Abstract
We recently reported that Alzheimer’s disease (AD) with amygdala Lewy bodies (ALB) is a distinct form of α-synucleinopathy that occurs in advanced AD. In AD/ALB the α-synuclein pathology correlated with tau pathology, but not amyloid plaques, and there was often co-localization of tau and α-synuclein in the same neuron. Given the anatomical connectivity of the anterior olfactory nucleus and the amygdala, which receives axonal projections from the olfactory bulb, we hypothesized that there might be a relationship between tau and α-synuclein pathology in the olfactory bulb and the amygdala in AD. We screened for α-synuclein pathology in the olfactory bulb in AD with and without ALB, and investigated its relationship with tau pathology. In 38 of 41 (93%) AD/ALB cases and 4 of 21 (19%) AD cases without ALB (AD/non-ALB), α-synuclein pathology was detected in the olfactory bulb. Double immunolabeling at the light and electron microscopic levels revealed co-localization of tau and α-synuclein in olfactory bulb neurons and neurites. The severity of tau pathology correlated with α-synuclein pathology in the olfactory bulb. In addition, α-synuclein pathology in the olfactory bulb correlated with α-synuclein pathology in amygdala. Tau pathology was greater in both the olfactory bulb and amygdala in AD/ALB than in AD/non-ALB, but there was no difference in tau pathology between the two groups in other brain regions assessed. The present study shows that in AD/ALB, the olfactory bulb is nearly equally vulnerable to tau and α-synuclein pathology as the amygdala and suggests that neurodegeneration in these two anatomical regions is linked.
Keywords: Alzheimer’s disease; amygdala; olfactory bulb; α-synuclein, tau
Introduction
Tau is a microtubule-associated protein that becomes abnormally phosphorylated in affected neurons of Alzheimer’s disease (AD) [11; 19] to form filamentous aggregates in neurofibrillary tangles (NFTs). Alpha-synuclein is a pre-synaptic protein that also forms filamentous inclusions as Lewy bodies (LBs) in affected neurons of Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) [26]. LBs can be found in the brain of AD, and the amygdala is the most commonly affected region [2; 13; 20]. We recently reported that AD with amygdala LBs (AD/ALB) is a distinct form of α-synucleinopathy that occurs in the setting of advanced AD [30]. In AD/ALB, LBs are relatively confined to amygdala, and the density of amygdala LBs correlates with density of amygdala NFTs, but not with senile plaques. Immunoelectron microscopy has demonstrated co-localization of tau and α-synuclein in the amygdala of AD/ALB, suggesting a close relationship between the two proteins in degenerating amygdala neurons [30].
In another neuropathologic cohort, we also reported that α-synuclein pathology is present in anterior olfactory nucleus of the olfactory bulb in all cases of Lewy body disease with concurrent NFTs [29]. Moreover, the severity of tau pathology in the olfactory bulb correlated with the density of amygdala LBs. Given the anatomical connectivity between the olfactory bulb and the amygdala, with anterior olfactory nucleus neurons projecting to the amygdala [24; 27], we hypothesized that there might be correlations between tau and α-synuclein pathology in olfactory bulb and the amygdala in AD.
To address this issue, we screened for α-synuclein pathology in the olfactory bulb of a series of cases of AD/ALB as well as AD cases without ALB and correlated these findings with quantitative measures of tau pathology. Other neuronal populations that are vulnerable to α-synuclein pathology were also screened for pathology. In a subset of cases, the changes in the olfactory bulb were further characterized by tau and α-synuclein double immunolabeling at the light and electron microscopic levels.
Materials and methods
Case selections
Cases with complete neuropathologic evaluations that also had histologic sampling of the olfactory bulb were obtained from Mayo Clinic Jacksonville brain bank. AD/ALB was defined as the presence of α-synuclein immunoreactive neuronal cytoplasmic inclusions in the amygdala with minimal or no α-synuclein pathology in other vulnerable brain regions. In some neurons the lesions resembled cortical Lewy bodies, but in other neurons the inclusions were not well defined on routine histologic stains. Nevertheless, such lesions were operationally referred to as “Lewy bodies” and fit the classification of AD/ALB. All cases met the pathological criteria for high likelihood AD according to NIA-RI criteria [14] and had Braak NFT stage [5] of V or greater. Cases were excluded if they had any other vascular pathology, hippocampal sclerosis or tauopathy (e.g., argyrophilic grain disease) [1; 9; 28]. A total of 41 cases of AD/ALB were matched to a consecutive series of 21 AD cases without ALB (AD/non-ALB) that were matched for age, sex, brain weight and Braak NFT stage [6] (Table 1).
Table 1.
Biographical and pathological data in AD/non-ALB and AD/ALB
| Pathological Dx. (n) | M:F | Mean age, years |
Mean Brain weight (g) |
Mean Braak NFT stage |
|---|---|---|---|---|
| AD/non-ALB (21) | 11:10 | 78 ± 2.4 | 1028 ± 26 | 5.7 ± 0.1 |
| AD/ALB (41) | 20:21 | 79 ± 1.4 | 1007 ± 21 | 5.6 ± 0.1 |
| P value | Ns. | Ns. | Ns. | Ns. |
Biographical and pathological data in AD/ALB and AD/non-ALB group. Dx: diagnosis, n: number, AD: Alzheimer disease, ALB: amygdala Lewy bodies, M: men, F: women, NFT: neurofibrillary tangle, Ns: not significant
Immunohistochemistry
Single immunostaining was the same as previously described with a monoclonal antibody to phospho-tau (CP13; 1:1000) [31] and a rabbit polyclonal antibody to α-synuclein (NACP; 1:3,000) [12]. The deparaffinized and rehydrated sections were pretreated with 95% formic acid for 30 minutes and then steamed in distilled water for 30 minutes for NACP; the formic acid pretreatment was omitted for CP13. Immunohistochemistry was performed with a DAKO Autostainer (DAKO, Carpinteria, CA) using 3, 3’-diaminobenzidine (DAB) as the chromogen. After immunostaining, the sections were lightly counterstained with hematoxylin.
Co-localization of α-synuclein and tau epitopes was studied using paraffin sections of the amygdala and olfactory bulb in 6 cases of AD/ALB. Deparaffinized sections were treated with hydrogen peroxide to block endogenous peroxidase activity, followed by normal serum to block nonspecific antibody binding. Sections were then incubated with a cocktail of primary antibodies: NACP (1:2,000) and mouse monoclonal IgG1 antibody to tau (PHF-1; 1:100; Dr. Peter Davies, Einstein College of Medicine, New York) [16]. They were then rinsed and incubated with a mixture of horseradish peroxidase conjugated anti-mouse IgG1 and alkaline phosphatase conjugated anti-rabbit IgG. Antibodies were visualized with DAB and then Sigma Fast™ 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (pH 9.5) as chromogens.
Evaluation of tau pathology
Thioflavin-S fluorescent microscopy was used for counting NFTs (at x400) in the cortical transitional region of the amygdala and in the association cortices, including mid-frontal, superior temporal and inferior parietal lobes [29; 30]. The olfactory bulb mainly projects to the cortical nuclei of amygdala, consistent with the region studied [24; 27]. The presence and severity of tau pathology (NFT and neuropil threads) were scored in the olfactory bulb on CP13-immunostained sections with a semi-quantitative method (0: none, 1: sparse to mild, 2: moderate, 3: severe). The scoring was performed blinded to pathologic diagnoses.
Evaluation of α-synuclein pathology
The presence and severity of α-synuclein pathology (LBs and Lewy related neurites) was scored in the olfactory bulb, amygdala, substantia nigra (SN), locus ceruleus (LC), dorsal motor nucleus of vagus (DMN), nucleus basalis of Meynert (nbM), entorhinal cortex (ERC) and anterior cingulate gyrus (CG) with a semi-quantitative method (0: none, 1: sparse to mild, 2: moderate, 3: severe). In addition to perikaryal LBs, this assessment also considered intra-axonal LBs, cortical-type LBs, pleomorphic LBs, and Lewy neurites. Scoring was performed blinded to pathologic diagnoses. Cases were assigned a Braak PD stage [6] based upon the distribution of Lewy bodies; however, many cases failed to fit the staging scheme and are so indicated in Table 2.
Table 2.
Distribution of α-synuclein pathology in AD/non-ALB and AD/ALB group
| Braak NFT | OB NFT | Braak PD | Αlpha-synuclein pathology | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OB | DMN | LC | SN | nbM | Amy | ERC | CG | ||||
| AD/non-ALB | VI | + | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | VI | + | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | V | + | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | VI | ++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | VI | ++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | VI | ++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | V-VI | ++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | VI | ++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | VI | ++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | V | ++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | V | ++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | VI | ++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | VI | +++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | VI | +++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | VI | +++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | VI | +++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | VI | +++ | 0 | − | − | − | − | − | − | − | − |
| AD/non-ALB | V | + | 1* | + | − | − | − | − | − | − | − |
| AD/non-ALB | VI | +++ | 1* | + | − | − | − | − | − | − | − |
| AD/non-ALB | VI | ++ | 1* | + | − | − | − | − | − | − | − |
| AD/non-ALB | V | +++ | 2* | + | − | + | − | − | − | − | − |
| AD/ALB | VI | ++ | 3* | − | − | − | − | − | + | − | − |
| AD/ALB | V | +++ | 3* | − | − | − | − | − | + | − | − |
| AD/ALB | V | +++ | 3* | − | − | − | − | − | + | − | − |
| AD/ALB | V | ++ | 3* | + | − | − | − | − | + | − | − |
| AD/ALB | V | ++ | 3* | + | − | − | − | − | + | − | − |
| AD/ALB | V-VI | ++ | 3* | + | − | − | − | − | + | − | − |
| AD/ALB | VI | +++ | 3* | + | − | − | − | − | + | − | − |
| AD/ALB | VI | +++ | 3* | + | − | − | − | − | + | − | − |
| AD/ALB | VI | ++ | 3* | ++ | − | − | − | − | + | − | − |
| AD/ALB | V-VI | ++ | 3* | ++ | − | − | − | − | + | − | − |
| AD/ALB | VI | +++ | 3* | ++ | − | − | − | − | + | − | − |
| AD/ALB | VI | +++ | 3* | ++ | − | − | − | − | + | − | − |
| AD/ALB | V | +++ | 3* | ++ | − | − | − | − | + | − | − |
| AD/ALB | VI | +++ | 3* | ++ | − | − | − | − | + | − | − |
| AD/ALB | V | +++ | 3* | +++ | − | − | − | − | + | − | − |
| AD/ALB | VI | +++ | 3* | +++ | − | − | − | − | + | − | − |
| AD/ALB | V | +++ | 3* | +++ | − | − | − | − | + | − | − |
| AD/ALB | VI | +++ | 3* | +++ | − | − | − | − | + | − | − |
| AD/ALB | VI | +++ | 3* | +++ | − | − | − | − | + | − | − |
| AD/ALB | VI | +++ | 3* | +++ | − | − | − | − | ++ | − | − |
| AD/ALB | VI | +++ | 3* | +++ | − | − | − | − | +++ | − | − |
| AD/ALB | VI | +++ | 3* | +++ | + | − | − | − | + | − | − |
| AD/ALB | VI | +++ | 3* | ++ | − | − | + | − | ++ | − | − |
| AD/ALB | VI | +++ | 3* | ++ | − | − | + | − | + | − | − |
| AD/ALB | VI | +++ | 3* | +++ | − | + | − | + | − | − | |
| AD/ALB | V | +++ | 3* | + | − | − | − | + | + | − | − |
| AD/ALB | VI | +++ | 3* | +++ | − | − | − | + | + | − | − |
| AD/ALB | VI | ++ | 3* | ++ | − | − | ++ | − | + | − | − |
| AD/ALB | V | +++ | 3* | ++ | − | + | + | − | + | − | − |
| AD/ALB | VI | +++ | 4* | ++ | − | − | + | + | +++ | + | − |
| AD/ALB | V | +++ | 4* | +++ | − | − | + | + | +++ | + | − |
| AD/ALB | V | +++ | 4* | +++ | + | − | + | + | ++ | + | − |
| AD/ALB | VI | +++ | 4* | + | + | − | + | + | +++ | + | − |
| AD/ALB | VI | +++ | 4* | +++ | + | − | + | + | +++ | + | − |
| AD/ALB | VI | +++ | 4* | +++ | − | + | + | + | +++ | + | − |
| AD/ALB | VI | +++ | 4* | +++ | ++ | ++ | − | + | ++ | + | |
| AD/ALB | V | ++ | 5* | + | − | − | − | − | + | + | + |
| AD/ALB | VI | +++ | 5* | ++ | − | − | − | + | ++ | + | + |
| AD/ALB | VI | +++ | 5* | ++ | − | − | − | + | ++ | + | + |
| AD/ALB | VI | +++ | 5 | ++ | + | + | + | ++ | +++ | + | + |
| AD/ALB | V | +++ | 5 | +++ | + | + | + | + | ++ | + | + |
| 100% | 68% | 11% | 10% | 21% | 21% | 66% | 19% | 8% | |||
Distribution of α-synuclein pathology in AD/non-ALB and AD/ALB groups. (−): none, (+): sparse to mild, (++): moderate, (+++): severe. OB=olfactory bulb, Amy=amygdala, DMN= dorsal motor nucleus of vagus, LC= Locus ceruleus, SN=substantia nigra, nbM=nucleus basalis of Meynert, ERC=entorhinal cortex, CG=cingulate gyrus. For Braak PD stage, * indicates imperfect fit to staging scheme, usually due to lack of Lewy bodies in one or more brainstem nuclei.
Immunoelectron microscopy (IEM)
Formalin-fixed olfactory bulbs were processed for embedding in LR White resin as previously described [30]. For double labeling, sections were incubated overnight at 4°C with NACP diluted 1:20 or with undiluted tau monoclonal antibody supernatants. After washing, they were labeled with a mixture of colloidal gold-conjugated goat antibodies against rabbit or mouse IgG (1:20 in PBS) for 30 min at room temperature. The goat anti-rabbit and anti-mouse IgG were conjugated with 5 nm and 18 nm gold particles (Amersham Biosciences; Piscataway, NJ, and Jackson Immuno Research Laboratories; West Grove, PA), respectively. After washing, the sections were briefly stained with uranyl and lead before examination with a Philips 208S electron microscope.
Statistical methods
Data were analyzed by using Sigma Stat for Windows, 3.0 (Systat Software, Inc. Point Richmond, CA), and a significance level was set at p<0.05. Demographics and pathological measures with for the two groups were analyzed with a Mann-Whitney U test or a student t-test, as appropriate. Spearman's rank order correlation was used to evaluate correlations between α-synuclein scores in the olfactory bulb with other pathological measures.
Results
Alpha-synuclein pathology in olfactory bulb
Alpha-synuclein immunoreactive pathology in the olfactory bulb was found in 4 of 21 AD/non-ALB (19%) and 38 of 41 AD/ALB (93%), respectively. The isolated α-synuclein pathology in the olfactory bulb in AD/non-ALB was predominantly sparse Lewy neurites rather than Lewy bodies, suggestive of an early stage of Lewy body pathology. The presence and distribution of severity of α-synuclein for all cases is shown in Table 2. In AD/non-ALB group, three cases had α-synuclein pathology confined to the olfactory bulb, and one case has α-synuclein pathology in both the olfactory bulb and the locus ceruleus, but not in other brain regions vulnerable to LBs. In contrast, in all but three cases of AD/ALB there was α-synuclein pathology in the olfactory bulb as well as the amygdala. In these three cases, α-synuclein pathology was sparse in the amygdala, but none was detected in the olfactory bulb. The frequency of α-synuclein pathology in olfactory bulb and amygdala was nearly the same (68% and 66%, respectively). Only 11% of all cases had α-synuclein pathology in DMN, where Lewy body pathology is proposed to begin in Parkinson’s disease (PD) according to the Braak PD staging scheme [7; 8]. More than half of AD/ALB cases had sparse α-synuclein pathology in either brainstem or limbic areas (Table 2). This pathology was not apparent with hematoxylin and eosin-stained sections as previously reported and was most often characterized by neurites and pale bodies [30].
Co-localization of tau and α-synuclein in olfactory bulb
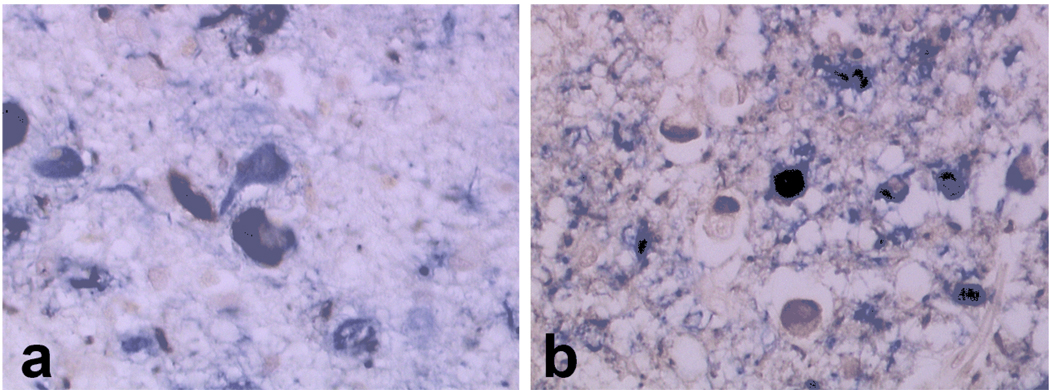
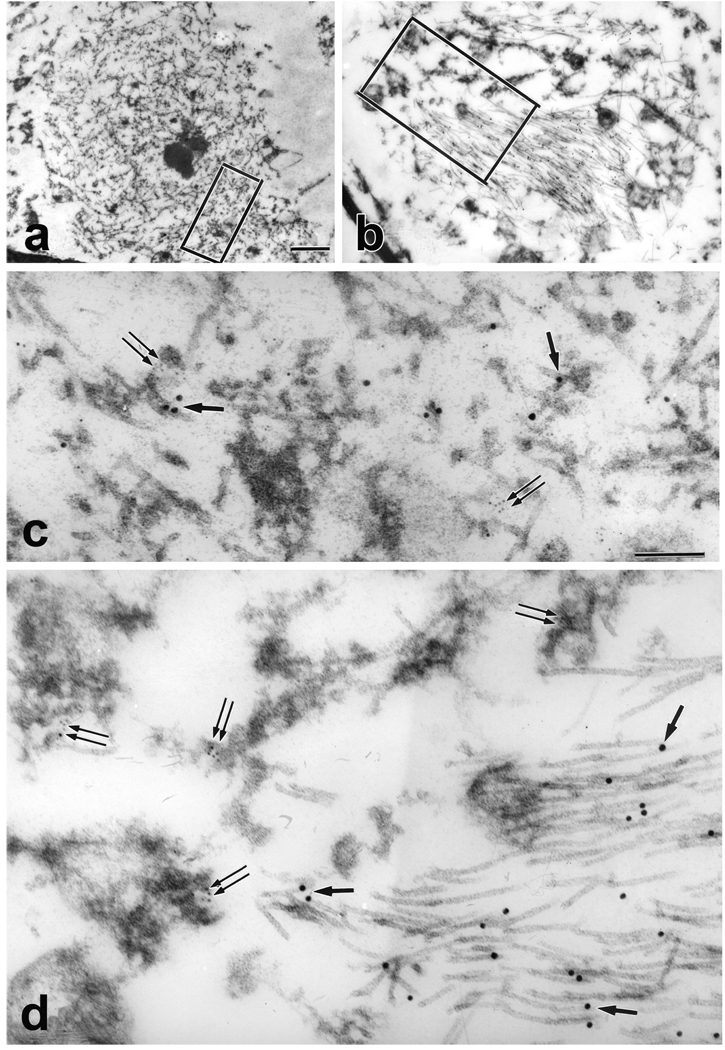
Double labeling IHC showed co-localization of tau and α-synuclein in many neurons in the amygdala (Fig. 1A) and in the olfactory bulb (Fig. 1B). The two proteins were either separate structures within the same neuron or partially overlapping. Only rarely did tau and α-synuclein immunoreactivities perfectly overlap. Ultrastructural analyses of olfactory bulb neurons showed that the tau-positive filaments were either paired helical filaments (PHF) or straight filaments (SF), while α-synuclein-positive structures were composed of granular dense material associated with filaments thinner than tau filaments (Fig. 2 and Fig. 3). This was true in both cell bodies (Fig. 2) and neurites (Fig. 3) in olfactory bulb. Most of the tau filaments formed tightly packed bundles, while α-synuclein-labeled filaments were dispersed and not bundled. Scattered tau filaments were occasionally found within α-synuclein aggregates, but α-synuclein filaments were largely excluded from the tau filament bundles. Double labeling results gave the same results when the size of the colloidal gold conjugates was reversed. The findings in olfactory bulb were similar to those in amygdala neurons [30].
Fig.1.
Immunohistochemical co-localization of tau (PHF1, blue) and α-synuclein (NACP, brown) epitopes in amygdala (A) and olfactory bulb (B). Note overlapping and non-overlapping labeled areas in cell bodies and neurites.
Fig.2.
ImmunoEM showing double labeling of tau (PHF 1, 18 nm gold particles) and α-synuclein (NACP, 5 nm gold particles) epitopes. A neuron with lipofuscin (Lf), peripherally placed nucleus (N) and a cytoplasmic inclusion composed of electron dense granular material and filaments (*) (inset; Bar = 2 µm). Higher magnification of the boxed area of the inclusion shows the junction between tau filaments (arrows) and α-synuclein-labeled granule-coated filaments (double arrows). Bar = 0.15 µm
Fig.3.
A neurite composed of disorganized filaments and granular material (A) Bar = 1 µm (for A and B). Higher magnification of boxed area in (A) shows a mixture of tau- (arrows) and α-synuclein- (double arrows) immunoreactive granulofilamentous structures. A neurite with electron dense granular material and bundles of filaments (B) Higher magnification of boxed area in (B) shows tau-immunoreactive SFs (arrows) and α-synuclein-labeled filaments with electron dense granular material (double arrows). Bar = 0.15 µm (for C and D).
Comparison with the severity of tau pathology in olfactory bulb
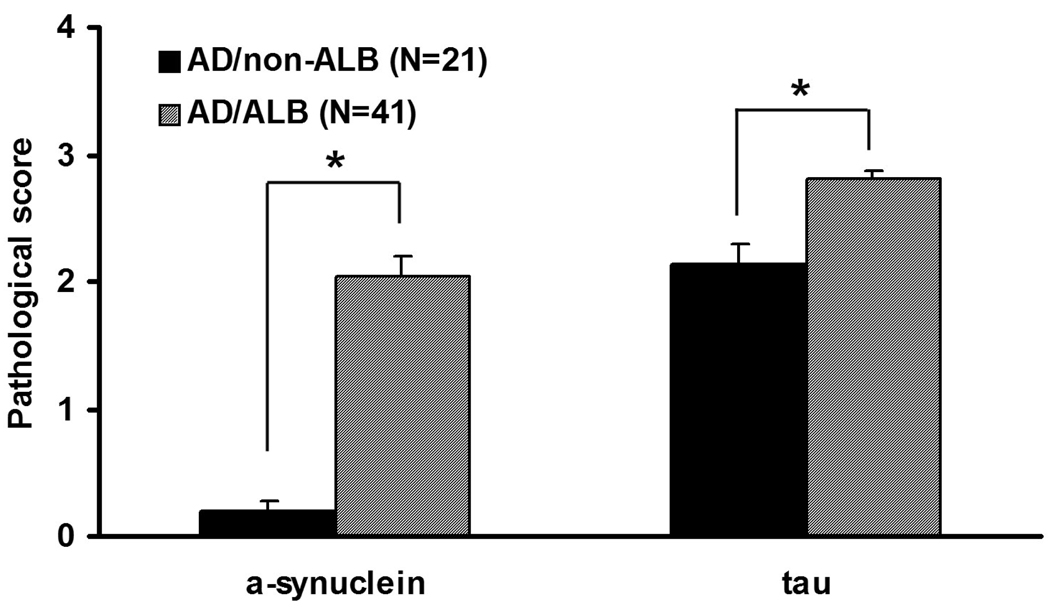
The density of NFTs in the cortical transitional zone of the amygdala were significantly greater in AD/ALB compared to AD/non-ALB (p<0.05) (Fig. 4), which is similar to our previous report [30]. In contrast, the average NFT density in association cortices and the Braak NFT stage were not different between two groups (Fig. 4). All cases had CP13 immunoreactive tau pathology in the olfactory bulb. Semi-quantitative scores for tau pathology and α-synuclein pathology in the olfactory bulb were both greater in AD/ALB than in AD/non-ALB (p<0.001) (Fig. 5).
Fig.4.
Comparison of NFT counts in amygdala and association cortices between AD with and without amygdala Lewy bodies (ALB). The NFT count in amygdala was significantly higher in AD with ALB (AD/ALB) than those in AD without ALB (AD/non-ALB) (*p<0.05), while there was no difference in NFT count in association cortices AD/ALB and AD/non-ALB. MF: mid frontal cortex, ST: superior temporal cortex, IP: inferior parietal cortex.
Fig.5.
Comparison of mean α-synuclein and tau score in olfactory bulb between AD with and without amygdala Lewy bodies (ALB). Alpha-synuclein and tau scores in olfactory bulb are greater in AD/ALB than those of AD/non-ALB (*p<0.001).
Correlation between α-synuclein pathology in olfactory bulb and other pathological measures
The relationship between the semi-quantitative score for α-synuclein pathology in olfactory bulb was correlated with other pathologic variables in various brain regions with Spearman rank order correlation. The α-synuclein score in olfactory bulb correlated with the tau score in olfactory bulb (r=0.44, p<0.01), but not with density of NFT in the amygdala or association cortices. The α-synuclein score in olfactory bulb also correlated with the α-synuclein score in amygdala (r=0.33, p<0.05), but not with α-synuclein score in brainstem nuclei or limbic cortices.
Discussion
In this study, most AD/ALB cases had α-synuclein pathology in the olfactory bulb, while the majority of AD/non-ALB cases did not, suggesting a close relationship of α-synuclein pathology between olfactory bulb and amygdala in AD/ALB. This is the first study to examine α-synuclein pathology in both olfactory bulb and amygdala in a relatively large number of AD cases. The results suggest that the olfactory bulb, in addition to the amygdala, is vulnerable to α-synuclein pathology in AD. Braak and co-workers hypothesized that Lewy body pathology begins in the DMN and olfactory bulb in Parkinson’s disease (PD) [7]. Bloch and co-workers examined incidental Lewy body disease, which is regarded as a presymptomatic phase of PD, and found that 100% of cases had α-synuclein pathology in the olfactory bulb and DMN, while 69% also had involvement of the amygdala, consistent with PD Braak scheme [4]. In the current study, the distribution of α-synuclein pathology in AD/ALB brains does not fit with the Braak PD staging scheme [7], and is consistent with other studies of α-synuclein pathology in AD [5; 17; 21; 22; 23; 30]. We found isolated α-synuclein pathology in either the olfactory bulb or amygdala in a few cases, but in most cases, both regions were affected. When only one region was affected, the pathology was mostly neuritic and sparse, suggesting that it was an early pathological process. The results suggest when α-synuclein pathology occurs in the setting of advanced AD, the olfactory bulb and amygdala are affected simultaneously, and not directly linked to pathology in vulnerable nuclei at lower levels of the neuraxis. When the process widens, the limbic lobe appears to have equal or greater vulnerability to brainstem nuclei affected in PD.
The relationship between tau and α-synuclein in the same neuron is similar in the amygdala and olfactory bulb at the light and electron microscopic levels. In the present study, tau and α-synuclein filaments were detected not only in neuronal perikarya, but also in neurites. The major finding is that the lesions are characterized by two populations of filaments, with no evidence supporting copolymers formed tau and α-synuclein molecules, despite the in vitro evidence that tau and α-synuclein are able to reciprocally seed filament formation [10]. While tau filaments can occasionally be intermingled with α-synuclein filaments, the reverse is not true, especially for tightly backed bundles of tau filaments; in most cases, they are separate or juxtaposed aggregates. This pattern of co-localization of tau and α-synuclein is similar to those reported in hippocampal and brainstem neurons in LBD [3; 16].
The tau filaments were composed of both SFs and PHFs. In contrast, most NFTs in AD are composed of PHFs. Whether the increased frequency of SFs is due to α-synuclein remains unknown. In vitro studies showed α-synuclein could bind to tau and stimulate tau phosphorylation by protein kinase A [18]. On the other hand, Souza and co-workers showed α-synuclein inhibited aggregation of insulin, suggesting a negative chaperone-like activity [25]. Similarly, the ill-defined LBs could be due to the presence of abnormal tau hindering formation of well-defined LBs. In another immunoelectron microscopic study, Iseki et al. identified non-filamentous components in LBs which may be an early stage of LB formation [15].
We showed brains of AD/ALB cases had more severe tau pathology in amygdala and olfactory bulb than brains of AD/non-ALB cases. In contrast, cortical NFT burden was similar as assessed by average NFT counts in association cortices and the Braak NFT stage. This difference is not related to any obvious demographic variable, as cases were matched (Table 1). The findings suggest that a subset of AD may have disproportionately severe limbic system neurofibrillary degeneration that makes them uniquely vulnerable to α-synuclein pathology.
Acknowledgment
Supported by NIH grants P50-AG16574, P01-AG-17216, P50-NS40256 and R01-AG15866
References
- 1.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai Y, Yamazaki M, Mori O, Muramatsu H, Asano G, Katayama Y. Alpha-synuclein-positive structures in cases with sporadic Alzheimer's disease: morphology and its relationship to tau aggregation. Brain Res. 2001;888:287–296. doi: 10.1016/s0006-8993(00)03082-1. [DOI] [PubMed] [Google Scholar]
- 3.Arima K, Mizutani T, Alim MA, Tonozuka-Uehara H, Izumiyama Y, Hirai S, Ueda K. NACP/alpha-synuclein and tau constitute two distinctive subsets of filaments in the same neuronal inclusions in brains from a family of parkinsonism and dementia with Lewy bodies: double-immunolabeling fluorescence and electron microscopic studies. Acta Neuropathol (Berl) 2000;100:115–121. doi: 10.1007/s004010050002. [DOI] [PubMed] [Google Scholar]
- 4.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered. Mov Disord. 2006;21:2042–2051. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 8.Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 9.Dickson DW. Tau and synuclein and their role in neuropathology. Brain Pathol. 1999;9:657–661. doi: 10.1111/j.1750-3639.1999.tb00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, Trojanowski JQ, Lee VM. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300:636–640. doi: 10.1126/science.1082324. [DOI] [PubMed] [Google Scholar]
- 11.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwinn-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen SH, Hardy J, Dickson DW. Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol (Berl) 2000;99:663–672. doi: 10.1007/s004010051177. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton RL. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Iseki E, Marui W, Kosaka K, Ueda K. Frequent coexistence of Lewy bodies and neurofibrillary tangles in the same neurons of patients with diffuse Lewy body disease. Neurosci Lett. 1999;265:9–12. doi: 10.1016/s0304-3940(99)00178-0. [DOI] [PubMed] [Google Scholar]
- 16.Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- 17.Jellinger KA. Alpha-synuclein pathology in Parkinson's and Alzheimer's disease brain: incidence and topographic distribution--a pilot study. Acta Neuropathol (Berl) 2003;106:191–201. doi: 10.1007/s00401-003-0725-y. [DOI] [PubMed] [Google Scholar]
- 18.Jensen PH, Hager H, Nielsen MS, Hojrup P, Gliemann J, Jakes R. alpha-synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. J Biol Chem. 1999;274:25481–25489. doi: 10.1074/jbc.274.36.25481. [DOI] [PubMed] [Google Scholar]
- 19.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci U S A. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ. Antibodies to alpha-synuclein detect Lewy bodies in many Down's syndrome brains with Alzheimer's disease. Ann Neurol. 1999;45:353–357. doi: 10.1002/1531-8249(199903)45:3<353::aid-ana11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Parkkinen L, Pirttila T, Alafuzoff I. Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol. 2008;115:399–407. doi: 10.1007/s00401-008-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkkinen L, Soininen H, Alafuzoff I. Regional distribution of alpha-synuclein pathology in unimpaired aging and Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:363–367. doi: 10.1093/jnen/62.4.363. [DOI] [PubMed] [Google Scholar]
- 23.Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M, Arai T, Nagura H, Yamanouchi H, Hasegawa M, et al. Accumulation of phosphorylated alpha-synuclein in aging human brain. J Neuropathol Exp Neurol. 2003;62:644–654. doi: 10.1093/jnen/62.6.644. [DOI] [PubMed] [Google Scholar]
- 24.Sims KS, Williams RS. The human amygdaloid complex: a cytologic and histochemical atlas using Nissl, myelin, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate diaphorase staining. Neuroscience. 1990;36:449–472. doi: 10.1016/0306-4522(90)90440-f. [DOI] [PubMed] [Google Scholar]
- 25.Souza JM, Giasson BI, Lee VM, Ischiropoulos H. Chaperone-like activity of synucleins. FEBS Lett. 2000;474:116–119. doi: 10.1016/s0014-5793(00)01563-5. [DOI] [PubMed] [Google Scholar]
- 26.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 27.Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 28.Togo T, Cookson N, Dickson DW. Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol. 2002;12:45–52. doi: 10.1111/j.1750-3639.2002.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuboi Y, Wszolek ZK, Graff-Radford NR, Cookson N, Dickson DW. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein epsilon4. Neuropathol Appl Neurobiol. 2003;29:503–510. doi: 10.1046/j.1365-2990.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- 30.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–697. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver CL, Espinoza M, Kress Y, Davies P. Conformational change as one of the earliest alterations of tau in Alzheimer's disease. Neurobiol Aging. 2000;21:719–727. doi: 10.1016/s0197-4580(00)00157-3. [DOI] [PubMed] [Google Scholar]