Abstract
Orbitofrontal cortex (OFC) damage produces impaired decision-making, impulsivity, and perseveration and potentially contributes to compulsive drug seeking in cocaine users. To further explore this phenomenon, we assessed the role of the lateral OFC (lOFC) in drug context-induced cocaine-seeking behavior in the reinstatement model of drug relapse. Rats were trained to lever press for intravenous cocaine infusions in a distinct environmental context (cocaine-paired context) followed by extinction training in a different context (extinction-paired context). Reinstatement of cocaine seeking (non-reinforced lever presses) was assessed in the cocaine context in the absence of response-contingent stimuli. In experiment 1, we evaluated whether acute inhibition of lOFC output alters context-induced cocaine-seeking behavior by infusing the GABAB+A agonists, baclofen+muscimol, or vehicle into the lOFC immediately before exposure to the cocaine-paired context. In experiments 2–3, we assessed how prolonged loss of lOFC output affects drug context-induced cocaine seeking by administering bilateral NMDA or sham lesions of the lOFC either before or after self-administration and extinction training. Remarkably, OFC functional inactivation attenuated, post-training lesions failed to alter, and pre-training lesions potentiated drug context-induced cocaine seeking without altering responding in the extinction context. These results suggest that neural activity in the lOFC promotes context-induced cocaine-seeking behavior. However, prolonged loss of lOFC output enhances the motivational salience of cocaine-paired contextual stimuli likely by eliciting compensatory neuroadaptations, with the effects of post-training lOFC lesions reflecting an intermediate state of compensatory neuroplasticity. Overall, these findings support the idea that OFC dysfunction may promote cue reactivity and enhance relapse propensity in cocaine users.
Keywords: cocaine, functional inactivation, lesion, relapse, self-administration
Drug addiction manifests as a chronic relapsing disorder characterized by compulsive drug seeking and drug craving that can be precipitated by exposure to drug-associated explicit cues or environments even after prolonged abstinence (Rohsenow et al., 1990; Ehrman et al., 1992; Foltin & Haney, 2000; Volkow & Fowler, 2000). The transition from recreational drug use to drug addiction may be related to either neural sensitivity predisposing one to drug addiction or neural plasticity resulting from drug exposure (Volkow et al., 1992; Franklin et al., 2002; Volkow et al., 2002). In particular, structural, physiological, and functional abnormalities in the frontal cortex may facilitate addictive behavior. Cocaine users exhibit abnormalities in frontal cortical regions, including decreased gray matter density in the orbitofrontal cortex and anterior cingulate, diminished baseline blood glucose metabolism in the frontal cortex, and enhanced cue-evoked activation of the orbitofrontal cortex, some of which are proportional to drug use (Volkow et al., 1991; London et al., 2000; Volkow & Fowler, 2000; Franklin et al., 2002; Bolla et al., 2003; Matochik et al., 2003). Thus, from an addiction-treatment perspective, it is important to understand whether frontal cortical mechanisms contribute to loss of control over drug seeking.
In the reinstatement model of drug relapse, lOFC lesions greatly potentiate cocaine-primed reinstatement to cocaine seeking in rats during a reinstatement test session held in the previously cocaine-paired operant chamber (Fuchs et al., 2004b). Interestingly, however, lOFC functional inactivation decreases, while lesions fail to enhance, reinstatement elicited by an explicit, response-contingent conditioned stimulus (CS) (Fuchs et al., 2004b). However, the negative effects of lOFC lesions on CS-induced reinstatement may stem from ceiling effects related to steady cocaine-seeking behavior in the sham control group in response to conditioned reinforcement. Thus, it remains to be investigated whether lOFC lesions augment the incentive motivational effects of cocaine-paired stimuli in the absence of conditioned reinforcement.
To test this hypothesis, the present study investigated the effects of bilateral lOFC functional inactivation (experiment 1), pre-training lOFC lesions (experiment 2), and post-training lOFC lesions (experiment 3) on the reinstatement of cocaine-seeking behavior upon re-exposure to a distinct cocaine-paired environmental context after extinction training in a different context. Response-contingent stimuli were not presented to the subjects during training or testing to eliminate the influence of conditioned reinforcement on drug-seeking behavior. Based on our previous study (Fuchs et al., 2004b), we hypothesized that lOFC functional inactivation would attenuate context-induced cocaine seeking, but that pre-training lOFC lesions would enhance this behavior due to lesion-induced compensatory neuroadaptations. Furthermore, we predicted that post-training lOFC lesions would either A) produce similar effects as lOFC functional inactivation if loss of lOFC output during self-administration training critically underlies the effects of pre-training lOFC lesions or B) have similar effects as pre-training lOFC lesions if lesion-induced neuroadaptations are sufficient to enhance context-induced motivation for cocaine.
Methods and Materials
Subjects
Male Sprague-Dawley rats (n = 62), weighing 250–300 g at the start of the experiment, were individually housed in a temperature- and humidity-controlled vivarium on a reversed light-dark cycle. Rats were maintained on 20–25 gm of rat chow per day with water available ad libitum. The housing and treatment of the rats followed guidelines outlined in the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Sciences, 1996).
Food Training
Rats were acclimated to handling 2 days before being trained to press a lever on a fixed ratio 1 (FR1) schedule of food reinforcement (45 mg pellets; Noyes, Lancaster, NH, USA) in sound-attenuated operant conditioning chambers (26 × 27 × 27 cm high; Coulbourn Instruments, Allentown, PA, USA) during a 16-h overnight food training session. During the food training session, stimuli subsequently used for contextual cocaine conditioning were not present. Each active lever response resulted in delivery of one food pellet only; inactive lever responses had no programmed consequences. Food pellet dispensers were removed from the chambers after food training.
Surgery
At least 48-h after food training, rats were pre-anesthetized using ketamine hydrochloride and xylazine (66.6 and 1.33 mg/kg, i.p., respectively). Full anesthesia was maintained during surgery with pentobarbital sodium (50mg/kg, i.p.) in all rats so that ketamine would not inhibit the development of N-methyl-D-aspartic acid (NMDA)-induced excitotoxic lesions. Chronic indwelling catheters were constructed in-house using bent-steel cannulae with a screw-type connector (Plastics One, Roanoke, VA, USA), SILASTIC tubing (Dow Corning, Midland, MI, USA), prolite monofilament mesh (Atrium Medical Corp., Hudson, NH, USA), and cranioplastic cement, as described before (Fuchs et al., 2007). The end of the catheter was inserted into the right jugular vein. The catheter ran subcutaneously and exited the back between the scapulae. Immediately following catheterization, all rats were placed into a stereotaxic instrument (Stoelting, Wood Dale, IL, USA) and bilateral stainless-steel guide cannulae (26 gauge; Plastics One) were aimed dorsal to the lOFC (+3.5 mm AP, +/−3.0 mm ML, −3.4 DV, relative to bregma) or the mOFC (control brain region; +4.2 mm AP, +/−0.6, mm ML, −4.2 DV, relative to bregma) using standard stereotaxic procedures. The guide cannulae were secured to the skull using three screws and cranioplastic cement. All rats received guide cannula implants regardless of experimental manipulation so that differences in surgical history could not account for potential differences across the experiments.
To extend catheter patency during the recovery period, catheters were flushed daily with 0.1 ml of an antibiotic solution of cefazolin (10.0 mg/ml; Schein Pharmaceuticals, Albuquerque, NM, USA) dissolved in heparinized saline (70 U/ml; Baxter Health Care Corp, Deerfield, IL, USA). Thereafter, catheters were flushed with 0.1 ml of heparinized saline (10 U/ml) before each self-administration session and with 0.1 ml of the cefazolin solution and 0.1 ml of heparinized saline (70 U/ml) after each session. Stylets (Plastics One) were placed in catheters and cannulae to prevent occlusion. Catheter patency was checked periodically using propofol (1mg/0.1ml, i.v. Eli Abbott Lab, North Chicago, IL, USA), a fast-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously.
Excitotoxic lesions and intracranial drug infusions
For intracranial manipulations, stainless-steel injection cannulae (33 gauge; Plastics One) were inserted to a depth of 2 mm (lOFC, exp. 1–3) or 1 mm (mOFC, exp. 1) below the tip of the guide cannulae. The injection cannulae were connected to 10 μl Hamilton syringes (Hamilton, Reno, NV, USA) mounted on an infusion pump (KD Scientific, Holliston, MA, USA). Infusions were administered bilaterally into the lOFC or mOFC over 2 min at a volume of 0.6 or 0.3 μl per hemisphere, respectively. The injection cannulae were left in place for 1 min before and 1 min (inactivation) or 4 min (lesion) after the infusion to minimize diffusion dorsally along the cannulae shaft.
Contextual Stimuli
Cocaine self-administration and extinction sessions were conducted in operant conditioning chambers configured to one of two unique environmental contexts that differed along four sensory modalities. Context 1 consisted of a continuous red house light (0.4 fc brightness) on the wall opposite the levers, an intermittent pure tone (80 dB, 1 kHz, 2 sec on, 2 sec off), a pine-scented air freshener strip (4.5 × 2 cm, Car Freshener Corp, Watertown, NY, USA), and wire mesh flooring (26 × 27 cm). Context 2 consisted of an intermittent white stimulus light above the inactive lever (1.2 fc brightness, 2 sec on, 4 sec off), a continuous pure tone (75 dB, 2.5 kHz), a vanilla-scented air freshener strip (4.5 × 2 cm, Sopus Products, Moorpark, CA, USA), and ceramic tile bisecting the steel bar flooring (19 cm × 27 cm). Rats had no exposure to these contextual stimuli prior to self-administration training. As in our previous studies, these stimuli were presented throughout each session independent of responding (Fuchs et al., 2005; Fuchs et al., 2007; Fuchs et al., 2008).
Self-Administration Training
Subjects were assigned randomly to undergo cocaine self-administration training in Context 1 or 2. Training was conducted during the rats’ dark cycle during daily 2-h sessions. The rats’ indwelling catheters were connected to liquid swivels (Instech, Plymouth Meeting, PA, USA) via polyethylene 20 tubing that was encased in steel spring leashes (Plastics One). The swivels were suspended above the operant conditioning chambers and were connected to infusion pumps (Coulbourn Instruments, Allentown, PA, USA). Responses on one (active) lever were reinforced on an FR1 schedule of cocaine reinforcement (0.2 mg/0.05 ml of cocaine hydrochloride infusions, duration 4 s, i.v.; NIDA, Research Triangle Park, NC, USA). Responses on the other (inactive) lever were recorded but had no programmed consequences. A 20-sec time-out period followed each infusion during which lever responses were recorded, but had no programmed consequences. Training continued until the rats successfully obtained ≥10 cocaine infusions per session on at least 10 training days (i.e., acquisition criterion).
Extinction Training
After meeting the acquisition criterion, rats underwent daily 2-h extinction training sessions in the environmental context that distinctly differed from the cocaine self-administration training context. Active and inactive lever presses were recorded, but had no programmed consequences. Extinction training continued for a minimum of 7 sessions plus additional extinction training sessions, as needed, until the rats reached the extinction criterion (≤25 active lever presses per session on 2 consecutive sessions).
Reinstatement Testing
After meeting the extinction criterion, rats were re-exposed to the cocaine-paired environmental context in the absence of cocaine reinforcement in order to assess drug context-induced motivation for cocaine. During the reinstatement test session, active and inactive lever presses were recorded, but had no programmed consequences. The duration and number of reinstatement tests varied in experiments 1–3, as described below.
Locomotor Activity Testing
Motor side effects of intracranial manipulations can affect instrumental behavior. To assess the general motor effects of the intracranial manipulations, locomotor activity was measured in a novel Plexiglas chamber (42 × 20 × 20 cm) equipped with an array of eight photodetectors and corresponding light sources. A computerized activity system (San Diego Instruments, San Diego, CA) recorded the number of consecutive photobeams interrupted by rats moving in the activity chamber during a 2-h test session. Locomotion was assessed within 72-h of the onset of reinstatement testing, as described below.
Experiment 1
Experiment 1 was designed to evaluate whether baclofen/muscimol-induced functional inactivation of the lOFC would alter drug context-induced reinstatement of cocaine-seeking behavior. Rats underwent self-administration training in one context and extinction training in a different context, as described under general methods. On extinction day 4, rats were acclimated to the intracranial infusion procedure. During the adaptation procedure, rats were held gently by the experimenter and injection cannulae were bilaterally inserted into the rats’ guide cannulae and left in place for 4 minutes, but no drug was infused. Immediately following the adaptation procedure, rats were placed into the operant chamber for an extinction session.
After the rats reached the extinction criterion, reinstatement of cocaine-seeking behavior was assessed in the cocaine-paired context or extinction context over the course of 4 test sessions. Immediately prior to each test session, rats received bilateral infusions of the GABAB+A agonist cocktail baclofen+muscimol (BM; 1.0 and 0.1 mM, respectively; pH ~7.0) or phosphate buffered saline vehicle (VEH) into the lOFC at a volume of 0.6 μl per hemisphere. The order of testing in the previously cocaine-paired versus extinction contexts and the order of intracranial treatments (BM, VEH) were counterbalanced based on previous cocaine intake. The dose of BM was selected based on previous research indicating that this intra-lOFC dose of BM impairs CS-induced cocaine-seeking behavior (Fuchs et al., 2004b; Fuchs et al., 2008). Because BM spread cannot be visualized, anatomical control groups received BM or VEH infusions into the mOFC (0.3 μl/hemisphere) to assess whether the effects were OFC sub-region specific. Session length was 1-h to allow for repeated testing without significant extinction learning in the cocaine-paired context. Subjects received additional extinction sessions in the extinction context between test sessions until they re-obtained the extinction criterion (≤25 lever presses per session for 2 consecutive days). Twenty-four h after the last test session, rats were given two 1-h locomotor activity test sessions. Immediately before each locomotor test, rats received either a BM or VEH infusion consistent with the order of treatment received during reinstatement testing.
Experiment 2
Experiment 2 was designed to evaluate the effects of pre-training lOFC lesions on drug context-induced reinstatement of cocaine-seeking behavior. Immediately after stereotaxic surgery, injection cannulae were inserted into the rats’ guide cannulae. Rats received infusions of either NMDA (0.1 M; pH ~7.0) or phosphate buffered saline vehicle (VEH) into the lOFC at a volume of 0.6 μl per hemisphere, with lesion group assignment randomized. The dose of NMDA was selected based on previous research showing this intra-lOFC dose of NMDA results in selective lesions of the lOFC, which enhances drug-primed cocaine-seeking behavior (Fuchs et al., 2004b). Rats were given a 7-day post-operative recovery period to allow the lesions to develop followed by self-administration training in one context and extinction training in a different context, as in experiment 1. After the rats reached the extinction criterion, reinstatement of cocaine-seeking behavior was assessed in the cocaine-paired context during a single 2-h test session. Responding in the extinction context 24-h before the cocaine-context reinstatement test served as the measure of lesion effects on baseline operant responding. Seventy-two hours prior to the reinstatement test, locomotor activity was assessed in all rats in order to examine the effects of the lesion and sham manipulations on general activity at the approximate time of reinstatement testing.
Experiment 3
To determine whether the differential effects of lOFC lesions and functional inactivation on drug context-induced reinstatement stemmed from the timing of the manipulation relative to associative learning processes, experiment 3 was designed to evaluate the effects of post-training lOFC lesions on context-induced reinstatement. Rats were trained to self-administer cocaine in one context and received extinction training in a different context, as in experiments 1–2. After reaching the extinction criterion, rats received bilateral infusions of either NMDA or VEH into the lOFC using the procedures described under experiment 2, with assignment to lesion group counterbalanced based on previous cocaine intake during self-administration training. Rats were given a 7-d post-operative recovery period to allow the lesions to develop. Rats then received a minimum of 2 extinction sessions to re-establish pre-surgery extinction baselines and eliminate spontaneous recovery. Thereafter, reinstatement testing and a locomotor testing were conducted using procedures identical to those employed in experiment 2.
Histology
Immediately following the last test session, rats were euthanized and their brains were dissected out. Brains were sectioned and stained using Cresyl violet (Kodak, Rochester, NY, USA). The extent of the lesions and cannula placements were verified on the brain sections under a light microscope. The pattern of cell loss or the most ventral point of each cannula track was mapped onto schematics of the appropriate plates from the rat brain atlas of Paxinos & Watson (1997).
Statistical Analysis
Only data from rats with correctly placed lesions and cannula placements were included in data analysis. In experiments 1–3, separate mixed-factorial ANOVAs were used to analyze active and inactive lever responses and cocaine intake during self-administration training and lever responding during extinction training with lesion (sham, lesion) and group (sham, lesion or BM, VEH) as between-subjects factors and time (day) as the within-subjects factor, where appropriate. In experiment 1, repeated measures ANOVAs were used to analyze lever responses on the test days with treatment (BM, VEH), context (cocaine context, extinction context), and time (20-min intervals) as factors, where appropriate. Locomotor activity data were analyzed using repeated measures ANOVA with treatment and time as factors. In experiments 2–3, mixed-factorial ANOVAs were used to analyze lever responses on the reinstatement test day and preceding extinction day with lesion as the between-subjects factor and context and time as within-subjects factors, where appropriate. Locomotor activity data were assessed using mixed-factorial ANOVAs with lesion as the between-subjects factor and time as the within-subjects factor. Significant main and interaction effects were investigated using simple main effects tests or Tukey HSD post hoc tests. Alpha was set at 0.05.
Results
Histology
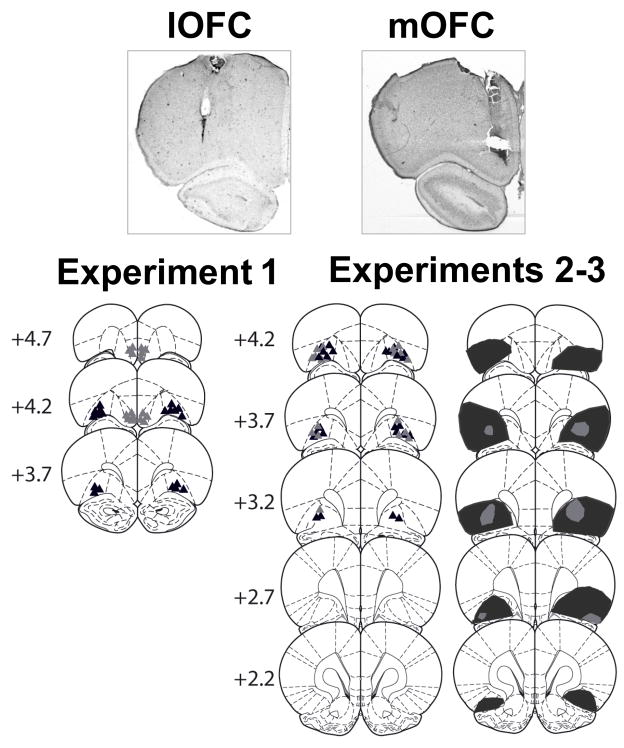
Fig 1 depicts photomicrographs of representative cannula placements within the lOFC and mOFC, schematic diagrams of the distribution of cannula placements in experiments 1–3, and schematics of the extent and the location of the smallest and largest lesions in experiment 2–3. The lOFC target region was defined as an aggregate of the lateral and ventrolateral subregions of the OFC, whereas the mOFC target region was defined as the combination of medial and ventromedial subregions of the OFC (Paxinos & Watson, 1997). The most ventral points of the cannula tracts were bilaterally located within the lOFC or mOFC for all rats whose data were included in the analyses. Furthermore, after lOFC lesions, cell loss was observed in the ventrolateral and lateral regions of the lOFC as well as in adjacent regions of the agranular insular (AIC) and frontal cortices in a subset of rats. Data obtained from rats with extensive lesion in unintended brain regions were excluded from analysis. The resulting groups (sample sizes) were as follows: lOFC functional inactivation (n = 10), mOFC functional inactivation (n = 8), pre-training lOFC lesion (n = 12), pre-training lOFC sham (n = 12), post-training lOFC lesions (n = 9), post-training lOFC sham (n = 11).
Figure 1.
Photomicrographs depicting representative cannula placements in the lOFC and mOFC as well as schematic illustrations depicting cannula placements in experiments 1–3 and the extent of the largest (dark shaded areas) and smallest (light shaded areas) NMDA lesions in experiments 2–3. Symbols indicate the most ventral point of the injection cannula tracks (exp. 1: lOFC, black triangles; mOFC, gray triangles; exp. 2: black triangles; exp. 3: gray triangles). The numbers represent the approximate distance (in millimeters) from bregma, based on the atlas of Paxinos and Watson (1997).
Experiment 1
Self-Administration and Extinction Responding
The lOFC-cannulated and mOFC-cannulated rats exhibited stable responding for cocaine reinforcement (Fig. 2A and Fig. 2D). There was no pre-existing difference in active and inactive lever responding or in cocaine intake between groups as a function of subsequent treatment order (BM or VEH). For the lOFC-cannulated group, the mean active and inactive lever responding ± SEM was 33.97 ± 5.15 and 2.97 ± 1.56, respectively, while the mean cocaine intake ± SEM was 11.60 ± 0.83 mg/kg per session (17.40 ± 1.245 infusions). For the mOFC-cannulated group, the mean active and inactive lever responding was 34.00 ± 3.374 and 1.88 ± 1.38, respectively, while the mean cocaine intake was 15.58 ± 1.493 mg/kg per session (23.38 ± 2.421 infusions). There was also no pre-existing difference in active or inactive lever responding during extinction training as a function of treatment order (Fig. 2A and Fig. 2D). In lOFC-cannulated and mOFC-cannulated subjects, the mean number of days (mean ± SEM) to reach the extinction criterion was 7.00 ± 0.00.
Figure 2.
Functional inactivation of the lOFC – but not mOFC – attenuates drug context-induced reinstatement of cocaine-seeking behavior. The top panels shows active and inactive lever responses (mean/2h + SEM) for lOFC- (A) and mOFC-cannulated (D) rats during cocaine self-administration (SA, last 7 days) and extinction training (EXT, first 7 days). During self-administration training, active lever responses resulted in cocaine infusions (0.2 mg/0.1 ml per infusion) and inactive lever responses had no programmed consequences. During extinction training, lever responses had no programmed consequences. The middle panel shows the effects of intra-lOFC (B) and intra-mOFC (E) infusions of BM and VEH on non-reinforced active lever responses (mean/1h + SEM) during testing in the extinction context (EXT CTX) and previously cocaine-paired context (COC CTX). The bottom panels show the effects of intra-lOFC (C) and intra-mOFC (F) infusions of BM and VEH on the time course of active lever responses (mean + SEM) during the test session in the cocaine-paired context. Baclofen plus muscimol (BM) or vehicle (VEH) was infused into the lOFC or mOFC immediately before testing. Asterisks represents a significant difference relative to responding in the extinction context (B: ANOVA context simple main effect, Tukey test, p < 0.01; E: ANOVA context main effect, p = 0.004). Daggers represent a significant difference relative to VEH treatment (B: ANOVA treatment simple main effect, Tukey test, p < 0.01; C: ANOVA treatment main effect, p = 0.001).
Effects of lOFC Functional Inactivation on Drug Context-induced Reinstatement of Cocaine-seeking Behavior
Rats exhibited enhanced non-reinforced active lever responding in the previously cocaine-paired context relative to responding in the extinction context (Fig. 2B; context, F(1,9) = 39.439, p = 0.001). Furthermore, intra-lOFC BM pretreatment impaired active lever responding relative to VEH pretreatment in a context-specific manner (treatment × context, F(1, 9) = 52.494, p = 0.001; treatment, F(1,9) = 40.218, p = 0.001). Thus, re-exposure to the cocaine-paired context increased active lever responding following VEH pretreatment (Tukey test, p < 0.01), but not BM pretreatment, relative to responding in the extinction context. Moreover, intra-lOFC BM pretreatment attenuated active lever responding in the cocaine-paired context relative to VEH pretreatment (Tukey test, p < 0.01) without altering active lever responding in the extinction context. The time course analysis of active lever responding in the cocaine-paired context indicated that active lever responding was greatest during the first 20-min interval of the session after which it declined in both groups (Fig. 2C; time, F(2,18) = 5.926, p = 0.011; interval 1 > intervals 2–3, Tukey test, p < 0.05). Furthermore, BM decreased responding throughout the test session relative to VEH (treatment, F(2,18) = 64.310, p = 0.001; treatment × time, F(2.18) = 0.399, p = 0.677).
Rats exhibited negligible responding on the inactive lever in the cocaine-paired (1.850 ± 1.225) and extinction contexts (2.38 ± 1.431; data not shown). Exposure to the cocaine-paired context did not alter responding on the inactive lever relative to responding in the extinction context (context, F(1,9) = 0.455, p = 0.517). Furthermore, intra-lOFC BM pretreatment failed to alter inactive lever responding relative to VEH pretreatment in either context (treatment × context, F(1, 9) = 0.638, p = 0.139; treatment, F(1,9) = 0.098, p = 0.761).
Effects of mOFC Functional Inactivation on Drug Context-Induced Reinstatement of Cocaine-seeking Behavior
Re-exposure to the cocaine-paired context increased non-reinforced active lever responding in the mOFC-cannulated rats relative to responding in the extinction context (Fig. 2E; context, F(1,7) = 17.184; p = 0.004). Further, intra-mPFC BM pretreatment failed to alter active lever responding relative to VEH pretreatment in either context (treatment × context, F(1,7) = 0.370, p = 0.562; treatment, F(1,7) = 0.057, p = 0.819). The time course analysis of active lever responding in the cocaine-paired context indicated that responding declined over the course of the test session (Fig. 2F; time, F(2,14) = 9.088, p = 0.03; interval 1 > intervals 2–3, Tukey test, p < 0.05) and confirmed that BM pretreatment failed to alter responding relative to VEH pretreatment (treatment × time F(2,14) = 0.139, p = 0.872; treatment F(1,7) = 0.218; p = 0.650).
Similar to the lOFC-cannulated rats, mOFC-cannulated rats exhibited negligible inactive lever responding in the cocaine-paired (3.550 ± 1.225) and extinction contexts (0.400 ± 0.237; data not shown). Exposure to the cocaine-paired context did not alter inactive lever responding relative to responding in the extinction context (context, F(1,7) = 1.197, p = 0.310). Furthermore, intra-mOFC BM pretreatment failed to alter inactive lever responding relative to VEH pretreatment in either the cocaine-paired or the extinction context (treatment × context, F(1,7) = 0.517, p = 0.495; treatment, F(1,7) = 0.040, p = 0.847).
Locomotor Activity
Both the lOFC-cannulated rats and mOFC-cannulated rats exhibited a decrease in motor behavior during the locomotor activity test (Fig. 3; lOFC: time, F(2,18) = 61.162, p = 0.001; mOFC: time, F(2,14) = 54.306, p = 0.001). This was due to a decrease in motor behavior following the first 20-min interval of the locomotor test session (interval 1 > intervals 2–3, Tukey test, p < 0.01). Further, intra-lOFC BM pretreatment slightly attenuated motor activity during the locomotor activity test relative to VEH pretreatment (Fig. 3A; treatment, F(1,9)= 5.895, p = 0.038; treatment × time, F(2,18) = 1.367, p = 0.280). In contrast, intra-mOFC BM pretreatment failed to alter motor activity relative to VEH pretreatment (Fig. 3B; treatment × time, F(2,14) = 0.415, p = 0.668; treatment, (F(1,7)= 0.037, p = 0.853).
Figure 3.
Functional inactivation of the lOFC, but not mOFC, attenuates locomotor activity measured as the number of photobeam breaks triggered by the movement of subjects in a novel context. The panels show the effect of intra-lOFC (A) and intra-mOFC (B) infusions of BM and VEH on photobeam breaks (mean/1h + SEM). BM or VEH was infused into the lOFC or mOFC immediately before testing. The dagger represents a significant difference relative to VEH pretreatment (ANOVA treatment main effect, p = 0.038).
Experiment 2
Self-Administration and Extinction Responding
Pre-training lOFC lesions did not alter responding for cocaine reinforcement (Fig. 4A). Pre-training lOFC lesions did not alter active lever responding during the last 7 days of cocaine self-administration training relative to the sham lesion (lesion × time, F(6,132) = 2.188, p = 0.134; time, F(6,132) = 1.482, p = 0.189; lesion, F(1,22) = 0.929, p = 0.346). Similarly, lOFC lesions did not alter inactive lever responding during the last 7 days of self-administration training (lesion × time F(6,132 = 0.843, p = 0.539; lesion, F(1,22) = 0.013, p = 0.909; time, F(6,132 = 2.073, p = 0.061). Finally, the lOFC lesion failed to alter cocaine intake during the last 7 days of cocaine self-administration training relative to sham lesions (lesion × time, F(6,132) = 2.008, p = 0.069; time, F(6,132) = 1.097, p = 0.367; lesion, F(1,22) = 0.011, p = 0.917). On average, the lOFC lesion and sham group exhibited a mean daily cocaine intake of 16.378 ± 1.71 and 14.833 ± 1.05 mg/kg per session (24.58 ± 2.57 and 22.25 ± 1.58 infusions), respectively.
Figure 4.
Pre-training lOFC lesions potentiate drug context-induced reinstatement of cocaine-seeking behavior but fail to alter responding for cocaine reinforcement or extinction learning. Panel A shows active and inactive lever responses (mean/2h + SEM) during cocaine self-administration (SA, last 7 days) and extinction training (EXT, first 7 days). During self-administration training, active lever responses resulted in cocaine infusions (0.2 mg/0.1 ml per infusion) and inactive lever responses had no programmed consequences. During extinction training, lever responses had no programmed consequences. Panel B shows the effects of pre-training lOFC lesions on active lever responses (mean/2h + SEM) during the last day of extinction training (EXT CTX) and during testing in the previously cocaine-paired context (COC CTX). Panel C shows the effects of pre-training lOFC lesions on the time course of active lever responses (mean + SEM) during the test session in the previously cocaine-paired context. Asterisks represent a significant difference relative to responding in the extinction context (ANOVA context main effect, Tukey test, p < 0.01). Daggers represent a significant difference relative to the sham group (ANOVA lesion simple main effect, Tukey test, p < 0.01).
Pre-training lOFC lesions did not alter responding on the active or inactive lever upon removal of cocaine reinforcement (Fig. 4A). Active lever responding declined following removal of cocaine reinforcement (time, F(6,132) = 12.954, p = 0.0001; day 1 > day 2–7, Tukey test, p < 0.01) irrespective of lesion condition (lesion × time, F(6,132) = 0.608, p = 0.723; lesion, F (1,22) = 0.041, p = 0.841). Similarly, inactive lever responding declined over the course of extinction training (time, F(6,132) = 2.734, p = 0.015; day 1 > days 2–7, Tukey test, p < 0.01), irrespective of lesion condition (lesion × time, F(6,132) = 0.611 p = 0.611; lesion, F(1,22) = 0.483, p = 0.494). Finally, the lOFC lesion and sham controls groups did not differ in the mean number of days needed to reach the extinction criterion (t(22) = 1.000, p = 0.328; Sham mean = 7.33 + 0.333, Lesion mean = 7.00 + 0.00; data not shown).
Effects of Pre-training lOFC lesions on Reinstatement of Cocaine-seeking Behavior
Exposure to the cocaine-paired context increased active lever responding in all groups relative to responding in the extinction context (Fig. 4B; context, F(1,22) = 216.789, p = 0.001), and pre-training lOFC lesions altered active lever responding relative to the sham manipulation in a context-specific manner (context × lesion, F(1,22) = 11.670, p = 0.002; lesion, F(1,22) = 13.463, p = 0.001). Specifically, the pre-training lOFC lesion group exhibited greater active lever responding than the sham group in the cocaine-paired context (Tukey test, p < 0.01) but not in the extinction context. The time-course analysis of active lever responding in the cocaine-paired context indicated that responding declined during the test session (Fig. 4C; time, F(2,44) = 6.172, p = 0.004). Furthermore, pre-training lOFC lesions potentiated active lever responding relative to sham lesions during the first 20-min of the session (time × lesion, F(2,44) = 3.543, p = 0.037; interval 1 > intervals 2–3, Tukey test, p < 0.01; lesion, F(2,44) = 5.380, p = 0.026).
As in experiment 1, inactive lever responding was negligible in the cocaine-paired context (3.67±1.208) and in the extinction context (1.62±0.918; data not shown). Re-exposure to the cocaine-paired context did not alter responding in the inactive lever relative to inactive lever responding in the extinction context (context, F(1,22) = 1.762, p = 0.098). Furthermore, pre-training lOFC lesions failed to alter inactive lever responding relative to sham lesions (context × lesion, F(1,22) = 0.705, p = 0.410; lesion, F(1,22) = 0.036, p = 0.851).
Locomotor Activity
Both the pre-training lOFC lesion and sham groups exhibited a decrease in motor activity after the first 20-min interval of the locomotor test session (time, F(2,44) = 127.5630, p = 0.001; interval 1 > intervals 2–3, Tukey test, p < 0.01; data not shown). Furthermore, pre-training lOFC lesions failed to alter motor activity relative to sham lesions (lesion × time, F(2,44) = 0.543, p = 0.584; lesion, F(1,22) = 0.382, p = 0.543).
Experiment 3
Self-Administration and Extinction Responding
The lOFC-cannulated rats exhibited stable responding for cocaine reinforcement (Fig. 5A). There were no pre-existing differences in active or inactive lever responding or cocaine intake between the groups that subsequently received lOFC lesion or sham manipulation. The mean ± SEM daily cocaine intake for the post-training lOFC lesion and sham groups was 14.66 ± 1.40 and 16.40 ± 2.19 mg/kg per session (22.00 ± 2.10 and 24.60 ± 3.29 infusions), respectively.
Figure 5.
Post-training lOFC lesions fail to alter drug context-induced reinstatement of cocaine-seeking behavior. Panel A shows active and inactive lever responses (mean/2h + SEM) during cocaine self-administration (SA, last 7 days) and extinction training (EXT, first 7 days) by the BLA-cannulated groups prior to the lesion manipulation. During self-administration training, active lever responses resulted in cocaine infusions (0.2 mg/0.1 ml per infusion) and inactive lever responses had no programmed consequences. During extinction training, lever responses had no programmed consequences. Panel B shows the effects of post-training lOFC lesions on active lever responses (mean/2h + SEM) during the last day of extinction training (EXT CTX) and during testing in the previously cocaine-paired context (COC CTX). Panel C shows the effects of post-training lOFC lesions on the time course of active lever responses (mean + SEM) during the test session in the previously cocaine-paired context. Asterisks represent a significant difference relative to responding in the extinction context (ANOVA context main effect, Tukey test, p < 0.01)
There was also no pre-existing difference between groups that subsequently received the lOFC lesion or sham manipulation in active lever responding during extinction training (Fig. 5A). Furthermore, both groups needed a similar mean number of days to reach the extinction criterion (t(18) = 0.900, p = 0.380; Sham mean = 7.09 + 0.30, Lesion mean = 7.00 + 0.00; data not shown). Inactive lever responding declined across the first 7 extinction sessions (time, F(6,108) = 8.251, p = 0.001), and inactive lever responding was greater on extinction day 1 in the sham group relative to the group that subsequently received lOFC lesions (group × time, F(6,108) = 2.503, p = 0.026; Tukey test, p < 0.05; group, F(1,18) = 2.890, p = 0.106). However, there were no significant differences between groups on extinction days 2–7.
Importantly, following the induction of post-training lesions, there was no difference between the lesion and sham groups in active or inactive lever responding during the extinction sessions that were conducted to re-establish the extinction baseline (active lever: group × time, F(1,18) = 1.507, p = 0.318; group, F(1,18) = 0.443, p = 0.514; time, F(1,18) = 1.437, p = 0.246; inactive lever: group × time, F(1,18) = 12.898, p = 0.061; group, F(1,18) = 0.779, p = 0.389; time, F(1,18) = 0.525, p = 0.478). Furthermore, both groups required a similar mean number of days to re-obtain the extinction criterion (t(20) = 0.102, p = 0.920; Sham mean = 2.82 + 0.519, Lesion mean = 2.75 + 0.313).
Effects of Post-training lOFC Lesions on Drug Context-induced Reinstatement of Cocaine-seeking Behavior
Exposure to the cocaine-paired context elicited robust active lever responding in both the post-training lOFC lesion and sham groups (Fig. 5B). Both groups exhibited more active lever responding in the cocaine-paired context relative to responding in the extinction context (context, F(1,18) = 53.245, p = 0.001), and post-training lOFC lesions failed to alter active lever responding relative to sham lesions in either context (context × lesion, F(1,18) = 1.327, p = 0.264; lesion F(1,18) = 1.224, p = 0.283). Further, the time-course analysis of active lever responding in the cocaine-paired context indicated that responding declined at a similar rate in both the lOFC lesion and sham groups (Fig. 5C; time, F(2, 36) = 12.445, p = 0.001; interval 1 > intervals 2–3, Tukey test, p < 0.05) and confirmed that post-training lOFC lesions failed to alter active lever responding relative to sham lesions (time × lesion, F(2,36) = 0.177, p = 0.839; lesion, F(1,18) = 1.572, p = 0.226).
Inactive lever responding was negligible in the cocaine-paired context (2.45 ± 0.829) and in the extinction context (1.20 ± 0.408; data not shown). Thus, the groups did not exhibit a change in inactive lever responding in the cocaine-paired context relative to the extinction context (context, F(1,18) = 1.957, p = 0.178). Furthermore, post-training lOFC lesions failed to alter inactive lever responding relative to sham lesions (context × lesion, F(1,18) = 0.853, p = 0.368; lesion, F(1,18) = 0.118, p = 0.735).
Locomotor Activity
Both the post-training lOFC lesion and sham groups exhibited a similar decrease in motor activity following the first 20-min interval of the locomotor test session (time, F(2,36) = 1117.155, p = 0.001; interval 1 > intervals 2–3; Tukey test, p < 0.01). Furthermore, post- training lOFC lesions failed to alter motor activity relative to sham lesions (lesion × time, F(2,36) = 0.229, p = 0.797; lesion, F(1,18) = 0.049, p = 0.828; data not shown).
Discussion
The findings in the present study highlight the complex role that the lOFC – a structure functionally homologous to the human medial OFC – plays in guiding drug-seeking behavior and provide the first evidence that the lOFC is critical for regulating drug context-induced reinstatement of cocaine seeking (Krettek & Price, 1977; Gallagher et al., 1999). Functional inactivation of the lOFC – but not the mOFC – disrupted the ability of a cocaine-paired context to reinstate extinguished cocaine-seeking behavior (Fig. 2). In contrast to these findings, post-training lOFC lesions failed to alter (Fig 5), whereas pre-training lOFC lesions augmented (Fig. 4), drug context-induced reinstatement of cocaine seeking. While this complex pattern of effects may seem contradictory, it likely reflects the intricate constellation of cognitive impairments produced by OFC damage in humans, as will be discussed in the following paragraphs.
lOFC, but not mOFC, functional inactivation impairs drug context-induced reinstatement of cocaine seeking
The current finding that intracranial BM infusions affect drug context-induced cocaine seeking in an OFC sub-region specific manner is consistent with our previous findings that lOFC – but not mOFC – functional inactivation prevents explicit cocaine-paired CSs from eliciting cocaine seeking (Fuchs et al., 2004b). Taken together, these findings suggest that the rat OFC is a functionally heterogeneous brain region with respect to guiding cue-induced cocaine seeking regardless of cue type and imply that the lOFC is selectively involved in this behavior. It is unlikely that BM infusions into the lOFC decreased cocaine-seeking behavior due to non-specific reductions in motor behavior even though this manipulation slightly depressed motor activity in a novel context. First, decreased motor activity was not observed during the first 20-min interval of the locomotor test (Fig. 3A) when functional inactivation of the lOFC produced the most robust impairment in active lever responding (Fig. 2C). Second, lOFC functional inactivation failed to alter inactive lever responding. Overall, these findings lead us to conclude that neural activity in the lOFC is necessary for regulating the motivational significance of cocaine-conditioned stimuli, either directly, by mediating context-induced incentive motivation for cocaine, or indirectly, by affecting the recall or utilization of established context-cocaine-response associations that guide cocaine-seeking behaviors.
Pre-training lOFC lesions fail to alter cocaine self-administration and extinction learning
While the functional inactivation experiment provides critical information about the acute role of the lOFC in guiding cocaine seeking, cocaine users typically present with protracted structural, physiological, and functional abnormalities in prefrontal cortical regions (Volkow et al., 1991; London et al., 2000; Volkow & Fowler, 2000; Franklin et al., 2002; Bolla et al., 2003; Matochik et al., 2003). These abnormalities, which may be modeled using lOFC lesions, have been postulated to underlie pathological drug-seeking and drug-taking behaviors observed in former cocaine addicts (Volkow & Fowler, 2000). In accordance with our earlier study (Fuchs et al., 2004b), the present findings indicate that pre-training lOFC lesions fail to alter cocaine-reinforced instrumental behavior (Fig. 4A). Thus, long-term loss of lOFC output does not prevent primary reinforcement, consistent with previous studies examining the effects of lOFC lesions on the acquisition of cocaine self-administration as a function of cocaine dose (Hutcheson & Everitt, 2003; Schoenbaum & Shaham, 2008) or on the acquisition of responding for natural reinforcers (Gallagher et al., 1999; Schoenbaum et al., 2002; McDannald et al., 2005; Ostlund & Balleine, 2007).
Similar to the lack of effects of lOFC lesions on cocaine-reinforced lever responding, pre-training lOFC lesions failed to alter the extinction of lever responding in a novel context (Fig. 4A). While this finding is consistent with results from our previous study(Fuchs et al., 2004b), it appears to contrast with reports that OFC damage causes perseveration of non-rewarded responses in humans and impairs performance on reinforcer devaluation, reversal, and extinction learning tasks in animals (Bechara et al., 1994; Gallagher et al., 1999; Pickens et al., 2003; Izquierdo et al., 2004; Izquierdo & Murray, 2005; Izquierdo et al., 2005; Pickens et al., 2005). However, perseverative errors induced by lOFC lesions in devaluation and reversal tasks likely reflect an inability to shift behavioral responding to a previously unrewarded stimulus, which requires the modification of existing CS-no reward associations rather than inhibition of non-rewarded responses (Tait & Brown, 2007). In accordance with this, animals with lOFC damage are not impaired when performing a novel odor discrimination problem, are capable of performing strategy set-shift tasks, and exhibit normal extinction learning when this involves the formation of new “no CS-response-no reward” associations (Schoenbaum et al., 2002; Fuchs et al., 2004b; Ghods-Sharifi et al., 2008). Because lOFC damage induces behavioral impairments that appear to reflect a difficulty in updating previously established stimulus-no reward associations, lOFC-lesioned rats might have relied on an intact ability to either form new “novel context-response-no-reward” associations or utilize state-dependent learning, such as the presence or absence of cocaine-related interoceptive cues in the present study, to adaptively inhibit lever responding.
Pre-training lOFC lesions enhance context-induced reinstatement of cocaine-seeking behavior
In contrast to the effects of lOFC functional inactivation on drug context-induced cocaine seeking, pre-training lOFC lesions augmented drug context-induced reinstatement of cocaine-seeking behaviors relative to sham lesions (Fig. 4B). This effect appeared to stem from enhanced drug context-induced motivation for cocaine rather than perseverative responding. Consistent with this, lOFC lesions significantly potentiated responding during the first 20 minutes in the cocaine-paired context rather than decreasing the rate of decline, or extinction, in cocaine-seeking behaviors over the course of the test session (Fig. 4C).
Because findings from the lOFC functional inactivation experiment indicated that the lOFC regulates the motivational effects of cocaine-conditioned contextual cues, the mechanism by which pre-training lOFC lesions potentiated cue-induced reinstatement bears explication. Unlike transient, functional inactivation of the lOFC, NMDA-induced lesions permanently eliminate lOFC neural output to other elements of the relapse circuitry and thus, in turn, may elicit compensatory neural adaptations that, in turn, contribute to heightened context-induced incentive motivation for cocaine. Previous studies have suggested that other behavioral deficits commonly associated with lOFC damage, such as behavioral inflexibility, may stem from neuroplasticity in brain regions connected with the lOFC. For instance, electrophysiological evidence indicates that neural activity in the lOFC indirectly promotes behavioral flexibility by facilitating associative encoding in the amygdala (Saddoris et al., 2005). As a result, unilateral lesions of the lOFC impair cue-selective firing in the basolateral amygdala during reversal learning, and lOFC lesion-induced impairments in reversal learning are prevented by OFC plus BLA lesions (Schoenbaum et al., 1999; Stalnaker et al., 2007). Hence, compensatory neuroadaptations may develop in other brain regions within the mesocorticolimbic reward circuitry following lOFC lesions and these neuroadaptations may account for potentiated context-induced cocaine seeking observed in the present study, as well as enhanced cue-induced motivation for cocaine in former cocaine users (Bonson et al., 2002; McLaughlin & See, 2003; Fuchs et al., 2004b). In terms of cognitive function, loss of lOFC output during cocaine self-administration may also alter associative learning processes that underlie the formation of context-response-cocaine associations that subsequently drive reinstatement to cocaine seeking. Enhanced context-induced motivation may stem from differences in associative encoding in brain regions, including the basolateral amygdala, dorsal prefrontal cortex, and hippocampus, which are thought to mediate contextual associative learning and memory, (Tzschentke & Schmidt, 1999; Kruzich & See, 2001; Fuchs et al., 2002; Meyers et al., 2006; Atkins et al., 2008).
Interestingly, the behavioral effects of pre-training lesions reported here appear to contrast with our previous study in which lOFC lesions did not alter explicit CS-induced cocaine-seeking behaviors (Fuchs et al., 2004b). However, the differential effects of lOFC lesions on context-induced versus CS-induced cocaine seeking may stem from critical differences between these cues. While response-contingent explicit CSs can maintain drug seeking by providing conditioned reinforcement or by signaling imminent drug effects, contexts act as occasion setters or discriminative stimuli that signal drug availability contingent upon responding (Bouton & Bolles, 1979; Crombag & Shaham, 2002; Fuchs et al., 2005). Explicit CSs and contexts engage partially distinct neural systems to guide the expression of cocaine-seeking behavior (Hutcheson & Everitt, 2003; Fuchs et al., 2004a; Fuchs et al., 2005; Bossert et al., 2007). Thus, lOFC lesions may produce compensatory neuroadaptations that differentially affect these distinct neural systems. Accordingly, lOFC lesions appear to impair, as opposed to facilitate, behavior maintained by conditioned reinforcement given that lOFC lesions disrupt responding for cocaine on a second-order reinforcement schedule and produce insensitivity to CS omission on a second-order task (Hutcheson & Everitt, 2003; Pears et al., 2003; Ostlund & Balleine, 2007). In contrast, lOFC lesions do not prevent the processing of discriminative stimuli given that lOFC-lesioned rats exhibit normal acquisition of instrumental discrimination learning, perform odor discriminations in a go/no-go task, and displayed normal acquisition of lever pressing for unsignaled cocaine in the present study (Schoenbaum et al., 2002; Chudasama & Robbins, 2003). However, lOFC lesion-induced neuroadaptations may enhance context-induced motivation for cocaine reinforcement, which manifests differently depending on the presence or absence of an explicit cocaine-paired CS. Hence, the lOFC lesion-induced enhancement in context-induced motivation for cocaine may have been obscured in the previous study by lOFC lesion-induced attenuation in responding maintained by conditioned reinforcement whereas in the current study, the lOFC lesion group exhibited an overall augmentation of context-induced cocaine-seeking behaviors in the absence of a response-contingent CS.
Post-training lOFC lesions fail to alter context-induced reinstatement of cocaine-seeking behaviors
Unlike the effects of both pre-training lOFC lesions and post-training lOFC functional inactivation, post-training lOFC lesions failed to enhance drug context-induced cocaine-seeking behaviors relative to the sham lesions (Fig. 5B–C). Both the lOFC lesion and sham group exhibited more robust responding following exposure to the cocaine-paired context relative to that observed in the inactivation and pre-training lesion experiments. This increase in responding likely stemmed from incubation (Tran-Nguyen et al., 1998; Grimm et al., 2001), a reliable time-dependent increase in cocaine-seeking behavior following experimenter-imposed abstinence from cocaine (i.e., 7-day post-lesion period in the present study). It is possible that a ceiling effect resulting from high response rates in the control group obscured a post-training lOFC lesion-induced potentiation of drug context-induced cocaine seeking. However, this is somewhat unlikely given that the lesion group exhibited a trend for less responding relative to the sham control group. Methodological requirements make it impossible to eliminate incubation effects in the post-training lesion experiment. Nevertheless, the results of this experiment are critical for exploring whether pre-training lOFC lesions and post-training lOFC functional inactivation differentially altered context-induced cocaine seeking in the present study because 1) these manipulations occurred at different points relative to associative learning or 2) these manipulations produced fundamentally different neurochemical effects. The results of the post-training lOFC lesion experiment failed to support the first possibility given that post-training lOFC lesions, unlike post-training lOFC functional inactivation, failed to significantly attenuate context-induced cocaine-seeking behaviors relative to sham lesions. It is also unlikely that post-training lOFC lesions and functional inactivation differentially altered context-induced reinstatement solely due to differences in their neurochemical effects because this would not account for differences between the effects of post-training and pre-training lOFC lesions. Specifically, post-training lOFC lesions, unlike pre-training lOFC lesions, failed to potentiate context-induced cocaine-seeking behavior.
Based on these collective findings, we speculate that lOFC lesions trigger neuroadaptations and, perhaps related, alterations in associative learning that enhance context-induced motivation for cocaine. While incubation effects may obscure enhanced cocaine-seeking behavior in the post-training lOFC lesion group, it is also possible that the neuroadaptations require more time to develop than the period available between lesion induction and reinstatement testing in the post-training lesion experiment. Therefore, animals with post-training lOFC lesions might display an intermediate state of neuroplasticity that compensated for decreased cocaine-seeking behavior stemming from acute loss of lOFC function but was insufficient to increase motivation for cocaine relative to sham lesions. Taken together, the findings from the pre- and post-training lesion experiments indicate that loss of OFC output during the formation of stimulus-cocaine associations in humans may underlie enhanced cue-induced neural reactivity observed in former cocaine users.
The role of the OFC in drug relapse behaviors
Overall, the findings from the present study indicate that the lOFC exerts a complex regulatory influence over the incentive motivational effects of cocaine-paired environmental stimuli (Jentsch & Taylor, 1999). The finding that the lOFC may play a different role in explicit CS-induced and context-induced cocaine-seeking behavior is consistent with the idea that different reinstatement triggers induce drug-seeking behavior by recruiting partially distinct neural mechanisms. Because context-induced cocaine-seeking behavior is attenuated by acute lOFC functional inactivation, but is enhanced by chronic loss of lOFC output, neuroadaptations elicited in other elements of the relapse circuitry during associative learning processes may account for enhanced motivation for cocaine reinforcement. Importantly, the lOFC may regulate cocaine seeking via its robust connections with the BLA, hippocampus, prefrontal cortex, thalamus, basal ganglia, and nucleus accumbens (Krettek & Price, 1977; Carmichael & Price, 1995a;1995b; Haber et al., 1995; Groenewegen et al., 1997; Groenewegen & Uylings, 2000). Of these brain regions, the dorsal hippocampus plays a selective role in context-induced reinstatement (Fuchs et al., 2005; Fuchs et al., 2007), the amygdala is critical for context-induced and CS-induced reinstatement (See et al., 2001; Sun & Rebec, 2003; Fuchs et al., 2005; Lasseter et al., in prep), and the prefrontal cortex, ventral hippocampus, and nucleus accumbens are necessary for cocaine-primed, CS-induced, and context-induced reinstatement (McFarland & Kalivas, 2001; McLaughlin & See, 2003; Rogers & See, 2007; Fuchs et al., 2008; Lasseter et al., in prep). The differential effects of pre-training lOFC lesions on these forms of reinstatement suggest that different reinstatement triggers may engage distinct subcircuits within the lOFC, and these may, in turn, develop a different set of neuroadaptations following lOFC damage. We hypothesize that the existence of such subcircuits may explain the concomitant presence of chronic hypofrontality and enhanced cocaine-cue neural activation in the OFC in humans (Volkow et al., 1991; London et al., 2000; Volkow & Fowler, 2000; Franklin et al., 2002; Bolla et al., 2003; Matochik et al., 2003; Hearing et al., 2008; Zavala et al., 2008). In future studies, it will be of particular interest to systematically investigate the nature of lOFC lesion-induced neuroadaptive changes in the relapse circuitry and to assess the distinct contribution of these putative neuroadaptations to addictive behavior. Exploring how lOFC damage contributes to cognitive and behavioral impairments in the lOFC-lesioned rat may help elucidate potential treatment strategies for cocaine addiction.
Acknowledgments
The authors would like to thank KaiCee Ponds and Stephanie Kaszycki for their excellent technical assistance and insightful comments on an earlier version of this manuscript. The authors do not have any conflicts of interest to disclose. This work was supported by National Institute on Drug Abuse (NIDA) R01 DA017673, a NIDA R01 grant supplement to promote diversity in health-related research (DA017673-S1), NIDA T32 DA07244, the University of North Carolina at Chapel Hill Junior Faculty Development Award, and the Mason and Linda Stephenson Faculty Award.
References
- Atkins AL, Mashhoon Y, Kantak KM. Hippocampal regulation of contextual cue-induced reinstatement of cocaine-seeking behavior. Pharmacol Biochem Behav. 2008;90:481–491. doi: 10.1016/j.pbb.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant S, Contoreggi C, Links J, Metalfe J, Weyl LH, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995a;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995b;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to Pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Shaham Y. Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci. 2002;116:169–173. doi: 10.1037//0735-7044.116.1.169. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Weber SM, Rice HJ, Neisewander JL. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res. 2002;929:15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004a;176:495–496. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J Neurosci. 2004b;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiol Learn Mem. 2008;89:567–573. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Uylings HB. The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J Psychopharmacol. 1997;11:99–106. doi: 10.1177/026988119701100202. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kunishio K, Mizobuchi M, Lynd-Balta E. The orbital and medial prefrontal circuit through the primate basal ganglia. J Neurosci. 1995;15:4851–4867. doi: 10.1523/JNEUROSCI.15-07-04851.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Struct Funct. 2008;213:215–227. doi: 10.1007/s00429-008-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ. The effects of selective orbitofrontal cortex lesions on the acquisition and performance of cue-controlled cocaine seeking in rats. Ann N Y Acad Sci. 2003;1003:410–411. doi: 10.1196/annals.1300.038. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Bilateral orbital prefrontal cortex lesions in rhesus monkeys disrupt choices guided by both reward value and reward contingency. J Neurosci. 2004;24:7540–7548. doi: 10.1523/JNEUROSCI.1921-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. Eur J Neurosci. 2005;22:2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the ventral hippocampus in context-induced motivation for cocaine reinforcement (in prep) [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: Functional Imaging. Cereb Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. NeuroImage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- McDannald MA, Saddoris MP, Gallagher M, Holland PC. Lesions of orbitofrontal cortex impair rats’ differential outcome expectancy learning but not conditioned stimulus-potentiated feeding. J Neurosci. 2005;25:4626–4632. doi: 10.1523/JNEUROSCI.5301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meyers RA, Zavala AR, Speer CM, Neisewander JL. Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci. 2006;120:401–412. doi: 10.1037/0735-7044.120.2.401. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Pears A, Parkinson JA, Hopewell L, Everitt BJ, Roberts AC. Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates. J Neurosci. 2003;23:11189–11201. doi: 10.1523/JNEUROSCI.23-35-11189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland P, Schoenbaum G. Differential roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. J Neurosci. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Saddoris MP, Gallagher M, Holland PC. Orbitofrontal lesions impair use of cue-outcome associations in a devaluation task. Behav Neurosci. 2005;119:317–322. doi: 10.1037/0735-7044.119.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JL, See RE. Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem. 2007;87:688–692. doi: 10.1016/j.nlm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: theoretical and treatment implications. Int J Addict. 1990;25:957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Nugent SL, Saddoris MP, Setlow B. Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. Neuroreport. 2002;13:885–890. doi: 10.1097/00001756-200205070-00030. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry. 2008;63:256–262. doi: 10.1016/j.biopsych.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Grimm JW, Kruzich PJ. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology. 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Franz TM, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Sun WL, Rebec GU. Lidocaine inactivation of ventral subiculum attenuates cocaine-seeking behavior in rats. J Neurosci. 2003;23:10258–10264. doi: 10.1523/JNEUROSCI.23-32-10258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait DS, Brown VJ. Difficulty overcoming learned non-reward during reversal learning in rats with ibotenic acid lesions of orbital prefrontal cortex. Ann N Y Acad Sci. 2007;1121:407–420. doi: 10.1196/annals.1401.010. [DOI] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci. 1999;11:4099–4109. doi: 10.1046/j.1460-9568.1999.00834.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abuse. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78:610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN, Neisewander JL. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse. 2008;62:421–431. doi: 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]