Abstract
All cells release nucleotides and are in one way or another involved in local autocrine and paracrine regulation of organ function via stimulation of purinergic receptors. Significant technical advances have been made in recent years to quantify more precisely resting and stimulated adenosine triphosphate (ATP) concentrations in close proximity to the plasma membrane. These technical advances are reviewed here. However, the mechanisms by which cells release ATP continue to be enigmatic. The current state of knowledge on different suggested mechanisms is also reviewed. Current evidence suggests that two separate regulated modes of ATP release co-exist in non-excitable cells: (1) a conductive pore which in several systems has been found to be the channel pannexin 1 and (2) vesicular release. Modes of stimulation of ATP release are reviewed and indicate that both subtle mechanical stimulation and agonist-triggered release play pivotal roles. The mechano-sensor for ATP release is not yet defined.
Keywords: P2 receptor, Mechano-sensation, Exocytosis, Epithelia, ATP secretion, Biosensor, Luciferase
Introduction
The research field concerning purinergic signalling has stepped out of its infancy, and purines and their receptors are now widely accepted as an important local communication system within the body. Recent breakthroughs in this field include the role of adenosine triphosphate (ATP) as neurotransmitter and/or modulator in sensory transduction [1–10], the role of released ATP as a precursor signalling molecule in renal tubulo-glomerular feedback [11, 12], the role of released nucleotides for migrating neutrophils [13] and the crucial function of nucleotides in the control of thrombocyte aggregation and haemostasis [14]. The essential features of the purinergic signalling system are well characterised. The large family of G protein-coupled P2Y and ionotropic P2X receptors, their relevant agonists and the growing field of ecto-ATPases for the termination of the nucleotide signals are all defined.
In numerous, if not all, organ and cell systems, the purinergic system has been recognised as a local auto- and paracrine signalling network for intercellular communication. Experimental activity crossing numerous research discipline borders is rapidly extending our physiological understanding of regulated organ functions. However, one major gap prohibits us from truly appreciating the physiological and patho-physiological impact of the puringeric signalling system. This gap is our limited understanding of the pathway(s) of nucleotide release. Apparently, the source of extracellular ATP is the large pool of cytosolic ATP. In classically secreting cells like neurons and neuro-endocrine cells, ample evidence indicates that ATP release occurs via exocytosis [2, 15–17]. Cells of non-neuronal origin like epithelia, endothelial cells or astrocytes can also be stimulated to release nucleotides. The mechanism of nucleotide release from these types of cells is currently not sufficiently understood. An extensive list of mechanisms has been proposed as the general pathway for nucleotide release. The main intention of this review is to update the current state of knowledge on mechanism(s) of nucleotide release from non-neuronal or non-neuro-endocrine cells. The interested reader is also directed to previous review articles which have covered many relevant aspects of cellular nucleotide release [15, 18–21]. Some of the uncertainty in this field is a consequence of a limited technical repertoire to measure rapidly and precisely the concentrations of nucleotides near the plasma membrane. Therefore, this review will also describe and explain the basic principles of the different methodological approaches used so far to detect and quantify extracellular ATP. Standard biochemical methods for measuring ATP are not covered.
Methods for ATP measurements with biosensors
The principle of ATP biosensors exploits the binding or reacting property of ATP to biological molecules/receptors and a secondary signal coupled to this binding process. The biosensor can either be an ATP-dependent enzymatic reaction with subsequent measurement of the product development, an entire cell with its ATP receptors or an excised membrane patch containing ATP receptors. These three principles will be described in detail below.
The luciferin–luciferase technique
The standard technique to measure extracellular ATP in bulk solutions uses the bioluminescence of the ATP-dependent luciferase-mediated oxidation of luciferin [22]. This is a straightforward and robust method using commercially available luminometers to prove the presence of ATP in a given solution, with or without cells. Other relevant nucleotide signalling molecules like uridine triphosphate (UTP), uridine diphosphate (UDP) or adenosine diphosphate (ADP) are not detected. It is exquisitely sensitive, shows fast response times (milliseconds) and a broad linear detection range and with careful optimisation allows the detection of ATP concentrations down to the femtomolar range. There are, however, many factors that interfere with the luciferase activity. The enzymatic action has its optimum at room temperature (20°C), pH 7.8 and requires Mg2+ [23]. It is significantly inhibited by various anions (e.g. Br−, I−, NO−3, etc.) [24] and high salt concentrations in general [25]. The anion transport inhibitor DIDS [26], the cation channel blocker gadolinium [27] or P2 receptor antagonist suramin [28] inhibits the luciferase reaction. All these characteristics have proven to be significant obstacles in the characterisation of the ATP release pathway(s). In cells triggered to release nucleotides, bulk extracellular ATP concentrations are commonly found to lie in the nanomolar range. Table 1 summarises some examples of extracellular ATP measurements in bulk solutions. Evidently, this method may not be very suitable to measure the ‘true’ ATP concentrations that a cell may encounter near the plasma membrane and does not allow the disclosure of kinetic information on ATP release because the released ATP is diluted into the relatively large surrounding fluid compartment. It needs to be appreciated that the extracellular space surrounding a cell in the native setting is very small. This contrasts with most commonly used experimental settings of cultured cells or freshly isolated tissues in which an enormous, non-physiological extracellular space is necessary to maintain homeostatic control of the experiment. Attempts to reduce the size of the extracellular fluid compartment have proven successful and show, e.g. in airway cell surface liquid layer that the hypotonicity-stimulated ATP concentration increase can be as high as 614 nM [29]. Despite this essential shortcoming, this method supplies a necessary fundamental observation because it proves the molecular identity of ATP.
Table 1.
Quantification of extracellular ATP concentrations in bulk solutions and assayed with near-to-plasma membrane sensors
| Cell/tissue | Stimulus | Resting bulk ATP concentration | Resting near-membrane ATP concentration | Bulk ATP concentration increase | Stimulated near-membrane peak ATP concentration | Reference |
|---|---|---|---|---|---|---|
| Taste bud-containing epithelium (m) | Bitter taste | 0 nM | 1 nM | [3] | ||
| Airway epithelium h, Calu-3) | Hypotonic swelling (33%) | 7 nM | 34 nM | [29] | ||
| Astrocytes 132 1N1 (h) | Thrombin, carbachol | 1 nM | 2–4 nM | [30] | ||
| Bone cells (m) | Fluid flow stress | ~1 nM | 34 nM | [85] | ||
| Retina (r) | Cell poking | 78 µM | [35] | |||
| Thrombocytes | Thrombin | 15−20 µM | [31] | |||
| Astrocytes 132 1N1 (h) | Thrombin, carbachol | 1 nM | With ecto-ATPase inhibition 150–550 nM | [30] | ||
| Bronchial epithelial cells (h) | Hypotonic swelling | 5 nM | >1 µM | [32] | ||
| HEK 293 (h) | Bz-ATP | 80 nM | 250 µM | [34] |
h human, m mouse, r rat
Luciferase tagged to the plasma membrane
As indicated, measurements of ATP in bulk solutions do not provide sufficient information about the concentrations surrounding the purinergic receptors. In fact, measurements of ATP in bulk solution often provide no meaningful results unless ecto-ATPases are inhibited [30]. This problem has been tackled by binding firefly luciferase to surface proteins. In this way, the detecting enzyme is placed in close proximity to the plasma membrane [31, 32]. The technique exploits the strong protein binding property of bacterial protein A to IgG immunoglobulins. A protein A luciferase chimaera can be stably adsorbed onto the surface of intact cells via the binding of primary IgG antibodies directed against native surface antigens. This technique allows the detection of ten to 20 times higher peak concentrations of ATP. Thus, it has been possible to obtain a much more accurate estimate of the ATP concentrations near the plasma membrane [30, 31]. It has also provided convincing evidence that the actual concentrations of extracellular ATP during stimulation lie well within the range of nucleotide affinities of P2 receptors. Refinement of the isolation and purification procedure of the protein A chimaeric luciferase yielded a detecting enzyme with the same biological activity as the commercially available luciferase. This has significantly improved the method and allowed for robust measurements of resting and stimulated apical extracellular ATP concentrations in airway epithelial cells [32]. In addition, this study also demonstrated that reduction of the extracellular space to a minimum makes it possible to use soluble luciferase to measure the same physiological concentrations of ATP as those detected with the tagged luciferase [32]. A follow-up approach used genetically engineered biotin-linked luciferase, which was membrane-tagged via the biotin–streptavidin method [33].
Yet another approach was to create a GPI-anchored luciferase (pmeLUC), which can be stably transfected into target cells [34]. However, this luciferase construct displays low ATP sensitivity and only detects extracellular ATP values above 10 µM. Co-expression of pmeLUC with P2X7 receptors indicated very high extracellular ATP concentrations of around 250 µM when the cells were stimulated with Bz-ATP [34]. Such high values have never been seen in other studies. The values obtained with the other tethered luciferase approaches lie two orders of magnitude lower [30–32].
Imaging of ATP release
The luciferin/luciferase technique has been used successfully to visualise ATP release [23, 25, 26, 35, 36]. In this approach, the cells (or tissue) are bathed with the ATP-specific luminescence indicator and the ATP is imaged directly as it is being released after a given stimulus. The first successful ATP imaging was conducted by Wang and co-workers in 2000, demonstrating non-destructive cell poking-induced ATP release from astrocytes and quantifying the ATP travelling wave velocity [23]. Beautifully, this study also succeeded in semi-simultaneous detection of cellular [Ca2+]i with fluo-3 [23]. Also, poking of retina glia cells showed a luminescence ATP wave propagating at similar speed to that observed with [Ca2+]i imaging [35]. The luciferase-generated light intensity is very low and requires highly sensitive imaging equipment (e.g. a nitrogen-cooled charge-coupled device (CCD) camera), together with long temporal integration, to achieve meaningful data. The achievable images are diffuse, with low spatial resolution. In the initial study, a camera integration time of 0.5 s was sufficient to detect ATP concentrations as low as 10 nM [23]. The second study also used temporal integrations of 0.5 s, together with the 2 × 2 binning function of a high-quality CCD camera, which was sufficient to monitor the kinetics of a 30-s period of touch-induced ATP release from glial cells [35]. A third study used cultured astrocytes, integration time of 10 s and a liquid nitrogen CCD camera to show spontaneous point source bursts of released ATP when the extracellular Ca2+ concentration was lowered below normal physiological values (0.5 mM) [36]. Undoubtedly, this novel technical extension carries the strength of a specific signal which reports extracellular ATP concentrations directly. The low temporo-spatial resolution of the luminescence imaging technique is a significant limiting factor and may preclude the possibility of ‘zooming’ closer into the mechanism of ATP release. Resting or spontaneous ATP release has not so far been imaged by the luciferin–luciferase method.
Two other studies using alternative ATP-dependent enzymatic reactions were also able to detect and image extracellular ATP. One exploited the disappearance of light absorption of consumed luciferin (as substrate in the ATP-dependent luciferin–luciferase reaction) to detect muscarinic receptor-stimulated release of ATP from pancreatic acinar cells [37]. The other study imaged ATP at the leading edge of a migrating neutrophil with the use of a two-enzyme assay system which catalyses the conversion of NADP+ to NADPH in the presence of ATP. The real-time generation of NADPH was measured as the appearance of NADPH fluorescence [13, 38].
Biosensor cells and ATP detection via an increase of cytosolic Ca2+
The use of a biosensor cell placed in the direct vicinity of an ATP-releasing cell was first introduced in 1989 by Cheek et al. who used NIH-3T3 fibroblasts co-cultured around single bovine adrenal chromaffin cells. After stimulation with nicotine, the chromaffin cells released ATP, which was sensed via a P2 receptor-dependent [Ca2+]i increase by the neighbouring fibroblasts [39]. Extracellular ATP and other nucleotides commonly produce elevations of cytosolic Ca2+ via activation of either P2Y or P2X receptors [40]. Thus, the increase of [Ca2+]i is used as a read-out to measure extracellular ATP. Also, the pioneering study demonstrating the ATP dependency of travelling [Ca2+]i waves in rat basophilic leukaemia cells applied this biosensor technique to substantiate ATP as a paracrine factor [41]. Later, this approach was refined by Okada and colleagues and was applied to ATP-secreting B cells from the pancreas [42]. A detailed technical note describing the essentials and features was recently published [43]. A critical issue for the biosensor cell approach is the biosensor’s own susceptibility to stimuli. Thus, the experimental protocol must ascertain that an observed increase of [Ca2+]i is caused by the extracellular nucleotide and not by other unidentified factors. An indicator cell can also be moved freely within the bath chamber using a micromanipulator and thereby positioned in the vicinity of the cell of interest to detect whether it releases ATP upon a given stimulus. This technique enables the researcher to answer in a convincing way the temporo-spatial characteristics of ATP release in different cells. Cultured mouse mesangial smooth muscle cells have been used as biosensors to detect ATP release from the basolateral side of rabbit renal macula densa cells [11, 17, 44]. ATP release was stimulated via an elevation of the luminal NaCl concentration without changing the environment of the biosensor cell. These findings were confirmed by PC12 cells [44]. Thus, little doubt is left that the release of ATP from the macula densa cells after exposure to high NaCl in the luminal perfusate is a key component of the tubulo-glomerular feedback mechanism [11]. Yet another example is the use of microglia cells as detectors for mechanical-induced astrocytic ATP release [45].
P2X2 receptor-mediated ion currents as biosensors for extracellular ATP
A similar principle of ATP biosensing uses P2X receptor-expressing cells and patch clamp recordings to measure ATP-induced inward currents either in the whole-cell configuration or in outside-out excised patches [43, 46]. It was coined the ATP ‘patch sniffing’ method [47]. P2X2 receptor-expressing neuro-endocrine (PC12) cells, derived from rat phaeochromocytoma, are often used in this approach. In the PC12 cell biosensor technique, a single non-attached cell is patched and therewith hooked up to a glass pipette. After breaking the seal, ATP-induced whole-cell currents can be recorded when holding the cell at −50 mV. This technique has been successfully applied to measure stimulated ATP release from a variety of different cultured cells, including various epithelial cells (C127 mammary epithelial cancer cells, 407 intestinal epithelial cells, airway epithelial cells, renal macula densa cells), primary cultured myocardial cells and pancreatic B cells [43] and Xenopus oocytes [48]. The outside-out patch approach adds a higher degree of specificity to the system because the membrane patch, in contrast to an entire cell, is less likely to respond to other external signals and is unable to release ATP itself.
The P2X2 receptor tagged with enhanced green fluorescence protein can also be transfected heterologously into other cells of interest, which therewith become equipped with an ATP biosensor. This has been done in insulin-secreting INS1 cells and used to show that ATP is released via exocytosis in these cells. ATP release was detected as spontaneous spiking of suramin-sensitive inward currents. This signal is also described as ‘autaptic’ activation of the expressed P2X2 receptor [17]. In a similar fashion, clusters of PC12 cells display such ‘autaptic’ behaviour, as detected by spontaneous transient inward currents (STICs) through P2X2 receptors. There is firm evidence that this phenomenon reflects quantal exocytotic release of ATP from PC12 cells [2]. In addition, the discovery of pannexin 1 as an ATP-releasing channel in taste bud epithelia convincingly used the P2X2/3 biosensor approach [10]. Precise quantification of extracellular ATP concentration with the cell-based biosensor approaches can be difficult because the used ATP receptors often desensitise.
The amperometric ATP biosensor microelectrode
This novel method provides hope of increasing our knowledge of ATP as a secreted extracellular molecule. It is based on an ATP biosensor built by coating a platinum microelectrode with an ultra thin layer of various enzymes [49, 50]. Different enzymes and coating protocols have successfully been used to produce functional, robust and fast-acting ATP electrodes [49, 50]. The first ATP microelectrode used immobilised glucose oxidase and hexokinase and measured ATP concentrations between 10 and 200 nM [49]. A second study used glycerol kinase (GK) and glycerol-3-phosphatase (G3P) in the enzyme layer. In the presence of glycerol, ATP leads to GK-dependent formation of glycerol-3-phosphate, which together with O2 is converted to H2O2 and glycerone phosphate by G3P. Subsequently, H2O2 is oxidised at the platinum electrode (+500 mV) which provides a current signal directly proportional to the amount of consumed ATP [50]. ATP-induced current signals are linear over the physiologically relevant ATP concentration range (200 nM to 50 µM) and the microelectrode appears to be very sensitive: ~250 mA M−1 cm−2. It works robustly in physiological extracellular solutions, has the dimensions of 50 µm in diameter and 2 mm in length, and can be positioned with a micromanipulator next to the tissue. It has been used to detect ATP release during locomotor activity of the spinal cord of Xenopus embryos. It does not detect other nucleotides but may perceive disturbances from other electro-active compounds like 5HT or ascorbate [50]. Using appropriate controls with the uncoated non-sensor electrode or without glycerol, it is possible to verify that the current signals are ATP-specific. Possibly, this technique can be optimised to record only from a few or even single cells. This ATP electrode was instrumental in demonstrating recently that ATP is an important central sensory transmitter in the medulla oblongata, stimulating breathing after elevation of peripheral pCO2 [4]. Interestingly, in a very similar fashion, the same group has also produced an adenosine electrode [51]. This has the potential of recording the ATP breakdown product adenosine and detecting the temporo-spatial formation of ATP and adenosine in native tissues [51]. Adaptation of the ATP electrode to micromanipulator scanning devices or even an atomic force microscope cantilever is under technical development and may evolve into an electrode-based ATP imaging method applicable to physiological preparations [52, 53].
Other ATP biosensors
An interesting methodological approach used the atomic force microscope (AFM) to scan the surface of cystic fibrosis transmembrane conductance regulator (CFTR)-expressing respiratory epithelial cells [54]. In this technique, the luminal cellular surface was scanned with the AFM cantilever. The cantilever was ‘myosin-functionalised’, which caused this detecting device to move slightly during myosin-induced ATP hydrolysis. Why ATPase activity produces cantilever movements is not fully explained. The ATP-induced cantilever movements were taken to indicate ATP release, and results suggest several ATP release point sources on each cell. The ATP release could be blocked by glibenclamide and was not seen in non-CFTR-expressing epithelial control cells [54]. However, no follow-up studies with this technique have been published so far, probably reflecting the significant technical difficulty and expense of this method.
Another way to exploit an ATP-dependent enzymatic reaction to measure extracellular ATP was recently reported by Yegutkin and colleagues [55]. They made use of the fact that lymphocytes express surface adenylate kinase, which generates ATP/ADP from its precursor adenosine monophosphate (AMP) [55]. Using [3H]AMP and radio thin layer chromatography, they found that phosphotransfers to form ADP/ATP occurred spontaneously without added exogeneous ATP. This reaction must therefore have been driven by extracellular ATP provided by the lymphocytes themselves and was defined as the ‘intrinsic’ AMP phosphorylation property [56]. Quantification of this reaction can be used to calculate [3H]AMP phosphorylation capacities and close estimation of the ATP concentrations necessary for this reaction. They found ATP values around 2–5 µM, which must be present to drive this reaction, and suggested that a tonically released ATP ‘aura’ surrounds the lymphocytes. This method provides independent evidence that extracellular ATP concentrations can reach micromolar concentrations. However, the method is probably not applicable to all cells as it relies on the surface expression of extracellular ATP-regenerating enzymes like adenylate kinases. It is not suitable for kinetic evaluations of the ATP release process.
Suggested mechanisms for cellular nucleotide release
Cytosolic ATP concentrations are high (~3 mM) and provide the source for extracellular ATP [57]. In principle, four different mechanisms may potentially be responsible for ATP release: (1) cellular lysis, necrosis and apoptosis; (2) transport via a conductive pore (channel); (3) transport via a specific solute carrier; and (4) exocytotic release. Even though the first mechanism can be relevant in some pathological states, it will not be discussed here.
A nucleotide-conductive pore
ATP is a weak acid (pKa 6.9) and therefore mainly present in anionic form in the cytosol [58]. The large electrochemical, outwardly directed ATP gradient has led researchers to suggest that cells may be equipped with an ATP-conductive pore [59, 60]. This could potentially allow ATP to permeate in a regulated fashion through a plasma membrane anion/chloride channel. Molecular evidence for functionally relevant plasma membrane anion channels includes the members of the ClC chloride channel family, CFTR and the different receptor-operated chloride channels (e.g. glycine receptor) [61]. The molecular nature of the cell swelling-induced anion conductances and the Ca2+-activated anion conductances is still unresolved [61]. Crystal structure and electrophysiological data from ClC channels clearly exclude permeation of ATP. The large organic anion ATP is much too big to pass through the ClC pore [62, 63]. Likewise, high-resolution structural data from water and K+ channels confirm that the narrow conductive pore is a general feature of ion channels [64, 65]. Thus, the available structural information on molecularly and structurally defined ion channels in general, and ClC channels in particular, do not support the proposition that the large bulky organic ion ATP can be transported through these pores.
Cell swelling-activated anion conductance
Despite the above, there is some evidence that ATP release could occur through an anion channel or pore. Numerous studies have demonstrated that cellular swelling activates a robust and large ATP release [42, 66–68]. This coincides with the fact that all cells in general react to cellular swelling with the activation of volume-sensitive anion channels [69]. Three discernible types of anion conductance are known to be activated by cellular swelling: ClC-2 channels, the volume-sensitive organic osmolyte and anion conductance (VSOAC; synonyms: VRAC, VSOR) [69] and a ‘maxi’ or large anion conductance. Whereas the molecular nature of VSOAC remains undefined, it has been suggested that the maxi or large anion conductance is identical to the mitochondrial voltage-dependent anion conductance (VDAC) and thus also present at the plasma membrane [69]. The ClC-2 channel is most likely not ATP-permeable, as discussed above. In contrast, VSOAC and the large anion channel show broad selective conductance, allowing different small organic anions and osmolytes to pass. VSOAC, for example, is permeable for sorbitol, various amino acids (e.g. taurine) and the methylamine betaine [69]. Okada has presented evidence that the maxi anion conductance (VDAC-like—VDACL) is a plasma membrane ATP-conductive pore in mammary cancer cells [70] and in macula densa cells [44], and follow-up work shows that this conductance is inhibited by internal arachidonic acid [71]. VDAC is a mitochondrial ATP-permeable membrane pore, but has been suggested also to be present in the plasma membrane as a specific splice variant (pl-VDAC-1) [72]. Interestingly, VADC-1 knockout mouse tissue shows reduced mechanically stimulated ATP release and, vice versa, overexpression of pl-VDAC-1 protein in fibroblasts leads to increased mechanically stimulated ATP release [73]. Thus, these data support a role of this protein in ATP release. However, mechanically triggered ATP release continues to be present in the absence of pl-VDAC-1 [73]. Another conflicting finding regarding VDAC is its very long lag time before activation of the membrane current (>10 min) [70], a finding which argues against its involvement in swelling-induced ATP release that always occurs promptly [67]. In addition, the other smaller conductance, outwardly rectifying VSOAC, has been suggested to mediate swelling-induced ATP release from endothelial cells. The major argument was that blockers of VSOAC currents also block ATP release [66]. Interesting follow-up work by the same authors found that swelling-induced endothelial ATP release involves Rho-kinase and tyrosine-kinase [74] and suggests the following sequence of events: Cell swelling activates the RhoA/Rho-kinase pathway followed by tyrosine phosphorylation of FAK and paxillin, which subsequently leads to ATP release and actin reorganisation [75]. In summary, volume-activated anion conductances continue to be putative candidates for swelling-induced ATP secretion but this issue is far from being resolved.
The link to CFTR
The CFTR chloride channel has been suggested to conduct ATP itself or to modulate a related ATP-conductive pore [59, 60, 76, 77]. However, a significant number of studies failed to detect differences in extracellular ATP in normal and CFTR-deficient epithelial tissues [78–80]. It was also shown that swelling-induced ATP release was independent from CFTR [32, 46, 81]. Furthermore, more indirect approaches using CFTR blocking drugs failed to link agonist-stimulated ATP release to CFTR [26]. Thus, many researchers believe that CFTR is not critically involved in tonic or regulated ATP release from non-excitable cells. However, this issue continues to be controversial. Glutamate-stimulated ATP release from microglia cells is significantly attenuated in cells isolated from CFTR knockout mice [82] and erythrocytes isolated from CF patients are reported to show no mechanical-induced ATP release [83]. Thus, a final verdict on this issue is premature.
ATP release via the P2X7 receptor
It is established that in some cells P2X7 receptor activation with high ATP concentrations (>100–300 µM) triggers cell membrane permeabilisation to medium size molecular markers (<900 Da) [84]. It was therefore speculated that this receptor-mediated permeabilisation may also allow ATP to exit cells. Evidence for this was generated in cells which expressed membrane-bound luciferase and the P2X7 receptor [34]. Activation of the P2X7 receptor with Bz-ATP triggered very large increases of extracellular ATP to about 250 µM. The authors suggested that the route for ATP release is directly via the pore-forming P2X7 receptor, but firm proof on this issue is pending. These data are contradicted by results showing that osteoblasts release the same amount of ATP by fluid shear stress, regardless of whether they express P2X7 receptors or not [85]. It is noteworthy that the P2X7 receptor in several cell types is associated with the induction of necrosis and apoptosis [86]. This could inevitably cause nucleotide release by a cell death mechanism and may confound the interpretation of a more direct P2X7 receptor-mediated mechanism.
A link to connexins, pannexins and ATP-permeable hemichannels
Numerous cultured cells of different origin can be stimulated to display travelling [Ca2+]i waves, a phenomenon attributed to diffusion of IP3 through gap junctions and most intensely investigated in cultured astrocytes [87]. However, [Ca2+]i waves often cross small cell-free areas and are blocked by extracellular ATP scavenging or P2 receptor blockade [88–90]. Thus, travelling [Ca2+]i waves can also be caused by release of nucleotides, stimulation of P2 receptors and generation of further nucleotide release to feed wave propagation. Travelling [Ca2+]i waves appear to be dependent on the expression of gap junction proteins, raising the question of whether expression of connexins may modulate nucleotide release [90, 91]. Forced expression of different connexins (Cx43, Cx32, Cx26) caused a greatly increased UTP-stimulated ATP release in different cell types [90]. It was thus speculated that the tunnel-shaped connexons, which allow non-selective permeation of molecules up to the size of 1 kDa, may form a regulated exit pathway for ATP. This requires that connexin molecules, in addition to building gap junctions between cells, also localise in the plasma membrane as hemichannels. Using well-characterised antibodies against the extracellular loops of connexins, strong evidence for hemichannel localisation in cellular processes and filipodia of astrocytes has been provided [91]. Lowering extracellular Ca2+ triggered the uptake of small (>1 kDa) fluid phase fluorophores, and this could be blocked with antibodies against connexins [91]. Thus, hemichannels have been shown to be functionally present in the plasma membrane and as a consequence of their biophysical structure could allow the exit of ATP. After lowering extracellular Ca2+ in Cx43-expressing C6 astrocytes, ATP release from a point source cell could be imaged, and simultaneously, the entry of propidium into this ATP-releasing cell was shown [36]. These results strongly support the proposition that gap junctional hemichannels can be stimulated to open and induce ATP release. There are, however, several shortcomings in the hypothesis of connexin-mediated ATP release under physiological conditions. Most studies employing connexin activation protocols do so by removing or lowering extracellular divalent cations, a situation most unlikely to be found under physiological conditions [36, 90]. Also, very large, unphysiological depolarisation steps are necessary for the opening of these hemichannels. Firm, unequivocal proof is lacking as to whether connexin hemichannels may open and release ATP under physiological conditions.
Recently, the novel protein family of pannexin channels has entered this field and several properties of this tunnel protein support a role in cellular ATP release [92]. Pannexins are structurally homologous to connexins and can form plasma membrane channels in Xenopus oocytes [93–95]. Several properties and findings make pannexin 1 a very attractive candidate for an ATP-releasing channel: (1) It can be activated by membrane depolarisation in the physiological range and allows permeation of small molecules including ATP [93–95]; (2) it can be activated at normal extracellular Ca2+ concentrations [93]; (3) it is activated by mechanical perturbation [95]; (4) it may open under conditions of cellular energy depletion [96]; and (5) it can be activated by increase of intracellular Ca2+ [95, 97]. In addition, important evidence suggests that the long-sought P2X7-related pore structure may be pannexin 1 [98, 99]. In macrophages, pannexin 1 siRNA knockdown or a pannexin 1-mimetic inhibitory peptide blocked P2X7-mediated dye uptake, leaving the ion current of the P2X7 receptors unchanged. Overexpression of pannexin 1 increased P2X7-meditated dye uptake [98]. Finally, there is strong evidence that taste sensation in taste bud epithelia involves pannexin 1-mediated ATP release. ATP then further stimulates the release of 5-HT from presynaptic cells within the taste bud [10]. These findings provide significant evidence that pannexins and not connexin are physiologically or patho-physiologically relevant conductive pores for ATP release.
Vesicular release of nucleotides
Vesicular release of ATP from neuronal or neuro-endocrine cells is an established phenomenon and many studies have determined ATP as a co-transmitter in peripheral and central neurons as well as from various neuro-endocrine cells. The interested reader is directed to several reviews of this area [15, 100, 101]. It is thus established that, e.g. chromaffin cells and the closely related PC12 cells store large amounts of ATP (>100 mM) together with catecholamines [2, 102], that insulin secretion from B cells occurs together with ATP [17] and that α-dense granules in thrombocytes contain very high concentrations of ADP, a known key factor in thrombus formation [14]. There is strong evidence that astrocytic release of nucleotides under physiological conditions involves an exocytotic mechanism [45, 103, 104], in contrast to the above-mentioned controversial issue of connexon hemichannel-mediated ATP release from astrocytes under non-physiological situations [36]. It is noteworthy that the inhibition of vesicular release with tools like brefeldin A or bafilomycin effectively inhibits travelling [Ca2+]i wave propagation in astrocytes [104]. Both brefeldin A and bafilomycin have been shown to inhibit ATP release in numerous systems, supporting the notion that many non-excitable cells may be able to perform nucleotide release by this mechanism [28, 45, 82, 105]. Additional evidence supporting vesicular ATP release comes from experiments that interfere with the molecular players of constitutive and regulated exocytosis. Thus, knockdown of members of the soluble NSF attachment receptor family (SNAREs), or neurotoxins like tetanus toxin in astrocytes, inhibits nucleotide release [45, 103]. Of note is a recent observation of spontaneous autocrine quantal release of protons from HEK 293 cells, a phenomenon that may be suggested to coincide with ATP release from these vesicles [106]. Taken together, the above findings provide strong and unequivocal evidence that vesicular ATP release is a ubiquitous phenomenon of most, if not all, cells except erythrocytes [107]. The key challenge will be to unravel the physiological context in which the different organ systems ‘use’ vesicular ATP release to regulate a specific function. One example of this is the way in which tonic vesicular astrocytic purinergic signalling dampens neuronal synaptic networks in the CNS [103].
Nucleotide release
Spontaneous or constitutive nucleotide release
Very low concentrations (nanomolar range) of extracellular ATP surround most, if not all, cultured cells. As externally applied ATP is rapidly broken down by ecto-ATPases on the cell plasma membranes, the stable extracellular ATP levels must reflect a steady-state situation of tonic ATP release balanced by ATP degradation at the same rate [19, 32, 56, 74]. Tonic ATP release can be unmasked by non-specific inhibition of ecto-ATPases. Substances like ARL67156 and α,β-methylene-ATP, β,γ-methylene-ATP, ebselen or levamisole result in a slow and sustained increase of extracellular ATP concentrations [30, 32, 108–110]. Kinetic measurements of this increase allow calculation of actual cellular ATP release rates, which lie in the range of 10–500 fmol min−1 per 106 cells in different cell types [19]. Several findings suggest that spontaneous nucleotide release is of physiological significance. Scavenging of extracellular ATP with apyrase reduces the amount of tonically released arachidonic acid and lowers cAMP values in MDCK cells [111]. Slow spontaneous [Ca2+]i oscillations in intact and cultured epithelial cells are greatly reduced in cells without relevant P2 receptors and are abrogated by ATP scavenging or pharmacological P2 receptor blockade [109]. Thus, constitutive nucleotide release appears to provide a tonic set-point regulation of a given organ function. In this context, the renal phenotype of the P2Y2 receptor knockout mouse appears very interesting. These mice have hypertension, low plasma renin/aldosterone levels and renal hyperabsorption of Na+ [112]. It is established that P2Y2 receptor stimulation inhibits ENaC-mediated Na+ and aquaporin2-mediated water transport in the renal collecting duct [113–115]. The absence of this receptor in the collecting duct should therefore result in an increased Na+ and H2O reabsorption, leading to volume expansion and reduction of the ‘tonus’ of the renin–angiotensin–aldosterone system. Exactly, this was found in a recent publication [112]. This, however, would imply that the P2Y2 receptors receive ‘tonic’ signalling input, a phenomenon, which should be preceded by tonic nucleotide release. This tonic nucleotide release can indeed be observed in cultured and intact renal epithelia [109, 111]. In summary, it is likely that control of organ function is exerted by tonic stimulation of P2 receptors. Most certainly, adenosine receptors are equally important in this scenario.
Intracellular Ca2+ oscillations
Non-stimulated cells in culture and intact tissues often display spontaneous [Ca2+]i oscillations [88, 116–119]. The interpretation of this phenomenon is complex and putatively involves all components of the [Ca2+]i signalling network. One interesting observation that links [Ca2+]i oscillations to nucleotide release was made in 132 1N1 astrocytes, cells which by fate do not express P2 receptors. These cells display a stable baseline [Ca2+]i, but can be stimulated to increase [Ca2+]i with, for example, carbachol. In contrast, 132 1N1 cells stably transfected with various P2Y receptors show very lively [Ca2+]i oscillations. As indicated above, it is generally agreed that most cells, including 132 1N1 cells, release nucleotides constitutively [19]. Thus, it is speculated that cells ‘constructed’ to sense extracellular nucleotides by P2 receptor transfection display spontaneous [Ca2+]i oscillations because they spontaneously release nucleotides. [Ca2+]i oscillations in a variety of cell types have been found to be abrogated by extracellular ATP scavenging or blockade of P2 receptors [109, 117, 118]. Taken together, these findings suggest that spontaneous [Ca2+]i oscillations in different cellular systems may reflect the basal property of cells to release and sense ATP.
Stimulated nucleotide release
Nucleotide release can be stimulated to produce rapid and transient elevation of, e.g. ATP around the cells. Two main stimulation modes are described regarding this triggered ATP release: (a) mechanical perturbation of cells, and (b) through agonists, including nucleotides themselves. These two topics will be elaborated below.
Mechanically released nucleotides
Mechanical stimulation is a common trigger for nucleotide release from most, if not all, cells [89, 90, 105, 120, 121]. This may be unphysiological, as any mechanical stimulus (e.g. poking at a cell) may cause damage and release of cellular content including ATP from the compromised cell. However, subtle non-destructive stimuli like gently rotating a dish with cultured cells [122], increasing the flow over an epithelial/endothelial sheet, or other gentle mechanical stimuli, are prominent triggers for nucleotide release [81, 123–125]. The field of astrocyte research has provided important clues in the recognition that a single cell mechanical stimulus can lead to waves of [Ca2+]i increases spreading to neighbouring cells, which is caused by the spread of released nucleotides [36, 90]. The trigger for the mechanically stimulated nucleotide release is not yet known, but it has been suggested that members of the multi-sensory TRP channel family [126] could play a pivotal role.
Fluid flow as mechanical stimulus for nucleotide release In vascular biology, evidence has accumulated that an increase of fluid flow triggers the release of ATP and pyrimidine nucleotides [123, 127, 128]. It has been suggested that the released nucleotides participate in the shear stress-induced regulation of vascular tone [40, 128]. In this model, released nucleotides bind to endothelial P2 receptors, triggering NO release and subsequent smooth muscle relaxation. A recent study has highlighted the critical role of endothelial P2X4 receptors in flow-induced vasodilation [129]. In mice lacking P2X4 receptors, flow-induced increases of [Ca2+]i in the endothelial cells were absent, as was the endothelium-dependent vascular dilatation. Flow-induced increases in [Ca2+]i can also be elicited in renal epithelia [130–132]. This phenomenon has received much attention as it was hypothesised that an absence of flow sensing in renal epithelia causes polycystic kidney disease [133]. Intriguingly, the flow-induced release of ATP from endothelial cells and the flow-stimulated increase of [Ca2+]i in renal epithelia show striking similarities: They are transient, gradable by the amount of applied shear stress, cause refractoriness to subsequent stimuli and are inhibited by removal of extracellular Ca2+ [127, 134]. Recently, it was shown that the flow-induced [Ca2+]i increase in intact renal tubules was caused by released nucleotides and stimulation of P2Y2 receptors [124]. Renal research may also provide clues towards understanding the trigger mechanism for mechanically induced nucleotide release. Of note, the [Ca2+]i flow response is absent or reduced in epithelia lacking the TRP channel TRPP2 [135]. Thus, this channel could be a mechano-sensor to trigger an initial Ca2+ influx which subsequently induces nucleotide release.
Hypotonic cell swelling-induced nucleotide release Cell swelling experiments are commonly used to apply a controllable mechanical stimulus to a cell. However, these manoeuvres do not reflect a physiological stimulus. It is common to cells that swelling induces nucleotide release and often subsequent P2 receptor stimulation, which leads to increases in [Ca2+]i [46, 67, 68, 74, 136]. Swelling-induced peak ATP release rates in different cells have been found to range between 17 and 294 pmol min−1 per 106 cells [67] and are thus 1,000-fold higher than constitutive ATP release rates [19, 32]. Swelling-induced ATP release is tightly synchronised to [Ca2+]i elevations and is significantly inhibited by the intracellular Ca2+ chelator BAPTA and by low temperatures [67, 77, 137]. In different cells, it was also found to be inhibited by brefeldin A [77], cytochalasin B [137], the SNARE blocking clostridium toxin F, N-ethylmaleimide [138] and bafilomycin A1 [29]. These results strongly suggest that swelling-mediated ATP release involves an exocytotic process. The results are, however, not without conflict because it was also reported that ATP release rates in primary cultures of human bronchial epithelial cells after cell swelling were largely independent of cytosolic Ca2+ [32]; instead, these authors favoured the activation of an ATP-conductive pore.In conjunction with the above discussion on ATP release via volume-sensitive anion channels (“Cell swelling-activated anion conductance” section), it needs to be realised that the mechanism of cell swelling-induced ATP release remains unresolved and controversial. The issue is further clouded by recent data from erythrocytes, which are known to be free of vesicular structures and exocytotic membrane insertions. Erythrocytes can release ATP after cell swelling, which is not thought to result from cell lysis and has been proposed to occur via pannexin 1 [107] (“A link to connexins, pannexins and ATP-permeable hemichannels” section). It is not clear whether swelling-induced ATP release and that induced by other mechanical stimuli are essentially the same, although we are inclined to assume this to be the case.
Agonist-stimulated nucleotide release
An increasing number of studies has shown that membrane receptor stimulation triggers an increase of [Ca2+]i and release of ATP. Table 2 lists various examples.
Table 2.
Agonist-induced ATP release
| Tissue, cell line | Agonist | Receptor | Reference |
|---|---|---|---|
| 132 1N1 astrocytes | Thrombin and thrombin receptor activation peptide (TRAP) | PAR | [30] |
| 132 1N1 astrocytes | Carbachol | Muscarinic | [30] |
| Thrombocytes | Thrombin | PAR1 | [140] |
| RBL cells (rat basophilic leukaemia cells) | Antigen, FCε receptor, compound 48/80 | FCε receptor | [41] |
| Bovine aortic endothelial cells and human umbilical vein endothelial cells | LPA (lysophosphatidic acid) | LPA receptor | [26, 74, 75] |
| Compound 48/80 | |||
| Schwann cells | UTP | P2Y2 | [28, 75] |
| Astrocytes | UTP | P2Y | [90] |
| Microglia | Glutamate | AMPA | [82] |
| A6 cells | Aldosterone | ? | [139] |
Most of these receptors are GPCRs which couple to an increase of [Ca2+]i via the generation of IP3. Direct stimulation of G proteins with GTP-γ-S or the G protein activator compound 48/80 strongly suggests that G protein activation is a pre-requisite for agonist-stimulated ATP release [26, 41]. In addition, most of these studies clearly indicated that the intracellular Ca2+ chelator BAPTA strongly or completely inhibited the agonist-induced ATP release [26, 28, 30, 82]. Receptor-independent [Ca2+]i elevations with, e.g. ionomycin or A23187 have been shown to trigger ATP release, but these are apparently poor agonists [26, 29, 67]. Inhibition of signalling events in the IP3 pathway, such as phospholipase C using U73122, can inhibit agonist-triggered ATP release [26, 28]. Taking these findings together, it is likely that agonist signals can trigger ATP release from most, if not all, cells. The released nucleotides could then, in a positive feedback mode, amplify a cellular response. These data indicate that agonist-induced ATP release involves a vesicular release process or activates a [Ca2+]i-sensitive conductive nucleotide pore (e.g. pannexin 1). Of note is the finding that agonist- and swelling-induced ATP releases were found to be additive and may therefore involve different mechanisms [30]. Finally, it is worth mentioning that aldosterone could trigger the release of basolateral ATP from frog-derived renal epithelium (A6). This phenomenon was suggested by the authors to be important for the activation of ENaC-mediated Na+ absorption in the distal renal tubule [139].
Schematic summary
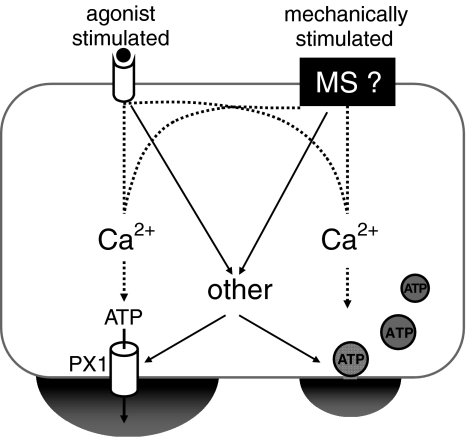
Figure 1 displays a simplified scheme of the cellular mode of ATP release from non-excitable cells. It assumes two major input signalling pathways to trigger ATP release. The mechano-sensor remains to be defined (black box, MS). It also assumes two major exit pathways for ATP, i.e. a conductive pore and exocytosis. The conductive pore is unknown but is shown as pannexin 1 (PX1). Intracellular Ca2+ is crucial for regulation of both of the pathways. The big ‘other’ remains to be identified as described in detail in the text. The inclusion of both mechanisms in one cell is certainly not true for all cells (e.g. erythrocytes) but may be the case for many cell types.
Fig. 1.
Working model for the ATP release mechanism from non-excitable cells
Acknowledgements
We greatly appreciate the critical input and proofreading from Holger Nilsson and Elvin Odgaard.
References
- 1.Spyer KM, Dale N, Gourine AV (2004) ATP is a key mediator of central and peripheral chemosensory transduction. Exp Physiol 89:53–59 [DOI] [PubMed]
- 2.Fabbro A, Skorinkin A, Grandolfo M, Nistri A, Giniatullin R (2004) Quantal release of ATP from clusters of PC12 cells. J Physiol 560:505–517 [DOI] [PMC free article] [PubMed]
- 3.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC (2005) ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 310:1495–1499 [DOI] [PubMed]
- 4.Gourine AV, Llaudet E, Dale N, Spyer KM (2005) ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436:108–111 [DOI] [PubMed]
- 5.Gourine AV, Llaudet E, Dale N, Spyer KM (2005) Release of ATP in the ventral medulla during hypoxia in rats: role in hypoxic ventilatory response. J Neurosci 25:1211–1218 [DOI] [PMC free article] [PubMed]
- 6.Hegg CC, Greenwood D, Huang W, Han P, Lucero MT (2003) Activation of purinergic receptor subtypes modulates odor sensitivity. J Neurosci 23:8291–8301 [DOI] [PMC free article] [PubMed]
- 7.Housley GD, Jagger DJ, Greenwood D, Raybould NP, Salih SG, Jarlebark LE, Vlajkovic SM, Kanjhan R, Nikolic P, Munoz DJ, Thorne PR (2002) Purinergic regulation of sound transduction and auditory neurotransmission. Audiol Neurootol 7:55–61 [DOI] [PubMed]
- 8.Newman EA (2006) A purinergic dialogue between glia and neurons in the retina. Novartis Found Symp 276:193–202 [PubMed]
- 9.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424:778–783 [DOI] [PubMed]
- 10.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD (2007) The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc Natl Acad Sci U S A 104:6436–6441 [DOI] [PMC free article] [PubMed]
- 11.Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD (2004) Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Renal Physiol 286:F1054–F1058 [DOI] [PubMed]
- 12.Schnermann J, Levine DZ (2003) Paracrine factors in tubuloglomerular feedback: adenosine, ATP, and nitric oxide. Annu Rev Physiol 65:501–529 [DOI] [PubMed]
- 13.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG (2006) ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314:1792–1795 [DOI] [PubMed]
- 14.Gachet C (2006) Regulation of platelet functions by p2 receptors. Annu Rev Pharmacol Toxicol 46:277–300 [DOI] [PubMed]
- 15.Bodin P, Burnstock G (2001) Purinergic signalling: ATP release. Neurochem Res 26:959–969 [DOI] [PubMed]
- 16.von Kügelgen I, Goncalves J, Driessen B, Starke K (1998) Corelease of noradrenaline and adenosine triphosphate from sympathetic neurones. Adv Pharmacol 42:120–125 [DOI] [PubMed]
- 17.Obermuller S, Lindqvist A, Karanauskaite J, Galvanovskis J, Rorsman P, Barg S (2005) Selective nucleotide-release from dense-core granules in insulin-secreting cells. J Cell Sci 118:4271–4282 [DOI] [PubMed]
- 18.Novak I (2003) ATP as a signaling molecule: the exocrine focus. News Physiol Sci 18:12–17 [DOI] [PubMed]
- 19.Lazarowski ER, Boucher RC, Harden TK (2003) Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64:785–795 [DOI] [PubMed]
- 20.Schwiebert EM, Zsembery A (2003) Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta 1615:7–32 [DOI] [PubMed]
- 21.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341 [DOI] [PMC free article] [PubMed]
- 22.Wood KV, Lam YA, McElroy WD (1989) Introduction to beetle luciferases and their applications. J Biolumin Chemilumin 4:289–301 [DOI] [PubMed]
- 23.Wang Z, Haydon PG, Yeung ES (2000) Direct observation of calcium-independent intercellular ATP signaling in astrocytes. Anal Chem 72:2001–2007 [DOI] [PubMed]
- 24.Denburg JL, McElroy WD (1970) Anion inhibition of firefly luciferase. Arch Biochem Biophys 141:668–675 [DOI] [PubMed]
- 25.Gruenhagen JA, Lovell P, Moroz LL, Yeung ES (2004) Monitoring real-time release of ATP from the molluscan central nervous system. J Neurosci Methods 139:145–152 [DOI] [PubMed]
- 26.Gruenhagen JA, Yeung ES (2004) Investigation of G protein-initiated, Ca2+-dependent release of ATP from endothelial cells. Biochim Biophys Acta 1693:135–146 [DOI] [PubMed]
- 27.Boudreault F, Grygorczyk R (2002) Cell swelling-induced ATP release and gadolinium-sensitive channels. Am J Physiol Cell Physiol 282:C219–C226 [DOI] [PubMed]
- 28.Liu GJ, Werry EL, Bennett MR (2005) Secretion of ATP from Schwann cells in response to uridine triphosphate. Eur J Neurosci 21:151–160 [DOI] [PubMed]
- 29.Okada, S., Paradiso, A. M., Lazarowski, E. R., and Boucher, R. C. A calcium-dependent pathway in swelling-induced ATP release from airway epithelial cells. J Physiol 567P, C53. 2006. Abstract
- 30.Joseph SM, Buchakjian MR, Dubyak GR (2003) Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem 278:23331–23342 [DOI] [PubMed]
- 31.Beigi R, Kobatake E, Aizawa M, Dubyak GR (1999) Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol 276:C267–C278 [DOI] [PubMed]
- 32.Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC (2006) Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 281:22992–23002 [DOI] [PMC free article] [PubMed]
- 33.Nakamura M, Mie M, Funabashi H, Yamamoto K, Ando J, Kobatake E (2006) Cell-surface-localized ATP detection with immobilized firefly luciferase. Anal Biochem 352:61–67 [DOI] [PubMed]
- 34.Pellegatti P, Falzoni S, Pinton P, Rizzuto R, Di Virgilio F (2005) A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell 16:3659–3665 [DOI] [PMC free article] [PubMed]
- 35.Newman EA (2001) Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci 21:2215–2223 [DOI] [PMC free article] [PubMed]
- 36.Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M (2002) Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci U S A 99:9840–9845 [DOI] [PMC free article] [PubMed]
- 37.Sorensen CE, Novak I (2001) Visualization of ATP release in pancreatic acini in response to cholinergic stimulus. Use of fluorescent probes and confocal microscopy. J Biol Chem 276:32925–32932 [DOI] [PubMed]
- 38.Corriden R, Insel PA, Junger WG (2007) A novel method using fluorescence microscopy for real-time assessment of ATP release from individual cells. Am J Physiol Cell Physiol 293:C1420–C1425 [DOI] [PubMed]
- 39.Cheek TR, Jackson TR, O’Sullivan AJ, Moreton RB, Berridge MJ, Burgoyne RD (1989) Simultaneous measurements of cytosolic calcium and secretion in single bovine adrenal chromaffin cells by fluorescent imaging of fura-2 in cocultured cells. J Cell Biol 109:1219–1227 [DOI] [PMC free article] [PubMed]
- 40.Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492 [PubMed]
- 41.Osipchuk Y, Cahalan M (1992) Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature 359:241–244 [DOI] [PubMed]
- 42.Hazama A, Hayashi S, Okada Y (1998) Cell surface measurements of ATP release from single pancreatic beta cells using a novel biosensor technique. Pflügers Arch Eur J Physiol 437:31–35 [DOI] [PubMed]
- 43.Hayashi S, Hazama A, Dutta AK, Sabirov RZ, Okada Y (2004) Detecting ATP release by a biosensor method. Sci STKE 2004:l14 [DOI] [PubMed]
- 44.Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y (2003) Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci U S A 100:4322–4327 [DOI] [PMC free article] [PubMed]
- 45.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C (2003) Storage and release of ATP from astrocytes in culture. J Biol Chem 278:1354–1362 [DOI] [PubMed]
- 46.Hazama A, Shimizu T, Ando-Akatsuka Y, Hayashi S, Tanaka S, Maeno E, Okada Y (1999) Swelling-induced, CFTR-independent ATP release from a human epithelial cell line: lack of correlation with volume-sensitive Cl− channels. J Gen Physiol 114:525–533 [DOI] [PMC free article] [PubMed]
- 47.Brown P, Dale N (2002) Spike-independent release of ATP from Xenopus spinal neurons evoked by activation of glutamate receptors. J Physiol 540:851–860 [DOI] [PMC free article] [PubMed]
- 48.King BF, Wildman SS, Unwin RJ (2006) Persistent activation of P2X2 receptors by locally released ATP. J Am Soc Nephrol 17:297A Abstract
- 49.Kueng A, Kranz C, Mizaikoff B (2004) Amperometric ATP biosensor based on polymer entrapped enzymes. Biosens Bioelectron 19:1301–1307 [DOI] [PubMed]
- 50.Llaudet E, Hatz S, Droniou M, Dale N (2005) Microelectrode biosensor for real-time measurement of ATP in biological tissue. Anal Chem 77:3267–3273 [DOI] [PubMed]
- 51.Llaudet E, Botting NP, Crayston JA, Dale N (2003) A three-enzyme microelectrode sensor for detecting purine release from central nervous system. Biosens Bioelectron 18:43–52 [DOI] [PubMed]
- 52.Kueng A, Kranz C, Lugstein A, Bertagnolli E, Mizaikoff B (2005) AFM-tip-integrated amperometric microbiosensors: high-resolution imaging of membrane transport. Angew Chem Int Ed Engl 44:3419–3422 [DOI] [PubMed]
- 53.Kueng A, Kranz C, Mizaikoff B (2005) Imaging of ATP membrane transport with dual micro-disk electrodes and scanning electrochemical microscopy. Biosens Bioelectron 21:346–353 [DOI] [PubMed]
- 54.Schneider SW, Egan ME, Jena BP, Guggino WB, Oberleithner H, Geibel JP (1999) Continuous detection of extracellular ATP on living cells by using atomic force microscopy. Proc Natl Acad Sci U S A 96:12180–12185 [DOI] [PMC free article] [PubMed]
- 55.Yegutkin GG, Henttinen T, Samburski SS, Spychala J, Jalkanen S (2002) The evidence for two opposite, ATP-generating and ATP-consuming, extracellular pathways on endothelial and lymphoid cells. Biochem J 367:121–128 [DOI] [PMC free article] [PubMed]
- 56.Yegutkin GG, Mikhailov A, Samburski SS, Jalkanen S (2006) The detection of micromolar pericellular ATP pool on lymphocyte surface by using lymphoid ecto-adenylate kinase as intrinsic ATP sensor. Mol Biol Cell 17:3378–3385 [DOI] [PMC free article] [PubMed]
- 57.Miller DS, Horowitz SB (1986) Intracellular compartmentalization of adenosine triphosphate. J Biol Chem 261:13911–13915 [PubMed]
- 58.Melchior NC (1954) Sodium and potassium complexes of adenosinetriphosphate: equilibrium studies. J Biol Chem 208:615–627 [PubMed]
- 59.Cantiello HF (2001) Electrodiffusional ATP movement through CFTR and other ABC transporters. Pflugers Arch 443(Suppl 1):S22–S27 [DOI] [PubMed]
- 60.Schwiebert EM, Egan ME, Hwang T-H, Fulmer SB, Allen SS, Cutting GR, Guggino WB (1995) CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell 81:1063–1073 [DOI] [PubMed]
- 61.Jentsch TJ, Stein V, Weinreich F, Zdebik AA (2002) Molecular structure and physiological function of chloride channels. Physiol Rev 82:503–568 [DOI] [PubMed]
- 62.Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R (2002) X-ray structure of a ClC chloride channel at 3.0 A reveals the molecular basis of anion selectivity. Nature 415:287–294 [DOI] [PubMed]
- 63.Rychkov GY, Pusch M, Roberts ML, Jentsch TJ, Bretag AH (1998) Permeation and block of the skeletal muscle chloride channel, ClC-1, by foreign anions. J Gen Physiol 111:653–665 [DOI] [PMC free article] [PubMed]
- 64.De Groot BL, Grubmuller H (2001) Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science 294:2353–2357 [DOI] [PubMed]
- 65.MacKinnon R (2003) Potassium channels. FEBS Lett 555:62–65 [DOI] [PubMed]
- 66.Hisadome K, Koyama T, Kimura C, Droogmans G, Ito Y, Oike M (2002) Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J Gen Physiol 119:511–520 [DOI] [PMC free article] [PubMed]
- 67.Boudreault F, Grygorczyk R (2004) Cell swelling-induced ATP release is tightly dependent on intracellular calcium elevations. J Physiol 561:499–513 [DOI] [PMC free article] [PubMed]
- 68.Wang Y, Roman RM, Lidofsky SD, Fitz JG (1996) Autocrine signaling through ATP release represents a novel mechanism for cell volume regulation. Proc Natl Acad Sci U S A 93:12020–12025 [DOI] [PMC free article] [PubMed]
- 69.Strange K, Emma F, Jackson PS (1996) Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol 270:C711–C730 [DOI] [PubMed]
- 70.Sabirov RZ, Dutta AK, Okada Y (2001) Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J Gen Physiol 118:251–266 [DOI] [PMC free article] [PubMed]
- 71.Dutta AK, Okada Y, Sabirov RZ (2002) Regulation of an ATP-conductive large-conductance anion channel and swelling-induced ATP release by arachidonic acid. J Physiol 542:803–816 [DOI] [PMC free article] [PubMed]
- 72.Bahamonde MI, Fernandez-Fernandez JM, Guix FX, Vazquez E, Valverde MA (2003) Plasma membrane voltage-dependent anion channel mediates antiestrogen-activated maxi Cl- currents in C1300 neuroblastoma cells. J Biol Chem 278:33284–33289 [DOI] [PubMed]
- 73.Okada SF, O’Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, Lazarowski ER, Boucher RC (2004) Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol 124:513–526 [DOI] [PMC free article] [PubMed]
- 74.Koyama T, Oike M, Ito Y (2001) Involvement of Rho-kinase and tyrosine kinase in hypotonic stress-induced ATP release in bovine aortic endothelial cells. J Physiol 532:759–769 [DOI] [PMC free article] [PubMed]
- 75.Hirakawa M, Oike M, Karashima Y, Ito Y (2004) Sequential activation of RhoA and FAK/paxillin leads to ATP release and actin reorganization in human endothelium. J Physiol 558:479–488 [DOI] [PMC free article] [PubMed]
- 76.Jiang Q, Mak D, Devidas S, Schwiebert EM, Bragin A, Zhang Y, Skach WR, Guggino WB, Foskett JK, Engelhardt JF (1998) Cystic fibrosis transmembrane conductance regulator-associated ATP release is controlled by a chloride sensor. J Cell Biol 143:645–657 [DOI] [PMC free article] [PubMed]
- 77.Reigada D, Mitchell CH (2005) Release of ATP from retinal pigment epithelial cells involves both CFTR and vesicular transport. Am J Physiol Cell Physiol 288:C132–C140 [DOI] [PubMed]
- 78.Reddy MM, Quinton PM, Haws C, Wine JJ, Grygorczyk R, Tabcharani JA, Hanrahan JW, Gunderson KL, Kopito RR (1996) Failure of the cystic fibrosis transmembrane conductance regulator to conduct ATP. Science 271:1876–1879 [DOI] [PubMed]
- 79.Watt WC, Lazarowski ER, Boucher RC (1998) Cystic fibrosis transmembrane regulator-independent release of ATP. Its implications for the regulation of P2Y2 receptors in airway epithelia. J Biol Chem 273:14053–14058 [DOI] [PubMed]
- 80.Donaldson SH, Lazarowski ER, Picher M, Knowles MR, Stutts MJ, Boucher RC (2000) Basal nucleotide levels, release, and metabolism in normal and cystic fibrosis airways. Mol Med 6:969–982 [PMC free article] [PubMed]
- 81.Grygorczyk R, Hanrahan JW (1997) CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol 272:C1058–C1066 [DOI] [PubMed]
- 82.Liu GJ, Kalous A, Werry EL, Bennett MR (2006) Purine release from spinal cord microglia after elevation of calcium by glutamate. Mol Pharmacol 70:851–859 [DOI] [PubMed]
- 83.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ (1998) Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol 275:H1726–H1732 [DOI] [PubMed]
- 84.North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067 [DOI] [PubMed]
- 85.Li J, Liu D, Ke HZ, Duncan RL, Turner CH (2005) The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem 280:42952–42959 [DOI] [PubMed]
- 86.Tsukimoto M, Maehata M, Harada H, Ikari A, Takagi K, Degawa M (2006) P2X7 Receptor-dependent cell death is modulated during murine T cell maturation and mediated by dual signaling pathways. J Immunol 177:2842–2850 [DOI] [PubMed]
- 87.Fry T, Evans JH, Sanderson MJ (2001) Propagation of intercellular calcium waves in C6 glioma cells transfected with connexins 43 or 32. Microsc Res Tech 52:289–300 [DOI] [PubMed]
- 88.Kono T, Nishikori T, Kataoka H, Uchio Y, Ochi M, Enomoto K (2006) Spontaneous oscillation and mechanically induced calcium waves in chondrocytes. Cell Biochem Funct 24:103–111 [DOI] [PubMed]
- 89.Enomoto K-I, Furuya K, Yamagishi S, Oka T, Maeno T (1994) The increase in the intracellular Ca2+ concentration induced by mechanical stimulation is propagated via release of pyrophosphorylated nucleotides in mammary epithelial cells. Pflügers Arch Eur J Physiol 427:533–542 [DOI] [PubMed]
- 90.Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M (1998) Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci U S A 95:15735–15740 [DOI] [PMC free article] [PubMed]
- 91.Hofer A, Dermietzel R (1998) Visualization and functional blocking of gap junction hemichannels (connexons) with antibodies against external loop domains in astrocytes. Glia 24:141–154 [DOI] [PubMed]
- 92.Barbe MT, Monyer H, Bruzzone R (2006) Cell-cell communication beyond connexins: the pannexin channels. Physiology 21:103–114 [DOI] [PubMed]
- 93.Bruzzone R, Barbe MT, Jakob NJ, Monyer H (2005) Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 92:1033–1043 [DOI] [PubMed]
- 94.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H (2003) Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A 100:13644–13649 [DOI] [PMC free article] [PubMed]
- 95.Bao L, Locovei S, Dahl G (2004) Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572:65–68 [DOI] [PubMed]
- 96.Thompson RJ, Zhou N, MacVicar BA (2006) Ischemia opens neuronal gap junction hemichannels. Science 312:924–927 [DOI] [PubMed]
- 97.Locovei S, Wang J, Dahl G (2006) Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 580:239–244 [DOI] [PubMed]
- 98.Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25:5071–5082 [DOI] [PMC free article] [PubMed]
- 99.Locovei S, Scemes E, Qiu F, Spray DC, Dahl G (2007) Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS Lett 581:483–488 [DOI] [PMC free article] [PubMed]
- 100.Pankratov Y, Lalo U, Verkhratsky A, North RA (2006) Vesicular release of ATP at central synapses. Pflügers Arch Eur J Physiol 452:589–597 [DOI] [PubMed]
- 101.North RA, Verkhratsky A (2006) Purinergic transmission in the central nervous system. Pflügers Arch Eur J Physiol 452:479–485 [DOI] [PubMed]
- 102.Njus D, Kelley PM, Harnadek GJ (1986) Bioenergetics of secretory vesicles. Biochim Biophys Acta 853:237–265 [DOI] [PubMed]
- 103.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG (2005) Astrocytic purinergic signaling coordinates synaptic networks. Science 310:113–116 [DOI] [PubMed]
- 104.Bowser DN, Khakh BS (2007) Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol 129:485–491 [DOI] [PMC free article] [PubMed]
- 105.Maroto R, Hamill OP (2001) Brefeldin A block of integrin-dependent mechanosensitive ATP release from Xenopus oocytes reveals a novel mechanism of mechanotransduction. J Biol Chem 276:23867–23872 [DOI] [PubMed]
- 106.Lalo U, Pankratov Y, North RA, Verkhratsky A (2007) Spontaneous autocrine release of protons activates ASIC-mediated currents in HEK293 cells. J Cell Physiol 212:473–480 [DOI] [PubMed]
- 107.Locovei S, Bao L, Dahl G (2006) Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A 103:7655–7659 [DOI] [PMC free article] [PubMed]
- 108.Beigi RD, Dubyak GR (2000) Endotoxin activation of macrophages does not induce ATP release and autocrine stimulation of P2 nucleotide receptors. J Immunol 165:7189–7198 [DOI] [PubMed]
- 109.Geyti CS, Odgaard E, Jensen ME, Leipziger J, Praetorius HA (2008) Slow spontaneous [Ca2+]i oscillations reflect nucleotide release from renal epithelia. Pflugers Archiv 455(6):1105–1117 [DOI] [PubMed]
- 110.Joseph SM, Pifer MA, Przybylski RJ, Dubyak GR (2004) Methylene ATP analogs as modulators of extracellular ATP metabolism and accumulation. Br J Pharmacol 142:1002–1014 [DOI] [PMC free article] [PubMed]
- 111.Ostrom RS, Gregorian C, Insel PA (2000) Cellular release of and response to ATP as key determinants of the set-point of signal transduction pathways. J Biol Chem 275:11735–11739 [DOI] [PubMed]
- 112.Rieg T, Bundey RA, Chen Y, Deschenes G, Junger WG, Insel PA, Vallon V (2007) Mice lacking P2Y2 receptors have salt-insensitive hypertension and facilitated renal Na+ and water excretion. FASEB J 13:3717–3726 [DOI] [PubMed]
- 113.Lehrmann H, Thomas J, Kim SJ, Jacobi C, Leipziger J (2002) Luminal P2Y2 receptor-mediated inhibition of Na+ absorption in isolated perfused mouse CCD. J Am Soc Nephrol 13:10–18 [DOI] [PubMed]
- 114.Leipziger J (2003) Control of epithelial transport via luminal P2 receptors. Am J Physiol Renal Physiol 284:F419–F432 [DOI] [PubMed]
- 115.Rouse D, Leite M, Suki WN (1994) ATP inhibits the hydrostatic effect of AVP in rabbit CCT: evidence for a nucleotide P2U receptor. Am J Physiol 267:F289–F295 [DOI] [PubMed]
- 116.Sauer H, Hofmann C, Wartenberg M, Wobus AM, Hescheler J (1998) Spontaneous calcium oscillations in embryonic stem cell-derived primitive endodermal cells. Exp Cell Res 238:13–22 [DOI] [PubMed]
- 117.Kawano S, Otsu K, Kuruma A, Shoji S, Yanagida E, Muto Y, Yoshikawa F, Hirayama Y, Mikoshiba K, Furuichi T (2006) ATP autocrine/paracrine signaling induces calcium oscillations and NFAT activation in human mesenchymal stem cells. Cell Calcium 39(4):313–324 [DOI] [PubMed]
- 118.Hellman B, Dansk H, Grapengiesser E (2004) Pancreatic {beta}-cells communicate via intermittent release of ATP. Am J Physiol Endocrinol Metab 286(5):E759–E765 [DOI] [PubMed]
- 119.Espelt MV, Estevez AY, Yin X, Strange K (2005) Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: role of the inositol-1, 4, 5-trisphosphate receptor and phospholipases C beta and gamma. J Gen Physiol 126:379–392 [DOI] [PMC free article] [PubMed]
- 120.Lazarowski ER, Homolya L, Boucher RC, Harden TK (1997) Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J Biol Chem 272:24348–24354 [DOI] [PubMed]
- 121.Praetorius HA, Frokiaer J, Leipziger J (2005) Transepithelial pressure pulses induce nucleotide release in polarized MDCK cells. Am J Physiol Renal Physiol 288:F133–F141 [DOI] [PubMed]
- 122.Harden TK, Lazarowski ER, Boucher RC (1997) Release, metabolism and interconversion of adenine and uridine nucleotides: implications for G protein-coupled P2 receptor agonist selectivity. Trends Pharmacol Sci 18:43–46 [PubMed]
- 123.Bodin P, Burnstock G (2001) Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol 38:900–908 [DOI] [PubMed]
- 124.Jensen ME, Odgaard E, Christensen MH, Praetorius HA, Leipziger J (2007) Flow-induced [Ca2+]I increase depends on nucleotide release and subsequent purinergic signaling in the intact nephron. J Am Soc Nephrol 18:2062–2070 [DOI] [PubMed]
- 125.Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, Zhang L, Collins PL, Pickles RJ, Fredberg JJ, Boucher RC (2005) Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. J Biol Chem 280:35751–35759 [DOI] [PMC free article] [PubMed]
- 126.Voets T, Nilius B (2003) TRPs make sense. J Membr Biol 192:1–8 [DOI] [PubMed]
- 127.Bodin P, Bailey D, Burnstock G (1991) Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol 103:1203–1205 [DOI] [PMC free article] [PubMed]
- 128.Saiag B, Bodin P, Shacoori V, Catheline M, Rault B, Burnstock G (1995) Uptake and flow-induced release of uridine nucleotides from isolated vascular endothelial cells. Endothelium 2:279–285
- 129.Yamamoto K, Sokabe T, Matsumoto T, Yoshimura K, Shibata M, Ohura N, Fukuda T, Sato T, Sekine K, Kato S, Isshiki M, Fujita T, Kobayashi M, Kawamura K, Masuda H, Kamiya A, Ando J (2006) Impaired flow-dependent control of vascular tone and remodeling in P2X4-deficient mice. Nat Med 12:133–137 [DOI] [PubMed]
- 130.Praetorius HA, Spring KR (2001) Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184:71–79 [DOI] [PubMed]
- 131.Praetorius HA, Spring KR (2003) Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 191:69–76 [DOI] [PubMed]
- 132.Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM (2003) Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285:F998–F1012 [DOI] [PubMed]
- 133.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33:129–137 [DOI] [PubMed]
- 134.Praetorius HA, Spring KR (2005) A physiological view of the primary cilium. Annu Rev Physiol 67:515–529 [DOI] [PubMed]
- 135.Okuhara DY, Geng L, Sfakianos JN, Mellman I, Somlo S (2006) Flow-induced Ca2+ Signaling in MDCK Cells. J Am Soc Nephrol 17:705A Abstract
- 136.Mitchell CH, Carre DA, McGlinn AM, Stone RA, Civan MM (1998) A release mechanism for stored ATP in ocular ciliary epithelial cells. Proc Natl Acad Sci U S A 95:7174–7178 [DOI] [PMC free article] [PubMed]
- 137.van der Wijk T, De Jonge HR, Tilly BC (1999) Osmotic cell swelling-induced ATP release mediates the activation of extracellular signal-regulated protein kinase (Erk)-1/2 but not the activation of osmo-sensitive anion channels. Biochem J 343:579–586 [DOI] [PMC free article] [PubMed]
- 138.van der Wijk T, Tomassen SF, Houtsmuller AB, De Jonge HR, Tilly BC (2003) Increased vesicle recycling in response to osmotic cell swelling. Cause and consequence of hypotonicity-provoked ATP release. J Biol Chem 278:40020–40025 [DOI] [PubMed]
- 139.Gorelik J, Zhang Y, Sanchez D, Shevchuk A, Frolenkov G, Lab M, Klenerman D, Edwards C, Korchev Y (2005) Aldosterone acts via an ATP autocrine/paracrine system: the Edelman ATP hypothesis revisited. Proc Natl Acad Sci U S A 102:15000–15005 [DOI] [PMC free article] [PubMed]
- 140.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R (2001) Proteinase-activated receptors. Pharmacol Rev 53:245–282 [PubMed]