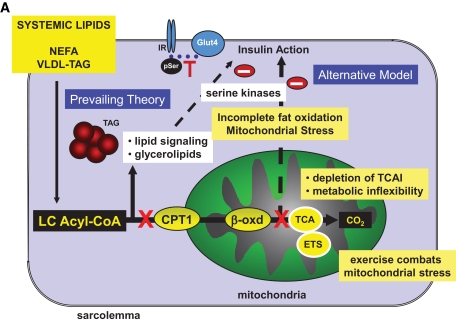
FIG. 4.
Mechanistic models of lipid-induced impairment of muscle insulin action and supporting metabolomics data. Feeding of diets high in fat results in muscle insulin resistance, and recent studies suggest the operation of two possible mechanisms for this effect (A). A prevailing theory is that increased delivery of fat to muscle saturates the capacity for mitochondrial β-oxidation, leading to accumulation of bioactive lipid-derived metabolites such as diacylglycerols and ceramides in the extramitochondrial space and activation of stress/serine kinases that interfere with insulin action. More recent studies have shown that fatty acid oxidation is actually increased in muscle in response to high-fat feeding but with no coordinate increase in TCA cycle activity. This results in accumulation of incompletely oxidized lipids in the mitochondria and depletion of TCA cycle intermediates, possibly resulting in mitochondrial stress and interference with insulin actions. The metabolic changes that underpin this new mechanism were identified by targeted GC-MS of organic acids and MS-MS analysis of acylcarnitines in muscle extracts from lean and obese animals, as summarized in B (data reprinted from ref. 51 with permission). Note that these mechanisms are not mutually exclusive and could work in concert to impair muscle insulin action. CPT1, carnitine palmitoyltransferase 1; ETS, electron transport system; NEFA, nonesterified fatty acid; TCAI, TCA cycle intermediates.