Figure 1.
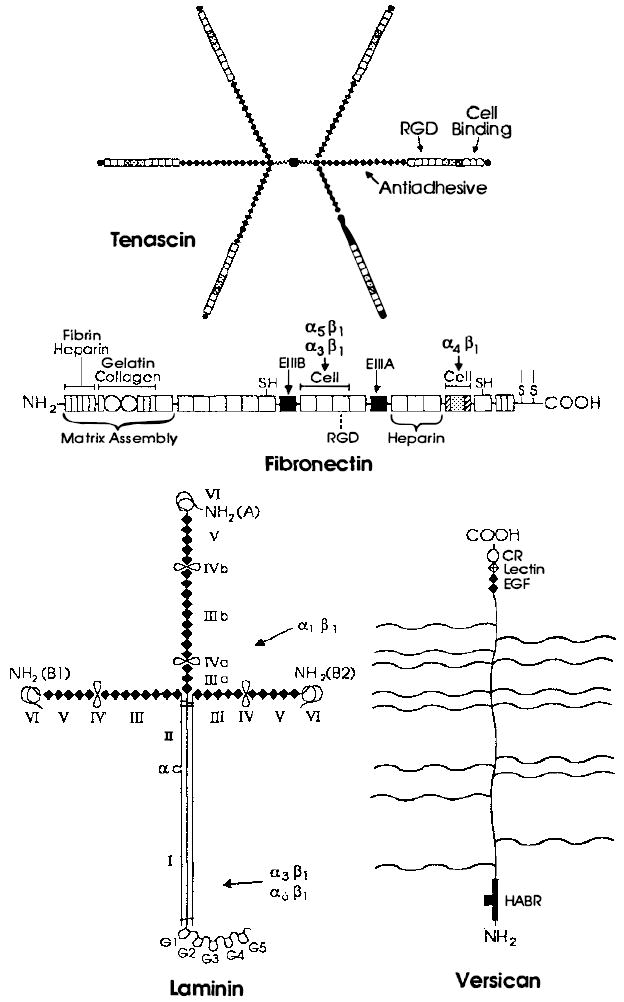
Examples of extracellular matrix glycoproteins and proteoglycans. Schematic structures for the adhesive glycoproteins tenascin, fibronectin, and laminin and for the proteoglycan versican arc presented. Fibroncctin is a dimer, but only one subunit is depicted. The sulfhydryl residues near its carboxyl terminus mediate its dimerization. The positions of FN type I [ ] FN type II [○], and FN type III [□] repeats are indicated in fibronectin. FN type III repeats are also found in tenascin, where their positions are marked by the same symbol [□]. Shaded FN type III repeats in fibronectin and tenascin are encoded by differentially spliced exons and are thus missing from some forms of these glycoproteins. The positions of domains homologous to those in the EOF-precursor, the EOF repeats [◆], are indicated in tenascin, laminin, and versican. The positions of domains homologous to functional domains in lectins [
] FN type II [○], and FN type III [□] repeats are indicated in fibronectin. FN type III repeats are also found in tenascin, where their positions are marked by the same symbol [□]. Shaded FN type III repeats in fibronectin and tenascin are encoded by differentially spliced exons and are thus missing from some forms of these glycoproteins. The positions of domains homologous to those in the EOF-precursor, the EOF repeats [◆], are indicated in tenascin, laminin, and versican. The positions of domains homologous to functional domains in lectins [ ], complement regulatory proteins [
], complement regulatory proteins [ ], and hyaluronic acid-binding proteins [
], and hyaluronic acid-binding proteins [ ] are indicated in versican. Note that many of these domains are also found in receptors, some of which are illustrated in Figure 2. In tenascin, the positions of the ROD sequence, the major cell-binding domain, and the region implicated in the anti-adhesive activity of this protein are shown. Similarly, in fibronectin, the positions of the RGD sequence and domains mediating interactions with fibrin, heparin, gelatin, collagen, and cells are indicated. Both the N-terminal region and the major cell attachment site containing the RGD sequence are required for assembly of fibronectin into the extracellular matrix. The regions of fibronectin recognized by the integrins α5β1, α3β1 and α4β1 are indicated by arrows. Note that the integrin α4β1 recognizes a sequence located in a differentially spliced exon that is not present in all forms of fibronectin. In laminin, homologous domains present in the A, B1, and B2 chains are indicated by roman numerals. Loops indicate the locations of putative globular domains, many of which have been visualized in the electron microscope. Domains I and II in the long arm of laminin’s cruciform structure represent regions in which the three subunits associate in a triple coiled rod. The major proteolytic fragments of laminin E3, E8, E1, and E1-4 described in the text contain approximately the following domains: E3 (G4 and G5), E8 (G1, G2, G3, and most of I), E1 (part of II, III, IV, IIIa, IVa, IIIb, IVb), EI-4 (part of II, III, IIIa, IIIb, IV, IV’, IVa, IVb, V, and VI). As indicated, the binding domains of the integrins α1β1, α3β1 and α6β1 have been roughly mapped by using these fragments. The site utilized by the integrin α2β1 has not yet been defined. The wavy lines in versican indicate the positions of several chondroitin sulfate side chains. All of these glycoproteins contain N-linked carbohydrate, but this is not shown.
] are indicated in versican. Note that many of these domains are also found in receptors, some of which are illustrated in Figure 2. In tenascin, the positions of the ROD sequence, the major cell-binding domain, and the region implicated in the anti-adhesive activity of this protein are shown. Similarly, in fibronectin, the positions of the RGD sequence and domains mediating interactions with fibrin, heparin, gelatin, collagen, and cells are indicated. Both the N-terminal region and the major cell attachment site containing the RGD sequence are required for assembly of fibronectin into the extracellular matrix. The regions of fibronectin recognized by the integrins α5β1, α3β1 and α4β1 are indicated by arrows. Note that the integrin α4β1 recognizes a sequence located in a differentially spliced exon that is not present in all forms of fibronectin. In laminin, homologous domains present in the A, B1, and B2 chains are indicated by roman numerals. Loops indicate the locations of putative globular domains, many of which have been visualized in the electron microscope. Domains I and II in the long arm of laminin’s cruciform structure represent regions in which the three subunits associate in a triple coiled rod. The major proteolytic fragments of laminin E3, E8, E1, and E1-4 described in the text contain approximately the following domains: E3 (G4 and G5), E8 (G1, G2, G3, and most of I), E1 (part of II, III, IV, IIIa, IVa, IIIb, IVb), EI-4 (part of II, III, IIIa, IIIb, IV, IV’, IVa, IVb, V, and VI). As indicated, the binding domains of the integrins α1β1, α3β1 and α6β1 have been roughly mapped by using these fragments. The site utilized by the integrin α2β1 has not yet been defined. The wavy lines in versican indicate the positions of several chondroitin sulfate side chains. All of these glycoproteins contain N-linked carbohydrate, but this is not shown.
