Abstract
Changes in the distribution of language function in the brain have been documented from infancy through adulthood. Even macroscopic measures of language lateralization reflect a dynamic process of language development. In this review, we summarize a series of functional MRI studies of language skills in children ages of 5 to 18 years, both typically-developing children and children with brain injuries or neurological disorders that occur at different developmental stages, with different degrees of severity. These studies used a battery of fMRI-compatible language tasks designed to tap sentential and lexical language skills that develop early and later in childhood.
In typically-developing children, lateralization changes with age are associated with language skills that have a protracted period of development, reflecting the developmental process of skill acquisition rather than general maturation of the brain. Normative data, across the developmental period, acts as a reference for disentangling developmental patterns in brain activation from changes due to developmental or acquired abnormalities. This review emphasizes the importance of considering age and child development in neuroimaging studies of language.
Keywords: Language development, fMRI, pediatric, speech, perinatal stroke, traumatic brain injury, epilepsy, verb generation, brain development, child language
Introduction
Language is a cognitive function of the highest order. Language deficit may be one of the earliest indicators of neurological impairment, often presenting as the first sign of a developmental delay. Additionally, a number of acquired neuropathologies can adversely impact language, including epilepsy, stroke, tumor, or traumatic brain injury. Recent functional magnetic resonance imaging (fMRI) studies in adults have supported the results of much older lesion studies, revealing that language functions are primarily based in neocortical areas in inferior frontal and posterior temporoparietal areas. However, normal developmental changes in the cortical representation of language capabilities have just begun to be mapped.
In this review, we summarize results of neuroimaging studies of language in children, with a focus on a large-scale study in more than 300 healthy children. We used fMRI to map the developmental trajectories of the neural substrates underlying language development in children ages 5 to 18 and to assess hemispheric lateralization trends across age that are associated with developing language skills. We then compared these results with similar fMRI studies examining some of the same neural networks in children with stroke, traumatic brain injury, and epilepsy. These studies allow for examination of a central issue concerning the biological foundations of language: whether typical left hemisphere lateralization of language results from pre-existing functional asymmetries or emerges in association with the development of language skills. Additionally, comparisons with data from children with varying degrees of brain injury during development provide insights about the neuroplasticity of language.
(Levistsky & Geschwind, 1968) first suggested that a structural asymmetry of the posterior language area supported the left hemisphere lateralization of language. Subsequent work revealed a leftward structural asymmetry for the anterior language regions as well (Falzi et al., 1982; Albanese et al., 1989). These asymmetries emerge as a function of prenatal brain development (Wada et al., 1975; Chi et al., 1977), suggesting that the bias for left hemisphere language lateralization is present early in development. However, how these structural asymmetries relate to functional lateralization over the course of language development has only recently been investigated. A recent functional MRI study by (Dehaene-Lambertz et al., 2002) demonstrated that left hemisphere dominance of language function may already be present in infancy, and an additional study from (Pena et al., 2003) revealed some evidence for left-lateralization in neonates (using Optical Topography). Also supporting the notion of early functional specialization are magnetoencepholography results (Kuhl et al., 2006), showing that the left inferior frontal region discriminated speech sounds as early as 6 months of age. In older children (ages 5−7), (Ahmad et al., 2003) found a left-lateralized pattern of activation using fMRI comparing listening to speech to a backward speech control condition. (Balsamo et al., 2002) found similar results with an auditory semantic decision task in 5−10 year olds. (Blumenfeld et al., 2006) used a semantic judgment task in a group of children ages 9−11, and examined correlations between level of activation and performance accuracy on the task. Increased accuracy was associated with increased fMRI signal ininferior and middle temporal gyri; decreased performance accuracy correlated with increased activation in inferior and middle frontal gyri. These patterns of activation were left-lateralized in the frontal cortex, but not in the temporal cortex.
All of these imaging studies suggest that some degree of left-lateralization is present throughout development; however, they were not designed to reveal changes in functional organization across development. A few studies (including the data we will review below) examine changes in the neural basis of language that occur with age. (Wood et al., 2004) used fMRI to test 48 children ages 6−15 and 17 adults performing verb generation (described below) and verbal fluency tasks (generate words in response to a presented initial letter). Forty children were judged to have a left-lateralized activation pattern (one child right-lateralized and seven were bilateral), but degree of lateralization (right or left) increased with age only in the verbal fluency task. (Chou et al., 2006) tested children ages 9−15 performing a semantic judgment task and found a bilateral, but left-dominant, activation pattern. The left superior and middle temporal gyri, right inferior frontal gyrus, and left medial frontal gyrus showed increased activation with age when making judgments on related pairs of words. The authors attribute these changes to the emergence of more extensive networks of semantic information as children learn. Most recently, using data from typically-developing children as a reference point, researchers are investigating how brain injuries that occur with varying severity and at varying points during language development affect the neural substrates of language (Booth et al., 2000; Staudt et al., 2001; Staudt et al., 2002)
Language Development in Normal Children
Using fMRI we can examine the physiological correlates of the progression of language acquisition in children. By selecting skills for study that are acquired relatively early and skills that require a protracted time period for proficiency, we can examine the extent to which lateralization is affected by stage of acquisition. A range of language tasks is required to fully assess development of the various aspects of language, whether for clinical evaluation or basic neurobiological investigations (Wilke et al., 2006). In order to contrast age with skill acquisition, we designed four tasks, two of which test language skills acquired prior to age five, and two of which test skills that continue to develop after age five. These tasks focus on word-level (lexical) and sentence-level (sentential) processing skills. Specifically, the tasks involve (1) syntactic prosody, (Blonder et al.) story processing, (3) word-picture matching, and (Blonder, Bowers et al.) verb generation. The developmental and theoretical framework for the four proposed language tasks is diagrammed below in Figure 1. Note that the contrast of early and late acquired skills and the secondary contrast of syntactically and semantically-loaded tasks offers a meaningful way to contrast language domains with early versus late age of acquisition. In this review, we do not examine the recent literature on neuroimaging of the orthographic and phonemic aspects of language. The reader is referred to the works of (Booth et al., 2004) (Binder et al., 2006; Pugh, 2006), and others for further insights about developing neural substrates supporting these facets of language so critical for reading proficiency.
Figure 1.
Schematic diagram showing the developmental and theoretical framework for the four fMRI language paradigms selected for this study. Rows group the tasks developmentally, and columns group tasks by language domain.
Components of Language
Sensitivity to syntactic prosody (i.e., the component of spoken language that involves modulation of pitch and timing in conjunction with syntactic structure) is among the earliest language skills to develop, before the age of five in normally developing children. The ability of infants to perceive the prosodic characteristics of speech is believed to assist them in segmenting the acoustic stream into words, clauses, and sentences (Gerken et al., 1994; Mandel et al., 1994; Mandel et al., 1996; Jusczyk, 1997); though, note that a full mapping of prosody to syntactic structure continues to develop in older children (Choi & Mazuka, 2003) Other aspects of sentence-level processing have a longer time course for development. Although preschool children are able to comprehend simple sentences, correct interpretation of sentences with complex syntax develops into the school years (Emerson, 1979; Bridges, 1980). Therefore, a listening task that is designed to include complex syntactic constructs would tax young listeners who are still in the process of mastering these structures.
Vocabulary and semantic knowledge, although clearly having developed to some extent by the age of five, continue to develop through the school-age years. Vocabulary develops throughout the life span, both in terms of the number of lexical items and the range of semantic properties associated with those items. However, by developing a picture-based, vocabulary identification task that uses words commonly acquired prior to age three (Fenson et al., 1993), we can create a lexical task that reflects early developing vocabulary skill. In contrast, a task that requires a child to use semantic associations (e.g., verb generation) among words demands skills that continue to develop through childhood.
In addition to educing a representative range of language functions, the tasks have been designed to have relevance to clinical testing of language and to be compatible with fMRI scanning. In particular, the verb generation task selected has now been used widely to assess hemispheric dominance for language with fMRI in studies of adults (Xiong et al., 1998; Benson et al., 1999) and children with epilepsy (Hertz-Pannier et al., 1997; Holland et al., 2001; Liegeois et al., 2002). We have also demonstrated previously that the verb generation task reveals changes in hemispheric lateralization of the supporting neural substrates with age in typically-developing children (Holland, Plante et al., 2001). The Story Processing task is also now used for clinical fMRI procedures involving language mapping because it has been shown to produce a bilateral activation of language association areas and is well suited to identifying pathological asymmetries in language activation associated with neurological pathologies (Wilke et al., 2003).
Here we present data from the literature and from our own, large-scale normative study of language development in children from ages 5 to 18. To emphasize changes in the neural substrates of language processing in the developing brain we focus on age-related changes in regions classically associated with language functioning. Specifically, we focus on changes in lateralization involving Broca's and Wernicke's areas and their right hemisphere homologues. The picture emerging from the collective functional neuroimaging data is a dynamic one in which language lateralization is a function of maturation, language domain, and rate acquisition of specific language skills. Some evidence also supports biological specialization of the dominant hemisphere for language, though this remains an open question.
Language Networks in Children with Neuropathology
Results from typically-developing children offer a framework in which to view the effects of neuropathologies on the neural basis of language. To this end we will describe a study of pediatric patients where the left hemisphere is profoundly injured very early in development. We then examine more subtle injuries occurring somewhat later during language development and finally examine the effects of chronic seizure activity. All of these data, considered alongside the normal results presented above, shed light on the flexibility of the left-hemisphere specialization for language.
Pediatric patients with left middle cerebral artery infarctions occurring in the perinatal period were examined using fMRI and the verb generation task to investigate patterns of language organization (Staudt, Grodd et al., 2001; Jacola et al., 2006). In adult patients with aphasia-producing lesions of a similar etiology, functional recovery is marked by increases in activation in either right hemisphere homologues of classical left hemisphere language cortex or in areas outside of the infarcted area ipsilateral to the stroke hemisphere. The limited available studies in children suggest that early insult to the left hemisphere may induce a right hemisphere reorganization in children similar to adults (Muller et al., 1998; Booth, MacWhinney et al., 2000) (Brizzolara et al., 2002) (Papanicolaou et al., 2001), but some incongruities exist among studies. Based on this literature, we hypothesize that left MCA stroke patients will show preferential right hemisphere activation, because a major left-hemisphere injury early in development has made the full extent of left-lateralization (as shown in the normal population) impossible.
Brain injuries occurring later in child development may also have an impact on language networks, but reduced neuroplasticity as a function of age may result in decreased potential for reorganization. Another study from our group examined the effects of traumatic brain injury (Naidich & Brightbill) on the functional networks supporting language, attention, and working memory in children(Karunanayaka et al., In Press). Although language skills have received relatively less attention than other aspects of behavioral and neuropsychological recovery following TBI, they may underlie observed problems in academic performance, social competence, and peer integration. Previous investigators have documented deficits in expressive and receptive language skills (Anderson et al., 1997; Morse et al., 1999) following TBI in children. Unlike the data on recovery from stroke, existing data suggest that TBI in a younger child results in potentially more severe sequelae than is the case for older children (Anderson, Morse et al., 1997; Ewing-Cobbs et al., 1997). Because language skills are undergoing rapid change during early childhood, they may be particularly vulnerable to disruption by brain trauma.
Children with mild, moderate or severe TBI, who were six years of age (or older), and at least 12-months post injury were selected for the fMRI study. fMRI results from this group of pediatric TBI patients were compared with results from the same fMRI paradigms in our normal cohort of healthy children. In this case, we expected to see more subtle differences in functional networks, suggesting less reorganization than in the MCA stroke cohort.
The neural circuitry of language may also be affected by chronic neuropathology, in particular, seizure activity in epilepsy. Unlike stroke or TBI patients whose injury occurs at a particular point in development, seizure activity may produces a more protracted, though lower dose of injury to the brain. Adult data suggests that epilepsy can exert profound and chronic impact on language functions, and one manifestation of such impact is the “atypical” language distribution pattern that has been observed in many adult fMRI studies (Springer et al., 1999; Billingsley et al., 2001; Gaillard et al., 2002; Adcock et al., 2003; Brazdil et al., 2005). Differences in the neural substrates of language between pediatric epilepsy patients and healthy children have recently been highlighted by Yuan et al. using the same fMRI language paradigm as in the normative group described above (Yuan et al., 2006). We will review language lateralization and reorganization patterns in the epilepsy group using a quantitative group comparison to contrast fMRI imaging results between the pediatric epilepsy patients and healthy control subjects.
The review of functional neuroimaging data from the same language paradigm in children over an equivalent age span but with neurological status varying from normal to devastating left hemisphere disruption provides a clinical laboratory for studying neuroplasticity of language during development. By examining this spectrum of neuropathologies and development using the same fMRI language paradigms, we can construct a complete picture of language development in the brain under various influences.
Methods
Subjects
Normal, healthy control subjects included 313 children (152 boys, 161 girls) who were successfully scanned as part of this IRB-approved study and yielded high-quality neuroimaging data for the analyses described here. Exclusion criteria were: previous neurological illness; learning disability; head trauma with loss of consciousness; current or past use of psychostimulant medication; pregnancy; birth at 37 weeks gestational age or earlier; or abnormal findings at a routine neurological examination performed by an experienced pediatric neurologist. All subjects were native monolingual English speakers. A complete age and gender breakdown of the subjects is detailed in Table 1. Two hundred and eighty seven of the subjects were right-handed, 23 were left-handed, and 3 were ambidextrous according to the Edinburgh test for handedness (Oldfield, 1971). The racial/ethnic background of the subjects was: 279 Caucasian, 22 African-American, 2 Asian, 2 Hispanic, 1 Native American, 7 Multi-Ethnic. All subjects were pre-screened for any conditions (such as the presence of orthodontic braces) which would prevent an MRI scan from being acquired. All subjects received the Wechsler Intelligence Scale for Children, Third Edition (WISC-III) or the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) and the Oral and Written Language Scales (OWLS) (Carrow-Woolfolk, 1995). Mean age = 11.9 ± 3.75 yrs. (range = 4.9 − 18.9 yrs.); Mean Wechsler Full-Scale IQ = 111.3 ± 14.1 (range = 70 − 147); Mean OWLS = 107.5 ± 14.1 (range = 66 − 151)(Schmithorst & Holland, 2006).
Table 1.
Number and gender of subjects successfully completing at least one of the fMRI language tasks and included in the lateralization growth curves in Figure 3 and composite language maps shown in Figure 2.
| 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 7 | 8 | 10 | 15 | 10 | 10 | 18 | 16 | 16 | 9 | 10 | 9 | 11 | 3 | =152 |
| F | 7 | 11 | 16 | 11 | 13 | 10 | 9 | 10 | 21 | 10 | 11 | 10 | 11 | 11 | =161 |
In addition to the normal cohort, we present data from a group of ten patients with an infarction of the left middle cerebral artery distribution (MCA stroke, n=10) during the perinatal period. Each of these patient's symptoms were consistent with perinatal left MCA infaction and resulted in a referral to a pediatrician or pediatric neurologist. All of these patients were native English speakers and were included in the study based on history, clinical examination, and radiological evidence of left MCA chronic ischemic stroke. A control group of healthy subjects was constructed from our normal cross-sectional cohort described above and matched for age, sex, and handedness when possible.
A group of eight children with traumatic brain injury (TBI, n=8) occurring between 36 to 84 months of age were also tested using fMRI. There was no documentation in the medical chart or in parent interview of child abuse as a cause of the injury in this group. English was the primary spoken language in the home for all eight children. Children in the TBI group must have had a TBI requiring overnight admission to the hospital and either evidence of altered neurological status on the Glasgow Coma Scale (GCS) (i.e., total score < 15) or abnormalities on imaging (MRI or CT scan). Thus, the lowest GCS score recorded at the time of hospitalization provided a measure of injury severity in the TBI group. GCS scores are generated by combining ratings of eye opening, best verbal response, and best motor response at the time of evaluation. Consistent with previous investigations, severe TBI was defined as one resulting in a GCS score of 8 or less at any point since injury, and moderate TBI was defined as a GCS score of 9 − 12 or a score of 13−15 accompanied by evidence of brain insult (on CT scan, MRI, or neurological exam). Mild TBI was defined as a GCS score of 13 or 15 with no imaging abnormalities. However, based on the recruitment criteria described above, children with a GCS score of 15 and normal imaging were not enrolled in the present study. Children who sustained non-blunt head trauma (e.g., projectile wounds, strokes, drowning) were excluded. Details of the TBI cohort can be found in(Karunanayaka, Holland et al., In Press).
Eighteen pediatric patients (age 8−18 years, 10 males, 8 females) diagnosed with epilepsy (Epilepsy, n=18) by a pediatric neurologist are also included in this review of fMRI of language development. Patients’ demographic and clinical information is summarized in Yuan et al. (Yuan et al., 2006). Among the eighteen subjects who completed the fMRI scanning, seventeen had a complete history of information about seizure onset age, EEG, MRI structure, and epilepsy classification. Fifteen subjects were diagnosed with partial epilepsy (including two with secondary generalized seizures). Two subjects had the diagnosis of idiopathic generalized seizure with normal MR imaging and bisynchronous epileptiform potentials on EEG. The majority (n=15) of this patient group had focal or multifocal epileptiform discharges on EEG, with the other two subjects failing to present any EEG abnormalities. MRI imaging was abnormal in seven, and MRI observation showed close agreement (with one exception) with the abnormality localized by EEG when lesions were present.
MRI Scanning
MRI scans for the normal control group, the MCA stroke cohort, and the TBI cohort were obtained using 3 Tesla MR imaging systems located at Cincinnati Children's Hospital Medical Center. An MRI-compatible audiovisual system was used for presentation of the stimuli as well as for a movie during the preparation (e.g. shimming) and acquisition of the whole-brain anatomical scans. Details of the techniques used to obtain fMRI data from younger children, as well as the success rates, are given in Byars et al. (Byars et al., 2002). EPI-fMRI scan parameters were: TR/TE = 3000/38 ms; BW = 125 kHz; FOV = 25.6 × 25.6 cm; matrix = 64 × 64; slice thickness = 5 mm. Multiple axial slices covering the entire cerebrum were acquired (the first 10 were discarded to allow the spins to reach relaxation equilibrium) for a total scan time of 5 min. 30 sec during the verb generation task. Techniques detailed elsewhere (Byars, 2002 #11) were used to acclimatize the subjects to the MRI procedure and render them comfortable inside the scanner. In addition to the fMRI scans, whole-brain T1-weighted MP-RAGE scans were acquired for anatomical coregistration.
MRI/fMRI scans for the epilepsy patient group were performed on a 1.5 Tesla, GE Signa Horizon MRI. Each fMRI scan consisted of 100 single-shot EPI gradient echo images acquired with TR/TE = 3000/40 msec, FOV=220 × 220 mm, matrix = 64 × 64 (Yuan, Holland et al., 2006). Six sagittal slices were acquired in each hemisphere for each time point, leading to a total of 1200 slices of fMRI data. Slice thickness was 5 mm with a 1 mm gap. The slices were positioned such that the outermost slice on each hemisphere extended to the most lateral aspect of the temporal lobe.
Language Activation Tasks
The fMRI tasks were designed to have relevance to clinical testing of language and to be MRI-compatible. In particular, the verb generation task (described below) has been used widely to assess hemispheric dominance for language with PET and fMRI in studies of adults and children (Petersen et al., 1988; Desmond et al., 1995; Worthington et al., 1997; Benson, FitzGerald et al., 1999; Hertz-Pannier et al., 2000). The difficulty of the language tasks used in this study were tailored for the youngest in the cohort (5 year olds) because all subjects are required to perform the same task. Finally, all fMRI language testing paradigms used in this study were based on a block-periodic design in which the language task of interest was alternated with a control task. The specifics of each task are as follows:
Story Processing Task.(Karunanayaka et al., 2007)
The Story Processing task involved auditory presentation of five simple stories, each composed of 10 sentences. One story was presented during each 30-s task period. The 30-s task periods were interleaved with 30-s rest, or control, periods, during which the subject performed an appropriate control task as described below. Stories contained vocabulary used as part of the Word-Picture Matching task (described below) and contained sentences of varied syntactic constructions. The subject was instructed to listen to the stories so that he or she could answer questions about them after the scans. Performance data were obtained at the end of the scanning session by asking the subject to answer two multiple choice questions about each story.
Although story comprehension obviously involves multiple skills, the inclusion of complex syntactic structures was designed to increase the relative processing load for this aspect of language. Thus, we refer to this sentential task as one that is syntactically-loaded for children.
Syntactic Prosody Task.(Plante et al., 2006)
The Syntactic Prosody task involved the auditory presentation of a target sentence (taken from the stories in the Story Processing task) to the subject, who was required to pick the target sentence out of a set of sentences (also from the stories) that were low-pass filtered (400 Hz cutoff) so that the words were not recognizable but syntactic prosody was preserved. Each target sentence was presented between two and four times in a set of 10 sentences, the balance of which were foils. The subject pressed a push button each time a target sentence was recognized; responses were recorded by computer.
Word-Picture Matching Task.(Schmithorst et al., In Press)
The Word-Picture Matching task involved the simultaneous visual presentation of two simple line drawings of common objects. The objects were selected from the MacArthur Communicative Development Inventories (Fenson, Dale et al., 1993) in order to ensure that the majority of 5-year-old children would be familiar with them. Simultaneously with the visual presentation of the pairs of object line drawings, the name of one of the objects was presented via the headphones. The subject used the push buttons to indicate whether the picture on the right or the left matched the name that was heard. Button responses were recorded by computer. Picture pairs and names were presented at 3 s intervals.
Verb Generation Task. (Holland, Plante et al., 2001)
The Verb Generation task is based on a task initially developed for PET imaging (Petersen, Fox et al., 1988) that involves the auditory presentation of a series of concrete nouns. The subject hears a noun every five seconds and is required to covertly generate as many verbs associated with that noun as possible during the 5 second interval. For example, if the noun “ball” were presented, the subject might generate the verbs “throw,” “kick,” and “hit.” The subject was instructed to think the verbs silently, without saying them, in order to minimize the motion artifact associated with speech.
Control Tasks
The control period tasks are critical to the interpretation of the activation data. As Binder (Binder, 1997) and others have noted, cognitive activity continues during rest periods and the careful design of the control interval task can result in fMRI data that is much more succinct and easy to interpret. The control interval activity has three purposes: a) to control for sensory and motor components of the stimulation task activity (for example, the auditory processing involved in listening to words or stories and the visual processing involved in looking at pictures); b) to distract the subject from unintentional continued engagement in the activation task during the control interval and; c) continue to engage the subjects’ attention without stimulating language behaviors. With these goals in mind the control tasks were designed to replicate, as nearly as possible, the sensory and motor demands of the activation tasks in order to control for non-language related contamination of the activation maps.
Tone Tasks
This control task involved the random auditory presentation of pure tones of 150, 200, 250, 500, 700, 900, and 1000 Hz. During control intervals for the Story Processing task, the subject listened to tones of 1 s duration played at unequal intervals of 1 to 3 s. In the Syntactic Prosody control periods, the subject was given a distinct target tone (frequency modulated beeper tone) and was asked to press the left push button each time the target tone was heard within a larger series of pure tones.
Image Discrimination Task
The Image Discrimination Task was used during the control interval for the Word-Picture Matching task. It involved the presentation of pairs of images of unnamable designs (line drawings). During task training, the target drawing was identified to the subject. During the scan, the subject was required to press the push button corresponding to the side on which the target image was displayed, similar to the task requirements in Word-Picture Matching. Tones, as described above, accompanied the images displayed at regular 3 s intervals during the Image Discrimination Task in order to control for the auditory stimulation provided by the spoken word in the Word-Picture Matching task.
Bilateral Finger Tapping Task
This task was used as the control task for the verb generation task. Like verb generation, the sensory-motor response to bilateral finger tapping has been extensively investigated in the clinical literature and in previous studies of epilepsy patients (Schapiro et al., 2004). In order to parallel the initiation of response when a noun is presented in the Verb Generation task, the subject is asked to tap his or her fingers for one cycle when a target tone is heard. This control task accomplished three objectives: 1) to control for the auditory stimulation present in the Verb Generation task; 2) to prevent the subject from continuing to generate verbs during the control period; and 3) to provide a reference area of activation within the motor strip as an independent means of validating subject compliance.
Stimulus Presentation and Response Recording
Audio stimuli were digitally recorded at a professional recording studio and visual stimuli were white line drawings on black background. Audio and video stimuli were presented to the subjects using an MRI compatible audio/video system with binocular goggles. For the Syntactic Prosody task and the Word-Picture Matching task, stimuli were presented and responses were recorded using the MacStim program (Version 2.5.7, White Ant Software, West Melbourne, VIC). Stimuli for the Story Processing task and Verb Generation task were presented from a prerecorded CD running synchronously with the MRI scanner. Performance on these two tasks was assessed by administering two recognition memory tests after scanning had ended, one for the content of the stories and one for the nouns that were presented.
fMRI Processing & Data Analysis
Image reconstruction, post-processing, and the group statistical analysis were performed with modern methodology using Cincinnati Children's Hospital Imaging Processing Software (CCHIPS@) written in Interactive Data Language (IDL) (Research Systems Inc., Boulder, CO, (Schmithorst et al., 2000). The EPI images were corrected for Nyquist ghosting and geometrical distortion (due to B0 field inhomogeneity) based on a multi-echo phase reference scan (Schmithorst et al., 2001). The reconstructed EPI-fMR images were corrected for drift using a quadratic baseline correction on a pixel-by-pixel basis. Artifacts due to subject motion were also corrected using a pyramid iterative co-registration algorithm (Thevenaz et al., 1998). The images were then transformed into Talairach coordinates (Talairach & Tournoux, 1988) using a linear affine transformation previously validated for the age range 5−18 years (Muzik et al., 2000; Wilke et al., 2002).
The fMRI data was post-processed using a general linear model (GLM) (Worsley et al., 2002) and implemented in CCHIPS/IDL on a pixel-by-pixel basis to identify the activated brain regions in a given experiment. For each subject, a pixel-by-pixel Pearson's correlation coefficient (between BOLD signal and the HRF-convoluted time course) was calculated and converted to the corresponding z-score maps using the Fisher's z-transformation. A composite group activation map was then computed from the individual z maps based on a one-sample t-test (for each pixel across all subjects) for the significance of a pixel in the group. A statistical parametric map was generated based on these t-statistics. The composite t-score map can be used to identify brain regions for the entire group with the most significant contrast between the active (language) and the control tasks as shown in Figure 2. This methodology accounts for both intra-subject and inter-subject variance and is commonly referred to as a random-effects analysis. A cluster method was used to improve the specificity by adjusting the inflated alpha for multiple comparisons (Xiong et al., 1995). In addition, Monte-Carlo simulation was used to determine the p-value corresponding to a specific combination of cluster size and z-threshold.
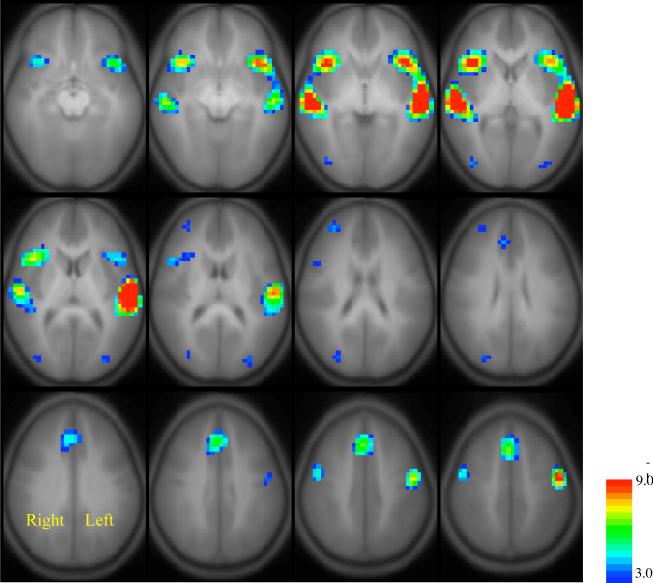
Figure 2.
Average composite maps of all subjects (N=313) undergoing each fMRI language task: A) Syntactic Prosody task, n=273; B) Story Processing, n=269; C) Word-Picture Matching task, n=304; and D) Verb Generation task, n=285. Color bars indicate the range of negative, base-10, log p-values depicted in the color overlays. All colored pixels meet the criteria that the average Z-score is significant at the level of p<0.001. Pixels are labeled from blue to red on a chromatic scale by the negative base-10 logarithm of the p-value as indicated by the color bars. The lowest value = 3.0 (i.e. p<10−3) corresponds to dark blue, and the highest value = 9.0 (i.e. p<10−9) corresponds to bright red. Images are presented in the Radiological convention with the right side of the brain projected on the left side of the images and visa versa.
An age and sex matched group of subjects was selected as a subset from our cross-sectional study of language development in children as a reference group for comparison with the pathlogical populations (Wilke et al., 2003; Schmithorst et al., 2005; Plante, Holland et al., 2006; Szaflarski et al., 2006; Szaflarski et al., 2006). For each pathological cohort of children, we then performed an analysis as described previously in our group studies (Yuan, Szaflarski et al., 2006) to test the difference in BOLD signal activation between the normal cohort and brain injured groups.
Regional Lateralization Index Calculation
A lateralization index (LI) was also calculated for each subject based on the individual z-score maps. The LI represents a relative hemispheric difference for an individual that is self-normalizing in terms of relative BOLD activity corresponding to the neuronal recruitment necessary to perform the language task. This index is non-dimensional and provides a convenient means of comparing groups of subjects across different scanning sessions and scanners. As noted later, the brain areas included for LI calculation were determined so that they were consistent across the populations while still conforming to the traditional standard of ROI selection for language lateralization. Every effort was made to insure that the methodologies applied in the LI calculation of two subject groups were compatible.
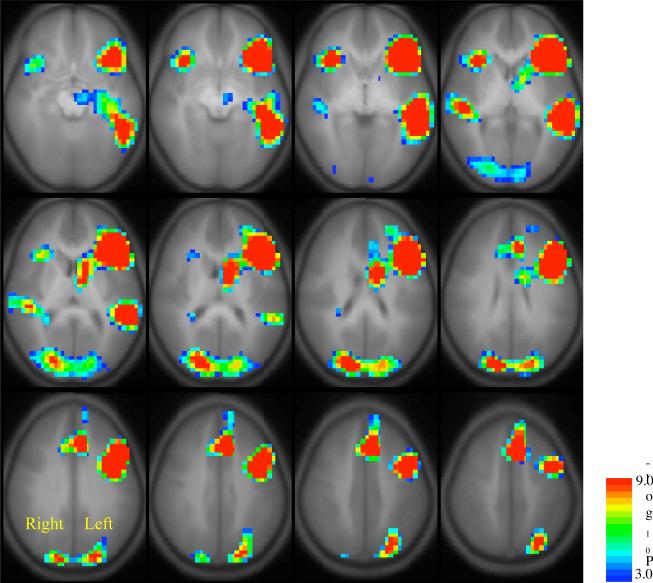
Universal regions of interest (ROIs) were defined for LI calculation based on the composite map of activation obtained from the fMRI Verb Generation task from all subjects in the cohort of normal children and adolescents (N=313). These ROIs included a large region of interest that encompasses Broca's area in the frontal lobe and the anterior and mediolateral aspects of Wernicke's area in the temporal lobe, areas considered critical for language in the classic models of cortical language representation. Because we retained all active voxels within and contiguous to these areas, the frontal ROI included the entire inferior frontal gyrus (BA 44, 45, 47) and extensions into additional dorsolateral prefrontal regions, including portions of the middle frontal gyrus (BA 46, 48, 49) and precentral gyrus (BA 46). The temporal ROI extended from the temporal plane (BA 41, 42) inferiorly through the middle temporal gyrus to the margin of the inferior temporal gyrus (including portions of BA 22, 21, 37) but did not include posterior aspects of Wernicke's area (BA 22, 39). ROIs for the right hemisphere homologues were established by reflecting the coordinates about the Y-axis (interhemispheric fissure). Figure 3 displays outlines of the ROIs defined using this procedure, which included Broca's and Wernicke's areas as well as portions of the anterior cingulate and fusiform gyri. LIs were then computed separately for each region as well as for the combined Broca and Wernicke ROIs.
Figure 3.

Regions of interest (ROIs) used for computation of the lateralization index (LI) for Broca's Area, Wernicke's Area, and the sum of these areas (hemispheric LI). These ROIs are constructed from an outline of composite activation for the normal cohort performing the Verb Generation task (Fig. 2A, n=285) and reflection of the activated volume from the left to the right hemisphere.
Only voxels with z-scores greater than or equal to the mean z-score within an ROI for each individual subject were used in the calculation of LIs. Pixels above this mean z-score threshold were counted and a LI was defined as the difference in the number of activated voxels, summed independently for the left and right ROIs, divided by the summed total of active voxels in the left and right regions of interest. According to this formula, a positive LI indicated left hemisphere lateralization and a negative number indicated right lateralization. Numbers close to zero (i.e. −0.1 ≤ LI ≤ 0.1) indicated a bilateral language distribution. These LI values were used to construct the lateralization growth curves for each task shown in Figure 4 as well as the histograms shown in Figure 5.
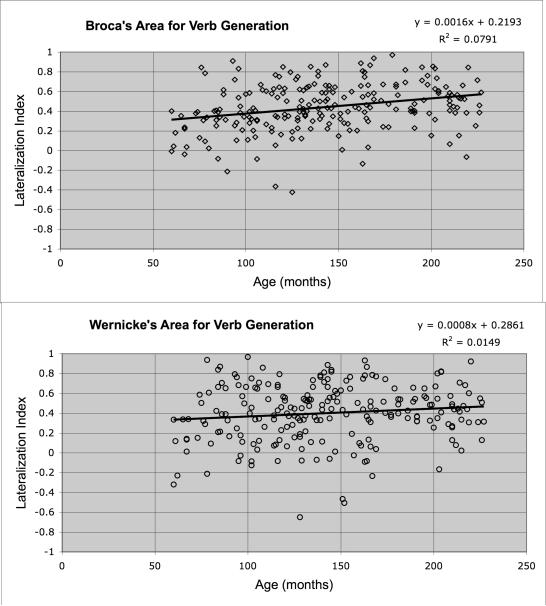
Figure 4.
Lateralization index as a function of age for 313 children, ages 5−18, included in the composite maps shown in Figure 2. LI values are computed using total z-scores extracted from the ROIs shown in Figure 2. Solid black lines indicate the linear regression fit to the data samples with the equation of best fit and the R2 value of the fit shown on the figures. A) Syntactic Prosody task, n=273; B) Story Processing, n=269; C) Word-Picture Matching task, n=304; and D) Verb Generation task, n=285.
Figure 5.

Histograms of LI values for pediatric epilepsy patients (n-18), MCA stroke patients (n=10) and TBI patients (n=8) plotted together with the combined group of age-gender-handedness matched healthy control subjects for each group. Note that LI=1 corresponds to left lateralization while LI=−1 corresponds to right lateralization.
Results
Overall Patterns
Figure 2 presents separate composite activation maps for each of the language tasks described above. Not all of the 313 children performed all four fMRI language tasks, consequently the composites were the average of slightly different numbers of subjects as follows: (1) Syntactic Prosody (n=273), (Blonder, Bowers et al.) Story Processing (n=269), (3) Word-Picture Matching (n=304), and (Blonder, Bowers et al.) Verb Generation (n=285). Some general observations can be made concerning activation within the classic language regions and their right hemisphere homologues as seen in Figure 2. Note that these images were presented using the Radiology convention where the brain is viewed from the perspective of looking up through the patient's feet while lying supine in the scanner. This causes the right side of the brain to project on the left side of the images and vice versa.
Both tasks that involve syntactic processing (i.e. Syntactic Prosody and Story Processing, Figures 2A & B) clearly produced a more bilateral distribution of activation in both the anterior and posterior language areas than the tasks that involve semantic processing (Figures 2C and D). Furthermore, the tasks that target language skills that develop in early childhood (i.e., Syntactic Prosody and Word-Picture Matching, Figures 2A &C) generated less activation on average than the tasks designed to reflect later developing skills (i.e., story processing and verb generation, Figures 2B & D). This finding is consistent with studies of motor, visual, and auditory activity showing that the degree of activation corresponds to the degree of difficulty of the task (Wexler et al., 1997; Metzner et al., 1999) (Thickbroom et al., 1999). Although the activation produced by the “easier” (i.e., early-developing skills) tasks was mostly encompassed within the activation produced by the more difficult task of each type, several unique areas of activation were also noted.
Age-Related Changes in Lateralization
In Figure 4 the LI for each individual is plotted as a function of age (in months) for each of the ROIs (Broca's and Wernicke's areas) for each of the four tasks. Comparisons of the lateralization plots across tasks revealed some overall trends. First, lateralization for lexical-semantic tasks was stronger overall than lateralization for syntactically-loaded tasks. Both the Word-Picture Matching task and the Verb Generation task resulted in left lateralization for both Broca's and Wernicke's areas. This finding can be contrasted with the results for the syntactically-loaded tasks. Lateralization for the Story Processing task varied with ROI. The anterior language area showed less lateralization than the posterior ROI. Finally, both ROIs showed largely bilateral representation in the Syntactic Prosody task.
Age-related changes in lateralization also varied across tasks. The largest change occurred in the anterior language area for the verb generation task (R=.31100; p < .01). In this region, age-related change accounted for 9.67% of the variance in the laterality index. Age-related change in the posterior ROI was more modest, accounting for 1.65% of the variance (R=.1285, p < .01). The Story Processing task also resulted in an age-related change (R=.1619; p < .01, 2.62% of the variance) in the anterior language region. The posterior language areas did not show a significant change with age (R=.0225, p = n.s.) for the Story Processing task. The Syntactic Prosody and Word-Picture Matching tasks were associated with age-related shifts that accounted for less than 1 percent of the variance in the laterality indices for both the anterior and posterior language areas. The Word-Picture Matching task showed little change with age in either anterior (R=.0424; p =n.s.) or posterior (R=.0031; p = n.s.) language regions. Similarly, the laterality indices for the posterior language regions were relatively stable with age (R=.0781, p = n.s.) for the Syntactic Prosody task. However, the Syntactic Prosody task was unique in that the anterior language region showed a marginally significant trend towards increasing rightward lateralization (R=.09165; p < .01). Additional, task-specific activations were found for other regions but discussion of these is beyond the scope of this report.
Verb generation in Children with Perinatal Stroke
For the left MCA stroke patients described above, we also computed the composite activation maps (not shown) and LI values. Several differences in the activation patterns compared to the typically-developing group were evident for in the MCA stroke group. Notably, activation in the MCA stroke group was concentrated in the frontal lobe of the right hemisphere. These activated cortical areas mirrored the left hemisphere activation patterns for this task seen in the healthy control group. No significant difference in size and strength of activation was seen for the right hemispheric regions in the MCA stroke group compared with the homologous regions in the left hemisphere of the control group, as measured by the number of voxels and respective z-scores. However, activation in the MCA stroke group did appear to be more concentrated in the dorsal part of the right frontal lobe (BA 46 and 9) compared with the control group. It is noteworthy that Weschler full-scale (FSIQ) and verbal IQ (VIQ) values in the MCA stroke cohort were more than a standard deviation below the values in the matched control group with: FSIQ= 81 + 14.6 vs. 105 +11.5 and VIQ = 84 +14.3 vs. 104 + 12.4 in the MCA stoke patients vs. matched controls.
We also found a difference in the activation pattern in the temporal lobe. In the control group this activation was stronger in the left medial temporal gyrus (MTG), while the distribution was more bilateral in the MCA stroke group, with an anterior shift relative to the normal left hemisphere pattern. This shift is most likely due to the structural changes with displacement of the remaining vital left hemisphere structures in the stroke patients.
A comparison of the LI values for the MCA stroke cohort with the normal control group revealed quantitative changes in the brain lateralization of language in these patients. The greatest shift in lateralization occurred in the frontal lobes where only one MCA stroke subject was left hemisphere dominant within this ROI, two were symmetric ( |LI|<0.1), and seven subjects were right hemisphere dominant. For the Broca's Area ROI, the difference in LI was significant between the patients and controls (p = 0.0014). A similar difference was observed in the temporal lobes where the difference in the LI computed for the Wernicke's ROI was highly significant (p = 0.004). Figure 5 demonstrates the lateralization shift between the MCA stroke patients and the healthy control groups graphically for the combined ROIs within the right and left hemispheres. Here one can clearly visualize the rightward shift in the LI for the MCA stroke group (D) compared with the control group (A).
|LI|<0.1), and seven subjects were right hemisphere dominant. For the Broca's Area ROI, the difference in LI was significant between the patients and controls (p = 0.0014). A similar difference was observed in the temporal lobes where the difference in the LI computed for the Wernicke's ROI was highly significant (p = 0.004). Figure 5 demonstrates the lateralization shift between the MCA stroke patients and the healthy control groups graphically for the combined ROIs within the right and left hemispheres. Here one can clearly visualize the rightward shift in the LI for the MCA stroke group (D) compared with the control group (A).
Verb generation in Children with Traumatic Brain Injury
Statistical parametric maps for the verb generation task in TBI group (not shown) and age-gender matched control subjects without brain injury revealed activation in left inferior frontal gyrus including Broca's area ( BA areas 44, 45 and 46), the left superior temporal gyrus and the left middle temporal gyrus including Wernicke's area (BA 21 and BA 22), as well as some homologues that were located contra-laterally in the right hemisphere. A strong left hemispheric dominance of frontal and temporal language activation, consistent with our previous studies (Holland, Plante et al., 2001; Szaflarski, Holland et al., 2006), was observed in the composite maps for both the TBI and selected control group.
Although the TBI cohort showed similar left hemispheric activation (in Broca's area), the right superior temporal gyrus (BA 22) and the right middle temporal gyrus (BA 21) showed significantly different activation patterns. We found several regions where the control group activation was greater than in the TBI group, including language related brain regions: superior/medial temporal gyrus and angular gyrus (nominal z = 5.5, cluster = 15, corrected p< 0.001). We also found language-related brain regions with greater activation intensity in the TBI group as compared with the control group (nominal z = 4, cluster = 25, corrected p< 0.02) in the right parietal-occipital- temporal junction, left middle temporal gyrus, left inferior frontal gyrus, and the dorsolateral prefrontal cortex.
Correlation between BOLD Activation and neuropsychological test scores was performed on a voxel-wise basis within and between groups in the TBI study. To test the significance of the relationship between the verbal fluency score (NEPSY) and the language-related BOLD signal activation in the combined population (both control and TBI), we conducted a simple correlation analysis. Several key cortical areas demonstrated significant negative associations in this analysis: the inferior frontal gyrus and superior/middle temporal gyrus bilaterally as well as posterior cingulate cortex. A linear regression between the average z-score of BOLD activation showed a significant negative correlation in these brain regions with the corresponding NEPSY score (R = −0.5, p = 0.036) for all OI and TBI subjects. Notably no significant positive correlations between BOLD signal and NEPSY score were found.
Similarly, a correlation analysis between the DAS verbal IQ (DAS-VIQ) score and the z-score of the BOLD signal activation in the combined population was performed. DAS-VIQ was negatively correlated with brain activation in the same brain regions as the NEPSY (this was expected as NEPSY and DAS-VIQ scores were significantly correlated for the 23 subjects recruited for the study). However, activation in the left angular gyrus showed a positive correlation with the DAS-VIQ (R = 0.6, p = 0.01) and (R = −0.67, p = 0.003), respectively.
We also conducted a within group correlation analysis between the GCS score and the BOLD signal z-score (language-related areas) in the TBI group. Once again there was correlated activation in the inferior frontal gyrus, middle and superior temporal gyrus, and the posterior cingulate, and in addition, the angular gyrus also showed a negative correlation with Glasgow Coma Scores (R = −0.82, p= 0.013), indicating that more severe injuries (i.e., lower GCS score) were associated with greater activation in these regions.
Parameterizing brain activation during the verb generation task using the hemispheric LI as defined above allows us to compare the subtle changes in language lateralization for the TBI group with typical distributions of this parameter for the normal controls and MCA stroke groups. The distribution of the hemispheric LI computed within the ROIs displayed in Figure 2 for the TBI group, is shown in the histogram of Figure 5 (C). Even with a relatively small sample of TBI patients (n=8), the shift in LI nearly reached significance with a two sample t-test (one-sided; p=0.066) and this shift is apparent in the displacement of the distribution of the TBI group in Figure 5 (C).
Verb generation in Children with Epilepsy
For both pediatric epilepsy patients and age-gender-handedness matched healthy children cortical activation during the verb generation task was observed in all subjects in the classical language areas including the inferior frontal gyrus (BA 44, 45), superior and middle temporal gyri (BA 41, 42, 21, 22), angular gyrus (BA 39), and supramarginal gyrus (BA 40). In general, brain activation in Broca's area and Wernicke's areas was stronger and more concentrated in healthy children than that in children with epilepsy.
Several notable differences were evident in the quantitative subject group comparison of lateralization index (LI), summarized in Figure 5. Among the 18 children with epilepsy examined in this study, six patients (33.3%) had bilateral language distributions (−0.1< LI < 0.1); eight patients (44.4%) had right-side language dominance (LI < −0.1) and only four patients (22.2%) demonstrated left-side language dominance (LI > 0.1). In contrast, for the same number of healthy children, only two subjects (11.1%) were bilateral while the vast majority (n=16, 88.89%) were categorized as left dominant for language (LI>+0.1). When we combined the bilateral and right-side dominant subjects together, they constituted the portion of subjects who had “atypical” language lateralization. The percentage of subjects with “typical” and “atypical” language dominance in the patient group were significantly different (χ2=16.2, p<0.001) than that of age-gender-handedness matched healthy children. The Welch Modified Two-sample t-test showed that the mean LI for the epilepsy patients was −0.038 (SD=0.152, n=18), a value significantly different (t=6.490, P<0.0001) when compared with the age-gender-handedness controlled healthy children (Mean LI=0.257, SD=0.120, n=18).
Figure 5 shows the histogram comparing the distribution of LI values between 18 epilepsy patients (B) and healthy subjects (A) selected from the normal group that performed the same Verb Generation task. The lateralization indices from the two subject groups form two distinct distributions. The distribution of LI for the epilepsy patients is shifted to the right hemisphere so that its peak is close to zero; representing a bilateral distribution. This shift suggests relatively more language activation in the right hemisphere for the pediatric epilepsy patients than healthy controls.
A linear regression model was used to examine the relationship between lateralization index (LI) and age. LI was found to increase with age with moderate strength of correlation (R2 =0.15) in the age-gender-handedness matched healthy children group, which was in line with our previous report that included a larger cohort (Holland et al., 2004; Schapiro et al., 2004). However, the correlation in our smaller group of healthy children did not reach statistical significance (p=0.11). On the other hand, no correlation between LI and age was found for the epilepsy patients (R2<0.004, p=0.80). Comparison of residuals from the linear regression found both groups were unbiased with a mean value of zero. No increasing or decreasing spread about the regression line was observed as the age increased. The standard deviation of the residual for the children with epilepsy (SD=0.15) was larger than the age-gender-handedness matched healthy children group (SD=0.12).
Linear regression analysis was also conducted to study whether the age of seizure onset age imposed any influence on the distribution of language function. No statistically significant association was observed between LI and seizure onset age (n=17, R2 = 0.11, p=0.19). However, LI was closely correlated (n=17, R2 = 0.234, p<0.05) with the duration between seizure onset and fMRI scanning.
Discussion
In this review, we have discussed a series of studies that focus on the neural basis of language during development, beginning with a large-scale study of typically-developing children from age 5 to 18 years. In that study, we surveyed multiple language domains to provide a more complete picture of developing language representation in the brain. Traditional models of language that suggest a strictly left-hemisphere function are visibly challenged by these data. Consistent with neuroimaging results in adults, language in children appears to be left-lateralized rather than left-localized. However, the degree of lateralization is task- and area-dependent. In the study discussed, lexical/semantic tasks tended to yield stronger patterns of lateralization in anterior and posterior language regions than did tasks designed to emphasize syntactic/prosodic processing. Therefore, models of brain lateralization must be qualified with reference to the type of language task employed and the region of interest examined.
The normative results also make apparent that the degree of lateralization associated with specific language regions and tasks can shift with age. In other words, younger healthy children are more likely to have atypical (relative to adult findings) language distribution than older healthy children, indicating an increasing specialization of language functions to the left hemisphere as age increases. However, lateralization changes were more closely tied to the period of acquisition for language tasks than to general maturation. The largest changes in lateralization occurred for the task that shows the most protracted period of development (verb generation). Children continue to improve their ability to form and retrieve semantic associations into adulthood. Likewise, the Story Processing task, which required children to process syntactic structures that are mastered in mid-childhood, also showed age-related changes in lateralization. In contrast, effect sizes for lateralization change with age were smallest (and statistically non-significant) for tasks designed to reflect early-acquired language skills.
We are able to use these data as a reference point for the results found for the verb generation task in children with perinatal stroke(Jacola, Schapiro et al., 2006; Tillema et al., In Press), traumatic brain injury (Karunanayaka, Holland et al., In Press), or epilepsy (Yuan, Szaflarski et al., 2006) respectively. The earliest and most severe injuries to the left hemisphere (left MCA stroke) showed the most drastic pattern of reorganization: the patients relied much more on their right hemisphere as reflected in Figure 5. These results provide evidence that when the left hemisphere is damaged early in language development, language processing mechanisms can be established in/reorganized to the right hemisphere. When language tasks produced activation in the right hemisphere in our stroke patients, these areas were homologues of the traditional left hemisphere language areas. As the amount of right hemisphere activation varied among patients, these findings suggest that language processing is redistributed to different degrees among homologous brain regions in children who have suffered stroke involving left hemisphere language areas. Thus, in children, either hemisphere can be activated by language tasks and provide support for language development. This is similar to results demonstrated in adults with similar injuries.
This increased use of the contralateral hemisphere was not found for patients with more subtle injuries later in development (e.g. TBI) ; though, their patterns of activation during the Verb Generation task were significantly altered compared to control participants. In contrast with the MCA stroke cohort children with TBI showed activation in the expected brain areas for this language based task. This suggests that the severity and nature of the traumatic injuries were not sufficient to require total neural reorganization. However, we did find significant differences in activation between the children with TBI and typically developing children during the Verb Generation task. Specifically, we found increased activation in the left inferior frontal gyrus (Broca's area) and dorsolateral prefrontal cortex, likely associated with monitoring and manipulation in working memory (Braver et al., 2001; Schmithorst, 2005). Children with TBI may need to draw upon language and working memory resources not usually required to perform the task. We also found decreased activation in the TBI group in the left/right middle temporal gyrus (BA21), left/right superior temporal gyrus (BA 22/STG), and the left/right angular gyrus relative to the comparison group. STG involvement has previously been reported in semantic processing (Kuperberg et al., 2000; Friederici et al., 2003), as well as processing of auditory input (Scott et al., 2000; Schmithorst, Holland et al., 2005), and the angular gyrus has been involved in higher order semantic processing (Price, 2000), memory rehearsal (Ravizza et al., 2004), and attention to verbal material. Overall, these results demonstrate that disruption of functional language networks persists for months to years after a TBI that occurs during peak years for language development.
Comparison of language lateralization during development in epilepsy patients and healthy controls revealed yet another scenario for neuroplasticity of language in the developing brain. Our results from the Verb Generation task showed that the language distribution patterns in epilepsy patients differed significantly from those found in healthy control subjects in both mean value and the variability of lateralization index. This pattern was also distinct from the stroke and TBI groups who experienced a single discrete brain injury, either perinatally or later in childhood. Intractable epilepsy in the left temporal lobe inflicts a chronic injury on key language areas at a lower dose than can be expected from TBI or left MCA infarction. Concomitantly we find that pediatric epilepsy patients have higher incidence of atypical (bilateral or right hemispheric) language dominance than healthy controls, an observation that has been consistently reported in previous adult studies. Unlike the conclusions by that suggested seizure onset age is a significant predictor of language lateralization (Springer, Binder et al., 1999; Saltzman et al., 2002), but in agreement with other studies, e.g.; (Janszky et al., 2003; Brazdil, Chlebus et al., 2005; Thivard et al., 2005), our results did not demonstrate any correlation between these two variables. However, duration since seizure onset was a more reliable predictor for LI.
Another interesting finding in our studies is the possible divergence between healthy controls and patients with epilepsy in the LI- versus-Age relationship. As discussed above, LI in healthy children increases with age during development, indicating an increasing specialization of language functions to the left hemisphere as age increases. From the perspective of neural development, the lack of correlation of LI with age in epilepsy patients suggested a disruption caused by epilepsy to the left hemisphere specialization of language functional areas as seen in normal development. The variability of seizure onset age, the inhomogeneous epilepsy pathologies, various lesion sites, intelligence, and socioeconomic status may have been possible confounding factors due to the limited sample size in our study, but this overall pattern is strongly supported.
The question remains as to whether the atypical language lateralization in epilepsy patients is due to the chronic effect of seizures on the brain, or whether preceding brain pathologies cause both. The fact that the degree of atypical lateralization, as quantified by LI, shows a strong correlation with the epilepsy duration seems to suggest a possible causal relation in which the seizure activity in epilepsy subjects causes redistribution of language function in order to compensate for injury to traditionally left dominant language areas or connections to them.
More work is needed to clarify the link between the hemispheric redistribution of language networks and ultimate level of language skill. Perinatal stroke patients did not demonstrate aphasic deficits. However, these patients did exhibit lower verbal and full-scale IQ than the control group, suggesting that left hemisphere mechanisms may be necessary for completely normal language skills. This finding supports very early (or even innate) specialization of these networks. In the TBI patients, a similar relationship was observed between use of typical language circuitry and language skill. Activation levels in most of the language circuitry were negatively correlated with test performance on measures of verbal intelligence and fluency. These findings suggest that additional neural recruitment directed at performing the task translated into poorer rather than better performance.
Taken together, these results support the notion that the left-hemisphere functional specialization for language begins early in development (even prenatally) and becomes more focused over time as children's language skills become increasingly complex. If this process is interrupted by a severe injury or seizures, right hemisphere mechanisms may be used to support language processing, though it remains unclear whether the same level of language skill can be attained with these mechanisms. Less severe brain injuries also affect language skill and its neural underpinnings but do not necessarily drive engagement of the contralateral hemisphere.
Further investigation of these patterns of lateralization and reorganization with a full range of language tasks, as well as more specific patient populations, will help to build an even more detailed picture of the neural basis of language throughout development with the goal of better assessment and treatment of language problems associated with neurological injuries and disorders.
Acknowledgements
This work was supported in part by grants from the Children's Hospital Research Foundation Trustees (P.I. SK Holland), the National Institute of Child Health and Human Development (R01-HD38578, P.I. SK Holland and R01-HD044279, P.I. SL Wade) the National Institute of Neurological Disorders and Stroke (K23-NS01467; P.I. AW Byars), and the National Institute of Mental Health (R01-MH073764, P.I. SL Wade). We would also like to acknowledge the contributions of many collaborators on the work summarizes here including: Richard H. Strawsburg, MD, Mekibib Altaye, Ph.D., Blaise V. Jones, MD, Linda J. Michaud, MD, Nicolay Chertkoff-Walz, Ph.D., Mark B. Schapiro, MD, and Jerzy P. Szaflarski, MD, PhD.
References
- Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Ahmad Z, Balsamo LM, Sachs BC, Xu B, Gaillard WD. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60:1598–1605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- Albanese E, Merlo A, Albanese A, Gomez E. Anterior speech region. Asymmetry and weight-surface correlation. Arch Neurol. 1989;46:307–310. doi: 10.1001/archneur.1989.00520390073019. [DOI] [PubMed] [Google Scholar]
- Anderson VA, Morse SA, Klug G, Catroppa C, Haritou F, et al. Predicting recovery from head injury in young children: a prospective analysis. J Int Neuropsychol Soc. 1997;3:568–580. [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Grandin CB, Petrella JR, Braniecki SH, et al. A functional magnetic resonance imaging study of left hemisphere language dominance in children. Arch Neurol. 2002;59:1168–1174. doi: 10.1001/archneur.59.7.1168. [DOI] [PubMed] [Google Scholar]
- Benson RR, FitzGerald DB, LeSueur LL, Kennedy DN, Kwong KK, et al. Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology. 1999;52:798–809. doi: 10.1212/wnl.52.4.798. [DOI] [PubMed] [Google Scholar]
- Billingsley RL, McAndrews MP, Crawley AP, Mikulis DJ. Functional MRI of phonological and semantic processing in temporal lobe epilepsy. Brain. 2001;124:1218–1227. doi: 10.1093/brain/124.6.1218. [DOI] [PubMed] [Google Scholar]
- Binder JR. Neuroanatomy of Language Processing Studied with Functional MRI. Neuroscience. 1997;4:87–94. [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage. 2006;33:739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonder LX, Bowers D, Heilman KMB. The role of the right hemisphere in emotional communication. Brain. 1991;114:1115–1127. doi: 10.1093/brain/114.3.1115. 114, 115−1127. [DOI] [PubMed] [Google Scholar]
- Blumenfeld HK, Booth JR, Burman DD. Differential prefrontal-temporal neural correlates of semantic processing in children. Brain Lang. 2006;99:226–235. doi: 10.1016/j.bandl.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, et al. Development of brain mechanisms for processing orthographic and phonologic representations. J Cogn Neurosci. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, MacWhinney B, Thulborn KR, Sacco K, Voyvodic JT, et al. Developmental and lesion effects in brain activation during sentence comprehension and mental rotation. Dev Neuropsychol. 2000;18:139–169. doi: 10.1207/S15326942DN1802_1. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb. Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Brazdil M, Chlebus P, Mikl M, Pazourkova M, Krupa P, et al. Reorganization of language-related neuronal networks in patients with left temporal lobe epilepsy - an fMRI study. Eur J Neurol. 2005;12:268–275. doi: 10.1111/j.1468-1331.2004.01127.x. [DOI] [PubMed] [Google Scholar]
- Bridges A. SVO comprehension strategies reconsidered: the evidence of individual patterns of response. Journal of child language. 1980;7:89–104. doi: 10.1017/s0305000900007042. [DOI] [PubMed] [Google Scholar]
- Brizzolara D, Pecini C, Brovedani P, Ferretti G, Cipriani P, et al. Timing and type of congenital brain lesion determine different patterns of language lateralization in hemiplegic children. Neuropsychologia. 2002;40:620–632. doi: 10.1016/s0028-3932(01)00158-0. [DOI] [PubMed] [Google Scholar]
- Byars AW, Holland SK, Strawsburg RH, Bommer W, Dunn RS, et al. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J Child Neurol. 2002;17:885–890. doi: 10.1177/08830738020170122201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrow-Woolfolk E. Oral and Written Language Scales. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of the temporal speech areas of the human fetus. Arch Neurol. 1977;34:346–348. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- Choi Y, Mazuka R. Young children's use of prosody in sentence parsing. Journal of psycholinguistic research. 2003;32:197–217. doi: 10.1023/a:1022400424874. [DOI] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Burman DD, Bitan T, Bigio JD, et al. Developmental changes in the neural correlates of semantic processing. Neuroimage. 2006;29:1141–1149. doi: 10.1016/j.neuroimage.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Desmond J, Sum J, Wagner A, Demb J, Shear P, et al. Functional MRI Measurment of Language Lateralization in Wada-Tested Patients. Brain. 1995;118:1411–1419. doi: 10.1093/brain/118.6.1411. [DOI] [PubMed] [Google Scholar]
- Emerson HF. Children's comprehension of ’because’ in reversible and non-reversible sentences. Journal of child language. 1979;6:279–300. doi: 10.1017/s0305000900002300. [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L, Fletcher JM, Levin HS, Francis DJ, Davidson K, et al. Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. J Int Neuropsychol Soc. 1997;3:581–591. [PubMed] [Google Scholar]
- Falzi G, Perrone P, Vignolo LA. Right-left asymmetry in anterior speech region. Arch Neurol. 1982;39:239–240. doi: 10.1001/archneur.1982.00510160045009. [DOI] [PubMed] [Google Scholar]
- Fenson L, Dale PS, Resznick JS, Thal D, Bates E, et al. The MacArthur Communicative Development Inventories. Singular Publishing Group; San Diego, CA: 1993. [Google Scholar]
- Friederici AD, Ruschemeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, et al. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59:256–265. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- Gerken LA, Jusczyk PW, Mandel DR. When prosody fails to cue syntactic structure: Nine-month-olds’ sensitivity to phonological vs syntactic phrases. Cognition. 1994;51:237–265. doi: 10.1016/0010-0277(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Gaillard WD, Mott SH, Cuenod CA, Bookheimer SY, et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology. 1997;48:1003–1012. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Lehericy S, Cordoliani YS, Le Bihan D, Marsault C, et al. [Brain functional MRI: physiological, technical, and methodological bases, and clinical applications]. J Radiol. 2000;81:731–733. [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars AM, Strawsburg RH, Schimthorst VJ, et al. Normal Brain Activation Patterns in Children Performing a Verb Generation Task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Jacola LM, Schapiro MB, Schmithorst VJ, Byars AW, Strawsburg RH, et al. Functional magnetic resonance imaging reveals atypical language organization in children following perinatal left middle cerebral artery stroke. Neuropediatrics. 2006;37:46–52. doi: 10.1055/s-2006-923934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janszky J, Jokeit H, Heinemann D, Schulz R, Woermann FG, et al. Epileptic activity influences the speech organization in medial temporal lobe epilepsy. Brain. 2003;126:2043–2051. doi: 10.1093/brain/awg193. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW. How Attention to Sound Properties May Facilitate Learning Other Elements of Linguistic Organization. In: Jusczyk PW, editor. The Discovery of Spoken Language. MIT Press; Cambridge, MA: 1997. [Google Scholar]
- Karunanayaka PR, Holland SK, Altaye M, Cecil KM, Walz NC, et al. Abnormalities in language circuitry in children with traumatic brain injury, an fMRI study. NeuroRehabilitation. 2007 In Press. [Google Scholar]
- Karunanayaka PR, Holland SK, Schmithorst VJ, Solodkin A, Chen EE, et al. Age-related connectivity changes in fMRI data from children listening to stories. Neuroimage. 2007;34:349–360. doi: 10.1016/j.neuroimage.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Kuhl PK, Stevens E, Hayashi A, Deguchi T, Kiritani S, et al. Infants show a facilitation effect for native language phonetic perception between 6 and 12 months. Developmental science. 2006;9:F13–F21. doi: 10.1111/j.1467-7687.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, McGuire PK, Bullmore ET, Brammer MJ, Rabe-Hesketh S, et al. Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. J Cogn Neurosci. 2000;12:321–341. doi: 10.1162/089892900562138. [DOI] [PubMed] [Google Scholar]
- Levistsky W, Geschwind N. Asymmetries of the right and left hemisphere in man. Transactions of the American Neurological Association. 1968;93:232–233. [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Salmond CH, Gadian DG, Vargha-Khadem F, et al. A direct test for lateralization of language activation using fMRI: comparison with invasive assessments in children with epilepsy. Neuroimage. 2002;17:1861–1867. doi: 10.1006/nimg.2002.1327. [DOI] [PubMed] [Google Scholar]
- Mandel DR, Jusczyk PW, Kemler Nelson DG. Does sentential prosody help infants to organize and remember speech information? Cognition. 1994;53:155–180. doi: 10.1016/0010-0277(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Mandel DR, Kemler Nelson DG, Jusczyk PW. Infants remember the order of words in a spoken sentence. Cognitive Development. 1996;11:181–196. [Google Scholar]
- Metzner R, Meyer H, Baudendistel K, Essig M, Schroder J, et al. fMRI: Different Degrees of Cortical Activation under Controlled Motoric Stimulation. Proc. of the ISMRM. 1999;7:772. [Google Scholar]
- Morse S, Haritou F, Ong K, Anderson V, Catroppa C, et al. Early effects of traumatic brain injury on young children's language performance: a preliminary linguistic analysis. Pediatric rehabilitation. 1999;3:139–148. doi: 10.1080/136384999289405. [DOI] [PubMed] [Google Scholar]
- Muller RA, Rothermel RD, Behen ME, O. M., T.J. M., et al. Differential Patterns of language and Motor Reorganization Following Early Left Hemisphere Lesion. Archives of Neurology. 1998:55. doi: 10.1001/archneur.55.8.1113. [DOI] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. Neuroimage. 2000;12:538–549. doi: 10.1006/nimg.2000.0651. [DOI] [PubMed] [Google Scholar]
- Naidich TP, Brightbill TC. Systems for localizing fronto-parietal gyri and sulci on axial CT and MRI. International J of Neurorad. 1996;2:313–338. [Google Scholar]
- Oldfield RC. OThe assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papanicolaou AC, Simos PG, Breier JI, Wheless JW, Mancias P, et al. Brain plasticity for sensory and linguistic functions: a functional imaging study using magnetoencephalography with children and young adults. J Child Neurol. 2001;16:241–252. doi: 10.1177/088307380101600403. [DOI] [PubMed] [Google Scholar]
- Pena M, Maki A, Kovacic D, Dehaene-Lambertz G, Koizumi H, et al. Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci U S A. 2003;100:11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomography studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–586. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Plante E, Holland SK, Schmithorst VJ. Prosodic processing by children: An fMRI study. Brain Lang. 2006;97:332–342. doi: 10.1016/j.bandl.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of anatomy. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh K. A neurocognitive overview of reading acquisition and dyslexia across languages. Developmental science. 2006;9:448–450. doi: 10.1111/j.1467-7687.2006.00528.x. discussion 451−443. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22:562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Saltzman J, Smith ML, Scott K. The impact of age at seizure onset on the likelihood of atypical language representation in children with intractable epilepsy. Brain Cogn. 2002;48:517–520. [PubMed] [Google Scholar]
- Schapiro MB, Schmithorst VJ, Wilke M, Byars AW, Strawsburg RH, et al. BOLD-fMRI signal increases with age in selected brain regions in children. NeuroReport. 2004;15:2575–2578. doi: 10.1097/00001756-200412030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometrical distortion artifacts in using a multiecho echo reference scan. IEEE Trans on Medical Imag. 2001;20:535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst V, Holland SK, Dardzinski BJ. CCHIPS: Cincinnati Children's Hospital Imaging Processing Software. 2000 http://www.irc.chmcc.org/chips.htm.
- Schmithorst VJ. Separate cortical networks involved in music perception: preliminary functional MRI evidence for modularity of music processing. Neuroimage. 2005;25:444–451. doi: 10.1016/j.neuroimage.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK. Functional MRI Evidence for Disparate Developmental Processes Underlying Intelligence in Boys and Girls. Neuroimage. 2006;31:1366–1379. doi: 10.1016/j.neuroimage.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E. Cognitive modules utilized for narrative comprehension in children: a functional magnetic resonance imaging study. Neuroimage. 2005 doi: 10.1016/j.neuroimage.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Plante E. Object Identification and Lexical/Semantic Access in Children: A Functional Magnetic Resonance Imaging Study of Word-Picture Matchin. Hum Brain Mapp. 2006 doi: 10.1002/hbm.20328. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123(Pt 12):2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, et al. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125:2222–2237. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Niemann G, Wildgruber D, Erb M, et al. Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology. 2001;57:122–125. doi: 10.1212/wnl.57.1.122. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006;27:202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59:796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic Atlas of the Human Brain. Thieme Mecial; New York: 1988. [Google Scholar]
- Thevenaz P, Ruttiman UE, Unser M. A Pyramid Approach to Sub-Pixel Registraion based on Intensity. IEEE Trans. Image Processing. 1998;7:27–41. doi: 10.1109/83.650848. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Phillips BA, Morris I, Byrnes ML, Sacco P, et al. Differences in functional magnetic resonance imaging of sensorimotor cortex during static and dynamic finger flexion. Experimental Brain Research. 1999;126:431–438. doi: 10.1007/s002210050749. [DOI] [PubMed] [Google Scholar]
- Thivard L, Hombrouck J, du Montcel ST, Delmaire C, Cohen L, et al. Productive and perceptive language reorganization in temporal lobe epilepsy. Neuroimage. 2005;24:841–851. doi: 10.1016/j.neuroimage.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Tillema JM, Byars AW, Jacola LM, Schapiro MB, Schmithorst VJ, et al. Reorganization of Language Networks in Children following Perinatal Left MCA Stroke. Brain and Language. 2007 doi: 10.1016/j.bandl.2007.07.127. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada JA, Clarke R, Hamm A. Cerebral hemispheric asymmetry in humans. Cortical speech zones in 100 adults and 100 infant brains. Arch Neurol. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- Wexler B, Fulbright R, Lacadie C, Skudlarkski P, Kelz M, et al. An fMRI Study of the Human Cortical Motor System Response to Increasing Functional Demands. Magn. Reson. Imag. 1997;14:385–396. doi: 10.1016/s0730-725x(96)00232-9. [DOI] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Ball WS., Jr. Language processing during natural sleep in a 6-year-old boy, as assessed with functional MR imaging. AJNR Am J Neuroradiol. 2003;24:42–44. [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Lidzba K, Staudt M, Buchenau K, Grodd W, et al. An fMRI task battery for assessing hemispheric language dominance in children. Neuroimage. 2006;32:400–410. doi: 10.1016/j.neuroimage.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Wilke M, Myseros JS, Holland SK, Schmithorst VJ, Ball J. WS. Clinical functional magnetic resonance imaging in pediatrics: problems, pitfalls, and benefit. NeuroPediatrics. 2003;24:42–44. doi: 10.1055/s-2003-43260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AG, Harvey AS, Wellard RM, Abbott DF, Anderson V, et al. Language cortex activation in normal children. Neurology. 2004;63:1035–1044. doi: 10.1212/01.wnl.0000140707.61952.ca. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, et al. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- Worthington C, Vincent DJ, Bryant AE, Roberts DR, Vera CL, et al. Comparison of functional magnetic resonance imaging for language localization and intracarotid speech amytal testing in presurgical evaluation for intractable epilepsy. Preliminary results. Stereotact Funct Neurosurg. 1997;69:197–201. doi: 10.1159/000099874. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao J-H, Lancaster JL, Fox PT. Clustered Pixels Analysis for Functional MRI Activation Studies of the Human Brain. Human Brain Mapping. 1995;3:287–301. [Google Scholar]
- Xiong J, Rao S, Gao J-H, Wondorff M, Fox P. Evaluation of Hemispheric Dominance for Language Using Functional MRI: A Comparison With Positron Emission Tomography. Human Brain Mapping. 1998;6:42–58. doi: 10.1002/(SICI)1097-0193(1998)6:1<42::AID-HBM4>3.0.CO;2-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Holland SK, Cecil KM, Dietrich KN, Wessel SD, et al. The impact of early childhood lead exposure on brain organization: a functional magnetic resonance imaging study of language function. Pediatrics. 2006;118:971–977. doi: 10.1542/peds.2006-0467. [DOI] [PubMed] [Google Scholar]
- Yuan W, Szaflarski JP, Schmithorst VJ, Schapiro M, Byars AW, et al. fMRI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 2006;47:593–600. doi: 10.1111/j.1528-1167.2006.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]