Abstract
The extensive body of literature regarding the interaction of polychlorinated biphenyls (PCBs) with transcription factors (receptors) has great value to understand similarities and distinctions and in formulating hypotheses regarding the activity of polybrominated diphenyl ethers (PBDEs) toward those same receptors. Our goal is to present the most comprehensive overview of PBDE effects on AhR, CAR, PXR, ER, AR, PR, DHT, TH, T3, T4 and IGF, as well as hypothetical biological activities of PPAR, RyR, GR and GABA. Aside the influence of the conformation of the ligand, we discuss its constitution influencing the binding affinity: size and polarizability, hydrophilicity, Gibbs free energy of solvation, inductive and mesomeric effects. We evaluate the techniques to determine the biologically relevant conformation of these halogenated hydrocarbons, including computation methods, X-ray and microwave spectroscopy. A novel fluoro-tagged ligand approach holds promise as tools for illuminating the steric and electronic effects in ligand-receptor interaction. Based on our assessment, we predict that PBDEs do not exhibit AhR activity themselves, but impurities are responsible for these effects.
Keywords: polybrominated diphenyl ethers, PBDEs, polychlorinated biphenyls, PCBs, AhR, CAR, PXR, ER, AR, PR, DHT, TH, T3, T4, IGF, PPAR, RyR, GR, GABA, Conformation, Constitution, Binding affinity, Polarizability, Hydrophilicity, Gibbs free solvation energy, Inductive effects, Mesomeric effects
1 Introduction
The ‘chemical links’ between Polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), the ‘PCBs of 21st century’ and Polybrominated biphenyls (PBBs) are:. each one consists of 209 individual congeners, distinguishable by the number and position of halogen substituents in two connected aromatic rings. The substitution by bromine or chlorine and the connection by a direct carbon-carbon bond or an ether function distinguish PBDEs, PCBs, and PBBs. These factors have strong influence on the physico-chemical properties, e.g. solubility, boiling point, vapor pressure, dipole momentum, London force, stability during light radiation and combustion, molecular volume, and constitution and conformation.
An analysis of transcription factor activation by PBDEs, in comparison to that of PCBs, is based on a three dimensional (3D) quantitative structure activity relationship (QSAR) method that can be either receptor or ligand based. Receptor-based 3D QSAR requires knowledge of protein quaternary structure. Alternatively, based on the QSAR of various ligands, an idealized receptor structure can be developed. Subsequently, the contour map generated from the model can provide some information about the molecular binding domain of the receptor (Oprea et al., 1994). Despite decades of scientific inquiry employing a variety of approaches, the quaternary structure of most receptors remains an elusive target. This review will focus on the comparison of the structural, steric, electrostatic and hydrophobic similarities and distinctions of PCBs and PBDEs to understand their biological activity as receptor agonists and antagonists, generally called ligands.
Receptor interaction initiates a cellular response, e.g. the transcription of specific genes, among them genes encoding proteins responsible for the metabolism of xenobiotics. The interaction of the ligand and receptor is not a covalent or long-lived interaction; but in fact a dynamic and often transient effect. Ligands that bind but do not activate the receptor are called antagonists, in contrast to the agonists. Inverse agonists have the ability to reduce the constitutive activity of a receptor and ability to preferentially bind to an inactive form of the receptor. Orphan receptors share structural features found in the nuclear receptor family, but lack known physiologic ligands (Kretschmer and Baldwin, 2005). The extensive literature on PCBs is worthwhile to be extrapolated onto PBDEs, due to their structural similarities, for the purpose of formulating hypotheses on ligand activity with several well-known receptors. Our exploration of receptor activity begins through careful analysis of known ligands, i.e. constitution, substitution pattern, and configuration. We will also discuss the limitations of the applied techniques to determine the biologically relevant conformation. Our goal is to present, for the first time, a complete overview of known and hypothetical receptor activities of PBDEs.
2 Structural characteristics of PCBs and PBDEs
2.1 Planarity and the influence of dihedral and torsion angles
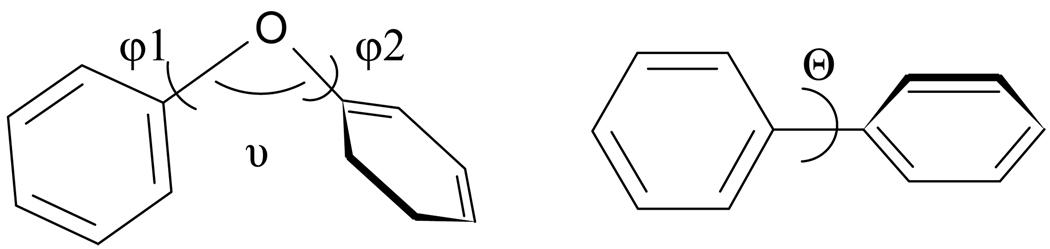
The fit of the ligand into the receptor depends on its constitution and conformation, which is determined by the substitution patterns on the aromatic rings. Distinct from the constitution, the conformation of the molecule is in most cases dynamic. For PCBs and PBBs the conformation is described by the dihedral angle Θ between the benzene rings of the biphenyl system (see Figure 1). The conformation of the PBDEs on the other hand is described by two torsion angles φ1 (C1’-O-C1-C2(6)) and φ2 (C2’(6’)-C1’-O-C1) between the C1–O–C1′ plane and planes of the phenyl rings and the angle υ of the ether bond C1–O–C1′. The angles are defined as positive when the rotation is clockwise looking down the C4–C1 and C4′–C1′ axes toward the oxygen (Figure 1). Theoretically, there are four possible types of conformations of diphenyl ethers: planar (φ1 = φ2 = 0°), butterfly (φ1 = φ2 = 90°), skew (φ1= 0°, φ2= 90°) and twist (φ1, φ2 > 0°).
Figure 1.
Definition of bond angles in diethyl ether (left) φ1 (C1’-O-C1-C2(6)), φ2 (C2’(6’)-C1’-O-C1) between the C1–O–C1′ plane and planes of the phenyl rings and the angle υ of the ether bond C1–O–C1′, and of biphenyl (right) with the dihedral angle Θ (C1-C1’-C2(6)) between the benzene rings of the biphenyl system.
For PCBs, the dihedral angle Θ is highly dependent on substitution in the ortho-positions (2, 2’, 6 and 6’). According to our review of the literature, the experimental dihedral angles determined for non-ortho-chloro substituted PCBs are 35–45°, mono-ortho 47–51°, di- 58–68° and tetra 86–87° (Luthe et al., 2007). Based on these findings, the descriptor of PCBs as ‘coplanar’ is in fact a misnomer. Even for PCBs with no ortho-chloro substitutions there is only a weak approximation of planarity.
A variety of experimental and theoretical studies have shown that the unsubstituted diphenyl ether itself has a twist conformation in that φ1 and φ2 lie in the vicinity of 25–50° (Schaefer et al., 1988). In general, PBDE congeners prefer a skew or twist conformation depending on the number of the ortho-bromo substituents (Klösener et al., 2008). ‘Planar’ or ‘co-planar’ PBDEs do not exist. It is crucial to understand that the values for angles describe energy minima of rotation barriers, dependent on intra- and intermolecular forces. The weak point of contemporary models is their emphasis on the lowest energy conformation of both the receptor and the ligand. This assumption may not always be valid. Depending on the energy of the rotation barrier, the molecule can be temporally or episodically changed to another conformation. Intramolecular factors affecting this are defined by the general substitution pattern. Greater ortho-substitution increases the energy barrier for conformational change, such that unsubstituted ortho- is the lowest and tetra-ortho-chloro biphenyl the highest.
Furthermore, the size of the substituent is important as well, since an increased molecular volume determined on the C(aromatic)-substituent bond length of the substituent, i.e. chloro (1.747 Å) versus bromo (1.899 Å), results in an increased steric interaction and therefore an increased energy barrier. But the substituents in meta- and para-positions influence the rotation barrier as well by a buttressing effect (Luthe et al., 2007). In addition, a stacking interaction operating in the molecular recognition event is hypothesized. Here, the most highly halogenated ring would be considered to be the preferred stacking plane for asymmetrically substituted molecules (McKinney et al., 1985). Conformational properties of variously substituted diphenyl ethers have been studied using semi-empirical or ab initio calculations (Nevalainen and Rissanen, 1994; Luthe et al. 2007) and for PBDEs as well (Klösener et al., 2008).
2.2 The search for the molecular conformation in the liquid state: X-ray versus semi empirical and ab initio computation and microwave spectroscopy
Intermolecular influences are dependent on the environment with the exception of the determination of conformation in a vacuum by microwave spectroscopy or computation. X-ray determinations, computations with semi-empirical self consistent field molecular orbital calculations (SCF-MO), ab initio calculations and comparative molecular field analysis (CoMFA) give different results, since the environment and the assumptions are different. X-ray determination of the conformation in a crystal is influenced by π…π stacking and C-H…π phenyl interactions, halogen-halogen interactions, hydrogen bonds and van der Waals interactions. All these factors deform the energetic optimal conformation (Luthe et al. 2007; Klösener et al., 2008). The programs used in computational chemistry are based on many different quantum-chemical methods that solve the molecular Schrödinger equation associated with the molecular Hamiltonian (Dewar et al., 1985). Ab initio methods that do not include any empirical or semi-empirical parameters in their equations are being derived directly from theoretical principles, with no inclusion of experimental data. None of these methods can stand alone; they are all approximate quantum mechanical calculations. Therefore, a comparison of the various methods needs to be carried out and the results evaluated on the basis of the environment of the ligand. Rotational or microwave spectroscopy studies the absorption and emission of electromagnetic radiation, typically in the microwave region associated with a corresponding change in the rotational quantum number of the molecule. Rotational spectroscopy (Townes and Schawlow, 1975) is only really practical in the gas phase where the rotation motion is quantized. In solids or liquids the rotational motion is usually quenched due to collisions. However, none of these techniques determines the true conformation of the molecule in a liquid matrix, which is of greatest biological relevance. Even the possibility of determining the conformation in liquid state by nuclear magnetic resonance spectrometry will not result in a true picture of the conformation of the guest molecule ligand in the receptor host. Only co-crystallization and X-ray determination of the guest-host agonist/antagonist-receptor complex will reveal the biologically relevant active confirmation. And even then, the binding site may be expected to be more complex, i.e. constituted of amino acids with more or less flexible side chains, which may impose more or less strict steric restrictions in various positions on the ligand for their high affinity binding.
2.3 Molecular constitution and receptor affinity
The constitution of the ligand is defined by its backbone and substitution pattern. In general, the xenobiotic receptor activity is thought to depend on the three dimensional fit of the ligand to its endogenous receptor. But often the constitution of the ligand itself is less important than the steric factors influencing its conformation (see 1.2). There are some intriguing findings in PCB receptor activity indicating that the constitution plays not just an indirect role keeping the larger molecule at the correct dihedral angle, but has a direct influence on the receptor activities. 2,3’,4,4’,5-Pentachlorobiphenyl (PCB 118) has a mixed functionality, inducing both aryl hydrocarbon (AhR) and constitutive androstane (CAR) receptor activities. The substitution pattern for the non-prime ring is identical to the symmetric 2,2’,4,4’,5,5’-hexachlorobiphenyl (PCB 153), a typical CAR receptor agonist (Denomme et al., 1983), while the prime ring is identical with the symmetric 3,3’,4,4’-tetrachlorobiphenyl (PCB 77) isomer, an AhR agonist (Bandiera, et al., 1982). Nearly all AhR inducers are more co-planar, but PCB 118 does not fit this description. Depending on the energy of the rotation barrier, the molecule might temporally achieve more co-planar properties. There are some other examples of mixed CAR and pregnane X (PXR) ligand activities for 2,4,4’-trichlorobiphenyl (PCB 28) and 2,2’,4,4’-tetrachlorobiphenyl (PCB 47) (Ludewig et al., 2007). However, this finding for PCBs 28 and 47 is anticipated; since these ligands exhibit structure characteristics for both receptors and do not radically differ in conformation.
As the constitution seems vital for the portability of findings to PBDEs, one must take the increased size and molecular volume of the bromo substituent into consideration. Communication between ligand and receptor is based solely on electrostatic interaction. The bromo substituent may be larger (C(aromatic)-Cl 1.747 Å versus (C(aromatic)-Br 1.899 Å)), but it exhibits an increased ‘chemical weakness’ according to the Hard and Soft Acid and Basis (HSAB) model (Pearson, 1963) compared to the chloro substituent. Therefore, the strength of the electrostatic interaction must be evaluated on both the orbital size and the strength of the electrostatic interaction between the interacting areas of ligand and receptor. Studies have shown that the most important explanation for the observed difference in binding affinity is the decrease in relative free energy of binding (ΔΔG(binding)), compared with the Gibbs free energy of solvation (ΔG(solv.)) in the unbound state. Receptor-ligand binding in solution must take solvation into consideration, which can be calculated using Monte-Carlo simulations (see Figure 2). Recent developments in and applications of free energy perturbation methods have emphasized the fact that if the relative difference in free energy in receptor binding of ligands are small relative to the differences in free energy of solvation, the latter determines the strength of the ligand-receptor interactions. In other words, if the binding site is conceived as being delineated by predominantly hydrophobic amino acids, then less water-soluble ligands will bind more strongly to the receptor.
Figure 2.
Thermodynamic cycle describing the determination of the relative free Gibbs energy (ΔΔG(binding)) of receptor (R) ligand (L) binding in solution by solvation (solv.) via separate solvation of R and L (ΔG(solv.)) followed by the binding in solvation (ΔGlig(solv.)) and the theoretical pathway of the formation of a transition state (‡) binding (R…L‡) followed by the binding in solvation at ground state (R…L(solv.)). Adapted from Gillner et al. (1993).
These different factors may all discretely influence ligand-receptor communication and must be examined alone and in concert to see synergistic effects. A promising tool for illuminating these effects lies in our novel concept of fluorine-tagging (F-tagging) PCBs and PBDEs.
3 PCBs and PBDEs as receptor ligands
3.1 AhR
The aryl hydrocarbon receptor (Ah receptor or AhR) is a cytosolic (Ashek et al., 2006) ligand-activated transcription factor involved in the regulation of several genes. These include xenobiotic-metabolizing enzymes such as cytochrome P450 (CYP) 1A and 1B. Ligands for the AhR include a variety of aromatic hydrocarbons, including chlorinated dioxins and co-planar PCBs (Hahn, 1998). An endogenous ligand for the AhR has not been identified and it is possible that its natural ligand is of exogenous nature. As no receptor structural information is available for the AhR, ligand based QSAR model have been developed (Ashek et al., 2006). The activity of various halogenated aromatics is structure dependent (Poland and Knutson, 1982, Safe, 1990). On the other hand, it is known that halogens are required for receptor activation by halogenated dioxins, dibenzofurans and PCBs. It is not only the planarity of the molecule that determines affinity for the receptor. Moreover, examples have been found, e.g. chloro-methyl-dibenzo-p-dioxin, that can be fitted into both mentioned patterns but have virtually no activity. This gives clear indication that neither of these patterns describes sufficiently the requirements for high-affinity binding. Other factors, such as hydrophobicity of the ligand, are also important determinants for AhR binding (Maitland et al., 1987). AhR binding of PCBs and dioxins has also been correlated with polarizability and stacking parameters (McKinney et al., 1985).
The findings for AhR active PCBs are the so called ‘co-planar’ meta-, and/or para-substituted which are not ortho-substituted (Bandeira et al., 1982). This is in agreement with the conformational finding, that ‘co-planar’ PCBs give a better steric fit, and that bulky groups in the 3 and 4 positions increase activity. There are reports in literature regarding weak to moderate AhR activity of the PBDEs 3, 15, 17, 28, 47, 49, 66, 71, 75, 77, 85, 99, 100, 119,126, 152, 153, 154, 183 and the metabolite 6-OH-PBDE 47 (Wang et al., 2006; Chen and Bruce, 2003; Peters et al., 2006a; Peters et al., 2006b; Germer et al., 2006). PBDE 47 and PBDE mixture (D-71) were found to be a weak AhR antagonist (Kuiper et al., 2004).
These findings have been questioned. Polybrominated dibenzofurans (PBDFs) and polybrominated dibenzodioxins (PBDDs) are found as impurities in individual PBDEs components and the mixture DE71 (Sanders et al., 2005). These impurities of PBDFs and PBDDs have been suggested as responsible for most, if not all, AhR receptor activity observed by PBDEs (Sanders et al., 2005). That AhR activity of 6-OH-PBDE 47 might as well be based on impurities by the formation of the corresponding dioxin due to ring closure via the hydroxyl group or to weak electrostatic interactions between the hydroxyl and bromo substituents.
Correlations of AhR affinity also seem to stress the importance of steric factors. This finding would be supported by the hypothesis that the conformation plays the main role in the activity and the theoretical studies of Ashek et al. (2006). Their CoMFA studies on TCDD analogues show that the biological activity is diminished by a sterically disfavored non-planarity between the benzene rings. Since PBDEs are all out-of-plane (non co-planar) they should not exhibit AhR activity. It can be hypothesized based on mixed receptor activities like in PCB 118, that the ether bond in PBDEs allows an interaction between AhR and one phenyl ring, while the other ring does not interact. In our considerations mixed activities are just found due to low rotational barriers between the rings. However, PBDEs do not approach a co-planar conformation, even though this seems probable due to the large distance between the two aromatic rings compared to PCBs, and the ether bond possesses more effective degrees of freedom.
3.2 Constitutive androstane (CAR) and pregnane X receptors (PXR)
The constitutive androstane receptor (CAR) received its name from the androstanes, which act as inverse agonists. CAR is a member of the orphan receptor subfamily, like the pregnane X receptor (PXR). Both PXR and CAR are activated by xenobiotics, controlling regulators of phase I through III enzymes involved in the detoxification and elimination of steroids, bile acids, and xenobiotics (Udea et al., 2002; Rosenfeld et al., 2003; Kretschmer and Baldwin, 2005; Waxman, 1999). Activated detoxification genes induced include several phase I CYPs (Lemaire, de Sousa and Rahmani, 2004), phase II enzymes such as uridine diphosphoglycoronosyltransferases (UDPGT), glutathione-S-transferases (GSTs) and sulfotransferases (SULTs) (Sugatani et al., 2001), and the phase III transporters such as bile salt export pump (BSEP) and the multidrug resistance-associated protein (MRP2) (Kast et al., 2002).
Initially, CAR was thought to regulate CYP 2B, while PXR controlled up regulation for CYP 3A. More recent investigations have shown that there is ‘cross-talk’ between CAR and PXR (Wei et al., 2002) which results in overlapping functions. Ortho- and para-substituted PCBs are known to induce CAR activity (Denomme et al., 1983), as well as poly-ortho-substituted PCBs (Hurst and Waxman, 2005; Schuetz et al., 1998). PBDE 71 and 79 are both probable activators of CAR and PXR (Kretschmer and Baldwin, 2005; Sanders et al. 2005; Germer et al., 2006). However, only PBDE 71 shows two substitutions in the ortho-positions, PBDE 79 none. The conformation of both PBDEs is very different, but the patterns in one ring of both isomers are identical. If PBDE 71 and 79 show CAR and PXR activity it must be due to the constitution, not the conformation.
3.3 Thyroid function (TH), thyroxin (T4) and triiodothyronine (T3)
The thyroid hormone thyroxin (T4) is produced by the thyroid gland. The main part of the T4 is bound to transport proteins, e.g. globulin (70%) and albumin (15-20%), circulating in the blood. Only the unbound form is biologically active. Thyroid hormones are essential to proper development and differentiation of all cells of the human body (Bianco et al., 2002). These hormones also regulate protein, fat and carbohydrate metabolism (Nussey and Whitehead, 2001). Numerous physiological and pathological stimuli influence thyroid hormone synthesis. In addition, T4 plays a role in the poorly understood mechanism resulting in inhibition of neuronal activity, e.g. hibernation cycles (Dratman and Gordon, 1996). To our knowledge, PCBs have only an indirect influence on the thyroid hormone (Gauger et al., 2004). However, a structural comparison of T3 and T4 with PBDEs reveal remarkable similarities, such that receptor activity seems likely. The common diphenyl ether forms the backbone of both molecules, while halogenation is also a common trait. In addition, the radii of iodo (C(aromatic)-I 2.095 Å) and bromo (C(aromatic)-Br 1.899 Å) substituents are of similar size. 2,3’,6-Tribromodiphenyl ether (PBDE 27) with triiodothyronine (T3), and 2,3’,5’,6-tetrabromodiphenyl ether (PBDE 73) with T4 exhibit the same backbone and substitution pattern (see Figure 3). It was determined that OH-PBDEs and PBDE 47 function as agonists (Kretschmer and Baldwin, 2005; Eslami et al., 2006; Hallgren and Darnerud, 2002, Zhou et al., 2002) and PBDE 71 and 79 as disruptors (Kretschmer and Baldwin, 2005). Based on structural similarity, the PBDE metabolite 4’hydroxyl-2,3’,6-tribromodiphenyl ether (4’OH-PBDE 27), 4hydroxyl-2,3’,5’,6-tetrabromodiphenyl ether (4OH-PBDE 73) are of initial interest for investigation. Furthermore, it has been determined that they reduce the serum level of T4 (Kretschmer and Baldwin, 2005; Stoker et al., 2004; Hooper and McDonald, 2000; Zhou et al., 2002; Eslami et al., 2006).
Figure 3.
Structural formulas of thyroxine (T4) and triiodothyronine (T3), with highlighted diphenyl ether backbone and substitution patterns of PBDEs 27 and 73.
3.4 Other activated receptors
For peroxisome proliferator activated receptors (PPARs) Ariyoshi et al. (1998) and Robertson et al. (2007) found that the highly toxic more co-planar PCB 126 acts as inverse agonist and reduces liver peroxisomal enzyme activity in the rat model. Other co-planar meta- and para-substituted PCBs should have the same potential (Ludewig et al., 2007). For PBDEs, PPAR activity has not been reported. Based on the here presented theoretical assessment, it is our hypothesis that PBDEs do not have potential for PPAR interaction. Pessah et al. (2006) established a structure-activity relationship for non co-planar PCBs toward the ryanodine receptor (RyR). It was shown that ortho-substituted PCBs, e.g. 2,2',3,5',6-pentachlorobiphenyl (PCB 95), alter microsomal calcium transport by direct interaction with the RyR of mammalian brain (Wong et al., 1997). Some phase I metabolites of PCBs demonstrated RyR interaction (Pessah et al., 2006). Nothing is reported in literature regarding the RyR activity of PBDEs or their metabolites. Based on the structure-activity relations by Pessah et al. (2006) it can be hypothesized that PBDEs exhibit some RyR activity, since PBDEs are all non co-planar and the molecular size of a hydroxyl substituent (C(aromatic)-OH 1.364 Å) is still comparable in size compared with the bromo substituent (C(aromatic)-Br 1.899 Å).
Recent studies have found that poly-ortho-substituted PCBs like 2,2’,6,6’-tetrachlorobiphenyl (PCB 54) are estrogenic (ER) (Arcaro et al., 1999, Bonefeld-Jorgensen et al., 2001). Hydroxylated phase I metabolites of PCBs were shown to have an indirect estrogenic effect by inhibiting estrogen sulfotransferase (Kester et al., 2000). For 2,2’,4,4’,5-pentabromodiphenyl ether (Ceccatelli et al., 2006) and mono- and dihydroxyl PBDEs (Kretschmer and Baldwin, 2005; Stoker et al., 2004; Meerts et al., 2001) an estrogenic activity was found as well. As for the RyR, a possible activation could be postulated to occur on the basis of the non co-planarity of PBDEs in general, especially ortho-substituted ones. The poly-ortho- substituted PCBs are active as antagonists for the AhR, just as with ER (Fang et al., 2003; Potigal et al., 2002; Schrader and Cooke, 2003).
This contrasts with the findings for PBDEs. The poly-ortho-substituted PBDEs 71 and 100 have shown androgen receptor (AR) suppression and inhibition and poly-ortho-substituted PBDEs 47, 99, 100, 153, 154 demonstrated competitive anti-androgenic (Stoker et al., 2005). Conner et al. (1997) reported that phase I metabolites of PCBs act as antagonists for progesterone based on structure-activity relationships. Ceccatelli et al., (2006) measured that progesterone receptor (PR) was down-regulated by poly-ortho substituted PBDE 99. Based on this finding, it can be assumed that more poly-ortho substituted PBDEs and their phase I metabolites could affect PR. Johansen et al. (1998) observed an interaction between methylsulfonyl PCBs (MeSO2-PCBs) and the glucocorticoid receptor (GR), where 4-substituted MeSO2-PCBs displayed only antagonistic effects. In an extensive study by Johansson et al. (2005), it was shown that substances with a structural resemblance via a methyl sulfonyl group, e.g. 4-MeSO2-2,5,6,2',4'-pentachlorobiphenyl (4-MeSO2-CB91), 4-MeSO2-2,3,6,2',4',5'-hexachlorobiphenyl (4-MeSO2-CB149) and the fungicide tolylfluanid inhibit glucocorticoid receptor-regulated gene transcription. We suggest that 4-substituted MeSO2–PBDEs may exhibit antagonistic GR activity as well. Thus far, MeSO2–PBDEs have not been detected, which may be due to their specific physico-chemical properties. Some studies on γ-Aminobutyric acid (GABA) have been carried out to determine the interactions of PCBs with GABA receptors (Mariussen and Fonnum, 2001; Wong et al., 1997; Wong et al., 1997). It was found that the non co-planar PCBs interact with the GABA receptor. We want to address the hypothesis that this might be the case as well for PBDEs, especially for the non meta- and para- substituted ortho-brominated PBDEs. Studies by Ceccatelli et al. (2006) have reported a weak up-regulation of insulin-like growth factor (IGF), especially IGF-I by 2,2’,4,4’,5-pentabromobiphenyl (PBDE 99).
4 Conclusions
In this review we present for the first time an overview of biological transcription factors activated by PBDEs. These include AhR, CAR, PXR, ER, AR, PR, DHT, TH, T3, T4 and IGF. In addition, we hypothesized regarding presently unknown activities of PBDEs based on comparisons with PCB receptor activities. Since all PBDEs and OH-PBDEs are not co-planar, we suggest that they are agonists for RyR and affect the Ca2+ channel function in analogy to non co-planar PCBs and OH-PCBs (Pessah et al., 2006). We addressed the suggestion that PBDEs can be metabolized to MeSO2-PBDEs like PCBs and repress by competitive antagonism GR function (Johansen, et al., 1998). By analogy to the non-meta- and para- but ortho-substituted PCBs (Wong et al., 1997), we hypothesize that analog substituted PBDEs are GABA agonists.
Second, we focused on the factors determining biological activity. While several models exclusively take the most energetically stable conformer into consideration, we discussed the influence of rotation barriers and dihedral versus torsion angles, along with the size of the substituents. A brief overview was given of the advantages and disadvantages of several techniques to determine the true molecular conformation of a ligand in solvation. Especially, conformation dynamics and 3D changes of ligand and receptor are extensively neglected.
Based on the assessment done in this review, we strongly support the conclusion that PBDEs are unlikely to exhibit AhR activity by themselves. The electronic interaction might be possible, but to do so the conformation of all PBDEs must be drastically changed to give the right fit for AhR, leading to an electrostatic interaction or receptor-ligand binding. The determination of purity is of critical nature in biological studies. Purity of the PBDEs is not easy to determine by gas chromatography coupled mass spectrometry (GC-MS), especially their discrimination from furan analogues. Both PBDEs and furan analogues form oxonium ion fragments indistinguishable in GC-MS. Bromine loss in the injector can also confound analytical determinations. Only clear carbon-hydrogen correlated nuclear magnetic resonance spectra (13C 1H COSY NMR) can definitively characterize a given PBDE.
It is evident that the 3D conformational fit of ligand and receptor does not exclusively lead to transcription. The molecular constitution influences the binding affinity by size and chemical hardness (polarizability) of the substituents, hydrophilicity, relative Gibbs free energy of solvation, inductive and mesomeric effects. The mesomeric effect of a given substituent seems to be critical compared with an inductive effect, either negative or positive. Only substituents with positive mesomeric effects seem to interact with the AhR. This finding might be explained on the basis of the extension of the aromatic system via hyperconjugation or mesomery. This would explain why phase I and II metabolites of xenobiotics – which do not have mesomeric effects - do not exhibit agonist activity, in fact demonstrating feedback inhibition against over-stimulation. Ligand-receptor relationships must be examined in concert with various synergistic influences and not on a basis of a competition of various models.
A novel fluoro-tagged ligand approach holds promise as a tool for illuminating the steric and electronic effects in ligand-receptor interaction. With the F-tagging approach it is possible to have several analogues of one specific PCB and PBDE congener available for receptor investigations. The fluoro-substituent alters the electronic distribution in the substrate and changes the conformation without affecting the main values (Luthe et al., 2007). These finely tuned changes in the electron distribution can provide detailed information of the electrostatic interaction with the receptor.
Table 1.
Receptor and function interactions of polychlorinated biphenyls (PCBs), diphenyl ethers (PBDEs) and their metabolites.
| PCBs |
PBDEs |
||||
|---|---|---|---|---|---|
| Receptor abbreviation |
Major Gene or function affected |
Ligands | Response and references | Ligands | Response and references |
| Aryl hydrocarbon (AhR) | CYP 1A | co-planar, meta-, para- PCBs | >20–30 increase (Bandeira, Safe and Okey, 1992) | PBDEs 3, 15, 17, 28, 47, 49, 66, 71, 75, 77, 85, 99, 100, 119,126, 152, 153154, 183, 6-OH-PBDE 47 | agonists, weak to moderate (Wang et al., 2006; Chen and Bruce, 2003; Peters et al., 2006a; Peters et al., 2006b; Germer et al., 2006;) |
| 47, PBDE mixture (D-71) | antagonist in fish, weak (Kuiper et al., 2004) | ||||
| PBDF impurities | no Ah activity by PBDEs, but from PBDFs impurity (Sanders et al., 2005) | ||||
| Constitutive androstane (CAR) | CYP 2B | ortho-, para- PCBs | >20–30 increase (Denomme et al., 1983) | PBDE mixtures DE-71, PBDE 79 | weak (Kretschmer and Baldwin, 2005; Sanders et al. 2005; Germer et al., 2006) |
| Pregnane X (PXR) | CYP 3A | poly-ortho- PCBs, PCBs 47, 184 | 5–10 increase (Hurst and Waxman, 2005; Schuetz, Brimer and Schuetz, 1998) | PBDE mixture D-71 | (Germer et al., 2006) |
| Peroxisome proliferator (PPAR) | CYP 4A | co-planar, meta-, para-PCBs | repression (Ariyoshi et al., 1998; Robertson et al., 2007) | Ah active PBDEs and potentially PBDFs | suggested repression in analogy to PCBs (Ariyoshi et al., 1998) |
| Ryanodine (RyR) | Ca2+ channel | PCB 95, OH- PCBs, PCB- catechols | 2–50 increased (Pessah et al., 2006; Pessah and Wong, 2001; Wong, Brackney and Pessah, 1997) | Non co-planar PBDEs and OH- PBDEs | suggested agonist in analogy to the findings in PCBs (Pessah et al., 2006) |
| Estrogen (ER) | Estrogen | poly-ortho- PCBs, OH-PCBs | agonist and antagonist (Arcaro et al., 1999; Bonefeld-Jorgensen et al., 2001; Connor et al., 1997) | OH-PBDE Di-OH-PBDE PBDE 99 | (Kretschmer and Baldwin, 2005; Stoker et al., 2004; Meerts et al., 2001; Ceccatelli et al., 2006) |
| Androgen (AR) | Androgen | poly-ortho-PCBs | antagonist (Fang et al., 2003; Portigal et al., 2002; Schrader and Cooke, 2003) | PBDE mixture DE-71, PBDEs 100 PBDEs 47, 99, 100 | Suppression and inhibition (Stoker et al., 2005) |
| 153, 154 | competitive anti-androgenic (Stoker et al., 2005) | ||||
| Progesterone (PR) | Progesterone | OH-PCB | antagonist (Conner et al., 1997) | PBDE 99 | suppression (Ceccatelli et al., 2006) |
| Dihydro-testosterone (DHT) | Dihydrotestosterone | PBDE 47, 100 | Inhibitor transcriptional activation (Stoker et al., 2005) | ||
| Thyroid function (TH) | Thyroid hormone | PCBs, OH-PCBs | indirect (Gauger et al., 2004) | OH-PBDEs PBDE 47 | Agonist (Kretschmer and Baldwin, 2005; Eslami et al., 2006; Hallgren, Darnerud, 2002, Zhou et al., 2002) |
| PBDE mixtures DE-71, 79 | disruptor (Kretschmer and Baldwin, 2005) | ||||
| Thyroxine (T4) Triiodothyronine (T3) | Thyroxine | PBDEs 71, 73, 125 4’OH-PBDE 27 | reducing serum T4 level (Kretschmer and Baldwin, 2005; Stoker et al., 2004; Hooper and McDonald, 2000; Zhou et al., 2002; Eslami et al., 2006) | ||
| Glucocorticoid (GR) | Glucocorticoid | MeSO2-PCBs | Competitive antagonist (Johansen, Nielsson and Lund, 1998) | MeSO2-PBDE | suggested repression by competitive antagonism in analogy to MeSO2-PCBs (Johansson et al., 1998) |
| γ-aminobutyric acid (GABA) | Neurotransmitter | non co-planar PCBs | (Mariussen and Fonnum, 2001; Wong, Brackney and Pessah, 1997; Wong et al., 1997). | non-meta- and para- but ortho- substituted PBDEs | Suggested agonist in analogy to findings (Wong et al., 1997). |
| Insulin-like growth factor (IGF) | Insulin-like growth factor | Xenobiotics in general | Scarth, 2006 | PBDE 99 | agonist, weak (Ceccatelli et al., 2006) |
Acknowledgments
This publication was made possible by the Alexander von Humboldt Foundation, Bonn, Germany, by grant number P42 ES 013661 from the National Institute of Environmental Health Sciences (NIEHS), and United States Environmental Protection Agency grant RD-82902102. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Humboldt Foundation, NIEHS or the EPA.
References
- Arcaro KF, Yi L, Seegal RF, Vakharia DD, Yang Y, Spink DC, Brosch K, Gierty JF. 2,2',6,6'-Tetrachlorobiphenyl is estrogenic in vitro and in vivo. J. Cell. Biochem. 1999;72:94–102. [PubMed] [Google Scholar]
- Ariyoshi N, Iwasaki M, Kato H, Tsusaki S, Hamamura M, Ichiki T, Oguri K. Highly toxic coplanar PCB126 reduces liver peroxisomal enzyme activities in rats. Environ. Toxicol. Pharmacol. 1998;5:219–225. doi: 10.1016/s1382-6689(98)00007-6. [DOI] [PubMed] [Google Scholar]
- Ashek A, Lee C, Park H, Cho SJ. 3D QSAR studies of dioxins and dioxin-like compounds using CoMFA and CoMSIA. Chemosphere. 2006;65:521–529. doi: 10.1016/j.chemosphere.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Bandiera S, Safe S, Okey AB. Binding of polychlorinated biphenyls classified as either phenobarbitone-3-methylcholanthrene- or mixed-type inducers to cytosolic Ah receptor. Chem. Biol. Interact. 1982;39:259–277. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, Cellular and Molecular Biology, and Physiological Roles of the Iodothyronine Selenodeiodinases. Endocr. Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jorgensen EC, Andersen HR, Rasmussen TH, Vinggaard AM. Effect of highly bioaccumulated polychlorinated biphenyl congeners on estrogen and androgen receptor activity. Toxicology. 2001;158:141–153. doi: 10.1016/s0300-483x(00)00368-1. [DOI] [PubMed] [Google Scholar]
- Ceccatelli R, Faass O, Schlumpf M, Lichtensteiger W. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology. 2006;220:104–106. doi: 10.1016/j.tox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Chen G, Bunce NJ. Polybrominated diphenyl ethers as Ah receptor agonists and antagonists. Toxicol. Sci. 2003;76:310–320. doi: 10.1093/toxsci/kfg236. [DOI] [PubMed] [Google Scholar]
- Conner K, Ramamoorrhy K, Moore M, Mustain M, Chen I, Safe S, Zacharewski T, Gillesby B, Joyeux A, Balaguer P. Hydroxylated polychlorinated biphenyls (PCBs) as estrogens and antiestrogens: Structure-activity relationships. Toxicol. Appl. Pharmacol. 1997;145:111–123. doi: 10.1006/taap.1997.8169. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Eriksen GS, Jóhannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ. Health Perspect. 2001;109:49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denomme MA, Bandiera S, Lambert I, Copp I, Safe L, Safe S. Biphenyls as phenobarbitone-type inducers of microsomal enzymes: Structure–activity relationships for a series of 2,4-dichloro-substituted congeners. Biochem. Pharmacol. 1983;32:2955–2963. doi: 10.1016/0006-2952(83)90402-1. [DOI] [PubMed] [Google Scholar]
- Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP. AM1: A new general purpose quantum mechanical model. J. Am. Chem. Soc. 1985;107:3902–3909. [Google Scholar]
- Dratman M, Gordon J. Thyroid hormones as neurotransmitters. Thyroid. 1996;6:639–647. doi: 10.1089/thy.1996.6.639. [DOI] [PubMed] [Google Scholar]
- Eslami B, Koizumi A, Ohta S. Large-scale evaluation of the current level of polybrominated diphenyl ethers (PBDEs) in breast milk from 13 regions of Japan. Chemosphere. 2006;63:554–561. doi: 10.1016/j.chemosphere.2005.09.067. [DOI] [PubMed] [Google Scholar]
- Fang H, Tong W, Branham WS, Moland CI, Dial sI, Hong H, Xie Q, Perkins R, Owens W, Sheelhan DM. Structure–activity relationships for a large diverse set of natural, synthetic, and environmental estrogens. Chem. Res. Toxicol. 2003;16:1338–1358. doi: 10.1021/tx000208y. [DOI] [PubMed] [Google Scholar]
- Gauger KJ, Kato Y, Haraguchi K, Lehmler HJ, Robertson LW, Bansal R, Zoeller RT. Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ. Health. Perspect. 2004;112:516–523. doi: 10.1289/ehp.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germer S, Fery Y, van der Ven L, Piersma AH, Kamyschnikow A, Schrenk D. Induction of Cytochrome P450 enzymes in Rat Liver and Rat Primary Hepatocytes by Polybrominated Diphenyl Ethers. Toxicol. Lett. 2006;164S:S159–S160. [Google Scholar]
- Gillner M, Bergman J, Cambillau C, Alexandersson M, Fernstrom B, Gustafsson J-A. Interactions of Indolo[3,2-b]carbazoles and related polycyclic aromatic hydrocarbons with specific binding sites for 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver. Mol. Pharmacol. 1993;44:336–345. [PubMed] [Google Scholar]
- Hahn ME. The aryl hydrocarbon receptor: a comparative perspective. Comp. Biochem. Physiol. 1998;121C:23–53. doi: 10.1016/s0742-8413(98)10028-2. [DOI] [PubMed] [Google Scholar]
- Hallgren S, Darnerud PO. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), and chlorinated paraffins (CPs) in rats-testing interactions and mechanisms for thyroid hormone effects. Toxicology. 2002;177:227–243. doi: 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- Hooper K, McDonald TA. The PBDEs: an emerging environmental challenge and another reason for breast-milk monitoring program. Environ. Health Perspect. 2000;108:387–392. doi: 10.1289/ehp.00108387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol. Environ. Chem. 2005;87:299–311. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- Johansson M, Johansson N, Lund BO. Xenobiotics and the glucocorticoid receptor: additive antagonistic effects on tyrosine aminotransferase activity in rat hepatoma cells. Basic Clin. Pharmacol. Toxicol. 2005;96:309–315. doi: 10.1111/j.1742-7843.2005.pto960406.x. [DOI] [PubMed] [Google Scholar]
- Johansson M, Nilsson S, Lund BO. Interactions between methylsulfonyl PCBs and the glucocorticoid receptor. Environ. Health Perspect. 1998;106:769–772. doi: 10.1289/ehp.98106769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stolz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor and constitutive androstane receptor. J. Biol. Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ. Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology. 2000;141:1897–1900. doi: 10.1210/endo.141.5.7530. [DOI] [PubMed] [Google Scholar]
- Klösener J, Swenson DC, Robertson LW, Luthe G. Electrostatic and aspheric influence of the fluoro-substitution of 4-bromodiphenyl ether (PBDE 3) Acta Cryst. 2008;B64:108–119. doi: 10.1107/S0108768107067079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer XC, Baldwin WS. CAR and PXR: xenosensors of endocrine disrupters? Chem. Biol. Interact. 2005;155:111–128. doi: 10.1016/j.cbi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Kuiper RV, Bergman A, Vos JG, van den Berg M. Some polybrominated diphenyl ether (PBDE) flame retardants with wide environmental distribution inhibit TCDD-induced EROD activity in primary cultured carp (Cyprinus carpio) hepatocytes. Aquat. Toxicol. 2004;68:129–139. doi: 10.1016/j.aquatox.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Lemaire G, de Sousa G, Rahmani R. A PXR reporter gene assay in a stable cell culture system: CYP3A4 and CYP2B6 induction by pesticides. Biochem. Pharmacol. 2004;68:2347–2358. doi: 10.1016/j.bcp.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Ludewig G, Esch H, Robertson LW. Polyhalogenierte Bi- und Terphenyle. In: Dunkelberg H, Gebel T, Hartwig A, editors. Handbuch der Lebensmitteltoxikologie. ch. 20. Weinheim, Germany: Wiley-VCH; 2007. pp. 1031–1094. [Google Scholar]
- Luthe G, Swenson DC, Robertson LW. Influence of fluoro-substitution on the planarity of 4-chlorobiphenyl (PCB 3) Acta Cryst. 2007;B 63:319–327. doi: 10.1107/S0108768106054255. [DOI] [PubMed] [Google Scholar]
- Maitland CG, Rigby M, Smith EB, Wakaham WA. Intermolecular Forces: Their Origin and Determination. Oxford, UK: Clarendon Press; 1987. Theoretical calculations of intermolecular forces; pp. 45–95. [Google Scholar]
- Mariussen E, Fonnum F. The effect of pentabromodiphenyl ether, hexabromocyclododecane and tetrabromobisphenol - A on dopamine uptake into rat brain synaptosomes. Toxicology. 2001;159:11–21. doi: 10.1016/s0300-483x(00)00374-7. [DOI] [PubMed] [Google Scholar]
- McKinney JD, Darden t, Lyerly MA, Pederson LG. PCB and Related Compound Binding to the Ah Receptor. Theoretical Model Based on Molecular Parameters and Molecular Mechanics. Quant. Struct.-Act. Relat. 1985;4:166–172. [Google Scholar]
- Meerts IATM, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, van der Burg B, Brouwer A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PBDEs, and polybrominated bisphenol A compounds. Environ. Health Perspect. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussey S, Whitehead S. The thyroid gland in Endocrinology. London, GB: An Integrated Approach by Published by BIOS Scientific Publishers Ltd.; 2001. [PubMed] [Google Scholar]
- Opera TI, Waller CL, Marshall GR. 3D-QSAR of human immunodeficiency virus (I) protease inhibitors. III. Interpretation of CoMFA results. Drug. Des. Discov. 1994;12:29–51. [PubMed] [Google Scholar]
- Pearson RG. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963;85:3533–3539. [Google Scholar]
- Pessah IN, Hansen IG, Alberson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW. Structure-Activity Relationship for Noncoplanar Polychlorinated Biphenyl Congeners toward the Ryanodine Receptor-Ca2+ Channel Complex Type 1 (RyR1) Chem. Re. Toxicol. 2006;19:92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- Peters AK, Nijmeijer S, Gradin K, Backlung M, Bergman A, Poellinger L, Denison MS, van den Berg M. Interactions of Polybrominated Diphenyl Ethers with the Aryl Hydrocarbon Receptor Pathway. Tocicol. Sci. 2006;91:133–142. doi: 10.1093/toxsci/kfj186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AK, Sanderson JT, Bergman A, van den Berg M. Antagonism of TCDD-induced Ethoxyresorufin-O-deethylation activity by Polybrominated Diphenyl Ethers (PBDEs) in Primary Cynomolgus Monkey (Macaca Fascicularis) Hepatocytes. Tocicol. Lett. 164:123–132. doi: 10.1016/j.toxlet.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Portigal CL, Cowell SP, Fedoruk MN, Butler CM, Rennie PS, Nelson CC. Polychlorinated Biphenyls Interfere with Androgen-Induced Transcriptional Activation and Hormone Binding. Toxicol. Appl. Pharmacol. 2002;172:185–194. doi: 10.1006/taap.2002.9371. [DOI] [PubMed] [Google Scholar]
- Robertson LW, Berberian I, Borges T, Chen LC, Chow CK, Glauert HP, Filser JG, Thomsa H. Supression of peroxisomal enzyme activities and cytochrome P4504A isozyme expression by congeneric polybrominated and polychlorinated biphenyls. PPAR Res. 2007;5 doi: 10.1155/2007/15481. doi:10.1155/2007/15481, Article ID 15481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld JM, Vargas R, Xie W, Evans RM. Genetic Profiling Defines the Xenobiotic Gene Network Controlled by the Nuclear Receptor Pregnane X Receptor. Mol. Endocrinol. 2003;17:1268–1282. doi: 10.1210/me.2002-0421. [DOI] [PubMed] [Google Scholar]
- Safe SH. Polychlorinated biphenyls (PCBs) and polybrominated biphenyls (PBBs): biochemistry, toxicology, and mechanism of action. Crit. Rev. Toxicol. 1990;21:51–88. doi: 10.3109/10408448409023762. [DOI] [PubMed] [Google Scholar]
- Sander JM, Burka LT, Smith CS, Black W, James R, Cunningham ML. Differential expression of CYP1A, 2B, and 3A genes in the F344 rat following exposure to a polybrominated diphenyl ether mixture or individual components. Toxicol. Sci. 2005;88:127–133. doi: 10.1093/toxsci/kfi288. [DOI] [PubMed] [Google Scholar]
- Scarth J. Modulation of the growth hormone-insulin-like growth factor (GH-IGF) axis by pharmaceutical, nutraceutical and environmental xenobiotics: An emerging role for xenobiotic-metabolizing enzymes and the transcription factors regulating their expression. A review. Xenobiotica. 2006;36:119–218. doi: 10.1080/00498250600621627. [DOI] [PubMed] [Google Scholar]
- Schaefer T, Penner GH, Takeuchi C, Tseki P. Remarks on the internal motion in diphenyl ether. Fluorophenyl ethers. Can. J. Chem. 1988;66:1647–1650. [Google Scholar]
- Schrader TJ, Cooke GM. Effects of Aroclors and individual PCB congeners on activation of the human androgen receptor in vitro. Reprod. Toxicol. 2003;17:15–23. doi: 10.1016/s0890-6238(02)00076-x. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Brimer C, Schuetz JD. Environmental Xenobiotics and the Antihormones Cyproterone Acetate and Spironolactone Use the Nuclear Hormone Pregnenolone X Receptor to Activate the CYP3A23 Hormone Response Element. Mol. Pharmacol. 1998;54:1113–1117. doi: 10.1124/mol.54.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetz EG, Wrighton SA, Safe SH, Guzelian PS. Regulation of Cytochrome P-450p by Phenobarbital and Phenobarbital-like inducers in adult rat hepatocytes in primary monolayer cultures and in vivo. Biochemistry. 1986;25:1124–1133. doi: 10.1021/bi00353a027. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Laws SC, Crofton KM, Hedge JM, Ferrell JM, Cooper RL. Assessment of DE-71, a Commercial Polybrominated Diphenyl Ether (PBDE) Mixture, in the EDSP Male and Female Pubertal Protocols. Toxicol. Sci. 2004;78:144–155. doi: 10.1093/toxsci/kfh029. [DOI] [PubMed] [Google Scholar]
- Sugatani H, Kojima H, Ueda A, Kakizaki s, Yoshimari QH. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology. 2001;33:1232–1238. doi: 10.1053/jhep.2001.24172. [DOI] [PubMed] [Google Scholar]
- Townes CH, Schawlow AL. Microwave Spectroscopy. New York City, USA: Dover Publications; 1975. [Google Scholar]
- Udea A, Hamadeh HK, Webb HK, Yamato T, Sueyoshi CA, Afsahari JM, Lehman M. Diverse Roles of the Nuclear Orphan Receptor CAR in Regulating Hepatic Genes in Response to Phenobarbital. Mol. Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao C, Ma W, Liu H, Wang Y, Jiang G. Quantitative structure–activity relationship for prediction of the toxicity of polybrominated diphenyl ether (PBDE) congeners. Chemosphere. 2006;64:515–524. doi: 10.1016/j.chemosphere.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Waxman DJ. P450 Gene induction by structurally diverse xenochemicals: Central Role of Nuclear receptors CAR, PXR and PPAR. Archives of Biochemistry and Biophysics. 1999;369:11–23. doi: 10.1006/abbi.1999.1351. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Dowhan DH, Han Y, Moore DD. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Nature. 2002;2:117–126. doi: 10.1038/sj.tpj.6500087. [DOI] [PubMed] [Google Scholar]
- Wong PW, Joy RM, Albertson TE, Schantz SL, Pessah IN. Ortho-substituted 2,2',3,5',6-pentachlorobiphenyl (PCB 95) alters rat hippocampal ryanodine receptors and neuroplasticity in vitro: evidence for altered hippocampal function. Neurotoxicology. 1997;18:443–456. [PubMed] [Google Scholar]
- Wong PW, Brackney WR, Pessah IN. ortho-Substituted Polychlorinated Biphenyls Alter Microsomal Calcium Transport by Direct Interaction with Ryanodine Receptors of Mammalian Brain. J. Biol. Chem. 1997;272:15145–15153. doi: 10.1074/jbc.272.24.15145. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental Exposure to Brominated Diphenyl Ethers Results in Thyroid Hormone Disruption. Toxicol. Sci. 2002;66:105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]