Abstract
Certain anesthetics exhibit neurotoxicity in the brains of immature but not mature animals. Gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the adult brain, is excitatory on immature neurons via its action at the GABAA receptor, due to a reversed transmembrane chloride gradient. GABAA receptor activation in immature neurons is sufficient to open L-type voltage gated calcium channels. As propofol is a GABAA agonist, we hypothesized that it and more specific GABAA modulators would increase intracellular free calcium ([Ca2+]i), resulting in the death of neonatal rat hippocampal neurons. Neuronal [Ca2+]i was monitored using Fura2-AM fluorescence imaging. Cell death was assessed by double-staining with propidium iodide and Hoechst 33258 at 1 h (acute) and 48 h (delayed) after 5 h exposure of neurons to propofol or the GABAA receptor agonist, muscimol, in the presence and absence of the GABA receptor antagonist, bicuculline, or the L-type Ca2+ channel blocker, nifedipine. Fluorescent measurements of caspase-3,-7 activities were performed at 1 h after exposure. Both muscimol and propofol induced a rapid increase in [Ca2+]i in day in vitro (DIV) 4, but not in DIV 8 neurons, that was inhibited by nifedipine and bicuculline. Caspase-3,-7 activities and cell death increased significantly in DIV 4 but not DIV 8 hippocampal neuronal cultures 1 h after a 5 h exposure to propofol, but not muscimol, and were inhibited by the presence of bicuculline or nifedipine. We conclude that an increase in [Ca2+]i, due to activation of GABAA receptors and opening of L-type calcium channels, is necessary for propofol-induced death of immature rat hippocampal neurons but that additional mechanisms not elicited by GABAA activation alone also contribute to cell death.
Keywords: calcium, apoptosis, anesthetic, caspase
Introduction
The immature brain differs from the adult brain in its enhanced neuronal excitability. In the adult rat brain, the neurotransmitter γ-amino butyric acid (GABA), acting at the GABAA receptor, induces chloride influx resulting in membrane hyperpolarization and inhibition of neuronal excitability. In contrast, in the immature brain, activation of the GABAA receptor results in chloride efflux and membrane depolarization due to a reversed chloride gradient compared to the adult 1,2. The resultant membrane depolarization activates voltage dependent calcium channels, in particular the L-type, increasing intracellular Ca2+ levels. The elevated intracellular Ca2+, due to spontaneous depolarizing GABAergic as well as glutamatergic activity, is required for both neuronal maturation and synaptogenesis during brain growth maturation. In the rat pup, the critical stage for the brain growth takes place at an early postnatal period, associated with neuronal depolarization by GABA during the first postnatal week. For the human infant, the critical time when the Cl− transport is as immature as in the rat spans the third trimester of pregnancy into the first 6 months after birth3. Disturbance of Ca2+ homeostasis during brain maturation, either by pharmacological blockade of glutamatergic neurotransmission or the activation of GABAA receptors, leads to neurodegeneration in the developing brain 4–9. Recent data from Jevtovic-Todorovic et al. 10–13 revealed that general anesthetics modulating both GABAergic and glutamergic neurotransmission (isoflurane, alone or in combination with midazolam and nitrous oxide, and ketamine) trigger widespread apoptotic neurodegeneration in many regions of developing rat brain. In addition, Warner et al. demonstrated that isoflurane induces a time- and maturity-dependent neuronal degeneration in vitro 14. However, the relative contribution of inhibition of glutamatergic neurotransmission or activation of depolarizing GABAergic neurotransmission to the anesthetic-induced neurotoxicity is unclear.
Propofol (2, 6-diisopropyl phenol), an intravenous hypnotic-sedative agent that selectively modulates GABAA receptor function by interacting with beta subunits, is widely used in pediatric anesthesia and intensive care practice. To reveal the GABAergic mechanism of anesthetic-induced neurotoxicity in the developing brain, we studied comparatively the effect of propofol and GABAA modulators on cytoplasmic free calcium ([Ca2+]i) and acute/delayed death of rat hippocampal neurons before and after the conversion of GABA receptor activity from depolarizing to hyperpolarizing.
Material and Methods
Materials
All cell culture reagents were from GIBCO-BRL, except B-27 supplement (Invitrogen, Carlsbad, CA, USA). Pure propofol was purchased from Alexis Biochemicals (San Diego, CA, USA) and freshly diluted in artificial cerebrospinal fluid (aCSF). Fura-2 AM was obtained from Molecular Probes Eugene (Eugene, OR, USA). Unless otherwise stated, all other chemicals were obtained from Sigma-Aldrich Inc. (St. Louis, MO, USA).
Cell preparation
Subjects were first-generation descendants of Sprague–Dawley albino rats from Charles River Laboratory (Wilmington, MA, USA). Female rats were bred in the University of Maryland School of Medicine animal colony. Animals were housed under a 12 h light/dark cycle with unlimited access to food and water. All animal procedures were carried out according to the University of Maryland, Baltimore IACUC and adhered to the NIH guidelines for the care and use of laboratory animals. The cages of pregnant females were checked daily for the presence of pups and the day of birth was designated as PND0. Hippocampi from PND0 male pups were used for primary cultures of hippocampal neurons. Pups are easily sexed at birth by examination of the external genitalia, providing a 99% degree of accuracy in correctly identifying the sex. Hippocampi were dissected into HBSS+ (88 ml sterile H2O, 10 ml Hanks' balanced salt solution (Ca2+- and Mg2+-free) 10×, 1 ml HEPES buffer, 1.0 M, pH 7.3, 1 ml Antibiotic/Antimycotic 100× liquid), then additional HBSS+ was added to the tube to a volume of 4.5 ml, with 0.5 ml trypsin (2.5%), and incubated in a 37 °C water bath for 15 min. The supernatant was discarded and the tissue washed with HBSS+. This procedure was repeated a second time. Cells were dissociated by trituration, with cell number and viability determined by trypan blue exclusion. Cells were plated on 25-mm poly-l-lysine (0.1 mg/ml, Sigma, St. Louis, MO, USA)-coated coverslips at a density of 30,000 cells per coverslip, and placed in 60-mm dishes containing 4 ml plating medium [86 ml MEM, 10 ml horse serum, 3 ml glucose (filter sterilized, 20%), 1 ml of 100 mM pyruvic acid]. Cells were allowed 4 h to adhere to the coverslips in a 37°C, 5% CO2 incubator. The coverslips were removed from the plating dishes and placed into 60-mm dishes filled with 3 ml neurobasal media, made by adding 1 ml B-27 supplement with 1 ml Antibiotic/Antimycotic 100×, with 125 µl l-glutamine and filled to 50 ml with neurobasal (phenol red free). One third of the neurobasal media was removed every other day and replaced with fresh media. Cells were used at 4 or 8 days in vitro (DIV).
Fluorescence microscopy
Intracellular free Ca2+ was monitored in hippocampal neurons by epifluorescence microscopy. Neurons were loaded with 2 µM concentration of Fura-2 AM (Molecular Probes, Eugene, OR) for 30 min followed by a 15 min dye de-esterification period at 37°C. The coverslips were mounted in the chamber of a Nikon Eclipse TE2000-S inverted microscope (SFluor 20 × 0.75 N.A.) in aCSF containing 120 mM NaCl, 3.5 mM KCl, 1.3 mM CaCl2, 0.4 mM KH2PO4, 1 mM MgCl2, 5 mM NaHCO3, 10 mM HEPES (pH 7.4), and 15 mM glucose. Single cell fluorescence of Fura-2 AM was imaged by alternate excitation at 340 and 380 nm (Polychrome IV, Till, Munich, Germany), and measurement of emission at 510 nm. Image sequences (10 sec/frame, 50 msec exposure time, 2×2 binning) were acquired by an ORCA-ER cooled digital CCD camera (Hamamatsu Photonics, Hamamatsu, Germany) and imaged with Metafluor 6.3 (Universal Imaging, West Chester, PA) imaging software.
All experiments were performed at 37°C. In-line heating (Harvard Apparatus, Inc., Holliston, MA, USA) of perfusate flow, solution reservoir heating by separate syringe warmers (Harvard Apparatus, Inc., Holliston, MA, USA), and chamber platform heating were all used in combination to provide efficient thermal regulation.
Cell Death and Caspase-3 and-7 staining
Cell death was assessed at either 1 h (acute) or 48 h (delayed) after 5 h of treatment with propofol and other GABAA modulators. Propidium Iodide (PI, 50 µM) (Sigma-Aldrich, St Louis, MO, USA) was used to label plasma membrane-permeable cells and Hoechst 33258 (40 µM) (Sigma-Aldrich, St Louis, MO, USA) to label all cell nuclei. Both PI-positive cells and fragmented/condensed nuclei were considered dead cells. Dead cells were scored by counting three random fields per slide. Green fluorescent inhibitor of caspases (FLICA) reagent (Molecular Probes, Eugene, OR, USA) was used 1 h after the 5 h treatments to detect caspase-3,-7 activation.
Data Analysis
All data are expressed as means ± S.E.M. of n = 3 – 9 coverslips. Statistical significance was assessed by one-way ANOVA with Dunnett post hoc test. Data with a heterogeneous variance were log transformed. For data that were not normally distributed, Kruskal-Wallis non-parametric ANOVA with Dunn’s post hoc test for multiple comparisons of unbalanced data was used. p < 0.05 was considered to be statistically significant.
Results
Muscimol and propofol elevate intracellular calcium in immature (DIV 4) hippocampal neurons
Changes in [Ca2+]i elicited by the GABAA agonist muscimol and propofol were measured in the absence and presence of the GABAA antagonist bicuculline using rat hippocampal neurons at both 4 and 8 DIV. Baseline [Ca2+]i level of DIV 4 and DIV 8 hippocampal neurons was the same. Exposure of neurons to muscimol (10 µM), induced a transient peak in [Ca2+]i that remained elevated in DIV 4 neurons at the end of the 10 min exposure period (ANOVA F = [2,8] 48.3, p < 0.001; Fig. 1 A). Perfusion of DIV 8 neurons with muscimol for 10 min did not cause a significant elevation in [Ca2+]i, and therefore remained significantly less than those observed with DIV 4 neurons (p < 0.001; Fig. 1 B,D). The increase in [Ca2+]i elicited by muscimol in DIV 4 neurons was significantly reduced by concurrent treatment with the L-type voltage-gated Ca2+ channel inhibitor, nifedipine, 10 µM (p < 0.001; Fig. 1 C,D). Perfusion of DIV 4 neurons with 5 µM propofol also caused a significant transient increase in [Ca2+]i (F = [3,11] 65.7, p < 0.001) to a level that was not different than that observed upon exposure to muscimol (Fig. 2A). In contrast to DIV 4 neurons, propofol infusion did not lead to a significant change in [Ca2+]i in DIV 8 neurons, resulting in fluorescent ratios that were significantly lower than those obtained during perfusion of DIV 4 neurons (p < 0.001, Fig. 2 B,E). In DIV 4 cultures, simultaneous application of nifedipine with propofol perfusion inhibited the propofol-induced rise in [Ca2+]i (p < 0.001, Fig. 2 D,E). Perfusion of DIV 4 neurons with the GABAA antagonist bicuculline (10 µM) completely abolished the propofol-induced initial rise in [Ca2+]i (p < 0.001, Fig. 2 C,E).
Fig. 1. GABAA receptor activation increases intracellular free Ca2+ in immature hippocampal neurons.
Fura-2 AM fluorescence (ratio at 340/380 nm excitation) measured in hippocampal neurons of different age in culture, DIV 4 (A) vs DIV 8 (B) induced by 10 µM muscimol applied for 10 min. (Each trace represents fluorescence monitored in a single neuron somata in the field). The muscimol-induced rise in the fluorescence ratio for DIV 4 hippocampal neuronal cultures was suppressed by the presence of nifedipine (10 µM) in the perfusate during muscimol perfusion (C). Values shown in (D) represent the means ± SEM for 3–4 independent experiments where fluorescence was quantified 40 sec after addition of muscimol using a total of 66 – 85 cells per group. * p < 0.001 when compared to control, n = 3 – 4 per group).
Fig. 2. Propofol increases intracellular free Ca2+ in immature hippocampal neurons.
Fura-2 AM fluorescence ratio in hippocampal neurons of different ages in culture, DIV 4 (A) vs DIV 8 (B) induced by 5 µM propofol applied for 10 min. The presence of 10 µM bicuculline (C) and 10 µM nifedipine (D) in the perfusate inhibited the propofol-induced increase in Fura-2 fluorescence ratio in DIV 4 hippocampal neuronal cultures. Values shown in (D) represent the means ± SEM for 3–4 independent experiments where fluorescence was quantified 30 sec after the addition of propofol using a total of 58 – 73 cells per group. * p < 0.001 when compared to control, n = 3 – 4 per group.
In vitro age-dependent propofol-induced neuronal death
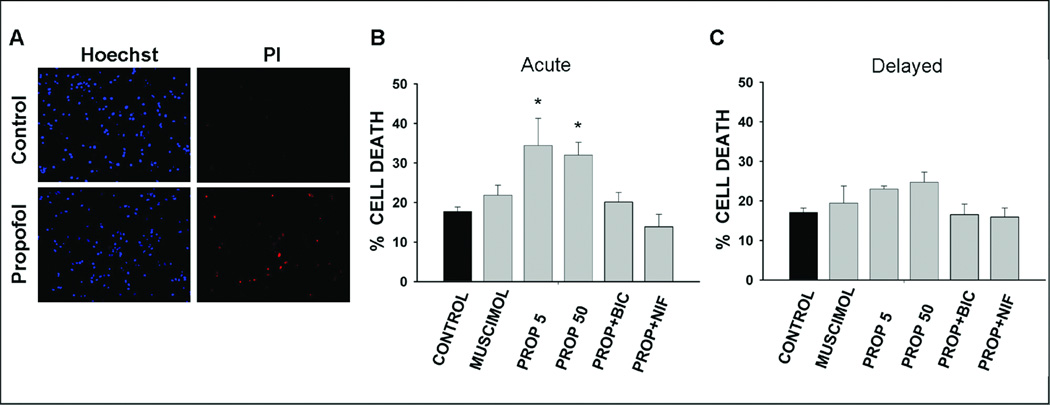
Treatment of DIV4 hippocampal neuronal cultures for 5 h with propofol induced a rapid and dose-independent increase in percentage of dead cells at 1 h post-treatment (Fig. 3 A,C). There was 94.4 % more cell death in cultures treated with propofol 5 µM and 80.8 % more in cultures treated with 50 µM propofol compared to untreated controls (K-W p = 0.004). However, the extent of the cell death induced by muscimol 10 µM was not significantly different than controls (23.4 % more than control; Fig.3 A). Simultaneous incubation of bicuculline 10 µM or nifedipine 10 µM with propofol prevented the propofol-induced increase in acute cell death (Fig. 3 A). The increase in delayed cell death in DIV 4 neuronal cultures at 48 h after treatment with propofol 5 and 50 µM was not significant (K-W p = 0.05; Fig. 3 B). Muscimol treatment for 5 h did not cause an increase in delayed cell death. Neither propofol nor muscimol induced acute or delayed cell death in DIV 8 hippocampal neuronal cultures (Fig. 4 A, B). Concurrent treatment of DIV 8 neuronal cultures with propofol and bicuculline or nifedipine affected neither acute nor delayed cell death percentage.
Fig. 3. Acute and delayed death of hippocampal neurons (4 days in vitro) following exposure to propofol and other GABAA receptor modulators.
Neurons were stained with propidium iodide (PI) and Hoechst at 1 h (Acute), and 48 h (delayed) after treatment for 5 h. Sample images of Hoechst and PI staining in untreated (Control) and propofol-treated DIV 4 hippocampal neuronal cultures (A). The acute (B) and delayed (C) cell death percentage in neuronal cultures of untreated (control) and treated with muscimol, 10 µM (MUSCIMOL), propofol, 5 µM (PROP 5), propofol, 50 µM (PROP 50), combined propofol 50 µM and bicuculline 10 µM (PROP+BIC) and, combined propofol ,50 µM and nifedipine, 10 µM (PROP+NIF) for 5 h. * p = 0.004 when compared to control. n = 3 – 9.
Fig. 4. Acute and delayed death of hippocampal neurons (8 days in vitro) following exposure to propofol and other GABAA receptor modulators.
Cell death in DIV8 hippocampal neuronal cultures treated with the artificial CSF vehicle (control), muscimol, 10 µM (MUSCIMOL), propofol, 5 µM (PROP 5), propofol, 50 µM (PROP 50), combined propofol 50 µM and bicuculline 10 µM (PROP+BIC) and, combined propofol, 50 µM, and nifedipine, 10 µM, (PROP+NIF) for 5 h. Neurons were stained with propidium iodide (PI) and Hoechst at 1 h (A), and 48 h (B) after treatment for 5 h. n = 4 – 6
Propofol induced caspase-3,-7 activation in DIV4 but not in DIV8 hippocampal neurons
Baseline level of caspase-3,-7-positive cells was 10.4 ± 2.2 % in untreated DIV 4 cultures and 6.5 ± 3.6 % in DIV8 untreated cultures. In propofol-treated DIV 4 cultures the percentage of caspase-3,-7-positive cells increased 110.6 % at 1 h post-treatment, when compared to untreated control cultures (21.9 ± 2.3 % vs 10.4 ± 2.2 %, F = [5,21] 3.39, p = 0.028; Fig. 5). Treatment of DIV 8 hippocampal cultures for 5 h with propofol had no effect on caspase-3 and-7 activities, nor did muscimol treatment change the percentage of caspase-3,-7 positive cells in cultures of either age.
Fig. 5. Caspase-3,-7 activity in hippocampal neurons following exposure to propofol and muscimol.
Sample images of Hoechst and green caspase-3,-7 stained DIV 4 neurons (A). Mean percentage of caspase-3,-7 positive cells in DIV 4 and DIV 8 cultures at 1 h after treatment with the artificial CSF vehicle (control), muscimol, 10 µM, and propofol, 5 µM, for 5 h (B). * p = 0.028 when compared to control group. n = 3 – 5
Discussion
The most important observation of this study is that a 5 h exposure to propofol, at a pharmacologically relevant dose of 5 µM, stimulates caspase activation and promotes acute death of neurons at an in vitro developmental age (DIV 4) when the propofol-sensitive GABAA receptor elicits cellular depolarization. GABAA-dependent propofol-induced depolarization was verified by detection of bicuculline-, and nifedipine inhibitable elevation of neuronal [Ca2+]i. Parallel inhibition of propofol toxicity by both bicuculline and nifedipine provides evidence that the rise in [Ca2+] is necessary to induce death. The rise in [Ca2+]i does not, however, appear to be a sufficient explanation for propofol toxicity as a comparable elevation generated upon exposure of DIV4 neurons to the GABAA agonist, muscimol, did not result in significant death. One limitation of this study is that changes in [Ca2+]i were measured during a 10 min exposure to propofol or muscimol and for 10 min thereafter whereas caspase activation and cell death were measured 1 hr after 5 hr of continuous exposure to these GABAergic agents. It is therefore possible that a delayed Ca2+ deregulation occurs in response to the early rise in [Ca2+]i, such as what is well established for glutamate excitotoxicity 15, and that this event is more robust with propofol than with muscimol.
Other known effects of propofol on cell physiology could contribute to the neuronal toxicity observed in our experiments. Clinically relevant concentrations of propofol induce a calcium-dependent change in the actin-cytoskeletal organization of neurons and glial cells 16,17, that is inhibited by bicuculline and the Ca2+ channel blocker, verapamil 17,18. Whereas propofol stimulates tyrosine phosphorylation of actin, this effect is not observed with muscimol, possibly due to the different GABA receptor β subunits with which these agents interact 19. Since cell death can be induced by alterations in actin dynamics 20, the differential sensitivity of actin to propofol and muscimol might explain the selective propofol-effects on viability observed in our study. Another effect of propofol that could promote Ca2+-dependent neuronal death is inhibition of respiration, as reported for macrophages 21, hepatocytes 22–24, cardiomyocytes 25,26, and brain synaptosomes 27,28. Effects of muscimol on neuronal respiration have not been reported, however. Since the toxicity of elevated intraneuronal Ca2+ is often ascribed to the multiple effects it has on mitochondrial bioenergetics 15, any additional impairment of mitochondrial respiration by propofol could therefore promote Ca2+-induced neurotoxicity.
Additional studies support the concept that propofol can be toxic to neurons within the immature brain. In aggregating cell cultures of fetal rat telencephalon, Honegger and Matthieu 29 report that application of >10 µM propofol for 8 h selectively produces dose-dependent irreversible structural changes in GABAA receptor containing GABAergic neurons; however, no protection was observed with GABAA receptor blockers 29. Moreover, exposure of immature GABAergic neurons to propofol (>5 µM) but not midazolam, induces a dose-dependent decrease in dendritic growth under physiological conditions 30. To more fully define the role of GABAA receptor modulation by propofol in immature neurons, we tested the effects of propofol and muscimol on DIV 4 hippocampal neurons compared to those observed with neurons cultured for 8 days, when the chloride gradient reverses and GABA receptor activation causes hyperpolarization instead of depolarization 1,31. As predicted, neither agent caused an increase in [Ca2+]i or cell death in DIV 8 neurons. Although there are no reports of toxicity to immature neurons by propofol administration in vivo, McCarthy et al. reported that administration of muscimol twice a day for two days in newborn rats increases cell death in the hippocampus measured on postnatal day 7 7, suggesting that other GABAergic stimuli such as propofol could also be toxic in vivo.
The degree to which our results obtained in vitro can be translated to the clinical scenario of propofol anesthesia is limited by several factors, including the relevance of propofol concentrations used in our experiments. Propofol is highly lipophilic and therefore concentrates in lipid-rich tissues such as brain 33. Thus, while the concentration of propofol in the CSF of patients is < 2 µM, studies in animals 33 and humans 34,35 indicate the measured/predicted brain concentration of propofol during maintenance of surgical anesthesia is above 22 µM (4 µg ml−1), and as high as 73 µM. The brain:blood partition coefficient for propofol of greater than 1.2 in both human and animals is in agreement with its high lipophilicity 33,35,36. The 5 – 50 µM concentration range used in our experiments are therefore within the range of concentrations that exist in the human brain during anesthesia.
While the results of our in vitro studies and those of other investigators suggest the possibility of propofol neurotoxicity to the immature brain, only careful dose-response experiments performed in vivo with more than one animal species, including primates, will resolve this question. The significance of our findings is therefore limited to the conclusion that pharmacologically relevant concentrations of propofol can be toxic to immature neurons in vitro and that this toxicity requires a GABA receptor-mediated rise in intracellular Ca2+. Additional studies are underway to determine what targets in addition to GABA receptors are responsible for the selective toxicity of propofol toward immature neurons.
Acknowledgements
This study was supported by NIH Grants NS07375(SK), NS34152 (GF), NS050525 (MMM) and HD16596 (GF)
References
- 1.Cherubini E, Gaiarsa JL, Ben Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Trombley PQ, van den Pol AN. Excitatory actions of GABA in developing rat hypothalamic neurones. J Physiol. 1996;494(Pt 2):451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–1213. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 4.Hilton GD, Bambrick LL, Thompson SM, McCarthy MM. Estradiol modulation of kainic acid-induced calcium elevation in neonatal hippocampal neurons. Endocrinology. 2006;147:1246–1255. doi: 10.1210/en.2005-1258. [DOI] [PubMed] [Google Scholar]
- 5.Hilton GD, Ndubuizu AN, McCarthy MM. Neuroprotective effects of estradiol in newborn female rat hippocampus. Brain Res Dev Brain Res. 2004;150:191–198. doi: 10.1016/j.devbrainres.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Hilton GD, Nunez JL, McCarthy MM. Sex differences in response to kainic acid and estradiol in the hippocampus of newborn rats. Neuroscience. 2003;116:383–391. doi: 10.1016/s0306-4522(02)00716-9. [DOI] [PubMed] [Google Scholar]
- 7.Nunez JL, Alt JJ, McCarthy MM. A new model for prenatal brain damage. I. GABA A receptor activation induces cell death in developing rat hippocampus. Exp Neurol. 2003;181:258–269. doi: 10.3201/eid0906.030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikonomidou C, Bittigau P, Ishimaru MJ, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- 9.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–74. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 10.Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 11.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jevtovic-Todorovic V, Wozniak DF, Benshoff ND, Olney JW. A comparative evaluation of the neurotoxic properties of ketamine and nitrous oxide. Brain Res. 2001;895:264–267. doi: 10.1016/s0006-8993(01)02079-0. [DOI] [PubMed] [Google Scholar]
- 13.Jevtovic-Todorovic V, Todorovic SM, Mennerick S, Powell S, Dikranian K, Benshoff N, Zorumski CF, Olney JW. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med. 1998;4:460–463. doi: 10.1038/nm0498-460. [DOI] [PubMed] [Google Scholar]
- 14.Wise-Faberowski L, Zhang H, Ing R, Pearlstein RD, Warner DS. Isoflurane-induced neuronal degeneration: an evaluation in organotypic hippocampal slice cultures. Anesth Analg. 2005;101:651–657. doi: 10.1213/01.ane.0000167382.79889.7c. table. [DOI] [PubMed] [Google Scholar]
- 15.Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Jensen AG, Lindroth M, Sjolander A, Eintrei C. Propofol induces changes in the cytosolic free calcium concentration and the cytoskeletal organization of cultured human glial cells and primary embryonic rat brain cells. Anesthesiology. 1994;81:1220–1229. doi: 10.1097/00000542-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Oscarsson A, Massoumi R, Sjolander A, Eintrei C. Reorganization of actin in neurons after propofol exposure. Acta Anaesthesiol Scand. 2001;45:1215–1220. doi: 10.1034/j.1399-6576.2001.451007.x. [DOI] [PubMed] [Google Scholar]
- 18.Garib V, Lang K, Niggemann B, Zanker KS, Brandt L, Dittmar T. Propofol-induced calcium signalling and actin reorganization within breast carcinoma cells. Eur J Anaesthesiol. 2005;22:609–615. doi: 10.1017/s026502150500102x. [DOI] [PubMed] [Google Scholar]
- 19.Bjornstrom K, Eintrei C. The difference between sleep and anaesthesia is in the intracellular signal: propofol and GABA use different subtypes of the GABA(A) receptor beta subunit and vary in their interaction with actin. Acta Anaesthesiol Scand. 2003;47:157–164. doi: 10.1034/j.1399-6576.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 20.Gourlay CW, Ayscough KR. A role for actin in aging and apoptosis. Biochem Soc Trans. 2005;33:1260–1264. doi: 10.1042/BST0331260. [DOI] [PubMed] [Google Scholar]
- 21.Chen RM, Wu CH, Chang HC, Lin LL, Chang CC, Chang HC, Wu CH. Propofol suppresses macrophage functions and modulates mitochondrial membrane potential and cellular adenosine triphosphate synthesis. Anesthesiology. 2003;98:1178–1185. doi: 10.1097/00000542-200305000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Branca D, Roberti MS, Vincenti E, Scutari G. Uncoupling effect of the general anesthetic 2,6-diisopropylphenol in isolated rat liver mitochondria. Arch Biochem Biophys. 1991;290:517–521. doi: 10.1016/0003-9861(91)90575-4. [DOI] [PubMed] [Google Scholar]
- 23.Rigoulet M, Devin A, Averet N, Vandais B, Guerin B. Mechanisms of inhibition and uncoupling of respiration in isolated rat liver mitochondria by the general anesthetic 2,6-diisopropylphenol. Eur J Biochem. 1996;241:280–285. doi: 10.1111/j.1432-1033.1996.0280t.x. [DOI] [PubMed] [Google Scholar]
- 24.Branca D, Roberti MS, Lorenzin P, Vincenti E, Scutari G. Influence of the anesthetic 2,6-diisopropylphenol on the oxidative phosphorylation of isolated rat liver mitochondria. Biochem Pharmacol. 1991;42:87–90. doi: 10.1016/0006-2952(91)90684-w. [DOI] [PubMed] [Google Scholar]
- 25.Branca D, Vincenti E, Scutari G. Influence of the anesthetic 2,6-diisopropylphenol (propofol) on isolated rat heart mitochondria. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1995;110:41–45. doi: 10.1016/0742-8413(94)00078-o. [DOI] [PubMed] [Google Scholar]
- 26.Schenkman KA, Yan S. Propofol impairment of mitochondrial respiration in isolated perfused guinea pig hearts determined by reflectance spectroscopy. Crit Care Med. 2000;28:172–177. doi: 10.1097/00003246-200001000-00028. [DOI] [PubMed] [Google Scholar]
- 27.Marian M, Bindoli A, Callegarin F, Rigobello MP, Vincenti E, Bragadin M, Scutari G. Effect of 2,6-diisopropylphenol and halogenated anesthetics on tetraphenylphosphonium uptake by rat brain synaptosomes: determination of membrane potential. Neurochem Res. 1999;24:875–881. doi: 10.1023/a:1020910131237. [DOI] [PubMed] [Google Scholar]
- 28.Marian M, Parrino C, Leo AM, Vincenti E, Bindoli A, Scutari G. Effect of the intravenous anesthetic 2,6-diisopropylphenol on respiration and energy production by rat brain synaptosomes. Neurochem Res. 1997;22:287–292. doi: 10.1023/a:1022438805337. [DOI] [PubMed] [Google Scholar]
- 29.Honegger P, Matthieu JM. Selective toxicity of the general anesthetic propofol for GABAergic neurons in rat brain cell cultures. J Neurosci Res. 1996;45:631–636. doi: 10.1002/(SICI)1097-4547(19960901)45:5<631::AID-JNR12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 30.Vutskits L, Gascon E, Tassonyi E, Kiss JZ. Clinically relevant concentrations of propofol but not midazolam alter in vitro dendritic development of isolated gamma-aminobutyric acid-positive interneurons. Anesthesiology. 2005;102:970–976. doi: 10.1097/00000542-200505000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Ben Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spahr-Schopfer I, Vutskits L, Toni N, Buchs PA, Parisi L, Muller D. Differential neurotoxic effects of propofol on dissociated cortical cells and organotypic hippocampal cultures. Anesthesiology. 2000;92:1408–1417. doi: 10.1097/00000542-200005000-00032. [DOI] [PubMed] [Google Scholar]
- 33.Riu PL, Riu G, Testa C, Mulas M, Caria MA, Mameli S, Mameli O. Disposition of propofol between red blood cells, plasma, brain and cerebrospinal fluid in rabbits. Eur J Anaesthesiol. 2000;17:18–22. doi: 10.1046/j.1365-2346.2000.00573.x. [DOI] [PubMed] [Google Scholar]
- 34.Ferreira DA, Nunes CS, Antunes L, Lobo F, Amorim P. Practical aspects of the use of target controlled infusion with remifentanil in neurosurgical patients: predicted cerebral concentrations at intubation, incision and extubation. Acta Anaesthesiol Belg. 2006;57:265–270. [PubMed] [Google Scholar]
- 35.Ludbrook GL, Visco E, Lam AM. Propofol: relation between brain concentrations, electroencephalogram, middle cerebral artery blood flow velocity, and cerebral oxygen extraction during induction of anesthesia. Anesthesiology. 2002;97:1363–1370. doi: 10.1097/00000542-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Dutta S, Matsumoto Y, Muramatsu A, Matsumoto M, Fukuoka M, Ebling WF. Steady-state propofol brain:plasma and brain:blood partition coefficients and the effect-site equilibration paradox. Br J Anaesth. 1998;81:422–424. doi: 10.1093/bja/81.3.422. [DOI] [PubMed] [Google Scholar]
- 37.Mazoit JX, Samii K. Binding of propofol to blood components: implications for pharmacokinetics and for pharmacodynamics. Br J Clin Pharmacol. 1999;47:35–42. doi: 10.1046/j.1365-2125.1999.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takizawa E, Hiraoka H, Takizawa D, Goto F. Changes in the effect of propofol in response to altered plasma protein binding during normothermic cardiopulmonary bypass. Br J Anaesth. 2006;96:179–185. doi: 10.1093/bja/aei293. [DOI] [PubMed] [Google Scholar]
- 39.Costela JL, Jimenez R, Calvo R, Suarez E, Carlos R. Serum protein binding of propofol in patients with renal failure or hepatic cirrhosis. Acta Anaesthesiol Scand. 1996;40:741–745. doi: 10.1111/j.1399-6576.1996.tb04521.x. [DOI] [PubMed] [Google Scholar]