Abstract
Management of yellow fever is focused on the prevention of illness by the use of the yellow fever virus (YFV) 17D vaccine. The role of neutralizing antibodies in protection is generally accepted with YFV-specific T cells likely contributing to the control of viral replication. We studied CD8+ T-cell responses to four defined human leucocyte antigen-B35-restricted epitopes in YFV vaccine recipients as a model of the kinetics of cytotoxic T-lymphocyte responses to an acute human viral infection. Multiple features of these epitope-specific responses were analysed after vaccination including magnitude, cytokine production, phenotype and T-cell receptor repertoire. Peak peptide-specific interferon-γ (IFN-γ) responses of almost 1% of CD8+ T cells were seen as early as 2 weeks post-vaccination; however, dominant responses varied between donors. Peptide-specific responses were still detectable at 54 months post-vaccination. Tetramer-positive cells, at high frequencies, were detected as early as 7–9 days, before detectable IFN-γ-producing cells, suggesting a defect in the functional capacity of some antigen-specific cells early post-vaccination. The predominant memory phenotype of the tetramer-positive population was a differentiated effector (CD45RA+ CCR7− CD62L−) phenotype. The T-cell receptor Vβ analysis revealed a diverse oligoclonal repertoire in tetramer-positive T-cell populations in two individuals. These characteristics of the YFV-specific T-cell response could contribute to vaccine effectiveness.
Keywords: CD8 T cells, human, vaccine, viral, yellow fever
Introduction
Yellow fever virus (YFV) is a single-stranded RNA virus belonging to the family Flaviviridae, whose members include clinically relevant viruses such as Japanese encephalitis virus, West Nile virus and Dengue virus. Treatment for yellow fever is supportive and efforts have focused on the prevention of illness by the use of the YFV 17D vaccine. This live attenuated vaccine has been administered to over 400 million people and YFV-specific antibodies can be detected as long as 35 years after vaccination.1 Based on evidence that CD8+ T cells are important in viral clearance in mouse models such as lymphocytic choriomeningitis virus and influenza and in human systems such as human immunodeficiency virus (HIV), cytomegalovirus (CMV) and Epstein–Barr virus (EBV),2–8 we analysed the YFV-specific cytotoxic T-cell response in vaccine recipients and defined the first four human YFV CD8+ T-cell epitopes,9 suggesting that YFV 17D vaccine was capable of inducing strong cytotoxic CD8 T-cell responses.
Studies on the kinetics of human virus-specific CD8+ T-cell responses have mostly been carried out for chronic viral infections such as HIV, CMV and EBV.10 Some acute viral infections have been studied, such as hantavirus and vaccinia virus infections.11,12 However, results in some acute viral infections can be difficult to generalize because of uncontrolled exposure (e.g. hantavirus), re-exposure to the virus (e.g. influenza virus), or localized rather than systemic infection (e.g. vaccinia virus). We studied the kinetics of an acute systemic human viral infection using YFV 17D vaccine as a model. This model has two major advantages. First, there is no known chronic or latent stage of the illness as seen in EBV, CMV and HIV. Second, this virus is not endemic in the United States; therefore there is little chance of re-exposure unless travel is undertaken to an overseas endemic area. We quantified the frequency of YFV-specific CD8+ T cells in three YFV vaccine recipients using intracellular cytokine staining and human leucocyte antigen (HLA)-peptide tetramers; we also examined the functional capacity, phenotype and T-cell receptor (TCR) Vβ repertoire of these epitope-specific cells over the post-vaccination period.
Materials and methods
Subjects
Blood was obtained from three healthy HLA-B35+ individuals who received the live attenuated YFV 17D vaccine. The study protocol was approved by the institutional review board of the University of Massachusetts Medical School and written informed consent was obtained from all subjects. Donors A and B were described in our previous report.9 Peripheral blood mononuclear cells (PBMC) were obtained pre-vaccination and at various time-points for up to 54 months post-vaccination. They were purified by Ficoll–Hypaque density gradient centrifugation and cryopreserved until studied.
Peptides
We previously defined four HLA-B35-restricted CD8+ T-cell epitopes on the YFV E (IPVIVADDL), NS1 (HAVPFGLVSM), NS2b (HPFALLLVL) and NS3 (TGHDWILA) proteins.9 All peptides were synthesized in the University of Massachusetts Medical School (UMMS) Peptide Core Facility.
Monoclonal antibodies
Anti-human monoclonal antibodies (mAb) and their respective isotype controls were obtained from BD Pharmingen (Franklin Lakes, NJ): purified murine immunoglobulin G (mIgG1) κ, allophycocyanin-conjugated (-APC) anti-interferon-γ (IFN-γ), phycoerythrin-conjugated (-PE) anti-CD45RO, fluorescein isothiocyanate-conjugated (-FITC) anti-CD38 or anti-CD38-PE, anti-CD69-FITC, anti-CD27-FITC, anti-CD28-PE, and anti-CCR7-PE; or from BD Biosciences (San Jose, CA): anti-CD3-FITC, anti-CD8-peridinin chlorophyll protein Cy5.5, anti-CD3-Pacific blue, and anti-CD45RA-FITC.
Intracellular cytokine staining (ICS)
The PBMC (1 × 106 to 2 × 106) were thawed and washed with 2–3 ml RPMI-1640 with 10% heat-inactivated human AB serum and then resuspended in 1 ml of the same medium. Peptides were added at a final concentration of 5 μg/ml. Staphylococcal enterotoxin B (1 μg/ml) was used as a positive control. After incubation for 2 hr at 37°, 10 μl Golgi Plug (BD Pharmingen) was added and the cells were incubated for an additional 4 hr at 37°. Cells were then washed with fluorescence-activated cell sorting (FACS) buffer (2% fetal bovine serum, 0·1% sodium azide in phosphate-buffered saline) and centrifuged at 1400 g for 5 min. After decanting, 10 μl of purified mouse IgG1κ (50 μg/ml) was added and incubated for 15 min at 4°. Cells were then stained for surface markers by incubating at 4° for 30 min with one or more of these mAb: CD3-FITC, CD8-PerCP or isotype control antibodies. Cells were then washed with 2 ml cold FACS buffer and centrifuged at 450 g. After the supernatant was decanted, cells were incubated with 250 μl Cytofix/Cytoperm (BD Pharmingen) for 20 min at 4°. Cells were then washed with 2–3 ml PermWash (BD Pharmingen) and stained with 10 μl mAb CD69-PE and IFN-γ-APC (25 μg/ml) and incubated in the dark for 30 min at 4°. Cells were washed with 2–3 ml PermWash and then resuspended in 0·32 ml FACS buffer for analysis at the UMMS Flow Cytometry Facility. Flow cytometry was performed using FACS Aria (BD Biosciences) and FACSVantage (BD Biosciences) flow cytometers and data were analysed using flo-jo software (Tree Star Inc., Ashland, OR).
There was limited availability of PBMC samples so replicate experiments or duplicates in single experiments were performed only on selected PBMC samples.
Major histocompatibility complex–peptide tetrameric complexes
The HLA-B*3501-NS1 tetramer was generated according to the method described by Altman et al.13 and Kilpatrick et al.14 The HLA-B*3502-E tetramer was synthesized by the National Institutes of Health tetramer facility at Emory University and the HLA-B*3501-NS2b tetramer was synthesized at the UMMS Tetramer Core Facility.
The PBMC were first stained with a saturating concentration of tetramer at room temperature or at 4° (based on optimization experiments for each tetramer) for 20 min and then with either or both mAb to CD3 and CD8 (BD Pharmingen). Cells were then washed, fixed and analysed in the UMMS Flow Cytometry Facility. The specificity of the NS1, NS2b and E tetramers was confirmed by testing epitope-specific clones or epitope-stimulated bulk cultures as positive controls and using clones specific for an unrelated HLA-B*3501-restricted epitope or bulk cultures stimulated with a different YFV epitope as negative controls.
Phenotypic analysis
The PBMC (1 × 106 to 2 × 106) were divided into aliquots, washed with 1 ml FACS buffer and then incubated with a pre-titrated concentration of tetramer for 20 min at room temperature or 4°. The choice of tetramer in each donor was based on the predominant epitope response observed in intracellular cytokine staining experiments. The cells were then stained for surface markers by incubation on ice in the dark with 10 μl anti-CD3 mAb and/or anti-CD8 mAb and then with conjugated mAb to the following (alone or in combination): CD27, CD28, CD45RO, CD45RA, CCR7, CD38, HLA-DR, CD69, CD62L, or the corresponding isotype controls. Cells were then washed twice with 1 ml FACS buffer and fixed with 200 μl of 1% paraformaldehyde in phosphate-buffered saline for 5–7 min on ice. After washing with 1 ml FACS buffer, cells were resuspended in 0·35 ml FACS buffer and analysed.
Determination of TCR Vβ usage
The PBMC (1 × 106 to 2 × 106) were stained with tetramer as described above. Cells were then stained with anti-human CD8-APC and with a panel of 24 anti-human Vβ chain antibodies (IO Test® Beta mark kit; Beckman Coulter, Fullerton, CA) in the dark at 4° for 30 min, washed with FACS medium twice and then resuspended in FACS medium for analysis. This was a single experiment given the limited availability of cells.
Results
Kinetics of the response to HLA-B35-restricted epitopes in YFV vaccine recipients
ICS was performed to examine the response to four HLA-B35-restricted T-cell epitopes in serial PBMC samples from three YFV 17D vaccine recipients (Fig. 1). Background staining for each of the YFV epitopes in pre-vaccination samples from Donors A, B and C was less than 0·04% of CD8+ T cells except in the case of the NS2b and NS3 peptides in Donor B, which had values of 0·09% and 0·06% of CD8+ T cells, respectively.
Figure 1.
Kinetics of interferon-γ (IFN-γ) response to human leucocyte antigen (HLA) B35 restricted yellow fever virus (YFV) epitopes in YFV 17D vaccinated individuals. Intracellular cytokine staining (ICS) assays were performed on peripheral blood mononuclear cell (PBMC) obtained from three YFV vaccine recipients (Donor A, B and C) at various time-points post-immunization and results were analysed by flow cytometry. Connected line represents the cumulative responses for the epitopes measured at each time-point. Frequencies represent the percentage of IFN-γ-producing CD3+ CD8+ cells (minus the background staining of unstimulated samples).
In the PBMC from Donor A, YFV peptide-specific responses to all four epitopes were detected at 14 days, but not at 7 days, post-vaccination. Dominant responses were to the NS1 and NS2b epitopes (0·43% and 0·24% of circulating CD8+ T cells, respectively) with the total response representing 0·90% of CD8+ T cells. At 1 month post-vaccination, peak YFV peptide-specific responses (Fig. 1) representing almost 2% of CD8+ T cells were detected, and the responses to the dominant NS1 and NS2b epitopes (0·77–0·84% of CD8+ T cells) were approximately equal. From 1 month to 54 months post-vaccination, while responses to the subdominant E and NS3 epitopes remained fairly stable, total responses declined markedly (2% to 0·36% of CD8+ T cells).
In the Donor B PBMC (Fig. 1), the E epitope responses were detected at 7 and 14 days post-vaccination. At 1 month post-vaccination, responses were detected to all four epitopes representing 1·3% of CD8+ T cells, with peak responses to the NS2b and NS3 epitopes (0·64% and 0·47% of CD8+ T cells, respectively). Time-points from 1 year to 2 years post-vaccination were marked by detectable but low responses to the E and NS1 epitopes and by a marked decline in the response to the NS2b and NS3 epitopes. However, at 36 months post-vaccination an increase in response to all four epitopes was detected without any history of intercurrent illness or travel.
In the Donor C PBMC (Fig. 1), the frequency of YFV peptide-specific responses was of a lower magnitude than seen in Donors A and B. Surprisingly, initial responses to any epitope were detected late post-vaccination, at the 2-month time-point. From 5 months to 1 year post-vaccination, responses were marked by changes in the hierarchy of the NS1 (0·17–0·03%) and the NS2b (0·18–0·36%) responses and low but detectable NS3 responses. Responses to the NS1 and ND2b epitopes declined at 33 months post-vaccination (0·01–0·05% of CD8+ T-cell responses). In contrast, E epitope responses were predominant at these later time-points (0·15–0·21% of CD8+ T cells).
In summary, peptide-specific responses were detected in all donors at 14 days post-vaccination though responses were detected as early as 7 days. Peak responses were seen 1 month post-vaccination in two donors but at 12 months in another donor. Responses to dominant epitopes varied among donors but were persistent as late as 54 months post-vaccination. The hierarchy of the most dominant epitopes over the post-vaccination period was relatively consistent; however, there were several time-points (e.g. 7 months and 1 year for Donor A and 3 years for Donor B) at which a change in the epitope hierarchy was noted (Fig. 1).
Comparison of YFV-specific CD8+ T-lymphocyte frequency by tetramer and intracellular cytokine staining
To quantify the YFV-specific T-cell responses independent of cytokine secretion, we used HLA–peptide tetramers to detect CD8+ T cells specific for the immunodominant NS1 epitope in Donor A, the NS2b epitope in Donor B and the E epitope in Donor C. The NS1-specific cells in Donor A were detected by tetramer staining as early as 7 days post-vaccination (0·14% of CD8+ T cells, Table S1). The NS1-specific response reached a peak at 2 weeks post-vaccination as detected by tetramer staining (1·5% of CD8+ T cells, Table S1), declined slightly at 1 month post-vaccination, and then declined further over the 4 years of study. The YFV peptide-specific T-cell frequencies detected by tetramer staining were substantially higher than those reported previously using IFN-γ ELISPOT assays. The frequencies reported by tetramer staining were also higher than those detected by ICS at all time-points tested (Table S1).
In Donor B, because of the limitations on PBMC availability, only three time-points (7 days, 27 days and 26 months) were tested. At 7 days post-vaccination, 1·7% of CD8+ T cells stained with the NS2b tetramer. The peak of the NS2b response by both tetramer and ICS staining was seen at 1 month post-vaccination (2·7% of CD8+ T cells). At 26 months, this response decreased dramatically to 0·27%.
In Donor C, E tetramer-positive cells were detected at 9 days post-vaccination (0·37% of CD8+ T cells, Table S1). At 2 months post-vaccination, more than 1% of cells (1·18%) were tetramer positive. Surprisingly, at 33 months, YFV peptide-specific T-cell frequencies were still high (0·9% of CD8+ T cells). In summary, at all time-points in all donors tested, the peptide-specific T-cell frequencies detected by functional staining (ICS) were lower compared with tetramer staining.
Phenotypic characteristics of YFV-specific T cells
Three memory differentiation schemes were used to examine the phenotype of YFV-specific CD8+ T cells at several time-points post-vaccination. The PBMC were directly stained with tetramer (NS1 for Donor A, NS2b for Donor B and E for Donor C) along with surface staining for CD27/CD28, CD45RO/CD45RA, CCR7/CD45 and the activation markers CD38 and CD69. These samples were tested once because of the limited availability of cells.
High levels of the activation marker CD38 (50–99%) at early time-points post-vaccination (7–28 days) were detected in Donor A and Donor B but not in Donor C, which may be related to the time-points tested in this Donor (Table S1). In contrast, CD69 expression was seen only at a low frequency (5%) at one early time-point (1 month) in Donor A (Data not shown).
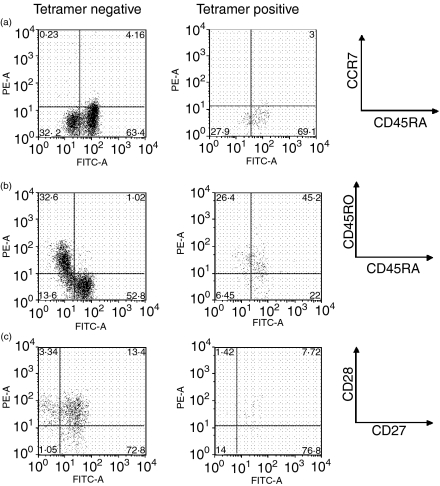
The CD45RO+ phenotype was seen in Donors A and C during the acute phase (Days 7–14) in the NS1 and E tetramer-positive populations at frequencies of 30–60%. The CD45RA+ phenotype could be detected as early as 7–9 days post-vaccination in Donors A and B (22–43%) but was most consistently seen in all donors at late time-points post-vaccination (> 2 months) in 40–60% of tetramer-positive cells (Figs 2 and 3).
Figure 2.
Kinetics of phenotypic marker expression by yellow fever virus (YFV) tetramer-positive population over the post-vaccination period. Serial peripheral blood mononuclear cells from three YFV 17D-vaccinated donors were analysed as described in the Materials and methods section with all events gated on a CD3+ CD8+ population and then on the tetramer-positive population. Values shown represent the number of tetramer-positive CD8+ T cells expressing the indicated phenotypic markers at each time-point (a) NS1 tetramer-positive population in Donor A, (b) NS2b tetramer-positive population in Donor B, (c) E tetramer-positive population in Donor C. CCR7+ CD45RA staining not performed in this donor.
Figure 3.
Representative phenotypic marker staining of yellow fever virus (YFV)-specific cells in Donor A, B and C. Donor A, B and C peripheral blood mononuclear cells (PBMC) taken at specified time-points post-vaccination were stained for CD8, for tetramer-positive cells and then for combinations of phenotypic markers. (a) NS1 tetramer-positive cells from Donor A PBMC taken 46 months post-vaccination stained with the CDRA and CCR7 markers, (b) NS2b tetramer-positive cells from Donor B PBMC taken 7 days post-vaccination stained with CD45RO and CD45RA markers (c) E tetramer-positive cells from Donor C taken 2 months post-vaccination stained with CD27 and CD28 markers
We also documented shifts in distribution between the ‘naïve’, central memory (CD45RA− CCR7+) and effector memory (CD45RA− CCR7−) subsets in Donors A and B. The effector memory subset comprised the majority of tetramer-positive cells at time-points up to 2 years post-vaccination in Donor A with peak expression seen at 14 days post-vaccination (85%). A shift was seen to the terminally differentiated phenotype (CCR7− CD45RA+) at 4 years post-vaccination. In contrast, for the NS2b tetramer-positive population of Donor B, the ‘naïve’ phenotype (CD45RA+ CCR7+) predominated at Day 7 with the effector memory phenotype (CD45RA− CCR7−) detected in the majority of cells at Day 27. At later time-points (26 months) for Donor B, tetramer-positive cells showed a relatively even distribution between a ‘naïve’ (34%) and a terminally differentiated phenotype (49%) (Fig. 2).
Using the costimulatory receptors CD27 and CD28 to further define these tetramer-positive populations, the patterns were clearer. In general, the ‘early’ CD27+ CD28+ phenotype predominated at late time-points post-vaccination, comprising up to 94% of tetramer-positive cells in Donor A. The intermediate CD27+ CD28− phenotype (49–77%) was seen at the 1–2 month time-points for Donors B and C before returning to the ‘early’ CD27+ CD28+ phenotype.
TCR Vβ family usage in YFV-specific CD8+ T cells
Flow cytometric analysis of Vβ usage in Donor A HLA-B*3501-NS1 and in Donor C HLA B*3502-E tetramer-positive T cells at selected time-points revealed no dominant Vβ family that persisted from the effector to the memory phases (Fig. 4). A broad range of TCR Vβ usage was seen throughout the vaccination period for both tetramer-positive populations with the repertoire for the E epitope appearing narrower than the NS1 epitope. Certain Vβ families, such as Vβ14 (16%) in Donor A and Vβ4 (14·9%) in Donor C, predominated only in the acute phase (9–14 days) while other Vβ families such as Vβ3, Vβ9 and Vβ13.2 predominated only in the memory phase. In some instances, such as for Vβ21.3 (13%) in Donor A and Vβ7.1 (14·5%) in Donor C, particular families were seen only at single time-points in the memory phase. The diverse usage of TCR Vβ subtypes in both individuals for different YFV epitopes highlights the variability in human T-cell responses. The distribution of the peptide-specific TCR repertoire did not remain stable in the post-vaccination period and only at rare time-points did a single Vβ subset predominate.
Figure 4.
Analysis of T-cell receptor (TCR) Vβ usage in NS1 and E tetramer-positive populations after yellow fever virus (YFV) 17D vaccination. (a) Immune cells from Donor A at four post-vaccination time-points were stained with NS1 tetramer and with antibodies to CD8 and a panel of Vβ families; (b) immune cells from Donor C at three post-vaccination time-points were stained with E tetramer and antibodies to CD8 and a panel of Vβ families. Data are summarized in a pie chart where each slice of the pie represents the proportion of CD8 T cells expressing a particular Vβ subtype. Individual Vβ subsets shown represented at least 8% of the total Vβ subsets detected at any one time-point. All other Vβ subsets were grouped in the Other category. Results are from one experiment.
Discussion
We have performed the first analysis of the evolution of the YFV-specific CD8+ T-cell response from the acute to the memory phase by examining epitope-specific T-cell frequency, phenotype, TCR repertoire and functional responses over the post-vaccination period. The YFV 17D model allows us to investigate the initial and memory human T-cell response to an acute, non-persistent systemic viral infection without antigenic re-exposure. Our data demonstrate a highly variable but persistent response that is more heterogeneous than what has been previously described in murine models of acute viral infection.
The kinetics of the IFN-γ cytokine CD8+ T-cell response differed among donors as assessed by ICS. Memory responses were preferentially directed toward different HLA-B35-restricted epitopes in each donor. Responses were detected as early as 14 days post-vaccination and persisted for at least 33–54 months post-vaccination. In acute non-persistent infections with hantavirus12 or after smallpox vaccination11,15,16 long-lasting memory responses have been demonstrated as late as 15 years and 75 years after initial exposure, respectively. Peak responses were detected at 1 month post-vaccination in two of three donors; however, in a third donor peak responses were delayed until 12 months post-vaccination. In that donor, dominant memory responses were not established before the peak of infection. Switches in epitope immunodominance have similarly been reported in the acute phase of other infections such as hepatitis C virus.17,18 Changes in hierarchy and increases in epitope-specific T-cell populations at later time-points could also be related to heterologous stimulation by unrelated viruses as seen in murine models.19 Our ICS data illustrate both the durability and the variability of human CD8+ T-cell responses to YFV 17D vaccine.
A slightly different picture was obtained using major histocompatibility complex class I tetramers specific for the same immodominant epitopes. Peak epitope-specific responses were detected earlier (14 days versus 1 month) when compared with ICS staining. Miller et al.20 also reported the peak CD8+ T-cell response to YFV 17D vaccine occurring at 2 weeks post-vaccination; however, they identified virus-specific cells based on an activated effector phenotype (positive for CD38, HLA-DR, Ki-67 and Bcl-2) rather than by the use of epitope-specific tetramers. A majority of YFV tetramer-positive cells in our study were CD38+ at early time-points, with our data indicating that the method of Miller et al. probably underestimated the frequency of YFV-specific T cells. Tetramer frequencies were also consistently higher than those measured by ICS. The discordance was greatest at early time-points (7 days to 2 months) corresponding to the peak of the CD8+ T-cell response, suggesting that a significant fraction of epitope-specific cells were unable to produce IFN-γ after peptide stimulation. This may reflect the heterogeneity of normal virus-specific cells,21 but such discordance has been seen in other acute17,18,22,23 and chronic viral infections.21 During acute EBV infection, highly activated antigen-specific cells, which are unable to produce IFN-γ after re-exposure to antigen in vitro,24 may enter the apoptotic pathway.22 After vaccination with the YFV 17D vaccine, peak viraemia levels have been detected within 5–7 days and viraemia is normally cleared by ∼ 7 days.20,25 Therefore, in vivo activation of YFV-specific T cells during the period of high viraemia may affect their functional status, leading to unresponsiveness to in vitro stimulation.
Recent murine studies have addressed the relationships between TCR diversity and the age-associated decline in CD8+ T-cell immunity26 and between TCR diversity and CD8+ T-cell functionality.27 However, human studies of the longitudinal diversity of the TCR repertoire in an acute viral infection have been limited to analyses of the whole CD4+ and CD8+ T-cell populations following hepatitis B vaccination.28,29
The most studied longitudinal model is murine lymphocytic choriomeningitis virus infection, in which the responding TCR repertoire showed narrow TCR Vβ family usage and remained stable from the acute to the memory state.30,31 Our analysis showed heterogeneous use of TCR Vβ families as well as the contraction and expansion of individual Vβ families over time. Similar findings have been described in the EBV-specific T-cell response.32 We found that certain Vβ families are present at all time-points, potentially representing ‘immunological scars’33 in the TCR repertoire. Future studies can address whether these patterns of TCR Vβ usage are shared between unrelated individuals sharing the same HLA allele (public specificity) or are unique to each individual (private specificity).34 Though our data show similarities with murine models of influenza virus infection, such as predominance of certain Vβ families phenotypes,35 the data highlight a diversity and plasticity of the response from the acute to the memory stage that has not been previously described.
Models of memory CD8+ T-cell differentiation have been derived primarily in murine models and persistent human infections such as CMV, EBV and HIV. We characterized the phenotype of YFV tetramer-positive cells using three commonly used classification schemes: naïve (CD45RA+ CD45RO−) versus memory (CD45RA− CD45RO+) phenotypes; early (CD27+ CD28+), intermediate (CD27+ CD28−) and late (CD27− CD28−) differentiated phenotypes; and central (CCR7+ CD45RA−) and effector (CCR7− CD45RA−) memory phenotypes.36 CD45RO+ expression was seen in a significant fraction of tetramer-positive cells only at early time-points (days 7–14). However, the majority of YFV-specific cells at all time-points tested were CD45RA+. This ‘revertant’ or effector memory T-cell phenotype has been described in chronic infections such as CMV and EBV24,37–40 as well as in acute viral infections such as vaccinia virus20,41 and in acute EBV.42 Two additional phenotypes, CD45RA+ CD45RO+ and CD45RA− CD45RO−, were seen in a high percentage of tetramer-positive cells, but only at selected time-points. The double-negative CD45RA− CD45RO− phenotype (Donor A) may identify cells that are undergoing programmed cell death;43 these cells were CD38+, suggestive of recent activation. The CD45RA+ CD45RO+ phenotype (Donor B) may represent cells that have been recently recruited, as described in primary CMV infection.38,40,44
Using an alternative classification, the majority of YFV-specific cells were CD45RA+ CCR7−, a phenotype proposed to represent ‘terminally differentiated’ cells with limited proliferative potential.45 However, 5,6-carboxyfluorescein diacetate succinimidyl ester-labelled CD45RA+ vaccinia virus-specific T cells have been shown to proliferate and secrete IFN-γ and interleukin-2 after 6 hr peptide stimulation.20
In the CD27/CD28 differentiation model, memory cells lose expression of costimulatory molecules CD27 and CD28 and acquire increasing cytotoxic potential and decreasing proliferative potential. The ‘early’ differentiation phenotype (CD27+ CD28+) detected in YFV-specific cells was seen in the primary stages of EBV and hepatitis C virus infections as well as in resolved acute viral infections such as respiratory syncytial virus and influenza virus.46,47 Evidence suggests that protective immunity may be conferred by these early differentiated cells.48,49 In summary, YFV-specific CD8+ T cells generated early post-vaccination were heterogeneous in terms of their phenotype, distributed between the ‘naïve’ CCR7+ CD45RA+ CD27+ CD28+, the differentiated effector CD45RA+ CCR7− and the effector memory EM1 CD45RA− CCR7− CD27+ CD28+ and EM2 CD45RA− CCR7− CD27+ CD28− phenotypes. The EM1 subset, similar to central memory cells, has been shown in other studies to have high levels of CD127, essential for memory cell survival, and low levels of effector molecules such as perforin, IFN-γ and granzyme B, while the EM2 subset has increased effector functions.50 At memory time-points, the predominant phenotype of YFV tetramer-positive cells was CCR7− CD45RA+ CD27+ CD28+. This phenotype does not fit neatly into the proposed paradigms, but vaccinia virus-specific cells with a similar phenotype have been described as polyfunctional, able to degranulate and produce IFN-γ, interleukin-2, macrophage inflammatory protein 1β and tumour necrosis factor α.41 These YFV-specific memory T cells may therefore be important for long-term memory.
The mechanisms by which the live attenuated YFV 17D vaccine induces protection have not been well investigated. The role of neutralizing antibodies is generally accepted1 and recent publications have highlighted the ability of the vaccine to induce components of the innate immune response including the inflammasome, complement, Toll-like receptor and interferons51–53 A recent paper using computational analyses has identified gene signatures that predict the CD8 T-cell and antibody responses, involving the gene for the complement protein C1QB and the gene for the B-cell growth factor TNFRSF17, respectively.54 The fact that increases in the frequency of YFV-specific CD4 and CD8 T cells have been demonstrated during the first 14 days after YF vaccination before the onset of neutralizing antibody production suggests that YFV-specific CD8+ T cells may contribute to the control of viral replication.53,55 Limitations of the current study include the small number of donors studied, the limitation to HLA-B35-restricted epitopes, and the differences in time-points studied among the donors. Nevertheless, the durable YFV-specific CD8+ T-cell responses with a broad and diverse T-cell repertoire and an effector phenotype described in this study may offer clues on the mechanisms that contribute to the long-lasting effectiveness of this vaccine, as well as general insights on the kinetics, magnitude and specificity of human CD8+ T-cell responses to acute systemic viral infections.
Acknowledgments
We wish to thank all the vaccine recipients who provided blood for this study, Dr Tomoko Maeda for her technical assistance on the phenotypic assays, Drs Marlou Mangada and Hema Bashyam for technical assistance on the ICS assays, and Marcia Woda and the UMMS Flow Cytometry Facility for the processing of samples. We also wish to thank Drs Francis Ennis and Sharone Green for critical reading of the manuscript. This study was supported by NIH grants U19 AI57319 and P01 AI49320.
Glossary
Abbreviations:
- APC
allophycocyanin
- CMV
cytomegalovirus
- EBV
Epstein–Barr virus
- FACS
fluorescence-acitvated cell sorting
- FITC
fluorescein isothiocyanate
- HIV
human immunodeficiency virus
- HLA
human leucocyte antigen
- ICS
intracellular cytokine staining
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- TCR
T-cell receptor
- YFV
yellow fever virus
Disclosures
The authors have no potential conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1. Comparison of CD8+ yellow fever virus-specific frequencies using human leucocyte antigen (HLA) peptide tetramers and intracellular cytokine staining.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any Supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Poland JD, Calisher CH, Monath TP, Downs WG, Murphy K. Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bull World Health Organ. 1981;59:895–900. [PMC free article] [PubMed] [Google Scholar]
- 2.Cheynier R, Langlade-Demoyen P, Marescot M, et al. Cytotoxic T lymphocyte responses in the peripheral blood of children born to human immunodeficiency virus 1 infected mothers. Eur J Immunol. 1992;22:2211–7. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- 3.Pinto LA, Sullivan J, Berzofsky JA, Clerici M, Kessler HA, Landay AL, Shearer G. Env-specific cytotoxic T lymphocyte responses in HIV seronegative health care workers occupationally exposed to HIV contaminated body fluids. J Clin Invest. 1995;96:867–76. doi: 10.1172/JCI118133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha MA, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–41. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 5.Rooney CM, Smith CA, Ng CY, Loftin S, Li C, Krance RA, Brenner MK, Heselop HE. Use of gene modified virus specific T lymphocytes to control Epstein–Barr-virus related lynphoproliferation. Lancet. 1995;345:9–13. doi: 10.1016/s0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 6.Rowland-Jones SL, Dong T, Fowke KR, et al. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J Clin Invest. 1998;102:1758–65. doi: 10.1172/JCI4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowland-Jones SL, Nixon DF, Aldhous MC, et al. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–1. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- 8.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–44. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 9.Co MD, Terajima M, Cruz J, Ennis FA, Rothman AL. Human cytotoxic T lymphocyte responses to live attenuated 17D yellow fever vaccine: identification of HLA-B35-restricted CTL epitopes on nonstructural proteins NS1, NS2b, NS3, and the structural protein E. Virology. 2002;293:151–63. doi: 10.1006/viro.2001.1255. [DOI] [PubMed] [Google Scholar]
- 10.Cohen GB, Islam SA, Noble MS, et al. Clonotype tracking of TCR repertoires during chronic virus infections. Virology. 2002;304:474–84. doi: 10.1006/viro.2002.1743. [DOI] [PubMed] [Google Scholar]
- 11.Demkowicz WE, Jr, Littaua RA, Wang J, Ennis FA. Human cytotoxic T-cell memory: long-lived responses to vaccinia virus. J Virol. 1996;70:2627–31. doi: 10.1128/jvi.70.4.2627-2631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van EppsHL, Terajima M, Mustonen J, Arstila TP, Corey EA, Vaheri A, Ennis FA. Long-lived memory T lymphocyte responses after hantavirus infection. J Exp Med. 2002;196:579–88. doi: 10.1084/jem.20011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 14.Kilpatrick ED, Terajima M, Koster FT, Catalina MD, Cruz J, Ennis FA. Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J Immunol. 2004;172:3297–304. doi: 10.4049/jimmunol.172.5.3297. [DOI] [PubMed] [Google Scholar]
- 15.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 16.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 17.Lechner F, Gruener NH, Urbani S, et al. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J Immunol. 2000;30:2479–87. doi: 10.1002/1521-4141(200009)30:9<2479::AID-IMMU2479>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 18.Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selin LK, Nahill SR, Welsh RM. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179:1933–43. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JD, van der Most RG, Akondy RS, et al. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity. 2008;28:710–22. doi: 10.1016/j.immuni.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Scott-Algara D, Buseyne F, Porrot F, Corre B, Bellal N, Rouzioux C, Blanche S, Riviere Y. Not all tetramer binding CD8+ T cells can produce cytokines and chemokines involved in the effector functions of virus-specific CD8+ T lymphocytes in HIV-1 infected children. J Clin Immunol. 2005;25:57–67. doi: 10.1007/s10875-005-0358-3. [DOI] [PubMed] [Google Scholar]
- 22.Catalina MD, Sullivan JL, Bak KR, Luzuriaga K. Differential evolution and stability of epitope-specific CD8(+) T cell responses in EBV infection. J Immunol. 2001;167:4450–7. doi: 10.4049/jimmunol.167.8.4450. [DOI] [PubMed] [Google Scholar]
- 23.Xiong Y, Luscher MA, Altman JD, Hulsey M, Robinson HL, Ostrowski M, Barber BH, MacDonald KS. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8(+) T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J Virol. 2001;75:3028–33. doi: 10.1128/JVI.75.6.3028-3033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catalina MD, Sullivan JL, Brody RM, Luzuriaga K. Phenotypic and functional heterogeneity of EBV epitope-specific CD8+ T cells. J Immunol. 2002;168:4184–91. doi: 10.4049/jimmunol.168.8.4184. [DOI] [PubMed] [Google Scholar]
- 25.Wheelock E, Sibley W. Circulating virus, interferon, and antibody after vaccination with the 17D strain of yellow fever virus. N Engl J Med. 1965;273:194. doi: 10.1056/NEJM196507222730404. [DOI] [PubMed] [Google Scholar]
- 26.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;2053:711–23. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.La Gruta NL, Thomas PG, Webb AI, et al. Epitope-specific TCRbeta repertoire diversity imparts no functional advantage on the CD8+ T cell response to cognate viral peptides. Proc Natl Acad Sci U S A. 2008;105:2034–9. doi: 10.1073/pnas.0711682102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hohn H, Neukirch C, Freitag K, Necker A, Hitzler W, Seliger B, Maeurer MJ. Longitudinal analysis of the T-cell receptor (TCR)-VA and -VB repertoire in CD8+ T cells from individuals immunized with recombinant hepatitis B surface antigen. Clin Exp Immunol. 2002;129:309–17. doi: 10.1046/j.1365-2249.2002.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soroosh P, Shokri F, Azizi M, Jeddi-Tehrani M. Analysis of T-cell receptor beta chain variable gene segment usage in healthy adult responders and nonresponders to recombinant hepatitis B vaccine. Scand J Immunol. 2003;57:423–31. doi: 10.1046/j.1365-3083.2003.01256.x. [DOI] [PubMed] [Google Scholar]
- 30.Sourdive DJ, Murali-Krishna K, Altman JD, et al. Conserved T cell receptor repertoire in primary and memory CD8 T cell responses to an acute viral infection. J Exp Med. 1998;188:71–82. doi: 10.1084/jem.188.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blattman JN, Sourdive DJ, Murali-Krishna K, Ahmed R, Altman JD. Evolution of the T cell repertoire during primary, memory, and recall responses to viral infection. J Immunol. 2000;165:6081–90. doi: 10.4049/jimmunol.165.11.6081. [DOI] [PubMed] [Google Scholar]
- 32.Annels NE, Callan MF, Tan L, Rickinson AB. Changing patterns of dominant TCR usage with maturation of an EBV-specific cytotoxic T cell response. J Immunol. 2000;165:4831–41. doi: 10.4049/jimmunol.165.9.4831. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 34.Kedzierska K, Day EB, Pi J, Heard SB, Doherty PC, Turner SJ, Perlman S. Quantification of repertoire diversity of influenza-specific epitopes with predominant public or private TCR usage. J Immunol. 2006;177:6705–12. doi: 10.4049/jimmunol.177.10.6705. [DOI] [PubMed] [Google Scholar]
- 35.Deckhut AM, Allan W, McMickle A, Eichelberger M, Blackman MA, Doherty PC, Woodland DL. Prominent usage of V beta 8.3 T cells in the H-2Db-restricted response to an influenza A virus nucleoprotein epitope. J Immunol. 1993;151:2658–66. [PubMed] [Google Scholar]
- 36.Sallusto F, Langenkamp A, Geginat J, Lanzavecchia A. Functional subsets of memory T cells identified by CCR7 expression. Curr Top Microbiol Immunol. 2000;251:167–71. doi: 10.1007/978-3-642-57276-0_21. [DOI] [PubMed] [Google Scholar]
- 37.Waller EC, Day E, Sissons JG, Wills MR. Dynamics of T cell memory in human cytomegalovirus infection. Med Microbiol Immunol. 2008;197:83–96. doi: 10.1007/s00430-008-0082-5. [DOI] [PubMed] [Google Scholar]
- 38.Wills MR, Carmichael AJ, Weekes MP, Mynard K, Okecha G, Hicks R, Sissons JG. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhighin vivo: CD45RAhighCD8+ T cells comprise both naive and memory cells. J Immunol. 1999;162:7080–7. [PubMed] [Google Scholar]
- 39.Gillespie GM, Wills MR, Appay V, et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J Virol. 2000;74:8140–50. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arlettaz L, Barbey C, Dumont-Girard F, Helg C, Chapuis B, Roux E, Roosnek E. CD45 isoform phenotypes of human T cells: CD4(+)CD45RA(–)RO(+) memory T cells re-acquire CD45RA without losing CD45RO. Eur J Immunol. 1999;29:3987–94. doi: 10.1002/(SICI)1521-4141(199912)29:12<3987::AID-IMMU3987>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Precopio ML, Betts MR, Parrino J, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405–16. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunne PJ, Faint JM, Gudgeon NH, et al. Epstein–Barr virus-specific CD8(+) T cells that re-express CD45RA are apoptosis-resistant memory cells that retain replicative potential. Blood. 2002;100:933–40. doi: 10.1182/blood-2002-01-0160. [DOI] [PubMed] [Google Scholar]
- 43.McElhaney JE, Pinkoski MJ, Meneilly GS. Changes in CD45 isoform expression vary according to the duration of T-cell memory after vaccination. Clin Diagn Lab Immunol. 1995;2:73–81. doi: 10.1128/cdli.2.1.73-81.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rentenaar RJ, Vosters JL, van Diepen FN, Remmerswaal EB, van Lier RA, ten Berge IJ. Differentiation of human alloreactive CD8(+) T cells in vitro. Immunology. 2002;105:278–85. doi: 10.1046/j.0019-2805.2002.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Champagne P, Ogg GS, King AS, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–11. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 46.de Bree GJ, Heidema J, van Leeuwen EM, van Bleek GM, Jonkers RE, Jansen HM, van Lier RA, Out TA. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J Infect Dis. 2005;191:1710–8. doi: 10.1086/429695. [DOI] [PubMed] [Google Scholar]
- 47.Hoji A, Rinaldo CR., Jr Human CD8+ T cells specific for influenza A virus M1 display broad expression of maturation-associated phenotypic markers and chemokine receptors. Immunology. 2005;115:239–45. doi: 10.1111/j.1365-2567.2005.02135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papagno L, Spina CA, Marchant A, et al. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS Biol. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sacre K, Carcelain G, Cassoux N, et al. Repertoire, diversity, and differentiation of specific CD8 T cells are associated with immune protection against human cytomegalovirus disease. J Exp Med. 2005;201:1999–2010. doi: 10.1084/jem.20042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero P, Zippelius A, Kurth I, et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–9. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 51.Barba-Spaeth G, Longman RS, Albert ML, Rice CM. Live attenuated yellow fever 17D infects human DCs and allows for presentation of endogenous and recombinant T cell epitopes. J Exp Med. 2005;202:1179–84. doi: 10.1084/jem.20051352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Querec TD, Pulendran B. Understanding the role of innate immunity in the mechanism of action of the live attenuated Yellow Fever Vaccine 17D. Adv Exp Med Biol. 2007;590:43–53. doi: 10.1007/978-0-387-34814-8_3. [DOI] [PubMed] [Google Scholar]
- 53.Gaucher D, Therrien R, Kettaf N, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Querec TD, Akondy RS, Lee EK, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–25. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reinhardt B, Jaspert R, Niedrig M, Kostner C, L’Age-Stehr J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol. 1998;56:159–67. doi: 10.1002/(sici)1096-9071(199810)56:2<159::aid-jmv10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.