by the addition of CSAT (■).
Because TASC recognizes β1-class integrins and promotes their function with respect to attachment kinetics and dose-dependence of ligand, and in cells where their function has been downregulated, we propose that TASC binding increases receptor affinity for laminin, collagen I and collagen IV by inducing a conformational change in specific β1-class integrin heterodimers. Several monoclonal antibodies against integrin α subunits that promote heterodimer function have recently been described, suggesting that antibody-induced changes influence the conformation of associated α and β subunits14-16. Precedence for the rapid regulation of integrin avidity without a corresponding change in levels of receptor expression has been established in circulating blood cells and is likely to depend on intracellular signalling mechanisms3,17-20. On macrophage, for example, interferon-γ and phorbol esters induce cell binding to laminin through the α6β1 heterodimer, and binding is coincident with the phosphorylation of α6 and association of the receptor with the cytoskeleton21. The results presented here show that β1-class integrins can occupy multiple functional states on the neuronal cell surface. This suggests that receptor avidity is also modulated on non-circulating cells whose movements within tissues may require rapid and dynamic regulation.
Acknowledgments
We thank K. Jones, M. Ignatius, C. Murphy-Erdosh and K. Tomaselli for their insights during this work; C. Damsky, C. Emmett, Z. Hall and F. Lefcort for comments on the manuscript; and C. Emmett and K. Venstrom for setting up the monoclonal screen. L.F.R. is an investigator of the Howard Hughes Medical Institute.
References
- 1.Akiyama SK, Nagata K, Yamada KM. Biochim biophys Acta. 1990;1031:91–110. doi: 10.1016/0304-4157(90)90004-v. [DOI] [PubMed] [Google Scholar]
- 2.Hemler ME. A Rev Immun. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 3.Springer TA. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 4.Hall DE, Neugebauer KM, Reichardt LF. J Cell Biol. 1987;104:623–634. doi: 10.1083/jcb.104.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reichardt LF, Tomaselli KJ. A Rev Neurosci. 1991;14:531–570. doi: 10.1146/annurev.ne.14.030191.002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierschbacher MD, Ruoslahti E. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 7.Buck CA, Shea E, Duggan K, Horwitz AF. J Cell Biol. 1986;103:2421–2428. doi: 10.1083/jcb.103.6.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz AF, et al. In: Monoclonal Antibodies and Functional Cell Lines. Kennett RH, Bechtol KB, McKearn TJ, editors. Plenum; 1984. pp. 103–118. [Google Scholar]
- 9.Santoro SA, Lawing WJ., Jr Cell. 1987;48:867–873. doi: 10.1016/0092-8674(87)90083-3. [DOI] [PubMed] [Google Scholar]
- 10.Elices MJ, et al. Cell. 1990;60:577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 11.Frelinger AL, et al. J biol Chem. 1990;265:6346–6352. [PubMed] [Google Scholar]
- 12.Neugebauer KM, Tomaselli KJ, Lilien J, Reichardt LF. J Cell Biol. 1988;107:1177–1187. doi: 10.1083/jcb.107.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen J, Nurcombe V, Jeffrey P, Edgar D. Development. 1989;107:381–387. doi: 10.1242/dev.107.2.381. [DOI] [PubMed] [Google Scholar]
- 14.Keizer GD, Visser W, Vliem M, Figdor CG. J Immun. 1988;140:1393–1400. [PubMed] [Google Scholar]
- 15.Bednarczyk JL, Mclntyre BW. J Immun. 1990;144:777–784. [PubMed] [Google Scholar]
- 16.Gulino D, Ryckewaert J-J, Andrieux A, Rabiet M-J, Marguerie G. J biol Chem. 1990;265:9575–9581. [PubMed] [Google Scholar]
- 17.Dustin ML, Springer TA. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 18.Plow EF, Ginsberg MH. Prog Hemost Thromb. 1989;9:117–156. [PubMed] [Google Scholar]
- 19.Figdor CG, van Kooyk Y, Keizer GD. Immun Today. 1990;11:277–280. doi: 10.1016/0167-5699(90)90112-m. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu Y, Van Seventer GA, Horgan KJ, Shaw S. Nature. 1990;345:250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- 21.Shaw LM, Messier JM, Mercurio AM. J Cell Biol. 1990;110:2167–2174. doi: 10.1083/jcb.110.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yatogo T, Izumi M, Kashiwagi H, Hayashi M. Cell Struct Funct. 1988;13:281–292. doi: 10.1247/csf.13.281. [DOI] [PubMed] [Google Scholar]
- 23.Timpl R, et al. J biol Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- 24.Bodary SC, Napier MA, McLean JW. J biol Chem. 1989;264:18859–18862. [PubMed] [Google Scholar]
- 25.Neugebauer KM, Emmett CJ, Venstrom K, Reichardt LF. Neuron. in the press. [Google Scholar]


