Summary
The omentum is a site of B1 lymphopoiesis and immune responsiveness to T-independent antigens. However, it is unknown whether it supports immune responses independently of conventional lymphoid organs. We show that the omentum collects antigens and cells from the peritoneal cavity and supports T-dependent B cell responses, including isotype switching, somatic hypermutation and limited affinity maturation, despite the lack of identifiable follicular dendritic cells. The omentum also supports CD4 and CD8 responses to peritoneal antigens and recruits effector T cells primed in other locations. Unlike conventional lymphoid organs, milky spots in the omentum develop in the absence of lymphoid tissue inducer cells, but require CXCL13. Although the lymphoid architecture of milky spots is disrupted in lymphotoxin-deficient mice, normal architecture is restored by reconstitution with lymphotoxin-sufficient hematopoietic cells. These results indicate that the milky spots of the omentum function as unique secondary lymphoid organs that promote immunity to peritoneal antigens.
Introduction
The omentum is a fatty tissue that connects the spleen, stomach, pancreas and colon (Williams and White, 1986) and often occludes wounds in the peritoneal cavity, including hernias, inflamed appendices, tumors and other infected or inflamed sites (Morrison, 1906). Surgeons appreciate the immunological and wound healing properties of the omentum and take advantage of these properties in reconstructive procedures or to close large surgical incisions (Williams and White, 1986). The advantages of the omentum for surgical closure include its enormous angiogenic potential (Goldsmith et al., 1984), large surface area (Das, 1976) and apparent immunological activity (Roberts, 1955; Walker and Rogers, 1961).
The omentum contains milky spots (MS), which are clusters of leukocytes embedded in the omental tissue (Krist et al., 1995a). The MS also collect fluids, particulates and cells from the peritoneal cavity (Fedorko et al., 1971; Gerber et al., 2006; Hodel, 1970), and the frequency and size of MS increase in the omenta of patients undergoing peritoneal dialysis (Beelen et al., 2005; Di Paolo et al., 2005). Plasma cell responses to some T-dependent antigens are observed in the omenta of mice immunized i.p. (Dux et al., 1977; Dux et al., 1986; Hajdu et al., 1972) and the surgical removal of the omentum in rabbits reduces the antibody response to i.p. SRBC by 75% (Portis, 1924), suggesting that the MS may be secondary lymphoid organs. However, the MS of naive animals consist primarily of macrophages and B1 cells, with few T cells (Beelen et al., 1980; Krist et al., 1995b; Van Vugt et al., 1996). Since they also seem to lack interdigitating dendritic cells and follicular dendritic cells (FDCs)(Van Vugt et al., 1996), and some studies were unable to elicit T-dependent immune responses in the omentum (Szaniawska, 1974; Szaniawska, 1975), some investigators conclude that MS are not true secondary lymphoid tissues (Szaniawska, 1974; Szaniawska, 1975; Van Vugt et al., 1996). Moreover, even in studies showing omental plasma cell responses, it is unclear whether these cells were originally primed in the omentum or in other secondary lymphoid organs. Thus, the immunological function of the MS is unclear.
Other data indicate that B1 cells initially develop from hematopoietic progenitors in the fetal omentum and fetal liver and are then maintained by a process of self-renewal in the peritoneal cavity (Solvason et al., 1992; Solvason and Kearney, 1992). In fact, the leukocytes in the MS are similar in composition to those in the peritoneal cavity, with a predominance of B1 cells and macrophages (Ansel et al., 2002; Beelen et al., 1980). Importantly, B1 cells express a unique repertoire of antigen receptors, including the T15 idiotype, which recognizes phosphorylcholine, a cell surface component of some bacteria (Benedict and Kearney, 1999; Vakil et al., 1991). Intestinal leakage or the intraperitoneal delivery of bacteria leads to rapid activation of B1 cells and promotes T independent antibody responses (Ansel et al., 2002; Ha et al., 2006). Moreover, cells in the MS are highly responsive to bacterial products like LPS (Cui et al., 2002; Ha et al., 2006), suggesting that B1 cells in the peritoneal cavity and omentum are specialized to provide natural immunity to bacterial pathogens. Consistent with this idea, Cxcl13−/− mice make poor antibody responses to bacterial antigens in the peritoneal cavity and have low levels of natural antibody, including the T15 idiotype (Ansel et al., 2002). Thus, the cell types in the peritoneal cavity and omentum appear specialized to provide innate immunity to T independent bacterial antigens,
Despite the immunological potential of the MS, many investigators use intraperitoneal injection to immunize laboratory mice with T cell dependent antigens and then assay those responses in the spleen or perithymic lymph nodes (LNs) - ignoring any possible role for the omentum. More importantly, the MS of the omentum collect metastasizing tumor cells (Gerber et al., 2006; Krist et al., 1998), fluid from peritoneal dialysis (Beelen et al., 2005), bacteria from intestinal perforations (Ha et al., 2006) and antigens from abdominal injuries (Morrison, 1906) - particularly when the omental tissue is used in reconstructive surgeries (Williams and White, 1986). Thus, it is essential that we understand the immunological properties of the MS.
Results
Mice lacking conventional lymphoid organs generate B cell responses to peritoneal antigens
To test whether functional local lymphoid tissues were formed in response to antigenic challenge in the peritoneal cavity, we intraperitoneally (i.p.) immunized Wild Type (WT) as well as Spleen Lymph node and Peyer’s patch deficient (SLP) mice with (4-hydroxy-3-nitrophenyl)-acetyl(15)-OVA (NP-OVA) adsorbed to alum and measured the serum titers of NP-specific antibody. We found that WT and SLP mice produced similar titers of NP-specific IgM that peaked early after immunization and were subsequently maintained at low levels (Figure 1A). The titers of NP-specific total IgG rapidly increased in both WT and SLP mice and stayed very high for nearly two months after immunization (Figure 1A). Both WT and SLP mice predominantly generated NP-specific IgG1 and to a lesser extent IgG2a (Figure 1B). Very little IgG2b or IgG3 were observed in either WT or SLP mice.
Figure 1. Antibody responses to peritoneal antigens in the absence of conventional lymphoid organs.

A–B. WT and SLP mice were i.p. immunized with NP-OVA. The serum titers of NP-specific IgM and total IgG (A) as well as NP-specific IgG1, IgG2a, IgG2b and IgG3 (B) were determined by ELISA. This experiment is representative of two experiments with 5 mice in each group. C. WT and SLP mice were immunized with TNP-KLH. The serum titers of TNP-specific IgG were determined by ELISA and the concentration of TNP-specific IgG was determined by comparison to a monoclonal anti-TNP standard. This experiment was performed twice with similar results. D. C57BL/6 and splenectomized Lta−/− mice were immunized with NP-OVA. The serum titers of NP-specific IgG were determined by ELISA
We also immunized WT and SLP mice with 2,4,6-trinitrophenyl(30)-KLH (TNP-KLH) adsorbed to alum and boosted the mice on day 28. As observed in the previous experiment, the SLP mice were slower to generate antigen-specific IgG, but the titers were equivalent by day 14 after immunization (Figure 1C). In addition, the titers of TNP-specific IgG rapidly increased after the booster immunization in both groups (Figure 1C). Since IgG titers do not give a good sense of how much antigen-specific IgG was produced, we repeated the ELISAs with anti-TNP standards and found that the SLP mice were slow to generate high levels of anti-TNP IgG, but that both groups ultimately generated more than 1 mg/ml of serum anti-TNP IgG. Together, these data demonstrate that conventional lymphoid organs - spleen, LNs and Peyer’s patches - are not necessary for B cell responses to haptenated proteins in the peritoneal cavity.
We were initially surprised that SLP mice made such a robust antibody response to NP-OVA, given that lymphotoxin-deficient (Lta−/−) mice make such poor antibody responses to peritoneal antigens (Banks et al., 1995; Matsumoto et al., 1996b) due to their lack of lymph nodes and disrupted splenic architecture (Banks et al., 1995; de Togni et al., 1994). To reconcile our data with published results, we i.p. immunized C57BL/6 and splenectomized Lta−/− mice with NP-OVA adsorbed to alum and measured the titers of NP-specific IgG. We found that the IgG response in splenectomized Lta−/− mice was both delayed and significantly reduced compared to that in C57BL/6 mice (Figure 1D). Since both SLP and splenectomized Lta−/− mice lack spleen and lymph nodes, we conclude that the major immune defect in Lta−/− mice is due to the loss of LTα, rather than the loss of spleen and lymph nodes.
The lymphoid areas of the omentum exhibit characteristics of secondary lymphoid organs
Although spleen and lymph nodes are absent from SLP mice, it was possible that local lymphoid tissues, such as MS, remained that could be responsible for generating antibody responses to peritoneal antigens. To test whether MS were present in the omentum of naïve SLP mice, we examined whole mounts of omentum. We found MS that consisted of large clusters of B cells with PNAd-expressing vessels in the omenta of both WT and SLP chimeric mice (Figure 2A–B). To test whether the MS collected haptenated OVA, we injected OVA labeled with Alexa Fluor-594 into the peritoneal cavity and examined the MS by immunofluorescence. We found that labeled OVA was easily detected in the MS as early as 2 hours after injection (Figure 2C) and was highly concentrated by 4 hours after injection (Figure 2D).
Figure 2. The milky spots of the omentum collect peritoneal cells and antigens.

A–B. Omenta were obtained from naïve WT and SLP mice and whole mounts were probed with antibodies to B220 and PNAd. Images were obtained as optical sections using the Zeiss Apotome. C–D. C57BL/6 mice were i.p. immunized with ovalbumin coupled to Alexa-594. Omenta were collected 2 or 4 hours later and whole mounts were probed with antibodies to CD11b. E. 1 × 106 CFSE-labeled SRBC were i.p. injected and the omenta were collected 4 hours later. Whole mounts were probed with antibodies to CD11b. The high magnification image is an optical section obtained with the Zeiss Apotome. F. Omental cells from SRBC-injected and control mice were analyzed by flow cytometry. The plots show all events collected. The inset in the top panel shows the profile of CFSE-SRBC prior to injection. The numbers in each plot refer to the percentage ± standard deviation of events in the CFSE-SRBC gate. G. Omental cells from SRBC-injected and control mice were analyzed by flow cytometry. The plots show live leukocytes and gated into 5 populations based on CD11c and CD11b expression. H. Total cells from the omenta of SRBC-injected and control mice were enumerated and the number of cells in each of the gated populations was calculated. I. GFP-expressing EL4 cells were cultured in vitro for 2 hours with or without pertussis toxin and then i.p. injected into C57BL/6 recipients. Omenta were collected 2 or 4 hours later and whole mounts were examined by fluorescence microscopy. J. Whole mounts of omenta from EL4-GFP-injected mice were probed with B220 to visualize MS. Images are optical sections obtained using the Zeiss Apotome. K. The frequency of PTX-treated and untreated EL4-GFP cells in the omenta was determined by flow cytometry. L The number of PTX-treated and untreated EL4-GFP cells in the omenta was calculated. There were 4–5 mice per group. M. B cells were purified from GFP-transgenic mice and mixed 2:1 with purified, bodipy-labeled T cells from C57BL/6 mice. The cell mixture was cultured for 2 hours with or without pertussis toxin and 1 × 107 total cells/mouse were i.p. injected into C57BL/6 recipients. The omenta were collected 2 and 4 hours after injection and whole mounts were analyzed by fluorescence microscopy. N. The frequencies of PTX-treated and untreated B and T cells in the omenta were determined by flow cytometry. O The numbers of PTX-treated and untreated B and T cells in the omenta were calculated. There were 4–5 mice per group.
To determine whether particulates in the peritoneal cavity were actively carried to the MS via phagocytic cells or were passively collected, we next transferred CFSE-labeled SRBCs to the peritoneal cavity. We found that peritoneal SRBCs rapidly collected in the MS (Figure 2E). At higher magnification, it was clear that many of the CFSE-labeled SRBCs had been engulfed by CD11b-expressing phagocytic cells (Figure 2E arrows). Flow cytometry revealed that the majority of SRBCs in the omentum were internalized by phagocytic cells (Figure 2F) although a small proportion of SRBCs were not associated with other cells (Figure 2F gated population). The SRBC transfer also changed the composition and cellularity of the omentum (Figure 2G), with the largest increase in cell frequency occurring in the CD11cnegCD11bhi population (Figure 2G box E). The total number of cells in the omentum increased about 4-fold upon SRBC administration, with significant increases occurring in all cell populations - even the lymphocyte population (Figure 2G box C), which was the only population that did not phagocytose SRBCs (Supplemental Figure 1). These data confirm previous reports that activation of peritoneal cells promotes their migration to the omentum (Ha et al., 2006) and demonstrate that at least some particulates can be passively collected by the omentum in addition to being captured by phagocytic cells.
To further test the idea that there is bulk flow from the peritoneal cavity through the MS, we injected EL4 lymphoma cells that had been transfected with green fluorescent protein (GFP). We found that EL4-GFP cells rapidly accumulated in the omentum (Figure 2I) and were found in close association with B cells in the MS (Figure 2J). This accumulation was largely unaffected by pretreatment with pertussis toxin (PTX) (Figure 2I–J), although there was a small, but significant decrease in the frequency and number of PTX-treated EL4 cells that accumulated in the omentum (Figure 2K–L). To test whether this was also true of lymphocytes, we purified B cells from green fluorescent protein (GFP) transgenic mice and purified T cells from C57BL/6 mice, which we labeled with BODIPY 558/568. These cells were mixed, incubated with or without PTX and injected into the peritoneal cavity. We found that while B and T cells accumulated in the omentum regardless of whether they were pretreated with PTX, the untreated cells rapidly entered MS and segregated into B and T cell areas (Figure 2M). In contrast, cells pretreated with PTX collected in the omentum, but the B and T cells were scattered and did not form compact follicular structures. As with the EL4 cells, the frequency and number of lymphocytes that migrated to the omentum was reduced by PTX-treatment (Figure 2N–O). Thus, peritoneal cells clearly use PTX-sensitive mechanisms to actively migrate to the omentum, but can also accumulate in the omentum by other mechanisms. However, the segregation of B and T cells and the formation of follicular structures in the MS are controlled by PTX-sensitive mechanisms.
Milky spots support T dependent immune responses to peritoneal antigens
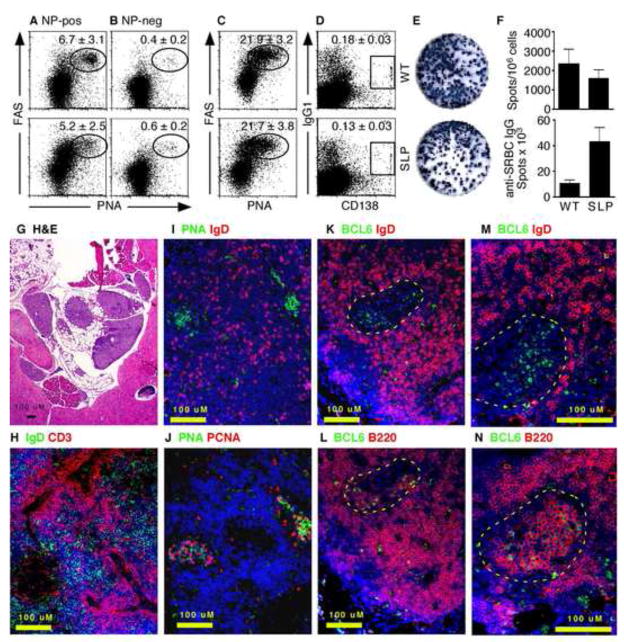
Since peritoneal antigens and cells rapidly collected in the MS, we next wanted to test whether the MS supported immune responses to T cell dependent antigens. Therefore, we i.p. immunized WT and SLP mice with NP-OVA adsorbed to alum and examined the response of NP-specific B cells in the omentum 7 days later. Using NP-conjugated allophycocyanin to identify NP-specific B cells, we found that both WT and SLP mice were able to generate NP-specific germinal center B cells in the omentum (Figure 3A). We also gated on B cells that were not NP-specific in these same animals and found that the frequency of germinal center B cells was much lower (Figure 3B). In fact, the frequency of germinal center B cells in the NP-non-specific B cell population was similar to that seen in omental B cells from naïve mice (Supplemental Figure 2). We also immunized WT and SLP mice with SRBC and boosted them on day 28. Five days after the boost, we found very high frequencies of germinal center B cells (Figure 3C) and isotype-switched plasma cells (Figure 3D) in the omenta of both groups. Although we could not demonstrate antigen-specificity of these cells by flow cytometry, we found similar frequencies of SRBC-specific IgG-secreting cells in the omenta of both groups (Figure 3E) and significantly higher numbers of IgG secreting cells in SLP mice (Figure 3F).
Figure 3. Antigen-specific B cell responses occur in the omentum.
A–B. WT and SLP mice were i.p. immunized with NP-OVA and omental cells were analyzed by flow cytometry on day 8 for NP-specific FAS+PNA-binding germinal center B cells (A) and NP-non-specific FAS+PNA-binding germinal center B cells (B). Cells in A–B were gated on CD19 expression and the ability to bind NP-APC (A) or the failure to bind NP-APC (B). The numbers in each plot refer to the percentage ± standard deviation of NP-specific B cells in the gates indicated by ovals or boxes. C–D. WT and SLP mice were i.p. immunized with SRBC, boosted on day 14 and omental cells were analyzed 5 days later by flow cytometry for FAS+PNA-binding germinal center B cells (C) and IgG1+CD138+ plasma cells (D). Cells in C–D were gated on CD19 expression. The numbers in each plot refer to the percentage ± standard deviation of CD19+ B cells in the gates indicated by ovals or boxes. E. WT and SLP mice were i.p. immunized with SRBC, boosted on day 14 and omental cells were analyzed by SRBC-specific ELISPOT. F. The frequency and total number of SRBC-specific IgG ELISPOTs was determined. There were 4–5 mice in each group. G. C57BL/6 mice were immunized with SRBC and omenta were collected on day 14. Omenta were embedded in pieces of liver to aid in sectioning. Paraffin sections were stained with H&E. H–N. C57BL/6 mice were immunized with NP-OVA and omenta were collected on day14 and frozen in OCT. Cryosections were stained with the indicated antibodies and counterstained with DAPI (blue). Germinal centers are outlined by yellow in panels K–N.
We next tested whether we could define germinal centers in MS by histology. To examine the histology of immunized omenta, we folded individual omenta in pieces of liver, fixed them in formalin and embedded them in paraffin. Sections of omenta embedded with this method could be easily stained with H&E to see the bluish areas of the lymphocyte-filled MS and the lacy areas of fatty tissue surrounded by the supporting liver (Figure 3G). Using cryosections, we also found separated B and T cell areas in omenta from SRBC-immunized mice (Figure 3H), as well as clusters of IgDnegPNA-binding B cells (Figure 3I). In serial sections, we found that the clusters of PNA-binding B cells were also proliferating as demonstrated by PCNA expression (Figure 3J). To confirm the presence of bona fide germinal centers in the omenta of immunized mice, we also probed cryosections with antibodies to BCL6 (Figure 3K–N), which is a marker of germinal center B cells (ref). We found clusters of BCL6posIgDneg cells surrounded by BCL6negIgDpos cells (Figure 3K and 3M). Serial sections revealed that these clusters consisted of large blast cells that expressed B220 on the cell surface and BCL6 in the nucleus (Figure 3L and 3N). These data demonstrate that the MS support local germinal center B cell responses to peritoneal antigens.
We next tested whether CFSE-labeled OVA-specific CD4 T cells could respond in the MS. We found that OTII cells in both WT and SLP mice immunized with alum and LPS (no OVA) predominantly expressed low levels of CD25 (Figure 4A top row) and high levels of CD62L (Figure 4B top row). In contrast, OTII cells in WT and SLP mice that were immunized with NP-OVA 36 hours previously rapidly increased their expression of CD25 and reduced their expression of CD62L (Figure 4A–B middle row). Changes in surface expression of these markers coincided with the first cell divisions of the OTII cells, although cell division was not required for either increased CD25 expression or decreased CD62L expression (Figure 4A–B middle row). OTII proliferation continued rapidly in both WT and SLP mice and most cells had divided once or twice by 42 hours after immunization (Figure 4C). We also tested whether CD8 T cells responded to NP-OVA. As shown in Figure 4D, OVA-specific CD8 T cells could be found in the MS of both WT and SLP chimeras 7 days after immunization with NP-OVA in alum. The frequency of OVA-responding CD8 T cells was relatively low, probably because NP-OVA is an exogenous antigen that is poorly presented by cross-priming. These data demonstrate that T cell responses can also occur in the MS of the omentum, even in mice that lack spleen, LNs and Peyer’s patches.
Figure 4. Antigen-specific T cell responses in the omentum.
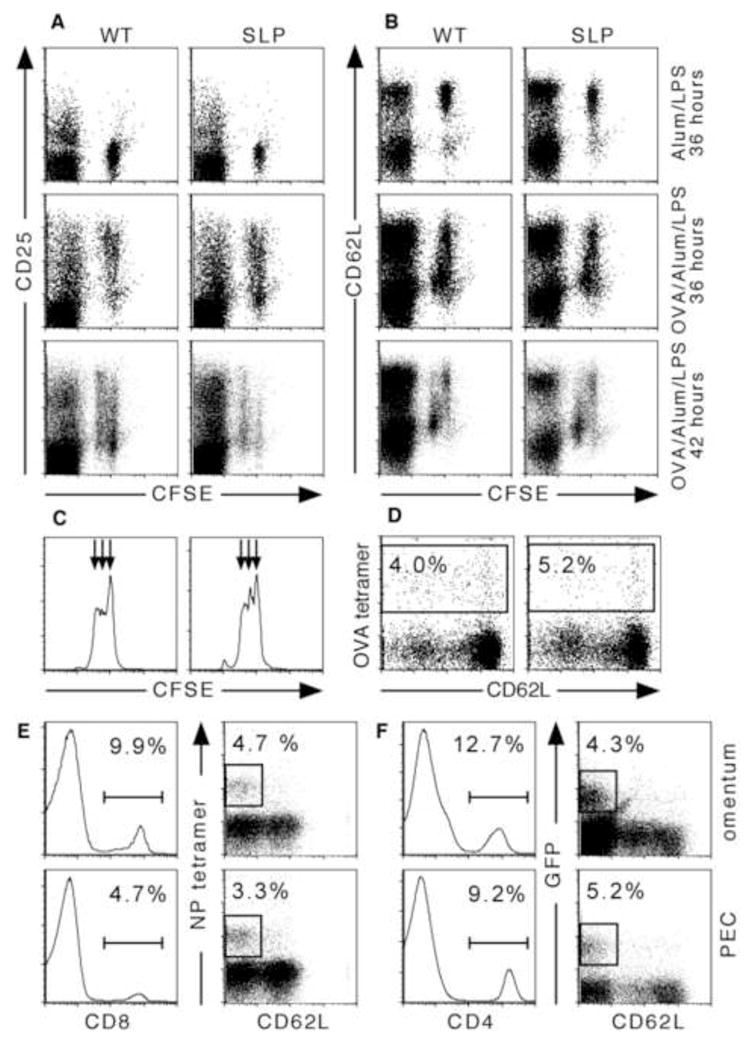
A–C. Purified OTII CD4 T cells were labeled with CFSE and i.p. injected into WT and SLP recipient mice. Recipients were immunized 4 hours later with 100 μg OVA and 10 μg LPS adsorbed to alum or with 10 μg LPS and alum as indicated. Omenta were obtained 36 or 42 hours later and omental cells were analyzed by flow cytometry for CD4, CFSE and CD25 (A) or CD4, CFSE and CD62L (B). The plots shown are gated on CD4+ cells. CFSE dilution in the transferred CD4 T cells was measured by flow cytometry (C). There were 4–5 mice per group and omenta were pooled prior to analysis. The data shown is representative of 3 independent experiments. D. WT and SLP mice were immunized with 100 μg OVA and 10μg LPS adsorbed to alum. OVA-specific CD8 T cells were identified by tetramer binding in the omenta of immunized mice. The plots shown are gated on CD8 T cells. E. C57BL/6 mice were intranasally infected with influenza and single cell suspensions from peritoneal cavity and omenta were analyzed by flow cytometry 21 days after infection. Histograms show the frequency of CD8 cells in the live leukocyte population. Dot plots were gated on CD8 cells and the boxes indicate influenza nucleoprotein-specific cells. F. 4-get IL-4 reporter mice were orally infected with H. polygyrus larvae and single cell suspensions from peritoneal cavity and omenta were analyzed by flow cytometry 28 days after infection. Histograms show the frequency of CD4 cells in the live leukocyte population. Dot plots were gated on CD4 cells and the boxes indicate Th2 cells based on GFP expression (B).
We next wanted to determine whether effector or memory T cell generated in other locations recirculated through the peritoneal cavity and the omentum. To test this possibility, we first intranasally infected C57BL/6 mice with influenza virus and looked in the peritoneal cavity and omentum for recirculating influenza-specific CD8 T cells. We found influenza-specific (tetramer-binding) CD8 T cells in both the peritoneal lavage and omentum of mice that had been infected with influenza 21 days previously (Figure 4E). We also orally infected IL-4 reporter (4-get) mice with larvae of the intestinal helminth, Heligmosomoides polygyrus, and looked in the peritoneal lavage and omentum for recirculating Th2 cells. Since the 4-get mice are transgenic for a reporter construct that expresses GFP under the control of the IL-4 promoter (Mohrs et al., 2001), Th2 cells should express GFP. We found GFP-expressing CD4 cells in both the peritoneal lavage and omentum on day 28 after infection (Figure 4F). Thus, antigen-experienced CD4 and CD8 cells recirculate through the peritoneal cavity and omentum, even when these cells were primed outside of the peritoneal cavity.
Development and architecture of milky spots
We next examined the role of lymphotoxin-α (LTα) and the homeostatic chemokines, CXCL13, CCL21 and CCL19, in the development and organization of the MS. We found that the MS were well-developed in both C57BL/6 and plt/plt mice, but were much smaller or even absent in Lta−/− and Cxcl13−/− mice (Figure 5A–C), consistent with previous observations (Ansel et al., 2002). In particular, the omenta of Lta−/− and Cxcl13−/− mice had very small or absent B cell areas and lacked detectable PNAd+ HEVs (Figure 5C). Given that MS are found in SLP mice (Figure 2B), it seems that the reconstitution of Lta−/− mice with normal bone marrow restores the structure of the MS, suggesting that the defects in the MS of Lta−/− mice are not due to an absolute blockade in development.
Figure 5. Milky spot development requires LTα and CXCL13, but not LTi cells.
A. Omenta were obtained from naïve C57BL/6, Lta−/−, Cxcl13−/− or plt/plt mice as indicated and whole mounts were probed with antibodies to B220 and CD11b. B. Paraffin sections of omenta were stained with H&E. C. Paraffin sections of omenta were probed with antibodies to B220 and PNAd. D. RNA was extracted from the omenta of naïve C57BL/6, Lta−/−, Cxcl13−/− or plt/plt mice as indicated and assayed for the expression of CXCL12, CXCL13, CCL19, CCL21, LTβ and TNFα by quantitative PCR. The expression of each mRNA was normalized first to GAPDH and then normalized to the expression in WT animals, which was set at 1. E. Omenta were obtained from naïve Rorc−/− or Id2−/− mice as indicated and paraffin sections were stained with H&E (E) or probed with antibodies to B220 and CD11b (F) or B220 and CD31. Images are representative of 3–5 mice per group.
Since the MS in the omenta of Lta−/− and Cxcl13−/− mice were architecturally abnormal, we hypothesized that LTα and CXCL13 were involved in a positive feedback loop similar to that described in other lymphoid organs (Ansel et al., 2000). To test this possibility, we extracted RNA from the omenta of naïve C57BL/6, Lta−/−, Cxcl13−/− and plt/plt mice and analyzed chemokine mRNA expression by quantitative PCR. To our surprise, we found that the expression of CXCL12, CCL21, CCL19 and CXCL13 as well as LTβ and TNFα, was essentially normal in the omentum of Lta−/− mice (Figure 5D). In contrast, we found that while the expression of CXCL12 and CCL21 was normal in the omenta of Cxcl13−/− mice, the expression of CCL19, CXCL13, LTβ and TNFα were very reduced (Figure 5D). Finally, we found that the expression of CCL19 and CCL21 was reduced in the omenta of plt/plt mice, consistent with the plt mutation, but that the expression of the other chemokines and cytokines that we tested was normal in the omentum of plt/plt mice (Figure 5D). These data demonstrate that even though CXCL13 is very important for the development of the MS, its expression is not controlled by LTα.
We next tested whether the formation of the MS required LTi cells, which are absent in mice deficient in retinoic acid related orphan receptor-γ (Rorc−/− mice) and in mice that lack the inhibitor of differentiation-2 (Id2−/− mice) (Sun et al., 2000; Yokoto et al., 1999). We found well-developed MS with large B cell follicles in both Rorc−/− mice and Id2−/− mice (Figure 5E–F), suggesting that the development of MS in the omentum occurs independently of LTi cells. Since it is possible that some types of LTi cells could develop in the absence of RORγ and Id2, we also looked for CD45+CD4+CD3− cells in the developing MS. Although we were able to identify lymphocytes in the omenta from 3-day old pups, we did not observe IL-7Rα+CD45+CD4+CD3− cells (not shown).
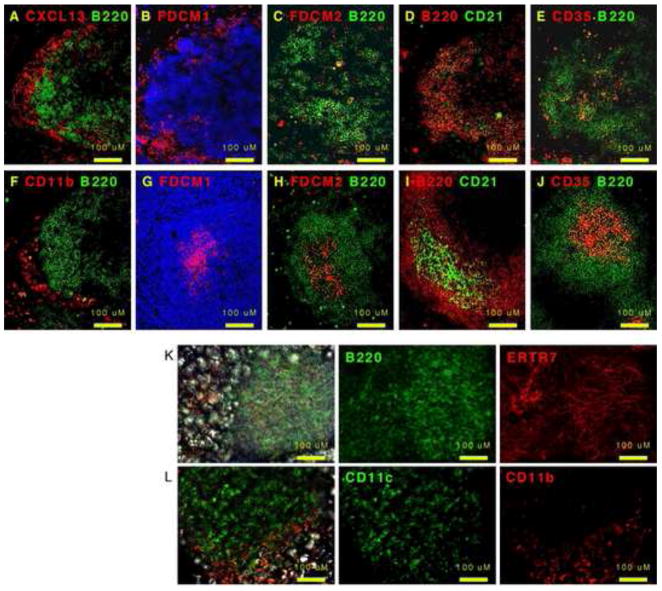
Since CXCL13 was so important for the structure of the MS, we next used immunofluorescence to examine where CXCL13 was expressed. As shown in Figure 6A, CXCL13 is primarily expressed in an area that surrounds the B cell follicle in a reticular pattern, suggesting some type of stromal cells. In fact, CXCL13 appeared to be expressed in a 3-dimensional basket that envelops the B cell area (Supplemental Figure 3A). Despite the unusual pattern of CXCL13 staining, it seemed to be specific as no red signal was observed when Cxcl13−/− omenta were stained with the same antibodies used in Figure 6A (Supplemental Figure 3B). In addition, we did not observe any red signal when C57BL/6 omenta were stained with either the secondary antibody alone (Supplemental Figure 3C) or with a different primary antibody followed by the secondary antibody (Supplemental Figure 3D). Since CXCL13 expression is often associated with FDCs (Cyster et al., 2000), we next tested whether markers of FDCs co-localized with CXCL13 using serial sections (Figure 6A–F). We found that FDCM1 was expressed in a reticular pattern surrounding the B cell area (Figure 6B) similar to where we observed CXCL13 expression. However, we only observed scattered staining with FDCM2 (Figure 6C). Moreover, CD21 was primarily expressed on B cells and was only expressed on a few scattered non-B cells (Figure 6D). Interestingly, a few CD35-expressing cells were observed in the center of the B cell area (Figure 6E). Despite the highly unusual staining patterns of FDC markers in omentum, the same combination of antibodies used at the same time on splenic sections revealed typical staining patterns of FDCs in the center of B cell follicles (Figure 6G–J). Despite the apparent absence of conventional FDC networks however, we did observe a network of ERTR7+ stromal cells throughout the MS (Figure 6K). Interestingly, CD11b+ macrophages, which are known to express CXCL13 in the peritoneal cavity (Ansel et al., 2002), were found around the outside of the MS (Figure 6F and L), while CD11c+ cells were found in the center of the MS similar to where the B cells are located (Figure 6D). Thus, the cellular architecture and pattern of chemokine expression in the MS are different than in conventional lymphoid organs.
Figure 6. CXCL13 is expressed surrounding the B cell areas.
A–F. Omenta were obtained from naïve C57BL/6 mice and cryosections were probed with the indicated antibodies. G–J. Spleens were obtained from naïve C57BL/6 mice and cryosections were probed with the indicated antibodies. Cryosections in panels B–E were stained at the probed at the same time, under the same conditions as cryosections in panels G–J. K–L. Omenta were obtained from naïve C57BL/6 mice and whole mounts were probed with the indicated antibodies. Images were generated using a combination of fluorescence (optical sections using the Zeiss apotome) and DIC. Control primary and secondary antibodies were negative. Images are representative of 3–5 mice.
Milky spots support somatic hypermutation and affinity maturation
The lack of a well-defined conventional FDC network made us question whether B cells responding to antigen could be selected for high affinity antigen receptors in the MS. To test whether somatic hypermutation and affinity selection were occurring in the MS of the omentum, we immunized WT and SLP mice with NP-OVA on day 0 and amplified NP-specific IgG1 heavy chain V regions using primers specific for V186.2 and the IgG1 constant region on day 7, day 14 and day 19 (5 days after boosting) and sequenced individual clones. Although we observed only a few mutations in the V regions of IgG heavy chains from either WT or SLP mice on day 7, more sequences in both groups had accumulated mutations by days 14 and 19 after immunization (Figure 7A). However, more of the sequences from WT mice had mutations and the number of mutations in each V region was also higher in sequences from WT mice than those from SLP mice on days 14 and 19 (Figure 7A). Furthermore, 16/25 sequences from WT mice on day 14 exhibited the W to L mutation at position 33 that confers about a 10-fold higher affinity for NP (Supplemental Figure 4A–B). In contrast, only 5/22 sequences from SLP mice exhibited this mutation at this time. Strikingly, 11/13 sequences from WT mice exhibited the W to L mutation at day 19, while 0/12 sequences from SLP mice had this mutation (Supplemental Figure 4A–B). The differences in sequence were not limited to the patterns of mutations, since 16/25 sequences from WT mice on day 14 had the YYYG motif at the V-D junction, which is characteristic of NP-binding sequences, while only 7/22 sequences from SLP mice had this motif. Interestingly, all of the W to L mutations in sequences from SLP mice were from those that contained the YYYG motif. Thus, while somatic hypermutation is still evident in SLP mice, the process of clonal selection seems to be very different.
Figure 7. Somatic hypermutation and affinity selection occurs in the absence of conventional secondary lymphoid organs.
A. WT and SLP mice were i.p. immunized with NP-OVA and boosted on day 14. RNA was extracted from omenta on days 7, 14 and 19 after immunization. The V regions of B cells with V186.2 heavy chains that had switched to IgG1 were amplified by PCR, subcloned and sequenced. The pie charts indicate the relative proportion of sequences that were unmutated (0) or contained the indicated number of mutations. B. The titers of NP-specific total IgG were determined using ELISA plates coated with either NP16-BSA and with NP2-BSA. The relative affinity of the NP-specific IgG is expressed as a ratio of binding to the NP2-BSA versus NP16-BSA. There were 5 mice in each group and this experiment is representative of 3 independent experiments.
To directly determine whether B cells responding to NP-OVA in the MS underwent affinity maturation, we performed NP-specific ELISAs using plates coated with either NP2BSA or with NP16BSA and compared the ratio of IgG that bound to each as a measure of the relative affinity of the antibody. As shown in Figure 7B, the affinity of NP-specific IgG from both WT and SLP mice was relatively low at day 10 after immunization. The affinity of NP-specific IgG increased rapidly in WT mice and increased somewhat more slowly in SLP mice (Figure 7B). Ultimately, however, the relative affinity of NP-specific IgG reached similar levels in both groups as measured in this assay. Together, these data demonstrate that the MS of the omentum support some aspects of T dependent B cell responses, although the process of clonal selection is unusual.
Discussion
Previous work suggested that the omentum had an immunological function and showed that plasma cells could be found in the omentum after intraperitoneal immunization with T-independent antigens (Ansel et al., 2002; Ha et al., 2006) as well as classic T-dependent antigens (Dux et al., 1977; Hajdu et al., 1972; Van Vugt et al., 1996). Nevertheless, some authors have concluded that the MS should not be classified as secondary lymphoid organs (Szaniawska, 1974; Szaniawska, 1975; Van Vugt et al., 1996). Given the lack of FDC networks in MS (Van Vugt et al., 1996), the prevalence of B1 cells in the peritoneal cavity and omentum (Ansel et al., 2002) and the altered repertoire and affinity selection of B cells responding to antigen in the MS of mice that lack conventional lymphoid organs, we would agree with Van Vugt (Van Vugt et al., 1996) that the MS are not conventional secondary lymphoid organs. However, Van Vugt’s conclusion that they should rather be classified as perivascular infiltrates is, in our opinion, too limiting. Our data clearly show that the MS of the omentum are present prior to immunization and support B cell responses (albeit unusual ones) as well as CD4 and CD8 T cell responses to antigens that are administered i.p.. Moreover, B cells as well as CD4 and CD8 T cells primed in distal locations recirculate through the peritoneal cavity and omentum, indicating that the MS of the omentum are sites of immune surveillance for a wide spectrum of antigens. Together, these data suggest that the omentum functions much more broadly as a secondary lymphoid organ than previously realized, even though it is structurally, developmentally and functionally unique.
Unlike encapsulated LNs, which acquire antigen via afferent lymphatics, or mucosal lymphoid tissues, which transport antigen across mucosal epithelium, the MS of the omentum open directly to the peritoneal cavity and collect cells and antigens suspended in the peritoneal fluid (Cui et al., 2002; Gerber et al., 2006; Hodel, 1970). Although peritoneal B1 cells activated by LPS clearly use chemokine receptors to rapidly migrate to the MS (Ha et al., 2006), i.p. administered naïve B and T cells also collect in the MS - even when treated with PTX, suggesting that at least some of the movement from the peritoneal cavity through the omentum occurs via bulk flow rather than specific homing. Consistent with this, free SRBC in the peritoneal cavity can be collected in the omentum, although most are rapidly phagocytosed by macrophages and DCs. In addition, the presence of PNAd-expressing HEVs in the MS suggests that naïve B and T cells can also be recruited to the MS from the blood. In fact, a recent study shows that B2 cells use α4β7-MAdCAM interactions to enter the MS of the omentum from the blood (Berberich et al., 2008) and other studies show that fibronectin as well as various cell adhesion molecules are important for the entry and migration of peritoneal cells (Cui et al., 2002). Together, these data suggest a model in which recirculating T and B cells entering the MS from the blood are confronted with a parade of antigens and cells from the peritoneal cavity, such as those released by abdominal surgical procedures, peritoneal dialysis, peritoneal tumor metastases and peritoneal infections. This intersection of recirculating lymphocytes and peritoneal drainage makes the MS ideal sites for the initiation of local immune responses.
Unlike conventional lymphoid organs, which have B cell follicles arranged around networks of FDCs (Endres et al., 1999; Tew et al., 1997), the B cell areas of the MS lack appear to lack central FDC networks and instead are surrounded by reticular networks of CD11b+ and FDCM1+ cells. Although CXCL13 is normally associated with a central reticular network in the B cell follicles of conventional lymphoid organs (Cyster et al., 2000), CXCL13 is expressed around the outside of the B cell areas of MS in the same area that contains CD11b+ cells. It is likely that the CD11b+ cells are macrophages, which are known to express CXCL13 in the peritoneal cavity (Ansel et al., 2002), but it is unclear whether the FDCM1+ cells are also macrophages, FDC precursors or some other cell type. Given that other FDC markers do not stain in the same area and also do not detect a well-defined reticular network in the center of the B cell areas, it is difficult to make a firm conclusion regarding the status of FDCs in the omentum. However, the structure of the MS seems inside-out relative to that of conventional lymphoid organs or even ectopic lymphoid follicles.
Despite the unusual architecture of the milky spots, we observe somatic hypermutation and some degree of affinity maturation in B cell responses from mice that lack conventional lymphoid organs, but retain MS. Interestingly, the repertoire of V186.2 IgG heavy chains from the omenta of NP-OVA-immunized SLP mice contains some unusual features, such as a relative paucity of sequences with the YYYG motif at the V-D junction and very few sequences that encode the W to L mutation, despite the relatively robust accumulation of mutations overall. While it is tempting to conclude that affinity maturation failed in these animals, the relative avidity for NP2BSA does increase in SLP mice over the first 21 days after immunization, albeit more slowly than in WT mice. Unfortunately, the ELISA-based assay of relative affinity has a relatively narrow dynamic range and cannot differentiate between antibodies with affinities higher than about 10−7M. Thus, the NP-specific antibodies generated in WT mice could have much higher affinities than those generated in SLP mice, even though both groups showed a similar increase in affinity over the first few weeks. Interestingly, the few sequences from SLP mice that acquired the W to L mutation all had the canonical YYYG motif at the V-D junction. Thus, an alternative explanation is that the initial repertoire of B cells recruited into the NP response is different in WT and SLP mice and that many of the heavy chains from SLP mice lacking the YYYG motif and the W to L substitution, accumulate different mutations that also confer higher affinity for NP.
The difference in frequency of W to L substitutions between sequences from WT and SLP mice is also striking in another way. If we assume that the sequences obtained from SLP mice are typical of the V regions that are selected in the omentum, then the high frequency of B cells with the W to L mutation in WT mice must mean that those B cells were originally selected in other lymphoid organs and that their progeny recirculated back to the omentum. Since the sequences that have the W to L substitution vastly outnumber those that lack the W to L substitution in WT mice, this suggests that the clones that are selected in conventional lymphoid organs and recirculate to the omentum out-compete those clones that are selected in situ. If competitive fitness is determined by relative affinity (Dal Porto et al., 2002), then the B cells selected in conventional lymphoid organs containing the W to L substitution would be of higher affinity that those selected locally. If this were true, however, we should still observe in SLP mice an increase in the frequency of sequences with the W to L mutation between day 14 and 19 after immunization. The fact that we do not observe this increase means that clones with the W to L mutation are not selectively advantaged in SLP mice and that they are competing with clones of equivalent affinity or that affinity selection is not occurring. Although some level of affinity maturation can occur in the absence of either germinal centers or immune complex deposition on FDCs (Hannum et al., 2000; Koni and Flavell, 1999), and can occur in the disorganized spleens of Lta−/− and Ltbr−/− mice (Futterer et al., 1998; Matsumoto et al., 1996a), it has long been assumed that immune complexes trapped on the surface of FDCs facilitate B cell selection and affinity maturation. Thus, the lack of clearly identifiable FDCs in MS may contribute to poor affinity-based selection.
Our data showing that antigen accumulates in MS, that antigen-specific germinal center B cells, proliferating B cells and plasma cells are found in MS and that CD4 T cells are primed in MS after peritoneal immunization of SLP mice, together strongly suggest that the MS can act as secondary lymphoid organs without input from conventional secondary lymphoid organs. Moreover, our data showing antigen-specific germinal center B cells in the omentum suggest that somatic hypermutation and isotype switching are occurring directly in the omentum. Although it is possible that other sites, like the liver or bone marrow (Smith et al., 1997), may support somatic mutation and affinity maturation, it is more difficult to explain why such distal locations would be preferentially used instead of the MS, which trap peritoneal antigens and have many of the hallmarks of secondary lymphoid tissues. In addition, we were unable to find any ectopic lymphoid tissues in the peritoneal cavity that could have been induced by immunization (not shown). Thus, the simplest explanation is that the MS directly support B cell responses, isotype switching, somatic mutation and at least some level of affinity maturation.
Unlike the development of the majority of secondary lymphoid organs, the development of the MS occurs independently of RORγ-dependent or Id2-dependent LTi cells, suggesting that MS use alternative cell types to trigger the differentiation of local mesenchymal cells into stromal cells that support lymphoid architecture. Of course the stromal elements in the MS are significantly different than those in conventional lymphoid tissues, since the MS lack clearly identifiable FDCs and maintain only an ERTR7+ stromal network. Another unusual feature of milky spot development and organization is the minimal role for LTα. Although the MS are clearly compromised in the absence of LTα, their structure is completely restored in adult mice after reconstitution with Lta+/+ hematopoietic cells, suggesting that the defects in the MS of Lta−/− mice are due to non-developmental defect, such as the poor differentiation of HEVs (Browning et al., 2005). This is reminiscent of the Nasal Associated Lymphoid Tissue (NALT), which develops independently of LTα, but requires LTα to maintain normal architecture due to its ability to promote the expression of chemokines and trigger the differentiation of PNAd-expressing HEVs (Fukuyama et al., 2002; Harmsen et al., 2002; Rangel-Moreno et al., 2005). Surprisingly, however, the expression of homeostatic chemokines is normal in the omenta of Lta−/− mice, just like the induced expression of these chemokines in the lungs of Lta−/− mice during influenza infection (Moyron-Quiroz et al., 2004). However, despite the minimal role of LTα in the expression of CXCL13, CCL19 and CCL21, CXCL13 is very important for the development of the MS. Essentially no B cells are found in the omenta of Cxcl13−/− mice and the expression of CCL19, LTβ and TNFα is severely impaired (Ansel et al., 2002).
Taken altogether, these data demonstrate that the MS of the omentum are unique secondary lymphoid organs that sample antigens from the peritoneal cavity and promote local, albeit unusual, immune responses. These results will be important for the understanding of how the omentum functions in response to antigens from abdominal surgeries, peritoneal dialysis, intestinal perforations and even peritoneal tumor metastases.
Experimental Procedures
Mice and generation of bone marrow chimeras
C57BL/6 mice and C57BL/6.129Ltatm1Dch (Lta−/− mice)(de Togni et al., 1994) were obtained from the Jackson laboratory. Cxcl13−/− (Ansel et al., 2000) and plt/plt mice (Nakano et al., 1997) were a kind donation of Dr. J Cyster (UCSF). Rorc−/− (Sun et al., 2000) and Id2−/− mice (Yokoto et al., 1999) were obtained from Dr. D. Littman (NYU). IL-4 reporter (4-get) mice (Mohrs et al., 2001) were obtained from Dr. M. Mohrs (Trudeau Institute). WT and SLP chimeric mice were generated by lethally irradiating C57BL/6 and splenectomized Lta−/− mice and reconstituting them with 1 × 107 whole bone marrow cells from C57BL/6 mice as described (Moyron-Quiroz et al., 2006; Moyron-Quiroz et al., 2004). Mice were irradiated with 1000 rads from a 137Cs source at 93 rad/min in two doses. Mice were allowed to reconstitute for at least 6 weeks prior to immunization. All gene-targeted mice were on the C57BL/6 background and bred in the animal facility of Trudeau Institute. All procedures involving animals were approved by the Trudeau Institute Institutional Animal Care and Use Committee and were conducted according to the principles outlined by the National Research Council.
Immunization and infection
Mice were immunized i.p. with 100 μg NP-OVA or 100 μg TNP-KLH adsorbed to alum or with 1 × 106 SRBCs in PBS or with 100 μg Alexa-594-OVA. Mice were intranasally infected with 100 egg infectious units of A/PR8/34 influenza or orally infected with 200 Heligmosomoides polygyrus larvae.
Isolation of RNA, PCR and sequencing
RNA was extracted from whole omentum using an RNeasy kit (Qiagen). 2 μg of DNase treated RNA were reverse transcribed with random hexamers and Superscript II (Invitrogen). Quantitative PCR was performed using Taqman master mix, following the Applied Biosystems protocol. Primers and probes for CXCL12, CXCL13, CCL19, LTβ, TNFα and AID were obtained from Applied Biosystems. Primers for CCL21 (5′-AGACTCAGGAGCCCAAAGCA-3′ and 5′-GTTGAAGCAGGGCAAGGGT-3′) and GAPDH (5′CTCGTCCCGTAGACAAAATGG-3′ and 5′-AATCTCCACTTTGCCACTGCA-3′) were synthesized by IDT. The probes for CCL21 (5′FAM-CCACCTCATGCTGGCCTCCGT-BHQ-3′) and for GAPDH (5′-FAM-CGGATTTGGCCGTATTGGGCG-BHQ-3′) were synthesized by Biosearch Technology. The ABI Prism 7700 Taqman instrument was available through the molecular biology core facility at the Trudeau Institute. CDNAs of VH regions from NP-specific BCRs were amplified using a forward primer specific for V186.2 NP 5-1 CATGCTCTTCTTGGCAGCAACAGC and a reverse primer specific for IgG1 NP 3-1 GTGCACACCGCTGGACAGGGATCC. PCR reactions were performed for 1 minute at 94°C, 1.5 minute at 58°C and 2 minutes at 72°C for 25 cycles using Expand High-Fidelity Taq polymerase from Roche. The PCR product was cloned into TOPO vector (Invitrogen) and DNA from individual colonies was purified and sequenced. Sequencing was performed by Polymorphic DNA Technologies and sequence data was aligned using Sequencher software.
Cell purification and labeling
B cells, total T cells and CD4 T cells were purified by positive selection using MACS beads. For B cells and CD4 T cells, anti-B220 or anti-CD4 magnetic beads (Miltenyi Biotec) were added directly to single cell suspensions at 25 μl beads per 1×108 total cells in 100 μl final volume and incubated on ice for an additional 15 minutes on ice. For total T cells, cells were first incubated with biotinylated anti-CD4 and anti-CD8 followed by streptavidin magnetic beads (Miltenyi Biotec). Cells were washed, filtered and passed over a type LS+ magnetic column (Miltenyi Biotec). After washing, the magnetically bound cells were eluted from the column with the supplied plunger. Purified OTII CD4 T cells were labeled with 5 μM carboxyfluoroscein succinimidyl ester (CFSE) in PBS for 10 minutes at 37°C. Total T cells were labeled with 0.2 μM 4, 4 difluoro-5(2thienyl)-4-bora-3a, 4a-diaza-s-indacene-3-propionic acid, succinimidyl ester (BODIPY 558/568) in PBS for 10 minutes at 37°C. In some cases, cells were incubated at 3 × 107/ml with 100 ng/ml pertussis toxin for 2 hours at 37°C in complete RPMI with 2% FBS, 10 mM HEPES, 2 mM glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin.
Flow cytometry
Omenta were digested with Collagenase D (Roche) at 37°C for 1 hour, mechanically disrupted by passage through a wire mesh and live leukocytes were obtained by density gradient centrifugation using Lympholyte-Poly (Cedarlane). Fc receptors were blocked with 10 μg/ml 2.4G2, followed by staining with antibodies or MHC class I tetramers. The H-2Db class I tetramer containing NP366–374 peptide were generated by the Trudeau Institute Molecular Biology Core Facility as described (Flynn et al., 1998; Murali-Krishna et al., 1998). NP-specific B cells were identified using NP-allophycocyanin (NP-APC). Fluorochrome-labeled antibodies to CD8, CD4, CD62L, CD138, FAS and CD19 were obtained from BD Biosciences. FITC labeled PNA was obtained from Sigma.
ELISAs and ELISPOTs
Plates were coated with NP(16)-BSA, NP(2)-BSA or TNP(5)-BSA at 1 μg/ml and blocked with 10 mg/ml BSA. Serum samples were initially diluted 100-fold in PBS with 10 mg/ml BSA and 0.1% Tween-20 and then serially diluted in 3-fold steps in the same buffer and applied to the coated plates. Bound Ig was detected with horseradish peroxidase-conjugated goat anti-mouse IgM or goat anti-mouse IgG from Southern Biotechnology Associates. IgG1, IgG2a, IgG2b and IgG3 were detected using HRP-conjugated isotype specific antibodies from BD Biosciences. Purified monoclonal anti-TNP antibodies (A111-3 and G155-178 BD Biosciences) were used as standards for the anti-TNP ELISAs. Antibody titers are defined as the dilution required to reduce a positive signal to 3-fold above background. ELISPOTs were performed as described (Rangel-Moreno et al., 2005).
Immunofluorescence
Whole omenta were placed in 24 well plates and blocked with 10 μg/ml 2.4G2 in PBS with 5% BSA for 1 hour at 4°C while rocking. Fluorochrome-conjugated antibodies were then added for an additional 4 hours at 4°C while rocking. Omentums were washed 3 times for 15 minutes each in PBS with 5% BSA and then wet mounted in PBS with 5% BSA. Omenta were also prepared for histology by folding strips of omentum into a piece of liver, fixing the tissue with 10% neutral buffered formalin and embedding in paraffin. Paraffin sections were treated with Antigen Retrieval Solution (DAKO) at 96°C for 20 minutes and then cooled at room temperature for 20 minutes before blocking and staining as described above. Sections probed with fluorescent antibodies were counterstained with DAPI. Cryosections were obtained by cutting tissues in the presence of dry ice so that the fatty tissue of the omentum could be cut. All tissues were viewed with a Zeiss Axiovert 200m microscope with Apotome. Green and red fluorescence was captured using appropriate filter sets and in some cases, combined with images obtained using DIC. Some images were obtained as optical sections using the Apotome. Images were recorded with a Zeiss AxioCam digital camera using Zeiss AxioVision 4.5 software and saved as TIFF files. Antibodies to CD21, CD11b, CD11C, B220, CD35 and PNAd were obtained from BD Biosciences and conjugated to either AlexaFluor 488 or AlexaFluor using kits from Invitrogen. Purified goat anti-mouse CXCL13 (R&D Systems) was detected using Alexa Fluor 594-conjugated donkey anti-goat IgG (Molecular Probes).
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Frances Lund for helpful discussions during the course of this work. This work was supported by the Trudeau Institute and NIH grants HL69409 AI072689 and AI061511.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- Ansel KM, Ngo VN, Hayman PL, Luther SA, Forster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin a deficient mice: effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- Beelen RH, Fluitsma DM, Hoefsmit EC. The cellular composition of omentum milky spots and the ultrastructure of milky spot macrophages and reticulum cells. J Reticuloendothel Soc. 1980;28:585–599. [PubMed] [Google Scholar]
- Beelen RH, Oosterling SJ, van Egmond M, van den Born J, Zareie M. Omental milky spots in peritoneal pathophysiology (spots before your eyes) Perit Dial Int. 2005;25:30–32. [PubMed] [Google Scholar]
- Benedict CL, Kearney JF. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity. 1999;10:607–617. doi: 10.1016/s1074-7613(00)80060-6. [DOI] [PubMed] [Google Scholar]
- Berberich S, Dahne S, Schippers A, Peters T, Muller W, Kremmer E, Forster R, Pabst O. Differential molecular and anatomical basis for B cell migration into the peritoneal cavity and omental milky spots. J Immunol. 2008;180:2196–2203. doi: 10.4049/jimmunol.180.4.2196. [DOI] [PubMed] [Google Scholar]
- Browning JL, Allaire N, Ngam-Ek A, Notidis E, Hunt J, Perrin S, Fava RA. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. 2005;23:539–550. doi: 10.1016/j.immuni.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Cui L, Johkura K, Liang Y, Teng R, Ogiwara N, Okouchi Y, Asanuma K, Sasaki K. Biodefense function of omental milky spots through cell adhesion molecules and leukocyte proliferation. Cell Tissue Res. 2002;310:321–330. doi: 10.1007/s00441-002-0636-6. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Ansel KM, Rief K, Hyman PL, Tang HL, Luther SA, Ngo VN. Foliicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000;176:181–193. doi: 10.1034/j.1600-065x.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- Dal Porto JM, Haberman AM, Kelsoe G, Shlomchik MJ. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J Exp Med. 2002;195:1215–1221. doi: 10.1084/jem.20011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK. The size of the human omentum and methods of lengthening it for transplantation. Br J Plast Surg. 1976;29:170–144. doi: 10.1016/0007-1226(76)90045-x. [DOI] [PubMed] [Google Scholar]
- de Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Di Paolo N, Sacchi G, Garosi G, Sansoni E, Bargagli L, Ponzo P, Tanganelli P, Gaggiotti E. Omental milky spots and peritoneal dialysis--review and personal experience. Perit Dial Int. 2005;25:48–57. [PubMed] [Google Scholar]
- Dux K, Janik P, Szaniawska B. Kinetics of proliferation, cell differentiation and IgM secretion in the omental lymphoid organ of B10/Sn mice following intraperitoneal immunization with sheep erythrocytes. Cell Immunol. 1977;32:97–109. [Google Scholar]
- Dux K, Rouse RV, Kyewski B. Composition of the lymphoid cell populations from omental milky spots during the immune response in C57BL/Ka mice. Eur J Immunol. 1986;16:1029–1032. doi: 10.1002/eji.1830160828. [DOI] [PubMed] [Google Scholar]
- Endres R, Alimzhanov MB, Plitz T, Futterer A, Kosco-Vilbois MH, Nedospasov SA, Rajewski K, Pfeffer K. Mature follicular dendritic cell networks depend on expression of lymphotoxin b receptor by radioresistant stromal cells and of lymphotoxin b and tumor necrosis factor by B cells. J Exp Med. 1999;189:159–167. doi: 10.1084/jem.189.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko ME, Hirsch JG, Fried B. Studies on transport of macromolecules and small particles across mesothelial cells of the mouse omentum. II. Kinetic features and metabolic requirements. Exp Cell Res. 1971;69:313–323. doi: 10.1016/0014-4827(71)90230-8. [DOI] [PubMed] [Google Scholar]
- Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- Fukuyama S, Hiroi T, Yokota Y, Rennert PD, Yanagita M, Kinoshita N, Terawaki S, Shikina T, Yamamoto M, Kurono Y, Kiyono H. Initiation of NALT organogenesis is independent of the IL-7R, LTbetaR, and NIK signaling pathways but requires the Id2 gene and CD3(−)CD4(+)CD45(+) cells. Immunity. 2002;17:31–40. doi: 10.1016/s1074-7613(02)00339-4. [DOI] [PubMed] [Google Scholar]
- Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin b receptor controls organogenisis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Gerber SA, Rybalko VY, Bigelow CE, Lugade AA, Foster TH, Frelinger JG, Lord EM. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathol. 2006;169:1739–1752. doi: 10.2353/ajpath.2006.051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HS, Griffith AL, Kupferman A, Catsimpoolas N. Lipid angiogenic factor from omentum. Jama. 1984;252:2034–2036. [PubMed] [Google Scholar]
- Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, Fagarasan S. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdu I, Holub M, Trebichavsky I. The sequence of appearance of antibodies in mouse omentum plasma cells. Exp Cell Res. 1972;75:219–230. doi: 10.1016/0014-4827(72)90539-3. [DOI] [PubMed] [Google Scholar]
- Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med. 2000;192:931–942. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen A, Kusser K, Hartson L, Tighe M, Sunshine MJ, Sedgwick JD, Choi Y, Littman DR, Randall TD. Organogenesis of Nasal Associated Lymphoid Tissue (NALT) occurs independently of lymphotoxin-α (LTα) and retinoic acid receptor-related orphan receptor-γ, but the organization of NALT is LTα-dependent. J Immunol. 2002;168:986–990. doi: 10.4049/jimmunol.168.3.986. [DOI] [PubMed] [Google Scholar]
- Hodel C. Ultrastructural studies on the absorption of protein markers by the greater omentum. Eur Surg Res. 1970;2:435–449. doi: 10.1159/000127543. [DOI] [PubMed] [Google Scholar]
- Koni PA, Flavell RA. Lymph node germinal centers form in the absence of follicular dendritic cells networks. J Exp Med. 1999;189:855–864. doi: 10.1084/jem.189.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krist LF, Eestermans IL, Steenbergen JJ, Hoefsmit EC, Cuesta MA, Meyer S, Beelen RH. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995a;241:163–174. doi: 10.1002/ar.1092410204. [DOI] [PubMed] [Google Scholar]
- Krist LF, Kerremans M, Broekhuis-Fluitsma DM, Eestermans IL, Meyer S, Beelen RH. Milky spots in the greater omentum are predominant sites of local tumour cell proliferation and accumulation in the peritoneal cavity. Cancer Immunol Immunother. 1998;47:205–212. doi: 10.1007/s002620050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krist LF, Kerremans M, Koenen H, Blijleven N, Eestermans IL, Calame W, Meyer S, Beelen RH. Novel isolation and purification method permitting functional cytotoxicity studies of macrophages from milky spots in the greater omentum. J Immunol Methods. 1995b;184:253–261. doi: 10.1016/0022-1759(95)00096-s. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Lo SF, Carruthers CJL, Min J, Mariathasan S, Huang G, Plas DR, Martin SM, Geha RS, Nahm MH, Chaplin DD. Affinity maturation without germinal centers in lymphotoxin a deficient mice. Nature. 1996a;382:462–466. doi: 10.1038/382462a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. Role of lymphotoxin and the type 1 TNF receptor in the formation of germinal centers. Science. 1996b;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- Morrison R. On the functional aspects of the greater omentum. Br Med J. 1906;1:76–78. [Google Scholar]
- Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, Lefrancois L, Cauley LS, Harmsen AG, Lund FE, Randall TD. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity. 2006;25:643–654. doi: 10.1016/j.immuni.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- Nakano H, Tamura T, Yoshimoto T, Yagita H, Miyasaka M, Butcher EC, Nariuchi H, Kakiuchi T, Matsuzawa A. Genetic defect in T lymphocyte-specific homing into peripheral lymph nodes. Eur J Immunol. 1997;27:215–221. doi: 10.1002/eji.1830270132. [DOI] [PubMed] [Google Scholar]
- Portis B. Role of omentum of rabbits, dogs and guinea-pigs in antibody production. J Infect Dis. 1924;34:159–185. [Google Scholar]
- Rangel-Moreno J, Moyron-Quiroz J, Kusser K, Hartson L, Nakano H, Randall TD. Role of CXC Chemokine Ligand 13, CC Chemokine Ligand (CCL) 19, and CCL21 in the Organization and Function of Nasal-Associated Lymphoid Tissue. J Immunol. 2005;175:4904–4913. doi: 10.4049/jimmunol.175.8.4904. [DOI] [PubMed] [Google Scholar]
- Roberts KB. Antibody formation in the omentum. Br J Exp Pathol. 1955;36:357–362. [PMC free article] [PubMed] [Google Scholar]
- Smith KGC, Light A, Nossal GJV, Tarlington DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. EMBO J. 1997;16:2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solvason N, Chen X, Shu F, Kearney JF. The fetal omentum in mice and humans. A site enriched for precursors of CD5 B cells early in development. Ann N Y Acad Sci. 1992;651:10–20. doi: 10.1111/j.1749-6632.1992.tb24589.x. [DOI] [PubMed] [Google Scholar]
- Solvason N, Kearney JF. The human fetal omentum: a site of B cell generation. J Exp Med. 1992;175:397–404. doi: 10.1084/jem.175.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORg in Thymocyte Survival and Lymphoid Organ Development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- Szaniawska B. Changes in the greater omentum of mice of different strains after intraperitoneal immunization with sheep erythrocytes. I. Production of IgM immunoglobulins in milky spots. Arch Immunol Ther Exp (Warsz) 1974;22:585–593. [PubMed] [Google Scholar]
- Szaniawska B. Changes in the greater omentum of mice of different strains following intraperitoneal strains following intraperitoneal immunization with sheep erythrocytes. III. Determination of the percentage of thymus-dependent cells in the omental milky spots in mice by the application of anti-o serum. Arch Immunol Ther Exp (Warsz) 1975;23:19–24. [PubMed] [Google Scholar]
- Tew JG, Wu J, Qin D, Helm S, Burton GF, Szakal AK. Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol Rev. 1997;156:39–52. doi: 10.1111/j.1600-065x.1997.tb00957.x. [DOI] [PubMed] [Google Scholar]
- Vakil M, Briles DE, Kearney JF. Antigen-independent selection of T15 idiotype during B-cell ontogeny in mice. Dev Immunol. 1991;1:203–212. doi: 10.1155/1991/45352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vugt E, Van Rijthoven EA, Kamperdijk EW, Beelen RH. Omental milky spots in the local immune response in the peritoneal cavity of rats. Anat Rec. 1996;244:235–245. doi: 10.1002/(SICI)1097-0185(199602)244:2<235::AID-AR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Walker FC, Rogers AW. The greater omentum as a site of antibody synthesis. Br J Exp Pathol. 1961;42:222–231. [PMC free article] [PubMed] [Google Scholar]
- Williams R, White H. The greater omentum: its applicability to cancer surgery and cancer therapy. Curr Probl Surg. 1986;23:789–865. doi: 10.1016/0011-3840(86)90007-9. [DOI] [PubMed] [Google Scholar]
- Yokoto Y, Mansouri A, Mori S, Sugawara S, Adachi S, Nishikawa SI, Gruss P. Development of peripheral lymphoid organs and natual killer cells depends on the helix-loop-helix inhibitor Id2. Nature. 1999;397:702–706. doi: 10.1038/17812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.