Abstract
In obstructive sleep apnea (OSA), oxidative stress contributes to endothelial dysfunction in the peripheral circulation. In the lung, oxidative stress can lead to alveolar injury. We hypothesized that patients with obstructive sleep apnea would have biomarker evidence of increased alveolar wall permeability.
We examined sleep characteristics, brachial artery flow-mediated dilation, and plasma KL-6 levels in 11 otherwise healthy patients with OSA and 10 controls at our center.
Plasma KL-6 levels were higher in patients with OSA compared to controls: median 317 U/ml (interquartile range 232 to 506) versus 226 U/ml (interquartile range 179 to 257), respectively. Higher plasma KL-6 levels were associated with greater time spent in sleep with an oxyhemoglobin saturation < 90%, lower nadir saturation, more frequent desaturation of > 4% during sleep, and lower brachial artery flow-mediated dilation. Adjustment for nadir saturation or flow-mediated dilation attenuated the association between plasma KL-6 level and OSA.
Circulating KL-6 levels are elevated in some patients with OSA, possibly reflecting increased alveolar wall permeability.
Keywords: alveolar wall permeability, biological markers, hypoxia, obstructive sleep apnea
INTRODUCTION
Obstructive sleep apnea (OSA), a chronic disorder characterized by sleep-related upper airway obstruction [1], is associated with increased oxidative stress, which contributes to endothelial dysfunction in the peripheral circulation [2]. The injurious effects of oxidative stress in the lungs of patients with OSA, however, are largely unknown.
Oxidative stress in the alveolus can lead to epithelial and endothelial cell injury which increases permeability of the alveolar wall, which, when severe, causes pulmonary edema [3]. While pulmonary edema occurs routinely in a dog model of OSA [4], it has only rarely been reported in humans with OSA [4]. Therefore, if OSA injures the alveolus, such injury would be expected to be mild and lead to only modest increases in alveolar wall permeability. We hypothesized that we would be able to detect subclinical lung injury in OSA by measuring circulating levels of KL-6, a mucin-like integral membrane glycoprotein localized to type II alveolar epithelial cells that is consistently elevated in the blood of patients with lung injury [5–7].
METHODS
Study Design and Subjects
We retrospectively studied 11 OSA patients and 10 controls enrolled in a prospective study at our center (See reference [2] for details regarding the cohort). In brief, patients with newly diagnosed OSA, defined as an apnea-hypopnea index (AHI) of ≥ 5 obstructive events per hour of sleep, and free of conditions known to affect the vascular endothelium were eligible for the prospective study. Exclusion criteria included cardiovascular disease or risk factors, pulmonary disease, recent smoking, and medication use [2].
Control subjects were nonsmoking healthy adults recruited from the community through advertising matched to OSA patients by gender, age (within 4 years), and body mass index (BMI) (within 15%). All control subjects had a normal physical examination and laboratory tests and underwent nocturnal polysomnography.
In the current study, we included all study subjects who provided plasma during participation in the prospective study. The Columbia University Medical Center Institutional Review Board approved the study.
Study Protocol
All study participants underwent attended nocturnal polysomnography in our center, as previously described [2]. Apneas, hypopneas, and sleep staging were defined as outlined by the American Academy of Sleep Medicine [8]. The Epworth Sleepiness Scale score was calculated as previously described [9]. Blood sample collection and flow-mediated dilation (FMD) were performed between 9 and 11 AM within 48 hours of polysomnography while fasting (See online depository) [10]. Platelet-poor plasma was isolated from citrated blood by centrifugation at 700 rpm for 10 minutes followed by centrifugation at 1800 rpm for 6 minutes.
KL-6 Measurement
Plasma KL-6 was measured by a sandwich-type enzyme-linked immunosorbent assay using a KL-6 antibody kit (Eisai network group Sanko Junyaku Co., LTD, Japan) (See online depository for details). The mean ± SD KL-6 level in healthy individuals is 239 ± 102 U/ml with a reference range of 40 – 438 U/ml [11].
Analysis
Continuous data are presented as mean ± SD or median (interquartile range). Categorical data are presented as percentage. We used Wilcoxon rank sum tests and Spearman correlation coefficients, as appropriate. We explored potential confounders and mediators of the association between KL-6 levels and OSA by regressing KL-6 level on OSA adjusting for a single covariate using linear regression. In light of our small sample size, we also used partial Spearman correlation coefficients to examine adjusted correlations with KL-6. Since both analyses resulted in similar findings, only the results of the linear models are shown.
Assuming a mean KL-6 level of 239 U/ml among controls and a standard deviation of 102 U/ml, we estimated that we would have 80% power to detect a mean level of 378 U/ml among those with OSA with alpha = 0.05 using a two-sided Wilcoxon-rank sum test [11]. Statistical significance was defined as two-tailed P-values <0.05. Analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
Eleven of the 30 subjects with OSA and 10 of the 15 controls in the prospective study had plasma available for analysis and were included in the current study (See Figure E1 in the online depository). The mean age was 40 ± 12 years and 52% were male. The mean BMI was 31 ± 6 kg/m2. Subjects were well matched by age, gender, and BMI (Table 1).
Table 1.
Participant characteristics
| Obstructive sleep apnea | Controls | |
|---|---|---|
| No. | 11 | 10 |
| Age, years | 40 ± 14 | 40 ± 9 |
| Male | 6 | 5 |
| Body mass index, kg/m2 | 32 ± 6 | 30 ± 7 |
| Apnea hypopnea index, events/h of sleep | 40 ± 37 | 1 ± 2 |
| ODI4, events/h of sleep | 11 (0 – 28) | 0 (0 – 0) |
| SpO2 nadir, % | 85 ± 9 | 96 ± 3 |
| Time SpO2 < 90%, % of total sleep time | 1 (0 – 16) | 0 (0 – 0) |
| Epworth sleepiness scale score | 5 ± 5 | 5 ± 4 |
| Brachial artery flow-mediated dilation, % | 4 ± 3 | 10 ± 3 |
Data are mean ± SD, median (interquartile range), and frequency.
ODI4 = Number of arterial oxyhemoglobin desaturation events ≥ 4% per hour of sleep;
SpO2 = oxyhemoglobin saturation.
Plasma KL-6 levels were significantly higher in those with OSA compared to controls: median value 317 U/ml (interquartile range 232 to 506) versus 226 U/ml (interquartile range 179 to 257) in OSA and controls, respectively (p = 0.03; Figure 1).
Figure 1.
Boxplots of plasma KL-6 levels in 11 subjects with obstructive sleep apnea and 10 controls. Dark horizontal lines = median values, upper and lower bounds of the box = interquartile ranges, whisker tips extend to the most extreme value within 1.5 times the interquartile range, black diamonds = individual KL-6 levels for the 21 study subjects. Wilcoxon ranks sum test p value = 0.03 for the comparison between groups. OSA = obstructive sleep apnea
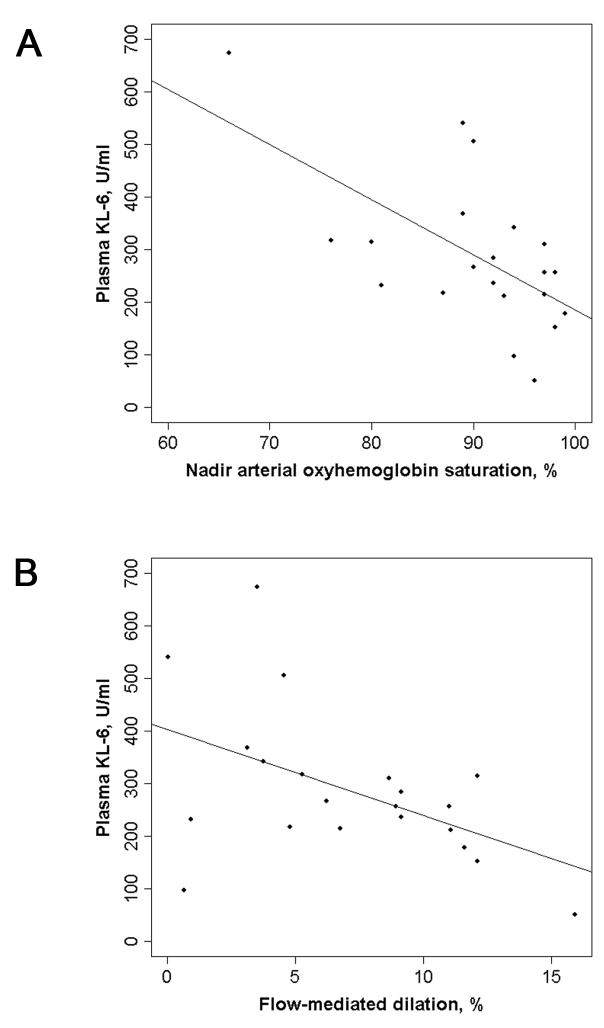
A higher plasma KL-6 level was associated with greater AHI, greater time spent with SpO2 < 90%, lower nadir SpO2, more frequent oxyhemoglobin desaturation > 4 % per hour of sleep (ODI4), and lower brachial artery FMD (Table 2 and Figure 2).
Table 2.
Spearman correlation coefficients between plasma KL-6 level and demographics, polysomnogram results, and flow-mediated dilation
| Variable | Spearman correlation coefficient with plasma KL-6 level | p value |
|---|---|---|
| Age | −0.10 | 0.68 |
| Body mass index | 0.04 | 0.87 |
| Polysomnography | ||
| Apnea hypopnea index | 0.61 | 0.003 |
| ODI4 | 0.49 | 0.02 |
| SpO2 nadir | −0.57 | 0.007 |
| Time SpO2 < 90% | 0.53 | 0.01 |
| Epworth Sleepiness Scale | −0.04 | 0.86 |
| Brachial artery flow-mediated dilation | −0.49 | 0.03 |
ODI4 = Number of arterial oxyhemoglobin desaturation events ≥ 4% per hour of sleep;
SpO2 = oxyhemoglobin saturation.
Figure 2.
Scatterplots of plasma KL-6 level with (A) nadir arterial oxyhemoglobin saturation during sleep and (B) brachial artery flow-mediated dilation. Solid lines are regression lines. Spearman correlation coefficient = −0.57 for nadir saturation (p = 0.007) and −0.49 for flow-mediated dilation (p = 0.03).
The means increase in KL-6 among those with OSA compared to controls was 136 U/ml (95% confidence interval 25 – 145, p = 0.03). Adjustment for nadir SpO2 or for FMD attenuated this difference, suggesting that hypoxemia and/or endothelial dysfunction might have contributed to higher KL-6 levels in OSA (Table 3). Nonparametric methods showed similar findings (Table E1).
Table 3.
Unadjusted and adjusted mean increases in plasma KL-6 level in subjects with obstructive sleep apnea compared to controls
| Model | Mean increase in plasma KL-6 in OSA compared to controls | 95% CI | p value |
|---|---|---|---|
| Unadjusted | 137 | 25 – 245 | 0.03 |
| Adjusted for: | |||
| Age | 136 | 24 – 248 | 0.03 |
| Gender | 139 | 27 – 251 | 0.03 |
| Body mass index | 133 | 17 – 149 | 0.04 |
| ODI4 | 153 | 25 – 280 | 0.03 |
| SpO2 nadir | 42 | −95 – 179 | 0.56 |
| Time SpO2 < 90% | 115 | −8 – 239 | 0.08 |
| Epworth sleepiness scale score | 137 | 23 – 251 | 0.03 |
| Brachial artery flow-mediated dilation | 75 | −92 – 242 | 0.39 |
ODI4 = Number of arterial oxyhemoglobin desaturation events ≥ 4% per hour of sleep;
SpO2 = oxyhemoglobin saturation.
Each mean increase in plasma KL-6 is adjusted for the covariate in the first column
One control subject had a plasma KL-6 level below the lower limit of detection. When we excluded this subject, the results were virtually identical. Similarly, when we excluded the subject with the highest KL-6 level (673 U/ml), our results remained significant.
DISCUSSION
In this preliminary study, we found that some patients with OSA had elevated circulating levels of KL-6, a marker of increased alveolar wall permeability, compared to age, gender, and BMI-matched controls. KL-6 levels correlated strongly with nocturnal hypoxemia and peripheral endothelial dysfunction as measured by brachial artery FMD. These same factors appeared to explain, at least in part, the higher levels of KL-6 in OSA patients.
Our results suggest that alveolar wall permeability may be increased in OSA. One mechanism by which OSA could increase the permeability of the alveolar wall is through the generation of oxidative stress arising from cyclic local hypoxia and reoxygenation in the alveolus. OSA is thought to cause peripheral endothelial dysfunction through this mechanism [2]. In our study, greater nocturnal hypoxemia was associated with higher circulating KL-6 levels, and the degree of nocturnal hypoxemia appeared to explain at least some of the elevation in KL-6 in OSA. Our results must be interpreted cautiously given our small sample size. Nevertheless, our study suggests a role for local hypoxia in the development of increased alveolar wall permeability in OSA.
In addition to hypoxia-reoxygenation, mechanical stretch is an intuitively appealing mechanism of epithelial or endothelial cell injury in the lung. Dynamic changes in intrathoracic pressure (Mueller maneuvers) accompany obstructive apneas and hypopneas and could conceivably deform alveolar epithelial cells and endothelial cells, mirroring the changes seen in ventilator-induced lung injury [12] and perhaps leading to stress failure of endothelial cells [13]. Our study was not designed to examine this mechanism.
The significance of the association between higher circulating KL-6 levels and greater endothelial dysfunction in the brachial artery is unclear. One explanation might be a common antecedent cause of both alveolar injury and peripheral endothelial injury, such as oxidative stress. Alternatively, this association may signal a role for pulmonary endothelial dysfunction in the development of increased alveolar wall permeability in OSA. Additional studies of pulmonary endothelial dysfunction and injury in OSA may clarify this finding.
Our results suggest that OSA may be a novel risk factor for subclinical lung injury. The consequences of such injury repeated nightly over many years are unknown. In susceptible individuals, we speculate that such injury might be followed by abnormal wound healing and local fibrosis in areas prone to local hypoxia and mechanical stretch during a Mueller maneuver. Intriguingly, an association between OSA and idiopathic pulmonary fibrosis, a disease characterized by peripheral lower lobe fibrosis, was recently reported [14]. Our findings should prompt further study of the mechanisms (and confounders) of this association.
Our study had several limitations. First and foremost, we examined only 11 patients with OSA at a single center, making chance and selection bias plausible explanations for our results. However, our cohort was recruited from consecutive patients with suspected sleep-disordered breathing, and circulating KL-6 levels were associated with nocturnal hypoxemia and endothelial dysfunction in mechanistically plausible directions concordant with our a priori hypothesis. Nevertheless, our results should be confirmed in a larger cohort with greater power to perform multivariate adjustment and examine markers of epithelial and endothelial injury and function. Second, we did not directly measure alveolar wall permeability. Instead, we relied on a circulating biomarker that has consistently been shown to be elevated in those with lung injury. Finally, the increase in circulating KL-6 in OSA patients was modest, albeit in a range similar to that of acute lung injury survivors [5].
In summary, we detected elevated circulating levels of KL-6 in patients with OSA, suggesting that alveolar wall permeability might be increased in OSA. Additional studies should focus on determining the site, mechanisms, and consequences of this finding.
Supplementary Material
Acknowledgments
We wish to acknowledge Sanko Junyaku and Eisai Company for kindly performing the KL-6 assays.
Funding: NIH K23 HL086714
References
- 1.Basner RC. Continuous positive airway pressure for obstructive sleep apnea. N Engl J Med. 2007;356:1751–1758. doi: 10.1056/NEJMct066953. [DOI] [PubMed] [Google Scholar]
- 2.Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–2278. doi: 10.1161/CIRCULATIONAHA.107.741512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cochrane CG, Spragg R, Revak SD. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983;71:754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhary BA, Ferguson DS, Speir WA., Jr Pulmonary edema as a presenting feature of sleep apnea syndrome. Chest. 1982;82:122–124. doi: 10.1378/chest.82.1.122. [DOI] [PubMed] [Google Scholar]
- 5.Ishizaka A, Matsuda T, Albertine KH, Koh H, Tasaka S, Hasegawa N, Kohno N, Kotani T, Morisaki H, Takeda J, Nakamura M, Fang X, Martin TR, Matthay MA, Hashimoto S. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1088–1094. doi: 10.1152/ajplung.00420.2002. [DOI] [PubMed] [Google Scholar]
- 6.Nathani N, Perkins GD, Tunnicliffe W, Murphy N, Manji M, Thickett DR. Kerbs von Lungren 6 antigen is a marker of alveolar inflammation but not of infection in patients with acute respiratory distress syndrome. Crit Care. 2008;12:R12. doi: 10.1186/cc6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato H, Callister ME, Mumby S, Quinlan GJ, Welsh KI, duBois RM, Evans TW. KL-6 levels are elevated in plasma from patients with acute respiratory distress syndrome. Eur Respir J. 2004;23:142–145. doi: 10.1183/09031936.03.00070303. [DOI] [PubMed] [Google Scholar]
- 8.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1. American Academy of Sleep Medicine; Westchester, IL: 2007. [Google Scholar]
- 9.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 10.Corretti MC, Anderson TJ, Benjamin EJ, Clearwater D, Chardonnay F, Creamer MA, Dean field J, Drexel H, Gerhard-Herman M, Herrington D, Valance P, Vita J, Vogel R for the International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilatation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Cull Cardio. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 11.Janssen R, Krait A, Gutters JOCK, Raven HE, Garretson WB, van den Bosch JAM. The mucking-1 568 adenosine to guanine polymorphism influences serum Krebs von den Lunge-6 levels. Am J Respire Cell Mol Boil. 2006;34:496–499. doi: 10.1165/rcmb.2005-0151OC. [DOI] [PubMed] [Google Scholar]
- 12.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respire Crit Care Med. 2000;162:357–362. doi: 10.1164/ajrccm.162.2.9807003. [DOI] [PubMed] [Google Scholar]
- 13.West JB, Mathieu-Costello O. Stress failure of pulmonary capillaries: role in lung and heart disease. Lancet. 1992;340:762–767. doi: 10.1016/0140-6736(92)92301-u. [DOI] [PubMed] [Google Scholar]
- 14.Lancaster LH, Mason WR, Parnell JA, Rice TW, Loyd JE, Milstone AP, Collard HR, Malow BA. Obstructive sleep apnea is common in IPF [abstract] Am J Respire Crit Care Med. 2008;177:A247. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.