Abstract
Objective
We investigated whether NADPH oxidase-dependent production of superoxide contributes to activation of NF-κB in endothelial cells by the saturated free fatty acid palmitate.
Methods and Results
Following incubation of human endothelial cells with palmitate at a concentration known to induce cellular inflammation (100 μM), we measured superoxide levels by using electron spin resonance spectroscopy and the spin trap 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH). Palmitate exposure induced a > 2-fold increase in superoxide levels, an effect associated with activation of NF-κB signaling as measured by phospho-IκBα, NF-κB activity, IL-6, and ICAM expression. Reduction in superoxide levels by each of three different interventions --- pretreatment with superoxide dismutase (SOD), diphenylene iodinium (DPI), or knockdown of NADPH oxidase 4 (NOX4) by siRNA -- attenuated palmitate-mediated NF-κB signaling. Inhibition of Toll Like Receptor-4 (TLR4) signaling also suppressed palmitate-mediated superoxide production and associated inflammation, whereas palmitate-mediated superoxide production was not affected by overexpression of a phosphorylation mutant IκBα (NF-κB super repressor) that blocks cellular inflammation downstream of IKKβ/NF-κB. Finally, high-fat feeding increased expression of NOX4 and an upstream activator, bone morphogenic protein (BMP4), in thoracic aortic tissue from C57BL/6 mice, but not in TLR4−/− mice, compared to low-fat fed controls.
Conclusions
These results suggest that NADPH oxidase-dependent superoxide production links palmitate-stimulated TLR4 activation to NF-κB signaling in endothelial cells.
Keywords: Toll like receptor 4, NADPH oxidase, endothelial cells, vascular inflammation
Introduction
Saturated FFAs such as palmitate readily induce endothelial inflammation, including increased IKKβ-NF-κB signaling, via a mechanism that involves activation of Toll-like receptors (TLR) that are key components of the innate immune system. Among the consequences of TLR4-induced activation of NF-κB is impaired vascular insulin signaling and reduced nitric oxide production.1 Based on these and other observations, elevated circulating concentrations of saturated free fatty acids (FFA) are implicated in the mechanism underlying obesity-associated inflammation and insulin resistance in endothelial cells, but the mechanism underlying this link has yet to be established.
One potential mechanism whereby exposure to saturated FFA induces cellular inflammation is via reactive oxygen species (ROS) such as superoxide (O2 •−)2 that can be generated by both mitochondrial electron transport and by cytosolic enzymes such as the NOX family of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases. These enzymes transfer electrons from NADPH across cell membranes and are a major source of cytoplasmic ROS. The electron acceptor for this reaction is oxygen, producing superoxide radicals. Of seven NOX homologues that have been identified in non-phagocytic cells, NOX4 is the major species expressed in endothelial cells, with NOX1, NOX2, and NOX5 being expressed at much lower levels. In vascular tissues of db/db mice, a genetic model of severe obesity and diabetes due to a mutation in the leptin receptor, expression of NOX1, NOX4, and p22phox (a smaller subunit which forms a stable heterodimer with NOX) are increased, as is NADPH oxidase-dependent superoxide generation, and these responses are implicated in the observed increase of inflammatory gene expression in the vasculature of these animals.3 Complementary studies using endothelial cell culture models report increased cellular NADPH oxidase levels following exposure to high levels of glucose, FFA, or insulin and are associated with increased superoxide production.4 These studies suggest that NADPH-mediated superoxide production has a central role in vascular inflammatory responses during nutritional excess.
Consistent with this hypothesis is recent evidence that NOX4 mediates lipopolysaccharide (LPS)-induced inflammatory responses in human endothelial cells.5 Since both LPS and palmitate activate IKKβ/NF-κB through TLR4 signaling, we sought to determine whether palmitate, like LPS, induces cellular inflammation via a mechanism involving NADPH oxidase-derived superoxide. In the current studies, we demonstrate that palmitate-activated TLR4 signaling results in NADPH oxidase-dependent superoxide production in endothelial cells, and that this sequence of events is required for the inflammatory response to palmitate in human endothelial cells. In addition, we report that high-fat feeding increases levels of both NOX4 mRNA and pro-inflammatory cytokines in vascular tissue from wild-type (WT) C57BL/6 mice compared to low fat-fed controls and that these effects are absent in mice lacking TLR4. Collectively, these findings suggest that TRL4-dependent activation of NADPH oxidase is necessary for the pro-inflammatory effects of palmitate in vascular tissue, and that NADPH oxidase- generated superoxide plays a key role to couple TLR4 activation to cellular signaling via NF-κB.
Methods
Materials
Anti-phospho-IκBα, anti-IκBα, anti-TLR4 antibodies were obtained from Cell Signaling (Beverly, MA); anti-BMP4, anti-NOX4 antibodies were obtained from Abcam Inc. (Cambridge, MA); anti-GAPDH and anti-NOX2 antibodies from Santa Cruz Biotechnology (Santa Cruz, CA) and anti-ICAM antibody, Human IL-6 ELISA kits from R and D Systems (Minneapolis, MN), and TransAM NF-kB p65 kit Active Motif (Carlsbad, CA). The spin trap 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH) was purchased from Alexis Biochemical (Lausen, Switzerland).
Superoxide dismutase (PEG-SOD), Diphenylene idonium (DPI), and LPS were obtained from Sigma-Aldrich. Dihydroethidium (DHE) was purchased for (Molecular Probes/Invitrogen, Eugene Oregon). Palmitic (C 16:0) fatty acids were obtained from Alltech Associates, Inc. (Deerfield, Illinois) and BSA (FFA-free) was purchased from Roche (Indianapolis, In). FFA were dissolved in 0.1 M NaOH at 70°C and then complexed with 10% BSA at 55°C for 10 min to achieve a final palmitate concentration of 100 μM as described previously6. Stock solutions of 5 mM FFA with 10% BSA and 10%BSA control solutions were prepared 1 d prior to experiments. Palmitate preparations were assessed for LPS contamination using Amebocyte Lysate Test (Biowhittaker).
Cell Culture
Human microvascular endothelial cells (HMEC) were purchased from (Invitrogen-Cascade Biological) and were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT) and 12 μg/ml of bovine brain extract (Clonetics, Walkersville, MD), L-glutamine (2 mM), sodium pyruvate (1 mM) and nonessential amino acids in the presence of penicillin (100 units/ml) and maintained at 37°C in 5% CO2. All Western bots were performed as described7, using equal amounts of total protein for each condition and experiment. SDS gel electrophoresis was performed using a 4% by 20% gradient gel. Generation of stably-transfected HMEC with a dominant negative myeloid differentiation factor-88 (DN-MyD88), interleukin-1 receptor-associated kinase (DN-IRAK), and phosphorylation-resistant mutant of IκBα that blocks NF-κB activation (NF-κB super repressor) was as previously described.8–10 The Silencer™ SiRNA Transfection Kit II (Ambion) was used to reduce expression of genes encoding TLR4, NOX4, NOX5. Silencer™ Negative Control siRNA was used as the scrambled control.
Total RNA extraction, quantitative RT_PCR
Thoracic aortic tissues from experiments described previously were used1. Briefly both C57BL6 and TLR4−/− mice were fed either a low fat or high fat diet for 8 wk after which thoracic aorta tissue were collected. Thoracic aortic tissue total RNA was extracted using RNeasy Mini Kit (Qiagen) and Mouse NOX4, NOX2, NOX1 and BMP4 primer pairs were purchased from Applied Biosystems.
Superoxide measurement
Endothelial superoxide radical was measured at room temperature by each of two different methods. The first of these employed electron spin resonance spectroscopy (ESR) using the spin trap (CMH)11–13. HMEC were processed by washing once with ice cold PBS and removed by scrapping. After centrifugation the cells were resuspended in Krebs-HEPES buffer and 0.1 mM diethylenetriamine-penta-acetic acid (DTPA) was used to inhibit iron-catalyzed oxidation of the CMH trap. Electron spin resonance spectroscopy (ESR) studies were performed on a table-top x-band spectrometer Miniscope (Magnettech, Germany). Recordings were made at room temperature using a small capillary tube. Instrument settings were biofield 3350, Sweep 60G, Microwave frequency 9.78 Ghz, microwave power 20 mW, and kinetic time of 10 min.
Superoxide measurement with dihydroethidium (DHE) was employed to provide a second, complementary approach to measure endothelial superoxide production. HMEC were grown on coverslips coated with 2% gelatin grown to 80% confluency and treated with palmitate (100 μM) for 2.5 h in phenol-red-free EBM. After the cells were incubated with DHE (2 μM) for 30 min, the following procedures were carried out in the dark: cells were washed twice with ice-cold PBS, fixed on ice with 2% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, PA) for 3 min and again washed twice with cold PBS. Cover slips were mounted onto slides with Gel Mount (Sigma, St. Louis, MO). Superoxide production was measured using an Olympus fluorescence microscope using a TRITC filter (excitation 528–553 nM, emission 600–660 nM). Fluorescence intensity of these images was quantified using Image J software (NIH). The mean fluorescent intensity of approximately 80–100 cells was measured and expressed as fluorescence arbitrary units.
Statistics
In all experiments, densitometry/ELISA measurements were normalized to controls incubated with vehicle and percent change relative to the control condition was calculated. Analysis of the results was performed using the STATA8 statistical package. Data are expressed as mean ± SEM, and values of p<0.05 were considered statistically significant. A two-tailed t-test was used to compare mean values from studies involving two experimental groups. To compare responses following treatment with BSA or BSA-palmitate across time points, during the use of different inhibitors of superoxide or changes in TLR4 signaling, data were analyzed by two-way analysis of variance using the Bonferoni-post-hoc comparison test.
Results
Effect of palmitate on superoxide production and NF-κB signaling in endothelial cells
Lipopolysaccharide is a known activator of NF-κB in most cell types and previous investigators have demonstrated that LPS treatment increases superoxide generation as measured by the dye, 2,7 dichlorofluorescein-diacetate (DCF-DA).5 To validate the ESR method of superoxide detection, HMEC were treated with LPS (5 ng/ml) for 1 h, followed by measurement of superoxide using the spin trap CMH. Consistent with previous results5, LPS increased superoxide production by 2.2 ± 0.4-fold (p < 0.05) compared to BSA-treated HMEC.
To extend our previous observation that incubation of endothelial cells with palmitate-BSA for 3 h increases phospho-IκBα protein levels (a marker of IKKβ-NF-κB signaling),14 we examined the effect of increasing palmitate-BSA concentrations (10–200 μM) on production of both superoxide and the NF-κB-dependent cytokine, IL-6. Palmitate-BSA increased both IL-6 and superoxide levels dose-dependently, with concentrations of 100 μM inducing a maximal > 4-fold increase in IL-6 expression (Figure 1A) and a 2-fold increase in superoxide production (Figure 1G). Analysis of the time course of the effect of palmitate (100 μM) on both cellular markers of NF-κB activation (IκBα phosphorylation, IκBα protein levels, NF-κB activity, IL-6 production, ICAM protein) and superoxide (O2 •−) production in HMEC showed that the latter is significantly elevated before IκBα is phosphorylated (Figure 1B, H), consistent with a model in which superoxide mediates IKKβ activation. Both IL-6 and ICAM protein levels also increased in response to palmitate, with increased IL-6 expression preceding the change of ICAM protein (Figure 1E–F). The effect of palmitate to increase superoxide production was confirmed using an independent method based on the fluorescent dye DHE (Figure 1I).
Figure 1. Time course of palmitate-dependent activation of NF-κB signaling and superoxide prodution.
A. IL-6 as determined by ELISA in HMEC treated with increasing concentrations of palmitate-BSA (0–200 μM) for 3 h. B–F. Measures of NF-κB signaling (phospho-IκBα, IκBα, NF-κB, IL-6, ICAM) in HMEC in response to 100 μM palmitate for time periods between 0–6 h. G–H. Superoxide levels as measured by ESR using the spin trap CMH in response to increasing concentrations of palmitate and for time period of 0–6 h using 100 μM of palmitate. I. DHE florescence in response to 3 h of palmitate-BSA (100 μM) or BSA control. * p<0.05 BSA vs palmitate-BSA, for each set of experiments n=(3–4).
Mechanisms linking palmitate-induced superoxide production to activation of endothelial NF-κB signaling
To determine whether superoxide is necessary for the effect of palmitate to increase phospho-IκBα and IL-6, HMEC were pre-treated with two different inhibitors of superoxide production, superoxide dismutase (SOD) and diphenyleneiodonium (DPI) which inhibits flavin-containing oxidases such as NADPH oxidase. At doses between 100–300 units, SOD blocked the effect of palmitate to increase levels of both phospho-IκBα (Figure 2A) and IL-6 (Figure 2D). As expected, palmitate-induced superoxide production in HMEC was also inhibited by SOD (100 units) pre-treatment (Figure 2G). Similarly, pre-treatment with DPI (25 μM) for 1 h blocked the stimulatory effect of palmitate on both phospho-IκBα and IL-6 production (Figure 2B, E), while also preventing palmitate-mediated superoxide production as measured by both ESR and the spin trap CMH (Figure 2H). These results collectively suggest that superoxide generation is required for palmitate-induced activation of IKKβ-NF-κB in endothelial cells.
Figure 2. Effect of superoxide inhibition on palmitate-dependent activation of endothelial cell NF-κB signaling.
HMEC were pretreated with either of two inhibitors of superoxide -- SOD (100, 200, or 300 Units/ml) or DPI 25 μM -- or they were transduced with siRNA to NOX4, NOX5, or with a negative scrambled siRNA (Scb). HMEC were then treated with 100 μM palmitate for 3 h. A,B,C. Phospho-IκBα Western blot representative of 3 independent experiments (C-BSA control, F-palmitate, T-TNF-α). GAPDH protein levels were used as a loading control. D,E,F. IL-6 levels as measured by ELISA. *, p<0.05, n=3. G,H,I. Fold increase in superoxide production in response to palmitate as measured by ESR and spin trap CMH, * p<0.05, (n=3).
Since NOX4 is the most abundant isoform of NADPH oxidase in vascular tissue, we hypothesized that palmitate-mediated activation of NF-κB and superoxide production are both NOX4-dependent. To test this hypothesis, we selectively reduced expression of either NOX4 or NOX5 (as a control, since NOX5 is not highly expressed in endothelial cells) in HMEC using siRNA. As expected, treatment with NOX4 siRNA, but not NOX5 siRNA, reduced NOX4 protein levels (Figure 2C). Moreover, palmitate-mediated increases of phospho-IκBα, IL-6 and superoxide production in HMEC were reduced following NOX4 siRNA treatment, whereas neither siRNA targeting NOX5 nor scrambled control siRNA had measurable effects (Figure 2C, 2F, 2H). Reduction of endothelial NOX2 protein by siRNA had no significant effect on palmitate-mediated increases in phospho-IκBα protein or on palmitate-mediated superoxide production as measured by ESR and the spin trap CMH (see supplementary data, Figure 1). These results demonstrate that endothelial NOX4 is necessary for palmitate-mediated superoxide generation and subsequent activation of inflammatory signaling in HMEC.
Role of TLR4/MyD88/IRAK signaling in palmitate-mediated superoxide production
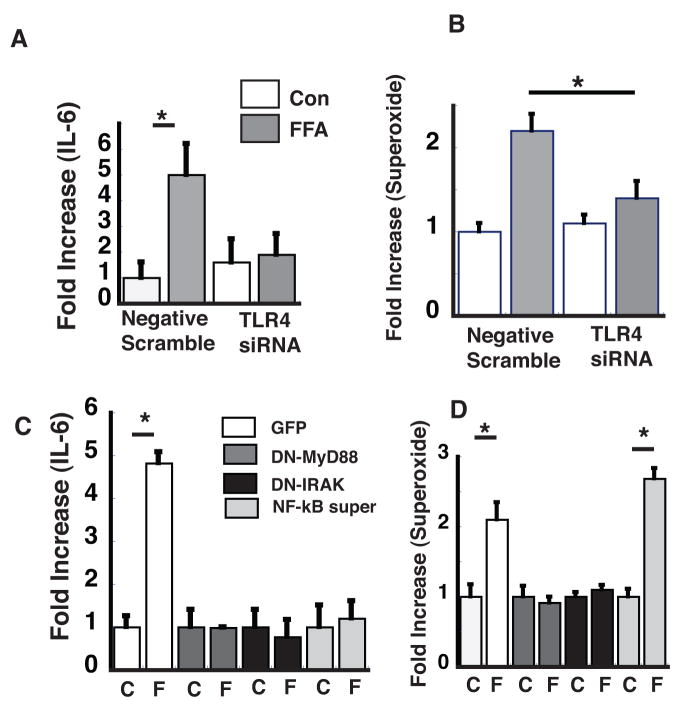
We have previously shown that in endothelial cells, palmitate-mediated activation of IKKβ is dependent on TLR4.1 To determine whether TLR4 also lies upstream of superoxide generation in response to palmitate, we determined whether the latter response is blocked by inhibition of TLR4 signaling. This was accomplished using siRNA to reduce TLR4 expression or with dominant negative constructs of MyD88 (DN-MyD88) and IRAK (DN-IRAK), previously shown to inhibit palmitate-dependent increases in NF-κB signaling.1 We found that reduction of TLR4 protein expression using siRNA prevented palmitate-mediated increases of both IL-6 production and superoxide production (Figure 3A, B). Similar results were obtained when HMEC were transfected with viral constructs expressing (DN-IRAK) or (DN-MyD88), which inhibit signal transduction downstream of TLR4 (Figure 3C, D), whereas transducing HMEC with NF-κB super-repressor to inhibit NF-κB activation1 (which inhibits palmitate-mediated increases of IL-6) had no effect on the increase of superoxide production induced by palmitate. Taken together, these findings suggest that in response to palmitate, superoxide generation occurs downstream of TLR4/MyD88/IRAK signaling but upstream of NF-κB signaling.
Figure 3. Effect of TLR4 signaling on palmitate-mediated superoxide production.
A. HMEC were transduced with siRNA to TLR4 or a scrambled siRNA (con) and then treated with palmitate (100 μM) for 3h (FFA). IL-6 was measured (to assess NF-κB signaling) by ELISA and B. superoxide production was measured by ESR and the spin trap CMH. C. HMEC were transduced with retrovirus expressing GFP, DN-MyD88, DN-IRAK, or phosphorylation resistant IκBα (NF-κB super repressor). Following treatment with palmitate-BSA (F) or BSA alone (C), IL-6 and D. superoxide was measured and fold increase from control was calculated. * p<0.05, n=3.
Role of TLR4 signaling in the induction of BMP4 and NOX4 by palmitate or HF-feeding
Bone morphogenetic protein 4 (BMP4) is reported to induce and activate NADPH oxidase in endothelial cells, and administration of BMP4 to mice induces hypertension and endothelial dysfunction via a mechanism that is NADPH oxidase-dependent15. We therefore investigated in HMEC 1) whether incubation in palmitate increases both BMP4 and NOX4 protein levels, and 2) if this effect is dependent on TLR4. Both NOX4 and BMP4 expression increased within 1 h of treatment with 100 μM palmitate, and inhibition of TLR4 signaling by siRNA attenuated both responses (Figure 4A, B). By comparison, reduction of NOX4 protein levels by NOX4 siRNA, had no effect on the palmitate-induced increase of BMP4 protein levels (Figure 4B), suggesting that TLR4 is upstream of both BMP4 and NOX4.
Figure 4. Effect of palmitate or HF-feeding on vascular BMP4 and NOX4 levels.
HMEC were treated with palmitate (100 μM) for time period between 0–6 h and 3 h in the presence or absence siRNA to TLR4. A. Western blot using anti-NOX4 antibody. Control (C), BSA alone, (F) palmitate-BSA. B. Same lysates were probed with anti-BMP4 antibody including addition of HMEC treated with NOX4 siRNA. Fold increase was normalized to GAPDH levels determined by Western blot (not shown) C. RNA was harvested from thoracic aortic from C57BL6 or TLR4 −/− mice fed either a low fat (10% saturated fat) or high fat (60% saturated fat) diet for 8 wk and mRNA content was assessed by quantitative PCR. Fold increase over LF group was calculated for BMP4, NOX4, NOX1 and NOX2. * p<0.05 (n=5 for each condition).
We next asked whether obesity induced by a high-fat diet in C57BL6 mice is associated with increased vascular expression of either BMP4 or NOX4 and whether this increase is dependent on TLR4 signaling. We examined stored thoracic mRNA samples from a previously described study1 in which C57BL6 (WT) and TLR4−/− mice were fed either a HF or a LF diet for 8 wk. In response to HF feeding, both WT and TLR4−/− mice displayed similar increases of body weight, body fat content, and serum insulin and free fatty acids (FFA) levels compared to mice fed a LF diet, and levels of phospho-IκBα, ICAM, and IL-6 (markers of vascular NF-κB signaling) were each increased in thoracic aorta extracts from WT mice but not from mice lacking TLR4 on the same diet.1 In WT mice fed a HF diet, levels of both BMP4 and NOX4 mRNA were also increased in thoracic aorta (as measured by quantitative PCR) compared to low-fat fed WT mice. By comparison, HF feeding did not increase NOX4 or BMP4 expression in TLR4 −/− mice, suggesting that TLR4 is necessary for induction of NOX4 in vascular tissue in this setting (Figure 4C). In contrast, HF-feeding was not associated with increased NOX1 or NOX2 expression in either WT or TLR4 −/− mice (Figure 4C), suggesting that the effect of HF feeding on the vasculature is specific to NOX4 expression in comparison to other NOX isoforms. HF-feeding was also associated with increased aortic NOX4 protein expression compared to the LF-fed group (see Supplementary Figure 2).
Discussion
In both cultured endothelial cells and vascular tissue in vivo, nutritional excess rapidly induces cellular inflammation at the level of NF-κB signaling. Growing evidence implicates vascular inflammation as a key mechanism linking cardiovascular disease to obesity and related metabolic disorders,16–18 and we have recently shown that during HF feeding, inflammation and insulin resistance develop in the vasculature of mice well before these changes are observed in liver, skeletal muscle or fat.19 The steady increase in prevalence of obesity and its metabolic sequelae20 heightens the need for an improved understanding of how nutrient excess affects the vasculature. In light of evidence of a key role for TLR4 signaling in the stimulation of inflammatory signaling in palmitate-treated endothelial cells,1 the present studies were undertaken to delineate the pathways linking TLR4 activation to the activation of IKKβ/NF-κB signaling in vascular tissue. We demonstrate in cultured human endothelial cells that superoxide production is required for palmitate to induce NF-κB signaling, and further, that palmitate increases superoxide production by activating NADPH oxidase through the TLR4/MyD88/IRAK pathway. Finally, in a mouse model of DIO and insulin resistance, expression of both BMP4 (which induces NADPH oxidase) and NOX4 (the predominant NADPH oxidase isoform in endothelial cells) increases in response to 8 wk of high fat feeding in thoracic aorta of C57BL6 mice, but not TLR4−/− mice, despite no differences in weight or adiposity. These results collectively suggest that the mechanism whereby palmitate-induced TLR4 signaling activates NF-κB in endothelial cells is dependent on NADPH oxidase-generated superoxide.
Palmitic, oleic, and linoleic acids constitute up to 70% of circulating FFA in humans, with each typically being present in concentrations between 10–50 μM.21, 22 Although the current cell culture studies relied upon concentrations of palmitic acid (100 μM) higher than those usually found in humans, lower concentrations of palmitic/BSA (50 μM) also increase endothelial levels of IL-6. Thus, the responses investigated in this paper can be elicited by palmitate at levels present in the circulation of normal humans, which reinforces the clinical relevance of these studies. Because long-term exposure to higher concentrations of palmitate (e.g., 100–300 μM for 24h) can trigger apoptosis in human endothelial cells, 23 we chose conditions that do not induce this effect.
Superoxide is an important participant in redox cell signaling and, when present in excess, is implicated in the development of vascular diseases such as hypertension and atherosclerosis. Superoxide, along with other ROS, is implicated in the activation of redox-sensitive transcription factors such as NF-κB,24 and also regulates inflammatory responses such as leukocyte adhesion25, 26 and induction of monocyte chemoattractant protein (MCP-1).27 Since both pharmacological and genetic interventions that reduce ROS also attenuate TNF-α-mediated activation of endothelial inflammatory signaling, these mediators appear to contribute to vascular inflammation in at least some conditions.
Interest in the role of ROS in cellular inflammation has grown with accumulating evidence that this mechanism may contribute to the effect of nutrient excess to cause insulin resistance in muscle, liver, fat and vascular tissue.1, 28 In a recent study, San Martin, et al., reported that NOX1, NOX4, p22phox mRNA levels and vascular superoxide production (measured by dihydroethidium fluorescence) are each increased in the aorta of db/db mice, a genetic model of obesity and diabetes. These findings extend our previous work in a murine DIO model showing that vascular inflammatory markers such as ICAM, VCAM, SOCS3, and IL-6 also increase in response to HF feeding.1, 19 Combined with the present findings of increased vascular expression of NOX4 and BMP4 in the aorta of DIO mice, these results support the hypothesis of a cause-and-effect relationship between ROS signaling and vascular inflammation at the level of NF-κB signaling. As TLR4−/− mice fed a high-fat diet are both protected from the development of vascular inflammation and fail to induce vascular NOX4/BMP4, we propose that HF feeding triggers vascular inflammation via a sequence of events that includes TLR4 activation and superoxide generation.
A potential link between LPS signaling, which like palmitate activates TLR4, and NADPH oxidase has been described in cultured phagocytic cells, in which LPS treatment leads to an NADPH-dependent oxidative burst 29. LPS also increases NADPH-dependent superoxide production, as measured by DCF fluorescence, in human endothelial5 and immune cells.30 Our current work extends these findings by demonstrating that TLR4 signaling is necessary for palmitate-mediated superoxide production in endothelial cells. Although Park and et al.5, demonstrated a direct interaction between TLR4 receptor and NADPH oxidase in endothelial cells, recent studies in immune cells suggest that IRAK4 may mediate the effect of TLR4 to activate NADPH oxidase.15 Thus, although growing evidence indicates that TLR4 activation induces NADPH oxidase in both endothelial and immune cells, the mechanisms underlying this effect remain incompletely defined and may differ between cell types.
Whereas LPS treatment leads to a rapid (<1 h) activation of TLR4/MyD88/IRAK signaling, our studies show that palmitate-dependent activation of IκBα signaling develops more slowly (3 h), suggesting that inflammation induced by LPS and palmitate may involve distinct mechanisms. One potential explanation for the slower onset of palmitate-mediated effects is that unlike LPS, palmitate does not bind the TLR4 receptor, but rather induces changes the composition of lipid rafts. Lipid rafts are dynamic assemblies of sphingolipids and cholesterol that are implicated as key components of cell surface signaling through their effect to physically concentrate receptors, downstream kinases, and adaptor proteins involved in signaling pathways. In macrophages and other immune cells, assembly of TLR4 and its accessory proteins (CD14, MD2) in lipid rafts is essential for LPS-mediated activation of NF-κB signaling.31, 32 Whether palmitate affects lipid raft formation or composition in endothelial cells, and in this way induces vascular inflammation, remains to be investigated.
The source of vascular superoxide generation during diabetes also remains incompletely understood. Several studies suggest that hyperglycemia increases ROS production via stimulation of mitochondrial respiration 33. Since, by comparison, specific inhibition of NADPH oxidase attenuated palmitate-induced superoxide generation in HMEC, our findings suggest a critical role for NADPH oxidase in this response. The present studies, however, do not exclude the mitochondria as a source of superoxide production in the presence of excess palmitate.
In summary, we conclude that endothelial inflammation induced by the saturated FFA palmitate is dependent on NADPH oxidase-generated superoxide and that the TLR4 signaling pathway connects palmitate to NADPH oxidase activation. Further in vivo studies are necessary to determine whether vascular dysfunction in DIO and other nutrient excess states is similarly dependent on endothelial NADPH oxidase activity.
Supplementary Material
Acknowledgments
This study was supported by NIH grants DK073878 (FK), U54 CA116847 (FK, MWS, IS, DMH), DK52989 and DK68384 (MWS)
Footnotes
Disclosure: none
References
- 1.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 2.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 3.San Martin A, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;292:H2073–2082. doi: 10.1152/ajpheart.00943.2006. [DOI] [PubMed] [Google Scholar]
- 4.Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 5.Park HS, Chun JN, Jung HY, Choi C, Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res. 2006;72:447–455. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Cousin SP, Hugl SR, Wrede CE, Kajio H, Myers MG, Jr, Rhodes CJ. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology. 2001;142:229–240. doi: 10.1210/endo.142.1.7863. [DOI] [PubMed] [Google Scholar]
- 7.Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR, Figeys D, Harrison DG, Berk BC, Aebersold R, Corson MA. Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem. 1999;274:30101–30108. doi: 10.1074/jbc.274.42.30101. [DOI] [PubMed] [Google Scholar]
- 8.Bannerman DD, Erwert RD, Winn RK, Harlan JM. TIRAP mediates endotoxin-induced NF-kappaB activation and apoptosis in endothelial cells. Biochem Biophys Res Commun. 2002;295:157–162. doi: 10.1016/s0006-291x(02)00638-1. [DOI] [PubMed] [Google Scholar]
- 9.Bannerman DD, Tupper JC, Erwert RD, Winn RK, Harlan JM. Divergence of bacterial lipopolysaccharide pro-apoptotic signaling downstream of IRAK-1. J Biol Chem. 2002;277:8048–8053. doi: 10.1074/jbc.M111249200. [DOI] [PubMed] [Google Scholar]
- 10.Ferri N, Garton KJ, Raines EW. An NF-kappaB-dependent transcriptional program is required for collagen remodeling by human smooth muscle cells. J Biol Chem. 2003;278:19757–19764. doi: 10.1074/jbc.M212714200. [DOI] [PubMed] [Google Scholar]
- 11.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dikalov SI, Li W, Mehranpour P, Wang SS, Zafari AM. Production of extracellular superoxide by human lymphoblast cell lines: comparison of electron spin resonance techniques and cytochrome C reduction assay. Biochem Pharmacol. 2007;73:972–980. doi: 10.1016/j.bcp.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKbeta. Arterioscler Thromb Vasc Biol. 2005;25:989–994. doi: 10.1161/01.ATV.0000160549.60980.a8. [DOI] [PubMed] [Google Scholar]
- 15.Miriyala S, Gongora Nieto MC, Mingone C, Smith D, Dikalov S, Harrison DG, Jo H. Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation. 2006;113:2818–2825. doi: 10.1161/CIRCULATIONAHA.106.611822. [DOI] [PubMed] [Google Scholar]
- 16.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 17.Williams IL, Wheatcroft SB, Shah AM, Kearney MT. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord. 2002;26:754–764. doi: 10.1038/sj.ijo.0801995. [DOI] [PubMed] [Google Scholar]
- 18.Yki-Jarvinen H. Insulin resistance and endothelial dysfunction. Best Pract Res Clin Endocrinol Metab. 2003;17:411–430. doi: 10.1016/s1521-690x(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 19.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mokdad AH, Serdula MK, Dietz WH, Bowman BA, Marks JS, Koplan JP. The spread of the obesity epidemic in the United States, 1991–1998. Jama. 1999;282:1519–1522. doi: 10.1001/jama.282.16.1519. [DOI] [PubMed] [Google Scholar]
- 21.Yli-Jama P, Meyer HE, Ringstad J, Pedersen JI. Serum free fatty acid pattern and risk of myocardial infarction: a case-control study. J Intern Med. 2002;251:19–28. doi: 10.1046/j.1365-2796.2002.00922.x. [DOI] [PubMed] [Google Scholar]
- 22.Knopp RH, Retzlaff B, Walden C, Fish B, Buck B, McCann B. One-year effects of increasingly fat-restricted, carbohydrate-enriched diets on lipoprotein levels in free-living subjects. Proc Soc Exp Biol Med. 2000;225:191–199. doi: 10.1046/j.1525-1373.2000.22524.x. [DOI] [PubMed] [Google Scholar]
- 23.Artwohl M, Roden M, Waldhausl W, Freudenthaler A, Baumgartner-Parzer SM. Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. Faseb J. 2004;18:146–148. doi: 10.1096/fj.03-0301fje. [DOI] [PubMed] [Google Scholar]
- 24.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 26.Chen XL, Zhang Q, Zhao R, Ding X, Tummala PE, Medford RM. Rac1 and superoxide are required for the expression of cell adhesion molecules induced by tumor necrosis factor-alpha in endothelial cells. J Pharmacol Exp Ther. 2003;305:573–580. doi: 10.1124/jpet.102.047894. [DOI] [PubMed] [Google Scholar]
- 27.Chen XL, Zhang Q, Zhao R, Medford RM. Superoxide, H2O2, and iron are required for TNF-alpha-induced MCP-1 gene expression in endothelial cells: role of Rac1 and NADPH oxidase. Am J Physiol Heart Circ Physiol. 2004;286:H1001–1007. doi: 10.1152/ajpheart.00716.2003. [DOI] [PubMed] [Google Scholar]
- 28.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remer KA, Brcic M, Jungi TW. Toll-like receptor-4 is involved in eliciting an LPS-induced oxidative burst in neutrophils. Immunol Lett. 2003;85:75–80. doi: 10.1016/s0165-2478(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 30.Ryan KA, Smith MF, Jr, Sanders MK, Ernst PB. Reactive oxygen and nitrogen species differentially regulate Toll-like receptor 4-mediated activation of NF-kappa B and interleukin-8 expression. Infect Immun. 2004;72:2123–2130. doi: 10.1128/IAI.72.4.2123-2130.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez JA, del Conde I, Shrimpton CN. Receptors, rafts, and microvesicles in thrombosis and inflammation. J Thromb Haemost. 2005;3:1737–1744. doi: 10.1111/j.1538-7836.2005.01463.x. [DOI] [PubMed] [Google Scholar]
- 32.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.