Abstract
PtdIns(3,5)P2 is vital in differentiation and development of multicellular organisms because the knockout of the PtdIns(3,5)P2-synthesizing enzyme PIKfyve or its associated regulator, ArPIKfyve, is lethal. In previous work with endogenous proteins we identified that Sac3, a phosphatase that turns over PtdIns(3,5)P2, associates with the PIKfyve-ArPIKfyve biosynthetic complex. However, whether the three proteins suffice for the organization/maintenance of this complex (referred to as PAS complex), how they interact with each other and what the functional relevance of this ternary association would be, remained unresolved. Using coimmunoprecipitation analyses in transfected mammalian cells with increased or decreased levels of the three proteins, singly or in double vs. triple combinations, we report here that the triad is sufficient to form and maintain the PAS complex. ArPIKfyve is the principal organizer interacting with both Sac3 and PIKfyve, whereas Sac3 is permissive for maximal PIKfyve-ArPIKfyve association in the PAS complex. We further identified that ArPIKfyve scaffolds the PAS complex through homomeric interactions, mediated via its conserved C-terminal domain. Introduction of the C-terminal peptide fragment of the ArPIKfyve-ArPIKfyve contact sites effectively disassembled the PAS complex and reduced the in vitro PIKfyve lipid kinase activity. Exploring insulin-regulated GLUT4 translocation in 3T3L1 adipocytes as a functional readout, a process that is positively regulated by PIKfyve activity and ArPIKfyve levels, we determined that ectopic expression of the ArPIKfyve C-terminal peptide inhibits GLUT4 surface accumulation. Our data indicate that the PAS complex is organized to provide optimal PIKfyve functionality and is maintained via ArPIKfyve homomeric and heteromeric interactions.
Keywords: PtdIns(3,5)P2; ArPIKfyve; PIKfyve; Sac3; Macromolecular interactions; GLUT4 translocation; PAS complex
Introduction
The phosphorylated derivatives of phosphatidylinositol (PtdIns), called collectively PIs, are eukaryotic cell membrane phospholipids, whose inositol head group is phosphorylated at positions D3, D4 or D5, singly or in all combinations, to yield seven PI metabolites1–3. Changes in the PI phosphorylation state, controlled by coordinated action of position-specific kinases and phosphatases, result in dynamic remodeling of cellular membranes. This ability to undergo acute and reversible phosphorylation makes the phosphoinositides versatile and indispensable membrane-anchored signaling molecules that, by recruiting distinct effector proteins onto the cytosol-exposed hydrophilic inositol head group, orchestrate diverse and essential cellular processes, including intracellular membrane trafficking and signaling4–9. Accumulated evidence indicates that PtdIns(3,5)P2, a low-abundance PI comprising as little as 0.8% of total PIs, mediates essential aspects of endosome plasticity. In mammalian cells, its function is required in coordinating fusion and fission events in the endosomal system10,11. Consistent with this role, perturbations in PtdIns(3,5)P2 production disrupt both the constitutive and regulated trafficking pathways that emanate from or traverse early endosomes12–16. In cultured mammalian cells of epithelial origin, defective PtdIns(3,5)P2 synthesis, achieved by several independent maneuvers, is phenotypically manifested by a progressive development of intracellular vacuoles in line with the essential role of PtdIns(3,5)P2 in maintaining proper endomembrane homeostasis15–17.
The enzymes and regulators involved in PtdIns(3,5)P2 synthesis and turnover are all large evolutionarily conserved proteins, products of single copy genes from yeast to humans8. PtdIns(3,5)P2 is synthesized from PtdIns(3)P through the action of mammalian PIKfyve or the yeast counterpart Fab1, both sole enzymes for PtdIns(3,5)P2 synthesis8,18,19. PIKfyve and Fab1, in turn, are activated by the orthologous proteins, mammalian ArPIKfyve20,21 and yeast Vac1422,23, to upregulate intracellular PtdIns(3,5)P2 production. PtdIns(3,5)P2 turnover is catalyzed, at least in part, by mammalian Sac310 or its yeast counterpart Fig424,25. Proper PtdIns(3,5)P2 synthesis is apparently essential for life as evidenced by the embryonic lethality of Drosophila melanogaster or Caenorhabditis elegans PIKfyve-null mutants26,27 and by the early postnatal death of mouse models with knockout of ArPIKfyve28.
Consistent with PtdIns(3,5)P2’s vital role in differentiation and development of multicellular organisms, the complex intracellular regulation of PtdIns(3,5)P2 homeostasis is a matter of intensive investigation. Several regulatory links have already emerged. Thus, PIKfyve displays a FYVE domain for PtdIns(3)P binding, which will place the kinase in a surrounding of high local PtdIns(3)P substrate concentration to efficiently increase the rate of PtdIns(3,5)P2 synthesis from this scarce substrate, when required29. Next, to secure optimal PtdIns(3,5)P2 production, PIKfyve physically interacts with its activator ArPIKfyve, as it has been recently seen in mammalian cells20. Furthermore, ArPIKfyve appears to also be engaged in the control of PtdIns(3,5)P2 turnover, as evidenced by the physical interactions of ArPIKfyve with Sac3, or Vac14 with Fig4, documented in mammalian and yeast cells, respectively10,22,24. Finally, coimmunoprecipitation analyses in several native mammalian cell types reveal unexpectedly, a physical association among PIKfyve, ArPIKfyve and Sac3 proteins, indicative of a tight coupling between PtdIns(3,5)P2 synthesis and turnover10. However, since triple interactions were identified with the endogenous proteins, important questions as to whether the three proteins suffice to form a single complex, and if yes, how and why they interact with each other remained to be answered. Using coimmunoprecipitation analysis in mammalian cells transfected to increase protein expression or deplete the endogenous proteins, in this study we report that the three proteins are sufficient to form the PAS (for PIKfyve-ArPIkfyve-Sac3) complex, in which the PIKfyve enzyme interacts with the ArPIKfyve-Sac3 core through an association with ArPIKfyve. We further report that ArPIKfyve homomeric interactions mediated through its C-terminal conserved domain, scaffold the PAS triad. Introduction of the C-terminal peptide fragment of the ArPIKfyve-ArPIKfyve contact sites effectively disrupts the PAS complex and reduces the PIKfyve lipid kinase activity. Exploring the insulin-regulated GLUT4 translocation in 3T3L1 adipocytes as a functional readout, we further report that ectopic expression of the ArPIKfyve C-terminal peptide inhibits GLUT4 surface accumulation. We conclude that the PAS complex is essential for optimal PIKfyve enzymatic activity and functionality.
RESULTS
Ectopically expressed PIKfyve, ArPIKfyve and Sac3 are sufficient to form the PAS complex
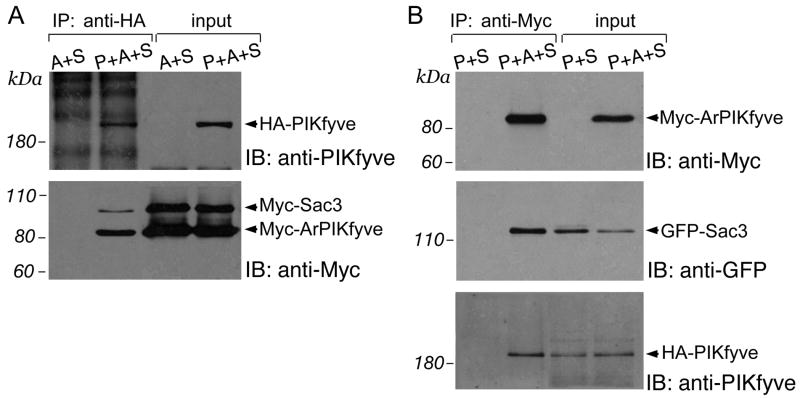
To begin mechanistically characterize the physical interactions among the PIKfyve, ArPIKfyve and Sac3, we sought to determine whether the ternary heteromeric association seen with the endogenous proteins10 could be reproduced with the ectopically expressed proteins by a similar immunoprecipitation analysis. A failure for such an interaction would indicate that the three overexpressed proteins are not sufficient to organize a ternary complex and that the triple interaction seen with the endogenous PIKfyve, ArPIKfyve and Sac3 is maintained by unknown endogenous protein(s). Examination of fresh RIPA lysates from COS cells triply transfected with HA-, Myc- and/or GFP-forms of the three proteins in various combinations revealed unequivocal copurification of the other two proteins with antibodies to either the HA-, Myc- or GFP-tag of the third protein. As documented in Fig. 1A, Myc-Sac3 and Myc-ArPIKfyve were coimmunoprecipitated with anti-HA-PIKfyve antibodies from the HA-PIKfyve/Myc-Sac3/Myc-ArPIKfyve triple transfection. Likewise, GFP-Sac3 and HA-PIKfyve were coimmunoprecipitated with anti-Myc-ArPIKfyve from the HA-PIKfyve/GFP-Sac3/Myc-ArPIKfyve triple transfection (Fig. 1B). Consistently, Myc-ArPIKfyve and Myc-PIKfyve were coimmunoprecipitated with anti-HA-Sac3 from the HA-Sac3/Myc-PIKfyve/Myc-ArPIKfyve triple transfection (Fig. 1C). Importantly, no coimmunoprecipitated bands were observed if the protein whose epitope-tag was targeted in the immunoprecipitation was left out from the transfection (Fig. 1A,B and C), demonstrating the specificity in the protein copurification. Likewise, there were no precipitated/coprecipitated bands corresponding to the ectopically expressed proteins with the control rabbit or mouse nonimmune IgG (not shown, and see further). Together, these data demonstrate that, just like the endogenous proteins in native cells, ectopically expressed PIKfyve, ArPIKfyve and Sac3 physically associate in triply transfected cells. Because it is highly unlikely that an unknown endogenous protein could be expressed at levels sufficient to organize the triad of overexpressed proteins, the data suggest that PIKfyve, ArPIKfyve and Sac3 are sufficient for the PAS complex formation.
FIGURE 1. Ternary heteromeric associations among overexpressed PIKfyve, ArPIKfyve and Sac3 in COS cells.
(A) Cells cotransfected with HA-PIKfyve + Myc-ArPIKfyve + Myc-Sac3 (lane 2) or with Myc-ArPIKfyve + Myc-Sac3 (lane 1) were immunoprecipiated with anti-HA antibodies. (B) Cells cotransfected with HA-PIKfyve + Myc-ArPIKfyve + eGFP-Sac3 (lane 2) or with HA-PIKfyve + eGFP-Sac3 (lane 1) were immunoprecipiated with anti-Myc antibody. (C) Cells cotransfected with Myc-PIKfyve + Myc-ArPIKfyve + HA-Sac3 (lane 2) or with Myc-ArPIKfyve + Myc-Sac3 (lane 1) were immunoprecipiated with anti-HA antibodies. (A-C) Fresh RIPA+ lysates (no freezing) were subjected to immunoprecipitations (IP). Following washes in the same buffer, the immunoprecipitates along with the inputs were analyzed by SDS-PAGE and immunoblotting with the indicated antibodies with a stripping step in between. Shown are chemiluminescence detections of a representative transfection experiment for each of tpanels A, B and C, out of four to nine with similar results. P, PIKfyve; A, ArPIKfyve; S, Sac3; IB, immunoblotting.
Binary associations of ArPIKfyve with Sac, and ArPIKfyve with PIKfyve
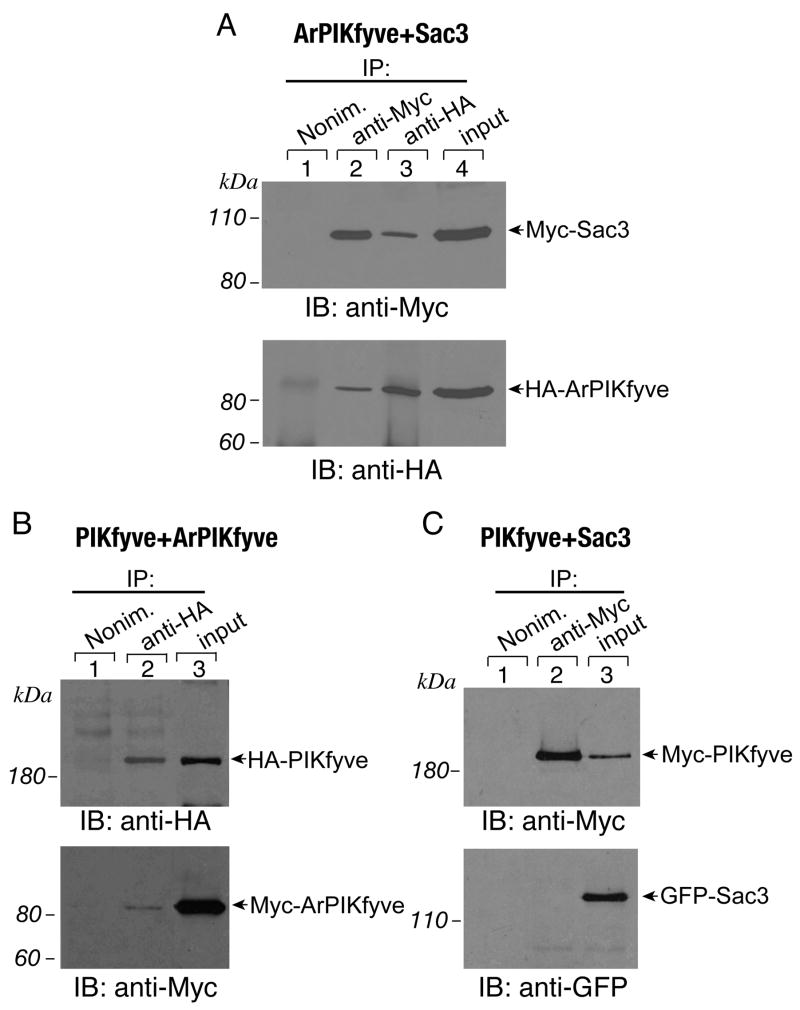
To gather further details about the associations among the three proteins in the PAS complex, we coexpressed the three proteins in a pair-wise manner, reasoning that, in case of heterodimeric interactions within a given pair, the pair-proteins should copurify with each other. COS cells were doubly transfected with the wild type constructs of PIKfyve and ArPIKfyve, ArPIKfyve and Sac3 or PIKfyve and Sac3, epitope tagged with either HA, Myc or GFP in various combinations, and then subjected to immunoprecipitation with antibodies against the epitope-tag. We detected a strong interaction between ectopically expressed ArPIKfyve and Sac3 as observed previously10, regardless of whether fresh or frozen RIPA lysates were used (Fig. 2A and not shown). There was also an interaction between ectopically expressed HA-PIKfyve and Myc-ArPIKfyve (Fig. 2B) or Myc-PIKfyve and HA-ArPIKfyve (not shown). However, unlike with expressed ArPIKfyve - Sac3, the interaction of ArPIKfyve with PIKfyve was less efficient as judged by its sensitivity to freezing or by the very minor fraction of Myc-ArPIKfyve coimmunoprecipitated with anti-HA-PIKfyve antibodies and vice versa, HA-PIKfyve coimmunoprecipitated with Myc-ArPIKfyve. Noteworthy, under the pair-wise expression, endogenous levels of the third protein were undetectable in the immunoprecipitates as revealed by immunoblotting with antibodies for the native proteins (data not shown). These data indicate that the observed binding between overexpressed ArPIKfyve and Sac3 or overexpressed ArPIKfyve and PIKfyve is not aided by the presence of the reciprocal endogenous protein and are compatible with a conclusion for direct binary interactions. By contrast, the two enzymes did not interact under the pair-wise analysis. Examination of the ectopically expressed PIKfyve-Sac3 protein pair failed to detect coimmunopurified Sac3 with PIKfyve or, vice versa, coimmunopurified PIKfyve with Sac3, using the HA-, Myc- or GFP-forms of the two proteins and the corresponding epitope-tag antibodies (Fig. 2C and not shown). The observation for undetectable PIKfyve - Sac3 association under pair-wise expression, yet a prominent PIKfyve - Sac3 interaction if ArPIKfyve is overexpressed (see Fig. 1) clearly indicates that the three proteins constitute a single complex, rather than three distinct heterodimers. Collectively, these data are consistent with the conclusion that the PAS complex incorporates heteromeric associations between ArPIKfyve and Sac3 or ArPIKfyve and PIKfyve, whereas the interaction of the two enzymes, PIKfyve and Sac3, utilizes an indirect binding mechanism.
FIGURE 2. Binary heteromeric associations of overexpressed ArPIKfyve with either Sac3 or PIKfyve, but not of PIKfyve with Sac3.
COS cells were doubly transfected with HA-ArPIKfyve + Myc-Sac3 (A), with HA-PIKfyve + Myc-ArPIKfyve (B) or with Myc-PIKfyve + eGFP-Sac3 (C). Fresh RIPA+ lysates were subjected to immunoprecipitations (IP) with the indicated antibodies or nonimmune rabbit IgG. Washed immunoprecipitates were analyzed along with inputs by SDS-PAGE and immunoblotting with the indicated antibodies, with a stripping step in between. Shown are chemiluminescence detections from representative transfection experiments (for each of the panels A, B and C) out of three to nine experiments with similar results.
Both ArPIKfyve and Sac3 are required for efficient heteromeric associations with PIKfyve
Based on data presented in Figs. 1 and 2, showing that ectopically expressed PIKfyve and Sac3 associate only in the presence of overexpressed ArPIKfyve, we considered ArPIKfyve as a candidate intermediary protein, scaffolding the interaction between the two enzymes in the PAS complex. This hypothesis was further supported by our data in several mammalian cell types where endogenous ArPIKfyve was knocked down by siRNAs. As illustrated in Fig. 3A for HEK293 cells, depletion of ArPIKfyve protein (by ~85%20) resulted in markedly lower levels (3 to 4-fold) of both PIKfyve and Sac3, coimmunoprecipitated with anti-ArPIKfyve antibodies, consistent with the key role of ArPIKfyve in organizing the endogenous PAS complex. Intriguingly, however, Sac3 depletion in these cells (by ~68%10) also elicited a decrease of 38±4% (p<0.001; n=3) in endogenous PIKfyve levels that coimmunoprecipitated with ArPIKfyve yet immunoprecipitated ArPIKfyve levels were similar to the control (Fig. 3A; upper panel, lane 3 vs. 1). These data suggest that whereas Sac3 does not directly interact with PIKfyve, as seen in Fig. 2C, it is permissive for an efficient PIKfyve association with ArPIKfyve in the PAS triad. Furthermore, the siRNAs-mediated PIKfyve depletion (by ~90%10) resulted in a marked loss of PIKfyve from the PAS complex yet no significant changes in the ArPIKfyve-Sac3 heteromeric association were noted, as evidenced by the control levels of Sac3 coprecipitated with anti-ArPIKfyve antibodies (Fig. 3A). These data thus demonstrate that ArPIKfyve-Sac3 heteromeric interactions occur in the absence of PIKfyve, and suggest that most likely the ArPIKfyve-Sac3 core is incorporated as a heterodimer in the PAS complex.
FIGURE 3. Sac3 is permissive for PIKfyve-ArPIKfyve heteromeric associations in the PAS complex.
(A) HEK293 cells were transfected by electroporation with the specific siRNAs to knockdown the individual endogenous proteins as described in Experimental Procedures. (B) COS cells were doubly or triply transfected with Myc-ArPIKfyve + Myc-Sac3 (lanes 1 and 4), with HA-PIKfyve + Myc-ArPIKfyve + Myc-Sac3 (lanes 2 and 5) or with HA-PIKfyve + Myc-ArPIKfyve (lanes 3 and 6). (A and B) Fresh RIPA+ lysates were immunoprecipitated (IP) with the indicated antibodies. Washed immunoprecipitates plus the indicated inputs were analyzed by SDS-PAGE and immunoblotting with the indicated antibodies, with a stripping step in between. Shown are chemiluminescence detections of a representative experiment out of four (A) or out of six independent transfection experiments (B) with similar results.
The conclusion that Sac3 facilitates the association between ArPIKfyve and PIKfyve in the PAS triad was further supported by data from our immunoprecipitation analyses in doubly vs. triply transfected COS cells. Importantly, we consistently observed greater amounts of Myc-ArPIKfyve coimmunoprecipitated with HA-PIKfyve if Sac3 was coexpressed and pulled down. Thus, quantitations of immunoblots from double (HA-PIKfyve/Myc-ArPIKfyve) vs. triple transfections (HA-PIKfyve/Myc-ArPIKfyve/Myc-Sac3) illustrated in Fig. 3B revealed about 3-fold greater Myc-ArPIKfyve amounts coimmunoprecipitated with anti-HA-PIKfyve antibodies under Myc-Sac3 coexpression/coimmunoprecipitation. Of note, this increase was manifested under even lower expression levels of Myc-ArPIKfyve in the triple vs. double transfections (Fig. 3B, input lanes P+A+S vs. P+A). Thus, these data are consistent with the idea that PIKfyve and ArPIKfyve interact efficiently with each other only in the presence of Sac3.
ArPIKfyve is involved in homomeric interactions
Usage of identical tags and, thus, the same antibodies for immunodetecting the coimmunoprecipitation proteins (as shown in Fig. 1A and C, and Fig. 3B) provides a means to reliably quantify the copurified protein amounts, because the diverse antibody efficiency is not an issue. These quantitative analyses revealed consistently that more Myc-ArPIKfyve than Myc-Sac3 was coimmunoprecipitated with overexpressed PIKfyve (see Figs. 1A and 3B). Likewise, there was more Myc-ArPIKfyve than Myc-PIKfyve coimmunoprecipitated with overexpressed Sac3 (see Fig. 1C). This important observation suggested to us that in the PAS complex, ArPIKfyve might be engaged in homomeric interactions.
To directly test whether ArPIKfyve could homomerize, we performed coimmunoprecipitation analyses in COS cells transfected with ArPIKfyve constructs tagged with two different epitopes. We selected GFP-HA-ArPIKfyve and Myc-ArPIKfyve because the electrophoretic mobility of expressed proteins differs substantially, 116 kDa vs. 84 kDa, respectively, thus facilitating reliable detection upon immunoblotting. As illustrated in Fig. 4A, Myc-ArPIKfyve coimmunoprecipitated with anti-GFP-HA-ArPIKfyve (upper panel, lane 1) and, vice versa, GFP-HA-ArPIKfyve coimmunoprecipitated with anti-Myc-ArPIKfyve (lower panel, lane 5) in the doubly transfected cells. The absence of bands for immunoreactive GFP-HA-ArPIKfyve and Myc-ArPIKfyve in anti-Myc and anti-HA immunoprecipitates, respectively, in the singly transfected cells (Fig. 4A, lanes 3 and 8) further evidences the specificity of our detection. Noteworthy, as emphasized above, neither endogenous Sac3 nor endogenous PIKfyve were detectable by immunoblotting with anti-Sac3 or anti-PIKfyve antibodies, indicating homomeric interactions of the ectopically expressed ArPIKfyve forms.
FIGURE 4. ArPIKfyve is engaged in homomeric interactions.
(A) COS7 cells were transfected with Myc-ArPIKfyve + eGFP-HA-ArPIKfyve (lanes 1 and 5), Myc-ArPIKfyve (lanes 3 and 7), eGFP-HA-ArPIKfyve (lanes 4 and 8), or the two empty vectors (lanes 2 and 6) as indicated. Presence of a specific Myc-ArPIKfyve band in anti-HA IPs (lane 1, upper panel) and a specific eGFP-HA-PIKfyve band in anti-Myc IPs (lane 5, lower panel) indicate that ArPIKfyve homomerizes. (B) COS7 cells, transfected with Myc-ArPIKfyve were cross-linked in vivo with a methanol-free formaldehyde as described in Experimental Procedures. (A and B) Fresh RIPA+ lysates were immunoprecipitated as indicated. Washed immunoprecipitates along with the inputs were analyzed by SDS-PAGE and immunoblotting with the indicated antibodies, with a striping step in-between. Shown are chemiluminescence detections of a representative experiment out of five (A) or three (B) independent experiments with similar results. Arrowheads in B depict the 170- and 250 kDa crosslinked forms, corresponding to homodimers and homotrimers, respectively.
To confirm the ArPIKfyve homomeric interactions by another approach, we performed in vivo crosslinking by a short exposure of the Myc-ArPIKfyve-expressing COS cells to a methanol-free formaldehyde reagent. Anti-Myc immunoprecipitates were then analyzed by immunoblotting. As demonstrated in Fig. 4B, we captured interactions corresponding to 170 kDa and ~ 250 kDa crosslinked bands that were immunoreactive with either anti-Myc or anti-ArPIKfyve antibodies. Noteworthy, the crosslinked bands did not display any immunoreactive signals for Sac3 or PIKfyve as revealed by reprobing with antibodies to the endogenous proteins (not shown). This result further substantiates an origin of the crosslinked species from homomeric interactions of overexpressed Myc-ArPIKfyve. Given an ArPIKfyve molecular mass of 82 kDa20, the 170- and 250- kDa crosslinked forms might represent ArPIKfyve homodimers and homotrimers, respectively. Together, these data indicate that ArPIKfyve is engaged in homomeric interactions that are essential for the association of the three proteins in the PAS complex.
The conserved C-terminal domain mediates ArPIKfyve homomeric interactions
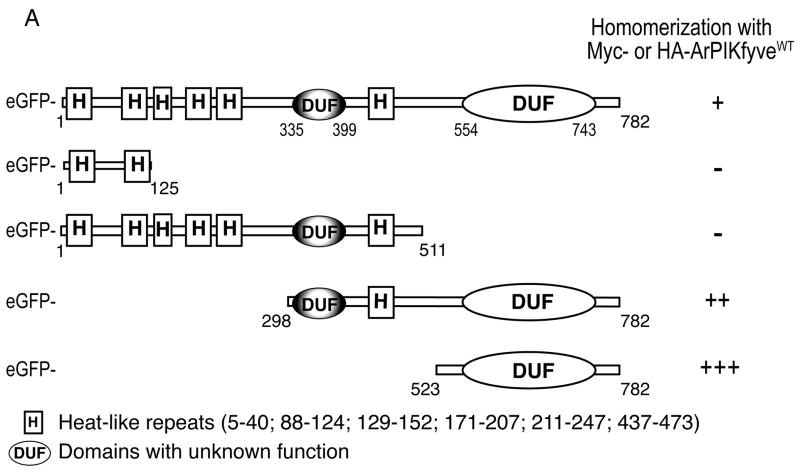
ArPIKfyve and its yeast ortholog Vac14 contain highly conserved domains at the N-terminal, the middle and the C-terminal parts of the molecules, with identity/similarity of 41/59%, 29/56% and 32/57%, respectively. Of significant interest are the N-terminally- and centrally-positioned conserved HEAT-like repeats (Fig. 5A), which are implicated in protein-protein interactions in other systems30,31. Therefore, we first considered the ArPIKfyve HEAT-like repeats as candidate domains responsible for the homomeric associations. N-terminal peptide fragments (residues 1–125 and 1–511) cloned in pEGFP vectors were tested for their ability to associate with ectopically expressed Myc-ArPIKfyveWT by coimmunoprecipitation analyses in RIPA lysates from transfected COS cells. As illustrated in Fig. 5B, the N-terminal peptide fragments constituting the first two or all six HEAT-like repeats did not associate with Myc-ArPIKyveWT. In contrast, the C-terminal peptide fragments (residues 298–782 and 523–782), like GFP-ArPIKfyveWT, displayed homomeric interactions with Myc-ArPIKyveWT (Fig. 5B). The conserved C-terminal peptide (residues 523–782; referred to hereafter as ArPIKfyveCt) that was devoid of HEAT-like sequences, but encompasses a conserved domain with unknown function, interacted with Myc-ArPIKfyveWT most efficiently, as quantified from the band intensity of the coimmunoprecipitated protein fragment vs. total overexpressed amounts (Fig. 5A, B and not shown). To confirm the interaction of ArPIKfyveCt with ArPIKfyveWT we also used an alternative GST pull-down assay. Immunoblotting illustrated in Fig. 5C revealed that a substantial fraction of Myc-ArPIKfyveWT binds specifically to GST-ArPIKfyveCt, but not to GST alone, following incubation of the purified GST proteins with RIPA lysates of Myc-ArPIKfyveWT-expressing COS cells. Together, these results show that the conserved ArPIKfyveCt domain is sufficient to mediate the homomeric interactions of ArPIKfyve.
FIGURE 5. Conserved C-terminal domain mediates ArPIKfyve homomeric interactions.
(A) Presented is a schematic diagram of ArPIKfyveWT and truncated mutants. Sequence analysis is performed by Pfam (http://pfam.wustl.edu and http://pfam.janella.org) at a medium stringency. The potency of the eGFP-based constructs to associate with HA- or Myc-ArPIKfyve is quantified based on data similar to those presented in B. (B) Fresh RIPA+ lysates derived from COS7 cells, transfected with Myc-ArPIKfyveWT alone or together with the indicated eGFP-based ArPIKfyve constructs were subjected to immunoprecipitation analysis with anti-Myc antibody, followed by SDS-PAGE and immunoblotting. Shown are chemiluminescence detections of a representative experiment out of three to five individual transfections for each truncated construct with similar results. Levels of the coimmunoprecipitated eGFP-based proteins are quantified (see panel A) relatively to their total expression amounts; (C) GST-ArPIKfyveCt (lanes 1 and 2) or GST alone (lanes 3 and 4), purified and immobilized (each at 1 μg) on GSH-agarose beads as described in Experimental Procedures, were incubated (18 h at 4°C) with RIPA+ lysates derived from Myc-ArPIKfyveWT (lane 5) or mock-transfected COS7 cells (not shown). Beads were washed in RIPA+ buffer and captured proteins were analyzed by SDS-PAGE and immunoblotting. Shown is a chemiluminescence detection of a representative experiment out of three pull-down experiments with similar results.
The ArPIKfyveCt peptide disassembles the PAS complex and reduces PIKfyve activity
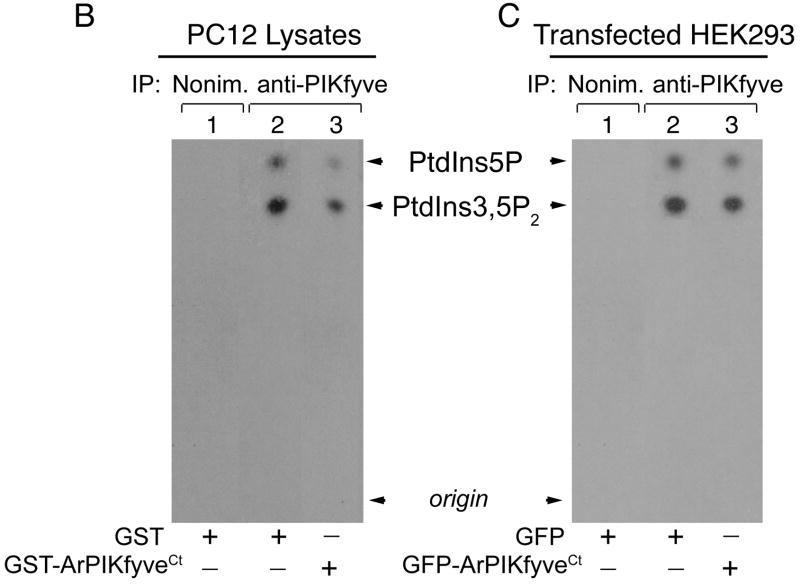
We suggested that ArPIKfyve homomeric interactions mediated via the ArPIKfyve C-terminal domain scaffold the PAS complex. We reasoned that if this is correct then the ArPIKfyveCt peptide fragment will inhibit the endogenous ArPIKfyve-ArPIKfyve interactions and disassemble the PAS complex due to the absence of the remaining 70% of the molecule, presumably interacting with the other two proteins. To test this, endogenous PAS complexes were immunopurified from PC12 cell lysates with anti-PIKfyve or anti-Sac3 antibodies in the presence of either GST-ArPIKfyveCt or GST alone (each at 36 nM) and then analyzed by western blotting (Fig. 6A). Quantitation of three separate experiments revealed that GST-ArPIKfyveCt induced a marked diminution in levels of ArPIKfyve (by 69 ± 7.4%; p<0.01; n=3) and PIKfyve (by 72 ± 8%; p<0.01; n=3) coimmunoprecipitated with anti-Sac3 as compared to GST alone, whereas immunoprecipitated Sac3 remained identical (Fig. 6A). Likewise, the presence of GST-ArPIKfyveCt substantially decreased the amounts of ArPIKfyve (by 57 ± 5.6%; p<0.01; n=3) and Sac3 (by 58 ± 6.6%; p<0.01; n=3) coimmunoprecipitated with anti-PIKfyve antibodies vs. GST alone yet immunoprecipitated PIKfyve remained the same (Fig. 6A). Together, these data demonstrate that a peptide fragment of the ArPIKfyve-ArPIKfyve contact sites disassembles the PAS complex consistent with the notion that the complex is maintained via ArPIKfyve homomeric interactions mediated by the C-terminal conserved domain of ArPIKfyve.
FIGURE 6. ArPIKfyveCt disrupts the PAS complex and reduces PIKfyve activity.
(A) Equal amounts of fresh RIPA+ lysates of PC12 cells were preincubated (3 h, 4°C) with purified GST-ArPIKfyveCt or GST proteins (each at 36 nM) and then, subjected to immunoprecipitations with the indicated antibodies. Following washes in RIPA+ buffer, the immunoprecipitates were analyzed along with the indicated GST-ArPIKfyveCt and Myc-ArPIKfyve standards by SDS-PAGE. Immunoblotting was performed with a stripping step between the antibodies. Note that the Sac3 band is not fully stripped and is still seen in the ArPIKfyve blot. Shown are chemiluminescence detections of a representative experiment out of three separate experiments with similar results. The broad band of ~90 kDa seen below Sac3 and above ArPIKfyve in the anti-PIKfyve IPs (indicted in anti-Sac and in anti-ArPIKfyve blots by arrows) is unspecific, originating from the anti-PIKfyve antibodies. (B) Equal protein amounts of PC12 cell-RIPA+ lysates were preincubated with purified GST-ArPIKfyveCt or GST proteins as indicted in A, and then subjected to immunoprecipitation with anti-PIKfyve antibodies or nonimmune rabbit IgG. Immunoprecipitates immobilized on protein A-Sepharose beads were analyzed by an in vitro lipid kinase assay as described in Experimental Procedures. Shown is an autoradiogram of the TLC-resolved lipids from a representative experiment out of three separate experiments. PIKfyve activity decreased to 42 ± 6% (p<0.01, n=3) of the control, due to GST-ArPIKfyveCt. (C) Equal protein amounts of fresh RIPA+ lysates, derived from HEK293 cells transfected with eGFP-ArPIKfyveCt or the empty vector, were immunoprecipitated as indicated in B. Immunoprecipitates immobilized on protein A-Sepharose beads were subjected to lipid kinase assay as described in Experimental Procedures. Shown is an autoradiogram of the TLC-resolved lipids from a representative experiments out of four separate experiments. PIKfyve activity decreased to 68 ± 6% (p<0.01%; n=4) of the control, due to GFP-ArPIKfyveCt.
Decrease of ArPIKfyve from the heteromeric association with PIKfyve achieved by siRNA-mediated protein knockdown or by protein stripping with low concentrations of ionic detergents markedly down-regulates the PIKfyve lipid kinase activity14,20. To examine whether ArPIKfyve homomeric interactions and, hence, the PAS complex integrity are relevant for the PIKfyve lipid kinase activity, we examined the in vitro PIKfyve activity in lysates of PC12 cells incubated with GST-ArPIKfyveCt or GST alone. We observed a decrease to 42 ± 6% (p<0.01; n=3) in PIKfyve lipid kinase activity due to the presence of GST-ArPIKfyveCt (Fig. 6B). We next examined whether such a decrease would be reproduced in vivo in cells expressing ArPIKfyveCt. For this reason, endogenous PIKfyve, immunopurified from HEK293 cells transfected with GFP-ArPIKfyveCt or GFP-ArPIKfyvewt was tested for its potency to function as a lipid kinase in vitro. We observed that, while expression of GFP-ArPIKfyveWT considerably up-regulated the endogenous PIKfyve lipid kinase activity, consistent with our previous observations20 (also this study, not shown), that of GFP-ArPIKfyveCt was inhibitory (Fig. 6C). Quantitation of four separate experiments revealed a decrease to 68 ± 6% (p<0.01; n=4) of the PIKfyve activity due to expressed GFP-ArPIKfyveCt. Given the relatively low transfection efficiency of the GFP-ArPIKfyveCt construct (~20% of total cells), this inhibition is in fact quite substantial. Collectively, the observed ArPIKfyveCt-dependent reduction of the in vitro PIKfyve lipid kinase activity suggests that ArPIKfyve homomeric interaction and, hence, PAS complex integrity are essential for optimal PIKfyve function.
ArPIKfyveCt inhibits GLUT4 surface translocation
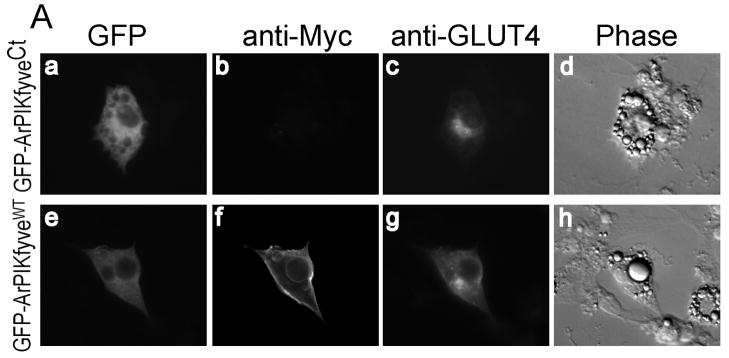
Our previous studies using siRNA-mediated protein knockdown have implicated both ArPIKfyve and PIKfyve as positive regulators in insulin-dependent activation of GLUT4 translocation and glucose transport14. To reveal whether the interaction of PIKfyve in the PAS complex is functionally relevant we tested the effect of ArPIKfyveCt on insulin responsiveness of GLUT4 translocation in 3T3L1 adipocytes. Cells were coelectroporated with eGFP-ArPIKfyveCt and GLUT4 engineered with the 7-Myc-epitope on the first exofacial loop, allowing the evaluation of the transporter cell surface translocation and insertion in non-permeabilized cells. We observed a marked decrease in cell surface Myc-GLUT4 by insulin due to expressed ArPIKfyveCt (Fig. 7A). Quantitation of the cells positive for exofacial Myc staining revealed that ~71% of control and ~84% of ArPIKfyveWT-expressing cells displayed a Myc-plasma membrane rim, while expression of ArPIKfyveCt resulted in only 38% of cells with exofacial Myc-staining (Fig. 7B). These results clearly indicate that PIKfyve’s functioning as a positive regulator of GLUT4 translocation in response to insulin requires not only ArPIKfyve, as found previously14, but also a proper assembly of PIKfyve within the PAS complex.
FIGURE 7. ArPIKfyveCt inhibits GLUT4 surface translocation by insulin.
3T3L1 adipocytes were cotransfected by electroporation with Myc7-GLUT4 + eGFP-ArPIKfyveCt or Myc7-GLUT4 + eGFP-ArPIKfyveWT cDNAs. After 24 h, serum-starved cells were stimulated with insulin or left untreated, then washed, fixed and processed for immunofluorescence microscopy with anti-Myc monoclonal antibody and Alexa568-conjugated anti-mouse IgG without permeabilization as described in Experimental Procedures. Cells were then permeabilized, incubated with the C-terminal GLUT4 antibodies (at a dilution detecting only overexpressed Myc7-GLUT4) followed by Alexa350-conjugated anti-rabbit IgG. (A) Typical images of insulin-stimulated 3T3L1 adipocytes depicting the GFP fluorescence of ArPIKfyve constructs (panels a and e), exofacial Myc (panels b and f), total Myc7-GLUT4 (panels c and g) and the phase contrast (panels d and h). (B) Quantitation of the cell-surface Myc fluorescence based on counting cells displaying a complete plasma membrane rim and presented as a percentage of the counted Myc7-GLUT4 expressing cells, with or without coexpression of the indicated ArPIKfyve constructs (~100 cells/condition) in three independent experiments (mean ± SEM *, different from the other two insulin-stimulated groups, p<0.01).
Discussion
Although the PIKfyve and Fab1 enzymes in mammals and yeast, respectively, have been known for a decade or more32,33, their regulation as well as that of the PtdIns(3,5)P2 product they synthesize is still elusive. Moreover, what we know thus far is associated with remarkable paradoxes. Thus, even though that PtdIns(3,5)P2 synthesis is markedly elevated, as in the case of the hyperosmotic stimulation in mouse 3T3L1 adipocytes, there is no measurable activation of PIKfyve enzymatic activity in vitro21. Furthermore, the regulatory proteins Vac14 or ArPIKfyve not only are required in basal and hyperosmotically activated PtdIns(3,5)P2 synthesis in yeast or mammalian cells, respectively20–23, but also facilitate the seemingly antagonistic process of PtdIns(3,5)P2 breakdown, as shown in yeast24,25. To further complicate matters, the acute hyperosmotic rise of PtdIns(3,5)P2 in yeast requires the PtdIns(3,5)P2-specific phosphatase Fig4, in addition to Fab1 and Vac1434,35. Next, deletion of Fig4 in yeast24,25,34,35 or the siRNA-mediated knockdown of the mammalian Fig4 counterpart, Sac310, exerts only a marginal increase, if any, in the PtdIns(3,5)P2 basal levels. Furthermore, the knockout of the Sac3 phosphatase, instead of up-regulating PtdIns(3,5)P2, is associated with decreased PtdIns(3,5)P2 basal levels in the mouse models36. Recent work in our lab shed some light on these perplexing yet concordant observations. Thus, we uncovered a physical association among the endogenous PIKfyve, ArPIKfyve and Sac3, suggesting that the activation of PtdIns(3,5)P2 synthesis and breakdown is coupled through complexation of the three proteins10. The aim of this study was to reveal first, how this protein triad is organized in a common complex and second, whether the integrity of the complex has any functional relevance. To this end, we have used coimmunoprecipitation analysis in cells transfected with cDNAs or siRNAs to increase or deplete, respectively, the intracellular levels of the three proteins. Comparison of the pair-wise vs. the triple protein overexpressions (Figs. 1 and 2) demonstrates that the three proteins indeed constitute a single complex, rather than three separate heterodimers, something what could have been inferred, at least theoretically, from our previous coimmunoprecipitation data in native cells10. Our results are compatible with the conclusion that in the PAS complex, ArPIKfyve interacts with both Sac3 and PIKfyve (Figs. 1, 2A and B), whereas the interaction between the two enzymes is indirect (Figs. 1 and 2C). Because ArPIKfyve interacts with Sac3 in the absence of PIKfyve (Fig. 3A) but PIKfyve requires Sac3 for an efficient association with ArPIKfyve (Figs. 3A and B), it is conceivable that the PAS complex incorporates the ArPIKfyve-Sac3 core formed independently of the PAS complex. We further determine that ArPIKfyve homomeric interactions, mediated by the conserved C-terminal domain, are necessary for the formation and maintenance of the PAS complex (Figs. 4–6A). Furthermore, we find the PAS complex’s integrity to be essential for PIKfyve functionality, because introduction of the ArPIKfyve C-terminal peptide fragment that disassembles the PAS complex (Fig. 6A) reduces the in vitro PIKfyve activity (Fig. 6B and C) and blunts insulin-stimulated GLUT4 translocation in 3T3L1 adipocytes (Fig. 7). These data provide new mechanistic and functional insights about the organization of the three proteins in the PAS complex and the physiological role of this complex for the catalytic activation of PIKfyve to support essential cellular functions.
One particularly important conclusion in our study is the maintenance of the PAS complex via ArPIKfyve homomerization. The ability of ArPIKfyve to be engaged in homomeric interactions is evidenced here not only by our data from coimmunoprecipitation and crosslinking experiments with overexpressed ArPIKfyve (Fig. 4) but also by data from yeast two-hybrid assays reported by Dove et al.22. Thus, the strongest interactions of the full-length Vac14 bait has been with Vac14 itself, suggesting the ability of ArPIKfyve/Vac14 to homomerize22. The C-terminal peptide fragment documented herein to mediate this ArPIKfyve-ArPIKfyve self-association (Fig. 5) is, except for the very last C-terminal 25 residues, highly homologous to the S. cerevisiae Vac14 sequence, as evidenced by ClustelW sequence analyses (data not shown). This indicates that a homologous region may mediate the Vac14 homomeric interactions observed by the two-hybrid assays. Intriguingly, of the three proteins we tested for homodimerization in a similar coimmunoprecipitation analysis with antibodies to the epitope-tags of overexpressed forms, only ArPIKfyveWT (Figs. 4 and 5), but not wild-type PIKfyve11 or Sac3WT (this study, data not shown) displays the ability for homomeric association. Based on crosslinking in cells overexpressing ArPIKfyveWT (Fig. 4B), the homomeric interactions are compatible with 2 and 3 monomers. Future studies should attempt to determine ArPIKfyve stoichiometry in the PAS complex and whether it is subjected to regulated fluctuations.
Of exceptional functional significance is our finding that an intact PAS complex is required for maximal PtdIns(3,5)P2 synthesis by PIKfyve. Thus, disruption of the ArPIKfyve-ArPIKfyve contact sites by the ArPIKfyve C-terminal peptide fragment not only disassembled the PAS complex (Fig. 6A) but also reduced the PIKfyve lipid kinase activity. Submaximal PIKfyve activity due to ArPIKfyveCt was documented both by in vitro addition to cell lysates (Fig. 6B) and by cellular ectopic expression (Fig. 6C), consistent with the conclusion that the ArPIKfyveCt-dependent PAS complex disassembly in intact cells is associated with reduced PIKfyve lipid kinase activity. The functional role of ArPIKfyveCt is further substantiated by data from the single-cell immunofluorescence microscopy assay of GLUT4 translocation in insulin stimulated 3T3L1 adipocytes (Fig. 7A), a process shown previously to require the PIKfyve activity and ArPIKfyve presence for an optimal performance12,14. The marked reduction of GLUT4 cell surface abundance by insulin due to expressed ArPIKfyveCt (Fig. 7B) is consistent with the conclusion that the PAS complex formation or maintenance is physiologically relevant. Together, our data support the notion that ArPIKfyve homomeric interactions mediated by ArPIKfyveCt scaffold the PAS complex whose formation is required for maximal PIKfyve activation.
Our studies also indicate that in addition to participating in the PAS complex, ArPIKfyve-Sac3 likely exists as an independent heteromeric complex. This is supported here by our observations with the ectopically expressed (Figs. 1 and 2A) or endogenously depleted PIKfyve protein (Fig. 3A), revealing insensitivity of the ArPIKfyve–Sac3 heteromeric associations to the presence or absence of PIKfyve. Furthermore, PIKfyve association within the PAS complex and ArPIKfyve-Sac3 heteromeric interactions are differentially sensitive to freeze-thaw. Thus, the integrity of the PAS complex, but not that of the ArPIKfyve-Sac3 interaction, with both the endogenous and overexpressed proteins was compromised by lysate freezing prior to coimmunoprecipitation analysis (Ref. 10 and this study). It is therefore conceivable, that, as with the yeast Vac14-Fig4 complex24, an ArPIKfyve-Sac3 heteromer outside the PAS complex controls PtdIns(3,5)P2 breakdown in mammalian cells and has to be addressed in future studies. Thus, it is highly likely that yet to-be-identified mechanisms regulate PIKfyve cycling on/off the PAS complex, which correlates with a switch in PtdIns(3,5)P2 synthesis/turnover, respectively. An apparent challenge for future studies is to unravel structure-activity details of these complexes and their regulation.
Based on data presented herein we propose a working model for the molecular organization of the PAS complex and its functioning in PtdIns(3,5)P2 synthesis, illustrated in Fig. 8. In this model, ArPIKfyve is necessary for activating PIKfyve, but Sac3 is permissive for an efficient ArPIKfyve association in the PAS complex. Therefore, Sac3 is a prerequisite for maximally activating PIKfyve, which conclusion explains the paradox with the Sac3 null mice synthesizing less PtdIns(3,5)P236. ArPIKfyve homomerization mediated by the C-terminal domain scaffolds the PAS complex. In addition to the PAS complex, we suggest that, as in yeast24,34,35, an ArPIKfyve-Sac3 independent heteromer executes PtdIns(3,5)P2 breakdown (Fig. 8). ArPIKfyve most likely stabilizes the phosphatase, as suggested by data for decreased Sac3/Fig4 levels in both mammalian and yeast cells under loss of ArPIKfyve/Vac1410,35. This conclusion may explain the similar neurodegenerative defects of ArPIKfyve and Sac3 null mice28,36 and awaits further verification in the ArPIKfyve null mice models. It remains to be seen whether PtdIns(3,5)P2 synthesis is under the control of a similar PAS complex in S. cerevisiae,* as is suggested here for mammalian cells. Likewise, whether Sac3-catalyzed PtdIns(3,5)P2 turnover is activated via association with ArPIKfyve, as proposed for budding yeast, remains to be addressed in mammalian cells.
FIGURE 8. Model for the molecular organization of the PAS complex and role in PtdIns(3)P-to-PtdIns(3,5)P2 synthesis.
Maximal PtdIns(3,5)P2 synthesis is achieved by PIKfyve assembly with ArPIKfyve and Sac3. ArPIKfyve is the principal organizer that is necessary but not sufficient for efficient PAS complex formation. The presence of Sac3 is permissive for ArPIKfyve full association with and activation of PIKfyve. ArPIKfyve homodimers or possibly, higher-order homomeric interactions, mediated by the conserved C-terminal domain, scaffold the PAS complex. Whether ArPIKfyve is required in Sac3-catalyzed PtdIns(3,5)P2 turnover, as seen in yeast, has to be validated for mammalian cells.
Materials and Methods
Constructs, siRNAs and antibodies
pCMV5-based cDNA constructs of mouse PIKfyve tagged with Myc- or HA-epitopes were described previously20,32. Full-length human ArPIKfyve in pEGFP-C2-HA and pCMV5-Myc, or the pGEX5X-3-hArPIKfyve(523–782) truncated mutant were described elsewhere20. The pEGFP-based truncated constructs of hArPIKfyve, namely, pEGFP-C2-HA-hArPIKfyve(1–125), pEGFP-C2-HA-hArPIKfyve(1–511), pEGFP-C2-hArPIKfyve(298–782) and pEGFP-C1-hArPIKfyve(523–782) were generated from the available pEGFP-C2-HA-hArPIKfyve construct, using convenient restriction enzymes and common subcloning techniques. pEGFP-C3-hSac3 and pEF-BOS-Myc-hSac3 vectors were described elsewhere10. pCMV5-HA-hSac3 was generated by releasing the XhoI-Sma1 fragment from pEGFP-C3-hSac3, which was subsequently ligated together with a double-stranded EcoR1-Xho1 flanked HA-epitope tag (AATTCATGTATCCATATGATGTTCCAGATTACGCTC) into EcoR1-Sma1 digest of pCMV5. All new constructs and peptide fragments were confirmed by restriction mapping and immunoblotting in transfected cells, respectively. pcDNA3.1-Myc7-GLUT4 construct, containing a 7-Myc epitope in the first exofacial loop to allow surface detection in nonpermeabilized cells37 was a gift by Dr. Kostya Kandror. Smart Pool™ siRNA duplexes targeting the human sequences of PIKfyve (M-005058-03), ArPIKfyve (M-015729-01) and Sac3 (M-019141-01) were designed, generated (Dharmacon) and characterized previously10,20. The typical protein silencing of these siRNAs in human HEK293, determined by immunoblotting vs. a control human cyclophilin B siRNA pool was ~90% for PIKfyve (10), ~85% for ArPIKfyve20, and ~68% for Sac310. Rabbit polyclonal antibodies against the endogenous PIKfyve (R-7069), ArPIKfyve (WS047) and Sac3 proteins were characterized previously10,20,38. They were used as protein A- and affinity-purified forms (PIKfyve and ArPIKfyve; Refs.10,20) or as a crude anti-serum (Sac3). Polyclonal anti-HA (R4289) and anti-GLUT4 C-terminal antibodies (R1288) (gifts by Dr. Mike Czech) were used as Protein A-purified IgG fractions. Anti-Myc monoclonal antibody was produced by 9E10.2 hybridoma cells (ATCC). Anti-GFP polyclonal antibodies (Ab290) were purchased from AbCam.
Cell cultures, transfections and crosslinking
HEK293, COS7 and PC12 cells were cultured under conditions described in our previous studies10,20. Culturing and differentiation of 3T3L1 fibroblasts to adipocyte phenotype was described elsewhere14,21. 3T3L1 adipocytes were transfected with the indicated cDNAs by electroporation. Twenty-four h post-transfection, cells were subjected to immunofluorescence microscopy analysis. HEK293 cells were transiently transfected with the human-specific siRNA duplexes (100 nM) by electroporation. Cell lysates, collected in RIPA+ buffer [50 mM Tris/HCl buffer, pH 8.0, containing 150 mM NaCl, 1% Nonidet P-40, 0.5% Na deoxycholate and 1X protease inhibitor cocktail (1 mM phenylmethylsulphonylfluoride, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin and 1 mM benzamidine)] were subjected to immunopecipitation 72–80 h post-transfection. HEK293 or COS7 cells were transfected with the indicated cDNAs by Lipofectamine 2000 (Invitrogen). Twenty-four h post-transfection, RIPA+ lysates were collected and subjected to immunoprecipitation. In some cases, lysates from the pCMV5-Myc-ArPIKfyve-transfected COS7 cells were collected subsequent to in-cell crosslinking carried out in the culture plates with 1% (w/v) methanol-free formaldehyde for 10 min at 25°C under constant rotation and quenched for 15 min with 100 mM glycine (pH 3.0; 10 mM final concentration). The methanol-free formaldehyde reagent was prepared fresh prior to each experiment from crystalline paraformaldehyde following described procedures39. Cells were washed several times in PBS before RIPA+ lysates were collected and subjected to immunoprecipitation.
Immunoprecipitation, Immunoblotting, and PIKfyve kinase activity
All immunoprecipitation analyses were performed in precleared (20,000 × g, 15 min, 4°C) cell lysates collected with RIPA+ buffer. Unless otherwise stated, cell lysates were immunoprecipitated immediately after harvesting without a freezing step, using antibodies indicated in the figure legends. Control immunoprecipitates with nonimmune rabbit or mouse IgG were run in parallel. Immunoprecipitations were carried out for 16 h at 4°C, with protein A-Sepharose CL-4B added in the final 1.5 h of incubation. Immunoprecipitates were washed five times with RIPA+ buffer and then processed by western blotting. Immunoblotting with the antibodies indicated in the figure legends was performed subsequent to protein separation by SDS-PAGE (typically on 6% gels) and electrotransfer onto nitrocellulose membranes as described previously10,20. A chemiluminescence kit (Pierce) was used to detect the horseradish-peroxidase-bound secondary antibodies. For the lipid kinase assay, washed PIKfyve immunoprecipitates were subjected to 15 min incubation at 37°C in an assay buffer (25 mM Tris, pH 7.4, 2.5 mM MgCl2 and 2.5 mM MnCl2) supplemented with 50 μM ATP, [γ–32P]ATP (12.5 μCi) and 100 μM PtdIns (from soybean, Avanti Polar Lipids Inc) as detailed elsewhere10,20,38. Lipids were extracted and analyzed by silica-gel TLC using an acidic solvent system (2M acetic acid/propanol, 1:2 v/v). Both the PtdIns(5)P and PtdIns(3,5)P2 products synthesized were monitored simultaneously as we detailed elsewhere20,38. Generated lipid products were detected by autoradiography.
Glucose transporter translocation and fluorescence microscopy – Myc7-GLUT4 translocation was evaluated by immunodetection with two antibodies, one to the exofacial Myc and another to the intracellular C-terminus, as used by others40. 3T3L1 adipocytes, coelectroporated with Myc7-GLUT4 and either GFP-ArPIKfyveCt or GFP-HA-ArPIKfyveWT plasmids (25 μg each) on day 5 of the differentiation program were seeded on 35-mm plates, as described previously12. Twenty-four h post-transfection, cells were serum-starved for 3 h in DMEM, then incubated in the presence or absence of insulin (100 nM; 20 min at 37°C), washed with PBS and fixed in freshly prepared methanol-free formaldehyde39 (2% in PBS) for 15 min. Myc-epitope detection (cell surface Myc7-GLUT4) was conducted in nonpermeabilized cells by staining with the anti-Myc-antibody followed by Alexa568-conjugated anti-mouse IgG (Invitrogen). Cells were then permeabilized with 0.5% Triton X-100 in PBS/1% FBS and incubated with the C-terminal GLUT4 antibodies, followed by Alexa350-conjugated goat anti-rabbit IgG (Invitrogen) (total Myc7-GLUT4). Cells were viewed in a Nikon Eclipse TE200 inverted fluorescence microscope (40x objective) using a Hoffman Modulation Contrast System (brightfield) and 3 standard fluorescence filter sets (i.e., green for GFP, red for anti-Myc/Alexa568, and UV for anti-GLUT4/Alexa350 signals. Images were captured with a SPOT RT slider charge-coupled device camera (Diagnostic Instruments, Sterling Heights, MI) and processed using SPOT and Adobe Photoshop 6.0 software. For quantitation, randomly selected GFP-/anti-GLUT4-positive adipocytes (at least 100 cells/condition) were evaluated for the presence of a complete Myc-plasma membrane rim on the red channel. Cells with an incomplete rim were considered negative. The percentage of positive cells was calculated.
GST-protein pull-down and in vitro displacement
The GST-ArPIKfyveCt-fusion peptide or GST alone were produced in transformed XA-90 E. coli strain as we described previously41. Briefly, bacteria were lysed with 1 mg/ml lysozyme in a buffer containing 50 mM Hepes, pH 7.4, 150 mM NaCl, 5 mM EDTA and 1 mM PMSF. The GST fusions were adsorbed (1 h, 4°C) on GSH-agarose beads (Sigma). Beads were washed (4 times in PBS supplemented with 1x protease inhibitor mixture and 2 times in RIPA+ buffer) and then incubated (18h; 4°C) with RIPA+ lysates of Myc-ArPIKfyve-transfected COS7 cells. Captured proteins were analyzed by immunoblotting. For the displacement experiments, the GST proteins were eluted from the beads and dialyzed against 50 mM Hepes, pH 7.4, prior to addition to fresh RIPA+ lysates of PC12 cells and analyses by immunoblotting or lipid kinase assays. The concentration and quality of the purified GST proteins, bound to or eluted from beads, were determined electrophoretically by the intensity of the Coomassie-stained protein bands versus bovine serum albumin standard (Pierce).
Others
Protein concentration was determined by bicinchoninic acid protein assay kit (Pierce). Protein or lipid levels were quantified from the intensity of the bands or spots with a laser scanner (Microteck) and UN-SCAN-IT software (Silk Scientific). Several films of different exposure times were quantified to assure the signals were within the linear range. Data are expressed as means ± SEM. Statistical analysis was performed by Student’s t test, with p<0.05 considered as significant.
Acknowledgments
We thank Dr. Ray Mattingly for insightful discussions, and Drs. Mike Czech and Kostya Kandror for the kind gifts of HA antibodies, GLUT4 antibodies and Myc7-GLUT4 cDNA. We thank Linda McCraw for the outstanding secretarial assistance. The senior author expresses her gratitude to Violeta Shisheva for her many years of support. This project was supported by National Institute of Health (DK58058) and American Diabetes Association Research grants (to AS).
Abbreviations
- PtdIns
phosphatidylinositol
- PI
phosphoinositides, PIKfyve, PhosphoInositide Kinase for position five containing a five domain
- ArPIKfyve
Associated regulator of PIKfyve
- GFP
green fluorescence protein
- eGFP
enhanced green fluorescence protein
- HA
hemagglutinin
- GST
glutathione S-transferase
- siRNA
short interfering RNA
- TLC
thin layer chromatography
- h
human
Footnotes
NOTE IN PROOF: The association of the yeast counterparts has just been confirmed (Botelho et al, 2008 Jul 23 [Epub ahead of print]).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemmon MA. Phosphoinositide recognition domains. Traffic. 2003;4:201–213. doi: 10.1034/j.1600-0854.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 2.Prestwich GD. Phosphoinositide signaling: from affinity probes to pharmaceutical targets. Chem Biol. 2004;11:619–637. doi: 10.1016/j.chembiol.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Shisheva A. Phosphoinositides in insulin action on GLUT4 dynamics: not just PtdIns(3,4,5)P3. Am J Physiol Endocrinol Metab. 2008:295. doi: 10.1152/ajpendo.90353.2008. [e-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth MG. Phosphoinositides in constitutive membrane traffic. Physiol Rev. 2004;84:699–730. doi: 10.1152/physrev.00033.2003. [DOI] [PubMed] [Google Scholar]
- 5.Balla T. Phosphoinositide-derived messengers in endocrine signaling. J Endocrinol. 2006;188:135–153. doi: 10.1677/joe.1.06595. [DOI] [PubMed] [Google Scholar]
- 6.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;1038:651–659. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 7.Shisheva A. Regulating GLUT4 vesicle dynamics by phosphoinositide kinases and phosphoinositide phosphatases. Front Biosci. 2003;8:s945–s967. doi: 10.2741/1101. [DOI] [PubMed] [Google Scholar]
- 8.Shisheva A. PIKfyve: partners, significance, debates and paradoxes. Cell Biol Int. 2008;32:541–604. doi: 10.1016/j.cellbi.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takenawa T, Itoh T. Membrane targeting and remodeling through phosphoinositide-binding domains. IUBMB Life. 2006;58:296–303. doi: 10.1080/15216540600732039. [DOI] [PubMed] [Google Scholar]
- 10.Sbrissa D, Ikonomov OC, Fu Z, Ijuin T, Gruenberg J, Takenawa T, Shisheva A. Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex. J Biol Chem. 2007;282:23878–23391. doi: 10.1074/jbc.M611678200. [DOI] [PubMed] [Google Scholar]
- 11.Ikonomov OC, Sbrissa D, Shisheva A. Localized PtdIns 3,5-P2 synthesis to regulate early endosome dynamics and fusion. Amer J Physiol Cell Biol. 2006;291:C393–C404. doi: 10.1152/ajpcell.00019.2006. [DOI] [PubMed] [Google Scholar]
- 12.Ikonomov OC, Sbrissa D, Mlak K, Shisheva A. Requirement for PIKfyve enzymatic activity in acute and long-term insulin cellular effects. Endocrinology. 2002;143:4742–4754. doi: 10.1210/en.2002-220615. [DOI] [PubMed] [Google Scholar]
- 13.Ikonomov OC, Sbrissa D, Foti M, Carpentier JL, Shisheva A. PIKfyve controls fluid-phase endocytosis but not recycling/degradation of endocytosed receptors or sorting of procathepsin D by regulating multivesicular body morphogenesis. Mol Biol Cell. 2003;14:4581–4591. doi: 10.1091/mbc.E03-04-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikonomov OC, Sbrissa D, Dondapati R, Shisheva A. ArPIKfyve-PIKfyve interaction and role in insulin-regulated GLUT4 translocation and glucose transport in 3T3-L1 adipocytes. Exp Cell Res. 2007;313:2404–2416. doi: 10.1016/j.yexcr.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutherford AC, Traer C, Wassmer T, Pattni K, Bujny MV, Carlton JG, Stenmark H, Cullen PJ. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J Cell Sci. 2006;119:3944–3957. doi: 10.1242/jcs.03153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefferies HBJ, Cooke FT, Jat P, Boucheron C, Koizumi T, Hayakawa M, Kaizawa H, Ohishi T, Workman P, Waterfield MD, Parker PJ. A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 2008;9:164–170. doi: 10.1038/sj.embor.7401155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikonomov OC, Sbrissa D, Shisheva A. Mammalian cell morphology and endocytic membrane homeostasis require enzymatically active phosphoinositide 5-kinase PIKfyve. J Biol Chem. 2001;276:26141–26147. doi: 10.1074/jbc.M101722200. [DOI] [PubMed] [Google Scholar]
- 18.Efe JA, Botelho RJ, Emr SD. The Fab1 phosphatidylinositol kinase pathway in the regulation of vacuole morphology. Curr Opin Cell Biol. 2005;17:402–408. doi: 10.1016/j.ceb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Michell RH, Heath VL, Lemmon MA, Dove SK. Phosphatidylinositol 3,5-bisphosphate: metabolism and cellular functions. Trends Biochem Sci. 2006;31:52–63. doi: 10.1016/j.tibs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Sbrissa D, Ikonomov OC, Strakova J, Dondapati R, Mlak K, Deeb R, Silver R, Shisheva A. A mammalian ortholog of Saccharomyces cerevisiae Vac14 that associates with and up-regulates PIKfyve phosphoinositide 5-kinase activity. Mol Cell Biol. 2004;24:10437–10447. doi: 10.1128/MCB.24.23.10437-10447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sbrissa D, Shisheva A. Acquisition of unprecedented phosphatidylinositol 3,5-bisphosphate rise in hyperosmotically stressed 3T3-L1 adipocytes, mediated by ArPIKfyve-PIKfyve pathway. J Biol Chem. 2005;280:7883–7889. doi: 10.1074/jbc.M412729200. [DOI] [PubMed] [Google Scholar]
- 22.Dove SK, McEwen RK, Mayes A, Hughes DC, Beggs JD, Michell RH. Vac14 controls PtdIns(3,5)P2 synthesis and Fab1-dependent protein trafficking to the multivesicular body. Curr Biol. 2002;12:885–893. doi: 10.1016/s0960-9822(02)00891-6. [DOI] [PubMed] [Google Scholar]
- 23.Bonangelino CJ, Nau JJ, Duex JE, Brinkman M, Wurmser AE, Gary JD, Emr SD, Weisman LS. Osmotic stress-induced increase of phosphatidylinositol 3,5-bisphosphate requires Vac14, an activator of the lipid kinase Fab1p. J Cell Biol. 2002;156:1015–1028. doi: 10.1083/jcb.200201002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudge SA, Anderson DM, Emr SD. Vacuole size control: regulation of PtdIns(3,5)P2 levels by the vacuole-associated Vac14-Fig4 complex, a PtdIns(3,5)P2-specific phosphatase. Mol Biol Cell. 2004;15:24–36. doi: 10.1091/mbc.E03-05-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gary JD, Sato TK, Stefan CJ, Bonangelino CJ, Weisman LS, Emr SD. Regulation of Fab1 phosphatidylinositol 3-phosphate 5-kinase pathway by Vac7 protein and Fig4, a polyphosphoinositide phosphatase family member. Mol Biol Cell. 2002;13:1238–1251. doi: 10.1091/mbc.01-10-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicot AS, Fares H, Payrastre B, Chisholm AD, Labouesse M, Laporte J. The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome maturation in Caenorhabditis elegans. Mol Biol Cell. 2006;17:3062–3074. doi: 10.1091/mbc.E05-12-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rusten TE, Rodahl LNW, Pattni K, Englund C, Samakovlis C, Dove S, Brech A, Stenmark H. Fab1 phosphatidylinositol 3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors. Mol Biol Cell. 2006;17:3989–4001. doi: 10.1091/mbc.E06-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Zolov SN, Chow CY, Slutsky SG, Richardson SC, Piper RC, Yang B, Nau JJ, Westrick RJ, Morrison SJ, Meisler MH, Weisman LS. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. PNAS. 2007;104:17518–17523. doi: 10.1073/pnas.0702275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sbrissa D, Ikonomov OC, Shisheva A. PtdIns 3-P-binding domains in PIKfyve: binding specificity and consequences to the protein endomembrane localization. J Biol Chem. 2002;277:6073–6079. doi: 10.1074/jbc.M110194200. [DOI] [PubMed] [Google Scholar]
- 30.Andrade MA, Petosa C, O’Donoghue SI, Müller CW, Bork P. Comparison of ARM and HEAT protein repeats. J Mol Biol. 2001;309:1–18. doi: 10.1006/jmbi.2001.4624. [DOI] [PubMed] [Google Scholar]
- 31.Budovskaya YV, Hama H, DeWald DB, Herman PK. The C terminus of the Vps34p phosphoinositide 3-kinase is necessary and sufficient for the interaction with the Vps15p protein kinase. J Biol Chem. 2002;277:287–294. doi: 10.1074/jbc.M109263200. [DOI] [PubMed] [Google Scholar]
- 32.Shisheva A, Sbrissa D, Ikonomov O. Cloning, characterization, and expression of a novel Zn2+-binding FYVE finger-containing phosphoinositide kinase in insulin-sensitive cells. Mol Cell Biol. 1999;19:623–634. doi: 10.1128/mcb.19.1.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto A, DeWald DB, Boronenkov IV, Anderson RA, Emr SD, Koshland D. Novel PI(4)P 5-kinase homologue, Fab1p, essential for normal vacuole function and morphology in yeast. Mol Biol Cell. 1995;6:525–539. doi: 10.1091/mbc.6.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duex JE, Nau JJ, Kauffman EJ, Weisman LS. Phosphoinositide 5-phosphatase Fig4p is required for both acute rise and subsequent fall in stress-induced phosphatidylinositol 3,5-bisphosphate levels. Eukaryotic Cell. 2006;5:723–731.0. doi: 10.1128/EC.5.4.723-731.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duex JE, Tang F, Weisman LS. The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover. J Cell Biol. 2006;172:693–704. doi: 10.1083/jcb.200512105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow CY, Zhang Y, Dowling J, Jin N, Adamska M, Shiga K, Szigeti K, Shy ME, Li J, Zhang X, Lupski JR, Weisman LS, Meisler MH. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448:68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi J, Kandror KV. Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3-L1 adipocytes. Dev Cell. 2005;9:99–108. doi: 10.1016/j.devcel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Sbrissa D, Ikonomov OC, Shisheva A. PIKfyve, a mammalian ortholog of yeast Fab1p lipid kinase, synthesizes 5-phosphoinositides. J Biol Chem. 1999;274:21589–21597. doi: 10.1074/jbc.274.31.21589. [DOI] [PubMed] [Google Scholar]
- 39.Manoonkitiwongsa PA, Schultz RL. Proper nomenclature of formaldehyde and paraformaldehyde fixatives for histochemistry. Histochem J. 2002;34:365–367. [PubMed] [Google Scholar]
- 40.Ishiki M, Randhawa VK, Poon V, JeBailey L, Klip A. Insulin regulates the membrane arrival, fusion, and C-terminal unmasking of glucose transporter-4 via distinct phosphoinositides. J Biol Chem. 2005;280:28792–28802. doi: 10.1074/jbc.M500501200. [DOI] [PubMed] [Google Scholar]
- 41.Ikonomov OC, Sbrissa D, Mlak K, Deeb R, Fligger J, Soans A, Finley RL, Jr, Shisheva A. Active PIKfyve associates with and promotes the membrane attachment of the late endosome-to-TGN transport factor Rab9 p40. J Biol Chem. 2003;278:50863–50871. doi: 10.1074/jbc.M307260200. [DOI] [PubMed] [Google Scholar]