Abstract
“The large scale remodeling of mitochondria during apoptosis is a necessary step for the complete release of cytochrome c” has been a tenet since 2002. However, more recent findings strongly indicate that the large-scale remodeling previously described actually takes place after the release of cytochrome c and in a caspase-dependent manner, bringing into question whether mitochondria remodeling is necessary. In a more recent article, however, it was shown that a much more subtle form of remodeling is taking place which is only observable by electron tomography. In the Bcl-2 inhibitable Bax/Bak-dependent intrinsic pathway of apoptosis, the release of cytochrome c from mitochondria is a consequence of two carefully coordinated events: formation of outer membrane pores and opening of crista junctions triggered by Opa1 oligomer disassembly, and both steps are necessary for the complete release of cytochrome c. We review the recent literature pertaining to the coordinated release of cytochrome c during cell death.
Keywords: Mitochondria, Crista junction, Electron tomography, Apoptosis, Cytochrome c, Opa1
1. Introduction
Once cytochrome c is released from mitochondria and activates apoptosomes in the cytosol, death is the certain outcome for the host cell. What happens to the cell's mitochondria, on the other hand, is turning out to be a very complicated story. Adding to the complexity is that this organelle has three distinct compartments defined by two distinct membranes [1] (Fig. 1) that separate functionality. Co-incubation of isolated mitochondria with recombinant BH3-only proteins or peptides triggers the rapid and complete release of cytochrome c [2–4]. Other proteins such as Htra2/Omi, SMAC and Opa1 are released at about the same time. Currently, the mitochondrial community has a very limited understanding of how BH3-only proteins initiate this process.
Fig. 1.
Model of mitochondria based on EM tomography. Mitochondria have three distinct compartments separated by two membrane systems. The inner membrane consists of two parts, the cristae membranes and the inner boundary membrane. Recent application of electron tomography produced the current view that these membrane domains are connected by narrow, tubular structures termed crista junctions. These junctions have been proposed to regulate the dynamic distribution of proteins, lipids, and metabolites between mitochondrial compartments. An example is the redistribution of cytochrome c and other mitochondrial proteins during apoptosis. (A) A surface-rendered view of an isolated liver mitochondrion generated from an electron tomographic volume. Scale=100 nm. (B) A model of the crista junction in relation to mitochondrial compartments.
It has been speculated that in order to release cytochrome c and other proteins, pores must be formed on the outer membrane of mitochondria [5, 6]. This pore is called the mitochondrial outer membrane pore (MOMP), its formation is triggered by tBid [7], and supposedly contains Bak or Bax proteins but VDAC channels are not involved [8]. It has also been speculated that cristae remodeling is a necessary part of cytochrome c release. There are a number of excellent reviews regarding the first speculation [9–11]. In this review, we will focus on the second speculation and address the question, “Is cristae remodeling necessary for the rapid and complete release of cytochrome c?”
2. The nature of cristae remodeling
Dramatic morphological changes of mitochondria are observed in cells undertaking apoptosis. Upon apoptotic stimulation in many situations, mitochondria change from a filamentous network into punctate fragments. Electron microscopic images revealed that the cristae, which are normally tubular or lamellar structures, become roundish and fragmented and then they disappear [12]. Blebbing of the matrix and extensive swelling of the mitochondria can occur, followed by the fragmentation of the mitochondria [2,5]. The release of cytochrome c from mitochondria activates apoptosome formation in the cytosol, and is a necessary step in mitochondrial-dependent apoptosis [13–16]. However, the question of whether large-scale morphological changes of mitochondria are necessary for the release of cytochrome c or the consequence of the released cytochrome c activating apoptosomes and altering mitochondria downstream has been a controversial issue. Proponents of the former model suggest that crista junctions, which are narrow tubular tunnels connecting cristae to the space between the outer and inner membranes, called the intermembrane space, must become wider to allow the passage of cytochrome c from the intracristal space to this intermembrane space. Once in the intermembrane space, cytochrome c can escape to the cytosol through the pores formed on the outer membrane. In this article, we present recent evidence with commonly used mechanisms to induce apoptosis favoring the latter model, being that more subtle forms of structural changes are taking place inside mitochondria. Among these changes are (1) narrowing of crista junction openings, and (2) disassembly of Opa1 protein complexes at the opening. We also discuss what this model suggests towards our understanding of mitochondria.
3. Timing of cytochrome c release
Doug Green and colleagues observed that when HeLa cells stably expressing GFP-Cyt C were stimulated with STS, Act D or UV to induce apoptosis, within each single cell the entire content of GFP-Cyt C was released from all the mitochondria within 10 min [17,18]. Furthermore, they found that transmembrane potentials of mitochondria were retained for at least 60 min from the time of cytochrome c release [19]. Several years later, Terry Frey et al. used correlated light microscopy and electron tomography to examine the 3D structures of mitochondria in cells that released GFP-Cyt C and found that in the presence of the pan-caspase inhibitor z-VAD, HeLa cells released the content of GFP-Cyt C from mitochondria with the same kinetics as in the absence of z-VAD [20]. Interestingly, little large-scale alterations of mitochondria took place in the presence of z-VAD. These recent observations suggest that large-scale morphological changes in mitochondria upon initiation of several forms of apoptosis are not necessary for the release of cytochrome c, but rather are the consequences of released cytochrome c activating cytosolic caspases. However, the warning was raised that immuno-fluorescent microscopy cannot distinguish different compartments of mitochondria. Thus, it was possible that GFP-Cyt C may be localized differently from endogenous cytochrome c, i.e., GFP-Cyt C could be residing only in locations adjacent to the outer membranes and thus remodeling of cristae was not required for release of GFP-Cyt C. The release of endogenous cytochrome c that resides in portions of cristae deep within mitochondria may still require cristae remodeling or other structural alterations. To address this question, researchers turned to in vitro experiments using isolated mitochondria incubated with the BH3-only proteins tBid, BimS and with BH3 peptides [4].
In in vitro experiments, isolated mouse liver mitochondria released the entire content of cytochrome c in 10–15 min in the presence of 10–20 nM tBid or BimS. However, the gross morphologies of treated and untreated mitochondria were virtually indistinguishable by transmission electron microscopy. Upon further analysis of mitochondria that released the entire content of cytochrome c using 3D tomography, it was found that the diameter of crista junctions was actually smaller. The release of cytochrome c was inhibited by the addition of excess anti-apoptotic proteins such as Bcl-XL and Mcl1 [4]. It has been estimated that over 85% of cytochrome c resides in cristae as opposed to the intermembrane space. The release of nearly 100% of cytochrome c must mean the release of most if not all the cytochrome c from within cristae within 10–15 min through narrowed crista junctions. Thus, essentially all cytochrome c can exit cristae and cross the outer membrane in as short as 10 min without large-scale structural alterations.
4. Molecular requirement for cytochrome c release at the mitochondrial outer membrane
How does cytochrome c traverse the mitochondrial outer membrane? The current model is that BH3-only pro-apoptotic proteins induce the formation of Bak and Bax oligomers, which then triggers pore formation through which cytochrome c and other intermembrane space proteins escape during apoptosis. Supporting this model is the observation that in the absence of Bak or Bax oligomerization, there is no cytochrome c release [21]. Examples are (1) in Bax/Bak double knockout cells tBid does not induce cytochrome c release from mitochondria [22], (2) addition of a recombinant mutant of tBid, G94E (tBid carrying a mutation in the BH3 domain) to mitochondria from wild-type mice does not induce Bak oligomerization, and does not induce apoptosis, (3) addition of excess anti-apoptotic proteins such as Bcl-XL or Mcl1 blocks tBid and BimS-induced Bak oligomerization and blocks apoptosis, and (4) chemical agents such as DCIC, MG132 and Ucf101 block Bak oligomerization and block apoptosis [4]. Experiments with mitochondrial outer membrane vesicles (OMV) loaded with GFP-dextran of various sizes up to several hundred kDa, also indicated the requirement of Bax or Bak for the tBid-induced dextran release from OMV [23].
It is not yet known what other proteins if any are involved in MOMP formation during apoptosis through which cytochrome c is released. It is understood, however, that the VDAC ion channel is not necessary for tBid-induced cytochrome c release [8]. The complication in determining the composition of the outer membrane pores rests with the fact that it is difficult to capture the images of cytochrome c being released through the pores [7].
5. Molecular events in the intermembrane space leading to the complete release of cytochrome c
The evidence we discussed so far points towards a simple model in which pro-apoptotic BH3-only proteins induce outer membrane pore formation through which the entire content of cytochrome c is released into the cytosol and large-scale alterations of mitochondrial morphologies are the consequences of cytochrome c activating apoptosomes in the cytosol. As we discuss in this section, however, subtle but significant discrepancies in this model were found. For example, it was found that the addition of MG132 blocked tBid-induced cytochrome c release by blocking Bak oligomerization [4]. When these mitochondria were analyzed by EM tomography, the average radius of crista junctions was 8.5 nm, about half the radius of crista junctions in untreated mitochondria (average 16 nm). In fact, the average radius was almost the same for crista junctions of mitochondria treated with tBid alone. Thus in the presence of MG132 and tBid, crista junctions narrowed while there were no pores on the outer membrane. When the accessibility of cytochrome c from the outer membrane was measured, it was found to be as accessible as cytochrome c in tBid-treated mitochondria. A search for a molecular marker for these subtly remodeled cristae found Opa1.
In isolated mitochondria, about 50% of Opa1 proteins are found in an oligomeric complex [4,24]. During tBid-treatment, oligomerized Opa1 complexes disappear rapidly, leaving only the monomeric form of Opa1 [4,24,25]. When isolated mitochondria were treated with MG132 and tBid, cytochrome c and Htra2/Omi as well as Opa1 were all retained, but there was no Opa1 complex. This led us to speculate that Opa1 complex assembly and disassembly regulate cytochrome c release from inside the cristae. One could imagine the Opa1 complex acting as a gate at crista junctions, retaining cytochrome c in the cristae under normal circumstances. During apoptosis, the Opa1 complex is disassembled by processes independent of caspases. When the gates collapse, they leave holes large enough to let through cytochrome c and many other proteins. But when there is no pore at the outer membrane, cytochrome c is still trapped inside the mitochondria. This model was tested by engineering a situation in which the outer membrane pores were formed but the Opa1 gates remained intact. To accomplish this, a point mutation in Opa1 was found which made the Opa1 protein resistant to disassembly. When this mutant protein was over-expressed in tissue culture cells and treated with apoptosis-inducing drugs, mitochondria released very little cytochrome c and became resistant to apoptosis. It was concluded that Opa1 controls passage of cytochrome c through crista junctions.
Thus how Opa1 oligomers are assembled and disassembled is a question central to understanding the molecular mechanism of tBid-induced cytochrome c release, and will be discussed later. But before we go on, we will address the differences between conflicting findings.
6. Two versions of the same incident — the Rashomon effect
Controversy is nothing new to Science. It often happens that competing camps with different models fight for a while only to find years later that no model was completely right. Further experimentation and/or analysis seems to always resolve these controversies. To resolve the different conclusions between Scorrano's group and our group, additional analysis is worthwhile because both groups used almost the same reagents [2,4,24]. Thus, we shall look more closely to the published articles and compared methods used and data derived from the experiments by Scorrano's group and ours.
Scorrano et al. used isolated mouse liver mitochondria as did we. However, they incubated the mitochondria with recombinant tBid protein in a buffer containing very little metal chelator (10 µM EGTA) compared to our buffer (1 mM EGTA and 1 mM EDTA) [2,4].1 We both saw a rapid release of cytochrome c. Using a different buffer, however, they saw changes in mitochondrial morphology, such as extensive vesiculation of cristae followed by swelling of mitochondria, whereas we saw only a subtle structural change — the narrowing of crista junction diameter from 16 nm to 8.7 nm, which was only observable with EM tomography. Scorrano et al. classified the features of altered mitochondria into 4 classes [2]; Class I being untreated mitochondria, Class II showing extensively vesiculated crista structures, Class III mitochondria having asymmetric blebbing of the matrix, often with partially ruptured outer membranes. Swollen mitochondria with ruptured outer membranes, completely devoid of cristae structures are classified as Class IV. Within 2–5 min of incubation with tBid, Classes II–IV become more predominant. Using EM tomography, they too measured the diameter of crista junctions. In untreated mitochondria, the diameter of crista junctions was approximately 18.6 nm, similar to ours. However, in later phases, the average diameter increased to as much as 56.6 nm.
The morphologies of isolated mitochondria treated with tBid in low EGTA buffer are significantly different from the mitochondrial remodeling in HeLa cells undergoing etoposide-induced apoptosis described by Frey et al. [20], who categorized the progression of mitochondria during apoptosis in three stages. In the correlated light and EM studies by Sun et al. [20], mitochondria in stages 2 and 3 had already released the entire content of cytochrome c. Furthermore, in the presence of the pan-caspase inhibitor z-VAD, mitochondria in HeLa cells released the entire content of cytochrome c without ever reaching stages 2 and 3. Indeed, they found that the average diameter of crista junctions in etoposide+z-VAD treated HeLa cells was not different than before the treatment. Thus, it appears that extensive remodeling of cristae structures including the widening of the crista junctions is unnecessary for the complete release of cytochrome c.
In a more recent article, Scorrano et al. found that over-expression of Opa1 delays the onset of cytochrome c release triggered by various apoptosis-inducers in tissue culture cells [24]. Indeed, when mitochondria isolated from Opa1 over-expressing cells were treated with tBid, there were considerably fewer mitochondria in Classes II–IV, and the average diameter of crista junctions remained almost unchanged (16.1±2.1 nm for untreated cells, 15.2±2.3 nm for Opa1 over-expressing cells and 17.1±2.1 nm for cells over-expressing K301A mutant). When isolated mitochondria from wild-type cells and Opa1 over-expressing cells were treated with tBid, the crista junction diameter widened only in mitochondria from wild-type cells (45.4 ± 3.2 nm for wt mouse embryonic fibroblasts (MEFs) and 20.2 ± 3.1 nm for Opa1 over-expressing MEFs). They interpreted this result as evidence that Opa1 over-expression blocked the widening of the crista junctions, preventing cytochrome c release. Another possible interpretation would be that in the presence of an increased amount of Opa1 protein, fewer mitochondria had released cytochrome c and transitioned to Classes II–IV mitochondria. Thus, the majority of mitochondria might have remained in Class I with relatively narrow crista junctions. It is worth mentioning that over-expression of Opa1 by itself does not change the average diameter of crista junctions, suggesting that cytochrome c is not sequestered in the cristae by making crista junctions narrower. The conclusion is that widening of crista junctions is not necessary for the release of cytochrome c.
Even if mitochondrial cristae remodeling takes place only after cytochrome c release, it would be of some interest to know what causes these changes. We can only speculate at this point. The obvious candidates to bring about morphological changes would be caspases because in HeLa cells, the presence of the pan-caspase inhibitor z-VAD blocked these changes. It would be interesting to test what effect z-VAD would have on isolated mitochondria in low EGTA buffer when cytochrome c is released by adding recombinant tBid. Would remodeling of mitochondria take place? If z-VAD were to block the remodeling then maybe a mitochondrial z-VAD binding protein could be identified. There are actually candidate binding proteins — two caspases. Caspase 9 has been found inside mitochondria [26] and caspase 3 has been shown to act on complex I on the mitochondrial inner membrane [26a]. Since the presence of 1 mM EGTA and 1 mM EDTA block mitochondria crista remodeling, caspases could be the metalloproteases present in mitochondria that influence the remodeling. One other possibility is the release of calcium from the endoplasmic reticulum (ER) to trigger these changes. Since the ER is tethered to mitochondria by Mitofusion 2 [27], it is possible and also likely that the ER is present in many isolated mitochondria preparations. Perhaps tBid could induce calcium release from the ER causing mitochondria to swell, although a mechanism for this is unknown. However, if this were the mechanism, then cyclosporine A (CsA) would block the swelling, and possibly the release of cytochrome c. Interestingly, Scorrano et al. reported that CsA attenuated the release of cytochrome c from isolated mouse liver mitochondria, and also blocked the remodeling of cristae structures in mitochondria [2]. Furthermore, the remodeling did not require the BH3 domain of tBid, and was independent of the Bak protein [2]. On the other hand, we could not block tBid-induced cytochrome c by CsA, and the same G94E mutant of tBid used by Scorrano et al. could not induce the release of cytochrome c, whereas other BH3 only proteins such as BimS and BH3 peptides could [4]. It is perhaps worth noting that Frey et al. also found that pre-treatment of HeLa cells with CsA could not block the swelling of mitochondria induced by etoposide [20].
Disagreement about the effectiveness of CsA as an inhibitor of apoptosis is not just between three groups but is more widespread among the community. For example, using mouse liver mitochondria, it was suggested that CsA did not inhibit tBid-induced cytochrome c release [28], whereas another concluded the opposite [29]. These articles reported the use of isolated mouse liver mitochondria2 with recombinant tBid and 1–3 µM of CsA. However, the buffers used for mitochondrial suspension were slightly different. Why such discrepancies among the scientific community is open for debate.
The anti-apoptotic effect of CsA on H2O2-treated cells is even more controversial. Some have claimed it to be inhibitory, whereas others have shown it to be ineffective [31,32]. The reason for the discrepancy may lie in the fact that H2O2 can initiate at least two different cell death pathways and which pathway is activated may depend on the cell type. The subject will be discussed later in this review. On the effectiveness of CsA related to cell death, however, there is at least one consensus and that is that CsA is effective in blocking nitrogen oxide-induced neural cells death [30].
7. The last step in the release of cytochrome c: how to break the cardiolipin–cytochrome c association?
BH3-only proteins induce outer membrane pore formation and open crista junctions by disassembling Opa1 oligomers, but many researchers now think that these events are not enough for the rapid and complete release of cytochrome c. In low salt buffer, they are enough to release intermembrane space proteins, such as short membrane-free Opa1 and Htar2/Omi [4], but not cytochrome c. Cardiolipin is a charged lipid almost exclusively found in the mitochondrial inner membrane where it constitutes about 20% of the total lipids [31]. There is an electrostatic force between cytochrome c and cardiolipin and unless this weak interaction is broken, it keeps cytochrome c inside mitochondria [32–34]. Indeed, when isolated mitochondria are incubated with recombinant tBid in buffer containing 10 mM KCl along with 300 M trehalose with or without metal chelators such as EGTA or EDTA, Opa1 oligomers are disassembled, opening crista junctions and releasing short-form Opa1, Htra2/Omi, and AK very rapidly [4,35]. However almost all cytochrome c remains within mitochondria. When the KCl concentration is raised to 60–80 mM, the complete release of cytochrome c is seen [4,36]. Intracellular concentration of K+ is estimated to be around 150 mM, more than enough to release cytochrome c from tBid-treated mitochondria. However, this concentration may include stored K+ in places such as mitochondria.
Supposing that we need to disrupt cardiolipin–cytochrome c interaction, one possibility is that calcium or other ions have entered the mitochondrial intracristal space, changing the ionic strength, and thus releasing cytochrome c from cardiolipin. K+ and Ca2+ are stored in the mitochondrial matrix [37]. However, since isolated mitochondria incubated with tBid do not release cytochrome c unless a large amount of KCl is added to the buffer, it seems reasonable to assume that the source of ion must come from outside the organelle. Another possibility is that cardiolipin is modified by reactive oxygen species (ROS) generated by altered respiratory chain reactions (both are reviewed in [38]). Here, we will briefly review the current knowledge regarding these two hypotheses.
Hypothesis 1
Calcium influx disrupts cardiolipin–cytochrome c association during tBid-induced apoptosis.
Apoptotic agents such as STS and etoposide that generate cleaved tBid also trigger influx of Ca2+ from the endoplasmic reticulum (ER). Could release of Ca2+ displace cardiolipin from cytochrome c and is it necessary for tBid-induced cytochrome c release?
When a large pool of Ca2+ is released from the ER, a significant fraction is quickly absorbed by mitochondria in very close contacts with the ER [39,40] and Ca2+ ends up in the matrix. In many cell types, transfer of Ca2+ from the ER to mitochondria seems to be a carefully coordinated event facilitated by the proximity of IP3-sensitive channels in the ER and calcium uniporters in mitochondria [39–41].
The Bcl-2 family of proteins in part regulates IP3-sensitive channels on the ER. For example, in PC-3, a prostate cancer cell line, STS-treatment can completely deplete Ca2+ from the ER and there is a concomitant increase in the Ca2+ store of mitochondria [42,43], and over-expression of ER-targeted Bcl-2 can block this. Another example is that Bax deficient cells are almost devoid of the stored pools of Ca2+ in either the ER or in mitochondria. Thus, the Bcl-2 family of proteins regulates Ca2+ stores on mitochondria and ER as well as the formation of the mitochondrial outer membrane pore.
There is one well-studied consequence of calcium influx: activation of mitochondria permeability transition pore (m-PTP), which is separate from tBid-induced cytochrome c release. When the mitochondrial calcium level reaches a high value, it can trigger the opening of the m-PTP, releasing all solutes less than 1500 Da from within mitochondria. Whether cytochrome c is also released through the m-PTP is controversial [44,45]. However, when components of the m-PTP such as cyclophilin-D and VDAC are depleted, cells still died from tBid-induced cytochrome c release. Thus, the m-PTP is dispensable for tBid-induced cytochrome c release. Interestingly, cyclophilin-D knock-out mouse embryonic fibroblasts (MEFs) treated with death-inducing agents died by necrosis.
This still leaves the possibility that calcium influx could participate in tBid-induced cytochrome c release by dissociating cytochrome c from cardiolipin. Bax deficient DU145 cells have very little store of Ca2+ in either the ER or in mitochondria. STS-treatment induced almost no changes in the amounts of stored Ca2+ in either organelle, but these cells released cytochrome c and died by apoptosis [42,46]. Expression of tBid in DU145 cells also induced cytochrome c release from mitochondria and cells died by apoptosis [46]. Thus, the huge influx of Ca2+ observed in STS and etoposide-treated normal cells is not required for tBid-induced cytochrome c release.
How much Ca2+ would be needed to release cytochrome c from cardiolipin? In isolated mitochondria, it takes 60–80 mM KCl. Thus, it could take as much as 30–40 mM Ca2+ in the intracristal space to dissociate cytochrome c from cardiolipin. Influx of huge Ca2+ waves observed during massive Ca2+ release from the ER in cardiac myotubes and other cell types was estimated to be in the range of 1–200 µM [47,48]. Of course, transient increases of micro-local concentration could be higher than 200 µM. Considering that DU145 cells have very little Ca2+ stored in the ER or mitochondria, it seems unlikely that they could generate a large enough influx of Ca2+ to dissociate cytochrome c from cardiolipin. Thus, it appears that the influx of Ca2+ is not necessary for the tBid-induced cytochrome c release from mitochondria. Our conclusion is that Hypothesis 1 is likely not valid.
Even though this review is focused on the Bax/Bak-dependent intrinsic pathway of apoptosis and the internal changes that must be made in mitochondria before cytochrome c can be released, a small detour is in order to discuss another pathway of cell death because of its dependence on the presence of calcium ions stored in the ER and its potential to affect mitochondria.
8. ER-Ca2+-dependent cell death pathway
Unlike developmentally regulated programmed cell death that uses Bak/Bax-dependent apoptosis, external injuries to tissues such as wound healing or ischemia/reperfusion activate the ER-Ca2+-dependent cell death pathway.
To distinguish Bax/Bak-dependent intrinsic pathway of apoptosis from ER-Ca2+-dependent cell death pathway, Scorrano et al. used Bak/Bax double knockout cells (DKO cells) [22]. These DKO cells have little stores of Ca2+ in either ER or mitochondria. The sarco/endoplasmic reticulum Ca2+-ATPase (SERACA) is a Ca2+ transporter. Over-expression of SERCA enhanced Ca2+ uptake by the ER and restored Ca2+ levels to normal in DKO cells. This restored the sensitivity to ceramide, arachidonic acid and H2O2, but cells remained resistant to tBid-induced cell death [22]. Transfection of mitochondria-targeted Bax to DKO cells, on the other hand, restored the sensitivity of DKO cells to STS, etoposide and BFA-treatment, without restoring Ca2+ stores in these cells. As expected, DKO cells transfected with mitochondria-targeted Bax died an apoptotic death when tBid was introduced. However, these cells remained resistant to ceramide, arachidonic acid and H2O2 [22]. Thus, these sets of experiments showed that there exist two independent pathways of programmed cell death. In mouse fibroblasts, ceramide, arachidonic acid, and H2O2 use an ER-Ca2+-dependent programmed cell death which does not relay on MOMP to release cytochrome c whereas the expression of tBid triggers an ER-Ca2+ independent, Bax/Bak-dependent apoptosis, and STS and etoposide activate both pathways.
Lastly, agents such as H2O2 and ceramide induce Ca2+ release from the ER, which is taken up by mitochondria, and when the Ca2+ level in mitochondria reaches a threshold concentration, it activates the mitochondria permeability transition pores through which cytochrome c escapes, activating caspases in the cytosol.3 But this is very different from tBid-induced cytochrome c that does not require calcium influx. In tBid-induced apoptosis, cytochrome c escapes from mitochondria through Bak/Bax pores formed on the outer membrane. Interestingly, over-expression of Opa1 seems to delay cytochrome c release in both pathways [49]. It is not known how or even if cristae are remodeled during H2O2-induced or ceramide-induced cytochrome c release, but by the time cytochrome c is released, mitochondria are all likely to be swollen. Thus, remodeling of mitochondria under ER-Ca2+-induced cell death appears to be very different from tBid-induced cytochrome c, yet Opa1 plays a role in both of them. For this review, however, we will remain focused on tBid-induced cytochrome c release.
Hypothesis 2
Modification of cardiolipin disrupts cardiolipin–cytochrome c association during tBid-induced apoptosis.
It has been reported that cardiolipin modification takes place during dexamethasone-induced thymocyte apoptosis [50], and p53-induced apoptosis [51]. It was assumed that reactive oxygen species generated during apoptosis cause peroxidation of cardiolipin. In these studies, however, 10-N-nonyl acridine orange (NAO) was used in FACS analysis to assay for amounts of cellular and mitochondrial cardiolipin. However, NAO is not as specific for cardiolipin as previously believed because cardiolipin deficient yeast cells are stained positive by NAO in in vitro and in vivo assays [52]. What is needed is a membrane permeable cardiolipin-specific dye to observe the loss of cardiolipin from mitochondria as cytochrome c is being released. Another useful tool would be the recently developed system for REDOX-sensitive GFP (roGFP), but targeted to the mitochondrial intracristal space to monitor how changes in REDOX state in the intracristal space would precede the loss of cardiolipin followed by the release of cytochrome c [53–55]. Because over-expression of Opa1 delays the release of cytochrome c when cells were treated with H2O2, it is possible that Opa1 has a role in protecting cardiolipin from being oxidized [24].
The only accurate measurement of cardiolipin currently available is by mass spectroscopy. Indeed, in STS-induced apoptosis, three anionic phospholipids: cardiolipin, phosphatidylserine and phosphatidylinositol all underwent oxidation as measured by mass spectroscopy [56], confirming lipid peroxidation of cardiolipin during apoptosis. Furthermore in actinomycin-D (ACD)-induced apoptosis in MEFs, oxidation of cardiolipin (6 h) preceded cytochrome c release (8 h), caspase3/7 activation (8 h), annexin V positivity (9 h) and a decrease in the transmembrane potential (12–14 h) after treatment with ACD [57], suggesting that peroxidation of cardiolipin is not the consequence of cytochrome c release nor a drop in the transmembrane potential.
What could be oxidizing cardiolipin? Borisenko et al. suggest that cytochrome c is 4. Using a phospholipase-based enzymatic assay for the presence of cardiolipin, they discovered that during apoptosis, the distribution of cardiolipin between monolayers of inner membrane changed from 60:40 (matrix-facing:intermembrane space-facing) to 30:70 [57]. Then using an in vitro assay, they showed that cytochrome c can act as a cardiolipin oxygenase. They reason that a two-fold increase in cardiolipin content directly facing the intermembrane space may be enough to begin peroxidation of cardiolipin by cytochrome c. The mechanism for the cardiolipin switch from the matrix side of the lipid bilayer to the intermembrane side is unknown. Taken altogether, it seems reasonable that a sudden emergence of cardiolipin molecules facing the intermembrane space triggers their own destruction, detaching cytochrome c, and preparing for the eventual release of cytochrome c from mitochondria. If this is true, then the intriguing question is, “How does tBid in the cytosol affect lipids in the matrix-facing inner membrane?”
Mass spectroscopy was also used to analyze the total lipid content of isolated mitochondria treated with tBid [58]. It was reported that these mitochondria lost more than 50% of their cardiolipin content. Composition of other lipids remained largely unchanged. A 50% reduction in cardiolipin content was also observed in mitochondria treated with tBid-G94E as well as mitochondria treated with tBid+ Bcl-XL. Thus, the reduction in cardiolipin content did not correlate with mitochondrial outer membrane pore formations or crista junction opening. Yin et al. interpreted the reduction in the amount of cardiolipin in their lipid scan as an indication of tight binding of tBid to cardiolipin [58]. Furthermore, they propose that this binding takes place at alpha helices α4–α9 of tBid, not through helix α3 that contains the BH3 domain. Lastly, they suggest that this binding of tBid through its C-terminal region to cardiolipin facilitates the translocation of tBid to mitochondria. Shortly after, Walensky et al. published evidence that a hydrocarbon-stapled BH3 helix can induce apoptosis very efficiently [59]. Thus, helices α4–α9 of tBid may be serving dual roles: one, as a mitochondrion localization signal, and two, a cardiolipin-binding site.
It is perhaps worth mentioning that tBid does not bind to pure cardiolipin in vitro. It does bind to cardiolipin-containing mixed-lipid liposomes, though [38]. Furthermore, it is believed that since cardiolipin is a charged lipid, it could attract a variety of charged proteins such as cytochrome c, protein kinase C, tBid and caspase-8 [23,60], and often serves as a platform to assemble large protein complexes on liposomes and on the inner membrane of mitochondria [23,61]. In particular, cardiolipin is enriched in mitochondrial contact sites where the mitochondrial outer and inner membranes adhere, and where VDAC, ANT and cyclophilin-D interact. Cardiolipin can induce hexagonal structures on liposomes in in vitro experiments and for this reason, it is speculated that hexagonal structures are formed at mitochondrial contact sites, exposing cardiolipins to the cytosol and thus to tBid [62,63]. In support, Lutter and coworkers found that tBid was localized to contact sites [64,65].
9. The role of Opa1 in maintaining cristae morphology
Mitochondria lacking Opa1 protein are largely devoid of cristae structures [66]. However, this finding is not unique. There are many more genes that affect the generation of cristae structures when their protein products are depleted [1]. One example gaining increasing interest is mitofilin [67]. The loss of specific isoforms of Opa1 can result in aberrant cristae morphogenesis and impaired cellular proliferation [68,69,66]. Furthermore, during siRNA treatment to inhibit Opa1 protein synthesis, the tubular network of mitochondria in cell lines becomes fragmented, indicating that Opa1 plays a role in mitochondrial fusion. Indeed, the yeast homologue of Opa1, Mgm1, is necessary for yeast mitochondrial inner membrane fusion [70]. However, we do not know exactly how Opa1 maintains cristae structures and how that is related to mitochondrial fusion activity.
Opa1 is a nuclear encoded gene and in humans, there are 8 splice variants of Opa1 [71,69]. In most tissue culture cells, at least two to three splice variants seem to be expressed. Each of these splice variants carry two to three cleavage sites by serine proteases. These sites were identified by Ishihara et al. [72] and designated MPP, S1 and S2. Once the product of a splice variant is imported into mitochondria, its mitochondrial localization signal peptide is cleaved at the MPP site, generating a mature membrane-bound Opa1 protein. Subsequently, second and possibly third cleavages can take place at either S1, S2 or both, producing shorter isoforms [72].
In most human and mouse cells, six isoforms of Opa1 are detectable by western blotting: two long membrane-bound isoforms and four short membrane-free isoforms. It is not known if each mitochondrion houses only long isoforms, only short isoforms or both. It is known, however, that the cells which had lost all the long isoforms of Opa1, such as found in patients suffering from mitochondrial myopathies or MEFs harboring an error-prone mitochondrial mtDNA polymerase α, contain fragmented mitochondrial networks and have reduced mitochondrial respiration rates [73]. Introducing various human Opa1 variants and their mutants into MEFs from Opa1 knockout mouse embryos, David Chan et al. demonstrated that at least one short isoform and one long membrane-bound isoform is necessary for the generation of fusion competent mitochondria [69]. Other approaches, such as over-expression of non-cleavable mutants or introduction of short isoforms, came to similar conclusions [68,71,74].
Because mitochondria need both long and short isoforms to support their fusion activity, knowing how conversion from long to short isoforms is regulated is of great interest. One could test if the same proteases cleave Opa1 during tBid-induced apoptosis. By knowing the identities of Opa1 processing enzymes, perhaps drugs could be developed to inhibit unwanted apoptosis by blocking Opa1 cleavage or do the opposite: prime tumor cells for apoptosis by activating Opa1 processing enzymes.
There are two conditions under which the complete conversion of all long isoforms of Opa1 to short isoforms takes place: one, during tBid-induced apoptosis and two, during the treatment of cells with the mitochondrial respiration inhibitor, CCCP. This latter process was inhibited by 1,10-phenanthroline, an inhibitor of metalloproteases, suggesting the involvement of m-AAA in the process [75]. Even though both processes convert Opa1 long isoforms to short isoforms, these two events differ in the way they alter cristae structures.
When isolated mitochondria are incubated with tBid, all the long isoforms of Opa1 are quickly converted to short isoforms and it is only the short isoforms that are released from mitochondria when Opa1 oligomers are disassembled [3]. However, the release of the short isoforms is not completed during the 30 min in which 100% of cytochrome c and Htra2/Omi molecules are both released. This might be because the membrane-free short isoforms are still attached to some other moieties in mitochondria, such as membrane-bound Parl. In tissue culture cells, the mitochondrial transmembrane potential is maintained for a few hours after the loss of cytochrome c [18,19]. On the other hand, CCCP-treatment of cells, as well as of isolated mitochondria, causes an immediate drop in the transmembrane potential and the long isoforms of Opa1 disappear from mitochondria in 10–30 min [75,66,69,76]. The mitochondrial network in these cultured cells becomes fragmented. The treatment does not cause immediate disassembly of Opa1 oligomers nor does it cause the loss of cristae structures. However, the long-term absence of Opa1 long isoforms seems to destabilize Opa1 complexes that eventually leads to the loss of cristae structures [68]. Thus, the changes to Opa1 brought on by two different treatments differ qualitatively — tBid-treatment causes immediate disassembly of Opa1 oligomers while CCCP-treatment cause only destabilization of Opa1 complexes.
10. Opa1 processing enzymes
Even though mitochondrial proteomes are slightly different from tissues to tissue, there are at most 20–30 serine proteases present in mitochondria. Three candidate serine proteases for processing mature membrane-bound Opa1 long isoforms are paraplegin, Yme1L and PARL. Mgm1 is cleaved by a rhomboid protease in mitochondria, Pcp1 [77]. When the mammalian homologue of Pcp1, PARL, is knocked out in mice, a wasting phenotype characterized by increased apoptosis was exhibited [49]. However, Parl−/− cells displayed normal mitochondrial morphology, did not show defects in mitochondrial fusion/fission, and did not display primary respiratory defects. Nevertheless, Parl−/− cells were more sensitive to apoptotic stimuli, such as etoposide, staurosporine and hydrogen peroxide. When exogenous Opa1 proteins were expressed in Parl−/− cells, they became less sensitized to apoptotic stimuli. Thus, it is suggested that increased sensitivity to apoptotic stimuli in Parl−/− cells may be due to altered Opa1 processing in these cells independent of mitochondrial fusion [24].
Yme1L (human Yme1-like) is an ATP-dependent membrane-inserted metalloprotease whose active site is in the intermembrane space thus classified as i-AAA [78]. David Chan's group showed that Yme1L cleaves Opa1 at S2 but not S1, They also discovered that the loss of transmembrane potential destabilized the long isoform of Opa1 and enhances the cleavage at S1 but not S2 [69]. Thus, Yme1L cannot be the sole protease that can generate short membrane-free Opa1 isoforms. van der Bliek's group showed that cells lacking either Parl or Yme1L responded to CCCP-treatment by cleaving the long isoforms of Opa1 [66]. Since Yme1L cannot cleave Opa1 at S1, their data imply that neither Parl nor Yme1L can cleave at S1. It was also found that Parl knockout did not affect Opa1 maturation nor did it affect induced proteolysis. Using a similar approach, Rojo's group also ruled out Parl and SPG7 (spastic paraplegia type 7) as serine proteases that generate short isoforms of Opa1 [76]. Reichert's group used yeast to reconstitute human Opa1 protein processing and found that human Parl expressed in yeast can cleave Mgm1 but not human Opa1 [79]. Ishihara et al. also expressed human Opa1 in yeast cells lacking various serine proteases and identified m-AAA as a candidate protease for Opa1 processing enzyme [72]. They also ruled out the involvement of paraplegin. In short, an S2 protease is known, but an S1 protease still has not been identified.
What could be the role of Parl? Mitochondria in Parl-depleted cells are more sensitive to apoptosis-inducing agents and their sensitivity is reversed by over-expression of Opa1. However, over-expression of Opa1 delays the induction of apoptosis in wild-type MEFs. Thus we do not know if Opa1 over-expression is restoring lost activity of Parl-processed Opa1 or simply protecting mitochondria by its abundant presence. Because Opa1 and Parl can co-precipitate each other [49], it is possible that the role of Parl is to stabilize Opa1 oligomers by providing an extra anchor. Stable Opa1 must be beneficial to the cell, making it more resistant to apoptosis. Not surprisingly, when wild-type Opa1 is over-expressed in MEFs, they become more resistant to apoptotic stimuli [24] while in Parl-depleted cells, Opa1 seems to make the cells less resistant [49,72].
The hypothesis that the role of Parl in cristae remodeling and apoptosis does not require its serine protease activity could be tested by expressing Parl point mutants defective in serine protease activity in Parl−/− cells. If expression of such a mutant in Parl−/− cells reverses the sensitivity, then Parl protease activity is not needed, and the role of Parl would be limited to its ability to associate with and stabilize Opa1 oligomers.
There is one more puzzle in this story. Prohibitins 1 and 2 are inner membrane proteins that form a large ring structure embedded in the inner membrane facing the intermembrane space, yet able to influence the matrix side. On the matrix side, the ring associates with an m-AAA protease complex [80]. It is known that knocking out one of the prohibitin genes destabilizes the protein product of the other, resulting in cells lacking both proteins. In prohibitin depleted cells, mitochondria are largely devoid of cristae much like Opa1 depleted cells, and both of the membrane-bound long isoforms of Opa1 are missing. The forced expression of a long isoform of Opa1 suppresses these defects. These data suggest that prohibitins regulate the serine protease(s) that cleaves membrane-bound long isoforms of Opa1.
11. Role of Opa1 in keeping cytochrome c inside crista
Using immuno-EM, Opa1 was found localized to the outer surface of the inner membrane seemingly throughout the intermembrane and intracristal spaces and not just at crista junctions [71,81]. Thus it seems that the Opa1 complex can be anywhere on the inner membrane.
Using yeast, Reichert et al. found that when protein synthesis and consequently mitochondrial import activities were arrested, Tim23, a part of the inner membrane portion of the import machinery that spans the mitochondrial inner membrane and outer membrane, was redistributed from predominantly inner boundary membrane localization to a more even distribution between inner boundary membranes and cristal membranes. They interpreted the results as follows: when mitochondrial protein import was arrested, it weakened the casual association between the N-terminus of Tim23 with the corresponding transporter complex of the outer membrane (a Tom), and since the C-terminus of Tim23 is embedded in the inner membrane, Tim23 diffused into the intracristal space. Their data suggest that there was no physical barrier blocking the lateral movement of inner membrane proteins across crista junctions [82].
If there is no barrier between inner boundary and cristal membranes, would there be lateral movement of cardiolipin across crista junctions as well? We do not know the distribution of cardiolipin since we do not yet have the means to separate inner boundary membranes from cristal membranes. But, since cardiolipin associating proteins such as cytochrome c and the oxidative phosphorylation (Oxphos) complexes are predominantly located in the cristal membranes, cardiolipin distribution might also be skewed towards cristal membranes. Reichert's group suggests that protein–protein association may also be a determinant factor for localization of cardiolipin [82]. Using their model, one could argue that once large complexes, such as the Oxphos complexes, are assembled, they would not easily be diffused and could even trap charged lipids like cardiolipin in the cristae. Furthermore, it is possible that the presence of Opa1 stabilizes Oxphos complexes and cytochrome c associating with them, thus keeping cytochrome c in the intracristal space. Indeed Frezza et al. showed that over-expression of wild-type Opa1 delayed the onset of H2O2-induced (ER-Ca2+-dependent) apoptosis [24].
What about soluble proteins and proteins that are loosely bound to membranes such as cytochrome c? Crista junctions in normal mitochondria can typically vary in mean diameter from 15 to 30 nm and vary much more in length. The mean diameter can be reduced to around 8 nm with tBid-treatment. Even at its narrowest, a hole of that size is big enough to let through 60–100 kDa proteins [83,84], hence it could let through cytochrome c molecules. At the same time, oligomerized Opa1 complexes may exceed several hundred kDa and they may be large enough to hinder free movement of soluble proteins across crista junctions.
12. Conclusion
“Happy families are all alike; every unhappy family is unhappy in its own way.” So begins Leo Tolstoy's novel, Anna Karenina. In many ways, studying apoptosis is like watching unhappy families. There are many ways leading to apoptotic cell death. Even if we restrict ourselves to the study of intrinsic forms of apoptosis, there are at least two distinct pathways: Bax/Bak-dependent pathway, which begins with the generation of BH3-only proteins that induce MOMP formation on mitochondria, and the ER-Ca2+-dependent pathway, which is triggered by calcium influx from the ER. This review focused on the molecular mechanism of tBid-induced cytochrome c release from mitochondria (Bax/Bak-dependent pathway) and internal changes that must take place before the release. Even though we briefly discussed the ER-Ca2+-dependent pathway for comparison purposes, we did not discuss how cristae are remodeled during Bax/Bak-independent ER-Ca2+-dependent cell death and the possible role of Opa1 in this pathway, nor did we examine the possible mechanisms of MOMP formation [7,9]. By narrowing the scope of this review, however, we were able to more completely cover what is known about the Bax/Bak-dependent pathway, identify key research issues and ongoing controversies, and to even propose experiments to help resolve these controversies. A model summarizing the cumulative knowledge of Opa1's role in tBid-induced apoptotic pathway is presented in Fig. 2. Even though tBid-induced cytochrome c takes place in the middle time frame of apoptosis, it does not happen in the way it was previously described: dying mitochondria first start to swell and then explode, spilling their entire contents throughout the cytosol. Instead, the evidence points to a complex interplay of events, causing subtle changes to mitochondrial structures. Three events necessary for the complete release of cytochrome c are the formation of mitochondrial outer membrane pores, the opening of crista junctions triggered by disassembly of Opa1 oligomers, and the dissolution of the cardiolipin–cytochrome c association. By examining the published evidence, one comes to understand the intricate timing of these events and the coordinated association of molecules that regulate this dynamic structural and functional correlation. Unresolved issues are (1) how Opa1 oligomers are assembled and disassembled and (2) how the electrostatic force between cardiolipin and cytochrome c is broken. The larger issue still to be addressed is how mitochondria cristae structure is generated and maintained. These are important questions because subtle defects in mitochondria are known to be the cause of premature aging and many age-related neurodegenerative diseases [85]. Furthermore, by better understanding this intricate organelle, we will learn how lipid and protein complexes can build and maintain very complex structures.
Fig. 2.
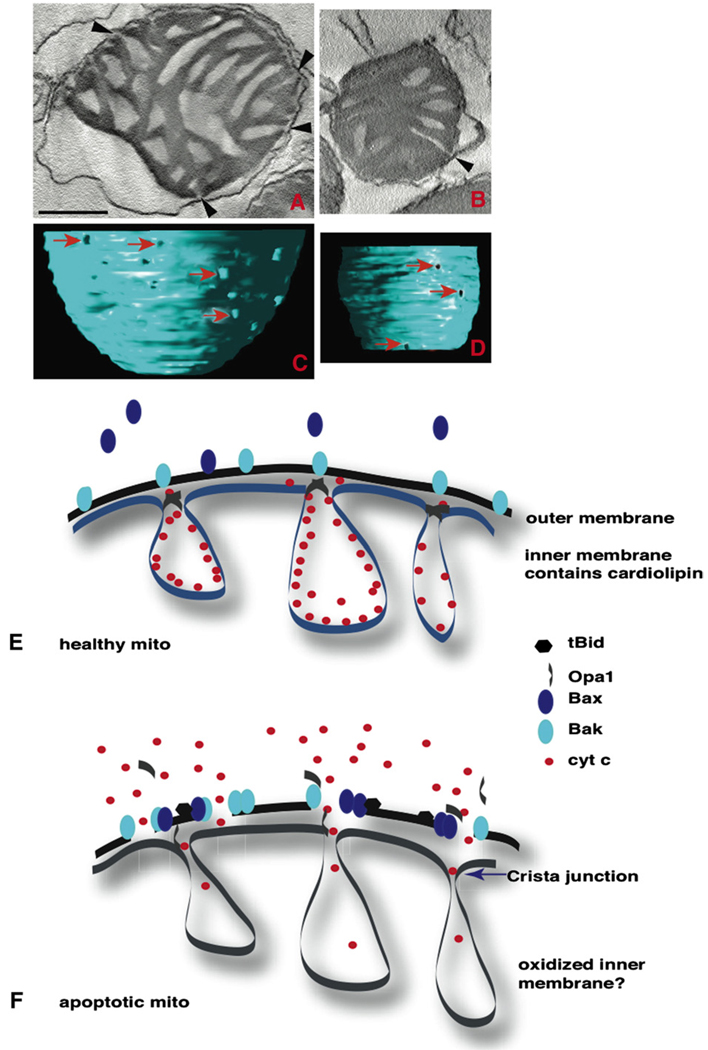
Crista junctions narrow upon Opa1 oligomer disassembly. (A–D) EM tomography of isolated mitochondria. Untreated mitochondria have relatively uniform crista junction openings (arrows and arrowheads). (A) A central slice through a tomographic volume and (C) the surface rendering of the inner boundary membrane. Upon dissolution of Opa1 oligomers in tBid-treated mitochondria, the crista junction diameter narrows to about half the size as demonstrated in (B) a central slice and (D) the rendered inner boundary membrane. Scale=100 nm for both A and B. The arrowheads point to the crista junctions in the tomographic slices and the arrows to the crista junctions in the surface-rendered volumes. E and F show a model of Opa1 oligomer disassembly, crista junction alteration, outer membrane permeability with the Bax + Bak pore, and subsequent cytochrome c release. Cardiolipin is almost exclusive in mitochondrial inner membranes. In this model (F), inner membranes are oxidized by ROS generated during apoptosis. By lipid peroxidation, cardiolipin is lost from the inner membrane, releasing cytochrome c from this membrane. Cytochrome c then exits the intracristal space through opened crista junctions and traverses the outer membrane through Bak/Bax pores into the cytosol.
Acknowledgements
We thank Prof. Lawrence Grossman ofWayne State University and Dr. Kate Welsh of the Burnham Institute for careful reading of the manuscript and many valuable suggestions. This publication was made possible by NIH NCRR grant P21 RR04050, grant ES010337 from the NIEHS, and NIH grant DK54441. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Our buffer composition was 300 mM Trehalose, 80–100 m MKCl, 20 mM Hepes pH 7.4, 1 mM EDTA, 1 mM EGTA, 0.1% BSA supplemented with ATP regenerating system (5 mM succinate, 2 mM ATP, 10 mM phosphocreatine, and 10 mg/ml creatine kinase). Scorrano's group used 125–150 mM KCl, 10 mM Tris–MOPS pH 7.4, 1 mM Pi, 5 mM glutamate, 2.5 mM malate, and 10 µM EGTA.
This is important since some laboratories use rat liver mitochondria which contain very little Bak or Bax, producing very different results.
This is suggested by Narita et al in Proc. Natl. Acad Sci USA 95 (1998). However, this may not always be the case in all cell types. There are data indicating that treatment of cells with H2O2 could cause caspase-independent necrotic cell death (Nakagawa et al., Nature 434 (2005) 652–658).
Nature Chemical Biology 1 (2005) 223–232.
References
- 1.Zick M, Rabl R, Reichert AS. Cristae formation-linking ultrastructure and function of mitochondria. Biochim. Biophys. Acta. 2009;1793:5–19. doi: 10.1016/j.bbamcr.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi R, Andreyev A, Murphy AN, Perkins GA, Ellisman MH, Newmeyer DD. Mitochondria frozen with trehalose retain a number of biological functions and preserve outer membrane integrity. Cell Death Differ. 2007;14:616–624. doi: 10.1038/sj.cdd.4402035. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi R, Lartigue L, Perkins G, Scott RT, Dixit A, Kushnareva Y, Kuwana T, Ellisman MH, Newmeyer DD. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol. Cell. 2008:557–569. doi: 10.1016/j.molcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed JC, Green DR. Remodeling for demolition: changes in mitochondrial ultrastructure during apoptosis. Mol. Cell. 2002;9:1–3. doi: 10.1016/s1097-2765(02)00437-9. [DOI] [PubMed] [Google Scholar]
- 6.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 7.Jonathan LPB, Lovell F, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Galluzzi L, Kroemer G. Mitochondrial apoptosis without VDAC. Nat. Cell Biol. 2007;9:487–489. doi: 10.1038/ncb0507-487. [DOI] [PubMed] [Google Scholar]
- 9.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev., Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 11.James DI, Martinou JC. Mitochondrial dynamics and apoptosis: a painful separation. Dev. Cell. 2008;15:341–343. doi: 10.1016/j.devcel.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat. Rev. Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ, Wang X, Williams RS. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell. 2000;101:389–399. doi: 10.1016/s0092-8674(00)80849-1. [DOI] [PubMed] [Google Scholar]
- 14.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 16.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein JC, Munoz-Pinedo C, Ricci JE, Adams SR, Kelekar A, Schuler M, Tsien RY, Green DR. Cytochrome c is released in a single step during apoptosis. Cell Death Differ. 2005;12:453–462. doi: 10.1038/sj.cdd.4401596. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat. Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- 19.Waterhouse NJ, Goldstein JC, von Ahsen O, Schuler M, Newmeyer DD, Green DR. Cytochrome c maintains mitochondrial transmembrane potential and ATP generation after outer mitochondrial membrane permeabilization during the apoptotic process. J. Cell Biol. 2001;153:319–328. doi: 10.1083/jcb.153.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun MG, Williams J, Munoz-Pinedo C, Perkins GA, Brown JM, Ellisman MH, Green DR, Frey TG. Correlated three-dimensional light and electron microscopy reveals transformation of mitochondria during apoptosis. Nat. Cell Biol. 2007;9:1057–1065. doi: 10.1038/ncb1630. [DOI] [PubMed] [Google Scholar]
- 21.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 23.Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 24.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J. Biol. Chem. 2005;280:35742–35750. doi: 10.1074/jbc.M505970200. [DOI] [PubMed] [Google Scholar]
- 26.Krajewski S, Krajewska M, Ellerby LM, Welsh K, Xie Z, Deveraux QL, Salvesen GS, Bredesen DE, Rosenthal RE, Fiskum G, Reed JC. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5752–5757. doi: 10.1073/pnas.96.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Ricci J-E, Munoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, Yadava N, Scheffler IE, Ellisman ME, Green DR. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the P75 subunit (Ndusf1) of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 27.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 28.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 29.Zamzami N, El Hamel C, Maisse C, Brenner C, Munoz-Pinedo C, Belzacq AS, Costantini P, Vieira H, Loeffler M, Molle G, Kroemer G. Bid acts on the permeability transition pore complex to induce apoptosis. Oncogene. 2000;19:6342–6350. doi: 10.1038/sj.onc.1204030. [DOI] [PubMed] [Google Scholar]
- 30.Hortelano S, Dallaporta B, Zamzami N, Hirsch T, Susin SA, Marzo I, Bosca L, Kroemer G. Nitric oxide induces apoptosis via triggering mitochondrial permeability transition. FEBS Lett. 1997;410:373–377. doi: 10.1016/s0014-5793(97)00623-6. [DOI] [PubMed] [Google Scholar]
- 31.de Kroon AI, Dolis D, Mayer A, Lill R, de Kruijff B. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and Neurospora crassa. Is cardiolipin present in the mitochondrial outer membrane? Biochim. Biophys. Acta. 1997;1325:108–116. doi: 10.1016/s0005-2736(96)00240-4. [DOI] [PubMed] [Google Scholar]
- 32.Rytomaa M, Kinnunen PK. Reversibility of the binding of cytochrome c to liposomes. Implications for lipid–protein interactions. J. Biol. Chem. 1995;270:3197–3202. doi: 10.1074/jbc.270.7.3197. [DOI] [PubMed] [Google Scholar]
- 33.Rytomaa M, Mustonen P, Kinnunen PK. Reversible, nonionic, and pH-dependent association of cytochrome c with cardiolipin-phosphatidylcholine liposomes. J. Biol. Chem. 1992;267:22243–22248. [PubMed] [Google Scholar]
- 34.Tuominen EK, Wallace CJ, Kinnunen PK. Phospholipid–cytochrome c interaction: evidence for the extended lipid anchorage. J. Biol. Chem. 2002;277:8822–8826. doi: 10.1074/jbc.M200056200. [DOI] [PubMed] [Google Scholar]
- 35.Uren RT, Dewson G, Bonzon C, Lithgow T, Newmeyer DD, Kluck RM. Mitochondrial release of pro-apoptotic proteins: electrostatic interactions can hold cytochrome c but not Smac/DIABLO to mitochondrial membranes. J. Biol. Chem. 2005;280:2266–2274. doi: 10.1074/jbc.M411106200. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb RA, Granville DJ. Analyzing mitochondrial changes during apoptosis. Methods. 2002;26:341–347. doi: 10.1016/S1046-2023(02)00040-3. [DOI] [PubMed] [Google Scholar]
- 37.Garlid KD, Paucek P. Mitochondrial potassium transport: the K(+) cycle. Biochim. Biophys. Acta. 2003;1606:23–41. doi: 10.1016/s0005-2728(03)00108-7. [DOI] [PubMed] [Google Scholar]
- 38.Iverson SL, Orrenius S. The cardiolipin–cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch. Biochem. Biophys. 2004;423:37–46. doi: 10.1016/j.abb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 40.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 41.Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. EMBO J;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nutt LK, Chandra J, Pataer A, Fang B, Roth JA, Swisher SG, O'Neil RG, McConkey DJ. Bax-mediated Ca2+ mobilization promotes cytochrome c release during apoptosis. J. Biol. Chem. 2002;277:20301–20308. doi: 10.1074/jbc.M201604200. [DOI] [PubMed] [Google Scholar]
- 43.Nutt LK, Pataer A, Pahler J, Fang B, Roth J, McConkey DJ, Swisher SG. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J. Biol. Chem. 2002;277:9219–9225. doi: 10.1074/jbc.M106817200. [DOI] [PubMed] [Google Scholar]
- 44.Rostovtseva TK, Tan W, Colombini M. On the role of VDAC in apoptosis: fact and fiction. J. Bioenerg. Biomembr. 2005;37:129–142. doi: 10.1007/s10863-005-6566-8. [DOI] [PubMed] [Google Scholar]
- 45.Kumarswamy R, Chandna S. Putative partners in Bax mediated cytochrome-c release: ANT, CypD, VDAC or none of them? Mitochondrion. 2009;9:1–8. doi: 10.1016/j.mito.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Rehm M, Dussmann H, Prehn JH. Real-time single cell analysis of Smac/DIABLO release during apoptosis. J. Cell Biol. 2003;162:1031–1043. doi: 10.1083/jcb.200303123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pacher P, Hajnoczky G. Propagation of the apoptotic signal by mitochondrial waves. EMBO J. 2001;20:4107–4121. doi: 10.1093/emboj/20.15.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat. Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D'Adamio L, Derks C, Dejaegere T, Pellegrini L, D'Hooge R, Scorrano L, De Strooper B. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 50.Petit PX, Lecoeur H, Zorn E, Dauguet C, Mignotte B, Gougeon ML. Alterations in mitochondrial structure and function are early events of dexamethasone-induced thymocyte apoptosis. J. Cell Biol. 1995;130:157–167. doi: 10.1083/jcb.130.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 52.Gohil VM, Gvozdenovic-Jeremic J, Schlame M, Greenberg ML. Binding of 10-N-nonyl acridine orange to cardiolipin-deficient yeast cells: implications for assay of cardiolipin. Anal. Biochem. 2005;343:350–352. doi: 10.1016/j.ab.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 53.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J. Biol. Chem. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 54.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 55.Merksamer PI, Trusina A, Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135:933–947. doi: 10.1016/j.cell.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tyurin VA, Tyurina YY, Feng W, Mnuskin A, Jiang J, Tang M, Zhang X, Zhao Q, Kochanek PM, Clark RS, Bayir H, Kagan VE. Mass-spectrometric characterization of phospholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis. J. Neurochem. 2008;107:1614–1633. doi: 10.1111/j.1471-4159.2008.05728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kagan VE, Tyurina YY, Bayir H, Chu CT, Kapralov AA, VlasovaI I, Belikova NA, Tyurin VA, Amoscato A, Epperly M, Greenberger J, Dekosky S, Shvedova AA, Jiang J. The “pro-apoptotic genies” get out of mitochondria: oxidative lipidomics and redox activity of cytochrome c/cardiolipin complexes. Chem. Biol. Interact. 2006;163:15–28. doi: 10.1016/j.cbi.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 58.Kim TH, Zhao Y, Ding WX, Shin JN, He X, Seo YW, Chen J, Rabinowich H, Amoscato AA, Yin XM. Bid-cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome C release. Mol. Biol. Cell. 2004;15:3061–3072. doi: 10.1091/mbc.E03-12-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Activation of apoptosis in vivo by a hydrocarbonstapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nobori K, Okimasu E, Sato EF, Utsumi K. Activation of protein kinase C with cardiolipin-containing liposomes in relation to membrane–protein interaction. Cell Struct. Funct. 1987;12:375–385. doi: 10.1247/csf.12.375. [DOI] [PubMed] [Google Scholar]
- 61.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzalvez F, Gottlieb E. Cardiolipin: setting the beat of apoptosis. Apoptosis. 2007;12:877–885. doi: 10.1007/s10495-007-0718-8. [DOI] [PubMed] [Google Scholar]
- 63.Epand RF, Tokarska-Schlattner M, Schlattner U, Wallimann T, Epand RM. Cardiolipin clusters and membrane domain formation induced by mitochondrial proteins. J. Mol. Biol. 2007;365:968–980. doi: 10.1016/j.jmb.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 64.Lutter M, Fang M, Luo X, Nishijima M, Xie X, Wang X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2000;2:754–761. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- 65.Lutter M, Perkins GA, Wang X. The pro-apoptotic Bcl-2 family member tBid localizes to mitochondrial contact sites. BMC Cell Biol. 2001;2:22. doi: 10.1186/1471-2121-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J. Cell Biol. 2007;178:757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.John GB, Shang Y, Li L, Renken C, Mannella CA, Selker JM, Rangell L, Bennett MJ, Zha J. The mitochondrial inner membrane protein mitofilin controls cristae morphology. Mol. Biol. Cell. 2005;16:1543–1554. doi: 10.1091/mbc.E04-08-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merkwirth C, Dargazanli S, Tatsuta T, Geimer S, Lower B, Wunderlich FT, von Kleist-Retzow JC, Waisman A, Westermann B, Langer T. Prohibitins control cell proliferation and apoptosis by regulating OPA1-dependent cristae morphogenesis in mitochondria. Genes Dev. 2008;22:476–488. doi: 10.1101/gad.460708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J. Cell Biol. 2007;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meeusen S, DeVay R, Block J, Cassidy-Stone A, Wayson S, McCaffery JM, Nunnari J. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127:383–395. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 71.Griparic L, van der Wel NN, Orozco IJ, Peters PJ, van der Bliek AM. Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J. Biol. Chem. 2004;279:18792–18798. doi: 10.1074/jbc.M400920200. [DOI] [PubMed] [Google Scholar]
- 72.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duvezin-Caubet S, Jagasia R, Wagener J, Hofmann S, Trifunovic A, Hansson A, Chomyn A, Bauer MF, Attardi G, Larsson NG, Neupert W, Reichert AS. Proteolytic processing of OPA1 links mitochondrial dysfunction to alterations in mitochondrial morphology. J. Biol. Chem. 2006;281:37972–37979. doi: 10.1074/jbc.M606059200. [DOI] [PubMed] [Google Scholar]
- 74.Misaka T, Murate M, Fujimoto K, Kubo Y. The dynamin-related mouse mitochondrial GTPase OPA1 alters the structure of the mitochondrial inner membrane when exogenously introduced into COS-7 cells. Neurosci. Res. 2006;55:123–133. doi: 10.1016/j.neures.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 75.Ishihara N, Jofuku A, Eura Y, Mihara K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem. Biophys. Res. Commun. 2003;301:891–898. doi: 10.1016/s0006-291x(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 76.Guillery O, Malka F, Landes T, Guillou E, Blackstone C, Lombes A, Belenguer P, Arnoult D, Rojo M. Metalloprotease-mediated OPA1 processing is modulated by the mitochondrial membrane potential. Biol. Cell. 2008;100:315–325. doi: 10.1042/BC20070110. [DOI] [PubMed] [Google Scholar]
- 77.Herlan M, Vogel F, Bornhovd C, Neupert W, Reichert AS. Processing of Mgm1 by the rhomboid-type protease Pcp1 is required for maintenance of mitochondrial morphology and of mitochondrial DNA. J. Biol. Chem. 2003;278:27781–27788. doi: 10.1074/jbc.M211311200. [DOI] [PubMed] [Google Scholar]
- 78.Leonhard K, Herrmann JM, Stuart RA, Mannhaupt G, Neupert W, Langer T. AAA proteases with catalytic sites on opposite membrane surfaces comprise a proteolytic system for the ATP-dependent degradation of inner membrane proteins in mitochondria. EMBO J. 1996;15:4218–4229. [PMC free article] [PubMed] [Google Scholar]
- 79.Duvezin-Caubet S, Koppen M, Wagener J, Zick M, Israel L, Bernacchia A, Jagasia R, Rugarli EI, Imhof A, Neupert W, Langer T, Reichert AS. OPA1 processing reconstituted in yeast depends on the subunit composition of the m-AAA protease in mitochondria. Mol. Biol. Cell. 2007;18:3582–3590. doi: 10.1091/mbc.E07-02-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Merkwirth C, Langer T. Prohibitin functionwithinmitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim. Biophys. Acta. 2009;1793:27–32. doi: 10.1016/j.bbamcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 81.Olichon A, Emorine LJ, Descoins E, Pelloquin L, Brichese L, Gas N, Guillou E, Delettre C, Valette A, Hamel CP, Ducommun B, Lenaers G, Belenguer P. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002;523:171–176. doi: 10.1016/s0014-5793(02)02985-x. [DOI] [PubMed] [Google Scholar]
- 82.Vogel F, Bornhovd C, Neupert W, Reichert AS. Dynamic subcompartmentalization of the mitochondrial inner membrane. J. Cell Biol. 2006;175:237–247. doi: 10.1083/jcb.200605138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kinnally KW, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis. 2007;12:857–868. doi: 10.1007/s10495-007-0722-z. [DOI] [PubMed] [Google Scholar]
- 84.Martinez-Caballero S, Dejean LM, Jonas EA, Kinnally KW. The role of the mitochondrial apoptosis induced channel MAC in cytochrome c release. J. Bioenerg. Biomembr. 2005;37:155–164. doi: 10.1007/s10863-005-6570-z. [DOI] [PubMed] [Google Scholar]
- 85.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]