Abstract
Objective
To assess mortality associated with mild cognitive impairment (MCI) and Alzheimer's disease (AD) in older African Americans and whites from an urban community.
Design
Longitudinal population-based observational study.
Setting
Four adjacent neighborhoods of Chicago, IL.
Participants
1,715 persons deemed free of dementia in a previous wave of data collection and sampled for detailed clinical evaluation: 802 had no cognitive impairment, 597 had MCI, 296 had AD, and 20 had other forms of dementia.
Main Outcome Measure
All-cause mortality.
Results
During up to 10 years of observation (mean = 4.7, SD = 3.0), 634 individuals died (37%). Compared to people without cognitive impairment, risk of death was increased by about 50% in those with MCI (hazard ratio [HR] = 1.48; 95% confidence interval [CI]: 1.22, 1.80) and was nearly threefold greater in those with AD (HR = 2.84; 95% CI:2.29, 3.52). These effects were seen in both African Americans and whites and did not differ by race. In MCI, risk of death increased with increasing severity of cognitive impairment and this effect did not vary by race. A similar effect was seen in AD, but it was slightly stronger for black than white persons. In both MCI and AD, the association of cognitive impairment with survival was stronger for perceptual speed than for other cognitive functions.
Conclusion
The presence and severity of MCI and AD are associated with reduced survival in African Americans and these effects are comparable to those seen in white persons.
Alzheimer's disease (AD) substantially reduces life expectancy [1-7] and has emerged as a leading cause of death in the United States [5,8]. Data from two national surveys suggest that life expectancy in the disease may be greater for African Americans than for white persons [9,10]. However, not all surveys have observed this difference [11]. Further, the diagnosis of AD in these surveys is not based on a uniform clinical evaluation but derived from medical records, increasing the likelihood of substantial variation in quality of diagnostic classifications.
Here we evaluate risk of death associated with incident AD in older African Americans and white persons residing in an urban community. Diagnoses were based on a uniform detailed clinical evaluation. We also examined survival in mild cognitive impairment (MCI), a precursor to AD that is common in older people [12,13]. To date, there have been relatively few population-based studies of survival in MCI [14-17]. Because these studies have primarily focused on white persons, little is known about survival in African Americans with MCI.
METHODS
Participants
Subjects are from the Chicago Health and Aging Project, a longitudinal study of aging and AD conducted in 3 adjacent neighborhoods in Chicago [18]. It began with a census of the community, after which all those aged 65 years or older were invited to participate in an in home interview that included brief tests of cognitive function. A stratified random sample of those interviewed was selected for a detailed clinical evaluation (see below). Approximately 3 years later, the population interview was repeated and a stratified random sample of those determined to be without dementia in the previous wave of data collection had a clinical evaluation. These two steps (interview of population, clinical evaluation of a new sample of persons previously deemed to be free of dementia) have been repeated at intervals of approximately 3 years, with the fifth wave of data collection now in progress. For these analyses, we included all persons who completed a clinical evaluation in the second wave of data collection or in a subsequent wave (i.e., third, fourth, or fifth) and monitored vital status after the date of the clinical evaluation. In the event that a person was sampled for more than one clinical evaluation, we used the earliest to maximize the length of the monitoring period. Those selected had a mean age of 80.1 (SD = 5.9) and had completed a mean of 12.7 years of education (SD = 3.6); 61.3% were women and 52.5% were African American.
Clinical Evaluation
Each participant had a structured uniform clinical evaluation that included a medical history, complete neurological examination, and cognitive function testing (see below), as previously described [19,20]. On the basis of this evaluation and an in person examination of the subject, an experienced physician classified individuals with respect to dementia and AD using the guidelines of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association [21]. The criteria require a history of cognitive decline and impairment in at least two cognitive domains. For the diagnosis of AD, one of the impaired domains must be memory.
MCI was diagnosed in a 2-step process, as previously described [22,23]. First, after review of all cognitive data, a neuropsychologist rated impairment in 5 cognitive domains. To maintain consistency in ratings, educationally adjusted cutoff scores on 11 tests and algorithmic ratings of each domain were provided [22]. Second, the clinician determined whether dementia criteria were met. Those with cognitive impairment but without dementia were deemed to have MCI. In other cohorts, persons meeting these criteria have shown intermediate levels of cognitive decline, AD pathology, and cerebral infarction relative to dementia and no cognitive impairment subgroups [24].
Apolipoprotein E genotyping was done by persons blinded to all clinical data using methods adapted from Hixon and Vernier [25].
Assessment of Cognitive Function
In an approximately 1-hour session, cognitive function was assessed with a battery of 18 tests. The Mini-Mental State Examination was used to describe level of global cognition but was not used in analyses. Episodic memory was assessed with 7 measures: immediate and delayed recall of the East Boston Story and story A from the Wechsler Memory Scale-Revised and Word List Memory, Recall, and Recognition. Semantic memory was assessed with a 15-item version of the Boston Naming Test, Verbal Fluency, and a 15-item version of the National Adult Reading Test. Digit Span Forward, Digit Span Backward, and Digit Ordering were used to measure working memory. Perceptual speed was measured with the oral version of the Symbol Digit Modalities Test and Number Comparison, and visuospatial ability was assessed with a 15-item version of Judgment of Line Orientation and a 12-item version of Standard Progressive Matrices.
To minimize floor and ceiling artifacts and other sources of measurement error, we used composite measures of two or more tests in analyses. A composite measure of global cognition was constructed using all 17 tests. To develop measures of specific cognitive functions, we hypothesized that the tests could be grouped into 5 domains: episodic memory, semantic memory, working memory, perceptual speed, visuospatial ability. In participants without dementia, we performed a factor analysis of the 17 tests with varimax rotation. The factor analytic grouping showed good agreement with the hypothesized grouping (Rand's statistic [26], a measure of goodness of fit ranging from -1 to 1, was 0.62, p<.001). Therefore, we used the hypothesized groupings to make summary measures of episodic memory (7 tests), semantic memory (3 tests), working memory (3 tests), perceptual speed (2 tests), and visuospatial ability (2 tests). For all composite scores, raw scores were converted to z scores, using the mean and standard deviation of the full group, and z scores on component tests were averaged to yield the composite, as previously described for other cohorts [27,28].
Determination of Vital Status
Information about death was obtained through multiple sources as previously reported [29]. Study personnel often learned of the death of a participant when attempting to schedule follow-up interviews. In addition, we regularly scanned local newspapers for obituaries and searched web sites such as www.ancestry.com. We also attempted to verify all deaths by acquiring a death record from the National Death Index.
Data Analysis
We analyzed the relation of diagnosis to risk of death in a series of proportional hazards models [30]. In these analyses, persons without cognitive impairment were used as a reference group that was contrasted with each diagnostic group. Terms were also included to control for age, sex, education, and race. In subsequent analyses, we added terms for comorbid medical conditions, possession of a copy of the apolipoprotein E ε4 allele, or socioeconomic status. We also conducted separate analyses in white and African American persons and repeated the original analysis with terms for the interaction of race with MCI and with AD.
In separate analyses within the MCI and AD subgroups, we examined the relation of severity of cognitive impairment to risk of death. The initial analyses included a term for level of global cognition at baseline. We then conducted separate analyses in black and white persons and repeated the initial analysis of the AD group with a term for the interaction of race with global cognitive score. In subsequent analyses, we substituted measures of specific cognitive functions for the global measure, first in separate models and then together in a single model.
Models were graphically and analytically validated. Programming was done in SAS [31].
RESULTS
A total of 802 people without cognitive impairment, 597 with MCI, 296 with AD, and 20 with other forms of dementia completed the clinical evaluation. As shown in Table 1, those with dementia were slightly older and less educated than those without dementia.
Table 1.
Descriptive information about the diagnostic subgroups at the time of the clinical evaluation*
| No Cognitive Imp. | Mild Cognitive Imp. | Alzheimer's Dis. | Other Dementia | |
|---|---|---|---|---|
| Characteristic | (n = 802) | (n = 597) | (n = 296) | (n = 20) |
| Age, y | 78.6 (5.3) | 80.7 (5.9) | 82.9 (5.9) | 82.6 (7.7) |
| Education, y | 13.4 (3.4) | 12.5 (3.6) | 11.5 (3.7) | 11.9 (3.0) |
| Women, % | 60.7 | 62.1 | 61.5 | 60.0 |
| African Am.,% | 43.3 | 60.0 | 61.5 | 70.0 |
| MMSE score | 27.9 (2.0) | 26.1 (2.8) | 19.5 (5.9) | 19.0 (7.2) |
| Deceased, % | 25.8 | 40.4 | 59.1 | 60.0 |
MMSE indicates Mini-Mental State Examination.
Dementia Diagnosis and Mortality
Vital status was monitored for up to 10 years (mean = 4.7, SD = 3.0). During this period, 634 individuals died (37.0%), including 25.8% of those without cognitive impairment, 40.4% of those with MCI, 59.1% of those with AD, and 60.0% of those with other forms of dementia. We examined the relation of diagnosis to risk of death in a series of proportional hazards models.
These and all subsequent analyses were adjusted for the potentially confounding influences of age, sex, race, and education. Individuals without cognitive impairment served as a reference group that was contrasted with each of the other diagnostic subgroups (i.e., MCI, AD, other dementia). As shown in the first column of Table 2, compared to those without cognitive impairment, risk of death was about 50% higher in those with MCI and nearly three times higher in those with AD or other forms of dementia. Results were similar after controlling for common chronic medical conditions (i.e., heart disease, hypertension, stroke, diabetes, cancer; hazard ratio [HR] for MCI = 1.44; 95% confidence interval [CI]:1.19,1.75; HR for AD = 2.81; 95% CI: 2.25, 3.50) possession of at least one apolipoprotein E ε4 allele (HR for MCI = 1.45; 95% CI:1.19, 1.77; HR for AD = 2.91; 95% CI: 2.32, 3.65), or socioeconomic indicators (i.e., occupation and income; HR for MCI = 1.49; 95% CI: 1.21, 1.82; HR for AD = 2.81; 95% CI:2.233, 3.55).
Table 2.
Relation of mild cognitive impairment and Alzheimer's disease to risk of death in all persons and racial subgroups*
| All Persons | African Americans | Whites | |
|---|---|---|---|
| Diagnosis | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Mild cognitive impairment | 1.48 (1.22,1.80) | 1.40 (1.04,1.88) | 1.57 (1.22,2.02) |
| Alzheimer's disease | 2.84 (2.29, 3.52) | 2.59(1.88,3.59) | 3.20 (2.40, 2.47) |
| Other dementia** | 2.69 (1.49,4.86) |
From proportional hazards models that controlled for age, sex, and education and either controlled for or stratified by race. HR indicates hazard ratio and CI indicates confidence interval.
There were too few cases of other dementia to estimate risk in racial subgroups.
Because little is known about racial differences in survival in AD, we conducted separate analyses for African American and white persons. In these analyses, the associations of MCI and AD with risk of death in African Americans (second column of Table 2 and upper panel of Figure 1) appeared slightly weaker than the same associations in whites (third column of Table 2 and lower panel of Figure 1). This apparent difference was not significant, however: in an analysis that included terms for the interactions of race with the indicators for MCI and AD, neither interaction was significant (for race × MCI, estimate = -0.29, SE = 0.19, p =.13; for race × AD, estimate = -0.36. SE = 0.21, p = .08).
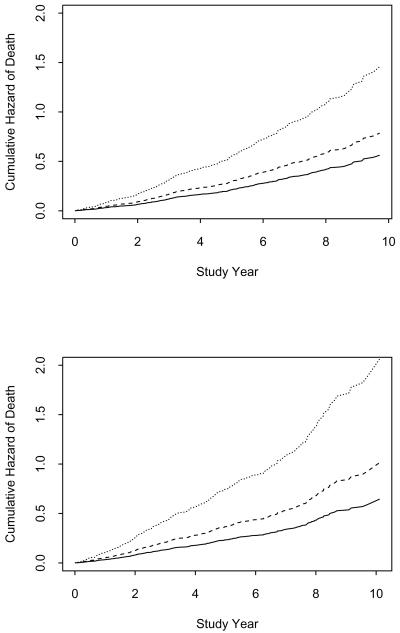
Figure 1.
Comparable risks of death in African Americans (upper panel) and white persons (lower panel) with mild cognitive impairment (dashed line) or Alzheimer's disease (dotted line) relative to those without cognitive impairment (solid line), adjusted for age, sex, and education.
Cognitive Impairment and Mortality Within Diagnostic Subgroups
MCI and AD are clinically heterogeneous conditions. One important way in which affected persons differ is in overall severity of cognitive impairment. To determine whether individual differences in the severity of these conditions added to the prediction of mortality, we conducted separate analyses within the MCI and AD subgroups. Each analysis used baseline score on the composite measure of global cognition (mean = 0.35, SD = 0.59 in full cohort) as an indicator of severity of cognitive impairment. Lower level of global cognition was associated with increased risk of death in both the MCI (HR = 2.65; 95% CI: 1.68, 4.16) and AD (HR = 2.29; 95% CI: 1.65, 3.19) subgroups. Thus, in MCI a relatively low global cognitive score (score = 0.01, 25th percentile in MCI group) was associated with a 58% increase in risk of death compared to a relatively high score (score = 0.48, 75th percentile in MCI group). Risk of death in AD was increased by about 58% for a low score (-0.70) versus a high score (-0.15). To determine whether these effects varied by race, we conducted separate analyses in black and white subjects. In MCI, the correlation of severity of cognitive impairment with mortality was equivalent in African Americans (HR = 2.54; 95% CI: 1.31, 4.94) and whites (HR = 2.44; 95% CI: 1.30, 4.57). In AD, severity of cognitive impairment had a slightly stronger association with mortality in black (HR = 3.38; 95% CI: 2.06, 5.53) than in white participants (HR = 1.62; 95% CI: 1.01,2.59), confirmed by an interaction between race and global cognitive score in a subsequent analysis (estimate = -0.64, SE = 0.31, p = .04).
Persons with MCI and AD also vary in the cognitive domains that are most compromised. Therefore, we conducted a final series of analyses to assess whether the association of severity of cognitive impairment with mortality in these conditions varied across domains of cognition. Within each diagnostic subgroup, we related baseline level of function in each of 5 cognitive domains to mortality, first with a separate model for each domain (Table 3, left columns) and then with all 5 domain scores simultaneously analyzed (Table 3, right columns). In the separate models, lower levels of function in nearly all cognitive domains were associated with increased risk of death in both MCI and AD, consistent with a global severity effect. By contrast, in the simultaneous models, only perceptual speed was related to increased mortality in both MCI and AD, with no associations in the other cognitive domains, suggesting a much more selective effect.
Table 3.
Relation of baseline level of cognitive function to risk of death*
| Mild Cognitive Impairment | Alzheimer's Disease | |||
|---|---|---|---|---|
| Cognitive Measure | Separate Models HR (95% CI) | Single Model HR (95% CI) | Separate Models HR (95% CI) | Single Model HR (95% CI) |
| Episodic memory | 1.48 (1.08, 2.04) | 1.29 (0.92, 1.80) | 1.60 (1.19,2.14) | 1.27 (0.87,1.86) |
| Semantic memory | 1.75 (1.30, 2.34) | 1.31 (0.94, 1.83) | 1.47 (1.16,1.86) | 0.95 (0.69, 1.31) |
| Working memory | 1.29 (1.03, 1.62) | 1.01 (0.79, 1.29) | 1.73 (1.35,2.23) | 1.16(0.84,1.62) |
| Perceptual speed | 1.66 (1.32, 2.09) | 1.51 (1.14, 2.00) | 1.87 (1.43, 2.43) | 1.56 (1.10, 2.21) |
| Visuospatial ability | 1.12 (0.90, 1.39) | 1.03 (0.80, 1.32) | 1.49 (1.21, 1.84) | 1.12 (0.85, 1.49) |
From proportional hazards models that controlled for age, sex, race, and education. HR, hazard ratio; CI, confidence interval.
COMMENT
In this study, older persons sampled from an urban community were clinically classified and vital status was monitored for up to 10 years. Persons with incident AD were nearly 3 times more likely to die than persons without cognitive impairment. This finding is consistent with previous population-based studies [1-7], further highlighting the malignant nature of the disease. MCI is widely recognized as a precursor to AD. In this study, risk of death was about 50% higher in those with MCI compared to those without cognitive impairment. This association has been previously observed in population-based samples [14-17]. Negative results have also been reported [32,33], possibly because of the modest size of the association.
Knowledge about the consequences of MCI and AD in African Americans is limited. In this biracial community, both the presence of MCI and AD and the severity of cognitive impairment within these conditions were associated with risk of death in African Americans. These associations did not reliably differ from the associations observed in white persons with one exception: the association of severity of cognitive impairment in AD with mortality was slightly stronger in black persons than in white persons. Overall, these results do not suggest strong racial differences in survival in MCI and AD. We are not aware of previous research on survival in African Americans with MCI. In national surveys of health care data, mortality rates in AD have been reported to be higher for whites than for African Americans [9,10]. Interpretation of these studies is difficult, however, because the quality of medical record data is unlikely to be uniform across racial subgroups [34], and results appear to depend in part on what data are used to establish dementia [11]. In recent analyses of cataloged data from AD Centers in the United States on more than 30,000 AD patients, African Americans were about 20% less likely to die than whites [35]. These data are subject to bias, as the authors acknowledge, but they are not necessarily inconsistent with the present results since we probably lack the statistical power to detect an effect of this size. If there are racial differences in the consequences of AD, it will be important to determine whether they are due to diagnostic bias or whether they reflect actual differences in the underlying neurobiology of the disease or in how affected individuals are cared for.
MCI and AD are clinically heterogeneous. In prior research, severity of cognitive impairment in AD has been associated with mortality [1,5,36,37]. We replicated this finding and extended it by showing a comparable effect in MCI which has not been observed in previous studies [15, 22], possibly owing to lack of statistical power. Although risk of death was higher in AD than MCI, individual differences in severity of cognitive impairment within each subgroup had comparable associations with mortality. Whether the association of severity of cognitive impairment with mortality in MCI and AD varies across domains of cognitive function has not been extensively studied [38,39]. In this cohort, using continuous measures of multiple cognitive domains, we found that lower level of function in any cognitive domain reduced survival, but the effect was strongest for perceptual speed in both MCI and AD. Thus, survival in people with MCI and AD appears to depend in part on relative preservation of attentional/executive abilities, suggesting that interventions targeting these abilities [40] should be considered in persons with these conditions or at risk of developing them.
This study has important strengths and limitations. Participants were sampled from a defined population making it more likely that a broad spectrum of affected persons was studied. Clinical classification of MCI, dementia, and AD was based on a uniform structured clinical evaluation and established criteria applied by an experienced clinician, minimizing the likelihood that diagnostic imprecision affected results. The availability of previously established composite measures of cognition allowed us to estimate the associations of severity and type of cognitive impairment with survival. The main limitation is that because individuals with evidence of cognitive impairment in the baseline phase of the study were eligible to be sampled for clinical evaluation, the MCI group includes both prevalent and incident cases.
In summary, MCI and AD were associated with reduced survival to a similar extent in older black and white persons. Further research is needed on the consequences of MCI and AD in racial and ethnic minorities.
ACKNOWLEDGMENTS
The authors thank the residents of Morgan Park, Washington Heights, and Beverly who participated in the study. They also thank Ms Ann Marie Lane for community development and oversight of project coordination, Ms Michelle Bos, Ms Holly Hadden, Mr Flavio LaMorticella, and Ms Jennifer Tarpey for coordination of the study, Mr Todd Beck for analytic programming, and the staff of the Rush Institute for Healthy Aging. This research was supported by National Institute on Aging grants AG 11101 and AG10161 and by National Institute of Environmental Health Sciences grant ES 10902. The funding agencies had no role in the design and conduct of this study; collection, management, analysis, or interpretation of the data; or in preparation, review, or approval of this manuscript.
REFERENCES
- 1.Evans DA, Smith LA, Scherr PA, Albert MS, Funkenstein HH, Hebert LE. Risk of death from Alzheimer's disease in a community population of older persons. Am J Epidemiol. 1991;134:403–412. doi: 10.1093/oxfordjournals.aje.a116102. [DOI] [PubMed] [Google Scholar]
- 2.Katzman R, Hill LR, Yu ES, et al. The malignancy of dementia. Predictors of mortality in clinically diagnosed dementia in a population survey of Shanghai, China. Arch Neurol. 1994;51:1220–1225. doi: 10.1001/archneur.1994.00540240064017. [DOI] [PubMed] [Google Scholar]
- 3.Aguero-Torres H, Fratiglioni L, Guo Z, Viitanen M, Winbald B. Mortality from dementia in advanced age: a 5-year follow-up study of incident dementia cases. J Clin Epidemiol. 1999;52:737–743. doi: 10.1016/s0895-4356(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 4.Helmer C, Joly P, Letenneur L, Commenges D, Dartigues J-F. Mortality with dementia: results from a French prospective community-based cohort. Am J Epidemiol. 2001;154:642–648. doi: 10.1093/aje/154.7.642. [DOI] [PubMed] [Google Scholar]
- 5.Tschanz JT, Corcoran C, Skoog I, et al. Dementia: the leading predictor of death in a defined elderly population: the Cache County Study. Neurology. 2004;62:1156–1162. doi: 10.1212/01.wnl.0000118210.12660.c2. [DOI] [PubMed] [Google Scholar]
- 6.Fitzpatrick AL, Kuller LH, Lopez OL, Kawas CH, Jagust W. Survival following dementia onset: Alzheimer's disease and vascular dementia. J Neurol Sci. 2005;229-230:43–49. doi: 10.1016/j.jns.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST. Alzheimer's disease and mortality: a 15-year epidemiological study. Arch Neurol. 2005;62:779–784. doi: 10.1001/archneur.62.5.779. [DOI] [PubMed] [Google Scholar]
- 8.Hoyert DL, Rosenberg HM. Alzheimer's disease as a cause of death in the United States. Public Health Reports. 1997;112:497–505. [PMC free article] [PubMed] [Google Scholar]
- 9.Hoyert DL. Mortality trends for Alzheimer's disease, 1979-1991. Vital Health Stat. 1996;20:1–23. [PubMed] [Google Scholar]
- 10.Gambassi G, Landi F, Lapane KL, et al. Predictors of mortality in patients with Alzheimer's disease living in nursing homes. J Neurol Neurosurg Psychiatry. 1999;67:59–65. doi: 10.1136/jnnp.67.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanska DJ. Dementia mortality in the United States: results of the 1986 National Mortality Followback Survey. Neurology. 1998;50:362–367. doi: 10.1212/wnl.50.2.362. [DOI] [PubMed] [Google Scholar]
- 12.Unverzagt FW, Gao S, Baiyewu O, et al. Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging. Neurology. 2001;57:1655–1662. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- 13.Graham JF, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 14.Johansson B, Zarit SH. Early cognitive markers of the incidence of dementia and mortality: a longitudinal population-based study of the oldest old. Int J Geriatr Psychiatry. 1997;12:53–59. doi: 10.1002/(sici)1099-1166(199701)12:1<53::aid-gps507>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Palmer K, Wang H-X, Backman L, Winblad B, Fratiglioni L. Differential evolution of cognitive impairment in nondemented older persons: results from the Kungsholmen Project. Am J Psychiatry. 2002;159:436–442. doi: 10.1176/appi.ajp.159.3.436. [DOI] [PubMed] [Google Scholar]
- 16.Ingles JL, Fisk JD, Merry HR, Rockwood K. Five-year outcomes for dementia defined solely by neuropsychological test performance. Neuroepidemiol. 2003;22:172–178. doi: 10.1159/000069891. [DOI] [PubMed] [Google Scholar]
- 17.Guehne U, Luck T, Busse A, Angermeyer M, Riedel-Heller SG. Mortality in individuals with mild cognitive impairment. Neuroepidemiol. 2007;29:226–234. doi: 10.1159/000112479. [DOI] [PubMed] [Google Scholar]
- 18.Bienias JL, Beckett LA, Bennett DA, Wilson RS, Evans DA. Design of the Chicago Health and Aging Project. J Alz Dis. 2003;5:349–355. doi: 10.3233/jad-2003-5501. [DOI] [PubMed] [Google Scholar]
- 19.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- 20.Wilson RS, Barnes LL, Bennett DA, et al. Proneness to psychological distress and risk of Alzheimer's disease in a biracial community. Neurology. 2005;64:380–382. doi: 10.1212/01.WNL.0000149525.53525.E7. [DOI] [PubMed] [Google Scholar]
- 21.McKahnn G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease: report of the NINCDS/ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 23.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer's disease and rate of cognitive decline. Neurology. 2005;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 24.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Mild cognitive impairment is related to Alzheimer's disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 25.Hixon JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with Hha I. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 26.Rand WM. Objective criteria for the evaluation of clustering methods. J Am Statis Assoc. 1971;66:846–850. [Google Scholar]
- 27.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 28.Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. J Clin Exp Neuropsychol. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- 29.Wilson RS, Krueger KR, Gu L, Bienias JL, Mendes de Leon CF, Evans DA. Neuroticism, extraversion, and mortality in a defined population. Psychosom Med. 2005;67:841–845. doi: 10.1097/01.psy.0000190615.20656.83. [DOI] [PubMed] [Google Scholar]
- 30.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;74:187–220. [Google Scholar]
- 31.SAS/STAT User's Guide. Version 8 SAS Institute Inc; Cary, NC: 2000. [Google Scholar]
- 32.Fisk JD, Merry HR, Rockwood K. Variations in case definition affect prevalence but not outcomes of mild cognitive impairment. Neurology. 2003;61:1179–1184. doi: 10.1212/01.wnl.0000089238.07771.c7. [DOI] [PubMed] [Google Scholar]
- 33.Fisk JD, Merry HR, Rockwood K. Outcomes of incident mild cognitive impairment in relation to case definition. J Neurol Neurosurg Psychiatry. 2005;76:1175–1177. doi: 10.1136/jnnp.2004.053751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg HM, Maurer JD, Sorlie PD, et al. Quality of death rates by race and Hispanic origin: a summary of current research. Vital Health Stat 2. 1999;128:1–13. [PubMed] [Google Scholar]
- 35.Mehta KM, Yaffe K, Perez-Stable EJ, et al. Race/ethnic differences in AD survival in US Alzheimer's disease centers. Neurology. 2008;70:1163–1170. doi: 10.1212/01.wnl.0000285287.99923.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larson EB, Shadlen M-F, Wang L, et al. Survival after initial diagnosis of Alzheimer's disease. Ann Intern Med. 2004;140:501–509. doi: 10.7326/0003-4819-140-7-200404060-00008. [DOI] [PubMed] [Google Scholar]
- 37.Wilson RS, Li Y, Aggarwal NT, et al. Cognitive decline and survival in Alzheimer's disease. Int J Geriatr Psychiatr. 2006;21:356–362. doi: 10.1002/gps.1472. [DOI] [PubMed] [Google Scholar]
- 38.Hui JS, Wilson RS, Bennett DA, Bienias JL, Gilley DW, Evans DA. Rate of cognitive decline and mortality in Alzheimer's disease. Neurology. 2003;61:1356–1361. doi: 10.1212/01.wnl.0000094327.68399.59. [DOI] [PubMed] [Google Scholar]
- 39.Yaffee K, Petersen RC, Lindquist K, Kramer J, Miller B. Subtype of mild cognitive impairment and progression to dementia and death. Dement Geriatr Cogn Disord. 2006;22:312–319. doi: 10.1159/000095427. [DOI] [PubMed] [Google Scholar]
- 40.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Nat Acad Sci U S A. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]