Abstract
The Family B G protein-coupled calcitonin receptor is an important drug target. The aim of this work was to elucidate the molecular mechanism of action of small-molecule agonist ligands acting at this receptor, comparing it with the action mechanism of the receptor's natural peptide ligand. cAMP responses to four non-peptidyl ligands and calcitonin were studied in COS-1 cells expressing wild-type and chimeric calcitonin-secretin receptors. All compounds were full agonists at the calcitonin receptor with no activity at the secretin receptor. Only chimeric constructs including the calcitonin receptor amino terminus exhibited responses to any of these ligands. We progressively truncated this domain and tested constructs for cAMP responses. Although calcitonin was able to activate the calcitonin receptor fully with the first 58 residues absent, its potency was 3 orders of magnitude lower than that at the wild-type receptor. After truncation of 114 residues, there was no response to calcitonin. In contrast, small-molecule ligands were fully active at receptors having up to 149 amino-terminal residues absent. Those compounds finally became inactive after truncation of 153 residues. Deletion and/or alanine replacement of the region of the calcitonin receptor between residues 150 and 153 resulted in marked reduction in cAMP responses to these compounds, with some compound-specific differences observed, supporting a critical role for this region. Binding studies further supported distinct sites of action of small molecules relative to that of calcitonin. These findings focus attention on the potential importance of the juxtamembranous region of the amino terminus of the Family B calcitonin receptor for agonist drug action.
Calcitonin (CT),3 a 32-amino acid peptide secreted by the thyroid gland in response to elevations in blood calcium levels, acts on bone and kidney to maintain calcium homeostasis (1, 2). CT is widely used therapeutically for the treatment of bone-related disorders such as osteoporosis, hypercalcemia of malignancy, and Paget disease (1, 2). This peptide must be administered parenterally, whereas small-molecule agonist ligands that can be administered orally have substantial clinical advantage, particularly for long-term treatment.
CT acts on the CT receptor, a Family B G protein-coupled receptor (GPCR). Members of this family include receptors for secretin, vasoactive intestinal polypeptide, parathyroid hormone, corticotrophin-releasing factor, glucagon, glucagon-like peptide 1, CT, and CT gene-related peptide (3), with each having a long extracellular amino-terminal domain containing six conserved cysteine residues that form three intradomain disulfide bonds. This region has been shown to play a critical role in natural peptide ligand binding and receptor activation (4–11).
Development of small-molecule ligands for Family B GPCRs represents an area of great interest. For the CT receptor, several such ligands have been developed (12–14). However, the mechanisms of their actions at their receptors remain unclear. Most recently, synthesis of a series of pyrazolopyridine CT receptor ligands described as partial agonists has been reported (15). Here, we explore the structural basis for their action at the CT receptor. Using CT-secretin receptor chimeric analysis, the amino terminus of the CT receptor was identified as a critical region for the action of these small-molecule ligands but with determinants that are distinct from those interacting with the natural ligand, CT. Truncation, deletion, and alanine replacement mutations of the CT receptor identified that three specific receptor residues, Tyr150, Leu151, and Ile153, within the juxtamembranous region of the amino-terminal domain of the CT receptor, play important roles in the actions of these small-molecule agonists. This study represents the first identification of this region as being critical for the action of small-molecule drugs acting at Family B GPCRs.
EXPERIMENTAL PROCEDURES
Materials
Human CT was purchased from Bachem (Torrance, CA). The human CT receptor cDNA was provided by GlaxoSmithKline. The solid-phase oxidant N-chlorobenzenesulfonamide (IODO-BEADS) was purchased from Pierce. Bovine serum albumin was from Serologicals Corp. (Norcross, GA). FetalClone II culture medium supplement was from HyClone Laboratories (Logan, UT). Rabbit polyclonal anti-CT receptor antibody raised against amino acids 411–490 at the carboxyl terminus of the human CT receptor was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Alexa Fluor 488-conjugated goat anti-rabbit IgG and tissue culture medium were from Invitrogen. All other reagents were analytical grade.
Small-molecule Compounds
Four non-peptidyl small-molecule ligands (Fig. 1), identified as 2d, 2e, 2f, and 2g based on their initial report (15), were provided by GlaxoSmithKline. The structures of compounds 2d, 2e, and 2f were described in that report (15), whereas compound 2g represents 2c with an additional para-fluoro group on its NH-R ring. These compounds were reported as partial agonists acting at the human CT receptor transiently expressed on Chinese hamster ovary cells along with the cAMP-responsive element-luciferase reporter system (15). All of these compounds were dissolved in dimethyl sulfoxide at concentrations of 10 mm and stored at −20 °C.
FIGURE 1.
Chemical structures of the small-molecule compounds used in this study. Illustrated are structures of compounds 2d, 2e, and 2f, as reported previously (15). Shown also is the structure of compound 2g, which represents compound 2c from the previous report (15) with an additional para-fluoro group on its NH-R ring.
Radioiodination of Human CT
CT was radioiodinated oxidatively at Tyr19, using 1 mCi of Na125I and a 15-s exposure to IODO-BEADS. The products were purified to homogeneity by reversed-phase high-pressure liquid chromatography, yielding a specific radioactivity of the product of ∼2,000 Ci/mmol (16).
Generation of CT-Secretin Receptor Chimeras
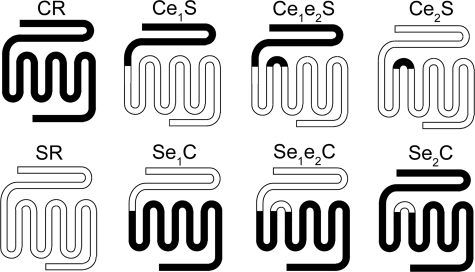
The chimeric receptor DNA constructs used in this study include portions of wild-type rat secretin and human CT receptors (Fig. 2 and Table 1). They were prepared by excising sequences of the wild-type receptor cDNAs and replacing them with the corresponding sequences of the other receptor. Receptor cDNA fragments of interest were joined together using unique naturally occurring and newly introduced restriction sites. The junctions of these constructs were verified by direct DNA sequencing (17), and when necessary, sequences were corrected by the QuikChange site-directed mutagenesis kit from Stratagene (La Jolla, CA).
FIGURE 2.
Schematic diagrams of chimeric receptors. Shown are the nomenclature and schematic diagrams of the wild-type and chimeric CT-secretin receptors. Black and white portions represent CT (C) and secretin (S) receptor sequences, respectively. For detailed sequences of the chimeric constructs, see Table 1.
TABLE 1.
Nomenclature of CT-secretin receptor chimeras
The numbers in parentheses represent the amino acid codons in the wild-type receptor proteins, which include the signal sequences of each receptor because the specific sites of processing and removal for the calcitonin receptor have not been established.
| Nomenclature | Construct sequences |
|---|---|
| Ce1S | CT receptor (1–153), secretin receptor (148–449) |
| Se1C | Secretin receptor (1–147), CT receptor (154–474) |
| Ce2S | Secretin receptor (1–193), CT receptor (200–221), secretin receptor (218–449) |
| Se2C | CT receptor (1–199), secretin receptor (194–217), CT receptor (222–474) |
| Ce1e2S | CT receptor (1–153), secretin receptor (148–193), CT receptor (200–221), secretin receptor (218–449) |
| Se1e2C | Secretin receptor (1–147), CT receptor (154–199), secretin receptor (194–217), CT receptor (222–474) |
Generation of Amino-terminal Truncation Constructs of the CT Receptor
CT receptor constructs with the amino terminus progressively truncated were prepared (Fig. 3). There is an internal EcoRI restriction site at the cDNA sequence of the CT receptor corresponding to Asn194. To generate the first truncation construct, Δ(1–47), the cDNA sequence encoding receptor fragment Met48–Asn194, which contained a HindIII restriction site at the start, was amplified by PCR and ligated to the pcDNA3 vector containing the CT receptor cDNA that had been digested by both HindIII and EcoRI to remove the cDNA sequence encoding Met1–Asn194. Progressive truncation of the receptor was achieved by using newly prepared truncated CT receptor constructs as templates and the QuikChange site-directed mutagenesis kit. PCRs were performed in a thermal cycler with PfuTurbo DNA polymerase, running 18 cycles of 95 °C for 30 s, 65 °C for 1 min, and 68 °C for 14 min.
FIGURE 3.
Amino acid sequence and predicted topological structure of the human CT receptor. Shown are the points of the truncation, deletion, and site mutations within the amino terminus of the CT receptor. The six functionally important Cys residues are highlighted in black circles with white lettering. Potential sites of glycosylation are indicated by forked symbols. Blank circles at the distal amino terminus indicate the potential signal sequence. The dashed line indicates the conserved disulfide bond between the first and second extracellular loops. The predicted transmembrane domains are shaded in gray.
Generation of Deletion and Site Mutation Constructs of the CT Receptor
Two deletion and three alanine replacement mutations in the juxtamembranous region of the amino terminus of the human CT receptor were prepared (Fig. 3). These represented deletion of segments 150–154 (Δ(150–154)) and 150–151 (Δ(150–151)) and substitutions of single amino acid residues Tyr150, Leu151, and Ile153 with an Ala (Y150A, L151A, and I153A). These were constructed using an oligonucleotide-directed approach with the QuikChange site-directed mutagenesis kit. PCRs were performed as described as above. Receptor constructs were subcloned into the eukaryotic expression vector pcDNA3 (Invitrogen). The sequences of all constructs were confirmed by direct DNA sequencing (17).
Receptor Expression
Receptor constructs were expressed transiently in COS-1 cells (American Type Culture Collection, Manassas, VA). In brief, 5 × 105 cells plated on tissue culture plasticware were transfected with 3 μg of DNA using a modification of the DEAE-dextran protocol that included 10% dimethyl sulfoxide shock and treatment with 0.1 mm chloroquine diphosphate (18). They were maintained in Dulbecco's modified Eagle's medium supplemented with 5% FetalClone II. Cells were used 48 h after transfection for radioligand binding and biological activity assays.
Ligand Binding Assay
CT binding to COS-1 cells transiently expressing the wild-type and mutant CT receptors was performed in a standard competition binding assay, using conditions established previously (19). In brief, ∼2 × 105 cells were incubated at room temperature for 1 h with a constant amount of 125I-CT radioligand (5–10 pm) in the absence and presence of varied concentrations of unlabeled CT or small-molecule compounds in Krebs-Ringer/HEPES medium containing 25 mm HEPES (pH 7.4), 104 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm KH2PO4, 1.2 mm MgSO4, 0.01% soybean trypsin inhibitor, and 0.2% bovine serum albumin. Separation of free from bound radioligand was performed by washing the cells twice with ice-cold Krebs-Ringer/HEPES medium. Cells were lysed with 0.5 m NaOH, and membrane-bound radioactivity was quantified with a γ-spectrometer. Nonspecific binding was assessed in the presence of 1 μm unlabeled human CT and represented <20% of total radioligand binding.
Biological Activity Assay
The biological activities of the COS-1 cells transiently expressing CT receptor mutants were assessed by measuring cAMP responses stimulated by CT or small-molecule compounds. Cells were stimulated with increasing concentrations of CT or small-molecule compounds at 37 °C for 30 min in Krebs-Ringer/HEPES medium supplemented with 1 mm 3-isobutylmethylxanthine (Sigma). The reaction was stopped by adding ice-cold perchloric acid. After adjusting the pH to 6 with KHCO3, cell lysates were cleared by centrifugation at 3,000 rpm for 10 min, and the supernatants were used in the assay employing a previously described competition binding assay for quantitation of cAMP (Diagnostic Products Corp., Los Angeles, CA) (20). Radioactivity was quantified in a Beckman LS6000 liquid scintillation counter.
Immunofluorescence Microscopy
For morphological assessment of expression of key CT receptor constructs on the cell surface, COS-1 cells transiently transfected with each construct were replated on coverslips and allowed to grow for 48 h. Cells were washed with phosphate-buffered saline (PBS) and fixed in 2% paraformaldehyde for 30 min. After two additional washes with PBS, cells were treated with 0.1% saponin in PBS for 10 min. Cells were then washed once with PBS and twice with 1% normal goat serum in PBS before being incubated with rabbit polyclonal anti-CT receptor antibody (1:50) for 1 h. Cells were washed three times with 1% normal goat serum in PBS and incubated with Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:200) for 1 h. Coverslips were then washed three times with PBS, mounted on slides, and examined with an LSM 510 confocal microscope (Zeiss, Thornwood, NY) with the following settings: excitation, 488-nm argon laser; emission, LP505 filter; and pinhole diameter, 2.6 airy units, Plan-Apochromat 63×/1.4NA oil. Background-subtracted images were assembled using Adobe Photoshop 7.0. All of the above procedures were performed at room temperature.
Statistical Analysis
All observations were repeated at least three times in duplicate in three independent experiments and are expressed as means ± S.E. Data were analyzed using the nonlinear least-squares curve-fitting program, LIGAND (21) and were graphed using Prism 3.0 (GraphPad, San Diego, CA).
RESULTS
Role of the Amino-terminal Domain of the CT Receptor in Natural Ligand Binding and Activation as Identified by Chimeric Receptor Analysis
Both wild-type receptors showed selectivity for their native agonist ligands. CT had no binding or biological activity at the secretin receptor, and secretin did not bind to or activate the CT receptor (Fig. 4 and Tables 2 and 3).
FIGURE 4.
Binding and biological characterization of the chimeric receptors by natural peptide ligands. Left, curves for CT (upper) and secretin (lower) to compete with their respective radioligands for binding to membranes from COS-1 cells transfected with the indicated constructs. Values represent percentages of maximal saturable binding of wild-type receptors that were observed in the absence of competitor and are expressed as means ± S.E. of duplicate data from three independent experiments. Right, intracellular cAMP responses to increasing concentrations of CT (upper) and secretin (lower) in COS-1 cells transfected with the indicated constructs. Values are expressed as means ± S.E. of data from three independent experiments performed in duplicate, with data normalized relative to the maximal response observed with wild-type CT (CR; upper) and secretin (SR; lower) receptors.
TABLE 2.
Binding properties of CT-secretin receptor chimeras and key CT receptor mutants by natural peptide ligands
Shown are the parameters of secretin or CT binding to COS-1 cells expressing each of the noted receptor constructs. All values are the means ± S.E. of data from three independent experiments performed in duplicate. CR, CT receptor; SR, secretin receptor.
| Construct |
Kd |
Bmax |
||
|---|---|---|---|---|
| Secretin | CT | Secretin | CT | |
| nm | binding sites/cell × 103 | |||
| CR | >1,000 | 1.1 ± 0.3 | 99 ± 25 | |
| SR | 3.3 ± 0.5 | >1,000 | 101 ± 31 | |
| Ce1S | >1,000 | >1,000 | ||
| Se1C | 270 ± 40 | >1,000 | 75 ± 19 | |
| Ce2S | 69 ± 21 | >1,000 | 87 ± 22 | |
| Se2C | >1,000 | 100 ± 27 | 78 ± 21 | |
| Ce1e2S | >1,000 | 288 ± 43 | 55 ± 18 | |
| Se1e2C | 160 ± 27 | >1,000 | 89 ± 29 | |
| Y150A-CR | 12 ± 3 | 123 ± 31 | ||
| L151A-CR | 6.6 ± 1.4 | 113 ± 28 | ||
| I153A-CR | 4.3 ± 1.1 | 72 ± 19 | ||
TABLE 3.
Biological properties (cAMP stimulation) of truncation, deletion, and Ala site mutation CT receptors
Shown are EC50 values for natural peptide or small molecule ligand-stimulated intracellular cAMP responses in COS-1 cells expressing each of the noted receptor constructs, as well as Emax values reflecting maximal intracellular cAMP concentrations achieved. The basal cAMP levels in these cells were between 2 and 4 pmol/million cells. All values are the means ± S.E. of data from three independent experiments performed in duplicate. CR, CT receptor; SR, secretin receptor.
A series of CT-secretin receptor chimeras (Fig. 2 and Table 1) were constructed and transiently expressed in COS-1 cells. CT did not bind to or elicit a cAMP response in Ce1S, Se1C, Ce2S, or Se1e2C constructs (Fig. 4 and Tables 2 and 3). Se2C and Ce1e2S constructs were able to bind CT, although with much lower affinity than the wild-type CT receptor. However, only the Se2C construct was able to accumulate intracellular cAMP in response to CT stimulation (Fig. 4 and Table 3). These constructs were also tested for secretin binding and signaling. Of all of the chimeric constructs, Se1C, Ce2S, and Se1e2C were able to bind secretin but with much lower affinity than the wild-type secretin receptor. Only the Ce2S construct was able to accumulate intracellular cAMP in response to secretin stimulation (Fig. 4 and Table 3).
These data suggest that both the amino terminus and the first extracellular loop regions of CT and secretin receptors play important roles in natural ligand binding, with the core helical bundle region required for receptor activation. These data are consistent with previous studies using chimeric secretin-VPAC1 (22) and CT-glucagon receptor (23) constructs.
Identification of the Role of the Amino-terminal Domain of the CT Receptor in Receptor Activation by Small-molecule Compounds by Chimeric Receptor Analysis
All four small-molecule CT receptor ligands were tested for their agonist activity at both wild-type CT and secretin receptors. They were all fully efficacious agonists of cAMP accumulation in COS-1 cells expressing the CT receptor but had no activity at the secretin receptor (Fig. 5 and Table 3). It should be noted that these compounds were previously reported as partial agonists in a cAMP-responsive element-luciferase reporter system in Chinese hamster ovary cells (15). These four small-molecule ligands were also tested for their agonist activity at the chimeric receptor constructs listed in Table 1. Interestingly, unlike natural CT, all four small-molecule agonists were able to elicit intracellular cAMP responses in COS-1 cells transfected with all chimeric constructs containing the amino terminus of the CT receptor, i.e. Ce1S, Se2C, and Ce1e2S (Fig. 5 and Table 3). This suggests that the amino terminus of the CT receptor contains the sites for action of these small molecules.
FIGURE 5.
Biological characterization of the chimeric receptors by small-molecule compounds. Shown are intracellular cAMP responses to increasing concentrations of small-molecule compounds in COS-1 cells transfected with the indicated chimeric constructs. Values are expressed as means ± S.E. of data from three independent experiments performed in duplicate, with data normalized relative to the maximal response observed with the wild-type CT receptor (CR). SR, secretin receptor.
Identification of Functionally Critical Residues of the CT Receptor for Small-molecule Agonists Using Truncation, Deletion, and Site Mutation Receptor Constructs
To localize the domain of action by the four small-molecule CT receptor agonists, a series of CT receptor truncation mutants were prepared that had residues at the receptor amino terminus progressively deleted (Fig. 3). The truncations were made around the six conserved cysteines within the amino terminus of the CT receptor. All of the constructs were expressed transiently in COS-1 cells and tested for their responses to stimulation by natural CT and small-molecule ligands.
Progressive truncation of the amino terminus of the CT receptor resulted in rapid loss of function in response to natural ligand. As shown in Table 4, deletion of the first 53 residues of the CT receptor resulted in a 30-fold reduction in potency while maintaining full efficacy. Deletion of the first 58 residues, interfering with the first disulfide bond, resulted in a 2,170-fold reduction in potency while still maintaining full efficacy. Deletion of the first 79 residues essentially abolished all cAMP responsiveness to CT.
TABLE 4.
Biological properties (cAMP stimulation) of truncation, deletion, and Ala site mutation CT receptors
Shown are EC50 values for CT or small molecule ligand-stimulated intracellular cAMP responses in COS-1 cells expressing each of the noted receptor constructs, as well as Emax values reflecting maximal intracellular cAMP concentrations achieved. The basal cAMP levels in these cells were between 1.7 and 4.2 pmol/million cells. All values are the means ± S.E. of data from three independent experiments performed in duplicate.
Truncation of the CT receptor was much better tolerated for stimulation by all four small-molecule agonists. As shown in Fig. 6 and Table 4, deletion of up to 149 residues at the amino terminus of the CT receptor did not result in significant losses of cAMP responses to stimulation by any of the four small-molecule agonists. However, deletion of one additional residue to include Tyr150 (Δ(1–150)) almost abolished the responses to stimulation by 2d and 2e, whereas responses to 2f and 2g continued to be fully efficacious with almost normal potency. Further deletion of one or more residues to include Leu151 (Δ(1–151), Δ(1–152), and Δ(1–153)) resulted in a dramatic reduction of efficacy and potency of cAMP responses to 2f and 2g as well. Normal surface expression of key truncation constructs Δ(1–150) and Δ(1–151) was confirmed by immunostaining using anti-CT receptor antibody against an epitope within the human CT receptor carboxyl-terminal domain (Fig. 7). These data suggest that Tyr150 plays an important role in actions of 2d and 2e, whereas Leu151 plays such a role in the action of 2f and 2g.
FIGURE 6.
Biological characterization of truncation, deletion, and site mutation CT receptor constructs by small-molecule compounds. Shown are intracellular cAMP responses to increasing concentrations of small-molecule compounds in COS-1 cells transfected with selected truncation, deletion, and site mutation constructs. Values are expressed as means ± S.E. of data from three independent experiments performed in duplicate, with data normalized relative to the maximal response observed with the wild-type (WT) CT receptor.
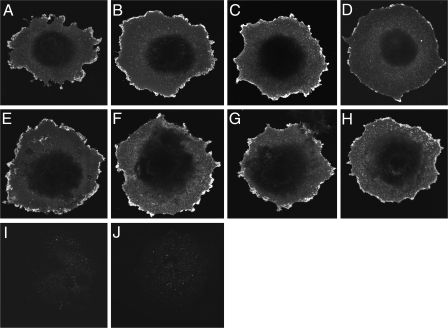
FIGURE 7.
Morphological evidence for normal cell surface expression of functionally impaired CT receptor constructs. Shown are representative examples of anti-CT receptor immunolabeling of COS-1 cells transfected with wild-type (A), Δ(1–150) (B), Δ(1–151) (C), Δ(150–154) (D), Δ(150–151) (E), Y150A (F), L151A (G), and I153A (H) CT receptors and cells transfected with the empty pcDNA3 eukaryotic expression vector (I). Shown also are COS-1 cells transfected with the wild-type CT receptor (J) but immunostained only with Alexa Fluor 488-conjugated goat anti-rabbit IgG. Images are representative of three repetitions.
To confirm the above observations further, two deletion CT receptor mutants, Δ(150–154) and Δ(150–151), were prepared with the remainder of the amino terminus intact. Deletion of residues 150–154 abolished the receptor's cAMP responses to all four small-molecule compounds (Table 4). The Δ(150–151) deletion mutant had markedly reduced efficacy and potency of cAMP responses to 2d, 2e, and 2g and had almost no response to 2f (Fig. 6 and Table 4). Both deletion mutants were confirmed for their cell surface expression by immunostaining (Fig. 7). It should be noted that the Δ(150–151) deletion mutant had a fully efficacious response to CT, although with significantly reduced potency, further confirming normal surface expression of this mutant. These data further suggest that both Tyr150 and Leu151 are critical for actions of the four small-molecule CT receptor agonists.
Three site mutants, Y150A, L151A, and I153A, were prepared to evaluate further the importance of Tyr150, Leu151, and Ile153 for actions by these small-molecule agonists. All three mutants were able to respond fully to CT, although Y150A and L151A had reduced potency compared with the wild-type CT receptor (Fig. 6 and Table 4). This suggests that all three receptor mutants were able to traffic normally to the cell surface, and this was confirmed by immunostaining (Fig. 7). Compared with the wild-type receptor, the Y150A mutant had markedly reduced efficacy of cAMP responses to 2d and 2e while having normally efficacious cAMP responses to 2f and 2g (Fig. 6 and Table 4). Compared with the wild-type receptor, the L151A mutant had cAMP responses similar to 2d and 2e although with reduced potency, whereas it had much reduced efficacy of responses to 2f and 2g (Fig. 6 and Table 4). The I153A mutant responded to all four small-molecule compounds fully but had reduced potency to 2d, 2e, and 2f compared with the wild-type CT receptor. This mutant responded to stimulation by 2g normally with potency and efficacy similar to those of the wild-type receptor (Fig. 6 and Table 4). These data suggest that the biological effects of 2d and 2e are affected predominantly by Tyr150 and Ile153, the effects of 2f are affected predominantly by Leu151 and Ile153, and the effects of 2g are affected predominantly by Leu151.
Evaluation of Potential Interactions between Natural Ligand CT and Small-molecule Compounds
Fig. 8 shows the ability of all four small-molecule agonists to compete with peptide radioligand, 125I-CT, for binding to membranes from COS-1 cells transfected with the wild-type, Y150A, L151A, and I153A CT receptor constructs. Although some partial competition of CT binding was observed for all compounds, all of these competition binding curves were shifted far to the right.
FIGURE 8.
Binding characterization of key site mutation CT receptor constructs. Shown are curves for CT and all four small-molecule agonists to compete with peptide radioligand, 125I-CT, for binding to membranes from COS-1 cells transfected with the indicated constructs. Values represent percentages of maximal saturable binding of CT receptor constructs that were observed in the absence of competitor and are expressed as means ± S.E. of duplicate data from three independent experiments. WT, wild-type.
Small-molecule compounds had no effects on the CT biological activity. Data in Fig. 9 show that CT and small-molecule compounds had independent actions at the CT receptor that were additive up to full efficacy, with neither agonist resulting in potentiation or inhibition of the action of the other. The maximal cAMP response observed was determined by the density of CT receptors.
FIGURE 9.
Biological activity of CT in the presence of small-molecule compounds. Shown are CT concentration-response curves for cAMP in COS-1 cells transiently transfected with the wild-type CT receptor, with each panel reflecting the concurrent presence of half-maximal and maximal fixed concentrations of 2d, 2e, 2f, and 2g. Values are expressed as means ± S.E. of data from three independent experiments performed in duplicate, with data normalized relative to the maximal response to CT.
These data suggest that these small molecules bind to distinct regions of the CT receptor from the natural peptide agonist, CT. Most likely, they bind to allosteric sites on the receptor independent of the orthosteric site of action of CT.
DISCUSSION
GPCRs represent one of the largest gene families in the human genome and have long been regarded as valuable targets for small-molecule drugs. Development of non-peptidyl or small-molecule therapeutics targeting GPCRs represents an area of substantial interest for pharmaceutical companies. Family B GPCRs contain many important drug targets, but development of small molecules targeting this family has lagged far behind the Family A members. Currently, a few such molecules are available for receptors for corticotrophin-releasing factor (24, 25), glucagon (26, 27), glucagon-like peptide 1 (28–30), CT (12–14), and CT gene-related peptide (31). However, the molecular mechanism of actions for these molecules remains unknown.
Understanding the molecular basis of drug action is extremely important, contributing to refinement of the specificity, affinity, and potency of lead compounds and even to the rational design of new drugs. In the present work, we attempted to elucidate the molecular interactions between non-peptidyl small-molecule agonist ligands and the CT receptor, comparing these with the mechanism of action of the natural peptide ligand, CT. By using CT-secretin receptor chimeras and CT receptor truncations, deletions, and site mutants, we identified a distinct region within the CT receptor that is critical for small-molecule agonist action. This is, to our knowledge, the first time that this juxtamembranous region of the amino terminus of a Family B GPCR has been identified as critical for small-molecule action.
Molecular Basis of Action of CT
Although there are strong data supporting the critical importance of the amino-terminal region of Family B GPCRs for natural peptide ligand binding and action (4–11), the details of this are not well established, and there are no clear insights into the molecular basis of binding or action of non-peptidyl small-molecule drug candidates. The current work started with chimeras of CT and secretin receptors to identify regions critical for natural ligand binding and activation by natural ligands and by the non-peptidyl agonists. The construct containing the amino terminus of the CT receptor alone (Ce1S) did not bind CT, but constructs containing the amino terminus with another part of the CT receptor (Se2C and Ce1e2S) were able to bind CT. CT receptor activation requires that the construct contain both the amino terminus and the body of the CT receptor (Se2C). However, the construct containing only the amino terminus of the secretin receptor (Se1C) was able to bind secretin. Like the CT receptor, secretin receptor activation by secretin required both the amino terminus and the body of the secretin receptor (Ce2S). Nevertheless, this work confirmed the importance of the amino terminus of both receptors for the binding of their respective natural ligands. Previous chimeric studies combining portions of the CT and glucagon receptors have identified that the amino terminus of the CT receptor contains binding determinants for CT (23). Photoaffinity labeling studies have also identified critical ligand-binding residues within this domain (6).
Truncation of the first 53 residues of the calcitonin receptor resulted in a 30-fold loss of potency. Further truncation of up to 58 residues to include the first conserved cysteine resulted in a 2,170-fold loss of potency, suggesting the importance of the disulfide bond known to involve this residue. The amino-terminal receptor truncation studies also suggested the importance of the residues within the distal amino terminus of the CT receptor, a critical binding region for the secretin receptor (5). This theme is consistent with photoaffinity labeling studies of the CT receptor that mapped Thr30 at the distal end of the receptor as the site of labeling for a photolabile position 26 probe (6).
Molecular Basis of Small-molecule Agonist Action
Most small-molecule ligands of GPCRs, agonists as well as antagonists, are thought to bind to sites within the transmembrane helical bundle domain (32). However, it is notable that the molecular basis for agonist and antagonist action in groups of what may be structurally similar molecules has remained unclear.
Small-molecule ligands for Family B GPCRs are not well developed, and there is little information available as to how these molecules interact with their receptors. To gain insights into this, in the current work, we used four small-molecule compounds (15) and tested them in COS-1 cells transfected with a series of CT-secretin receptor chimeras and CT receptor constructs that included truncations, deletions, and site mutations. It should be noted that these compounds were full agonists at the CT receptor expressed in the COS-1 cells. The improved potency from what had initially been described is likely caused by the use of a different cellular expression system and/or higher receptor density on the cell surfaces. Chimeric receptor analysis established that the juxtamembranous amino-terminal domain contributes important determinants for the action of each of these four compounds. Further amino-terminal truncation, residue deletion, and site mutation studies identified CT receptor residues Tyr150, Leu151, and Ile153 as providing critical determinants for the actions of these molecules. These residues are predicted to be located in the juxtamembranous portion of the amino-terminal domain of the CT receptor. However, it is not clear where these residues are located relative to the lipid bilayer because this interface has not been definitively mapped. It is also not yet possible to be certain that there are direct contacts between these residues and the small-molecule ligands. Their sites of binding may be distal to this region containing the determinants for their action.
Different mechanisms have been proposed for non-peptidyl antagonist action at various Family B GPCRs, such as the corticotrophin-releasing factor (24, 25), glucagon (26, 27), glucagon-like peptide 1 (28–30), and CT gene-related peptide (33, 34) receptors. Some non-peptidyl antagonists targeting the corticotrophin-releasing factor (24, 25) and glucagon (26, 27) receptors have been reported to act at sites within transmembrane helical bundle domains. Other small-molecule ligands have been reported to be affected by residues within the amino terminus of their receptors, such as the glucagon-like peptide 1 receptor antagonist that is affected by Trp33 (28). The binding determinants of the non-peptidyl antagonist BIBN4096BS are present within the extracellular amino-terminal region of RAMP1, the accessory protein that associates with the CT gene-related peptide receptor (33, 34). It is believed that all of these small-molecule ligands act at sites distinct from the orthosteric natural peptide ligand-binding regions. Indeed, in the current work, we have also demonstrated that all four small-molecule agonists of the CT receptor have critical determinants that are independent of the natural peptide ligand-binding site within the amino terminus.
This work was supported, in whole or in part, by National Institutes of Health Grant DK46577 (to L. J. M.). This work was also supported by a grant from GlaxoSmithKline (to L. J. M.).
- CT
- calcitonin
- GPCR
- G protein-coupled receptor
- PBS
- phosphate-buffered saline.
REFERENCES
- 1.Purdue B. W., Tilakaratne N., Sexton P. M. (2002) Receptors Channels 8, 243–255 [PubMed] [Google Scholar]
- 2.Sexton P. M., Findlay D. M., Martin T. J. (1999) Curr. Med. Chem. 6, 1067–1093 [PubMed] [Google Scholar]
- 3.Mayo K. E., Miller L. J., Bataille D., Dalle S., Göke B., Thorens B., Drucker D. J. (2003) Pharmacol. Rev. 55, 167–194 [DOI] [PubMed] [Google Scholar]
- 4.Cao Y. J., Gimpl G., Fahrenholz F. (1995) Biochem. Biophys. Res. Commun. 212, 673–680 [DOI] [PubMed] [Google Scholar]
- 5.Dong M., Lam P. C., Gao F., Hosohata K., Pinon D. I., Sexton P. M., Abagyan R., Miller L. J. (2007) Mol. Pharmacol. 72, 280–290 [DOI] [PubMed] [Google Scholar]
- 6.Dong M., Pinon D. I., Cox R. F., Miller L. J. (2004) J. Biol. Chem. 279, 1167–1175 [DOI] [PubMed] [Google Scholar]
- 7.Gourlet P., Vilardaga J. P., De Neef P., Waelbroeck M., Vandermeers A., Robberecht P. (1996) Peptides 17, 825–829 [DOI] [PubMed] [Google Scholar]
- 8.Holtmann M. H., Hadac E. M., Miller L. J. (1995) J. Biol. Chem. 270, 14394–14398 [DOI] [PubMed] [Google Scholar]
- 9.Jüppner H., Schipani E., Bringhurst F. R., McClure I., Keutmann H. T., Potts J. T., Jr., Kronenberg H. M., Abou-Samra A. B., Segre G. V., Gardella T. J. (1994) Endocrinology 134, 879–884 [DOI] [PubMed] [Google Scholar]
- 10.Stroop S. D., Nakamuta H., Kuestner R. E., Moore E. E., Epand R. M. (1996) Endocrinology 137, 4752–4756 [DOI] [PubMed] [Google Scholar]
- 11.Vilardaga J. P., De Neef P., Di Paolo E., Bollen A., Waelbroeck M., Robberecht P. (1995) Biochem. Biophys. Res. Commun. 211, 885–891 [DOI] [PubMed] [Google Scholar]
- 12.Funahashi Y., Kawamura N., Ishimaru T. (1996) Chem. Abstr. 126, 6553 [Google Scholar]
- 13.Katayama T., Furuya M., Yamaichi K., Konishi K., Sugiura N., Murafuji H., Magota K., Saito M., Tanaka S., Oikawa S. (2001) Biochim. Biophys. Acta 1526, 183–190 [DOI] [PubMed] [Google Scholar]
- 14.Su M. H., Hosken M. I., Hotovec B. J., Johnston T. L. (1998) Chem. Abstr. 128, 239489 [Google Scholar]
- 15.Boros E. E., Cowan D. J., Cox R. F., Mebrahtu M. M., Rabinowitz M. H., Thompson J. B., Wolfe L. A., 3rd (2005) J. Org. Chem. 70, 5331–5334 [DOI] [PubMed] [Google Scholar]
- 16.Powers S. P., Pinon D. I., Miller L. J. (1988) Int. J. Pept. Protein Res. 31, 429–434 [DOI] [PubMed] [Google Scholar]
- 17.Sanger F., Nicklen S., Coulson A. R. (1977) Proc. Natl. Acad. Sci. U.S.A. 74, 5463–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holtmann M. H., Hadac E. M., Ulrich C. D., Miller L. J. (1996) J. Pharmacol. Exp. Ther. 279, 555–560 [PubMed] [Google Scholar]
- 19.Hadac E. M., Ghanekar D. V., Holicky E. L., Pinon D. I., Dougherty R. W., Miller L. J. (1996) Pancreas 13, 130–139 [DOI] [PubMed] [Google Scholar]
- 20.Ganguli S. C., Park C. G., Holtmann M. H., Hadac E. M., Kenakin T. P., Miller L. J. (1998) J. Pharmacol. Exp. Ther. 286, 593–598 [PubMed] [Google Scholar]
- 21.Munson P. J., Rodbard D. (1980) Anal. Biochem. 107, 220–239 [DOI] [PubMed] [Google Scholar]
- 22.Holtmann M. H., Ganguli S., Hadac E. M., Dolu V., Miller L. J. (1996) J. Biol. Chem. 271, 14944–14949 [DOI] [PubMed] [Google Scholar]
- 23.Stroop S. D., Kuestner R. E., Serwold T. F., Chen L., Moore E. E. (1995) Biochemistry 34, 1050–1057 [DOI] [PubMed] [Google Scholar]
- 24.Kehne J., De Lombaert S. (2002) Curr. Drug Targets CNS Neurol. Disord. 1, 467–493 [DOI] [PubMed] [Google Scholar]
- 25.Hoare S. R., Brown B. T., Santos M. A., Malany S., Betz S. F., Grigoriadis D. E. (2006) Biochem. Pharmacol. 72, 244–255 [DOI] [PubMed] [Google Scholar]
- 26.Madsen P., Brand C. L., Holst J. J., Knudsen B. (1999) Curr. Pharm. Des. 5, 683–691 [PubMed] [Google Scholar]
- 27.Petersen K. F., Sullivan J. T. (2001) Diabetologia 44, 2018–2024 [DOI] [PubMed] [Google Scholar]
- 28.Tibaduiza E. C., Chen C., Beinborn M. (2001) J. Biol. Chem. 276, 37787–37793 [DOI] [PubMed] [Google Scholar]
- 29.Knudsen L. B., Kiel D., Teng M., Behrens C., Bhumralkar D., Kodra J. T., Holst J. J., Jeppesen C. B., Johnson M. D., de Jong J. C., Jorgensen A. S., Kercher T., Kostrowicki J., Madsen P., Olesen P. H., Petersen J. S., Poulsen F., Sidelmann U. G., Sturis J., Truesdale L., May J., Lau J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 937–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen D., Liao J., Li N., Zhou C., Liu Q., Wang G., Zhang R., Zhang S., Lin L., Chen K., Xie X., Nan F., Young A. A., Wang M. W. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 943–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doods H., Hallermayer G., Wu D., Entzeroth M., Rudolf K., Engel W., Eberlein W. (2000) Br. J. Pharmacol. 129, 420–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz T. W., Frimurer T. M., Holst B., Rosenkilde M. M., Elling C. E. (2006) Annu. Rev. Pharmacol. Toxicol. 46, 481–519 [DOI] [PubMed] [Google Scholar]
- 33.Brain S. D., Poyner D. R., Hill R. G. (2002) Trends Pharmacol. Sci. 23, 51–53 [DOI] [PubMed] [Google Scholar]
- 34.Mallee J. J., Salvatore C. A., LeBourdelles B., Oliver K. R., Longmore J., Koblan K. S., Kane S. A. (2002) J. Biol. Chem. 277, 14294–14298 [DOI] [PubMed] [Google Scholar]