Abstract
The map of the retina onto the optic tectum is a highly conserved feature of the vertebrate visual system and the mechanism by which this mapping is accomplished during development is a long-standing problem of neurobiology. The early suggestion by Roger Sperry that the map is formed through interactions between retinal ganglion cell axons and target cells within the tectum has gained significant experimental support and wide spread acceptance. Nonetheless, reports in a variety of species indicate that some aspects of retinotopic order exist within the optic tract, leading to the suggestion that this ‘pre-ordering’ of retinal axons may play a role in the formation of the mature tectal map. A satisfactory account of pre-target order must provide the mechanism by which such axon order develops. Insofar as this mechanism must ultimately be genetically determined, the mouse suggests itself as the natural species in which to pursue these studies. Quantitative and repeatable methods are required to asses the contribution of candidate genes in mouse models. For these reasons, we have undertaken a quantitative study of the degree of retinotopic order within the optic tract and nerve of wild type mice both before and after the development of the retinotectal map. Our methods are based on tract-tracing using lipophilic dyes and our results indicate that there is a reestablishment of dorsoventral but not nasotemporal retinal order when the axons pass through the chiasm, and that this order is maintained throughout the subsequent tract. Furthermore, this dorsoventral retinotopic order is well-established by the day after birth, long before the final target zone is discernible within the tectum. We conclude that pretarget sorting of axons according to origin along the dorsoventral axis of the retina is both spatially and chronologically appropriate to contribute to the formation of the retinotectal map, and suggest that these methods be used to search for the molecular basis of such order using available mouse genetic models.
Keywords: Development, chemoaffinity, retinotectal, superior colliculus, vision, retinotopy
Introduction
The pattern of topographic projection of the retina onto the optic tectum is shared by all vertebrates. This map has served as the main model of neural specificity, exhibiting the ability of a large number of projecting neurons to connect via their axons to a distant target in such a way as to reproduce the spatial layout of the projecting structure. Details of the retinotectal map have been characterized in fish, amphibians, birds, reptiles, and mammals, all of which are found to share many essential features. Nonetheless, after more than half a century of research, we still lack a full account of the developmental events and mechanisms by which this, or any other, retinotopic map is established.
Following the work of Roger Sperry (Sperry, 1963), most investigators have attempted to explain the development of retinotectal and other neural maps according to a chemoaffinity model. In this scheme, projecting neurons achieve their connectional specificity by virtue of chemical signatures borne on their navigating axons. Although this is often understood to be a principle that operates solely within the target tissue, Sperry initially contemplated the possibility that these chemical signatures might be recognized by landmarks en route, which might serve to organize the fiber tracts themselves (Attardi and Sperry, 1963). Sperry later revised his model to include matched chemical gradients between projecting neurons and their targets (Sperry, 1964). It is this form of the ‘chemoaffinity hypothesis’ that today has gained wide assent, with the receptor tyrosine kinase families of Eph receptors and their ephrin ligands having emerged as leading candidates to play the role of chemoaffinity cues.
Despite the current dominance of Sperry’s matched gradient chemoaffinity model, several studies have indicated that retinal ganglion cell (RGC) axons do exhibit some features of retinotopic order within the optic tract. It should be stated outright that a robust and precise retinotopy comparable to that within the tectum itself is definitely not discernible in either the optic nerve or optic tract of any species. What has been reported is a partial and approximate order, and this fact has complicated comparison of results obtained in different species, by different workers, and by use of different methods. Anamniotes exhibit the clearest and most extensive preservation of order in the retinal projection. In fish and amphibians, for instance, a considerable degree of retinotopy has been reported throughout the retinofugal projection (Bunt and Horder, 1983). An extensive study using retrograde transport of HRP in chicks (Rager et al., 1988) revealed a similar degree of order, with the authors interpreting the repeated reorganization of fibers in the axonal tract as a mechanism to prepare them for the retinotopic innervation of each successive retinal target.
Among mammals the pattern of pre-target order is more variable. But even within the mammals studied, various degrees of ‘preordering’ have been reported. In reviewing the scattered literature on this topic, meaningful comparisons are frequently precluded by the often anecdotal and qualitative descriptions of the phenomena involved. One study addressed the order of retinal axons in the optic nerve of the cat (Horton et al., 1979). This study used retrograde transport of HRP injected into a small region of the lateral geniculate nucleus, resulting in a small cluster of labeled retinal ganglion cells. It was observed that within the optic nerve, the labeled axons diverged widely from one another, and thus could not be confined to a retinotopic arrangement. In marsupials, however, there does appear to be rather good preservation of retinotopy even in the optic nerve (Dunlop et al., 2000). A study in rats (Simon and O’Leary, 1991) revealed an almost total loss of retinotopic order as axons reached the chiasm. In general, the conclusion from the literature is that an initial retinotopic order of axons as they exit the eyeball is greatly degraded along the course of the optic nerve, so that virtually no order remains upon reaching the chiasm.
Despite the evidence that retinal axons are more or less randomly distributed in the optic nerve in at least some mammals, it has been noticed that in those same animals dorsal and ventral axons show some degree of segregation after they have passed through the chiasm. This has been reported in the cat (Torrealba et al., 1982), ferret (Walsh and Guillery, 1985), monkey (Naito, 1989), and embryonic rat (Chan and Guillery, 1994). In the embryonic rat, for example, the authors concluded that dorsal and ventral axons become more or less segregated into the mediocaudal and rostrolateral aspects, respectively, of the ascending optic tract. The optic chiasm is well known as a point of axon rearrangement, mediating, for example, the sorting of ipsi- and contralaterally projecting axons. It is thus conceivable that additional mechanisms might exist at the chiasm to reestablish at least some aspects of retinal topography among the retinal axons as they pass through.
It is possible that both presorting mechanisms and within-target interactions are required for the proper formation of the retinotectal map. In this case, the objection that the observed presorting is ‘too crude’ to explain the final precision of the map may be beside the point. Perhaps only a rough pretarget sorting is required in order for within-target interactions to be successful. It has also been suggested that, while some pretarget order is present, at least in some species, this order is coincidental, and not a carefully regulated process that is required for map formation. Studies of the cat optic tract have particularly contributed to this view (Walsh et al., 1983). The dorsal retinal ganglion cells of the cat are generated before those in ventral retina, and it has been suggested that this fact accounts for the observed tendency of dorsal axons to lie on the medial aspect of the tract, with later-arriving ventral growing over the surface and thus laterally. This pattern, however, does not preclude the possibility that the post-chiasmatic ordering of dorsal and ventral axons is specifically regulated by dorsoventral origin and not merely time of arrival. A careful study of another carnivore, the ferret, has suggested that an active sorting mechanism redistributes dorsal and ventral RGC axons to their own territories within the optic tract after exiting the chiasm (Reese and Baker, 1993).
If the order that has been described in the pretectal tract does make an instructive contribution to the development of the retinotectal map, then we would expect that this is a genetically regulated process. That this is the case in the zebrafish, there can be little doubt, given the recent report (Lee et al., 2004) that disruption of heparan sulfate synthesis results in a marked disordering of the normally well-segregated dorsal and ventral axons in the ascending optic tract. Heparan sulfate is a known regulator of signal recognition for several receptor-ligand systems, including the ephrins (Lee et al., 2004). The genetic basis of axon order in the optic tract has not been studied in any mammal.
Given wide inter-species variability in observed pre-ordering, and considering that the mouse has emerged as the primary mammalian genetic model for neural development, it would seem useful to have a detailed characterization of the pretarget order in the murine retinofugal system. One report (Hindges et al., 2002), with data confined to the brachium of the superior colliculus, has suggested that such order may be significant, but the chronologic and spatial development of this order must be established to make this claim. To this end, we undertook a detailed and quantitative axon tracing study in the widely studied C57BL/6 mouse strain. Our results indicate that fiber populations originating from the dorsal and ventral retina become segregated from one another immediately after the chiasm, as reported in some other species, and that this segregation is accomplished by postnatal day 1 (PND1), well before target zones are formed in the superior colliculus. On the other hand, we found no evidence of axon sorting according to the position of the parent cell along the nasotemporal axis of the retina. This data suggests that pre-target axon ordering in the optic tract may play an important role in the development of retinotopic maps in the LGN and Superior Colliculus.
Methods
Animals
We examined mice of the C57BL/6 strain bred in our colony. Two age groups were studied, PND0-1, and PND11-21. Birth time was usually noted within 6 hours, but never more than 24 hours. Procedures were carried out in accordance with approved protocols from Baylor College of Medicine and followed NIH guidelines.
Retinal labeling
Pups were anesthetized with an IP injection (0.7 ml/kg) of a combination anesthetic (Ketamine- 4.28mg/ml, Xylazine- 0.82mg/ml, Acepromazine- 0.07mg/ml). After surgically opening the eyelid, the eye was protruded and a small injection (2.6nL) of dye (‘DiI’ or ‘DiAsp’ (Molecular Probes), 10% in dimethylformamide) was made beneath the sclera. The injection was through a glass pipette attached to a microinjector (Microject II, Drummond Scientific). Animals were allowed to recover from the anesthesia and were put back with their mother, then sacrificed after 48 hours, or 24 hours for neonatal (PND0) mice. Upon sacrifice, the injected eye was fixed in 10% buffered formalin for later examination to localize the injection site relative to the four major eye muscles (the superior rectus (SR), medial rectus (MR), inferior rectus (IR), and lateral rectus (LR)). Animals with dye injections that spread beyond a focal spot in the retina were eliminated from further analysis. The injection position along the perimeter of the retina was reliably localized to a given one-third of each muscle or inter-muscle space, yielding 24 possible injection sites. The inter-muscle spaces and the spans of the insertion of the four muscles are not equal; they were measured in three PND14 animals and the means are indicated schematically in Fig. 1.
Figure 1. Ocular muscle anatomy and the retinofugal projection of the mouse.
A. Vitreal injections of DiI in both eyes reveals the gross form of the retinofugal projection. The overlying cortex and the eyes have been removed from this whole mount fluorescent image. B. Schematic of relevant anatomical features of the retinofugal projection in the mouse. Drawing is with approximately the same perspective and scale as A. C. Schematic showing the pattern of terminations of dorsal, nasal, ventral, and temporal retinal ganglion cell axons in the dorsal and ventral lateral geniculate nuclei (dLGN and vLGN) and contralateral superior colliculus. Axons enter the targets perpendicular to the mediolateral target axes, corresponding to the dosoventral axis of the retina. D. Schematic of muscle insertion points around the globe of the eye. Oblique muscles are very small in rodent and are not considered. The sizes of both the muscle (sectors 1–3 for superior rectus, 7–9 for medial rectus, 13–15 for inferior rectus and 19–21 for lateral rectus) and intermuscle regions (4–6; 10–12; 16–18 and 22–24) are drawn to scale. E, F, G, H. Injection of dye into the four principal muscles leads to spots of label around the perimeter of the superior colliculus. In E, dye was injected into sector 2 of the superior rectus (SR). In F, dye was injected into sector 8 of the medial rectus (MR). In G, dye was injected into sector 15 of the inferior rectus (IR). In H, dye was injected into sector 20 of the lateral rectus (LR). White lines show outline of the superior colliculus. Scale bars in A and E are 1 mm. Scale bar in E applies to E, F, G and H, and medial is to the right, caudal is up in these panels.
Distribution of label in whole mount preparations
At the time of sacrifice, the brain was removed and the cerebral cortex was dissected away. Digital images of dye label were acquired under epifluorescent illumination using a CCD camera and associated software (Epix, Inc., Houston, TX).
The superior colliculus was imaged using a 2.5X objective. The position of the target zone of the labeled retinal ganglion cells (RGCs) in the superior colliculus was quantified by first aligning the digital image of the colliculus on a standard coordinate system using the midline and the point at which the medial edges of the two colliculi diverge rostrally (Fig. 2A). The center of mass of the fluorescent label (thresholded at 75% of the maximum fluorescence) in the superior colliculus was calculated. The position of the center of mass is then considered to be the collicular location of the focal retinal projection, or the ‘target zone’. Images were also taken focused on the rostral edge of the SC in order to assess the distribution of axons as they passed into the SC from the brachium.
Figure 2. The collicular projection as a function of retinal location.
Using the coordinates defined by the eye muscles in Figure 1D, we performed a series of retinal injections around the periphery of the retina and determined the location of their collicular target zones. A. All of the retinal injection sites (n=45) are shown in eye muscle coordinates. B. An example collicular target zone for a temporal retinal injection, with axes (white lines) used to quantify target zone location. C. A standard coordinate system, depicted here by black lines, was used to identify the location of the target zone in the superior colliculus. All target zones were measured with respect to the position of the point of divergence of the rostral superior colliculus from the midline (intersection of vertical and horizontal white lines in B). This is analogous to the ‘lambda’ point in skull coordinate systems. Shown are the positions of all the target zones from the retinal injection sites shown in A, with colors representing retinal location of injection in muscle coordinates as shown in A. Scale bar in B is 500 μm.
After the superior colliculus and brachium were digitally imaged, the brain was bisected mid-sagittally and the half contralateral to the injected eye was oriented with the lateral side up so that the uppermost part of the optic tract could be visualized. This is the region just before the tract reaches the ventral lateral geniculate nucleus, and is referred to here as the delta of the optic tract (deltaOT, Fig. 1B). Fluorescent digital images of the deltaOT were obtained using a 5X objective.
We quantified the axon distribution in the deltaOT and brachium of the SC using software written in IDL (Research Systems Inc) and Matlab. First, a path was defined crossing the tract from the medial to the lateral edge. The fluorescence of the pixels in this path was weakly smoothed with a Gaussian filter and recorded as a fluorescence profile (FP), which gives the filtered fluorescence as a function of mediolateral position in the tract. The FP was background subtracted and normalized by the total area under the FP curve. The position of labeled retinal axons in the tract was quantified by calculating the center of mass of fluorescent label along the defined mediolateral path. A center of mass value of 0 would indicate that all axons lie on the medial edge of the tract, and a value of 1 would indicate that all axons lie on the lateral edge of the tract.
All results are reported as mean +/− SEM. Error bars in figures represent SEM. Means are compared with a Students t-test, and corrected for multiple comparisons where appropriate. Results are considered significant at the P=0.05 level.
Distribution of label in tissue sections
Some mice received both dorsal and ventral injections on the day of birth (PND0), using DiI and DiAsp, in the same eye. On the following day, the animal was anesthetized and sacrificed and the skin and skull above the SC were removed. At this age, nearly the entire SC is visible because the cortex has not yet made the extensive backward expansion that will eventually obscure much of the SC. Whole mount fluorescence images were taken with filters designed to admit emission from only DiI or DiA. The pair of retinal injections was judged to be successful if the labeled axons from both the dorsal and ventral focal injections were not spread diffusely across the mediolateral extent of the colliculus. In a small number of cases with poor retinal injections the axons from one of the injections were broadly distributed, so the sample was discarded. The entire head of the animal, with the SC exposed, was then fixed in 10% buffered formalin and cryoprotected in 30% sucrose, frozen, and cut at 20μm, either coronally (to study the optic nerves) or horizontally (to study the optic tract) on the cryostat. The sections were then photographed at 100× or 200× using filters designed to reveal DiI and DiA label.
Horizontal sections were used to study the distribution of dorsally and ventrally labeled RGC axons in the basal optic tract. This plane of section cut the axons approximately normal to their direction of travel. All quantified sections were within 200 μm of the chiasm. Axon distribution in the optic nerve was similarly studied in coronal sections, also taken within 200 μm of the chiasm.
The basal optic tract in horizontal section has the shape of a thick comma with a wide blunt rostral end and a tapering, pointed caudal end. In order to quantify the distribution of labeled retinal axons in the section, the bOT was outlined and an axis was drawn across the widest point, approximately in the mediolateral direction. The midpoint of this axis was then connected to both the rostral and caudal ends, which divided the tract into roughly equal sized medial and lateral hemisections. The dorsal and ventral retinal labels were thresholded at 75% of maximum intensity and the fraction of labeled pixels in each hemisection was calculated. The same quantification procedure was followed for the optic nerve, except we looked for segregation of label in any of 8 hemifields (dorsal, ventral lateral, medial, dorsal-lateral, ventral-medial, dorsal-medial and ventral-lateral).
Results
The retinofugal projection of the mouse
We examined the retinotopy of retinofugal axons at several anatomical locations, including the optic nerve, optic tract and brachium of the superior colliculus in early neonatal (PND 0) and two week old (PND 14) mice (Figure 1A, B). The three major topographic targets of the retinofugal projection, viz. vLGN, dLGN, and SC, are presented to the optic tract in series, and the tract traverses them along the axis of nasotemporal mapping (Figure 1C). This anatomical, which is true for all vertebrate species examined, implies that the targeting of the nasotemporal coordinate must be reestablished anew in each target. On the other hand, the spatial representation of the dorsoventral axis in each of these targets lies perpendicular to the axons’ trajectories in the optic tract. Once dorsoventral order has been established in the first target, it could be sufficient for subsequent targets, as long as it is not lost in the intervening tract.
To quantitatively analyze the routes taken by RGC axons in the retinal projections from different locations within the eye, we established a system of retinal coordinates based upon the location of the focal retinal injections relative to the insertion point of the principal eye muscles on the globe of the eye. We targeted the retinal injections to the periphery of the retina, with 34 of 47 injections within 500 μm of the outer edge of the retina, 12 within 1000 μm, and one between 1000 and 1500 m of the outer edge of the retina (438 +/−34.7 μm from outer edge on average). We then used the insertions of the principal eye muscles (Fig 1D) to define a coordinate system for the injections around the circumference of the retina. Focal retinal injections of dye in two week old mice, which have an anatomically mature retinotopic map (Hindges et al., 2002), produced a small spot of label in the superior colliculus (Fig 1 E–H). We examined the distribution of labeled retinal ganglion cell axons at different levels of the optic nerve and ascending optic tract (Figure 1B), including in the optic nerve just before the chiasm, in the optic tract just after the chiasm, in the ‘delta of the optic tract’ just before the LGN, in the brachium of the superior colliculus just before axons enter the superior colliculus, and finally in the superior colliculus itself.
Retinal locations defined in ‘eye muscle coordinates’ (Figure 1D) have a predictable and reproducible projection pattern to the superior colliculus (Figure 2). For example, temporal injections (yellow in Figure 2, in region of lateral rectus muscle (LR)), reproducibly produce target zones on the rostral side of the superior colliculus. As the injection location moved clockwise from lateral to dorsal retina, (red in Figure 2), the target zone moved to the lateral colliculus. Further clockwise movement of the injection in eye ‘muscle coordinates’ produced commensurate clockwise movement of the target zone in the superior colliculus. The muscle coordinate system therefore provides a reliable way of identifying the retinal location of labeled RGCs.
Magnification of the Ventrotemporal projection
In the course of characterizing the collicular targets produced by focal injections around the periphery of the retina, we noticed that ventral injections frequently produced target zones that were dramatically elongated along the rostrocaudal axis. Typical focal injections in the retina produced roughly circular target zones in the colliculus. For example (Figure 3), injections into dorsal retina (sectors 1–3 in Figure 1D) produced target zones that were modestly longer on the rostrocaudal axis than the mediolateral axis of the colliculus (ratio of 1.95 +/−0.18; n=7; Figure 3B and D). In contrast, injections into ventrotemporal retina (sectors 15–18) produced target zones that were very elongated along the rostrocaudal axis of the colliculus (ratio of 8.23 +/−1.99; n=6; P < 0.01; Figure 3A and D). Injections into neighboring ventronasal retina (sectors 13–14) produced target zones (ratio of 2.84 +/−0.48; n=8; Figure 3C and D) that were not significantly elongated relative to dorsal retina (sectors 1–3), and were much less elongated (P< 0.01) than ventrotemporal retina (sectors 15–18). Thus, a very limited region of the ventrotemporal retina produced target zones that were much more elongated than other regions of the retina, even nearby injections into ventronasal retina. It is interesting to observe that the region of dramatically elongated projections to the colliculus corresponds to the field of binocular vision that receives input from both ipsilateral and contralateral retina.
Figure 3. Elongation of ventrotemporal retinal targets in superior colliculus.
A. The collicular target zone from axons originating from ventrotemporal retinal (sectors 15–18 in Figure 1D) shows a characteristic elongation along the rostrocaudal axis. B. Target zones originating from neighboring ventronasal retina (sectors 13–14 in Figure 1D) are much less elongated. C. Target zones from axons originating from dorsal retina (sectors 1–3) are also much less elongated than those from ventrotemporal retina. D. Quantification of the target zone elongation along the rostrocaudal relative to mediolateral dimension due to injections in ventrotemporal (n=6; black bar), ventronasal (n=8; grey bar) and dorsal (n=7; white bar) retina. Medial is to the right, caudal is up. 500 μm scale bar in C applies to all panels.
Retinotopic order of dorsoventral axons in the ascending optic tract
We first examined, in mice with mature retinotopic maps (PND 11–21), the distribution of axons from different retinal locations at the brachium of the superior colliculus, which is just before axons enter their final target. Order at the brachium of the superior colliculus has been previously noted in the rat (Simon and O’Leary, 1992) and in the mouse (Hindges et al., 2002). Using eye muscle coordinates to quantify this order (Figure 4), we found that the mediolateral order of retinal axons in the brachium of the superior colliculus reflects their dorsoventral (DV) origin in the retina. At PND11-21, axons from the dorsal retina are highly lateralized in the brachium (Fig 4A, E), while axons from the ventral retinal run in the most medial aspect of the brachium (Fig 4B, E). Axons originating from nasal or temporal retinal origins tended to have a similar distribution within the center of the brachium (Fig 4 C, D, E). A summary of these data is shown in Figure 4 F, where the center of mass (CofM) of axon label in the brachium (See Methods) is shown as a function of retinal origin in eye muscle coordinates. RGC Axons originating near the superior rectus (dorsal axons) are lateralized (CofM = 70% +/−2% of mediolateral distance; n=7) in the brachium relative to axons originating near the inferior rectus (ventral axons; CofM = 26% +/−1%; n=10; P<0.001). In contrast, axons originating near the medial or lateral rectus run centrally in the brachium with a statistically indistinguishable center of mass location (P=0.34).
Figure 4. The mediolateral order of axons in the brachium of the superior colliculus reflects their dorsoventral origin in the retina.
A. Axons originating from dorsal retina (SR) are strongly biased to enter the SC (outlined) from the lateral side. The diagonal line (red) indicates the path across the brachium in which axonal labeling was measured. B. Axons originating from temporal retina (LR) are not biased to either the lateral or medial colliculus on entry. C. Axons from ventral retina (IR) are strongly biased to enter the colliculus on the medial edge. D. Axons originating from the nasal retina (MR) show no medial-lateral bias upon entry into the colliculus. E. Normalized distribution of fluorescent label in the brachium for the four animals represented in A–D. F. Average position of the center of mass of the distribution of fluorescent label in the brachium as a function of retinal location of the injection. Position of the center of mass of the fluorescent label is shown as a function of total medial-lateral width of the brachium, such that 0% means the average center of mass is on the medial edge, and 100% means the average center of mass of the distribution of fluorescent label is on the lateral edge. The location of the retinal injection (abscissa) is defined in muscle coordinates. The four principal ocular muscles (SR, MR, IR and LR) are indicated, along with the intervening inter-muscle regions (MS, MI, LI, LS). The number of animals (injections) appears next to the corresponding point in the plot. 1 mm scale bar in A applies to all panels. Medial is to the right and caudal is up.
We wondered whether ordering of axons at the brachium of the colliculus could possibly be an indirect effect of interactions between axons and their nearby target in the superior colliculus (or even interactions at the LGN, which the axons already passed through). We therefore analyzed the topography of axons at the delta of the optic tract (deltaOT), which lies before the axons reach the LGN or any other synaptic target (Figure 5). At PND 11–21, axons from dorsal and ventral retina are well sorted in the deltaOT (Fig. 5A, C, E), with dorsal axons running on the lateral edge of the contralateral optic tract (Fig. 4A). Ventral axons that originate from the middle third of the inferior rectus run on the medial edge of the deltaOT (Fig. 5C). In contrast, axons from the nasal and temporal retina (from the middle third of the medial rectus and lateral rectus, respectively) are overlapping in the middle of the delta of the optic tract (Figure 5B, D, F). The distribution of axons across the delta of the optic tract for axons originating from dorsal and ventral retina are shown in Fig. 5E, where it is apparent that the two groups are well segregated in the optic tract. In contrast, axons originating from nasal or temporal retinal are overlapping and more broadly distributed across the center of the optic tract. A summary quantification of the center of mass of these distributions (Fig. 5G) clearly shows that dorsal and ventral axons run on the lateral (CofM = 76% +/−2% of mediolateral distance; n=3) and medial (CofM = 30% +/−2%; n=5) edge of the optic tract, respectively. Dorsal (SR) axons are significantly more lateralized than ventral (IR) axons (P<0.001). In contrast, nasal (MR) and temporal (LR) axons have a similar distribution (CofM = 56% +/−2% for n=2 nasal injections; CofM = 63% +/−2%; for n=4 temporal injections; P=0.36) in mid-optic tract that is significantly different than the distribution of dorsal or ventral axons (P<0.05).
Figure 5. Position of RGC axons in the delta of the Optic Tract as a function of retinal origin.
Axons originating from dorsal (A) or ventral (C) retina travel in lateral or medial optic tract, respectively, whereas axons from nasal (D) or temporal (B) retina show a broader, more central distribution.. The target zone in ventral LGN is visible in A and D. E. Distribution of label in the optic tract for ventral (red, n=4) and dorsal (blue, n=3) injections. Dorsal (blue) injections are lateralized, ventral (red) injections are in medial optic tract. F. Distribution of label in optic tract for nasal (red, n=2) and temporal (blue, n=4) injections. No difference in the distribution of label in the optic tract for the nasal (red) and temporal (blue) injections is apparent. G. Quantification of axon order in optic tract. Position of the center-of-mass of labeled axons for the four ‘polar’ retinal injection locations. Injections in the dorsal retina (SR sector 2; green bar) result in label that is much more (P<0.001) lateralized than injections into ventral retina (IR sector 14, red bar). No difference in label distribution is seen for nasal (MR sector 8, light blue) and temporal (LR sector 20, dark blue) injections, but the center of mass of label for the nasal and temporal injections is different than the either the dorsal or ventral injections (P<0.01). 200 μm scale bar in A applies to all panels.
Sorting of RGC axons occurs prior to target formation
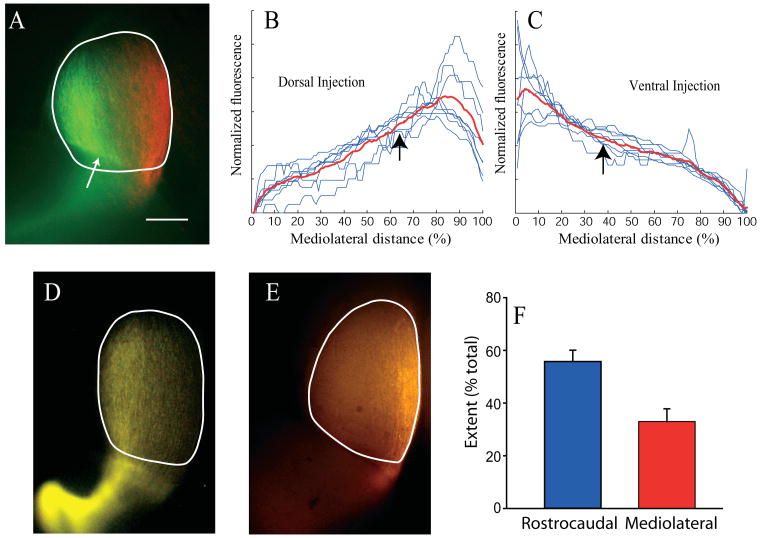
We have demonstrated that at a time when the retinotopic map is anatomically mature in the superior colliculus (PND11-21), the dorsoventral origin of retinal axons is reflected in their position in the optic tract both at the brachium of the superior colliculus and delta of the optic tract. In contrast, axons originating from the nasal and temporal retina show no positional bias. In order for this pretarget sorting to be instructional, it must precede the formation of target zones in the colliculus. We therefore investigated whether pretarget order exists at PND1, a time when axons have invaded the colliculus but have not yet arborized or formed target zones (Fig 6A; (Hindges et al., 2002)). Although target zones have not formed at PND1, there was still a clear tendency for axons originating from the dorsal retina to enter the superior colliculus on its lateral edge and run parallel to the midline (Fig. 6A, 6B). Similarly, axons from the ventral retina enter the colliculus on its medial edge and run up the midline on the opposite side of the colliculus from dorsal axons (Fig. 6A, 6C). Summary quantification of this separation in singly injected animals confirms this effect, with RGC axons originating from dorsal retina significantly more lateralized than ventral originating axons in the superior colliculus (mean centers of mass, ventral 38 ± 0.9% of SC mediolateral width; n=8; dorsal, 63% ± 1.94% of SC mediolateral width; n= 8; P<0.001). Axons from the dorsal or ventral retina at PND1 are more confined along the ML axis of the colliculus than along the RC axis. Examination of dorsal (Figure 5D) and ventral (Figure 5E) injections reveals that the mediolateral extent of the axon label for a dorsal or ventral injection is much less (33.0 +/−4.8% of SC width) than the rostrocaudal extent (55.8 +/−4.3% of SC length; P<0.001 for n=12 injections). Thus, axon targeting in the SC is already quite accurate at PND1 across the mediolateral axis, but much coarser across the rostrocaudal axis.
Figure 6. Segregation of DV axons in Superior Colliculus at PND1.
The day after birth (PND1), the majority of RGC axons have invaded the SC, but the future target zone of a focally labeled population of RGC axons is not yet discernible. Nonetheless, the mediolateral extent of the presumptive target zone is already defined by the mediolateral extent of the invading axons. A. Example of a superior colliculus labeled by a doubly injected retina (dorsal green, ventral red) at PND1. Overlying cortex partly obscures the rostral colliculus at the white arrow. Axons labeled after focal injection into dorsal (B) or ventral (C) retina travel in lateral or medial SC, respectively. Black arrows indicate the average center of mass of these distributions. Axons from dorsal retina are more lateral (P<0.001) than axons from ventral retina. D. Example of PND1 SC after a dorsal retinal injection illustrates that axons are quite confined in the mediaolateral plane, but stretch nearly the entire rostrocaudal extent of the SC. E. Example of a PND1 SC after a ventral injection, showing again that axons are confined mediolaterally, but extend nearly the entire rostrocoaudal length of the SC. F. Quantification of the difference between the mediolateral and rostrocaudal extent of axon label after confined dorsal or ventral injections into retina. The mediolataral spread (width at ¾ height) of axon label in the SC is much less (P<0.001) than the rostrocaudal spread. Medial is to the right, caudal is up in all panels. 500 μm scale bar in A applies to all panels.
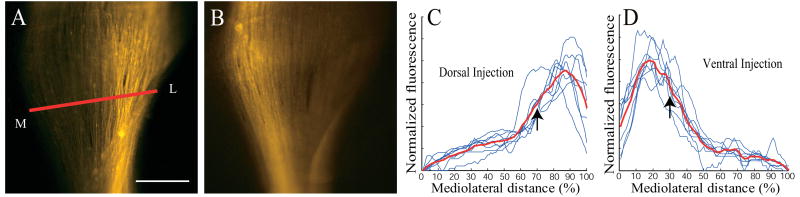
Segregation of dorsal and ventral RGC axons at PND1 in the ascending optic tract is also apparent when examining the delta of the optic tract (Figure 7). Dorsal axons (Fig. 7A, C) are strongly biased toward the lateral side of the optic tract (center of mass of label at 70 ± 1.4% of the medial-lateral extent; n=8), whereas ventral axons (Fig. 7B, D) are biased toward the medial edge of the tract (center of mass of label at 31% ± 1.0% of the mediolateral extent; n=8; P<0.001). Thus, the distribution of axons in the optic tract before they have reached a central target reflects their dorsoventral origin in the retina.
Figure 7. Segregation of DV axons in the optic tract (deltaOT) at PND1.
Axons labeled after focal injection into dorsal or ventral retina travel in lateral or medial optic tract, respectively. A. Example of axon label in the delta of the optic tract after a dorsal retinal injection. Red line indicates where quantification of fluorescent label across the optic tract is performed. Medial is left, lateral is right. B. Example of axon label in the delta of the optic tract after a ventral injection. Again medial is left, lateral is right. Note the label is shifted toward the medial side of the tract. C. Profile of the fluorescent label in the delta of the optic tract for (n=8) dorsal injections. D. Profile of the fluorescent label in the delta of the optic tract for (n=8) ventral injections. Black arrows in C and D mark the mean of the center of mass of the fluorescent label, which is much different for the dorsal (C) and ventral (D) injections (P<0.001). Scale bar is 250 μm in A and B.
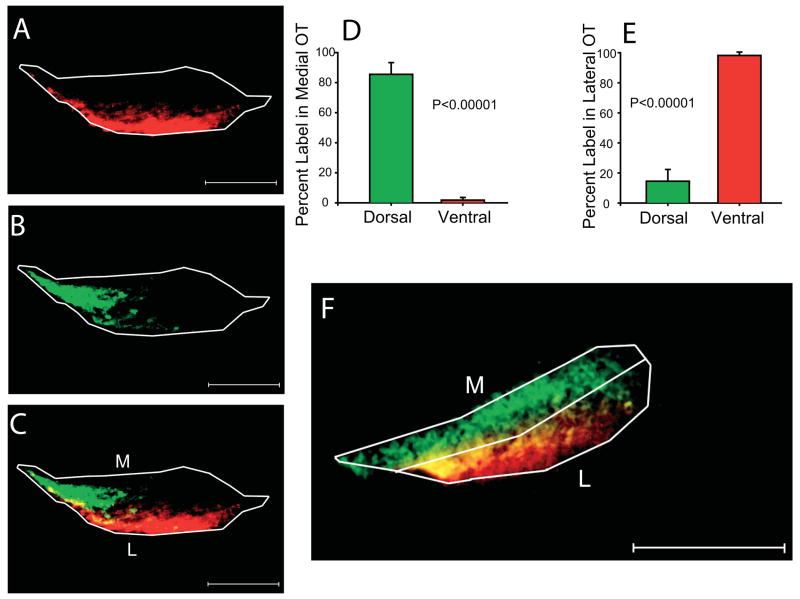
We examined whether the segregation of axons observed in the delta of the optic tract, near the LGN, is already established at a point where the axons exit the optic chiasm at the very base of the optic tract at PND1 (Fig. 8). This was more difficult to assay than at more central locations in the optic tract, as the nerve is quite compact near the chiasm, and twists as it ascends the side of the brain. We used horizontal sections, which roughly transect the nerve near the base of the brain. We also used paired injections of different fluorescent tracers (DiI and DiA) in the dorsal and ventral retina, which was more reliable because it allowed us to compare the distribution of both dorsal and ventral axons in the same optic tract. We found in these sections that axons originating from the ventral retina (Fig 8A) tended to lie in the lateral aspect of the optic tract, whereas axons from the dorsal retina (Fig. 8B) were in the medial tract. A second example of this pattern is shown in Figure 8F. On average (n=5 in all cases), 86+/−7.8% of the dorsal retinal ganglion cell label was in medial optic tract (see Methods), whereas only 1.8+/−1.8% of the ventral retinal ganglion cell label was in medial optic tract (Figure 8D; P<0.00001). In contrast, only 14.5+/−7.8% of the dorsal retinal ganglion cell label was in lateral optic tract, whereas 98+/−1.8% of the ventral retinal ganglion cell label was in lateral optic tract. Ventral retinal ganglion cell label was not restricted to either rostral or caudal tract, but there was a tendency for dorsal retinal ganglion cell label to preferentially localize to caudal tract (89+/−6%; P<0.0001). Thus, just past the chiasm, dorsal and ventral axons tend to occupy distinct zones within the ascending optic tract.
Figure 8. Position of dorsal and ventral axons in basal optic tract at PND1.
Animals received paired ventral (A, red) and dorsal (B, green) injections in one eye on the day of birth. Horizontal sections of the optic tract within 200μm of the optic chiasm reveal a spatial segregation of the two labels (A, B, overlay in C). Another example is shown in F, to illustrate the division of the tract into medial and lateral sectors for quantitative comparison. Rostral is to the right in A,B,C, and F. Quantification of the distribution of label for the top quartile of fluorescence shows a differential distribution of dorsal and ventral label in the medial (D) and lateral (E) halves of the tract, with dorsal axons running preferentially in the medial optic tract, and ventral axons in the lateral optic tract. Scale bar is 125 μm in panels A, B, C and F.
No order in the optic nerve
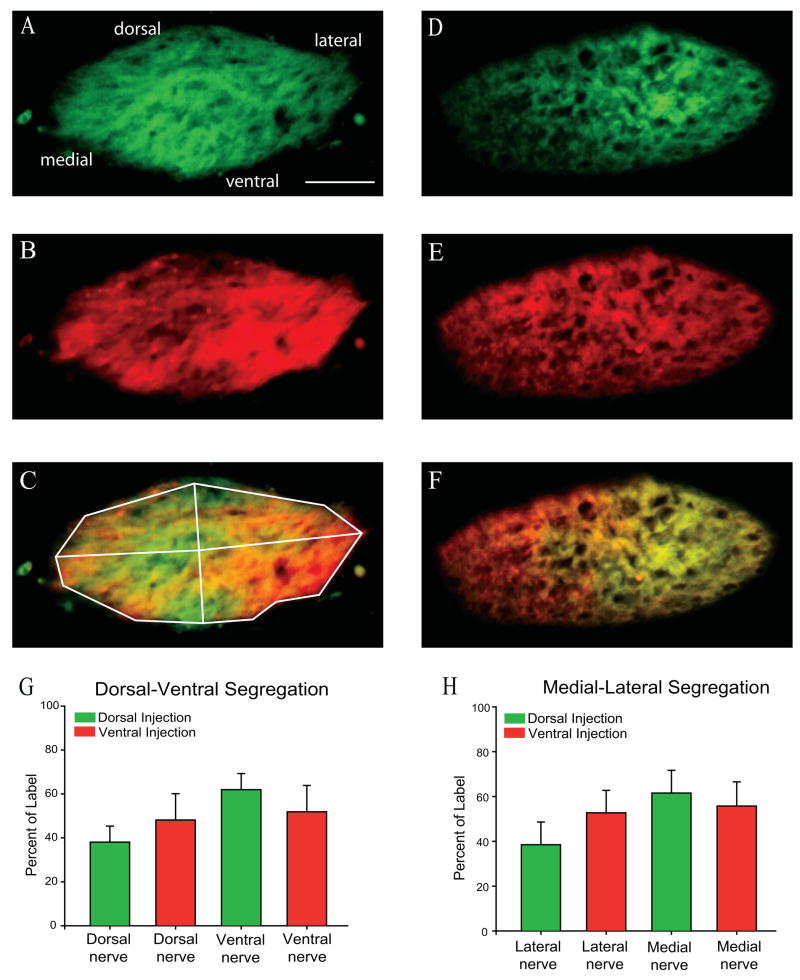
In contrast to the optic tract, axon order was not visible in the optic nerve at a point just proximal to the optic chiasm (Fig. 9). Using the same double labeling methodology and quantification as was used in the optic tract, we examined the distribution of axons from dorsal and ventral retina within the last 200 lm of the optic nerve before reaching the optic chiasm at PND1. Axons in the optic nerve near the chiasm are quite broadly distributed, without any consistent order or segregation within the nerve, and with a highly variable distribution from case to case. In the optic tract (Figure 8), segregation between medial and lateral tract was obvious by visual inspection. In contrast, there was no obvious restriction of either dorsal or ventral label to any particular region of the optic nerve. We checked for segregation of dorsal (Figure 9A; 9D) and ventral (Figure 9B; 9F) axons in dorsal nerve (19+/−7% dorsal retinal label; 24+/−6% ventral retinal label), ventral nerve (31+/−6% dorsal retinal label; 26+/−7% ventral retinal label), medial nerve (19+/−4% dorsal retinal label; 22+/−7% ventral retinal label), and lateral nerve (31+/−6% dorsal retinal label; 28+/−6% ventral retinal label) hemisections (n=5), and none was found (Figure 9G and 9H). Division of the optic nerve into dorsal and ventral or medial and lateral hemisections is arbitrary. Thus, we also examined segregation in oblique hemisections (dorsal-lateral, dorsal-medial, ventral-lateral and ventral-medial). Again, no restriction of label was found. Axon order with respect to dorsal or ventral origin is therefore absent in the optic nerve, and appears to emerge at or just after the optic chiasm.
Figure 9. Position of dorsal and ventral axons in optic nerve near the chiasm at PND1.
The same analysis of the distribution of label done for the optic tract (Figure 8) was performed on the optic nerve within 200 μm of the optic chiasm, with no consistent preference for label of dorsal (shown in green) or ventral (shown in red) axons in any sector of the nerve. Panels A, B and C show the pattern of axons in the optic tract of a PND1 mouse whose eye was injected dorsally (A, green) and ventrally (B; red) on the day of birth. C is the overlay. Panels D, E and F illustrate a similar series of data for a second animal and reveal a very different distribution of label, with no consistent pattern between animals. Whether the optic nerve is divided into dorsoventral (G) or mediolateral (H) halves, no statistically significant difference in the distribution of the labels can be distinguished. 50 μm scale bar and orientation of section (dorsal-up, ventral-down, medial-left, lateral-right) shown in A applies to all panels.
Discussion
In this report, we have demonstrated that axonal order in the optic tract, representing the dorsoventral origin of axons in the mouse retina, emerges just after the chiasm. In order to quantitatively exam this order, we developed a ‘muscle map’ of the eye, which allowed us to definitively determine the retinal origin of labeled axons in the optic nerve and tract. At the time of a ‘mature’ tectal map (PND 14), we showed that there is clear axon order or segregation in the brachium and the delta of the OT for dorsal and ventral axons but not nasal and temporal axons. We also showed that before map maturation (PND 1), when RGC axons have only just arrived at the SC and have not begun to branch, axons are already sorted in the optic tract with respect to origin along the dorsoventral axis of the retina, but not the nasotemporal axis. We showed that this order is not apparent in the optic nerve before axons enter the chiasm, but axons are segregated just after they emerge from the chiasm, demonstrating that sorting occurs at or near the chiasm. Finally, we demonstrated that the chronological development of this axonal ordering is such that it may play an important role in the development of synaptic topography in the retinofugal targets.
Axon order emerges at the optic chiasm
When retinal ganglion cell axons exit the retina and enter the optic nerve head, they are initially sorted with respect to their retinal origin, with dorsal RGC axons dorsal in the nerve head, ventral axons ventral in the nerve head (reviewed in Jeffery, 2001). Interestingly, this order is soon lost, so that by the time axons near the optic chiasm, they are diffusely spread through the nerve and show no consistent order. Our data show that order emerges again after the axons exit the chiasm. It is curious that axon order is first lost and then regained. It may be that interactions between RGC axons and factors at the chiasm are responsible for both establishing retinotopic order and executing the crossing decision. Alternatively, there may be distinct signals mediating routing and sorting. For example, axons may change the expression or localization of receptors and/or ligands when they encounter the midline (Thomas, 1998), causing the re-emergence of retinotopic order after the midline. Evidence suggests that there may be two classes of ventrotemporal RGCs whose distinct transcription factor expression profiles determine whether they cross the midline (Herrera et al., 2003; Pak et al., 2004). By analogy with crossing at the midline in the insect that is mediated by a Comm-Robo interaction (Couch and Condron, 2002), one class of these axons is sorted by a midline signal upon approaching the chiasm, whereas the second class does not experience this sorting signal because it is suppressed. Once midline crossing occurs, the suppression of axon ordering signals in the optic tract is released, and order with respect to retinal origin reemerges. Implicit in this is that ‘crossing’ signals interact with ‘ordering’ signals in the RGC axons. A specific example of how this may occur is by the regulated expression and trafficking of EphB receptors by RGC axons interacting with an ephrinB sorting signal at the chiasm. In this model, repulsion at the midline by an EphB/ephrinB interaction produces ipsilaterally projecting axons that are pushed to the lateral optic tract. Contralateral projecting axons, in contrast, would not interact with the ephrinB signal at the midline until after they have crossed, when they would again be pushed to the lateral optic tract. Such a crossing dependent expression of EphB1 has been reported in the development of mouse spinal cord (Imondi and Kaprielian, 2001; Imondi et al., 2000).
Progressive emergence of DV order in the optic tract
Due to the anatomy of the retinofugal projection at the chiasm and ascending optic tract, we were forced to use separate measures of DV order in the optic tract just after the chiasm and the delta of the optic tract, near the entry into the LGN. Nonetheless, retinotopic order is more apparent at the delta of the optic tract than at the chiasm. This feature suggests that, while the initial segregation of dorsal and ventral axons emerges at the chiasm, further mechanisms are responsible for refining this order as the axons ascend the side of the brain. The retinotopic refinement may be due to interactions between dorsal and ventral axons themselves (Birgbauer et al., 2001), or they may be due to external guidance factors that influence axon order along the ascending tract. The former possibility is attractive given the complementary expression gradient of ephrinB ligand and EphB receptor along the DV axis of the retina (Peters, 2002), and this form of progressive fiber sorting by axon-axon interactions through inhibitory Eph-ephrin signaling has been described in the invertebrate (Kaneko and Nighorn, 2003). A complementary gradient of ephrinA ligand and EphA receptor also exists along the nasotemporal (NT) axis of the retina (Hornberger et al., 1999), but it is not thought to be causative in mapping of NT axis retinal ganglion cells onto the tectum (Yates et al., 2001). If EphB receptors are not trafficked to ventral RGC axon growth cones until after they have crossed the midline (Imondi and Kaprielian, 2001; Imondi et al., 2000), axon-axon interactions among dorsal and ventral retinal ganglion cell axons may also explain why retinotopic order does not emerge until after the optic chiasm.
Mechanisms
Recent experiments have identified some of the early morphogenic signals that are responsible for the establishment of DV polarity in the retina (Koshiba-Takeuchi et al., 2000). In particular, an early high-dorsal to low-ventral gradient of BMP4 in the eye is believed to be the primary dorsalizing cue in the retina. Experimental evidence suggests that a complex cascade of transcription factors and regulators culminates in the differential expression of specific proteins along the DV axis of the retina (Peters, 2002). These include the EphB/ephrinB receptor-ligand system, so it is tempting to speculate that these are also responsible for pretarget order in the optic tract. However, a completely separate molecular system may be responsible for pretarget order, with the role of EphB/ephrinB signaling in DV axis retinotopy limited to the target itself. Though the molecular mechanisms responsible for pretarget order are as yet unknown, recently it was demonstrated in zebrafish that disruption of heparan sulfate expression disturbs pretarget order in the optic tract (Lee et al., 2004). In vitro studies have shown that many receptor-ligand signaling pathways that directly influence axon guidance are modulated by heparan sulfate expression, including Eph/ephrin, Slit/Robo, Netrin/DCC and others (Lee and Chien, 2004). Furthermore, the sorting of ipsilateral and contralaterally projecting axons at the chiasm is strongly influenced by EphB/ephrinB signaling (Nakagawa et al., 2000; Williams et al., 2003). It is thus possible that pretarget sorting and chiasm sorting share common signaling pathways.
Pretarget sorting common to all vertebrate species
We presented evidence for substantial pretarget order in the optic tract for axons along the DV axis of the retina in the mouse. Similar pretarget order has been previously observed in many species, including the rat, ferret, cat, primate, marsupials, as well as all the sub-mammalian orders for which it has been examined (Bunt and Horder, 1983). In general, pretarget order is stronger in sub-mammalian orders, and has been described as weakest in rodents (Chelvanayagam et al., 1998), though we have shown here that it is quite robust. In the human, clinical literature suggests little fiber order in the optic nerve but an increasing degree of order in the ascending optic tract (Miller and Newman, 1998). In mammals in general (rat, ferret and cat), order of axons in the optic tract is similar to that reported here, with more obvious order of axons along the dorsoventral axis of the retina, and not along the nasotemporal axis. Generally though, the results appear to be dependent on the specific method used, with anecdotal evidence of order the norm. We have adopted a quantitative methodology that is amenable both to comparison across species and between different genotypes within the same species.
Model for Establishment of Retinotopy
It is striking that pretarget order appears to be limited to the DV axis of the retina. It is also interesting to note that evidence for Sperry-like interactions via EphA/ephrinA receptor-ligand signaling in the superior colliculus for axons along the NT axis of the retina is very strong (Wilkinson, 2001), but evidence for similar interactions (perhaps via EphB/ephrinB signaling) for retinal ganglion cells along the DV axis of the retina is relatively weak (Hindges et al., 2002). One possibility is that pretarget ordering of DV axis axons is only the first in a multi-step process that establishes precise retinotopy in the superior colliculus. In this model, fibers enter the colliculus within a ‘competence zone’ relative to the final target zone. Fibers within this zone are then competent to respond to classic within-target chemoaffinity cues, enabling them to undergo selective branching to form the topographically correct target zone. The implication is that neither local, target-based chemoaffinity nor pre-target sorting is sufficient for the proper formation of the retinotectal map. Axon-target interactions may be unable to exert their influence if the initial targeting of axons in the first step is grossly incorrect, and axons outside of this initial first step ‘competence zone’ would be eliminated (Simon and O’Leary, 1992). The final step in this process is activity dependent refinement of axons via spontaneous retinal waves (Feller, 2002; Grubb et al., 2003; Chandrasekaran et al., 2005). This multi-step process may be limited to only the final two steps for NT axis mapping, as pretarget order appears to be absent for these axons. One implication of this is that if activity based refinement (correction) were eliminated, then deficits in NT refinement should be much more severe than DV refinement, which is what has been reported in the literature (Grubb et al., 2003;Chandrasekaran et al., 2005).
Is pretarget order causative for retinotopy at the target?
The existence of retinal ganglion cell axon order in the optic tract strongly suggests that molecular factors influence the development of retinotopy well before axons reach their synaptic target. It is difficult to prove that axon order in the optic tract is causative for retinotopic mapping in the target (SC or LGN), because it is difficult to specifically disrupt pretarget order and not also affect mapping mechanisms at the target. On the other hand, the converse is also true: It is difficult to prove that pretarget order is not necessary for map formation, and experiments specifically disrupting molecular interactions at the target without disrupting pretarget order have also not been done. Thus, we cannot say definitively whether pretarget order is essential for the establishment of target retinotopy, but at a minimum, its existence implies that Sperry-like interactions at the target have less ‘work’ to do.
Supplementary Material
The radial position of each red circle represents the position of each retinal focal injection. Most of the injections (34/47) were made within 500 μm of the outer edge of the retina. Several of the injections (12/47) were made between 500 and 1000 μm of the outer edge of the retina, and one injection was a little more than 1000 μm from the outer edge of the retina.
Acknowledgments
We gratefully acknowledge John Maunsell, David Sparks and the members of our lab for helpful discussions and comments on the manuscript.
This work was supported by NIH grants R01 MH62639 and T32 EY07001, as well as grants from the Klingenstein Foundation, Merck Foundation, Sloan Foundation, the AHA and NARSAD (MCC). JL is supported by Discovery Lab through NIH Grant R25 RR18595 and a grant from the A+ Challenge.
Literature Cited
- Attardi DG, Sperry RW. Preferential selection of central pathways by regenerating optic fibers. Exp Neurol. 1963;7:46–64. doi: 10.1016/0014-4886(63)90093-1. [DOI] [PubMed] [Google Scholar]
- Birgbauer E, Oster SF, Severin CG, Sretavan DW. Retinal axon growth cones respond to EphB extracellular domains as inhibitory axon guidance cues. Development. 2001;128(15):3041–3048. doi: 10.1242/dev.128.15.3041. [DOI] [PubMed] [Google Scholar]
- Bunt SM, Horder TJ. Evidence for an orderly arrangement of optic axons within the optic nerves of the major nonmammalian vertebrate classes. J Comp Neurol. 1983;213(1):94–114. doi: 10.1002/cne.902130109. [DOI] [PubMed] [Google Scholar]
- Chan SO, Guillery RW. Changes in fiber order in the optic nerve and tract of rat embryos. J Comp Neurol. 1994;344(1):20–32. doi: 10.1002/cne.903440103. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran AR, Plas DT, Gonzalez E, Crair MC. Evidence for an instructive role of retinal activity in retinotopic map refinement in the superior colliculus of the mouse. J Neurosci. 2005 doi: 10.1523/JNEUROSCI.1470-05.2005. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelvanayagam DK, Dunlop SA, Beazley LD. Axon order in the visual pathway of the quokka wallaby. J Comp Neurol. 1998;390(3):333–341. doi: 10.1002/(sici)1096-9861(19980119)390:3<333::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Couch J, Condron B. Axon guidance: Comm hither, Robo. Curr Biol. 2002;12(21):R741–742. doi: 10.1016/s0960-9822(02)01253-8. [DOI] [PubMed] [Google Scholar]
- Dunlop SA, Tee LB, Beazley LD. Topographic order of retinofugal axons in a marsupial: implications for map formation in visual nuclei. J Comp Neurol. 2000;428(1):33–44. [PubMed] [Google Scholar]
- Feller MB. The role of nAChR-mediated spontaneous retinal activity in visual system development. J Neurobiol. 2002;53(4):556–567. doi: 10.1002/neu.10140. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Rossi FM, Changeux JP, Thompson ID. Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor. Neuron. 2003;40(6):1161–1172. doi: 10.1016/s0896-6273(03)00789-x. [DOI] [PubMed] [Google Scholar]
- Herrera E, Brown L, Aruga J, Rachel RA, Dolen G, Mikoshiba K, Brown S, Mason CA. Zic2 patterns binocular vision by specifying the uncrossed retinal projection. Cell. 2003;114(5):545–557. doi: 10.1016/s0092-8674(03)00684-6. [DOI] [PubMed] [Google Scholar]
- Hindges R, McLaughlin T, Genoud N, Henkemeyer M, O’Leary DD. EphB forward signaling controls directional branch extension and arborization required for dorsal-ventral retinotopic mapping. Neuron. 2002;35(3):475–487. doi: 10.1016/s0896-6273(02)00799-7. [DOI] [PubMed] [Google Scholar]
- Hornberger MR, Dutting D, Ciossek T, Yamada T, Handwerker C, Lang S, Weth F, Huf J, Wessel R, Logan C, Tanaka H, Drescher U. Modulation of EphA receptor function by coexpressed ephrinA ligands on retinal ganglion cell axons. Neuron. 1999;22(4):731–742. doi: 10.1016/s0896-6273(00)80732-1. [DOI] [PubMed] [Google Scholar]
- Horton JC, Greenwood MM, Hubel DH. Non-retinotopic arrangement of fibres in cat optic nerve. Nature. 1979;282(5740):720–722. doi: 10.1038/282720a0. [DOI] [PubMed] [Google Scholar]
- Imondi R, Kaprielian Z. Commissural axon pathfinding on the contralateral side of the floor plate: a role for B-class ephrins in specifying the dorsoventral position of longitudinally projecting commissural axons. Development. 2001;128(23):4859–4871. doi: 10.1242/dev.128.23.4859. [DOI] [PubMed] [Google Scholar]
- Imondi R, Wideman C, Kaprielian Z. Complementary expression of transmembrane ephrins and their receptors in the mouse spinal cord: a possible role in constraining the orientation of longitudinally projecting axons. Development. 2000;127(7):1397–1410. doi: 10.1242/dev.127.7.1397. [DOI] [PubMed] [Google Scholar]
- Jeffery G. Architecture of the optic chiasm and the mechanisms that sculpt its development. Physiol Rev. 2001;81(4):1393–1414. doi: 10.1152/physrev.2001.81.4.1393. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Nighorn A. Interaxonal Eph-ephrin signaling may mediate sorting of olfactory sensory axons in Manduca sexta. J Neurosci. 2003;23(37):11523–11538. doi: 10.1523/JNEUROSCI.23-37-11523.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba-Takeuchi K, Takeuchi JK, Matsumoto K, Momose T, Uno K, Hoepker V, Ogura K, Takahashi N, Nakamura H, Yasuda K, Ogura T. Tbx5 and the retinotectum projection. Science. 2000;287(5450):134–137. doi: 10.1126/science.287.5450.134. [DOI] [PubMed] [Google Scholar]
- Lee JS, Chien CB. When sugars guide axons: insights from heparan sulphate proteoglycan mutants. Nat Rev Genet. 2004;5(12):923–935. doi: 10.1038/nrg1490. [DOI] [PubMed] [Google Scholar]
- Lee JS, von der Hardt S, Rusch MA, Stringer SE, Stickney HL, Talbot WS, Geisler R, Nusslein-Volhard C, Selleck SB, Chien CB, Roehl H. Axon Sorting in the Optic Tract Requires HSPG Synthesis by ext2 (dackel) and extl3 (boxer) Neuron. 2004;44(6):947–960. doi: 10.1016/j.neuron.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Miller NR, Newman NJ, editors. Walsh & Hoyt’s Clinical Neuro-Ophthalmology. 5. Baltimore: Williams & Wilkins; 1998. [Google Scholar]
- Naito J. Retinogeniculate projection fibers in the monkey optic nerve: a demonstration of the fiber pathways by retrograde axonal transport of WGA-HRP. J Comp Neurol. 1989;284(2):174–186. doi: 10.1002/cne.902840203. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Brennan C, Johnson KG, Shewan D, Harris WA, Holt CE. Ephrin-B regulates the Ipsilateral routing of retinal axons at the optic chiasm. Neuron. 2000;25(3):599–610. doi: 10.1016/s0896-6273(00)81063-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak W, Hindges R, Lim YS, Pfaff SL, O’Leary DD. Magnitude of binocular vision controlled by islet-2 repression of a genetic program that specifies laterality of retinal axon pathfinding. Cell. 2004;119(4):567–578. doi: 10.1016/j.cell.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Peters MA. Patterning the neural retina. Curr Opin Neurobiol. 2002;12(1):43–48. doi: 10.1016/s0959-4388(02)00288-x. [DOI] [PubMed] [Google Scholar]
- Rager U, Rager G, Kabiersch A. Transformations of the retinal topography along the visual pathway of the chicken. Anat Embryol. 1988;179(2):135–148. doi: 10.1007/BF00304695. [DOI] [PubMed] [Google Scholar]
- Reese BE, Baker GE. The re-establishment of the representation of the dorso-ventral retinal axis in the chiasmatic region of the ferret. Vis Neurosci. 1993;10(5):957–968. doi: 10.1017/s0952523800006179. [DOI] [PubMed] [Google Scholar]
- Simon DK, O’Leary DD. Relationship of retinotopic ordering of axons in the optic pathway to the formation of visual maps in central targets. J Comp Neurol. 1991;307(3):393–404. doi: 10.1002/cne.903070305. [DOI] [PubMed] [Google Scholar]
- Simon DK, O’Leary DD. Influence of position along the medial-lateral axis of the superior colliculus on the topographic targeting and survival of retinal axons. Brain Res Dev Brain Res. 1992;69(2):167–172. doi: 10.1016/0165-3806(92)90155-p. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci U S A. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry RW. Problems outstanding in the evolution of brain function. The American Museum of Natural History 1964 [Google Scholar]
- Thomas JB. Axon guidance: crossing the midline. Curr Biol. 1998;8(3):R102–104. doi: 10.1016/s0960-9822(98)70058-2. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Guillery RW, Eysel U, Polley EH, Mason CA. Studies of Retinal Representations Within the Cat’s Optic Tract. The Journal of Comparative Neurology. 1982;211:377–396. doi: 10.1002/cne.902110405. [DOI] [PubMed] [Google Scholar]
- Walsh C, Guillery RW. Age-related fiber order in the optic tract of the ferret. J Neurosci. 1985;5(11):3061–3069. doi: 10.1523/JNEUROSCI.05-11-03061.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C, Polley EH, Hickey TL, Guillery RW. Generation of cat retinal ganglion cells in relation to central pathways. Nature. 1983;302(5909):611–614. doi: 10.1038/302611a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat Rev Neurosci. 2001;2(3):155–164. doi: 10.1038/35058515. [DOI] [PubMed] [Google Scholar]
- Williams SE, Mann F, Erskine L, Sakurai T, Wei S, Rossi DJ, Gale NW, Holt CE, Mason CA, Henkemeyer M. Ephrin-B2 and EphB1 mediate retinal axon divergence at the optic chiasm. Neuron. 2003;39(6):919–935. doi: 10.1016/j.neuron.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Yates PA, Roskies AL, McLaughlin T, O’Leary DD. Topographic-specific axon branching controlled by ephrin-As is the critical event in retinotectal map development. J Neurosci. 2001;21(21):8548–8563. doi: 10.1523/JNEUROSCI.21-21-08548.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The radial position of each red circle represents the position of each retinal focal injection. Most of the injections (34/47) were made within 500 μm of the outer edge of the retina. Several of the injections (12/47) were made between 500 and 1000 μm of the outer edge of the retina, and one injection was a little more than 1000 μm from the outer edge of the retina.