Abstract
Neuroimaging research over the past decade has revealed a detailed picture of the functional organization of the human brain. Here we focus on two fundamental questions that are raised by the detailed mapping of sensory and cognitive functions and illustrate these questions with findings from the object-vision pathway. First, are functionally specific regions that are located close together best understood as distinct cortical modules or as parts of a larger-scale cortical map? Second, what functional properties define each cortical map or module? We propose a model in which overlapping continuous maps of simple features give rise to discrete modules that are selective for complex stimuli.
Advances in brain imaging technology (especially functional MRI (fMRI)) have radically improved our understanding of the functional organization of the human brain (BOX 1). In this Review we describe the organization of the ventral visual pathway, which is characterized by strong selectivity for particular object categories (for example, faces and bodies) at the level of both individual neurons and larger cortical regions. We then consider two central questions: whether this organization reflects maps or modules, and what properties are mapped. In each case we derive clues from the literature on the primary sensory cortex, in which cortical maps have been studied extensively using electrophysiology in animals. We find that apparently modular cortical regions, such as orientation columns and face-selective regions, might be parts of larger maps, and show that it is a substantial challenge to determine the basic properties and dimensions that describe functional organization most parsimoniously. We then propose a new framework that reconciles the existence of graded cortical maps and distinct functional modules. In this framework, the strong category selectivity that exists for faces and other objects might arise from the nonlinear combination of multiple correlated maps for simpler stimulus properties.
The ventral visual pathway
The ventral visual pathway comprises a large cortical region that occupies the ventral and lateral surfaces of the occipital and temporal lobes (FIG. 1). A substantial proportion of fMRI voxels in this pathway are ‘object-selective’ — that is, they respond more strongly when people view images of objects than when people view scrambled versions of these objects or texture patterns. This object-selective region is often referred to as the lateral occipital complex (LOC)1. The LOC has little selectivity for particular stimulus categories2–4, but several regions of cortex near the LOC are selective for particular object categories: they respond at least twice as strongly to their ‘preferred’ stimuli than to other stimuli. For example, in essentially all humans cortical regions can be found that respond selectively to faces (the fusiform face area (FFA)5,6 and, in many individuals, the occipital face area (OFA))7,8, to places and — to a lesser extent — to buildings (the parahippocampal place area (PPA))9,10, to body parts (the extrastriate body area (EBA)11–13 and, in most people, the fusiform body area14,15) and to visually presented words or letter strings16–19 (FIG. 1). The location and functional properties of these regions are very similar across humans5,20.
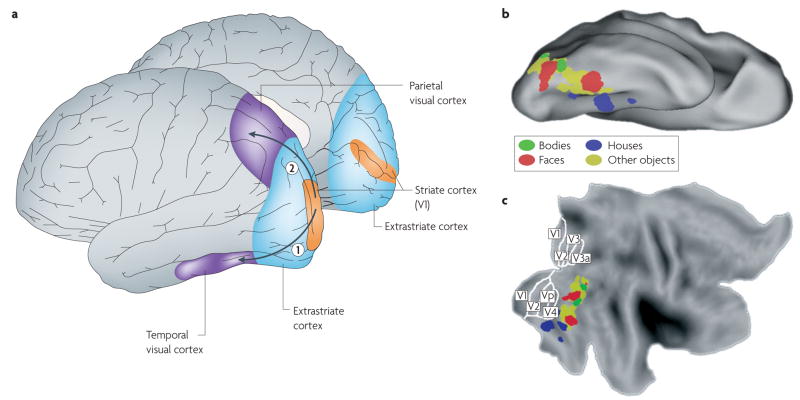
Figure 1. Typical locations of category-selective regions in the human ventral visual cortex.
a | The location of visual regions in the human cortex, including the primary visual cortex (area V1 in the striate cortex) and the extrastriate cortex in the occipital lobe, and the traditional distinction into two visual cortical pathways that start in area V1 and extend into the temporal lobe (the ventral ‘what’ or ‘object-vision’ pathway (1)) or into the parietal lobe (the dorsal ‘where’ pathway (2))137. b,c | Ventral pathway regions in one individual that were activated significantly at the voxel level (P < 0.0001, uncorrected) in the following contrasts: bodies > faces + houses (shown in green); faces > bodies + houses (shown in red); houses > bodies + faces (shown in blue). In addition, the yellow areas represent the regions that, in a group of people (n = 9), activated significantly in the contrast: intact objects > scrambled objects. All data were processed using SPM5 (Wellcome Department of Cognitive Neurology, London). Data are shown on top of the PALS human atlas (using CARET software138,139) in a ventral view of the inflated cortical surface (b) and in a flattened view of the cortical surface (c). The partitioning of retinotopic areas in the striate and extrastriate cortex is shown as included in the PALS atlas140.
Additional areas with weaker selectivity for some of these object categories7 have been described, but no selectivity of similar strength and spatial scale has been reported for other object categories21. This lack of selectivity for other categories does not mean that no such preferences exist in the cortex. First, ‘brain-reading’ algorithms (multi-voxel pattern analyses)22,23 can decode the category of an object from the distribution of activity across the object-selective cortex for a wide range of object categories (for example, cars, scissors and chairs)24–27. The success of these algorithms in the absence of selectivity that is localized to a few focal regions indicates that many voxels show weak selectivity, and that this spatially distributed pattern of selectivity replicates across repeated measurements. Second, scanning at higher resolution might ultimately reveal focal functional specificity that was not apparent at lower resolution15, although recent studies have not yet provided conclusive evidence for this possibility28,29. In sum, some clustering of preferences exists for a wide range of object categories, but large-scale spatial clustering of strong selectivities has so far been found only for faces, bodies, scenes and letter strings. For now we will refer to ‘category selectivity’, but later in this Review we consider the possibility that simpler properties, or a combination thereof, might explain part of this selectivity.
Box 1 | Recent advances through functional MRI
The power of functional MRI (fMRI) to investigate functional specificity at high resolution is demonstrated by its ability to replicate neurophysiological findings from animals non-invasively in humans. The left column in the figure illustrates the high-resolution data that can be obtained in monkeys with invasive techniques; the right column illustrates the quality of data that can be obtained in the human cortex with non-invasive imaging. Large-scale maps of the visual field (or ‘retinotopic maps’), which were first described physiologically in the primary visual cortex in macaques127,128, were obtained in humans with fMRI more than a decade ago129,130 (see figure, part a). At a finer scale, physiological studies carried out long ago determined that cortical area V1 in non-human primates contains ocular-dominance columns, which are elongated regions approximately 0.5 mm wide in which neurons receive input that is dominated by one eye131,132 (see figure, part b, left-hand panel). The first evidence for ocular-dominance columns in human cortical area V1 in vivo was obtained five years ago, by scanning at high spatial resolution (in-plane resolution of 0.5 mm)133 (see figure, part b, right-hand panel). Finally, early physiological studies showed that V1 in non-human primates contains orientation columns in which all cells have the same orientation preference; these columns134 are small enough for all orientations to be represented in less than 1.0 mm2 of the cortical surface60 — the size of a single high-resolution fMRI voxel (see figure, part c, left-hand panel). Although columnar-scale imaging has been reported in animals135, the small scale of this organization precludes imaging of the individual columns in humans with current methods (see figure, part c, right-hand panel; the colour scale represents preferred orientation with hue and strength of selectivity with colour saturation). Nevertheless, recent fMRI studies have been able to exploit subtle differences between voxels in their selectivity for oriented gratings to decode the orientation of the gratings from the distributed activation pattern in human V1, using multi-voxel pattern analyses 22,23,136 (see figure, part c, right-hand panel).

In addition to this organization by object category, a weak eccentricity bias that appears to be an extension of the eccentricity map in retinotopic visual areas has been reported in extrastriate and temporal visual cortex30,31. Evidence for fine-grained selectivity for other object properties, such as orientation and size, or for specific exemplars within a category is sparse. Some reports suggest, based on activity in the ventral visual pathway, that brain-reading algorithms have weak but above-chance classification performance on within-category discriminations (for example, for pigeons versus seagulls or for fearful versus happy faces)32–34. Conversely, even high-resolution scans have so far failed to find above-chance classification performance for discriminating the identity of faces based on activity in the FFA35, or for discriminating different body parts (for example, hands versus legs) from activity in the EBA (R. F. Schwarzlose and N.G.K., unpublished observations). nevertheless, future studies using high-resolution scans and/or multi-voxel pattern analyses might find further evidence for functional organization of object properties other than category membership.
If neural selectivities are clustered at a spatial scale that is smaller than the current minimum voxel size (~1 mm), a technique known as fMRI adaptation can be used to measure stimulus selectivity at a sub-voxel scale. Studies using fMRI adaptation5,20 have indicated that the FFA discriminates between different individual faces36 and the LOC discriminates between individual object exemplars37,38. Adaptation studies have also shown that these regions are partly insensitive to size, position and spatial scale39,40 but more sensitive to viewpoint and direction of illumination37,41,42. However, fMRI adaptation is an indirect measure of selectivity that might be linked only partially to neuronal selectivity43; moreover, the extent to which the sensitivity of the ventral visual cortex to object exemplars can be explained through sensitivity for low-level stimulus properties such as luminance, contrast, line orientation or texture has not been systematically explored.
Substantial evidence thus indicates that the human ventral visual pathway contains a small set of cortical regions, each of which responds selectively to a single category of visual stimuli (faces, places, bodies, scenes or letter strings), whereas patterns of selectivity for other object categories are more distributed across the ventral visual cortex. In addition, fMRI adaptation has revealed sensitivity to a range of within-category differences37,38,44.
Monkey studies
Studies in monkeys with fMRI and extracellular recordings have revealed striking similarities between the monkey and human ventral visual pathways. As in humans, fMRI in monkeys has revealed regions of the ventral visual pathway that are selective for a few object categories, including faces and bodies45,46. Electrophysiological recordings have recently confirmed the clustering of single-neuron response properties that underlie this fMRI selectivity47: in some subregions in the inferior temporal (IT) cortex almost all neurons respond more strongly to faces than to other objects. A distributed pattern of selectivity is also found for other object categories46.
In addition, category membership seems to be the main determinant of the population response in the IT. In a recent study in which monkeys were shown images of objects from many natural categories, the population of IT neurons was highly selective for category membership, especially for animate objects like faces and bodies48. More indirect evidence for category selectivity is the observation that single IT neurons are more selective for shape features that are useful for object categorization (‘non-accidental properties’) than for other shape features (‘metric properties’)49. Thus, the stimulus property that seems to be associated with the strongest selectivity in single cells in the IT cortex is object category.
Finally, IT neurons also show selectivity for other features, including object shape50,51, viewpoint52, position53,54 and size53. These results are in accordance with the findings from fMRI adaptation studies in humans. Overall, there seems to be a high degree of similarity in the neural mechanisms that underlie face and object processing in monkeys and in humans, as well as in the sensitivity for object properties that define category membership.
Over the years several controversies have arisen concerning the interpretation of category-selective regions in the brain. First, how distinct are these ‘regions’? Are they discrete modules or are they parts of a continuous selectivity map? Second, is ‘stimulus category’ really what these regions are selective for? In the following sections we tackle both questions, drawing on findings from the primary sensory cortex in animals, where similar questions have been addressed in great detail.
Are distinct regions parts of larger maps?
Are functionally specific regions that are located close together best understood as a set of distinct cortical modules or as part of a larger-scale cortical map? Specifically, does the ventral visual pathway contain a single large-scale map of object category in which the face and body areas constitute individual components, in the same way that the upper-left visual field forms one segment of the primary visual cortex? Or are the face and body areas self-contained and discrete functional units with relative spatial locations and functional specificities determined by factors that are unrelated to their location within a larger map of object shape or meaning?
Distinction between maps and modules
The word ‘map’ is generally used to refer to a gradient of selectivities along the cortical sheet. By contrast, ‘module’ — in the context of brain function — refers to the clustering of selectivities in discrete regions, with clear selectivity discontinuities at the boundaries of these regions. For now we will stick to this simple functional definition and not commit to further, as yet unresolved, questions about the size of a module (except for the notion that it is bigger than a column55) or further anatomical criteria. Note that modularity in cognitive science is a more complex, multi-faceted and theoretically committed concept56 than in neuroscience. Nevertheless, the two meanings of modularity are not completely distinct, and the existence of discrete brain regions with clear functional boundaries invites the question of whether the more extensive criteria for modularity in cognitive science also hold for these regions.
How can we empirically distinguish between maps and (brain) modules as defined here? Experimentally this enterprise requires a continuous variation of a stimulus parameter, for example, stimulus position, and an investigation of how the peak of activation shifts along the cortical surface in response to the varying stimulus parameter. If the continuous variation is associated with a gradual shift in the peak of activation, we have found a map. If the continuous variation is associated with discrete jumps in the peak of activation, we have evidence for a module. Note, however, that this method applies only to stimulus dimensions that vary along continual, or at least ordinal, scales. In addition, to provide convincing evidence of a map, the variable that determines the map must explain the strength of selectivity in each region. In the following sections we review the evidence for maps and modules in primary sensory cortices and then apply these insights to the ventral visual pathway.
Maps and modules in primary sensory cortex
Is there evidence for maps and modules in primary sensory cortex, and can the two types of organization be distinguished? The retinotopic organization in the primary visual cortex (area v1 in the striate cortex; see FIG. 1a) is a prototypical example of a map: the preferred stimulus position changes smoothly across the cortical surface57–59. The situation is less clear, however, for other stimulus parameters, such as orientation. Gradual variation of stimulus orientation produces a gradual shift of orientation preference in numerous v1 subregions60, but these regions are separated by singularities (pinwheel centres) in which the orientation preference shifts abruptly61–63. This pattern is sometimes referred to as a mosaic-like map64. Computational analyses have shown that the discontinuities that are found in a mosaic-like map are unavoidable whenever multiple stimulus properties (such as orientation, direction of motion and spatial frequency) are mapped onto the two-dimensional cortical sheet58,65–67. We will not use the term ‘module’ to refer to this kind of mosaic-like map for two reasons. First, the pinwheels where orientation preference changes abruptly are local exceptions in what is otherwise a smooth map. Second, in contrast to our definition of a module, the pinwheels do not divide the cortex into discontinuous regions that have no relationship in preferred values across their boundaries; instead, the preferred stimulus values depend on the direction in which the pinwheel is crossed. Note that this conclusion is based on the functional response properties in the cortex, and the relevance of certain relationships between functional properties, such as colour selectivity, and cytoarchitectonic features, such as ‘blobs’ and ‘interblobs’, is as-yet unclear68–70.
Does any primary sensory region contain functional modules? At first glance, the somatosensory cortex of animals with whiskers or similar organs comprised of discrete units, appears to be a plausible candidate. Indeed, the first-order cortical somatosensory representations in these animals are discontinuous. Typical examples are the barrels in the rat somatosensory cortex and the layout of the nasal appendages of the star-nosed mole rat71,72. However, even though the barrel cortex is sometimes cited as an example of columnar structure, it is in fact analogous to the retinotopic visual cortex55 because a barrel constitutes a first-order representation of a whisker that is isomorphic with the structure of the receptor organ. Furthermore, a clear spatial relationship holds across barrels, with the barrel array on the cortex reflecting the whisker array on the snout. Thus, the barrel cortex has an ordinal (although not continuous) mapping of whiskers. Finally, the selectivity in each barrel is as strong as one would expect given the stimulus property that is mapped across barrels: given that each barrel represents one whisker, it is not surprising that neurons in a barrel will respond only to stimulation of that particular whisker. From this perspective the barrel cortex contains a map, not modules. More generally, there is no evidence for modules (as defined here) in sensory cortex.
Maps and modules in the ventral visual pathway
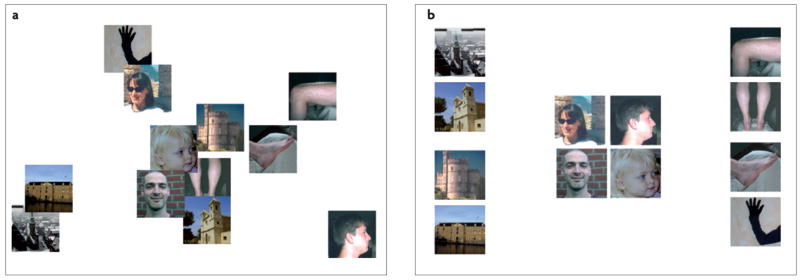
Should category-selective regions in the ventral visual pathway be regarded as stand-alone modules that are specialized for the recognition of a special category of objects6,73, or should they be regarded as part of a larger topographical organization that encompasses most or all of these regions13,31,74,75? As mentioned above, the best way to answer this question is to investigate how the pattern of selectivity in these regions shifts with a gradual change in object properties. Given that object category seems to be important for the organization of the object-vision pathway, it is object category that should change gradually. However, the investigation of this idea encounters two problems. First, in contrast to the stimulus properties that are represented in v1, category membership is not linked to variation in a simple physical parameter. Although the term ‘object category’ is intuitively clear, no analytic approach or computational model offers a convincing parameterization of ‘object category’. So what can we do without a physical standard for object category? The solution that has been adopted in numerous studies of categorization is to derive the complex properties by which objects are represented from the behaviour of humans when they rate the similarity between objects76–78 (FIG. 2). FIGURE 2a shows images of twelve objects in a spatial arrangement that reflects the physical pixel-based similarity between the images (as accurately as is possible with two dimensions). This spatial configuration is strikingly different from that which most closely reflects the similarity between these images as judged by a human observer (FIG. 2b). These judgments suggest that the relevant dimension in humans’ ‘mental object space’ is object category. Thus, object category dominates not only the functional organization in the object-vision pathway but also perceptual similarity. One fMRI study found good correspondence between the rated similarity among objects and the degree of overlap among their representations in the object-vision pathway79. This study illustrated how a detailed analysis of how humans perceive objects can provide at least a partial solution to the lack of a simple parameterization.
Figure 2. object category is an important factor in the mental space of objects that underlies similarity judgments.
a | A two-dimensional representation of the physical differences that exist between twelve images, as quantified by the luminance difference between corresponding pixels, summed across pixels. b | A two-dimensional representation of the perceived differences between the same twelve images, as indicated by a human observer. Note that there is no correspondence between this higher-order mental space and the physical space of part a. The two-dimensional representations were obtained by applying non-metric multidimensional scaling to the matrices, with pair-wise physical (a) and perceived (b) differences.
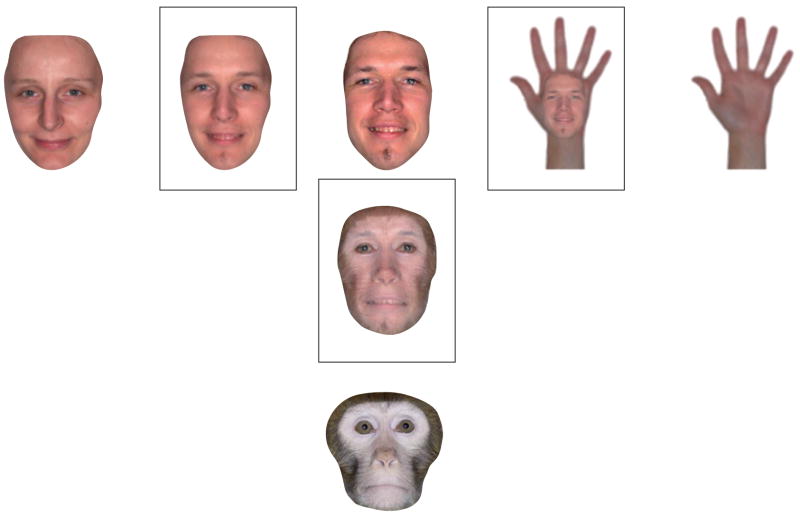
Another problem remains, however. In all stimulus sets that have been used in fMRI research, category membership was a discontinuous variable. The stimulus set shown in FIG. 2b is a representative example: there are several faces in this set, but there is no gradual morphing of a face into an exemplar of another category. This problem might be unsolvable, because the mental object space seems to be only locally continuous. Within each object category, individual objects’ shapes can be changed to move parametrically from one exemplar to another50,80–85: morphing the faces of two members of a species or of members of two different species is relatively straightforward, as the corresponding features in the two faces are immediately obvious (FIG. 3). However, the mental object space has sharp discontinuities that coincide with the boundaries between categories, and morphing across these boundaries is not straightforward. For example, what are the corresponding features of a hand and a face? What would a hand–face morph look like, and what are the odds of seeing such a morph in real life? This simple example suggests that the category ‘faces’ has relatively sharp boundaries. The same applies to other categories, with the exception of categories of objects that have similar shapes (for example, arms and legs, or bottles and vases). Thus, a few special cases excluded, category membership is an inherently discontinuous variable.
Figure 3. The clear boundaries between regions that are selective for different object categories reflect the clear boundaries that exist between object categories in mental object space.
This figure shows morphed images (in squares) that are, respectively, combinations of two human faces (left); a human face and a monkey face (bottom); and a human face and a human hand (right). For two human faces, or even for the faces of members of different species, corresponding points in the two figures can be easily found. However, it is not straightforward to identify corresponding points on objects that are more distant in object space, such as a face and a hand.
Returning to our original question, does the object-vision pathway contain a large-scale map, or does it contain a set of independent modules? We have seen above that in the barrel cortex even discrete stimulus parameters, such as different whiskers, can be represented in a continuous map: even though both whiskers and cortical barrels are discrete units, neighbouring whiskers are nevertheless mapped onto neighbouring barrels in the cortex. This represents the ordinal characteristic of a map. Similarly, even though faces and other objects constitute discontinuous categories in visual object space, and even though the corresponding cortical regions have relatively sharp boundaries, as is required of modules86, these regions can still be part of a map if there is any systematic relationship between their relative positions. Indeed, such a relationship has been suggested: high-level visual cortex exhibits a weak centre–periphery organization30, and it has been argued that the relative location of category-selective regions in this eccentricity map corresponds to the eccentricity at which the preferred stimuli of these regions are typically seen. These considerations raise the question of whether even a face-selective ‘module’ such as the FFA can be considered to be part of a larger topographic object category map.
Other data argue against this view of continuous functional organization in the ventral visual pathway, however. First, the relatively weak retinotopic organization in high-level visual cortex does not seem to be strong enough to explain the much stronger category selectivity that is observed. The retinotopic organization might have a role in determining where selectivity for a category will be found, but it does not seem sufficiently strong to function as the sole argument against modules. Further research is needed to establish whether there is another variable, or combination of variables (see below), that links the regions that have strong category selectivity. Second, it is not yet known whether category-selective regions such as the FFA and the LOC differ from one another in their cytoarchitecture or connectivity; if such differences exist and exceed the simple wiring differences of, for example, barrels, this would challenge the view that these regions are parts of a larger object-category map.
Finally, we use a minimal definition of a ‘module’ here, referring only to the functional neuroanatomical characteristics of an area — namely the strength of its functional specificity and the sharpness of its boundaries. More elaborate definitions of a ‘module’ in cognitive science include a list of additional properties, such as mandatory processing and a characteristic ontogeny56,87. Although the evidence is not conclusive for any of these properties, the FFA might satisfy some of them, and such findings could challenge the idea of a large-scale map. First, the FFA and the nearby LOC differ not only in terms of their selectivity, but also in terms of the computations that they conduct on their preferred stimuli. For example, the FFA responds similarly during discrimination of faces on the basis of face parts and on the basis of the spacing between parts. By contrast, the LOC responds much more strongly to part-based than spacing-based discrimination of both faces and houses88. Second, recent evidence suggests that the FFA and the PPA develop on a different timescale to the rest of the ventral visual pathway, so they seem to have a characteristic ontogeny89.
In sum, the category-selective regions in the ventral visual cortex can be considered part of a larger topographic organization that reflects the characteristics of the mental space of objects; however, further studies are needed to differentiate this perspective from a discontinuous, modular view of the ventral visual pathway.
What functional properties are mapped?
When we find maps or modules with spatially varying preferences for a functional property, can we determine whether this functional property is the ‘basic’ property or dimension that most parsimoniously describes the functional organization in that particular brain region? More specifically, if experiments reveal that the primary visual cortex and ventral visual cortex contain maps of orientation and object category, respectively, can we be sure that we have identified the basic functional properties of these brain regions? Or might we have identified irrelevant functional properties that merely happen to correlate with the actual basic properties?
Basic properties in the primary visual cortex
What functional properties are represented in primary sensory cortex? For some aspects of coding the answer is simple and uncontested. An obvious example is the first-order representation of receptor arrays (such as retinotopic maps) that determine the large-scale organization of primary sensory cortices. Although the fine characteristics of these maps (for example, their magnification factor, local smoothness and scatter) have been discussed in many studies, there is little debate about what these maps represent (for example, visual-field position in the case of retinotopy).
However, controversy arises once the clear link to the receptor array is lost and the maps reflect higher-order properties of the stimulus. The first demonstrations of more fine-scale functional organization in v1 were maps of ocular dominance10 and orientation preference90,91. Later studies provided evidence of clustering of several other functional-response properties in at least some species, for example, spatial frequency and direction of motion64. The traditional approach has been to consider these maps as feature maps with overlapping regions that give rise to selectivity for particular feature combinations58,92. However, this interpretation was a consequence of the experimental approach that was used: the different feature maps were discovered one by one, typically by mapping one feature at a time and averaging across all values of the other features.
By contrast, recent studies have included multidimensional stimulus manipulations. Using this approach, the map of orientation preference in v1 was found to depend heavily on several other stimulus properties, such as bar length, direction and speed93. These results cast doubt on the multiple-feature-map interpretation. Instead, it has been proposed that these multiple maps might arise from the mapping of only one property — spatiotemporal energy93. According to this view, the finding of independent maps for multiple features is an artefact of using stimuli that vary in only one feature at a time. This discussion illustrates that, even for seemingly simple functional properties, it is not an easy task to find the ‘basic’ dimensions that most parsimoniously describe a regions’ functional organization.
Basic properties in the ventral visual cortex
Even more controversy exists regarding how to describe the functional organization in the ventral visual cortex. Until now we have described it in terms of object category, and most studies that have targeted the object-vision pathway have presented exemplars from a wide variety of ‘everyday’ object categories. However, object category is potentially confounded by various other factors, such as shape characteristics, the way in which the stimuli are processed (for example, part-based versus holistic processing), semantic information and retinal eccentricity. In early studies on high-level visual cortex these properties were manipulated jointly, but recent studies have begun to isolate specific variables. So far, however, no individual variable has been able to explain a substantial proportion of the observed category selectivity. We next review the evidence for a variety of such candidate variables.
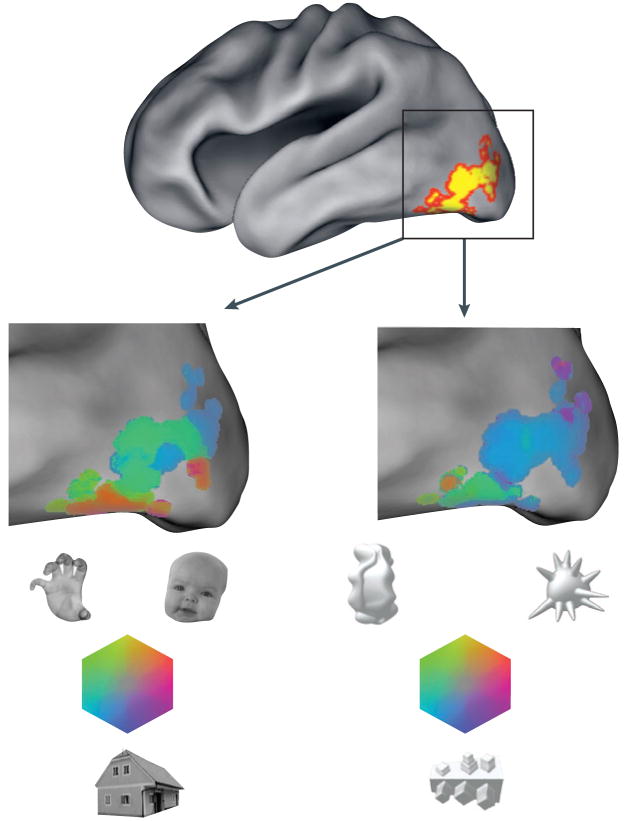
First, could high-level visual areas simply be selective for shape characteristics? In support of this view, unfamiliar artificial-object categories and relatively simple patterns elicit selective responses in the object- and face-selective cortex in humans94,95 (FIG. 4). Furthermore, it has been shown that single cells and columns in the monkey IT cortex are selective for moderately complex features96,97; that neurons in v4 and in the posterior IT cortex are tuned for simple shape characteristics98,99; and that IT neurons exhibit gradual tuning in simple shape spaces50,51. Finally, single-cell recordings and fMRI studies have provided evidence of selectivity for object parts and non-accidental properties (for example, symmetry, parallelism and collinearity) in the ventral temporal cortex49,100, as predicted by structural description theories of object recognition101,102.
Figure 4. Functional specificity for familiar categories of objects and for initially novel categories of objects.
Functional specificity in the lateral occipital and ventral occipitotemporal cortex (the ‘lateral occipital complex’, as defined by the contrast: intact objects > scrambled objects) is shown by a colour map for two sets of stimuli: three familiar objects (faces, body parts and houses; left-hand enlargement) and three novel objects (right-hand enlargement). Colour saturation represents the amount of selectivity; hue represents which object class is preferred. It is important to note that the colour scale is not given a threshold for statistical significance, and few individual voxels show significant specificity for the novel objects. Nevertheless, the pattern of selectivity across many voxels is replicable. In this individual the spatial correlation across voxels between independent subsets of the data (‘odd’ and ‘even’ runs) was 0.78 for familiar objects and 0.52 for novel objects.
These findings indicate that part of the functional specificity for familiar objects and faces might be due to differences in basic shape characteristics across categories. However, several lines of evidence limit the role of shape properties. First, the selectivity for unfamiliar shapes is weaker than that for familiar objects and faces. The pattern of selectivity is distributed and weak in individual voxels, at least for the unfamiliar-object classes that have been tested. For example, in a recent study with high spatial resolution, in the scattered regions that showed the most selectivity the strength of the regions’ response to their least-preferred novel objects was approximately two thirds of the response to a preferred novel object95. By contrast, the maximum selectivity for some familiar objects is far stronger, even at lower spatial resolution: the FFA, the PPA and the EBA all respond at least two to three times more strongly to their preferred object class (faces, houses and bodies, respectively) than to a wide range of non-preferred object classes21. Second, the strong and indistinguishable response of the body-selective regions to, for example, hands and legs, which have very different shapes, indicates that shape alone cannot account for all aspects of the selectivity of these regions12,26,46. Thus, simple shape characteristics can explain only part of the functional organization that exists in cortical responses to familiar-object classes. Third, it is possible that apparent selectivities for simple shape features are by-products of selectivities for more complex objects. In particular, the aforementioned studies that demonstrated selectivity for shape features cannot rule out that this selectivity is actually caused by a tuning for the whole shape of complex, familiar objects. Indeed, computational work has shown that empirical data that seem to favour an explanation in terms of part- or feature-based selectivity can be mimicked when simple shapes are presented to a hierarchical model with units that are tuned for the whole shape of complex objects103. Thus, some tuning and clustering for simple shape features is to be expected, even if the actual shape or object characteristics that determine tuning and clustering are more complex in nature.
Could functional organization be driven not by physical stimulus attributes but by the neural processing that is triggered by each stimulus104? According to this view, any object that engages a given process could strongly activate the relevant region. This idea has been mostly put forward for face-selective regions, based on evidence that faces are processed more holistically than other types of object105–108. The hypothesis that the face-selective cortex is not actually selective for faces per se, but rather for the neural processing that is triggered by our extensive expertise with faces, has been tested with other object categories for which some individuals have expertise109–111 — for example, cars and birds in car experts and ornithologists, respectively. However, recent evidence casts doubt on the idea that holistic processing occurs for any expert object category other than faces112, and all fMRI studies of expertise that investigated both the FFA and the LOC found that any increased responses to expert categories were larger in the LOC than in the FFA112,113. Further, as mentioned above, the FFA response to faces is largely unaffected by task participation, and when humans are induced to process non-face stimuli in a face-like fashion the face area does not respond strongly88. This means that either the face-selective responses are not caused by process differences or that the holistic processing of faces is so automatic and mandatory that there is no way to dissociate it from viewing a face. Finally, the hypothesis applies only to faces, and is not sufficiently specific to address the full pattern of functional specificity for a wider range of object categories. Thus, there is little evidence that category-selective responses are due only to differences in types of processing.
It has also been proposed that the association of object categories with non-visual information might determine part of the organization in the ventral visual pathway114,115. For example, the distribution of neural specificity for ‘tools’ or manipulable objects in the ventral visual pathway might be driven by the connectivity of some ventral regions with other brain regions that are involved in the coding of actions. Similarly, one might speculate that the neural specificity for faces in the fusiform gyrus is related to the functional connectivity that exists between this region and brain regions that are involved in affective reactions, such as the amygdala, or social cognition, such as the temporo-parietal junction116. Likewise, the lateralization of the visual word-form area in the left hemisphere might be caused by the left-hemisphere lateralization of language processing19. The evidence indicating that functional connectivity is a general organizing principle in the ventral visual pathway is currently mostly circumstantial, but this is a question that warrants further research.
One series of studies has suggested that there is a systematic relationship between the localization of category-selective regions and a weak eccentricity map in the object-selective cortex30,31. Object categories that tend to be seen at particular eccentricities evoke selective activity in these eccentricity bands. For example, faces, which are mostly foveated, activate regions that have a strong preference for foveal stimulation, rather than regions that are activated by scenes and houses. This relationship might explain the location of some category-selective regions in high-level visual cortex, although it does not by itself explain strong category selectivity (which is much stronger than the eccentricity biases).
In sum, many studies have tried to define more precisely what aspects of objects and faces drive functional specificity in the occipitotemporal cortex. Simple and quantitatively defined stimulus properties, such as shape characteristics, explain some of the observed specificity, but object category remains the most parsimonious criterion by which to explain functional organization in the object-vision pathway.
Object category as a basic property
Despite the parsimony of using one concept, object category, this criterion remains unattractive because of its subjectivity. Two routes may lead to a more systematic understanding of the selectivity for object category.
Computational modelling might provide a more mechanistic way to describe the functional organization in high-level visual cortex. This has been illustrated by the fresh perspective that computational modelling has afforded of the distinction between part-based and configural processing of objects and faces117. Computational evidence has also revealed that image fragments of intermediate complexity are more informative for object categorization than fragments of low or high complexity118,119, suggesting that neural coding in object-selective areas might be based on such intermediate-complexity shape features. However, a computational description of cortical organization in the ventral pathway remains a distant goal, because current neurophysiologically plausible models cannot yet predict the strong category selectivity that exists in the object-vision pathway48,120: for example, the dominance of object category in the selectivities of monkey IT neurons is not predicted by ‘standard’ hierarchical models48. Similar discrepancies might exist in the human brain100.
Adding learning processes to computational models might increase their power to explain the dominance of object category as an organizing principle in high-level visual cortex. Visual experience changes the visual processing of objects in various ways, and it might be an important factor for the development of the sensitivity to category distinctions and the emergence of category-selective regions. Part of this learning might occur in a bottom-up, unsupervised manner121. In addition, supervised category learning can change the perception and visual processing of objects118, leading to a biased processing of relevant dimensions122–126. Furthermore, learning to discriminate objects within categories changes the pattern of selectivity across the object-vision pathway95. Thus, learning about categories and their members might be responsible for some of the functional organization that exists with regards to object category.
A comprehensive framework
The evidence regarding the organization of the object-vision pathway leaves us with little overall agreement about the correct answer to our two central questions. There are regions with strong category selectivity, but it is not clear to what degree these regions are part of a larger-scale map. There is some selectivity in the ventral visual pathway for simpler functional properties than object category, but it is doubtful that this selectivity is strong enough to explain the strong category selectivity.
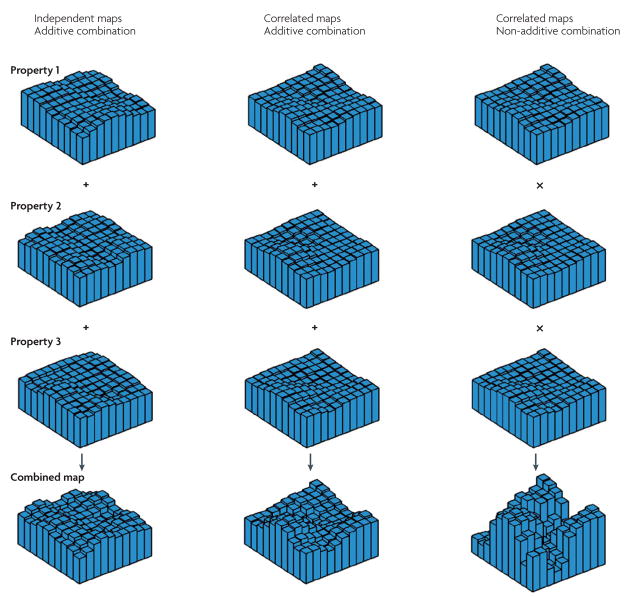
Here we propose a coherent framework to make sense of these data. The starting point is the idea that relatively weak selectivity maps might exist for many functional properties, for example, a shape map, a functional connectivity map, a process map and an eccentricity map. When one functional property is studied in isolation, such as the shape of novel objects, then only weak selectivity is found. However, when a stimulus combines several functional properties, multiple maps are activated and the eventual selectivity is a combination of these multiple maps. How can strong selectivity be achieved with such overlapping maps of weak selectivity? The crux of the matter is the extent to which the maps are independent and the question of whether they are combined additively. Several possibilities are illustrated in FIG. 5. If the maps are independent and simply added, the combined selectivity will be only slightly stronger than in each of the original maps. However, if the maps are spatially correlated — that is, if selectivity for one property (for example, compact, curved shapes) implies selectivity for another property (for example, foveal stimulation), then a simple addition of the maps results in a more pronounced selectivity profile. Such correlations might be based on naturally occurring coincidences in familiar objects (for example, faces are compact and curved and are mostly foveated). Finally, if multiple maps are combined non-additively, for example, by multiplication, then the combination of weak selectivity maps, which might or might not be correlated, can result in strong combined selectivity.
Figure 5. The existence of maps for multiple functional properties and different ways in which they might be combined.
Each plot in the top three rows shows the response profile of a hypothetical voxel set to a particular functional property. The bottom row illustrates different possibilities for the combination of these overlapping maps. If the individual maps are uncorrelated and their integration is additive (left-hand column), the resulting combined selectivity profile will be similar to those of the individual properties. If the individual maps are correlated and additively combined (middle column), the joint presence of all three features will lead to a more selective reponse profile. If the individual maps are correlated and combined nonlinearly (here, by multiplication; right-hand column), the resulting selectivity profile will be pronounced, with subsets of the voxel space responding strongly to the joint presence of two or more individual properties.
This framework incorporates all of the various hypotheses in the literature: these hypotheses posit the existence of maps for only one stimulus feature, for example, a process map. According to our framework, all of these single maps might coexist, much as maps of orientation selectivity, ocular dominance and direction selectivity coexist in the primary visual cortex. In addition, our framework provides a formal way to link these single maps to the hypothesis that focuses on strong selectivity in terms of object category — a hypothesis that is often called ‘domain specificity’73. This hypothesis can be re-phrased in terms of strongly correlated and non-additively combined maps. Finally, the framework opens up a clear route for the future because it raises questions that have been ignored in the literature. Future studies need to investigate the relative strength of the various selectivity maps, the correlations between these maps, the way in which the maps are combined when familiar objects such as faces and bodies are shown, and whether this combination is less additive for faces than for other, less familiar objects. It would be of particular interest to examine why any correlations between maps exist, how the maps arise developmentally, whether they arise in a particular order, and why focal regions with strong selectivity have been found for only a few object categories.
Conclusions and future directions
To fully understand the wealth of new data from fMRI about the functional organization of the human brain, cognitive neuroscientists have to grapple with a number of fundamental questions regarding the existence of maps and modules in any brain region. First, we noted that many cortical regions (for example, barrels and face areas) appear to be modular, in that they have strong selectivity and relatively sharp functional borders. However, in primary sensory cortices these apparently modular cortical regions (orientation columns, barrels, et cetera) turn out to be parts of larger maps. It remains to be seen whether the same will be found for the face-, place-, and body-selective areas of the ventral visual pathway — that is, whether a broader mapping scheme will be discovered that can subsume these regions, which would explain the location and, especially, the strong selectivity of each area as components of that larger map. Second, it is a substantial challenge to determine the basic properties and dimensions that describe functional organization most parsimoniously, even for relatively simple stimulus properties such as orientation and spatial frequency. With respect to the object-selective cortex, studies have not yet been able to explain the strong functional specificity that exists for (for example) faces and bodies by simpler or more unambiguously defined properties than the intuitive notion of object category. In this Review we have proposed a comprehensive framework in which the strong functional specificity for object category arises from the nonlinear combination of multiple correlated maps for simpler functional properties.
We hope that future fMRI studies will provide further information on the strength of maps for various functional properties in the ventral visual pathway, on the interactions between these maps, on their development, and on the role of experience in the construction and plasticity of these maps. At a more technical level, we expect to see more work conducted at a resolution close to 1 mm. These data are bound to provide new information about the extent and nature of functional specificity in the human brain15,28. Furthermore, parallel work in animals is needed to decipher the relationship between the spatial distribution of fMRI activation patterns and the spatial distribution of synaptic and output activity in single neurons. Together, these approaches will provide promising new opportunities for understanding both the neural code that underlies the recognition of complex visual stimuli and the relationship between maps and modules in the ventral visual pathway and other cortical areas.
Acknowledgments
We thank C. Baker, J. DiCarlo, B. Farley, G. Kayaert and J. Wagemans for their helpful comments on the manuscript. N.K. was supported by grant EY13455 and H.P.O. was supported by the Human Frontier Science Program, the Fund for Scientific Research Flanders, and grants IMPH/06/GHW and CREA/07/004.
- Map
A clustering of neurons with similar functional properties that is characterized by a gradual progression of preferred stimulus values across the cortical sheet
- Module
A clustering of neurons with similar functional properties that is characterized by discrete regions with clear boundaries across which there is no relation in preferred stimulus values
- Multi-voxel pattern analysis
Multivariate analysis of the spatial distribution of fMRI responses across large sets of voxels
- Eccentricity
The distance of the retinal stimulus position from the fovea (the central area of the retina that provides the best visual acuity)
- fMRI adaptation
A technique that makes use of the fact that the fMRI response to two sequentially presented stimuli is smaller (adapted) when the stimuli are identical or similar compared with when they are different
- Ocular dominance
The term that describes the characteristic of cells in the striate cortex to respond more strongly to input from one eye than from the other
- Foveation
Visual detection of an object by the fovea, the central area of the retina that provides the best visual acuity
Footnotes
FURTHER INFORMATION
Hans P. Op de Beeck’s homepage: http://ppw.kuleuven.be/~odbeeckh/
Nancy G. Kanwisher’s homepage: http://web.mit.edu/bcs/nklab/
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
References
- 1.Malach R, et al. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA. 1995;92:8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- 3.Grill-Spector K, et al. A sequence of object-processing stages revealed by fMRI in the human occipital lobe. Hum Brain Mapp. 1998;6:316–328. doi: 10.1002/(SICI)1097-0193(1998)6:4<316::AID-HBM9>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanwisher N, Woods RP, Iacoboni M, Mazziotta JC. A locus in human extrastriate cortex for visual shape analysis. J Cogn Neurosci. 1997;9:133–142. doi: 10.1162/jocn.1997.9.1.133. [DOI] [PubMed] [Google Scholar]
- 5.Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grill-Spector K. The neural basis of object perception. Curr Opin Neurobiol. 2003;13:159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- 8.Rossion B, et al. A network of occipito-temporal face-sensitive areas besides the right middle fusiform gyrus is necessary for normal face processing. Brain. 2003;126:2381–2395. doi: 10.1093/brain/awg241. [DOI] [PubMed] [Google Scholar]
- 9.Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- 10.Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- 11.Peelen MV, Downing PE. The neural basis of visual body perception. Nature Rev Neurosci. 2007;8:636–648. doi: 10.1038/nrn2195. This was the first detailed review of the processing of visually presented bodies in the visual system and beyond. [DOI] [PubMed] [Google Scholar]
- 12.Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2470–2473. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- 13.Downing PE, Wiggett AJ, Peelen MV. Functional magnetic resonance imaging investigation of overlapping lateral occipitotemporal activations using multi-voxel pattern analysis. J Neurosci. 2007;27:226–233. doi: 10.1523/JNEUROSCI.3619-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor JC, Wiggett AJ, Downing PE. Functional MRI analysis of body and body part representations in the extrastriate and fusiform body areas. J Neurophysiol. 2007;98:1626–1633. doi: 10.1152/jn.00012.2007. [DOI] [PubMed] [Google Scholar]
- 15.Schwarzlose RF, Baker CI, Kanwisher N. Separate face and body selectivity on the fusiform gyrus. J Neurosci. 2005;25:11055–11059. doi: 10.1523/JNEUROSCI.2621-05.2005. This study demonstrated the power of high-resolution fMRI to dissociate the selectivity of two adjacent functional regions (one face-selective, the other body-selective) that are lumped together as one larger region with less clear selectivity by standard-resolution fMRI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen L, et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- 17.Baker CI, et al. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proc Natl Acad Sci USA. 2007;104:9087–9092. doi: 10.1073/pnas.0703300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA. Differential sensitivity to words and shapes in ventral occipito-temporal cortex. Cereb Cortex. 2007;17:1604–1611. doi: 10.1093/cercor/bhl071. [DOI] [PubMed] [Google Scholar]
- 19.McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003;7:293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 20.Grill-Spector K, Malach R. The human visual cortex. Annu Rev Neurosci. 2004;27:649–677. doi: 10.1146/annurev.neuro.27.070203.144220. This paper provides a detailed review of what fMRI has taught us about the organization of the object-vision pathway in the human cortex. [DOI] [PubMed] [Google Scholar]
- 21.Downing PE, Chan AW, Peelen MV, Dodds CM, Kanwisher N. Domain specificity in visual cortex. Cereb Cortex. 2006;16:1453–1461. doi: 10.1093/cercor/bhj086. [DOI] [PubMed] [Google Scholar]
- 22.Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. This is an excellent review of the many recent fMRI studies that have made use of multi-voxel pattern analyses to understand the function and organization of the human brain. [DOI] [PubMed] [Google Scholar]
- 23.Haynes JD, Rees G. Decoding mental states from brain activity in humans. Nature Rev Neurosci. 2006;7:523–534. doi: 10.1038/nrn1931. [DOI] [PubMed] [Google Scholar]
- 24.Haxby JV, et al. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. This seminal paper introduced the basic thinking behind the now widely used multi-voxel pattern analyses. [DOI] [PubMed] [Google Scholar]
- 25.Cox DD, Savoy RL. Functional magnetic resonance imaging (fMRI) “brain reading”: detecting and classifying distributed patterns of fMRI activity in human visual cortex. Neuroimage. 2003;19:261–270. doi: 10.1016/s1053-8119(03)00049-1. [DOI] [PubMed] [Google Scholar]
- 26.Spiridon M, Kanwisher N. How distributed is visual category information in human occipito-temporal cortex? An fMRI study Neuron. 2002;35:1157–1165. doi: 10.1016/s0896-6273(02)00877-2. [DOI] [PubMed] [Google Scholar]
- 27.Hanson SJ, Matsuka T, Haxby JV. Combinatorial codes in ventral temporal lobe for object recognition: Haxby (2001) revisited: is there a “face” area? Neuroimage. 2004;23:156–166. doi: 10.1016/j.neuroimage.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 28.Grill-Spector K, Sayres R, Ress D. High-resolution imaging reveals highly selective nonface clusters in the fusiform face area. Nature Neurosci. 2006;9:1177–1185. doi: 10.1038/nn1745. [DOI] [PubMed] [Google Scholar]
- 29.Baker CI, Hutchison TL, Kanwisher N. Does the fusiform face area contain subregions highly selective for nonfaces? Nature Neurosci. 2007;10:3–4. doi: 10.1038/nn0107-3. [DOI] [PubMed] [Google Scholar]
- 30.Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center–periphery organization of human object areas. Nature Neurosci. 2001;4:533–539. doi: 10.1038/87490. This was the first of a series of studies that suggested that the retinal position at which objects from particular categories are commonly viewed might determine the anatomical location of category-selective brain regions. [DOI] [PubMed] [Google Scholar]
- 31.Hasson U, Levy I, Behrmann M, Hendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:479–490. doi: 10.1016/s0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- 32.Kim DJ, Tong F. Human ventral temporal areas contain flexible position-invariant information about subordinate-level objects. J Vis Abstr. 2005;5:855a. [Google Scholar]
- 33.Pessoa L, Padmala S. Quantitative prediction of perceptual decisions during near-threshold fear detection. Proc Natl Acad Sci USA. 2005;102:5612–5617. doi: 10.1073/pnas.0500566102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eger E, Ashburner J, Haynes JD, Dolan RJ, Rees G. fMRI activity patterns in human LOC carry information about object exemplars within category. J Cogn Neurosci. 2008;20:356–370. doi: 10.1162/jocn.2008.20019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriegeskorte N, Formisano E, Sorger B, Goebel R. Individual faces elicit distinct response patterns in human anterior temporal cortex. Proc Natl Acad Sci USA. 2007;104:20600–20605. doi: 10.1073/pnas.0705654104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eger E, Schweinberger SR, Dolan RJ, Henson RN. Familiarity enhances invariance of face representations in human ventral visual cortex: fMRI evidence. Neuroimage. 2005;26:1128–1139. doi: 10.1016/j.neuroimage.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Grill-Spector K, et al. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- 38.Kourtzi Z, Kanwisher N. Representation of perceived object shape by the human lateral occipital complex. Science. 2001;293:1506–1509. doi: 10.1126/science.1061133. [DOI] [PubMed] [Google Scholar]
- 39.Eger E, Schyns PG, Kleinschmidt A. Scale invariant adaptation in fusiform face-responsive regions. Neuroimage. 2004;22:232–242. doi: 10.1016/j.neuroimage.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 40.Macevoy SP, Epstein RA. Position selectivity in scene- and object-responsive occipitotemporal regions. J Neurophysiol. 2007;98:2089–2098. doi: 10.1152/jn.00438.2007. [DOI] [PubMed] [Google Scholar]
- 41.Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. Portraits or people? Distinct representations of face identity in the human visual cortex. J Cogn Neurosci. 2005;17:1043–1057. doi: 10.1162/0898929054475181. [DOI] [PubMed] [Google Scholar]
- 42.Epstein R, Graham KS, Downing PE. Viewpoint-specific scene representations in human parahippocampal cortex. Neuron. 2003;37:865–876. doi: 10.1016/s0896-6273(03)00117-x. [DOI] [PubMed] [Google Scholar]
- 43.Sawamura H, Orban GA, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: a single-cell study of the FMRI adaptation paradigm. Neuron. 2006;49:307–318. doi: 10.1016/j.neuron.2005.11.028. This study provided the first systematic investigation of what can and cannot be inferred about neuronal stimulus selectivity from neuronal adaptation. [DOI] [PubMed] [Google Scholar]
- 44.Kourtzi Z, Erb M, Grodd W, Bulthoff HH. Representation of the perceived 3-D object shape in the human lateral occipital complex. Cereb Cortex. 2003;13:911–920. doi: 10.1093/cercor/13.9.911. [DOI] [PubMed] [Google Scholar]
- 45.Pinsk MA, Desimone K, Moore T, Gross CG, Kastner S. Representations of faces and body parts in macaque temporal cortex: a functional MRI study. Proc Natl Acad Sci USA. 2005;102:6996–7001. doi: 10.1073/pnas.0502605102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nature Neurosci. 2003;6:989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. This study gave the first demonstration that a strong clustering of face-selective neurons underlies the strongly face-selective regions found with fMRI in both humans and monkeys. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiani R, Esteky H, Mirpour K, Tanaka K. Object category structure in response patterns of neuronal population in monkey inferior temporal cortex. J Neurophysiol. 2007;97:4296–4309. doi: 10.1152/jn.00024.2007. [DOI] [PubMed] [Google Scholar]
- 49.Kayaert G, Biederman I, Vogels R. Shape tuning in macaque inferior temporal cortex. J Neurosci. 2003;23:3016–3027. doi: 10.1523/JNEUROSCI.23-07-03016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Op de Beeck H, Wagemans J, Vogels R. Inferotemporal neurons represent low-dimensional configurations of parameterized shapes. Nature Neurosci. 2001;4:1244–1252. doi: 10.1038/nn767. [DOI] [PubMed] [Google Scholar]
- 51.Kayaert G, Biederman I, Op de Beeck HP, Vogels R. Tuning for shape dimensions in macaque inferior temporal cortex. Eur J Neurosci. 2005;22:212–224. doi: 10.1111/j.1460-9568.2005.04202.x. [DOI] [PubMed] [Google Scholar]
- 52.Logothetis NK, Pauls J. Psychophysical and physiological evidence for viewer-centered object representations in the primate. Cereb Cortex. 1995;5:270–288. doi: 10.1093/cercor/5.3.270. [DOI] [PubMed] [Google Scholar]
- 53.Op de Beeck H, Vogels R. Spatial sensitivity of macaque inferior temporal neurons. J Comp Neurol. 2000;426:505–518. doi: 10.1002/1096-9861(20001030)426:4<505::aid-cne1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 54.DiCarlo JJ, Maunsell JH. Anterior inferotemporal neurons of monkeys engaged in object recognition can be highly sensitive to object retinal position. J Neurophysiol. 2003;89:3264–3278. doi: 10.1152/jn.00358.2002. [DOI] [PubMed] [Google Scholar]
- 55.Horton JC, Adams DL. The cortical column: a structure without a function. Philos Trans R Soc Lond B Biol Sci. 2005;360:837–862. doi: 10.1098/rstb.2005.1623. This extensive review of decades of studies on cortical columns reaches the challenging conclusion that, despite the great amount of work that has been carried out, there is no clear evidence that a columnar structure serves any purpose. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fodor JA. Modularity of Mind: An Essay on Faculty Psychology. MIT Press; Cambridge, Massachusetts: 1983. [Google Scholar]
- 57.Van Essen DC, Newsome WT, Maunsell JH. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 1984;24:429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- 58.Yu H, Farley BJ, Jin DZ, Sur M. The coordinated mapping of visual space and response features in visual cortex. Neuron. 2005;47:267–280. doi: 10.1016/j.neuron.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Buzas P, Volgushev M, Eysel UT, Kisvarday ZF. Independence of visuotopic representation and orientation map in the visual cortex of the cat. Eur J Neurosci. 2003;18:957–968. doi: 10.1046/j.1460-9568.2003.02808.x. [DOI] [PubMed] [Google Scholar]
- 60.Hubel DH, Wiesel TN. Sequence regularity and geometry of orientation columns in the monkey striate cortex. J Comp Neurol. 1974;158:267–293. doi: 10.1002/cne.901580304. [DOI] [PubMed] [Google Scholar]
- 61.Blasdel GG. Orientation selectivity, preference, and continuity in monkey striate cortex. J Neurosci. 1992;12:3139–3161. doi: 10.1523/JNEUROSCI.12-08-03139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bonhoeffer T, Grinvald A. Iso-orientation domains in cat visual cortex are arranged in pinwheel-like patterns. Nature. 1991;353:429–431. doi: 10.1038/353429a0. [DOI] [PubMed] [Google Scholar]
- 63.Ohki K, et al. Highly ordered arrangement of single neurons in orientation pinwheels. Nature. 2006;442:925–928. doi: 10.1038/nature05019. [DOI] [PubMed] [Google Scholar]
- 64.Weliky M, Bosking WH, Fitzpatrick D. A systematic map of direction preference in primary visual cortex. Nature. 1996;379:725–728. doi: 10.1038/379725a0. [DOI] [PubMed] [Google Scholar]
- 65.Durbin R, Mitchison G. A dimension reduction framework for understanding cortical maps. Nature. 1990;343:644–647. doi: 10.1038/343644a0. [DOI] [PubMed] [Google Scholar]
- 66.Swindale NV. How different feature spaces may be represented in cortical maps. Network. 2004;15:217–242. [PubMed] [Google Scholar]
- 67.Aflalo TN, Graziano MS. Possible origins of the complex topographic organization of motor cortex: reduction of a multidimensional space onto a two-dimensional array. J Neurosci. 2006;26:6288–6297. doi: 10.1523/JNEUROSCI.0768-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu HD, Roe AW. Functional organization of color domains in V1 and V2 of macaque monkey revealed by optical imaging. Cereb Cortex. 2007 Jun;18 doi: 10.1093/cercor/bhm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bartfeld E, Grinvald A. Relationships between orientation-preference pinwheels, cytochrome oxidase blobs, and ocular-dominance columns in primate striate cortex. Proc Natl Acad Sci USA. 1992;89:11905–11909. doi: 10.1073/pnas.89.24.11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- 71.Catania KC. Early development of a somatosensory fovea: a head start in the cortical space race? Nature Neurosci. 2001;4:353–354. doi: 10.1038/85992. [DOI] [PubMed] [Google Scholar]
- 72.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- 73.Kanwisher N. Domain specificity in face perception. Nature Neurosci. 2000;3:759–763. doi: 10.1038/77664. [DOI] [PubMed] [Google Scholar]
- 74.Haxby JV, Ishai II, Chao LL, Ungerleider LG, Martin II. Object-form topology in the ventral temporal lobe. Response to I Gauthier (2000) Trends Cogn Sci. 2000;4:3–4. doi: 10.1016/s1364-6613(99)01423-0. [DOI] [PubMed] [Google Scholar]
- 75.Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci USA. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Edelman S. Representation is representation of similarities. Behav Brain Sci. 1998;21:449–467. doi: 10.1017/s0140525x98001253. [DOI] [PubMed] [Google Scholar]
- 77.Nosofsky RM. Attention, similarity, and the identification-categorization relationship. J Exp Psychol Gen. 1986;115:39–61. doi: 10.1037//0096-3445.115.1.39. [DOI] [PubMed] [Google Scholar]
- 78.Shepard RN. Toward a universal law of generalization for psychological science. Science. 1987;237:1317–1323. doi: 10.1126/science.3629243. [DOI] [PubMed] [Google Scholar]
- 79.Edelman S, Grill-Spector K, Kushnir T, Malach R. Toward direct visualization of the internal shape representation space by fMRI. Psychobiology. 1998;26:309–321. [Google Scholar]
- 80.Loffler G, Yourganov G, Wilkinson F, Wilson H. R fMRI evidence for the neural representation of faces. Nature Neurosci. 2005;8:1386–1390. doi: 10.1038/nn1538. [DOI] [PubMed] [Google Scholar]
- 81.Jiang X, et al. Evaluation of a shape-based model of human face discrimination using FMRI and behavioral techniques. Neuron. 2006;50:159–172. doi: 10.1016/j.neuron.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 82.Leopold DA, Bondar IV, Giese MA. Norm-based face encoding by single neurons in the monkey inferotemporal cortex. Nature. 2006;442:572–575. doi: 10.1038/nature04951. This systematic study of face neurons in the monkey brain showed that single neurons have monotonic tuning for a face with a specific identity and for how this face deviates from the average face. [DOI] [PubMed] [Google Scholar]
- 83.Rotshtein P, Henson RN, Treves A, Driver J, Dolan RJ. Morphing Marilyn into Maggie dissociates physical and identity face representations in the brain. Nature Neurosci. 2005;8:107–113. doi: 10.1038/nn1370. [DOI] [PubMed] [Google Scholar]
- 84.Newell FN, Bulthoff HH. Categorical perception of familiar objects. Cognition. 2002;85:113–143. doi: 10.1016/s0010-0277(02)00104-x. [DOI] [PubMed] [Google Scholar]
- 85.Panis S Perceived similarity between objects of the same category and prototypicality gradients in the lateral occipital complex. Society for Neuroscience Meeting; Washington DC. 2005. [Google Scholar]
- 86.Spiridon M, Fischl B, Kanwisher N. Location and spatial profile of category-specific regions in human extrastriate cortex. Hum Brain Mapp. 2006;27:77–89. doi: 10.1002/hbm.20169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wagemans JP. Modules in vision: a case study of interdisciplinarity in cognitive science. Acta Psychol (Amst) 1988;67:59–93. doi: 10.1016/0001-6918(88)90024-8. [DOI] [PubMed] [Google Scholar]
- 88.Yovel G, Kanwisher N. Face perception; domain specific, not process specific. Neuron. 2004;44:889–898. doi: 10.1016/j.neuron.2004.11.018. This paper provided a clear demonstration that the FFA is selective for a type of stimulus (namely faces), rather than for a type of processing (for example, configural processing) [DOI] [PubMed] [Google Scholar]
- 89.Golarai G, et al. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nature Neurosci. 2007;10:512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grinvald A, Lieke E, Frostig RD, Gilbert CD, Wiesel TN. Functional architecture of cortex revealed by optical imaging of intrinsic signals. Nature. 1986;324:361–364. doi: 10.1038/324361a0. [DOI] [PubMed] [Google Scholar]
- 92.Baker TI, Issa NP. Cortical maps of separable tuning properties predict population responses to complex visual stimuli. J Neurophysiol. 2005;94:775–787. doi: 10.1152/jn.01093.2004. [DOI] [PubMed] [Google Scholar]
- 93.Basole A, White LE, Fitzpatrick D. Mapping multiple features in the population response of visual cortex. Nature. 2003;423:986–990. doi: 10.1038/nature01721. [DOI] [PubMed] [Google Scholar]
- 94.Wilkinson F, et al. An fMRI study of the selective activation of human extrastriate form vision areas by radial and concentric gratings. Curr Biol. 2000;10:1455–1458. doi: 10.1016/s0960-9822(00)00800-9. [DOI] [PubMed] [Google Scholar]
- 95.Op de Beeck HP, Baker CI, DiCarlo JJ, Kanwisher NG. Discrimination training alters object representations in human extrastriate cortex. J Neurosci. 2006;26:13025–13036. doi: 10.1523/JNEUROSCI.2481-06.2006. This paper demonstrated that object-discrimination training alters the pattern of activity for trained objects across the extrastriate cortex in a way that is not predicted by either the pattern of activity before training or the distribution of face selectivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kobatake E, Tanaka K. Neuronal selectivities to complex object features in the ventral visual pathway of the macaque cerebral cortex. J Neurophysiol. 1994;71:856–867. doi: 10.1152/jn.1994.71.3.856. [DOI] [PubMed] [Google Scholar]
- 97.Tanaka K. Columns for complex visual object features in the inferotemporal cortex: clustering of cells with similar but slightly different stimulus selectivities. Cereb Cortex. 2003;13:90–99. doi: 10.1093/cercor/13.1.90. [DOI] [PubMed] [Google Scholar]
- 98.Pasupathy A, Connor CE. Population coding of shape in area V4. Nature Neurosci. 2002;5:1332–1338. doi: 10.1038/nn972. [DOI] [PubMed] [Google Scholar]
- 99.Brincat SL, Connor CE. Underlying principles of visual shape selectivity in posterior inferotemporal cortex. Nature Neurosci. 2004;7:880–886. doi: 10.1038/nn1278. [DOI] [PubMed] [Google Scholar]
- 100.Hayworth KJ, Biederman I. Neural evidence for intermediate representations in object recognition. Vision Res. 2006;46:4024–4031. doi: 10.1016/j.visres.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 101.Marr D, Nishihara HK. Representation and recognition of the spatial organization of three-dimensional shapes. Proc R Soc Lond B Biol Sci. 1978;200:269–294. doi: 10.1098/rspb.1978.0020. [DOI] [PubMed] [Google Scholar]
- 102.Biederman I. Recognition-by-components: a theory of human image understanding. Psychol Rev. 1987;94:115–147. doi: 10.1037/0033-295X.94.2.115. [DOI] [PubMed] [Google Scholar]
- 103.Knoblich U, Riesenhuber M. Stimulus simplification and object representation: a modeling study. MIT Computer Science and Artificial Intelligence Laboratory. 2007 [online] http://people.csail.mit.edu/knoblich/papers/AIM-2002-004.pdf.
- 104.Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nature Neurosci. 2000;3:764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- 105.Tanaka JW, Farah MJ. Second-order relational properties and the inversion effect: testing a theory of face perception. Percept Psychophys. 1991;50:367–372. doi: 10.3758/bf03212229. [DOI] [PubMed] [Google Scholar]
- 106.Farah MJ, Tanaka JW, Drain HM. What causes the face inversion effect? J Exp Psychol Hum Percept Perform. 1995;21:628–634. doi: 10.1037//0096-1523.21.3.628. [DOI] [PubMed] [Google Scholar]
- 107.Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychol Rev. 1998;105:482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- 108.Young AW, Hellawell D, Hay DC. Configurational information in face perception. Perception. 1987;16:747–759. doi: 10.1068/p160747. [DOI] [PubMed] [Google Scholar]
- 109.Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nature Neurosci. 2000;3:191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- 110.Xu Y. Revisiting the role of the fusiform face area in visual expertise. Cereb Cortex. 2005;15:1234–1242. doi: 10.1093/cercor/bhi006. [DOI] [PubMed] [Google Scholar]
- 111.Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nature Neurosci. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- 112.McKone E, Kanwisher N, Duchaine BC. Can generic expertise explain special processing for faces? Trends Cogn Sci. 2007;11:8–15. doi: 10.1016/j.tics.2006.11.002. This review of the recent literature indicates that classic face-selective effects that can be measured behaviourally and neurally cannot be accounted for in terms of generic expertise. [DOI] [PubMed] [Google Scholar]
- 113.Robbins R, McKone E. No face-like processing for objects-of-expertise in three behavioural tasks. Cognition. 2007;103:34–79. doi: 10.1016/j.cognition.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 114.Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- 115.Mahon BZ, et al. Action-related properties shape object representations in the ventral stream. Neuron. 2007;55:507–520. doi: 10.1016/j.neuron.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 117.Riesenhuber M, Jarudi I, Gilad S, Sinha P. Face processing in humans is compatible with a simple shape-based model of vision. Proc Biol Sci. 2004;271 (Suppl 6):S448–S450. doi: 10.1098/rsbl.2004.0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ullman S. Object recognition and segmentation by a fragment-based hierarchy. Trends Cogn Sci. 2007;11:58–64. doi: 10.1016/j.tics.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 119.Ullman S, Vidal-Naquet M, Sali E. Visual features of intermediate complexity and their use in classification. Nature Neurosci. 2002;5:682–687. doi: 10.1038/nn870. [DOI] [PubMed] [Google Scholar]
- 120.Kayaert G, Biederman I, Vogels R. Representation of regular and irregular shapes in macaque inferotemporal cortex. Cereb Cortex. 2005;15:1308–1321. doi: 10.1093/cercor/bhi014. [DOI] [PubMed] [Google Scholar]
- 121.Serre T, Oliva A, Poggio T. A feedforward architecture accounts for rapid categorization. Proc Natl Acad Sci USA. 2007;104:6424–6429. doi: 10.1073/pnas.0700622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jiang X, et al. Categorization training results in shape- and category-selective human neural plasticity. Neuron. 2007;53:891–903. doi: 10.1016/j.neuron.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sigala N, Logothetis NK. Visual categorization shapes feature selectivity in the primate temporal cortex. Nature. 2002;415:318–320. doi: 10.1038/415318a. [DOI] [PubMed] [Google Scholar]
- 124.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. A comparison of primate prefrontal and inferior temporal cortices during visual categorization. J Neurosci. 2003;23:5235–5246. doi: 10.1523/JNEUROSCI.23-12-05235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Goldstone R. Influences of categorization on perceptual discrimination. J Exp Psychol Gen. 1994;123:178–200. doi: 10.1037//0096-3445.123.2.178. [DOI] [PubMed] [Google Scholar]
- 126.Op de Beeck H, Wagemans J, Vogels R. The effect of category learning on the representation of shape: dimensions can be biased but not differentiated. J Exp Psychol Gen. 2003;132:491–511. doi: 10.1037/0096-3445.132.4.491. [DOI] [PubMed] [Google Scholar]
- 127.Van Essen DC, Newsome WT, Maunsell JH. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 1984;24:429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- 128.Tootell RB, Switkes E, Silverman MS, Hamilton SL. Functional anatomy of macaque striate cortex. II Retinotopic organization. J Neurosci. 1988;8:1531–1568. doi: 10.1523/JNEUROSCI.08-05-01531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sereno MI, et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- 130.Engel SA, et al. fMRI of human visual cortex. Nature. 1994:369–525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- 131.Hubel DH, Wiesel TN. Anatomical demonstration of columns in the monkey striate cortex. Nature. 1969;221:747–750. doi: 10.1038/221747a0. [DOI] [PubMed] [Google Scholar]
- 132.Hubel DH, Wiesel TN. Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B Biol Sci. 1977;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- 133.Cheng K, Waggoner RA, Tanaka K. Human ocular dominance columns as revealed by high-field functional magnetic resonance imaging. Neuron. 2001;32:359–374. doi: 10.1016/s0896-6273(01)00477-9. [DOI] [PubMed] [Google Scholar]
- 134.Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- 135.Zhao F, Wang P, Hendrich K, Kim SG. Spatial specificity of cerebral blood volume-weighted fMRI responses at columnar resolution. Neuroimage. 2005;27:416–424. doi: 10.1016/j.neuroimage.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 136.Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nature Neurosci. 2005;8:679–685. doi: 10.1038/nn1444. This paper provided one of the clearest demonstrations of the power of multi-voxel pattern analyses to decipher the organization of cortical areas in the absence of strong functional specificity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 138.Van Essen DC. Windows on the brain: the emerging role of atlases and databases in neuroscience. Curr Opin Neurobiol. 2002;12:574–579. doi: 10.1016/s0959-4388(02)00361-6. [DOI] [PubMed] [Google Scholar]
- 139.Van Essen DC, et al. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Van Essen DC, et al. Mapping visual cortex in monkeys and humans using surface-based atlases. Vision Res. 2001;41:1359–1378. doi: 10.1016/s0042-6989(01)00045-1. [DOI] [PubMed] [Google Scholar]