SUMMARY
The ventral spinal cord generates multiple inhibitory and excitatory interneuron subtypes from four cardinal progenitor domains (p0, p1, p2, p3). Here we show that cell-cell interactions mediated by the Notch receptor play a critical evolutionarily conserved role in the generation of excitatory v2aIN and inhibitory v2bIN interneurons. Lineage-tracing experiments show that the v2aIN and v2bIN develop from genetically identical p2 progenitors. The p2 daughter cell fate is controlled by Delta4 activation of Notch receptors together with MAML factors. Cells receiving Notch signals activate a transcription factor code that specifies the v2bIN fate, whereas cells deprived of Notch signaling express another code for v2aIN formation. Thus, our study provides insight into the cell-extrinsic signaling that controls combinatorial transcription factor profiles involved in regulating the process of interneuron subtype diversification.
INTRODUCTION
In the vertebrate spinal cord, sensory and motor functions are regulated by interneurons in the dorsal and ventral spinal cord, respectively. Most ventral interneuron subtypes, and all motor neurons, emerge from five distinct progenitor cell populations arrayed along the dorsal-ventral axis of the neural tube, termed the p0, p1, p2, pMN, and p3 domains (Goulding and Lamar, 2000; Jessell, 2000). These progenitors express unique combinations of transcription factors through exposure to a gradient of sonic hedgehog (Shh) along the dorsal ventral axis (Briscoe et al., 2000; Ericson et al., 1995; Roelink et al., 1995). They also represent dividing precursors that eventually give rise to the postmitotic interneurons and motor neurons in the adult ventral spinal cord (Goulding and Pfaff, 2005; Jessell, 2000; Kiehn, 2006). The process by which the postmitotic daughter cells arising from the cardinal progenitor domains become further diversified to acquire more specialized subtype properties, such as their physiological characteristics, remains poorly understood for interneurons.
The development of motor neuron subtypes has been more intensely characterized and is a gradual process where cell-intrinsic transcriptional codes are determined by extrinsic signals in separate phases. In the first phase, Shh and retinoic acid activities determine the transcriptional code for pMN progenitor cell specification (Briscoe et al., 2000; Ericson et al., 1995; Novitch et al., 2003; Roelink et al., 1995). In a second phase, retinoid signaling establishes the limb motor neuron subtype identity by regulating the expression of LIM-homeodomain factor Lim1 (Sockanathan and Jessell, 1998). In a third phase, the Hox-encoded motor pool identity is established by FGF, Gdf11, and retinoid activity (Dasen et al., 2003; Dasen et al., 2005; Liu et al., 2001). Thus, both the dividing progenitor cells (pMN) and immature postmitotic motor neurons are constantly influenced by their environment and respond by changing the expression of transcription factors that in turn determine more specific motor neuron subtype attributes.
Based on our understanding of motor neuron development, it is possible that interneuron lineages might also use extrinsic signals to generate functional neuronal subtypes present in the adult spinal cord. Such mechanisms would be critical for generating the cellular diversity necessary for constructing the complex motor circuits that reside within the spinal cord. Understanding such mechanisms might help to determine how cellular diversity is generated in other regions of the CNS.
Progenitors in the p2 domain generate excitatory v2aIN that are marked by their expression of the homeodomain protein Chx10, and these neurons regulate motor functions in zebrafish and mouse (Kimura et al., 2006; Line Lundfald and Ole Kiehn, personal communication). Inhibitory v2bIN also appear to arise from p2 progenitor cells during development and are marked by Gata3. Previous genetic studies have identified many transcription factors that are critical for v2aIN and v2bIN fates, notably FoxN4, Gata2, Lhx3, Mash1, and Scl (Ericson et al., 1997; Karunaratne et al., 2002; Li et al., 2005; Muroyama et al., 2005; Nardelli et al., 1999; Parras et al., 2002; Smith et al., 2002; Thaler et al., 2002; Zhou et al., 2000) (Figure 1A). Despite the identification of numerous transcription factors, a key unresolved problem is the logic by which p2 progenitor cells choose to activate the transcription factor programs that specify v2aIN or v2bIN fates.
Figure 1. Generation of v2aIN and v2bIN from Lhx3+ p2 Progenitors Is Regulated by Notch Receptor Signaling.
(A) Summary of v2IN development.
(B) Analysis of e11.5, Lhx3:IRES:CRE; β-actin-LNL-nLacZ mice. Transverse section shows the expression of β-galactosidase (blue), Chx10, and Gata3 in the p2 domain (boxed area). The midline is to the left and ventral is to the bottom in this and all subsequent panels. Blue cells outside the p2 domain are motor neurons. (B′ and B″) Higher-magnification view of the boxed area in panel (B). Colocalization of β-Gal with Chx10 ([B′], arrowheads) and Gata3 ([B″] arrowheads) is evident in many cells.
(C) Chx10 and Gata3 cell counts in wild-type mice (mean cell count ± SEM; n = 5 embryos). Fewer v2bIN are seen at e13.5 since Gata3 expression is lost during development, whereas Chx10 expression is maintained even in adult mice.
(D) Expression of Lhx3 and Gata2 in e11.5 wild-type mouse spinal cord. Colocalization of Lhx3 and Gata2 (yellow nuclei, arrow) is evident in the p2-VZ but not in p2-ML.
(E and F) Coexpression of Nicd and Lhx3 (E) or Gata2 (F) in some p2 progenitors (arrows) but not in others (arrowheads). The identity of the few Lhx3 and Gata2 cells that do not show detectable expression of Nicd is not clear (see Figure 8 for a possible explanation).
(G and G′) Expression of Nicd is greatly reduced in PS1−/− mice ([G′], n = 5 embryos) when compared to controls ([G], n = 6 embryos).
(H and H′) Delta4 expression is moderately increased in the PS1−/− mice (arrow, [H and H′]). Delta4 is also expressed in blood vessels located throughout the spinal cord (arrowhead in [H′]).
(I and I′) More Chx10 neurons are generated in the PS1−/− embryos (I′) compared to the controls (I).
(J and J′) Expression of Gata3 is seen in the p2-ML of the spinal cord from the PS1+/+ mice (J). Gata3 expression is lost in the PS1−/− mice (J′).
(K) Cell count of total v2IN (Chx10 + Gata3 cells), v2aIN, and v2bIN in PS1+/+ and PS1−/− mice (mean ± SEM; ten sections per embryo; n = 6 for PS1+/+ embryos; n = 5 for PS1−/− embryos). The total number of v2IN is increased (121% of the control number, p = 0.0004), whereas the number of v2aIN is almost doubled (217% of the control number, p = 1.12e−31). Note that v2bIN are not generated in PS1−/− mice.
Scale bar, 75 µm in (B), 50 µm in (B′)–(J′).
In many cell lineages in which neural progenitors select alternate cell fates, it is expected that factors exist to control the relative abundance of differentiated cell types. In the p2 lineage, null mutations in Mash1, FoxN4, and Scl factors generate more v2aIN and fewer or no v2bIN (Li et al., 2005; Muroyama et al., 2005). The temporal distinction in the function of these transcription factors is high-lighted by their expression patterns. Mash1 and FoxN4 are expressed by p2 progenitor cells, whereas Scl is also expressed in differentiated v2bIN (Li et al., 2005; Muroyama et al., 2005). A recent study has implicated quantitative differences in the levels of Mash1 expression in the development of excitatory versus inhibitory inter-neurons in the dorsal spinal cord (Mizuguchi et al., 2006). However, differences in the expression of Mash1 in p2 progenitor cells are not known. A key question is whether the p2 domain consists of homogeneous population of bipotential (v2aIN/v2bIN) progenitors or a mixed population of v2aIN and v2bIN progenitors.
In invertebrate model systems, the Notch signaling pathway is known to regulate neuronal differentiation as well as neuronal fate (Artavanis-Tsakonas et al., 1999). However, the role of Notch signaling in cell-fate specification in the vertebrate neural tube is often masked by its role in neuronal differentiation. During normal development, the pMN progenitors generate motor neurons before the p2 progenitors generate v2 interneurons. In the Notch1 conditional null mice, the differentiation of pMN progenitors is delayed, and as a result their progeny are thought to adopt the v2 interneuron fate (Yang et al., 2006). The timing of differentiation within the p2 domain might also determine whether v2aIN or v2bIN are generated. A prediction from such a mechanism would be that v2aIN and v2bIN are generated at different times during development. Alternatively, genetically similar p2 progenitors might generate different cell types using a combination of inductive signals and the Notch signaling pathway, as in the case of vulval precursor cells in the worm (Chen and Greenwald, 2004; Yoo et al., 2004).
In this study we address four key questions about the origins of interneuron diversity in the developing spinal cord. First, do distinct interneuron subtypes develop from genetically similar progenitors? Second, at which stage during development do progenitors acquire alternate fate? Third, what mechanisms are used to select alternate cell fates? Fourth, how are these selector mechanisms coordinated with the transcriptional codes that are known to determine interneuron cell fate? We use cre-recombinase-mediated lineage tracing and gene expression analysis to reveal that v2aIN and v2bIN originate from similar p2 progenitors. We show that postmitotic, but immature, neurons choose either v2aIN or v2bIN cell fate. We identify Delta4-activated Notch signaling as the binary switch that determines whether v2aIN or v2bIN is generated. Finally, we demonstrate that Notch in partnership with MAML factors activates a cell-intrinsic transcriptional program for generating distinct neuronal subtypes. Our studies in the fish, chick, and mouse show that the role of cell-cell interactions in generating excitatory v2aIN and inhibitory v2bIN is conserved through evolution.
RESULTS
Lhx3-Expressing p2 Progenitors Generate v2aIN and v2bIN
Since Lhx3 is expressed in differentiated v2aIN but not in v2bIN, we asked whether Lhx3 expression is restricted to those p2 progenitors that generate v2aIN. Previously, we made use of double-transgenic Lhx3:IRES:CRE;β-actin:LNL:nLacZ mouse embryos to indelibly trace the expression of Lhx3 in the pMN-derived, motor neuron lineage (Sharma et al., 1998). In the double-transgenic mice, nuclear β-galactosidase marks cells derived from lineages where Lhx3 is expressed. We took advantage of these animals to analyze the fate of Lhx3-expressing p2 progenitors. We find that throughout the rostral-caudal extent of the mouse spinal cord, nuclear β-galactosidase colocalizes with Chx10 (v2aIN marker) as well as Gata3 (v2bIN marker) in the p2 domain (Figures 1B, 1B′, and 1B″). These data show that Lhx3 is expressed in p2 progenitors and that expression of Lhx3 is maintained in the v2aIN lineage but lost in the v2bIN lineage. In the pMN lineages, motor neurons that retain the expression of Lhx3 are generated earlier than those in which expression of Lhx3 is lost (Sharma et al., 1998). We asked whether similar differences exist in the timing of v2aIN and v2bIN development. We counted v2aIN and v2bIN in the mouse spinal cord between embryonic days (e) 10 and 13.5 (Figure 1C). These cell counts suggest that the time of development does not correlate with the ability of p2 progenitors to maintain Lhx3 expression and generate v2aIN or to downregulate Lhx3 expression and adopt the v2bIN cell fate. Together, these observations suggest that the expression of Lhx3 is initiated in the v2a as well as v2b progenitors that differentiate at similar times.
Notch Signaling Is Required for the Generation of v2bIN
More Lhx3-expressing v2aIN are generated in the absence of Notch signaling (Yang et al., 2006). This finding was interpreted as a cell-fate switch from motor neurons to v2aIN (Yang et al., 2006). We considered the possibility that Notch signaling might regulate v2aIN versus v2bIN cell fate. We used Lhx3 and Gata2 expression to visualize developing v2aIN and v2bIN lineages, respectively. We find that many cells located in the ventricular zone (p2-VZ) coexpress Lhx3 and Gata2 (Figure 1D). In the mantle layer (p2-ML), expression of Lhx3 and Gata2 does not overlap (Figure 1D), as it is restricted to v2aIN and v2bIN, respectively. Whether Lhx3/Gata2 double-labeled cells in the p2-VZ are v2a, v2b, or bipotential v2a/v2b progenitors is not known. We asked whether Notch signaling is activated in Lhx3 or Gata2 cells in the p2-VZ. Using an antibody that binds to Nicd but not to the full-length Notch receptor (Tokunaga et al., 2004), we find that many but not all Lhx3 and Gata2 cells in the p2-VZ express Nicd (Figures 1E and 1F). The specificity of the Nicd antibody is confirmed by significant reduction of staining in the PS1−/− embryos that are known to be deficient in proteolytic processing of the Notch receptor (Figures 1G and 1G′). As shown before (Benedito and Duarte, 2005), progenitor cells in the p2-VZ also express Notch ligand, Delta4 (Figure 1H). In the PS1−/− embryos, the expression of Delta4 is moderately increased, confirming that the ligand is not a limiting factor in the PS1−/− mice.
Since Nicd expression is drastically reduced in the PS1−/− mutants, we used these mice to test whether Notch signaling regulates v2aIN versus v2bIN cell fate. In the PS1−/− mice, the number of v2aIN is almost doubled (Figures 1I′ and 1K), a finding consistent with the reported conditional Notch1 mutant phenotype (Yang et al., 2006). Additionally, the PS1−/− mice show complete absence of Gata3 expression, indicating that v2bIN fate is not specified (Figures 1J′ and 1K). We asked whether Notch signaling affects the development of other inhibitory interneuron subtypes. We find that the number of Evx-1+ v0 neurons is increased slightly, whereas the number of En-1+ v1 interneurons is noαt changed (see Figure S1 in the Supplemental Data available online). In addition, the number of motor neurons is not altered in the PS1−/− mice (Figure S1). In the complete absence of v2bIN in the PS1−/− mice (Figure 1K), all p2 progenitor-derived neurons adopt the v2aIN cell fate. These results indicate that Notch signaling promotes the inhibitory v2bIN cell fate instead of the excitatory v2aIN cell fate.
Notch Signaling Activates the Expression of Scl in v2bIN Lineage
To examine the stage at which Notch signaling is required for the generation of v2bIN, we first analyzed the expression of transcription factors known to regulate cell-fate decisions in the p2 domain. Representative micrographs (Figures 2A–2F) and average cell counts (Figure 2G) are presented. While the control mice express Lhx3 and Gata2 in the p2-VZ and p2-ML (Figures 2A and 2B), PS1−/− mutant mice show increased numbers of Lhx3 cells but very few Gata2 cells in the p2-ML (Figures 2A′ and 2B′). In the PS1−/− mutants, Gata2 expression in the p2-VZ is not affected as severely as seen in the p2-ML (Figure 2B′). These findings suggest that in the PS1−/− mutants, the p2 progenitors develop normally but fail to generate Gata2/Gata3-expressing v2bIN. It is known that the p2 progenitors express Mash1 and FoxN4 (Li et al., 2005). Both of these factors are expressed in p2 progenitors that generate v2aIN as well as v2bIN. Consistent with the idea that p2 progenitors are generated normally in the PS1−/− mutants, we find that the expression of FoxN4 and Mash1 is similar in the mutant and control embryos (Figures 2C, 2C′, 2D, and 2D′). Scl is a key factor in the development of v2bIN from p2 progenitors (Muroyama et al., 2005). In control mice, Scl is expressed in p2-VZ and also in the v2bIN located in the p2-ML (Figures 2E and 2F). In the PS1−/− mutants, the expression of Scl mRNA and protein is not detected (Figures 2E′ and 2F′). These data indicate that in the PS1−/− mutant mice, p2 progenitors are generated normally. More importantly, these data implicate Notch signaling in the further development of p2 progenitors, including the activation of the Scl expression and initiation of v2bIN cell-fate programs.
Figure 2. Reduced Notch Signaling in PS1−/− Embryos Leads to the Loss of Scl Expression in the p2 Domain.
(A–F) Expression of transcription factors in the p2 domain of e11.5 PS1+/+ and PS1−/− mice. In PS1+/+ embryos, Lhx3 (A), Gata2 (B), Mash1 (C), FoxN4 (D), Scl mRNA (E), and Scl protein (F) expression is evident in the p2 domain. In the PS1−/− embryos, Lhx3 (A′), Gata2 (B′), Mash1 (C′), and FoxN4 (D′) expression is retained, whereas Scl mRNA (E′) and Scl protein are not detected (F′). Note that Gata2 expression is reduced in the p2-VZ and absent from the p2-ML in PS1−/− embryos (B′).
(G) Cell counts (mean ± SEM; ten sections per embryo; n = 6 for PS1+/+ embryos; n = 5 for PS1−/− embryos) in the spinal cord of e11.5 PS1+/+ and PS1−/− embryos. The number of Lhx3 cells is increased (p = 8.6e−26), Gata2 cells are decreased (p = 1.6e−43), and the number of Mash1 or FoxN4 cells is not changed (p > 0.1). In the PS1−/− mice, Scl-expressing cells are not detected.
Scale bar, 50 µm.
Identification of Intermediate Precursors in v2aIN and v2bIN Lineages
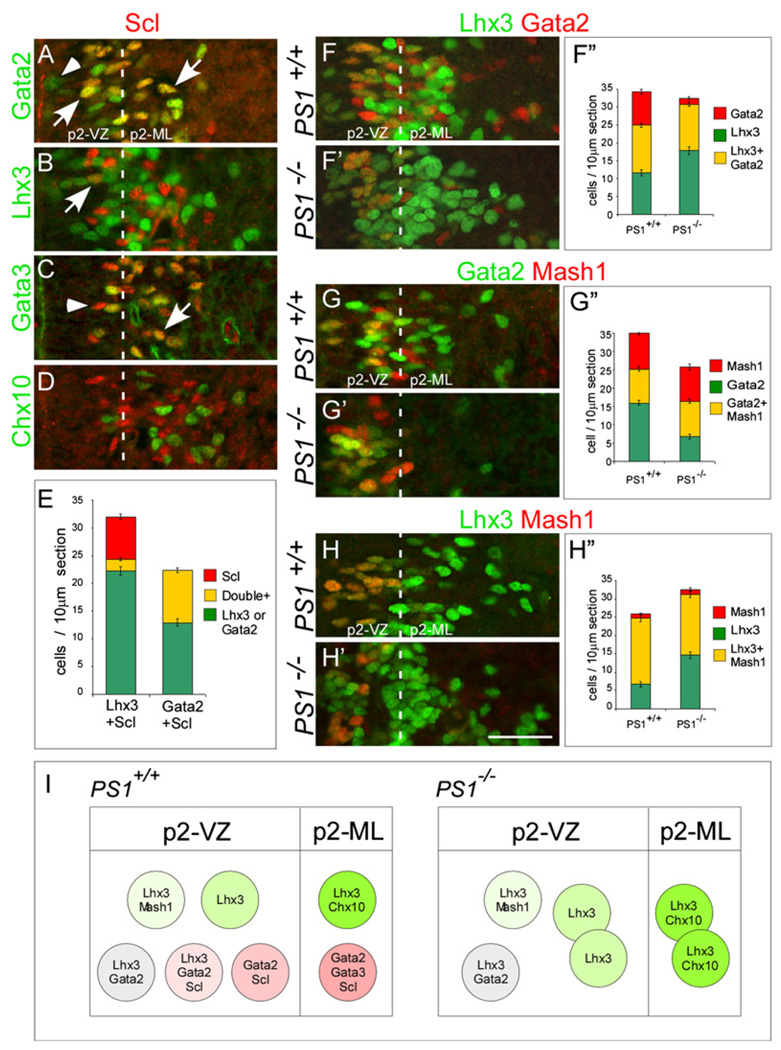
The genetic hierarchy revealed by the gene expression described above suggests that Scl expression is initiated in p2 progenitors at a later time point in the development of v2bIN. We asked which cells in the p2 domain express Scl during normal development. Double-labeling studies show that Scl is coexpressed with Gata2 and Lhx3 in the p2-VZ, Gata2 and Gata3 in the p2-ML, and it is not coexpressed with Chx10 (Figures 3A–3D). Since Notch signaling appears to regulate the initiation of Scl expression in the p2-VZ, we performed cell counts in the p2-VZ (Figure 3E). These data show that in the p2-VZ, all cells that express Scl also express Gata2 and that a small but significant proportion coexpress Lhx3 (Figure 3E). Based on these data we can identify two progenitor types in the control mice with the following gene express profile, the Lhx3/Gata2/Scl cells and Gata2/Scl cells (Figure 3I). It is likely that the Gata2/Gata3/Scl-expressing v2bIN are generated from these progenitor cell types. However, whether Lhx3/Gata2/Scl cells and Gata2/Scl cells represent two stages in the development of v2bIN is not clear from this analysis.
Figure 3. Changes in Combinatorial Gene Expression in the p2-VZ of PS1−/− Mice Reveal the Role of Notch Signaling in v2aIN versus v2bIN Cell-Fate Program.
(A–D) Expression of Scl protein in the p2 domain of e11.5 wild-type mice. Scl is coexpressed with Gata2 in the p2-VZ and p2-ML ([A], arrows). Lhx3 ([B], arrow) and Gata3 ([C], arrow), but not with Chx10 (D). Arrowheads indicate cells that express only Gata2 (A) or Scl (C).
(E) Cell counts of combinatorial expression of Scl/Lhx3 and Scl/Gata2 in the p2-VZ show that a small but significant number of Scl cells coexpress Lhx3 (21.56% of Scl cells, n = 388 cells analyzed in six embryos). In contrast, all Scl cells coexpress Gata2 (100% of Scl cells, n = 399 cells analyzed in six embryos).
(F–H) Lhx3/Gata2 (F and F′), Gata2/Mash1 (G and G′), and Lhx3/Mash1 (H and H′) are coexpressed in the p2 domain of e11.5 PS1+/+ and PS1−/− embryos. The numbers (mean ± SEM; ten sections per embryo; n = 6 embryos) of Lhx3/Gata2 ([F″], p = 0.56), Gata2/Mash1 ([G″], p = 0.61), and Lhx3/Mash1 ([H″], p = 0.19) and Mash1-alone cells ([G″], p = 0.67; [H″], p = 0.49) are not affected in the PS1−/− embryos. However, Lhx3-alone cells are increased ([F″], p = 5.6e−5; [H″], p = 8.7e−6) and Gata2-alone cells are decreased ([F″], p = 2.5e−14; [G″], p = 8.2e−9) in the p2-VZ of PS1−/− embryos.
(I) A schematic model that summarizes the p2 progenitor cell types identified in PS1+/+ and PS1−/−embryos.
Scale bar, 50 µm.
Since Scl is not expressed in the PS1−/− mutants, the fate of Lhx3/Gata2/Scl and Gata2/Scl progenitors could not be monitored directly. We asked whether these progenitor cells are replaced by progenitors that express Lhx3/Gata2 or Gata2 alone in the PS1−/− mutant mice. We find that in the p2-VZ of PS1−/− mutant mice, the number of Lhx3/Gata2 cells is not altered significantly (Figures 3F, 3F′, and 3F″). In contrast, the number of Gata2 cells is drastically reduced, and Lhx3 cells are significantly increased in the PS1−/− mice (Figures 3F, 3F′, and 3F″). We asked whether Lhx3/Gata2 cells are lineally related to cells that express Lhx3 or Gata2 alone. To test this we used Mash1 as a marker for earlier p2 progenitor since expression of Mash1 and FoxN4 is not altered in PS1−/− mice. In Gata2/Mash1 double-labeling experiments, we find similar numbers of double-positive Gata2/Mash1 and Mash1-only cells, but fewer Gata2-only cells in the PS1−/− mutant embryos (Figures 3G, 3G′, and 3G″). In Lhx3/Mash1 double-labeling experiments, we find similar numbers of Lhx3/Mash1 and Mash1-only cells, but more Lhx3-only cells in the PS1−/− mutant embryos (Figures 3H, 3H′, and 3H″0). Together these data demonstrate that in the absence of Notch signaling a cell-fate conversion takes place in late p2 progenitors lacking Mash1 (Figure 3I). It is important to note that the late Gata2 to Lhx3 cell-fate conversion correlates with the loss of Scl expression.
Coexpression of v2aIN and v2bIN Cell-Fate Determinants in Mitotic p2 Progenitors
Selective loss of Scl expression in a subset of p2 progenitors suggests that Scl expression is initiated after Lhx3, Gata2, and Mash1. We used successively longer pulses of BrdU to determine the temporal sequence of gene expression in dividing p2 progenitors. In cells labeled during a 1 hr pulse of BrdU, we find expression of Gata2, Lhx3, and Mash1 but not Scl (Figures 4A–4D). Expression of Scl is first seen after a 6 hr BrdU pulse (Figure 4F). We find the expression of Chx10 and Gata3 in BrdU-labeled cells after a 10 hr pulse (Figure 4G). These data suggest the following sequence of gene expression in dividing p2 progenitors: early expression of Lhx3, Gata2, and Mash1, followed by the expression of Scl, and finally the expression of Chx10 (v2aIN) and Gata3 (v2bIN). We find that 3 hr after BrdU incorporation, p2 progenitors begin to enter the M phase (Figure 4E). As expected, expression of Scl is not activated in mitotic p2 progenitors (Figure 4H). We find that all mitotic p2 progenitors coexpress Lhx3, Gata2, and Mash1 (Figures 4I–4K and Table S1). Our findings from gene expression studies in the p2 domain in PS1−/− mutant and control mice and in mitotic cells are consistent with the idea that a common p2 progenitor generates v2aIN and v2bIN (Figure 4L). This common progenitor undergoes a series of changes in gene expression that define intermediate states in the development of v2aIN and v2bIN. Whether this progenitor chooses the v2aIN or v2bIN lineage is regulated by Notch signaling. However, it is not clear why some p2 progenitors receive the Notch signaling and activate the expression of Scl while others do not.
Figure 4. Coexpression of Lhx3, Gata2, and Mash1, but Not Scl, in Mitotic p2 Progenitors.
(A–H) Temporal sequence of transcription factor expression in the p2 domain. Following 1 hr pulse, many BrdU-positive cells in the p2 domain coexpress Gata2 (A), Lhx3 (B), and Mash1 (C), but colocalization with Scl is not observed (D). The p2 progenitors enter the mitotic phase starting 3 hr after BrdU incorporation, as revealed by coexpression of Phospho-Histone H3 ([E], ph-H3, arrow). Coexpression of Scl is observed following a 6 hr BrdU pulse ([F], arrow). The v2aIN and v2bIN are labeled with BrdU only after a 10 hr pulse ([G], arrow), suggesting that Scl expression precedes the v2bIN cell fate. None of the mpm2-marked mitotic cells in the p2 domain express Scl ([H], arrowhead).
(I–K) Triple-labeling studies show coexpression of Lhx3/Gata2 (I), Lhx3/Mash1 (J), and Gata2/Mash1 (K) in mitotic cells labeled with mpm2 antibody (blue, arrow). Note that most mpm2-marked cells that express one factor also express the other. However, some mpm2-marked cells do not express either factor tested.
(L) Model shows the sequence of combinatorial gene expression in the p2 progenitors.
Scale bar, 50 µm.
Delta4 but Not Delta1 Activates Notch Signaling Required for v2bIN Cell Fate
A prediction from the aforementioned studies is that activated Notch would cell-autonomously promote the generation of v2bIN at the expense of v2aIN. To test this prediction we turned to chick electroporation studies. Like mice, similar numbers of v2aIN and v2bIN are generated in the chick spinal cord (Figure S1). In the chick spinal cord, Notch1, Delta1, and Delta4 are expressed in Lhx3 cells located within the p2-VZ (Figures 5A–5C), whereas Jagged1 expression is found in cells located dorsal to the p2 domain (Figure 5D). Although Delta1 is expressed throughout the neural tube, the expression of Delta4 is restricted to the p2 domain (Figure 5C and Figure S1). We electroporated expression plasmid containing the desired cDNA into the spinal cord of chick embryos and compared the development of v2aIN and v2bIN in the electroporated and control half of the spinal cord. We find that in embryos electroporated with the Notch1 receptor, similar numbers of v2aIN and v2bIN are generated in the electroporated and control sides (Figure 5K). From this experiment we hypothesized that, in the p2 domain, activation of the Notch receptor is tightly controlled, perhaps by the availability of the ligand(s). To test this hypothesis, we electroporated Delta1 and Delta4 expression constructs into the chick spinal cord. In embryos electroporated with Delta1, the number of v2aIN and v2bIN and the expression of Scl did not change significantly (Figures 5E–5G and 5K). In contrast, we find that the electroporation of Delta4 increases the number of v2bIN and decreases the number of v2aIN (Figures 5H, 5I, and 5K). Electroporation of Delta4 also increases Scl expression (Figure 5J). When Delta4 is electroporated at stage 13, reduction in the number of v2aIN is not completely compensated for by the increase in the number of v2bIN (Figure 5K). However, when electroporated in stage 20 embryos, Delta4-induced increase in the v2bIN completely compensates for the corresponding decrease in v2aIN (Figure S2). These findings suggest that the expression of Delta4 at earlier stages of development affects neuronal differentiation and neuronal subtype identity; later its function is restricted to specification of neuronal subtype identity alone. These data suggest that Delta4 but not Delta1 activates the Notch signaling required for p2 progenitors to activate Scl expression and promote the v2bIN cell-fate program.
Figure 5. Activation of Notch Signaling by Delta4 Promotes the v2bIN Cell Fate at the Expense of v2aIN Cell Fate in the Chick Spinal Cord.
(A–D) In stage 24 chick thoracic spinal cord, expression of Notch1, Delta1, and Delta4 is seen in Lhx3 cells within the p2-VZ ([A–C], arrowheads), but Jagged1 is expressed dorsal to the p2 domain (D).
(E–J) Electroporation studies in the chick spinal cord show that Delta4, but not Delta1, promotes v2bIN cell fate. Representative transverse sections from stage 25 chick thoracic spinal cord after electroporation of Delta1 and Delta4 at stage13. Electroporated side is to the right in these and subsequent panels. Expression of the transgene is shown in green ([E and F], GFP; [H and I], β-galactosidase). Electroporation of Delta1 does not alter the Chx10 (E), Gata2/3 (F), or Scl (G) expression. Electroporation of Delta4 results in the generation of fewer Chx10 (H) and more Gata2/3 neurons ([I]; arrowheads). Delta4 also upregulates the expression of Scl on the electroporated side ([J], arrowheads).
(K) Cell counts (mean ± SEM) in embryos electroporated with Notch1 (n = 5 embryos), Delta1 (n = 8 embryos), or Delta4 (n = 8 embryos).
(L and M) In situ hybridization for Delta4 or Delta1 in chick embryo spinal cord following electroporation of Mash1. Ectopic Mash1 promotes the expression of Delta4 expression ([E], n = 4 embryos) but inhibits the expression of Delta1 ([F], n = 4 embryos).
Scale bar, 20 µm in (A)–(D); 50 µm in (E)–(J), (L), and (M).
Additionally, we analyzed the requirement for Notch signaling in specifying v2aIN versus v2bIN fate in the zebrafish system by examining marker gene expression using whole-mount in situ hybridization. We found that the Notch signaling mutant, mindbomb (Itoh et al., 2003), lacks Scl and Gata3 expression in the spinal cord (Figure S2), suggesting that the v2bIN fate was not specified. Consistent with our findings in PS1−/− mice, zebrafish mindbomb mutants ectopically express the v2aIN marker, Vsx1 (Chx10), in the neural tube (Figure S3). These data indicate that the requirement for Notch signaling in v2bIN lineage commitment is evolutionarily conserved. In Xenopus and mice, Mash1 and FoxC proteins regulate the expression of Delta1 and Delta4 (Chitnis et al., 1995; Pattyn et al., 2004; Seo et al., 2006). In the p2 domain, FoxN4 regulates the expression of Mash1 (Li et al., 2005). We asked whether Mash1 regulates the expression of Delta4 in p2-VZ. In electroporation studies we find that ectopic Mash1 activates the expression of Delta4 throughout the ventricular zone (Figure 5L) but inhibits the expression of Delta1 (Figure 5M). It is possible that Mash1 directly regulates Delta4 expression, or it may affect neuronal differentiation. From these experiments it appears that, in the chick spinal cord, Mash1 either activates the expression of Delta4 in the p2-VZ or promotes the development of p2 progenitors such that they initiate the expression of Delta4. Expression of Delta4 triggers Notch signaling in neighboring cells, resulting in the activation of the Scl-dependent v2bIN cell-fate program (see Discussion). These findings suggest that in the presence of Delta4-expressing neighbors, p2 progenitors are more likely to activate the v2bIN cell-fate program.
Activated Notch Acts Cell-Autonomously to Specify v2bIN Cell Fate
To directly test the hypothesis that activation of the Notch receptor in a p2 progenitor activates the v2bIN cell-fate program, we generated an expression construct that includes the cDNA encoding only the Notch1 intracellular domain (Nicd). We tested the ability to alter v2aIN versus v2bIN fate, cell autonomy, and induction of Scl expression (Figure 6). We find that Nicd electroporation results in more v2bIN and fewer v2aIN in the electroporated half compared to the control half (Figures 6A, 6B, and 6S). However, the total number of v2IN (Chx10 + Gata3) does not change (Figure 6S). Along with the increase in v2bIN, more cells express Scl after Nicd electroporation (Figure 6C). We find that ectopic Nicd is preferentially localized to v2bIN (Figure 6S). Preferential localization of the myc-tag to v2bIN indicates that Nicd acts cell-autonomously to initiate the Scl-dependent v2bIN cell-fate program. Additionally, evolutionary conservation of this pathway for promoting v2bIN fate was demonstrated in the zebrafish (Figure S3). Using an inducible transgenic system in zebrafish, brief induction of Nicd resulted in a vast increase of Scl and Gata3 transcripts with a concomitant loss of Vsx1 expression (Figure S3). These data show that increased Notch signaling in the chick spinal cord and zebrafish embryo promotes the generation of Scl-expressing v2bIN.
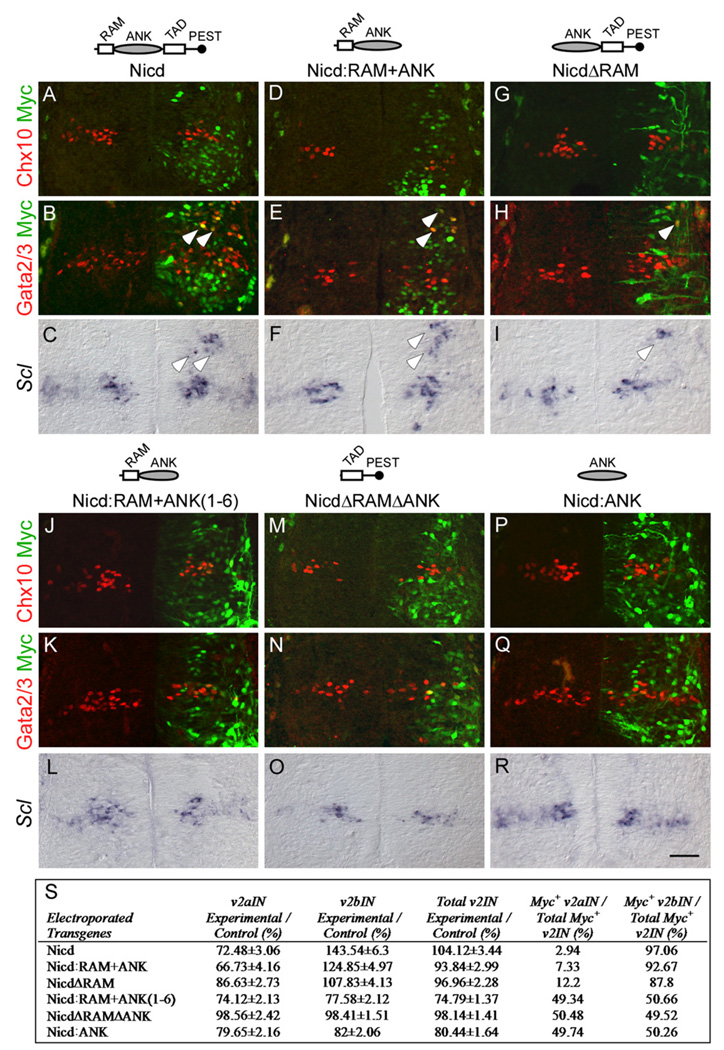
Figure 6. Deletion Analysis Reveals that the Ankyrin Repeat Seven Is Required for Nicd to Activate the v2bIN Cell-Fate Program.
Representative transverse sections from stage 25 chick thoracic spinal cord after electroporation of Nicd and Nicd-deletion constructs at stage 13. Double labels for Chx10 or Gata2/3 with the electroporated transgene and the expression of Scl are shown. (A–C) In embryos electroporated with Nicd ([A–C], n = 10 embryos), Nicd:RAM+ANK ([D–F], n = 10 embryos), and NicdΔRAM ([G–I], n = 10 embryos), fewer Chx10 and more Gata2/3 and Sclexpressing neurons are present in the electroporated side. In the electroporated side, some ectopic v2bIN appear to have migrated to a more dorsal location. Changes in v2IN cell fate are not observed in Nicd:RAM+ANK(1–6) ([J–L]; n = 8 embryos), NicdΔRAMΔANK ([M–O]; n = 7 embryos), or Nicd:ANK ([P–R]; n = 8 embryos). (S) Quantification of v2aIN and v2bIN cell counts in electroporated embryos. Changes in v2IN cell fate are presented as the ratio of the mean cell numbers on the electroporated side over the control side ± SEM, calculated from ten sections per embryo. Same embryos are analyzed for the number of myc+ v2aIN and v2bIN compared to the number of total myc+ v2IN population. Scale bar, 50 µm.
Ankyrin Repeat Seven Is Essential for the Activation of Scl-Dependent v2bIN Cell Fate
To understand how Notch signaling initiates the v2bIN cell-fate program, we generated a series of deletion constructs to determine which domains in the Nicd are required to promote v2bIN fate in the chick electroporation assay. We find that Nicd:RAM+ANK peptide promotes the v2bIN cell fate at the expense of v2aIN cell fate, preferentially localizes to the v2bIN, and upregulates Scl expression (Figures 6D–6F and 6S). These observations suggest that, in the v2IN lineage, Nicd does not require direct recruitment of coactivators through the TAD. Deletion of the RAM domain (NicdΔRAM) reduces the ability of Nicd to promote v2bIN cell fate and to upregulate Scl expression (Figures 6G–6I and 6S). However, the NicdΔRAM peptide preferentially localizes to v2bIN (Figure 6S), suggesting that the RAM domain potentiates Nicd function but is not necessary for promoting the v2bIN cell fate. We find that the deletion of the ankyrin repeat seven in Nicd:RAM+ANK(1–6) abolishes the ability to promote the v2bIN cell fate and to upregulate Scl expression (Figures 6J–6L and 6S). The Nicd:RAM+ANK(1–6) peptide also does not show preferential localization to v2bIN (Figure 6S), suggesting that factors that bind to ANK domain are required for the v2bIN cell fate. However, the ANK domain alone or the TAD+PEST domains fail to promote v2bIN cell fate and do not upregulate Scl expression (Figures 6M–6R) From these studies we conclude that, in the presence of Nicd, the p2 progenitors activate the expression of Scl and the v2bIN cell-fate program. Furthermore, we find that the ankyrin repeat seven is essential for the Nicd to activate the expression of Scl and the v2bIN differentiation program.
MAML1 Function Is Required for the Specification of v2bIN Cell Fate
The MAML family proteins bind to the ankyrin repeats three to seven (Nam et al., 2006; Wilson and Kovall, 2006). We find that MAML1 is expressed in the ventricular zone of the chick spinal cord (Figure S1). A 60 amino acid N-terminal fragment of MAML1 that contains the minimum Notch-binding domain acts as a dominant-negative (DN) form of MAML and inhibits Notch signaling (Maillard et al., 2004; Yedvobnick et al., 2004). To inhibit the function of endogenous MAML proteins, we electroporated the DN-MAML1 construct into the chick spinal cord. We find that DN-MAML1 promotes the v2aIN cell fate at the expense of the v2bIN cell fate and inhibits the activation of Scl expression (Figures 7A–7C and 7L). To confirm that DN-MAML1 affects the cell-fate decisions by inhibiting the function of Nicd, we coelectroporated DN-MAML1 and Nicd. In the coelectroporated embryos, fewer v2bIN and more v2aIN neurons are generated, and the expression of Scl is reduced (Figures 7D–7F and 7L). These coelectroporation studies confirm that DN-MAML1 inhibits the function of Nicd in activation of Scl expression and the v2bIN cell fate.
Figure 7. Nicd-MAML Complex Is Required for the Activation of the Scl-Dependent v2bIN Cell-Fate Program.
(A–K) Transverse sections from stage 25 chick thoracic spinal cord following electroporation with DN-MAML1 ([A–C], n = 8 embryos), DN-MAML1+ Nicd ([D–F], n = 8 embryos), DN-MAML1+Scl ([G–I], n = 6 embryos), and Scl ([J and K], n = 10 embryos). More Chx10 neurons and fewer Gata2/3 neurons are generated in embryos electroporated with DN-MAML1 (A and B). DN-MAML1 also inhibits Scl expression (C). Coelectroporation of DN-MAML1 and Nicd also results in more Chx10 (D) and fewer Gata2/3 (E) and Scl (F) expressing cells on the electroporated side. Coelectroporation of DN-MAML1 and Scl rescues the DN-MAML1 phenotype as more v2aIN and v2bIN are generated (G and H). Scl expression is also promoted in the ectopic v2bIN generated as the result (I). Electroporation of Scl alone results in fewer v2aIN and more v2bIN (J and K).
(L) Quantification of v2aIN and v2bIN cell count in electroporated embryos. Changes in v2IN cell fate are presented as the ratio of the mean cell numbers on the electroporated side over the control side ± SEM in one-half of the thoracic spinal cord, calculated from ten sections per embryo.
Scl Is Sufficient to Bypass the Loss of Notch Signaling for v2bIN Cell Fate
In all electroporation studies where Nicd (or deletion peptides) and DN-MAML1 affected the v2aIN and v2bIN cell fate, we recorded a parallel change in the expression of Scl. We reasoned that if the main function of the Notch signaling is to directly or indirectly activate the expression of Scl, then electroporation of Scl expression plasmid would rescue the lack of Notch signaling phenotype. We chose DN-MAML1 to inhibit the Notch signaling and coelectroporated Scl. Embryos coelectroporated with DN-MAML1 and Scl generate more v2aIN and v2bIN in the electroporated side (Figures 7G, 7H, and 7L). As shown previously (Muroyama et al., 2005), we find that Scl electroporation alone results in the generation of more v2bIN and fewer v2aIN (Figures 7J–7L). Surprisingly, in the coelectroporation experiments we find that DN-MAML1 prevents Scl from inhibiting the v2aIN cell-fate program as the number of v2aIN increases. Increase in the number of v2bIN in this experiment suggests that the expression of Scl might be sufficient to compensate for the loss of endogenous Notch signaling in p2 progenitors. The ability of Scl to activate the v2bIN cell-fate program is further evidenced by the increase of endogenous Scl expression in the coelectroporation experiment (Figure 7I). These data suggest that the expression of Scl is sufficient to overcome the DN-MAML1-mediated loss of Notch signaling and to promote the v2bIN cell-fate program.
DISCUSSION
The generation of distinct subtypes of excitatory and inhibitory interneurons in the ventral spinal cord is an essential step in the development of motor circuits. Many cell-fate decisions require combinatorial expression of transcription factors, but the extrinsic signals that establish the proper regulatory codes are poorly understood. Here we show that Notch signaling, initiated by the Delta4 ligand, is essential for establishing excitatory versus inhibitory interneuron cell fate. We show that activated Notch and its partner MAML regulate the selection of cell-type-specific transcription code. These findings allow us to generate a model of Notch-mediated v2IN cell-fate specification as shown in Figure 8. Below we discuss how genetically similar p2 progenitors use the Notch signaling pathway to activate the expression of transcription factors that specify excitatory v2aIN versus inhibitory v2bIN cell fate.
Figure 8. Schematic Model Diagram of Notch-Mediated v2aIN and v2bIN Cell-Fate Specification.
This model shows the development of p2 progenitors starting with Lhx3 expression, generation of identifiable transient progenitors and leading to the generation of v2aIN or v2bIN. Salient features of this lineage include the following: (1) lower expression of Nicd in the early common progenitor as it enters terminal differentiation; (2) coexpression of four transcription factors (Lhx3, Gata2, Mash1, and FoxN4) in the mitotic, common p2 progenitor; (3) likely expression of Delta4 in the common progenitors and during the initial steps in the v2aIN lineage; (4) activation of the Notch receptor in the v2bIN lineage, leading to high Nicd in v2bIN progenitors and low Nicd in v2aIN progenitors; (5) Nicd-and MAML-dependent activation of Scl expression in the v2bIN lineage. These four steps generate nearly equal numbers of v2aIN and v2bIN from the p2 progenitors in the mouse and chick spinal cord.
Distinct Interneuron Subtypes Develop from Genetically Similar Neural Progenitors
Our data provide insight into the issue of whether v2aIN and v2bIN neurons develop from genetically similar p2 progenitors. Lineage-tracing analysis shows that both v2aIN and v2bIN are generated from p2 progenitors that express Lhx3. At the time that v2aIN and v2bIN neurons are generated, most mitotic p2 progenitors coexpress Lhx3, Gata2, Mash1 (this study) and perhaps FoxN4 (Li et al., 2005). We find that v2aIN and v2bIN are generated over the same time period, arguing against a contribution of temporal changes in gene expression in progenitor cells that are known to determine daughter cell fates, as in the fly nervous system (Isshiki et al., 2001). A surprising finding is that the relatively low levels of Notch signaling in PS1−/− mice do not affect the generation of p2 progenitors that coexpress Lhx3/Gata2/Mash1/FoxN4. It appears that the expression of Lhx3, Gata2, Mash1, and FoxN4 is likely regulated by an “early” transcription factor code in the p2 progenitors involving Pax6, Nkx6.1, and Irx3 (Briscoe et al., 2000). Based on these observations, we infer that no obvious differences exist between mitotic p2 progenitors that would generate v2aIN or v2bIN. Thus, interneuron diversity can be generated by mechanisms that operate in immature postmitotic neurons. Our findings bear comparison to the cell-cell signaling mechanisms used to generate two subtypes of motor neurons within the lateral motor column. In the motor neuron system, cell birthdate is an important component of the retinoic acid-mediated program of neuronal diversification (Sockanathan et al., 2003), whereas in the p2 lineage Delta-Notch signaling drives diversity in the absence of temporal differences in neuronal production.
Role of Delta-Notch Signaling in the Determination of Interneuron Cell Fate
How do immature interneurons adopt a subtype identity? Several lines of evidence indicate that in the p2 lineage Delta4 and Notch1 regulate whether Lhx3/Gata2/Mash1/FoxN4 progenitor cells generate a v2aIN or v2bIN fate. In the Notch-hypomorphic PS1−/− mice, the number of v2aIN doubles as p2 progenitors fail to generate v2bIN. In the chick, electroporation of Nicd promotes v2bIN cell fate at the expense of v2aIN differentiation. In the zebrafish mindbomb mutant, the loss of v2bIN is likewise paralleled by increased numbers of v2aIN cells. From these findings, we conclude that in the p2 lineage the Delta-Notch signaling generates two distinct interneuron subtypes from a pool of genetically equivalent progenitors.
In the p2 lineage, the disruption of the Notch signaling alters interneuron cell-fate decisions but does not affect the total number of v2 interneurons. In a previous study, it was reported that, in the conditional Notch1 null mice, more v2 interneurons are generated at the expense of earlier-born motor neurons (Yang et al., 2006). It is important to note that the previous study considered the Chx10-expressing v2aIN the only progeny of the p2 progenitors. We also find more v2aIN in the PS1−/− mice, but our analysis of PS1−/− mice shows that the number of motor neurons is not altered significantly (p = 0.1; Figure S1). Moreover, we provide clear evidence that the Delta-Notch signaling regulates the v2aIN versus v2bIN cell fate.
While the role of Notch in neuronal differentiation has been well documented in the vertebrate systems (Conlon et al., 1995; de la Pompa et al., 1997; Lutolf et al., 2002), evidence for Notch function in neuronal cell-fate specification has been accumulating at a slower pace. Until recently, Notch signaling was thought to affect cell-fate decisions by regulating the timing of progenitor cell differentiation. For example, the timing of neuronal subtype birth is disrupted in the developing retina by the gain or loss of Notch signaling (Austin et al., 1995; Dorsky et al., 1997; Furukawa et al., 2000; Ohnuma et al., 2002). However, recent studies have found that more photoreceptors are generated at the expense of other cell types in conditional Notch1-deficient mice (Jadhav et al., 2006; Yaron et al., 2006). Perhaps the role of Delta-Notch signaling in the vertebrate nervous system is widespread but remains unappreciated due to the lack of detailed lineage information and marker availability.
Cross-Talk between Transcriptional Codes and Delta-Notch Signaling in Developing Interneurons
How is the Delta-Notch signaling coordinated vis-à-vis the cell-intrinsic transcriptional codes? In the p2 lineage, we find evidence for reciprocal interaction between these two mechanisms. It appears that the Delta4-Notch signaling is initiated by cell-intrinsic factors. We show that, in the chick spinal cord, Mash1 induces the expression of Delta4 but not Delta1. In the FoxN4 null mice, Mash1 as well as Delta4 expression is lost in the p2 domain (Li et al., 2005; William D. Richardson, personal communication). This finding raises the possibility that FoxN4 might regulate the expression of Delta4 via the activation of Mash1. The idea that cell-intrinsic factors set up the Delta-Notch signaling among equivalent progenitors is consistent with a recent finding in the dorsal spinal cord, where Mash1 expression activates Notch signaling in the neighboring cells to induce the dILB interneuron cell fate (Mizuguchi et al., 2006).
As the p2 progenitors generate daughter cells, the interaction between Delta-Notch signaling and transcription factor codes is reversed. Immature v2 interneurons have two choices. They can downregulate Gata2, maintain their expression of Lhx3, and activate the expression of Chx10 to adopt the v2aIN cell fate. Alternatively, they can downregulate Lhx3, maintain their expression of Gata2, and activate the expression of Scl and Gata3 to adopt the v2bIN cell fate. The choice of the transcriptional code is regulated by the Delta4-Notch signaling. We find that Delta4-Notch signaling directly regulates the combinatorial transcription code for v2bIN cell fate. Three lines of evidence suggest that Notch signaling facilitates the activation of the Scl-dependent v2bIN cell-fate program. First, in mice the loss of Notch signaling prevents the initiation of Scl expression. Second, in the chick spinal cord activation of the Notch signaling pathway induces Scl expression. Third, electroporation of Scl is sufficient to bypass the requirement for Notch signaling in the generation of v2bIN. It is important to note that Delta4 but not Delta1 activates the v2bIN cell-fate program, although both Delta1 and Delta4 are coexpressed with Lhx3 in the ventricular zone. As discussed above, Delta4 but not Delta1 expression is induced by transcription factors in the p2 progenitor cells.
These findings reveal that reciprocal interactions between transcriptional codes and Delta-Notch signaling generate v2aIN and v2bIN from a pool of equivalent, bipotential p2 progenitors.
Notch Partners with MAML Factors to Regulate Combinatorial Transcriptional Code
How does the activation of the Notch signaling pathway regulate combinatorial transcriptional codes? Our studies reveal that Delta4-Notch signaling regulates the expression of Scl, which is a critical transcription factor for establishing v2bIN cell identity (Muroyama et al., 2005). The deletion analysis suggests that the ANK domain is an important site for potential interactions that allow Nicd to activate the v2bIN cell-fate program. More importantly, the removal of the putative MAML1 binding site in Nicd: RAM+ANK(1–6) completely masks the ability of Nicd to participate in the cell-fate decision. Although it is tempting to suggest that the interaction within the ankyrin repeat seven is essential for v2 cell fate, it was shown that the presence of the ankyrin repeat seven is necessary for the stability of the entire ANK domain (Lubman et al., 2004). We show that MAML proteins are an essential component of the transcription complex that activates the v2bIN cell-fate program. The function of the Nicd-MAML complex is to regulate the activation of Scl expression in the v2bIN lineage.
Significance of Delta-Notch Signaling in the Diversification of Interneuron Identity
What are the functional consequences of Delta-Notch signaling in the diversification of interneuron identity? In the ventral spinal cord, neural progenitors use two distinct strategies for generating excitatory and inhibitory interneurons. In the first strategy, multipotential progenitor cells produce different subclasses of daughter cells, whereas in the second strategy progenitors are restricted to producing a single cell type at any given time in development. In this report, we find that a homogeneous population of bipotential p2 progenitors generates both excitatory v2aIN and inhibitory v2bIN. In contrast, progenitor cells in the p1 domain generate mostly inhibitory interneurons (Alvarez et al., 2005; Sapir et al., 2004). We find that the Delta-Notch signaling is essential for the generation of inhibitory interneurons (v2bIN) from bipotential progenitors (p2) but not for the development of inhibitory interneurons (v1) from unipotential progenitors (p1). Excitatory and inhibitory interneurons represent two fundamentally distinct neuronal types and are used in circuits throughout the nervous system. Although our studies have defined roles for Delta-Notch signaling within the ventral spinal cord, it is likely that this signaling pathway will play a critical role in the development of many neural circuits. The widespread use of the Delta-Notch signaling in neuronal diversification is supported by the recent finding that excitatory or inhibitory interneuron cell fate within the dorsal spinal cord also relies on Notch-mediated signaling (Mizuguchi et al., 2006).
Finally, our findings provide further support to the idea that Notch signaling plays a critical role in the generation of excitatory and inhibitory interneurons in the spinal cord. This study reveals that the activated Notch and its partner MAML regulate the combinatorial expression of transcription factors in developing neurons. The role of Delta4-Notch1 signaling in the p2 lineage is similar in design but distinct in mechanism from that of retinoids in the specification of motor neuron subtype identity (Sockanathan and Jessell, 1998). Both mechanisms operate in postmitotic neurons and regulate the combinatorial expression of transcription factors. We suspect that by regulating the binary cell-fate decision to generate excitatory v2aIN or inhibitory v2bIN neurons, Notch signaling plays a critical role in the development of functional motor circuits.
EXPERIMENTAL PROCEDURES
Animals
The Lhx3:IRES:CRE; β-actin:LNL:nLacZ transgenic mice and Presenilin1 knockout mice were described previously (Sharma et al., 1998; Wong et al., 1997). Embryos were harvested from timed-pregnant females according to the procedures approved by the IACUC committee at the University of Chicago. The day of vaginal plug is considered embryonic day 0.5 (e0.5).
Expression Constructs
The Nicd construct contains cDNA encoding the 1744–2531 aa of the mouse Notch1. Nicd and deletion constructs were generated by restriction digests and cloned into CMV-based, Myc-tagged expression vector pCS2-MT. Amino acid sequences of the deletion constructs are as follows: Nicd-RAM+ANK, 1744–2193 aa; Nicd-RAM+ANK(1–6), 1744–2097 aa; NicdΔRAM, 1875–2531 aa; NicdΔRAMΔANK, 2097–2531 aa; Nicd-ANK, 1875–2193 aa. Mouse Delta1 and Delta4 cDNA were provided by Dr. Chris Kintner. Delta4 was cloned into pCAGGs-IRES-nLacZ vector provided by Dr. Shanthini Sockanathan. Human DN-MAML1-GFP expression construct was obtained from Dr. Warren Pear (Maillard et al., 2004). Full-length rat Mash1 (a gift from Dr. Kathy Millen) was cloned into pCS2-MT vector. Mouse Scl expression construct was obtained from Dr. John Crispino.
Zebrafish Strains
Zebrafish were bred and maintained as described (Solnica-Krezel et al., 1994). The following lines were used: mind bombta56b (Jiang et al., 1996), Tg(uas:notch 1a-intra) (Scheer and Campos-Ortega, 1999), and Tg(hsp70:gal4) (Scheer and Campos-Ortega, 1999). Following fixation of 26 hr embryos, standard whole-mount in situ hybridization was performed with digoxygenin-labeled riboprobes.
Chick In Ovo Electroporation
Fertilized white leghorn chicken eggs (SPAFAS) were incubated at 39°C and 50%–60% humidity prior to electroporation. For electroporation, plasmid DNA was purified using QIAGEN maxiprep kit, dissolved in MilliQ H2O (2–4 µg/ml) and stored at −20°C. Plasmid DNA was mixed with Fast Green dye prior to injection into the central canal of the neural tube in stage13 chick embryos (Hamburger and Hamilton, 1992). Electroporation was performed in the thoracic spinal cord (five pulses at 25 V, each for 50 ms) via a pair of platinum electrodes (BTX Model 510) using square pulse electroporator (BTX Model ECM830). Eggs were sealed and returned to incubator. At specified times, embryos were retrieved from the egg, eviscerated, and fixed in 4% paraformaldehyde for immunohistochemistry and in situ hybridization.
Immunohistochemistry and In Situ Hybridization
Mouse and chick embryos were fixed and stained as previously described (Sharma et al., 1998). Primary antibodies were used at the following dilutions: guinea pig anti-Chx10 (1:20,000), guinea pig anti-Lhx3 (1:20,000), rabbit anti-Lhx3 (1:4000), guinea pig anti-Gata2/3 (1:8000), mouse anti-Gata3 (1:100, Santa Cruz #SC-268), rabbit anti-Mash1 (1:2000, Santa Cruz #SC-52), mouse anti-mpm2 (1:10,000, Upstate Biotech), mouse anti-phospho-histone H3 (1:5000, Upstate Biotech), rabbit anti-cleaved Notch1 (1:1000, Cell Signaling Technology #2421), mouse anti-myc (1:5000, Santa Cruz #SC-40), rabbit anti-Myc (1:5000, Santa Cruz #SC-789), Rat anti-BrdU (1:1000, Axyll, Accurate Chemical & Scientific Corp.), affinity-purified rabbit anti-SCL antibody (1:2000, obtained from Dr. Catherine Porcher). Antigen retrieval for staining with anti-cleaved Notch1 antibody follows a protocol previously described (Tokunaga et al., 2004). Rabbit anti-FoxN4 was provided by Dr. Michael Matise (1:50). For in situ hybridization, chick Notch1, Delta1, and Jagged1 mRNA probes were generated from partial cDNAs generously provide by Dr. Juan-Carlos Belmonte. Full-length Scl probe was kindly provided by Dr. John Crispino. Partial Delta4 sequence (1607–2677 bp) was amplified from HH stage 23 chick embryo cDNA and cloned into the pCR-Topo vector. Antisense probes were prepared using digoxigenin-11-rUTP (Roche) and T7 or Sp6 polymerases (GIBCO). 20 µm frozen sections of HH stage 23–25 chick embryos were used for the in situ hybridizations with AP-coupled anti-digoxigenin antibody, NBT, and BCIP (Roche).
5′-Bromo-2′-Deoxyuridine Incorporation
BrdU (Sigma) was dissolved in PBS at 50 mg/ml concentration and injected intraperitoneally into timed pregnant mice or injected into the heart of HH23–25 chick embryos. Duration of incorporation depends on the experimental design (1–12 hr), and embryos were fixed following incorporation as described.
Quantitation and Statistical Analysis
All cell counts where performed on 10 µm transverse sections taken from one-half of the thoracic spinal cord of e10.5–e13.5 mouse or HH stage 20–25 chick embryos. Cell counts were presented as mean ± SEM. In electroporation experiments, cell number of the experimental side over the control side is presented as percent of control ± SEM in one-half of the thoracic spinal cord. Significance of data was determined by two-sample Student’s t test.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Thomas Jessell and Martyn Goulding for numerous discussions and comments on previous versions of this manuscript. We thank Dr. David Rowitch for many discussions during the course of these studies and Dr. William Richardson for sharing data prior to publication. We also thank Drs. Bruce Appel, John Crispino, Chris Kintner, Kathy Millen, and Warren Pear for providing various cDNA constructs. Special thanks to Drs. Catherine Porcher and Paresh Vyas for the generous gift of affinity-purified anti-Scl antibody. Special thanks to Ms. Charity Goodman, Aida Pourbovali, and Jennifer Setlak for technical help. This work was supported by grants from Brain Research foundation, Basil O’Connor grant from March of Dimes, and NIH-RO1 NS045106 to K.S.
Footnotes
Supplemental Data
The Supplemental Data for this article can be found online at http://www.neuron.org/cgi/content/full/53/6/813/DC1/.
REFERENCES
- Alvarez FJ, Jonas PC, Sapir T, Hartley R, Berrocal MC, Geiman EJ, Todd AJ, Goulding M. Postnatal phenotype and localization of spinal cord V1 derived interneurons. J. Comp. Neurol. 2005;493:177–192. doi: 10.1002/cne.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Austin CP, Feldman DE, Ida JA, Jr, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. doi: 10.1242/dev.121.11.3637. [DOI] [PubMed] [Google Scholar]
- Benedito R, Duarte A. Expression of Dll4 during mouse embryogenesis suggests multiple developmental roles. Gene Expr. Patterns. 2005;5:750–755. doi: 10.1016/j.modgep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Chen N, Greenwald I. The lateral signal for LIN-12/Notch in C. elegans vulval development comprises redundant secreted and transmembrane DSL proteins. Dev. Cell. 2004;6:183–192. doi: 10.1016/s1534-5807(04)00021-8. [DOI] [PubMed] [Google Scholar]
- Chitnis A, Henrique D, Lewis J, Ish-Horowicz D, Kintner C. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature. 1995;375:761–766. doi: 10.1038/375761a0. [DOI] [PubMed] [Google Scholar]
- Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Liu JP, Jessell TM. Motor neuron columnar fate imposed by sequential phases of Hox-c activity. Nature. 2003;425:926–933. doi: 10.1038/nature02051. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, Aguilera RJ, Nakano T, Honjo T, Mak TW, Rossant J, Conlon RA. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development. 1997;124:1139–1148. doi: 10.1242/dev.124.6.1139. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Chang WS, Rapaport DH, Harris WA. Regulation of neuronal diversity in the Xenopus retina by Delta signalling. Nature. 1997;385:67–70. doi: 10.1038/385067a0. [DOI] [PubMed] [Google Scholar]
- Ericson J, Muhr J, Jessell TM, Edlund T. Sonic hedgehog: a common signal for ventral patterning along the rostrocaudal axis of the neural tube. Int. J. Dev. Biol. 1995;39:809–816. [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Mukherjee S, Bao ZZ, Morrow EM, Cepko CL. rax, Hes1, and notch1 promote the formation of Muller glia by postnatal retinal progenitor cells. Neuron. 2000;26:383–394. doi: 10.1016/s0896-6273(00)81171-x. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lamar E. Neuronal patterning: Making stripes in the spinal cord. Curr. Biol. 2000;10:R565–R568. doi: 10.1016/s0960-9822(00)00615-1. [DOI] [PubMed] [Google Scholar]
- Goulding M, Pfaff SL. Development of circuits that generate simple rhythmic behaviors in vertebrates. Curr. Opin. Neurobiol. 2005;15:14–20. doi: 10.1016/j.conb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Isshiki T, Pearson B, Holbrook S, Doe CQ. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell. 2001;106:511–521. doi: 10.1016/s0092-8674(01)00465-2. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev. Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jadhav AP, Mason HA, Cepko CL. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development. 2006;133:913–923. doi: 10.1242/dev.02245. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Brand M, Heisenberg CP, Beuchle D, Furutani-Seiki M, Kelsh RN, Warga RM, Granato M, Haffter P, Hammerschmidt M, et al. Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development. 1996;123:205–216. doi: 10.1242/dev.123.1.205. [DOI] [PubMed] [Google Scholar]
- Karunaratne A, Hargrave M, Poh A, Yamada T. GATA proteins identify a novel ventral interneuron subclass in the developing chick spinal cord. Dev. Biol. 2002;249:30–43. doi: 10.1006/dbio.2002.0754. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Locomotor circuits in the mammalian spinal cord. Annu. Rev. Neurosci. 2006;29:279–306. doi: 10.1146/annurev.neuro.29.051605.112910. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Okamura Y, Higashijima S. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J. Neurosci. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Misra K, Matise MP, Xiang M. Foxn4 acts synergistically with Mash1 to specify subtype identity of V2 interneurons in the spinal cord. Proc. Natl. Acad. Sci. USA. 2005;102:10688–10693. doi: 10.1073/pnas.0504799102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- Lubman OY, Korolev SV, Kopan R. Anchoring notch genetics and biochemistry; structural analysis of the ankyrin domain sheds light on existing data. Mol. Cell. 2004;13:619–626. doi: 10.1016/s1097-2765(04)00120-0. [DOI] [PubMed] [Google Scholar]
- Lutolf S, Radtke F, Aguet M, Suter U, Taylor V. Notch1 is required for neuronal and glial differentiation in the cerebellum. Development. 2002;129:373–385. doi: 10.1242/dev.129.2.373. [DOI] [PubMed] [Google Scholar]
- Maillard I, Weng AP, Carpenter AC, Rodriguez CG, Sai H, Xu L, Allman D, Aster JC, Pear WS. Mastermind critically regulates Notch-mediated lymphoid cell fate decisions. Blood. 2004;104:1696–1702. doi: 10.1182/blood-2004-02-0514. [DOI] [PubMed] [Google Scholar]
- Mizuguchi R, Kriks S, Cordes R, Gossler A, Ma Q, Goulding M. Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat. Neurosci. 2006;9:770–778. doi: 10.1038/nn1706. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujiwara Y, Orkin SH, Rowitch DH. Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature. 2005;438:360–363. doi: 10.1038/nature04139. [DOI] [PubMed] [Google Scholar]
- Nam Y, Sliz P, Song L, Aster JC, Blacklow SC. Structural basis for cooperativity in recruitment of MAML coactivators to Notch transcription complexes. Cell. 2006;124:973–983. doi: 10.1016/j.cell.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Nardelli J, Thiesson D, Fujiwara Y, Tsai FY, Orkin SH. Expression and genetic interaction of transcription factors GATA-2 and GATA-3 during development of the mouse central nervous system. Dev. Biol. 1999;210:305–321. doi: 10.1006/dbio.1999.9278. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Ohnuma S, Hopper S, Wang KC, Philpott A, Harris WA. Co-ordinating retinal histogenesis: early cell cycle exit enhances early cell fate determination in the Xenopus retina. Development. 2002;129:2435–2446. doi: 10.1242/dev.129.10.2435. [DOI] [PubMed] [Google Scholar]
- Parras CM, Schuurmans C, Scardigli R, Kim J, Anderson DJ, Guillemot F. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002;16:324–338. doi: 10.1101/gad.940902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn A, Simplicio N, van Doorninck JH, Goridis C, Guillemot F, Brunet JF. Ascl1/Mash1 is required for the development of central serotonergic neurons. Nat. Neurosci. 2004;7:589–595. doi: 10.1038/nn1247. [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Sapir T, Geiman EJ, Wang Z, Velasquez T, Mitsui S, Yoshihara Y, Frank E, Alvarez FJ, Goulding M. Pax6 and engrailed 1 regulate two distinct aspects of renshaw cell development. J. Neurosci. 2004;24:1255–1264. doi: 10.1523/JNEUROSCI.3187-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer N, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech. Dev. 1999;80:153–158. doi: 10.1016/s0925-4773(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev. Biol. 2006;294:458–470. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Sharma K, Sheng HZ, Lettieri K, Li H, Karavanov A, Potter S, Westphal H, Pfaff SL. LIM homeodomain factors Lhx3 and Lhx4 assign subtype identities for motor neurons. Cell. 1998;95:817–828. doi: 10.1016/s0092-8674(00)81704-3. [DOI] [PubMed] [Google Scholar]
- Smith E, Hargrave M, Yamada T, Begley CG, Little MH. Coexpression of SCL and GATA3 in the V2 interneurons of the developing mouse spinal cord. Dev. Dyn. 2002;224:231–237. doi: 10.1002/dvdy.10093. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Jessell TM. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Perlmann T, Jessell TM. Retinoid receptor signaling in postmitotic motor neurons regulates rostrocaudal positional identity and axonal projection pattern. Neuron. 2003;40:97–111. doi: 10.1016/s0896-6273(03)00532-4. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L, Schier AF, Driever W. Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics. 1994;136:1401–1420. doi: 10.1093/genetics/136.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell. 2002;110:237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Tokunaga A, Kohyama J, Yoshida T, Nakao K, Sawamoto K, Okano H. Mapping spatio-temporal activation of Notch signaling during neurogenesis and gliogenesis in the developing mouse brain. J. Neurochem. 2004;90:142–154. doi: 10.1111/j.1471-4159.2004.02470.x. [DOI] [PubMed] [Google Scholar]
- Wilson JJ, Kovall RA. Crystal structure of the CSL-Notch-Mastermind ternary complex bound to DNA. Cell. 2006;124:985–996. doi: 10.1016/j.cell.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Chen HY, Price DL, Van der Ploeg LH, Sisodia SS. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- Yang X, Tomita T, Wines-Samuelson M, Beglopoulos V, Tansey MG, Kopan R, Shen J. Notch1 signaling influences v2 interneuron and motor neuron development in the spinal cord. Dev. Neurosci. 2006;28:102–117. doi: 10.1159/000090757. [DOI] [PubMed] [Google Scholar]
- Yaron O, Farhy C, Marquardt T, Applebury M, Ashery-Padan R. Notch1 functions to suppress cone-photoreceptor fate specification in the developing mouse retina. Development. 2006;133:1367–1378. doi: 10.1242/dev.02311. [DOI] [PubMed] [Google Scholar]
- Yedvobnick B, Kumar A, Chaudhury P, Opraseuth J, Mortimer N, Bhat KM. Differential effects of Drosophila mastermind on asymmetric cell fate specification and neuroblast formation. Genetics. 2004;166:1281–1289. doi: 10.1534/genetics.166.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AS, Bais C, Greenwald I. Crosstalk between the EGFR and LIN-12/Notch pathways in C. elegans vulval development. Science. 2004;303:663–666. doi: 10.1126/science.1091639. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yamamoto M, Engel JD. GATA2 is required for the generation of V2 interneurons. Development. 2000;127:3829–3838. doi: 10.1242/dev.127.17.3829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.