Abstract
Older adults often show bilateral brain activation, compared to unilateral activation in younger adults, when performing tasks in domains of age-associated cognitive impairment, such as episodic and working memory. Less is known about activation associated with performance in cognitive domains that are typically unaffected by healthy aging. We used event-related functional magnetic resonance imaging to examine age-related patterns in brain activation associated with a form of implicit memory, repetition priming, which is typically preserved in healthy aging. Sixteen younger adults and 15 nondemented older adults performed semantic judgments (abstract/concrete) on single words in a study phase. In a test phase, identical judgments were made for repeated and new words. Younger and older adults showed similar response-time benefits (repetition priming) from repeated semantic classification. Repetition priming was associated with repetition-related reductions of prefrontal activation in both groups, but the patterns of activation differed between groups. Both groups showed similar activation reductions in dorsal left inferior prefrontal cortex (LIPFC), but older adults showed less reduction than younger adults in ventral and anterior LIPFC. Activation reductions were exclusively left-lateralized for younger adults, whereas older adults showed additional reductions in multiple regions of right frontal cortices. Right prefrontal activation reductions in older adults correlated with better repetition priming and better performance on independent tests of semantic processing. Thus, reduced asymmetry of prefrontal activation reductions in healthy aging was related to conceptual repetition priming, a form of learning that is spared in aging, and with the sparing of semantic memory.
Keywords: Conceptual repetition priming, Aging, fMRI, Semantic classification, Prefrontal cortex, Memory and Aging Project
Introduction
Aging disproportionately affects some cognitive abilities (e.g., long-term memory, working memory, fluid intelligence, processing speed), but does not affect other abilities (semantic knowledge, vocabulary; reviewed in Hedden and Gabrieli, 2004; Reuter-Lorenz and Lustig, 2005). Within long-term memory, aging is associated with reduced performance on explicit memory tests that require direct and conscious recollection of previously experienced events, such as tests of recall and recognition (Craik and Jennings, 1992; Light, 1991; Zacks et al., 2000). In contrast, age-invariance is often the case when memory is measured indirectly on implicit memory tests. Healthy older adults often perform at the level of younger adults on many kinds of implicit memory tests, including repetition priming in which performance is enhanced (i.e., decreased latency or increased accuracy) for repeated stimuli relative to new stimuli (see Cabeza et al., 2005; Fleischman, 2007; Fleischman and Gabrieli, 1998; LaVoie and Light, 1994; Light et al., 2000a,b; Mitchell and Bruss, 2003; Rybash, 1996, for reviews).
Neuroimaging has allowed the examination of the brain bases of compromised and spared cognitive functions in aging. To date, these studies have yielded mixed results, showing region-specific overactivation or underactivation in older adults, compared to younger adults, depending on task and material characteristics (reviewed in Cabeza, 2002; Reuter-Lorenz and Lustig, 2005; Rajah and D'Esposito, 2005; Reuter-Lorenz and Cappell, 2008). However, one pattern often observed in older adults is bilateral activation of prefrontal cortex (PFC) compared to unilateral recruitment in younger adults. Bilateral activations in older adults produce a reduction in hemispheric lateralization (i.e., reduced asymmetry) of activation observed in younger adults. For example, in tasks associated specifically with left-hemisphere prefrontal cortex (LPFC) activation in younger adults, such as verbal working memory and semantic encoding, older adults often show not only left prefrontal activation, but also contralateral recruitment of right-hemisphere PFC (RPFC; e.g., Cabeza et al., 2004; Logan et al., 2002; Morcom et al., 2003; Rosen et al., 2002; Stebbins et al., 2002). Likewise, in tasks associated specifically with RPFC activity in younger adults, such as visual encoding and episodic retrieval, older adults often show additional contralateral recruitment of LPFC (e.g., Bäckman et al., 1997; Cabeza et al., 1997, 2004; Madden et al., 1999).
Although the evidence for reduced lateralization in older adults is strong, the function of the phenomenon is still debated (Buckner, 2003; Buckner and Logan, 2003; Cabeza, 2002; Rajah and D'Esposito, 2005; Reuter-Lorenz and Cappell, 2008; Reuter-Lorenz and Lustig, 2005). Reduced lateralization may reflect an age-related compromise in recruiting specialized neural mechanisms (dedifferentiation hypothesis) or it may reflect a mechanism that aids in counteracting age-related neurocognitive decline (compensation hypothesis). Studies that provide support for the compensation hypothesis demonstrate that reduced lateralization is correlated with better performance on independent cognitive tests in older adults. For example, older adults who displayed a bilateral pattern of PFC activity were faster responders in a verbal working memory task than those who did not display the bilateral pattern (Reuter-Lorenz et al., 2000). Similarly, older adults with low memory scores recruited similar unilateral PFC regions as those recruited by younger adults during semantic encoding (LPFC; Rosen et al., 2002) or source memory (RPFC; Cabeza et al., 2002), but older adults with high memory scores recruited bilateral PFC regions in the service of these tasks. Thus, bilateral recruitment of PFC in older adults appears to provide additional neurocognitive resources that minimize deleterious effects of aging on working memory and explicit long-term memory.
Neuroimaging studies of healthy older adults have focused on domains of impairment such as working memory and episodic memory (encoding or retrieval; e.g., Logan et al., 2002). Only a few studies have focused on domains of preserved function such as repetition priming (e.g., Bäckman et al., 1997; Lustig and Buckner, 2004) in which memory is measured indirectly as facilitation in cognitive processing. Repetition priming in younger adults is associated with reduced activation for repeated relative to initial processing of a stimulus in specific brain regions recruited for processing the first exposure to the stimulus (reviewed in Schacter and Buckner, 1998; Henson, 2003). Repetition priming for conceptual information, as opposed to perceptual information (Roediger and McDermott, 1993), is most associated with reduced activation in the left inferior prefrontal cortex (LIPFC; Buckner et al., 2000; Demb et al., 1995; Gabrieli et al., 1996; Wagner et al., 1997, 2000; reviewed in Gabrieli, 1998; Gabrieli et al., 1998; Henson, 2003).
The lateralization of repetition-priming related PFC activations is of particular theoretical interest in older adults. On the one hand, one may expect that because conceptual repetition priming is behaviorally similar in younger and older adults, patterns of brain activation would also be similar in younger and older adults. In that case, one would expect similar LIPFC reductions in younger and older adults as the neural correlate of a form of learning that is not altered by healthy aging. This would support the view that the reduced lateralization of PFC activation often observed in older adults reflects compensatory mechanisms only for domains of cognition, such as explicit memory, that are altered by healthy aging. On the other hand, preserved repetition priming in older adults could be associated with reduced LIPFC activation and increased RPFC activation relative to younger adults. This finding would support the view that reduced lateralization of brain function occurs in healthy aging not only in domains where reduced ability requires compensatory recruitment of additional brain regions, but also in domains that are not affected behaviorally by aging.
Prior functional neuroimaging studies have not found altered lateralization for intact repetition priming in healthy older adults relative to younger adults. One PET study reported similar priming-related reductions for younger and older adults in right posterior visual cortex on a perceptual priming task (Bäckman et al., 1997; see also Daselaar et al., 2005). More similar to the present study, Lustig and Buckner (2004) examined priming-related activations on a conceptual task (judging whether words referred to living or nonliving things) in younger and older healthy adults and patients with Alzheimer's disease. The healthy younger and older adults exhibited similar behavioral magnitudes of repetition priming. In an a priori region of interest (ROI) analysis in LIPFC, younger and older adults showed similar magnitudes of activation. Right inferior prefrontal (RIPFC) activation was examined only in a single region selected as homologous to the left LIPFC ROI. Priming-related activation was observed in the RIPFC region, but this was symmetrical in left and right PFC for both younger and older healthy adults. Thus, Lustig and Buckner (2004) demonstrated intact LIPFC activation in healthy older adults, but no age-related change in hemispheric lateralization.
In the present study, we used event-related fMRI to examine conceptual priming-related patterns of activation for healthy younger and older adults. Participants judged whether words referred to abstract (hope, love) or concrete (wall, table) entities in a study-phase and these judgments were repeated in a test-phase along with judgments of new words. We hypothesized that younger and older groups would have equal magnitudes of behavioral repetition priming, measured as enhanced speed of response for repeated words relative to new words. Further, we hypothesized a reduction in LIPFC activation for repeated relative to new words in younger adults (Demb et al., 1995; Gabrieli et al., 1996), and a comparable reduction in older adults (Lustig and Buckner, 2004). The open question was whether or not healthy older adults would exhibit enhanced priming-related activation in RPFC and thus reduced hemispheric lateralization.
Methods
Participants
Older participants were recruited from continuous care retirement communities, retirement homes, and subsidized housing facilities in and around Cook County in northeastern Illinois as part of the Rush Memory and Aging Project, a longitudinal clinicopathologic study of aging and Alzheimer's disease (Bennett et al., 2005; Wilson et al., 2003). Each older participant underwent a structured, uniform evaluation that incorporated the procedures used by the Consortium to Establish a Registry for Alzheimer's Disease (Morris et al., 1989). The evaluation included a medical history, neurological examination, cognitive function testing, and a review of a brain scan when available. All older participants (N=15; 5 males; age M=78.7, SD=8.6, range=65–90; education M=14.3, SD=3.9, range=8–22 years; Mini-Mental Status Examination Score M=28.5, SD=1.1, range=27–30), included in this study were free of dementia and other neurological and psychiatric conditions. Younger participants (N=16; 5 males; age M=23.4, SD=2.9, range 18–28; education M=12.8, SD=1.9, range 12–18 years), who were healthy by self-report, were recruited from local colleges and paid $10.00 per hour for participation. All participants were right-handed and had normal or corrected to normal vision. The study was approved by the Institutional Review Board of Rush University Medical Center.
Clinical evaluation
Each older participant underwent the structured, uniform evaluation used in the Rush Memory and Aging Project (Bennett et al., 2005). The evaluation included a medical history, neurological examination, and cognitive function testing. Cognitive performance tests were reviewed by a board-certified neuropsychologist (see Cognitive function tests below). Participants were evaluated in person by a board-certified or board-eligible, neurologist, geriatrician or advanced practice geriatric nurse practitioner, with expertise in the evaluation of older persons with and without dementia. Based on this evaluation, participants were classified with respect to dementia, Alzheimer's disease, stroke, Parkinson's disease, and other common age-related neurologic conditions. The diagnosis of dementia was made by the examining physician based on NINCDS-ADRDA criteria (McKhann et al., 1984), which require a history of cognitive decline and evidence of impairment in at least two cognitive domains.
Cognitive function tests
Cognitive function of the older adults was assessed with a set of 19 tests (Wilson et al., 2003). The Mini-Mental State Examination was used for descriptive purposes only and one test, Complex Ideational Material (Goodglass and Kaplan, 1972) was used only in diagnostic classification. The remaining 17 tests were selected to assess five cognitive domains: episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability. There were seven episodic memory measures: Word List Memory, Recall, and Recognition (Morris et al., 1989), immediate and delayed recall of the East Boston Story (Albert et al., 1991), and Story A from Logical Memory (Wechsler, 1987). Semantic memory was assessed with three tests: a 15-item version (Morris et al., 1989) of the Boston Naming Test (Kaplan et al., 1983); Verbal Fluency (Morris et al., 1989), which involved generating exemplars from two semantic categories (animals; fruits and vegetables) in separate 1-min trials; and a 15-item reading test (Wilson et al., 2002, 2003) which requires reading aloud words with atypical spelling-sound correspondence (e.g., “impugn”). Working memory was assessed with Digit Span Forward and Digit Span Backward (Wechsler, 1987) and with Digit Ordering (Cooper and Sagar, 1993) administered in a span format (Wilson et al., 2002, 2003). Two tests of perceptual speed were given: the oral version of the Symbol Digit Modalities Test (Smith, 1982), and Number Comparison (Ekstrom et al., 1976) which involved rapidly classifying pairs of 3- to 10-digit numbers as same or different. Visuospatial ability was assessed with a 15-item version of Judgment of Line Orientation (Benton et al., 1994) and a 16-item version of Standard Progressive Matrices (Raven et al., 1992). Summary measures of these five cognitive domains were formed, based in part on a principal-components factor analysis, as previously described (Wilson et al., 2003). Raw scores on each test were converted to z scores (mean of 0, standard deviation of 1) and then the average z score among tests in a given domain was computed. At least half of the tests in each domain had to have a valid score or the summary measure was treated as missing. Older participants were additionally administered a modified version of the WAIS-R Vocabulary Subtest (Wechsler, 1987) in which they were asked to produce definitions to every other word taken from the full version of the subtest.
Materials
The stimuli consisted of 240 words, 3–11 letters long, half concrete (rating range 5.81–6.79) and half abstract (rating range 1.18–3.48), based on Paivio et al. (1968) (rating range 1.00–7.00), divided into four lists of 60 words to be used in four study-test runs. These lists were equated for word frequency, word length, and abstract–concrete ratings. Each list was further divided into two 30-word study lists (half abstract). This way, each of four study phases comprised 30 new words and each of four test phases comprised 30 studied, and 30 new unstudied words (half abstract). Words were counterbalanced across participants within a group such that repeated items for one participant were new items for another participant. The stimuli were presented in central vision, Geneva font in white capital letters on black background.
Behavioral procedure
The experiment consisted of four study-test runs, four study and four test phases, all of which were scanned. In each study phase, the participant made semantic judgments (abstract or concrete?) about each word twice in two separate blocks, responding with a button press. A new set of words was presented in each study phase. During each event-related fMRI test phase that followed, 30 new words and 30 words repeated from the study phase were randomly mixed with 30 baseline fixation trials. In each test phase, repeated, new, and fixation trials were randomly intermixed with first-order counterbalancing such that each trial type followed all trial types equally often to allow subtraction to cancel out the effects of hemodynamic overlap across adjacent trials (see Dale and Buckner, 1997; Buckner et al., 1998). In two of the test phases, participants repeated the abstract/concrete semantic judgment (implicit test). In the other two test phases, participants made a recognition judgment (explicit test), indicating for each word whether they had seen it in the preceding study phase (old word) or not (new word). Order of tests (the two explicit tests first vs. the two implicit tests first) and mapping of hands (right vs. left) to responses (old vs. new and abstract vs. concrete) were counterbalanced across the participants. The fMRI data reported in this paper came from the two implicit tests. Explicit memory fMRI data will be described in a separate report.
For both study and test phases, each trial lasted 3500 ms. Target words or fixation crosses were presented at central fixation for 2000 ms, followed by a fixation cross for 1500 ms. A colorized reminder of the mapping of the hands (left/right) to the task (abstract/concrete, old/new) was displayed on the screen below the word throughout each study and test phase. At the onset of the study and test phases of each run, a 10,000 ms task prompt (“abstract–concrete” or “old–new”) was presented at central fixation. Each study-test run lasted 9 min and 16 s, separated by approximately 3 min of rest.
fMRI procedure
MRI data were acquired using a 1.5 T General Electric Signa scanner. T1-weighted structural images were acquired first, including a high-resolution 3D SPGR image (TE/TR 7/34 ms, flip angle 35, 124 1.6 mm gapless slices) and a gradient echo image that was acquired at the same plane as the functional images (TE/TR 1000/500 ms, flip angle 84, 21 6 mm gapless slices). Functional images were then acquired using a T2*-weighted gradient echo spiral sequence sensitive to blood-oxygen level-dependant (BOLD) contrast (Glover and Lai, 1998). Each whole-brain acquisition consisted of 21 axial slices aligned parallel to the plane of the anterior commissure and the posterior commissure (6 mm gapless slices, 3.75 mm × 3.75 mm in-plane resolution, 240 mm FOV, 64 × 64 matrix, TE/TR 40/1991 ms, flip angle 84). A total of 276 volume images per run were taken continuously. The last 159 volume images of each run that corresponded to the two implicit test phases were included in the analyses.
To reduce burden on the older participants, the experiment was divided into two parts; each part included two study-test runs, with the test phases within a part being of the same type (either two implicit runs followed by two explicit runs or the reverse). Participants were taken out of the scanner after the first two study-test runs and after about an hour break went back into the scanner to go through the third and fourth study-test runs. An additional structural image was acquired in the plane of the functional runs to allow for localization of the functional activity in the second part of the experiment.
In both parts of the experiment, cushions and a tape on the forehead were used to minimize head movement. Ear-plugs were used to minimize scanner noise, and communication with the participant was enabled via a microphone and speaker placed in the scanner. A PowerBook G4 and Psyscope software (version 1.2.1; Cohen et al., 1993) controlled stimulus display and recorded response from a key-press device. Stimuli were projected to a screen at the head of the scanner and were viewable via a mirror attached to the head coil.
Data analysis
Behavioral
Behavioral responses on the implicit test were examined in a 2×2 ANOVA, with age (younger, older) as a between-subjects factor and word presentation (new, repeated) as a within-subject factor. Simple effects were examined using paired-sample t-tests. For the explicit test, corrected recognition scores (Hits-False Alarms) were compared between younger and older adults using an independent-sample t-test.
fMRI
SPM99 (Wellcome Department of Cognitive Neurology, London, UK) was used to process and analyze the functional data.
To correct for differences in acquisition time, all slices were resampled in time relative to the acquisition time of the middle slice, using sinc interpolation in time. All volumes were then realigned to the first volume of the first semantic judgment session (using sinc interpolation) to correct for motion. Estimated motion parameters computed by SPM99 were examined on a participant-by-participant basis; the amount of absolute motion did not exceed 2 mm in the implicit part of the experiment for any participant. The T1 structural volume was coregistered with the mean realigned functional volume and segmented to gray and white matter. The gray matter was then normalized to the MNI gray matter template (based on Montreal Neurological Institute reference brain). The functional volumes were normalized using the normalization parameters that were generated based on the normalization of the gray matter. Then, the functional volumes were smoothed with an 8 mm full-width half-maximum isotropic Gaussian kernel.
Differences between stimulus conditions were examined by using the general linear model (GLM; Friston et al., 1994), modeling the activation at each voxel using an HRF function. Statistical analysis was performed using a mixed effects model; fixed effects were used for single-subject analyses and random effects for group analysis (Holmes and Friston, 1998). For group analyses, contrast images were computed for each participant, then submitted to a one-sample t-test (Friston et al., 1999). These t-maps were thresholded at p<.001 (uncorrected for multiple comparisons) with a spatial extent threshold of 5 contiguous voxels. Group activation maps from this analysis were overlaid on the single-subject T1 image.
To directly compare and contrast priming-related reductions of activation in younger and older adults, we identified activations that were common and unique to the two age groups. Conjunction analysis of specific contrast, entered into a random effects analysis, was used to identify voxels showing statistically significant reductions across both younger and older groups (Nichols et al., 2005). A statistical threshold of p<.01 (uncorrected), with a spatial extent threshold of 5 contiguous voxels, was used for each group separately for each contrast. To identify regions that showed greater reductions for one age group compared to the other, we used a two-sample t-test thresholded at p<.001 (uncorrected for multiple comparisons).
Results
Behavioral
Due to technical problems, the behavioral responses of one younger participant were not fully recorded. Thus, the behavioral analyses included 15 younger and 15 older participants. Participants exhibited repetition priming by responding significantly faster to repeated words (M=976.7 ms, SE=33.6) than to new words (M=1128.6 ms, SE=42.7), main effect of word repetition, F(l,28)=72.87, p<.0001, MSE=4748.7. Younger participants (M=967.9 ms, SE=55.2) responded faster than older participants (M=1137.4 ms, SE=41.4), main effect of age, F(l,28)=6.04, p=.02, MSE=71390.3. Critically, there was no interaction between repetition priming and age(F<1, p=.87), indicating that the younger and older groups did not differ significantly in priming magnitude. Both the younger, t(14)=5.61, p<.0001, and the older, t(14)=6.53, p<.0001, groups showed independent and significant repetition priming (Table 1). There was no group difference in priming when calculating the relative improvement in RT for repeated versus new words (percent priming=[RTnew-RTold]/RTnew), t<l (Table 1).
Table 1.
Reaction time means for new and repeated words, priming scores, and percent priming scores (and standard errors) in the semantic judgment task, separately for younger and older groups
| New | Repeated | Priming | %Priming | |
|---|---|---|---|---|
| Young | 1042.3 | 893.5 | 148.9 | .14 |
| (63.3) | (49.3) | (26.5) | (.02) | |
| Old | 1214.9 | 1060.0 | 154.9 | .12 |
| (49.7) | (35.2) | (23.7) | (.02) |
The similar priming effects for younger and older groups stood in marked contrast to the age effect in explicit memory. The younger group performed significantly better (Hits-FA=.88; SE=.01) than the older group (Hits-FA=.64; SE=.04), t(28)=5.70, p <.0001. Presentation order of the implicit and explicit tests had no effect on priming or percent priming scores (all ps>.24).
fMRI
Four types of analyses were performed to characterize the effects of aging on repetition-related activation reductions. First, we contrasted new and repeated items to reveal regions that showed reduced activation for repeated items, separately for each age group. Second, we identified brain regions that were common (conjunction analysis) and unique (group contrast) to the two age groups and then directly contrasted repetition-related reductions between groups within these regions of interest (ROIs). Third, to further characterize repetition-related reductions in aging, we identified ROIs that showed significant correlations between magnitude of repetition-related activation reduction and behavioral priming. Finally, for the older participants, we examined associations between repetition-related activation reduction, behavioral priming, and performance on independent neuropsychological tests.
Because individual outliers may greatly influence the results of correlational analyses with small sample sizes (Wager et al., 2005), two younger participants with extreme scores (2.5 SD beyond the group mean) were excluded from the correlational analyses. One younger participant had an extreme behavioral priming score and the other had an extreme brain activation score in the LIPFC region. This resulted in a sample of 14 younger and 15 older participants for the brain activation analyses and a sample of 13 younger and 15 older participants for the brain-behavior correlational analyses. Excluding these outliers did not significantly change any of the behavioral priming effects.
Repetition-related activation reductions
To observe repetition-related reductions for the younger and older groups separately, whole brain analyses were performed for each of the age groups examining activations that were greater for new than for repeated items (Fig. 1, Table 2). Both age groups showed repetition-related reductions in LIPFC (BA 44/45 and 9 for younger group and BA 45/46 for older group). Repetition-related reductions were exclusively left-lateralized for the younger group, whereas the older group showed additional repetition-related reductions in right PFC regions, including right middle frontal cortex (BA 46), right superior frontal cortex (BA 9), medial frontal cortex (BA 8), and anterior cingulate (BA 24).
Fig. 1.
Whole-brain activation maps for younger adults (green) and older adults (red), showing regions of significantly greater activation for new compared to repeated words (superimposed on a 3D single-subject T1 image, using a threshold of p=.001, uncorrected). Frontal regions of activation were left lateralized for younger adults, but bilateral for older adults.
Table 2.
Maxima within regions demonstrating BOLD signal changes when contrasting new> repeated words, separately for younger and older groups
| Approximate gyral location | Regiona | Atlas coordinates |
Z value | Volumeb | ||
|---|---|---|---|---|---|---|
| Young | ||||||
| L inferior frontal gyrus | 44/45 | −50 | 16 | 3 | 3.96 | 248 |
| L inferior frontal gyrus | 45 | −50 | 22 | 15 | 3.30 | 48 |
| L inferior frontal gyrus | 9 | −42 | 7 | 25 | 3.17 | 48 |
| Old | ||||||
| Medial frontal gyrus | 8 | 2 | 26 | 45 | 3.93 | 424 |
| Anterior cingulate | 24 | 10 | 2 | 44 | 3.93 | 128 |
| Anterior cingulate | 24 | −2 | −2 | 41 | 3.33 | 64 |
| R superior frontal gyrus | 9 | 14 | 48 | 23 | 3.7 | 56 |
| L inferior/middle frontal gyrus | 45/46 | −42 | 26 | 23 | 3.61 | 56 |
| L inferior/middle frontal gyrus | 45/46 | −42 | 15 | 23 | 3.34 | 40 |
| R middle frontal gyrus | 46 | 38 | 34 | 22 | 3.38 | 56 |
Regions are named based on their approximate Brodmann Area (BA) in the Talairach and Tournoux (1988) atlas for cortical regions.
Volume reported in mm3.
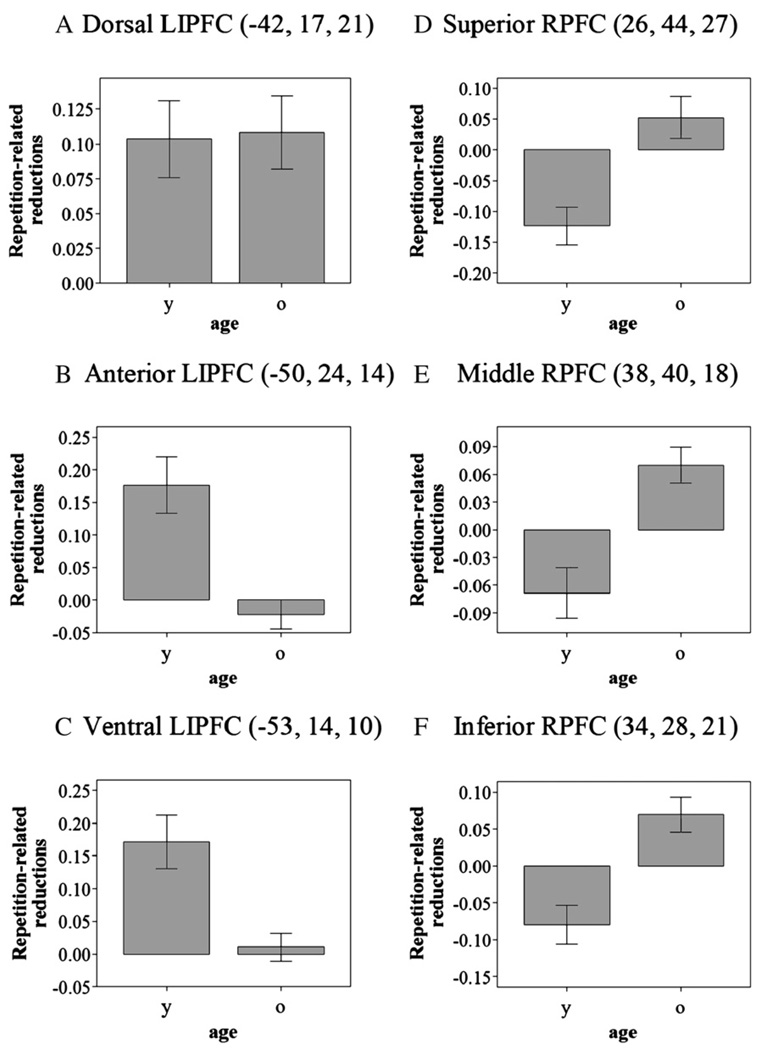
The conjunction analysis used to identify regions of common activation in older and younger groups identified one region of shared activation. The LIPFC (BA 44/46, Fig. 2a, Table 3) exhibited reduced activation for repeated relative to new words in both younger, t(13)=3.77, p=.002, and older, t(14)=4.11, p=.001, groups (Fig. 3A).
Fig. 2.
Regions of similar and dissimilar activation in younger and older adults. Dorsal LIPFC region showed common activation in younger and older groups (Panel A, p=.01 for each group separately). Anterior and ventral LIPFC regions showed greater activations for younger than older adults (Panel B, p=.001). Right frontal and other regions showed greater activations for older than younger adults (Panel C, p=.001).
Table 3.
Maxima within regions demonstrating BOLD signal changes when contrasting new> repeated words in regions common to young and old and in regions where this contrast was greater for young than old or greater for old than young
| Approximate gyral location | Regiona | Atlas coordinates | Z value | Volumeb | ||
|---|---|---|---|---|---|---|
| Common (conjunction)c | ||||||
| L inferior/middle frontal gyrus | 44/46 | −42 | 17 | 21 | 408 | |
| Young >O1dd | ||||||
| L inferior frontal gyrus | 45 | −50 | 24 | 14 | 3.62 | 32 |
| 44 | −53 | 14 | 10 | 3.55 | 8 | |
| Old > Youngd | ||||||
| R precentral gyrus | 6 | 38 | −16 | 32 | −4.07 | 128 |
| R cerebellum | 36 | −67 | −20 | −3.89 | 152 | |
| L lingual gyrus | 18 | −12 | −90 | −17 | −3.72 | 552 |
| Cingulate gyrus | 24 | 0 | −2 | 37 | −3.63 | 296 |
| L caudate | −16 | 26 | 10 | −3.63 | 152 | |
| R anterior cingulate | 32 | 16 | 32 | 19 | −3.57 | 40 |
| R inferior/middle frontal gyrus | 45/46 | 34 | 28 | 21 | −3.5 | 112 |
| R cingulate gyrus | 32 | 8 | 17 | 32 | −3.43 | 80 |
| L frontal lobe white matter | −28 | −2 | 35 | −3.42 | 224 | |
| R middle frontal gyrus | 10 | 38 | 40 | 18 | −3.42 | 48 |
| R superior frontal gyrus | 10 | 26 | 44 | 27 | −3.37 | 48 |
Regions are named based on their approximate Brodmann Area (BA) in the Talairach and Tournoux (1988) atlas for cortical regions.
Volume reported in mm3.
Thresholded at p<.01 (uncorrected for multiple comparisons) for each group separately.
Thresholded at p<.001 (uncorrected for multiple comparisons).
Fig. 3.
Repetition-related activations in three LIPFC ROIs and three RPFC ROIs. Younger adults (marked with y) showed repetition-related activations only in left lateralized regions, whereas older adults (marked with o) showed bilateral repetition-related activations. Error bars represent standard error of the mean.
The whole-brain, group contrast analysis revealed two LIPFC regions that showed greater reductions for the younger than for the older group (BA 45 and 44, Fig. 2b, Table 3). These regions were more anterior (−50, 24, 14) and ventral (−53, 14, 10) to the LIPFC region that showed common activation for younger and older groups. In both these regions, the younger group exhibited significantly reduced activation for repeated relative to new words, (ps<.002), whereas the older group did not exhibit reliable reductions in activation in either region (ps>.34; Fig. 3B, C). Thus, both younger and older groups demonstrated comparable and robust repetition-related reductions of activation in dorsal LIPFC, but only the younger group showed significant activations in more anterior and ventral LIPFC regions.
The reverse whole-brain group contrast analysis revealed regions in which the older group showed greater activation reductions than the younger group. This analysis revealed significantly greater reductions for the older group in multiple regions, including right inferior (BA 45/46), middle (BA 10), and superior frontal cortex (BA 10), right precentral gyrus (BA 6), left lingual gyrus (BA 18), and several anterior cingulate regions (BA 24 and 32; Fig. 2c, Table 3). Of these areas, repetition-related reductions were significant for the older adults in the right inferior (BA 45/46) and middle frontal (BA 10) regions, the anterior cingulate regions, the left lingual gyrus, the left caudate, and the cerebellum, all ps<.02. To further characterize differential RPFC reductions between groups, we compared repetition-related reductions in the three right lateral PFC regions that showed greater reductions for older than for younger groups (right inferior/middle frontal gyrus, BA 45/46; right middle frontal gyrus, BA 10; and right superior frontal gyrus, BA 10). Whereas the older group showed significant reductions in right middle (p=.003) and inferior frontal regions (p=.01; Figs. 3E, F), the young group showed significant increases of activations in all three RPFC regions (ps<.027; Figs. 3D–F). The old group did not reveal significant activation reduction in the superior RPFC ROI (p=.147; Fig. 3D), hence the between-group difference in this ROI was mostly driven by an increase of activation in the young group.
We examined the relations between left and right prefrontal activations separately in each group. For older adults, activation reductions in the anterior and ventral LIPFC ROIs did not correlate with activation reductions in any of the RPFC ROIs (all ps>.268). However, activation reductions in dorsal LIPFC ROI were positively correlated with activation reductions in the right middle frontal ROI, r=.56, p=.029, and the right inferior frontal ROI, r=.67, p=.006. For younger adults, activation reductions in the ventral LIPFC ROI were negatively correlated with activation reductions in the right superior frontal ROI, r=−.55, p=.041. All the other cross-hemisphere correlations were nonsignificant (all ps>.07).
Finally, we examined whether presentation order (implicit versus explicit test first) influenced activation findings. Activation in all ROIs was submitted to an ANOVA, with order of tests and age as between-subjects factors. Order of tests had no effect on repetition-related activation reductions in any of the ROIs, and there were no significant interactions with age (all ps>.27).
Behavior-fMRI correlations
To examine the relation between behavioral and neural repetition effects, we performed correlation analyses between behavioral priming and activation reductions, for each group separately, in 1) the ROI that was common to the two groups (dorsal LIPFC) for repetition reduction; 2) the two LIPFC ROIs that showed greater reductions for the younger than the older group; and 3) the right frontal ROIs that showed greater reductions for the older than the younger group. For both younger and older groups, there were significant positive correlations between behavioral priming and activation reductions in dorsal LIPFC, for both absolute priming scores, r=.79, p=.001 and r=.54, p=.037, respectively (Fig. 4, Panel A), and percent priming scores, r=.72, p=.005 and r=.57, p=.026, respectively. In both LIPFC ROIs that showed unique activation for the younger group, correlations were nonsignificant (all ps>.24).
Fig. 4.
Scatter plots of repetition-related activation as a function of behavioral priming for younger adults (filled squares) and older adults (empty circles), in dorsal LIPFC ROI (Panel A) and middle RPFC ROI (Panel B). Both age groups demonstrated positive correlations between behavioral priming and activation reductions in dorsal LIPFC and middle RPFC.
Additionally, a positive correlation between activation reductions and priming in right middle frontal cortex was found for younger, r=.56, p=.049, and for older, r=.59, p=.02, participants (Fig. 4, Panel B). These correlations were also significant for activation reductions with percent priming scores, r=.68, p=.011 and r=.58, p=.022, for younger and older participants, respectively. No significant correlations were found in the two other right frontal ROIs (all ps>.ll). Despite similar correlations for the younger and older groups in the right middle frontal ROI, examination of the data (Fig. 4, Panel B) revealed that whereas most older participants (13 out of 15; 87%) showed activation decreases associated with repetition (marked as positive scores on the y-axis), most younger participants (10 out of 13; 77%) showed activation increases associated with repetition (marked as negative scores).
Finally, we examined the correlation between repetition-related activation reductions and performance on the independent neuropsychological tests in the older group. Activation reductions in the dorsal LIPFC ROI showed a significant and positive association with the semantic domain measure (more activation reduction with better semantic ability; r=.67, p=.006). In addition, activation reductions in all three right frontal ROIs were positively correlated with Vocabulary score (Inferior frontal: r=.60, p=.02; Middle frontal: r=.69, p=.004; Superior frontal: r=.68, p=.005). No other neuropsychological measure was significantly correlated with repetition-related reductions in the left and right ROIs, all ps>.05.
Discussion
The findings from this study indicate that successful cognitive aging is characterized by increased bilateral PFC mediation of memory even in a domain of memory, conceptual repetition priming, that is behaviorally unaffected by healthy aging. Implicit memory, measured behaviorally by conceptual repetition priming, was similar in younger and older adults, whereas explicit memory, measured by recognition accuracy, was reduced in older adults. The similar magnitudes of behavioral repetition priming for the two groups were coupled with similar magnitudes of repetition-related activation reductions in dorsal LIPFC. Older adults, however, exhibited two differences in brain activation relative to younger adults. First, older adults had less repetition-related activation reductions than younger adults in two more ventral and more anterior LIPFC regions. Second, older adults had more repetition-related activation reductions than younger adults in right-hemisphere inferior and middle PFC, as well as in medial frontal cortex and anterior cingulate. These additional repetition-related activation reductions for the older adults in these two right PFC regions were associated with greater reductions in dorsal LIPC. Finally, both left and right PFC activation reductions were associated with better performance for the older adults on independent tests of semantic function.
As expected, there was an age-related dissociation between compromised explicit memory and spared implicit memory, with implicit memory measured via conceptual repetition priming. This finding is consistent with the frequently observed dissociation between explicit and implicit memory in aging (see Cabeza et al., 2005; Fleischman, 2007; Fleischman and Gabrieli, 1998; LaVoie and Light, 1994; Light et al., 2000a, 2000b; Mitchell and Bruss, 2003; Rybash, 1996, for reviews). In this study, these similar levels of conceptual repetition priming were associated with similar levels of repetition-related activation reduction in the region most associated with conceptual repetition priming based on studies of healthy younger adults, the LIPFC (Buckner et al., 2000; Demb et al., 1995; Gabrieli et al., 1996; Wagner et al., 2000). Although these studies all reported repetition-related reductions in LIPFC, the location of those reductions varied across LIPFC regions, including more anterior regions (BA 45/47) and more dorsal/posterior regions (BA 44/46), near our dorsal LIPFC ROI. Moreover, the finding of correlations between behavioral priming and repetition-related reductions in dorsal LIPFC for both age groups converges with the other studies reporting such correlations in frontal regions using various priming tasks in younger adults (Bergerbest et al., 2004; Orfanidou et al., 2006). For example, Maccotta and Buckner (2004) reported brain-behavioral correlations at −47, 17, 24 for repetition priming on living/nonliving judgments, proximate to our dorsal LIPFC region −42, 17, 21. Thus, older adults were similar to younger adults in terms of the magnitude of behavioral priming, the magnitude of priming-related activation reduction in dorsal LIPFC, and the relation between the magnitude of behavioral priming and the magnitude of reduced dorsal LIPFC activation across individuals.
Older and younger adults, however, differed in regards to repetition-related activation in two other more ventral and more anterior LIPFC regions. In these regions, younger adults exhibited significantly greater reductions in repetition-related activation than did older adults. Indeed, older adults failed to exhibit any repetition-related reductions in those two regions. This finding suggests that some left PFC regions that are associated with conceptual repetition priming in younger adults are no longer associated with such priming in older adults.
Older and younger adults also differed in regards to repetition-related activation in right PFC. Younger adults exhibited repetition-related activation reductions exclusively in left PFC, a left-lateralized activation that likely reflects the verbal nature of the conceptual classification task. Older adults exhibited extensive activation reductions in the right PFC, and these activation reductions were significantly greater in older than younger adults in three right PFC regions. The magnitude of activation reduction in one right PFC region correlated positively with the magnitude of behavioral priming in the older adults, and the magnitude of activation reductions in all three right PFC regions correlated positively with an independent vocabulary measure of semantic knowledge. These findings are consistent with prior studies reporting bilateral PFC engagement in older adults under conditions that yield unilateral, or asymmetric PFC engagement in younger adults (Bäckman et al., 1997; Cabeza et al., 1997, 2004; Logan et al., 2002; Madden et al., 1999; Morcom et al., 2003; Rosen et al., 2002; Stebbins et al., 2002), and also with prior studies reporting that an age-associated increase in contralateral PFC engagement is associated with successful cognitive aging (Cabeza et al., 2002; Reuter-Lorenz et al., 2000; Rosen et al., 2002). It is noteworthy that in the present study such bilateral engagement occurred for a form of memory unaffected by aging, whereas prior imaging studies found bilateral engagement in older adults in domains such as episodic and working memory that are compromised in aging (e.g., Cabeza et al., 2002; Reuter-Lorenz et al., 2000; Rosen et al., 2002).
Older adults may have recruited RPFC regions to compensate for reduced engagement of anterior and ventral LIPFC regions. Indeed, we found that greater repetition-related reductions in dorsal LIPFC were significantly, and specifically, associated with repetition-related reductions in two RPFC regions in the older group. Rajah and D'Esposito (2005) presented three criteria for determining the presence of age-related functional compensation (for similar ideas see Reuter-Lorenz and Lustig, 2005): 1) increased activation in non-task-related brain regions, which results in better behavioral performance, 2) reduced activation in task-related brain regions (as defined by brain regions engaged during task performance in younger adults) in older subjects compared to younger subjects, and that 3) activation in both task-related and non-task-related brain regions produces concomitant increases in behavioral performance and brain-behavior relationships in the older subjects. The data presented here meet these criteria.
The present findings may be related to those from other imaging studies that compared activations between younger and older groups for semantic memory. One study compared PFC activation in younger and older groups for semantic judgments relative to non-semantic judgments for words (Stebbins et al., 2002). Older adults, relative to younger adults, exhibited reduced activation in left PFC, thus demonstrating reduction in asymmetry compared to the younger adults, similar to that found in the current study. Two other studies have reported a pattern of decreased left PFC activation and increased right PFC activation, similar to the current results, when performing either a semantic judgment task (Logan et al., 2002) or a verb generation task (Persson et al., 2004). Thus, another domain of cognition that is spared in healthy aging, semantic memory or word knowledge, exhibits reduced left PFC activation and increased right PFC activation in older adults.
Conceptual priming may be interpreted as experience-dependent plasticity in the neural systems that mediate conceptual (i.e., semantic) processing. Indeed, it has been shown in young adults that a common left PFC region is associated with independent measures of conceptual processing and conceptual repetition priming (Gabrieli et al., 1996). Thus, the bilateral brain basis of conceptual repetition priming in older adults may reflect plasticity in the bilateral basis of conceptual processing in older adults. We hypothesize that if activations related to conceptual processing are decreased in left PFC and increased in right PFC in older adults (Logan et al., 2002; Persson et al., 2004; Stebbins et al., 2002), then conceptual repetition priming will exhibit the same age-associated alteration because the priming reflects plasticity in the same neural system.
Although multiple imaging studies have found increased activation of contralateral PFC in older adults, there has been little, if any, insight into why specific contralateral regions become recruited in older adults. In the present study, the same regions that exhibited greater activations for new than repeated words in the older group exhibited the opposite pattern of activation in the younger group (greater activation for repeated than novel words). The enhanced response for repeated words in younger adults may reflect incidental explicit recognition of repeated words (for a similar suggestion, see Donaldson et al., 2001) as many imaging studies have reported retrieval-related increase of activation for verbal material in RPFC (e.g., Donaldson et al., 2001; Tulving et al., 1994). Speculatively, it may be that areas sensitive to repetition in younger adults are potentiated to subserve semantic memory and repetition priming in older age. Perhaps these areas are also potentiated to subserve the recovery of language functions after left-hemisphere injury in patients with aphasia (Blasi et al., 2002; Rosen et al., 2000). It is suggested that the same region that is sensitive to repetition in young adults is also sensitive to repetition in older adults, albeit in opposite ways. Thus, properties of specific right prefrontal regions in younger adults may be identified as indicators of regions that are candidates for effective compensatory conceptual processing in older adults.
Our finding of similar repetition-related activation reductions in dorsal LIPFC for younger and older adults replicates a previous study that used a different semantic classification task (living/nonliving) and examined activation reductions in only four regions of interest in LIPFC that were identified on the basis of prior semantic encoding studies (Lustig and Buckner, 2004). Although that study did not find reliable differences in activation reductions between younger and older adults, it did note a trend in that direction in ventral LIPFC. In one predefined LIPFC ROI (BA 45/47; −45, 29, 6; similar to our anterior LIPFC ROI: −50, 24, 10), activation was reduced for repeated words such that it was no longer above zero relative to baseline for the younger adults. However, activation in this region remained above baseline in older adults and persons with Alzheimer's disease, leading the authors to conclude that “the effects of repetition on left prefrontal cortex are robust and present in aging and dementia but are not necessarily identical [to the effects in younger adults]” (Lustig and Buckner, 2004, p. 867). Thus, overall there is considerable convergence in the two studies, with both studies reporting equal activation reductions for younger and older people in dorsal LIPFC (e.g., their dorsal/posterior ROIs [−47, 17, 24] proximate to our dorsal LIPFC ROI [−42, 17, 21]), and both studies reporting either a trend (Lustig and Buckner, 2004) or a reliable reduction of activation in ventral and anterior LIPFC in older adults.
We found increased right PFC activation reductions in three clusters in older adults, whereas Lustig and Buckner (2004) reported no reliable difference in older and younger adults in right PFC activation. At least three major differences between the two studies could explain this discrepancy. First, different semantic tasks were used in the two studies, abstract/concrete versus living/nonliving. Wagner et al. (1997) found that repetition-related reductions in LIPFC were more extensive in the abstract/concrete task than in the living/nonliving task, and suggested that this could be due to differences in semantic processing demands of the two tasks. Indeed, RTs for making the abstract/concrete judgment in the current study were about 100 ms longer than RTs for making the living/nonliving judgment in Lustig and Buckner's study (however, this comparison should be interpreted with caution, as the inter-trial interval between words was longer in the current study). The abstract/concrete task used in the current study could thus be more demanding for the older adults, revealing age differences in repetition-related reductions that were not that pronounced in the Lustig and Buckner study. As noted earlier, it is unlikely that explicit memory is responsible for engaging these processes because 1) in the current study test presentation order was counterbalanced and there was no test order effect and 2) explicit contamination was more likely in the Lustig and Buckner study because implicit runs consistently followed explicit runs. Second, in the Lustig and Buckner study, the repeated words were a relatively small set of words (12 per test phase) that were studied three times and repeated three times during the test phase. In contrast, the current study used a larger set of repeated words (30 words per test phase) that were studied twice and were presented once during the test phase. The older group may have benefited disproportionately from more repetition in the Lustig and Buckner study leading to a smaller group difference in activation. Finally, different analysis strategies were used in the two studies. We performed a whole-brain analysis without prior assumptions about where in right PFC older adults may show repetition-related reductions in activation, whereas Lusting and Buckner selected a single region in the left PFC and examined only the homologous region in right PFC. It is unknown whether contralateral activations in older adults ought to occur in homologous regions. Our findings of age-related normal recruitment of dorsal LIPFC, under-recruitment of ventral LIPFC, and over-recruitment of regions of RPFC underscore the importance of viewing PFC as functionally heterogeneous and indicate that distinct patterns of age-related change may occur in distinct regions (Rajah and D'Esposito, 2005).
The recruitment of right PFC in older adults correlated positively with vocabulary scores, suggesting that the right-sided activation was associated with successful aging and consistent with other imaging studies in that regard (Cabeza et al., 2002; Grady et al., 2003; Morcom et al., 2003; Rosen et al., 2002; but see Persson et al., 2006). Because this was a cross-sectional rather than a longitudinal study, it cannot be determined whether the variation in right-lateralized activations reflect long-term differences among older adults (cognitive reserve) or more recent adaptations to cognitive aging.
This study had some limitations. First, the sample size was small. Nonetheless, there were reliable age-related differences in activation. Second, as with all fMRI studies, the analyses are correlational, and it is possible that repetition suppression and behavioral priming may co-occur without being directly linked (Sayres and Grill-Spector, 2006). However, research with repetitive transcranial magnetic stimulation (TMS) interference indicates in young adults that there is a causal link between LIPFC and conceptual repetition priming in an object classification task (Wig et al., 2005).
Prior studies reporting bilateral activation in successful aging have focused on domains that are most vulnerable to aging, such as long-term or episodic memory (e.g., Cabeza et al., 2002; Rosen et al., 2002). These changes in brain function may reflect adaptations to minimize the consequences of reduced functions of the neural systems that support episodic memory in younger adults. It could have been thought that semantic memory and conceptual repetition priming are spared in aging precisely because their neural bases are somehow protected from aging. Although it remains unclear whether the recruitment of contralateral regions on the part of older adults signals recruitment of additional brain regions to carry out the same processes or engagement of additional processes, compared to young adults, the present findings show that even in areas of well preserved learning, such as conceptual priming which is not reduced in aging, there is an age-related change in the neural systems that mediate the learning.
Acknowledgments
We thank the participants in the Rush Memory and Aging Project and staff of the Rush Alzheimer's Disease Center. This study was supported by grants R01AG17133 and RO1AG17917 from the National Institute on Aging. D. Bergerbest was supported by postdoctoral scholarships from the Fulbright Foundation and the Feldman Foundation to Stanford University.
References
- Albert M, Smith L, Scherr P, Taylor J, Evans D, Funkenstein H. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int. J. Neurosci. 1991;57:167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Almkvist O, Andersson J, Nordberg A, Winbald B, Reineck R, Langstrom B. Brain activation in young and older adults during implicit and explicit retrieval. J. Cogn. Neurosci. 1997;9:378–391. doi: 10.1162/jocn.1997.9.3.378. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Mendes de Leon CF, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25:163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, Hamsher K, de S, Varney NR, Spreen O. Contributions to Neuropsychological Assessment. 2nd ed. New York: Oxford University Press; 1994. [Google Scholar]
- Bergerbest D, Ghahremani DG, Gabrieli JDE. Neural correlates of auditory repetition priming: reduced fMRI activity in auditory cortex. J. Cogn. Neurosci. 2004;16:966–977. doi: 10.1162/0898929041502760. [DOI] [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;36:159–170. doi: 10.1016/s0896-6273(02)00936-4. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Functional-anatomic correlates of control processes in memory. J. Neurosci. 2003;23:3999–4004. doi: 10.1523/JNEUROSCI.23-10-03999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Logan JM. Frontal contributions to episodic memory encoding in the young and elderly. In: Parker AE, Wilding EL, Bussey T, editors. The Cognitive Neuroscience of Memory Encoding and Retrieval. Philadelphia: Psychology Press; 2003. pp. 57–81. [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, Rosen B, Dale AM. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–296. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Rosen BR. Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain. 2000;123:620–640. doi: 10.1093/brain/123.3.620. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, Mcintosh AR, Tulving E, Kapur S, Jennings J, Houle S, Craik FIM. Age-related differences in neural activity during memory encoding and retrieval: a positron emission tomography study. J. Neurosci. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McINtosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb. Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L, Park DC. Cognitive Neuroscience of Aging. New York: Oxford University Press; 2005. [Google Scholar]
- Cohen J, MacWhinney B, Flatt M, Provost J. Psyscope: an interactive graphical system for designing and controlling experiments in the Psychology laboratory using Macintosh computers. Behav. Res. Meth. Instrum. Comput. 1993;25:257–271. [Google Scholar]
- Cooper JA, Sagar HJ. Incidental and intentional recall in Parkinson's disease: an account based on diminished attentional resources. J. Clin. Exp. Neuropsychol. 1993;15:713–731. doi: 10.1080/01688639308402591. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Jennings JM. Human memory. In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Hillsdale, NJ: Erlbaum; 1992. pp. 51–100. [Google Scholar]
- Dale AM, Buckner RL. Selective activation of rapidly presented individual trials using fMRI. Hum. Brain Mapp. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SARB, Raaijmakers JGW, Jonker C. Aging affects both perceptual and lexical/semantic components of word stem priming: an event-related fMRI study. Neurobiol. Learn. Mem. 2005;83:251–262. doi: 10.1016/j.nlm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J. Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Buckner RL. Dissociating memory retrieval processes using fMRI: evidence that priming does not support recognition memory. Neuron. 2001;31:1047–1059. doi: 10.1016/s0896-6273(01)00429-9. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB, French JW, Harman HH, Kermen D. Manual for Kit of Factor-Referenced Cognitive Tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Fleischman DA. Repetition priming in aging and Alzheimer's disease: an integrated review and future directions. Cortex. 2007;43:889–897. doi: 10.1016/s0010-9452(08)70688-9. [DOI] [PubMed] [Google Scholar]
- Fleischman DA, Gabrieli JDE. Repetition priming in aging and Alzheimer's disease: a review of findings and theories. Psychol. Aging. 1998;13:88–119. doi: 10.1037//0882-7974.13.1.88. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Jezzard P, Turner R. Analysis of functional MRI time-series. Hum. Brain Mapp. 1994;1:153–171. [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. NeuroImage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE. Cognitive neuroscience of human memory. Annu. Rev. Psychol. 1998;49:87–118. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Desmond JE, Demb JB, Wagner AD, Stone MV, Vaidya CJ, Glover GH. Functional magnetic resonance imaging of semantic memory processes in the frontal lobes. Psychol. Sci. 1996;7:278–283. [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc. Natl. Acad. Sci. U. S. A. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Lai S. Self-navigated spiral fMRI: interleaved versus single-shot. Magn. Reson. Med. 1998;39:168–361. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. Philadelphia: Lea and Febiger; 1972. [Google Scholar]
- Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's Disease. J. Neurosci. 2003;23:986–993. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JDE. Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci. 2004;5:87–97. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Henson RN. Neuroimaging studies of priming. Prog. Neurobiol. 2003;70:53–81. doi: 10.1016/s0301-0082(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, random effects and population interference. NeuroImage: Abstr. 4th Int. Conf. Funct. Mapp. Hum. Brain. 1998;7:S754. [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- LaVoie D, Light LL. Adult age differences in repetition priming: a meta-analysis. Psychol. Aging. 1994;9:539–553. doi: 10.1037//0882-7974.9.4.539. [DOI] [PubMed] [Google Scholar]
- Light LL. Memory and aging: four hypotheses in search of data. Annu. Rev. Psychol. 1991;42:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- Light LL, Prull MW, Kennison RE. Divided attention, aging, and priming in exemplar generation and category verification. Mem. Cog. 2000a;28:856–872. doi: 10.3758/bf03198421. [DOI] [PubMed] [Google Scholar]
- Light LL, Prull MW, LaVoie D, Healy MR. Dual process theories of memory in older age. In: Perfect T, Maylor E, editors. Theoretical Debate in Cognitive Aging. Oxford, UK: Oxford University Press; 2000a. pp. 239–299. [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL. Under-recruitment and non-selective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lustig C, Buckner RL. Preserved neural correlates of priming in old age and dementia. Neuron. 2004;42:865–875. doi: 10.1016/j.neuron.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL. Evidence for neural effects of repetition that directly correlate with behavioral priming. J. Cogn. Neurosci. 2004;16:1625–1632. doi: 10.1162/0898929042568451. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Turkington TG, Provenzale JM, Denny LL, Hawk TC, Gottlob LR, Coleman RE. Adult age differences in functional neuroanatomy of verbal recognition memory. Hum. Brain Mapp. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mitchell DB, Bruss PJ. Age differences in implicit memory: conceptual, perceptual, or methodological? Psychol. Aging. 2003;18:807–822. doi: 10.1037/0882-7974.18.4.807. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Good CD, Frackowiak RSJ, Rugg MD. Age effects on the neural correlates of successful memory encoding. Brain. 2003;126:213–229. doi: 10.1093/brain/awg020. [DOI] [PubMed] [Google Scholar]
- Morris J, Heyman A, Mohs R, Hughes J, van Belle G, Fillenbaum G, Mellits E, Clark C, Investigators C. The consortium to establish a registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's Disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Brett M, Anderson J, Wager TD, Poline JB. Valid conjunction inference with the minimum statistic. NeuroImage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Orfanidou E, Marslen-Wilson WD, Davis MH. Neural response suppression predicts repetition priming of spoken words and pseudowords. J. Cogn. Neurosci. 2006;18:1237–1252. doi: 10.1162/jocn.2006.18.8.1237. [DOI] [PubMed] [Google Scholar]
- Paivio A, Yuille JC, Madigan SA. Concreteness, imagery, and meaningfulness values for 925 nouns. J. Exp. Psychol. 1968;76:1–25. doi: 10.1037/h0025327. [DOI] [PubMed] [Google Scholar]
- Persson J, Sylvester CYC, Nelson JK, Welsh KM, Jonides J, Reuter-Lorenz PA. Selection requirements during verb generation: differential recruitment in older and younger adults. NeuroImage. 2004;23:1382–1390. doi: 10.1016/j.neuroimage.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L, Lind J, Larsson A, Nilsson LG, Ingvar M, Buckner RL. Structure–function correlates of cognitive decline in aging. Cereb. Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Rajah NM, D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven's Progressive Matrices And Vocabulary: Standard Progressive Matrices. 1992 ed. Oxford: Oxford Psychologists Press; 1992. [Google Scholar]
- Reuter-Lorenz PA, Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr. Opin. Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz P, Jonides J, Smith ES, Hartley A, Miller A, Marshuetz C, Koeppe RA. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J. Cogn. Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Roediger HL, III, McDermott KB. Implicit memory in normal human subjects. In: Spinnler H, Boiler F, Boiler F, Grafman J, editors. Handbook of Neuropsychology. Vol.8. Elsevier: Amsterdam; 1993. pp. 63–131. [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber M, Snyder AZ, White D, Chapman L, Dromerick JA, Fiez JA, Corbetta M. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–1894. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O'Hara R, Race EA, Desmond JE, Glover GH, Yesavage JA, Gabrieli JDE. Variable effects of aging on frontal lobe contributions to memory. NeuroReport. 2002;13:2425–2428. doi: 10.1097/00001756-200212200-00010. [DOI] [PubMed] [Google Scholar]
- Rybash JM. Implicit memory and aging: a cognitive neuropsychological perspective. Dev. Neuropsychol. 1996;12:251–263. [Google Scholar]
- Sayres R, Grill-Spector K. Object-selective cortex exhibits performance-independent repetition suppression. J. Neurophysiol. 2006;95:995–1007. doi: 10.1152/jn.00500.2005. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL. Priming and the brain. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities Test Manual-Revised. Los Angeles: Western Psychological Services; 1982. [Google Scholar]
- Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, et al. Aging effects on memory encoding in the frontal lobes. Psychol. Aging. 2002;17:44–55. doi: 10.1037//0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Tulving E, Kapur S, Markowitsch HJ, Craik FIM, Habib R, Houle S. Proc. Natl. Acad. Sci. U. S. A. 1994 Neuroanatomical correlates of retrieval in episodic memory auditory sentence recognition;91:2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Sylvester C-YC, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. NeuroImage. 2005;27:323–340. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Desmond JE, Demb JB, Glover GH, Gabrieli JDE. Semantic repetition priming for verbal and pictorial knowledge: a functional MRI study of left inferior prefrontal cortex. J. Cogn. Neurosci. 1997;9:714–726. doi: 10.1162/jocn.1997.9.6.714. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Koutstaal W, Maril A, Schacter DL, Buckner RL. Task-specific repetition priming in left inferior prefrontal cortex. Cereb. Cortex. 2000;10:1176–1184. doi: 10.1093/cercor/10.12.1176. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised Manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Wig GS, Grafton ST, Demos KE, Kelley WM. Reductions in neural activity underlie behavioral components of repetition priming. Nat. Neurosci. 2005;8:1228–1233. doi: 10.1038/nn1515. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA. Individual differences in rates of change in cognitive abilities of older persons. Psychol. Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. J. Clin. Exp. Psychol. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Hasher L, Li KZH. Human memory. In: Craik FIM, Salthouse TA, editors. The Handbook of Cognition and Aging. Mahwah, NJ: Erlbaum; 2000. pp. 293–357. [Google Scholar]