Abstract
Angiotensin-converting enzyme (ACE) has been implicated in Alzheimer's disease (AD): ACE1 variations influence plasma ACE and risk of AD, and ACE is increased in AD brain. We measured frontal ACE level and activity in 89 AD and 51 control brains, and post-mortem CSF from 101 cases and 19 controls. Neuron-specific enolase (NSE) level and Braak stage were used to indicate neuronal preservation and disease progression. We genotyped the common ACE insertion/deletion polymorphism, rs4343, rs1800764 and rs4921. ACE activity was elevated in AD and correlated with Braak stage. Crude ACE levels were unchanged but adjustment for NSE suggested increased neuronal ACE production with Braak stage. Exposing SH-SY-5Y neurons to oligomeric Aβ1-42 increased ACE level and activity, suggesting Aβ may upregulate ACE in AD. In CSF, ACE level but not activity was reduced in AD. ACE1 genotype did not predict ACE level or activity in brain or CSF. ACE activity and neuronal production increase in AD brain, possibly in response to Aβ. Peripheral measurements do not reflect ACE activity in the brain.
Keywords: Angiotensin-converting enzyme, enzyme activity, Braak stage, ACE1, Alzheimer's disease, cerebrospinal fluid, neuron-specific enolase
Introduction
Angiotensin-converting enzyme (ACE) is an endopeptidase that consists of two catalytic domains and is normally expressed by endothelial, epithelial and neuronal cells [1]. It exists in both membrane-bound (ACE) and soluble (sACE) forms, the latter produced by the action of an as yet unidentified zinc metalloprotease (‘ACE secretase’) which cleaves mature, membrane-bound ACE at a juxtamembranous extracellular domain to release the large extracellular part of the enzyme [2, 3]. The traditional view of the function of ACE relates to the renin-angiotensin system (RAS) pathway, within which ACE catalyzes the formation of the vasoconstrictor octapeptide angiotensin II (AngII) from the its non-vasoactive precursor angiotensin I (AngI) and is also responsible for cleavage and inactivation of the vasodilator bradykinin [4]. The net result is vasopressor activity, which can be blocked by ACE-inhibitors – a standard treatment for hypertension [5].
More recently ACE has been shown to cleave amyloid-β (Aβ), the accumulation of which is central to the pathogenesis of Alzheimer's disease (AD) and cerebral amyloid angiopathy (CAA). ACE-mediated cleavage of Aβ has been demonstrated in vitro [6–9], ex vivo [10] and in some [10, 11] but not all [12, 13] recently studied animal models of AD. Variation in the efficiency of degradation of Aβ by ACE has been hypothesized to underpin the association between the ACE1 and AD [5, 14, 15]; inheritance of the DD genotype of a common Alu 237-bp insertion(I)/deletion(D) (indel) polymorphism (rs1799752) in intron 16 of the ACE1 gene [16] was reported to be associated with higher plasma levels of ACE [17] and reduced risk of AD [16]. The latter observation was confirmed in several meta-analyses [18–21] and more recently in whole-genome association studies (John Hardy, personal communication) [22, 23]. Indeed after APOE, the only widely accepted susceptibility gene for late-onset AD [24], ACE1 is probably the strongest candidate susceptibility gene for AD. The Aβ degradation hypothesis would explain this on the basis that differences in ACE1 genotype influence ACE levels and activity and these, in turn, affect Aβ accumulation and toxicity.
In most published studies of ACE protein levels and enzyme activity in human brain tissue, ACE was found to be elevated in AD [25–27]. We reported a positive association between ACE activity and parenchymal Aβ load [27]. Studies of ACE levels and activity in the cerebrospinal fluid (CSF) have yielded apparently inconsistent findings, some showing increased ACE activity in AD [28], others showing ACE protein levels to be unchanged [29] or reduced [30]. Most ACE within the brain is of neuronal origin [31], and a limitation of previous studies of ACE in brain tissue in AD has been the lack of adjustment for neuronal loss or damage. Furthermore, ACE1 genotypes have been ignored in studies of ACE in both brain and CSF; the assumption has been made that the relationship between ACE1 genotype and ACE expression is the same in the central nervous system as in the periphery, where the ACE1 DD genotype is associated with increased ACE levels and activity [17, 19, 32–36].
Our aims in this study were to measure ACE levels and activity in frontal cortex and CSF from a series of neuropathologically-confirmed AD and control cases, to examine the implications of adjusting the cortical measurements for neuronal loss or damage as indicated by a reduction in neuron specific enolase (NSE) [32], and also the impact of disease progression as indicated by Braak tangle stage. In addition, we wished to analyse the influence on ACE levels and activity (in both cortex and CSF) of ACE1 genotype – not only the AD-associated ACE1 indel polymorphism but also SNPs rs4291, rs4343 and rs1800764, which we previously showed to be associated individually and as haplotypes with increased risk of AD, increased CSF Aβ and earlier age of disease onset [19, 37].
Materials and Methods
We used brain tissue from the South West Dementia Brain Bank (Human Tissue Authority licence number 12273), University of Bristol, with local Research Ethics Committee approval. To ensure that we examined the full spectrum of disease severity, we included not only cases of ‘definite’ AD (assessed according to the criteria of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD)) and controls (showing the absence of AD or other neuropathological abnormalities), but also cases with AD pathology of intermediate severity (CERAD ‘possible’ and ‘probable’ AD). For analyses in which the comparison was simply between AD and control brains, we included the cases of possible, probable and definite AD in the AD group. For analysis according to Braak stage pathology the following groups were used: Braak stages 0-II, III-IV and V-VI. Braak stage was determined for each case after assessment of temporal tissue sections stained using mouse monoclonal PHF-Tau antibodies (AT8, clone BR03, Autogen Bioclear, Wiltshire, UK).
The age, gender and post-mortem delay data are summarised in Table 1. Information on the use of ACE-inhibitors (ACE-Is) was available for a small number of cases; ACE levels and activity were not significantly different between individuals who were and those who were not on ACE inhibitors (data not shown) and ACE-inhibitor usage was not therefore used to stratify data for subsequent analysis.
Table 1.
Summary of clinical features of left mid-frontal cortex tissue homogenates and post-mortem CSF
| Diagnosis AD:Control | Gender Male:Female | Age (Mean ± SEM) | Post-mortem delay (Mean ± SEM) | |
|---|---|---|---|---|
| Frontal Cortex | 89 AD | 46:53 | 79.2 ± 9.0 | 41.7 ± 23.2 |
| 51 C | 18:33 | 77.7 ± 10.8 | 44.9 ± 38.3 | |
| Cerebrospinal Fluid | 101 AD | 41:60 | 79.3 ± 9.6 | 44.1 ± 24.0 |
| 19 C | 12:7 | 82.6 ± 10.9 | 39.7 ± 23.4 |
ACE genotyping had been performed for a previous study [19]; the data are summarised in Table 2. Of note is the close linkage disequilibrium between rs4343 (situated in exon 17 of ACE1) and the ACE1 indel polymorphism (r2 =0.91), as previously described [19], whilst rs4291 and rs1800764 are in the ACE1 promoter and 5′ untranslated region (UTR) of the gene and were previously shown to be associated with AD and with ACE plasma levels [37].
Table 2.
Genotype and allelic frequencies of ACE-1 genotypes (Indel, rs1800764, rs4343 and rs4291) in Alzheimer's disease
| ACE1 | n | Genotype Frequencies | Allele Frequencies | |||
|---|---|---|---|---|---|---|
| Indel | DD (%) | ID (%) | II (%) | D allele | I allele | |
| Control | 49 | 22 (0.44) | 20 (0.41) | 7 (.0.14) | 0.67 | 0.33 |
| AD | 86 | 24 (0.28) | 39 (0.45) | 23 (0.27) | 0.51 | 0.49 |
| rs1800764 | CC | TC | TT | C | T | |
| Control | 43 | 12 (0.28) | 24 (0.56) | 7 (0.16) | 0.56 | 0.44 |
| AD | 60 | 12 (0.2) | 28 (0.47) | 20 (0.33) | 0.43 | 0.56 |
| rs4291 | AA | TA | TT | A | T | |
| Control | 43 | 12 (0.28) | 21 (0.49) | 10 (0.23) | 0.52 | 0.48 |
| AD | 59 | 9 (0.15) | 22 (0.37) | 28 (0.48) | 0.34 | 0.66 |
| rs4343 | GG | AG | AA | G | A | |
| Control | 43 | 16 (0.37) | 19 (0.44) | 8 (0.18) | 0.59 | 0.41 |
| AD | 62 | 16 (0.26) | 29 (0.47) | 17 (0.27) | 0.49 | 0.51 |
ACE activity assays
Fresh frozen frontal cortex (200mg) from the left mid-frontal region (Brodmann area 6) was homogenized in a Precellys automated homogeniser (Stretton Scientific, Derbyshire, UK) with 2mm ceramic beads (Thistle Scientific, Glasgow, UK) in 1ml of 1M Tris pH 7.6 buffer containing 1% SDS, 5M NaCl, and the protease inhibitors aprotinin (1μg/ml; Sigma Aldrich, Gillingham, UK) and PMSF (10uM; Sigma Aldrich) The homogenates were spun at 13000rpm for 15 minutes at 4°C and the supernatant was removed and stored at -80°C until used. Ventricular cerebrospinal fluid collected at the time of autopsy was spun and aliquoted and stored at -80°C until used.
A fluorogenic enzyme activity assay, using the ACE-specific fluorogenic peptide substrate Abz-FRK(Dnp)-P) (Biomol International, Exeter, UK), was used to measure ACE activity, as previously described [27]. Fluorescence was measured with excitation at 320nm and emission at 405nm, in a fluorescent plate reader (FLUOstar, BMG Labtech, Aylesbury, UK). The specificity of the assay was assessed for serial dilutions of recombinant human ACE (0, 31.125, 62.5, 125, 250, 500, 1000ng/ml) (R&D systems, Abingdon, UK), serial dilutions of brain tissue homogenates (1000μg/ml total protein, 500, 250, 125, 62.5 or 31.25μg/ml), and CSF; in all cases incubation with captopril (1mM) (Biomol International), an ACE-specific inhibitor, reduced enzyme activity by more than 90%.
Brain tissue homogenates were assayed at a concentration of 250μg/ml (total protein) and 10μl samples of CSF in a total volume of 50μl. Fluorescence was read in the presence and absence of captopril. Each sample was assayed on two separate occasions and the mean enzyme activity calculated. To prevent error resulting from plate-to-plate variation, each plate of samples included serial dilutions of recombinant human ACE that were used for calibration.
To test the post-mortem stability of ACE activity, we used several immediately adjacent samples of frontal cortex from one control brain that were incubated for 24, 48 or 72h at room temperature or 4°C before being homogenised in 1% SDS lysis buffer as described above. To assess the effect of different detergents on the results of the assay, we measured ACE activity in immediately adjacent samples of frontal cortex that were homogenised in Tris buffer containing 1% SDS, 0.5% NP-40 (Sigma Aldrich), 0.5% triton-X-100 (Sigma Aldrich), or in the absence of detergent. The tissue homogenates were spun at 13K rpm for 15 minutes at 4°C and the supernatants stored at -80°C until used.
ACE protein measurements
A commercially available sandwich ELISA (R&D systems) was used according to the manufacturer's guidelines to measure ACE concentration in brain tissue homogenates and CSF. Goat-anti human ACE (0.8μg/ml) was coated overnight on Costar EIA microplates (R&D systems), washed five times with phosphate-buffered saline (PBS)/0.05% tween 20 and blocked with 1% PBS/bovine serum albumin (BSA) (1%PBS/BSA) for 2h. After a further five washes, samples and recombinant human ACE standards were added to the plate for 2h with shaking. Guided by preliminary studies in which serially diluted samples of frontal tissue homogenate from a control case were used to determine the linear range of the assay, we added 150μg of (total) protein diluted in 100ul of 1%PBS/BSA to each well. For assays of ACE in CSF, we found that measurements in the linear range of the assay were obtained by adding 25μl of CSF in 100μl 1%PBS/BSA. After 2h the plates were washed and incubated with biotin-labelled anti-ACE rabbit polyclonal antibody (0.2μg/ml) for 2h. The plates were again washed, streptavidin-horseradish peroxidase (1:100) was added for twenty minutes in the dark, then tetramethylbenzidine (TMB) was added and emission read at 450nm. The assays were repeated in duplicate, with standards included on each plate to prevent error from plate-plate variation. ACE concentrations were determined by interpolation from the standard curve determined for each plate from the known concentrations of recombinant human ACE.
Measurement of neuron-specific enolase (NSE)
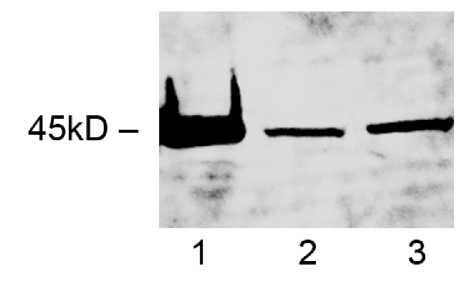
A western blot of NSE was performed to validate the specificity of the anti-NSE antibody (Abcam, Cambridge, UK), which was used for subsequent measurements. A single band was observed at 45 kDa in homogenates of human frontal cortex (Figure 1). NSE levels in brain homogenates were then measured by direct ELISA. Briefly, Costar EIA microplate wells were coated with 10μl of brain tissue homogenate (200mg wet brain in 1ml 1% SDS buffer, as above) for 2h, washed 5 times in PBS/tween 0.05% and incubated with anti-NSE (1:1000) for 2h. After further washes, mouse-HRP (1:100) (Vector Labs, Peterborough, UK) was added for 20 minutes in the dark, washed and TMB substrate added for 10 minutes. A serial dilution of recombinant human NSE (Biomol International, Exeter, UK) was used to construct a best-fit curve and NSE concentrations in each individual were calculated by interpolation. Each sample was run in duplicate and the mean determined. NSE level was significantly reduced in AD, particularly in Braak stages V-VI [38] and was used to provide a measure of the number of functionally intact neurons in tissue samples. Measurements of ACE levels and activity were adjusted for neuronal loss or damage according to the formula: ACEadjusted = ACEsample x NSEmean normal / NSEsample, where ACEadjusted was the level or activity of (neuronal) ACE after correcting for neuronal loss in the sample, ACEsample was the unadjusted level or activity of ACE in the sample, NSEmean normal was the mean level of NSE in the control cohort, and NSEsample was the measured level of NSE in the sample. To normalise the data, which were right-skewed in both the AD and control groups, all NSE-adjusted ACE protein and activity measurements were logarithmically transformed for statistical analysis.
Figure 1.
Representative western blot of neuron specific enolase in brain tissue homogenate prepared from the frontal cortex. Recombinant human NSE (5μg) was loaded in lane 1 and homogenates (30μg total protein) from an AD and control brain were loaded in lanes 2 and 3 respectively.
In vitro assays
SH-SY5Y cells were grown in DMEM (Sigma Aldrich) supplemented with 2mM glutamine (Sigma Aldrich) and 15% fetal calf serum (Autogen Bioclear, Wiltshire, UK) at 37°C in 5%CO2/95% air. The cells were differentiated in 10uM retinoic acid (Sigma Aldrich) for 6 days and exposed for 4h or 24h to 10μM monomeric Aβ1-42 (Anaspec, CA, U.S.A) or oligomeric Aβ1-42 (prepared by leaving the Aβ1-42 overnight in phosphate-buffered saline, as described) [39], or left in control medium (DMEM). Following treatment, cells were lysed in 1% SDS lysis buffer as above, and supernatants stored at -80°C until used. Total protein level, ACE activity and protein level were determined for each cell lysate, as above.
Statistical analysis
Data were analysed by independent-samples t-test, one way ANOVA with Bonferroni post-testing, or Spearman rank correlation analysis, as appropriate, with the help of Statistical Package for Social Science software (12.0.1). Values of p < 0.05 were considered significant.
Result
Effects of tissue storage and lysis buffer detergent on ACE activity
Incubation of tissue at room temperature or 4°C for 24–72h did not cause significant change in ACE activity (Figure 2A). ACE activity was comparable in all of the brain tissue homogenates that contained detergent (1% SDS, 0.5% triton X-100 or 0.5% NP1-40) but was significantly lower in tissue homogenised in the absence of detergent (Figure 2B).
Figure 2.
Bar charts showing the effect of tissue storage and lysis buffer detergents on ACE enzyme activity in frontal cortex. (A) Storage of brain tissue for up to 72h at 4°C or room temperature did not affect ACE activity. (B) Bar chart showing ACE activity in brain tissue homogenates prepared in lysis buffers containing different detergents (1% SDS, 0.5% NP-40 or 0.5% triton-X-100). Error bars indicate 1 standard error of the mean (SEM). The three detergents yielded similar measurements but measured ACE activity was lower in the absence of detergent.
ACE levels and activity in homogenates
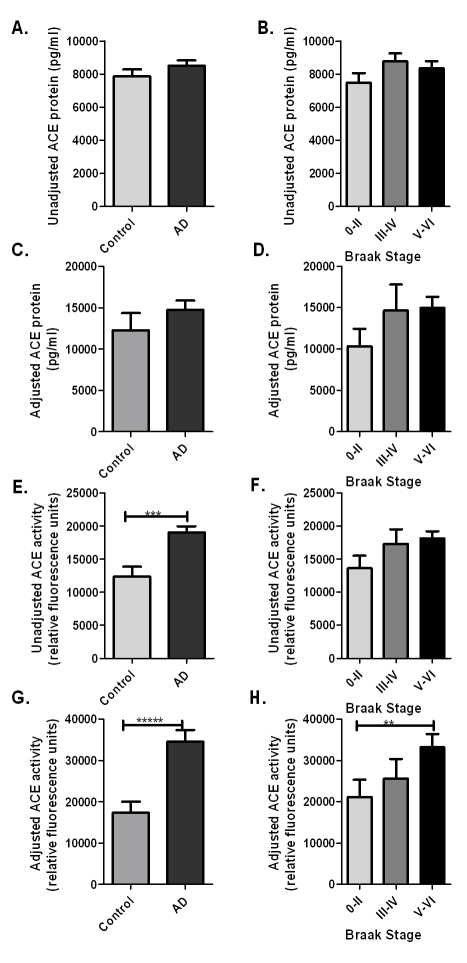
Unadjusted ACE protein levels were similar in AD (mean=8506 pg/ml, SE=312) and control cases (mean=7874 pg/ml, SE=414) and did not change with Braak stage (Figure 3A, B). After NSE adjustment for neuronal damage and loss, ACE levels were greater in AD cases than controls but not significantly so (in AD, mean=14741 pg/ml, SE=1114; in controls mean=12272 pg/ml, SE=2064; p=0.275) (Figure 3C). However, NSE-adjusted ACE levels correlated significantly with Braak stage (p=0.004, Spearman rank correlation coefficient rs=0.266) (Figure 3D), reflecting increased neuronal production of ACE with progression of disease, after correcting for neuronal damage and loss in severe AD.
Figure 3.
Bar charts showing ACE level and enzyme activity in frontal cortex in relation to diagnosis and Braak stage, both before (unadjusted) and after (adjusted) adjusting for neuronal damage as measured by the reduction in NSE level below the mean control value. Error bars indicate 1 SEM. (A-B) Unadjusted ACE level did not vary with AD or Braak stage. (C-D) Adjusted ACE level increased in AD and was positively associated with Braak stage (p=0.004, Spearman rs=0.266). (E-F) ACE activity increased significantly in AD (p=0.0001) and was positively associated with Braak stage (p=0.03, rs=0.201). (G-H) Adjusted ACE activity increased even more dramatically in AD (p=0000002) and with increasing Braak stage (p=0.005, rs = 0.260). Post hoc comparison between subgroups revealed significantly increased ACE activity in Braak stage V-VI compare to 0-II (ANOVA, p=0.005).
Unadjusted ACE activity was significantly increased in AD (mean=19063 relative fluorescence units (r.f.u.), SE=902) compared to controls (mean=12398 r.f.u., SE=1465; p=0.0001) (Figure 3E) and ACE activity correlated significantly with Braak tangle stage (p=0.03, rs=0.201) (Figure 3F). When the measurements were adjusted for NSE levels, to provide an indication of ACE activity per functioning neuron, the increase in ACE activity in AD was approximately two-fold (in AD, mean=34483 r.f.u, SE=2883; in controls mean=17357 r.f.u., SE=2650; p=0.0000002) (Figure 3G). Adjusted ACE activity correlated significantly with Braak tangle stage (p=0.005, rs=0.260). ANOVA revealed significant differences in ACE activity between Braak stage 0-II, III-IV and V-VI (p=0.004), Bonferroni post hoc analysis showing significantly increased ACE activity in Braak stages V-VI than 0-II (p=0.005) (Figure 3H). ACE levels and activity did not correlate with post-mortem delay and did not vary with gender, age or APOE genotype (data not shown).
ACE levels and activity in CSF
As for the tissue homogenates, the CSF samples were analysed for correlation between PM delay and ACE levels and activity. Within the CSF, ACE activity did not correlate with post-mortem delay but CSF ACE levels did show a significant positive correlation with post-mortem delay (rs=0.215, p=0.011) (data not shown). For subsequent analyses, we used the best-fit linear regression equation to adjust measurements of CSF ACE levels for post-mortem delay. We did not adjust CSF ACE levels or activity for NSE levels in the frontal cortex.
There was an unexpected disparity between the level and activity of ACE in the CSF. In AD, ACE levels were significantly lower in AD (mean=1633.0 pg/ml, SE=334.4) than controls (mean=661.7 pg/ml, SE=150.0; p=0.030) (Figure 4A). ACE levels were significantly lower in Braak stages V-VI than 0-II (ANOVA, p=0.04) and the reduction in ACE correlated with Braak tangle stage (rs =-0.252, p=0.010) (Figure 4B). In contrast ACE activity was higher in AD (mean=16611.7 r.f.u, SE=1286.3) than controls (mean=11864.1 r.f.u., SE=2071.8; p=0.060) but the difference was not statistically significant (Figure 4C). There was a non-significant trend towards elevated ACE activity in intermediate and higher Braak stages (Figure 4D).
Figure 4.
Bar charts showing ACE level and activity in post-mortem CSF samples in relation to AD and disease progression. Error bars indicate 1 SEM. (A-B) ACE level was significantly lower in AD than control CSF (p=0.030) and correlated inversely with Braak stage (p=0.010, rs =-0.252). ACE levels were significantly lower in Braak stages V-VI than 0-II (p=0.04). (C-D) ACE activity was greater in AD than control CSF but the difference did not reach significance (p=0.060).
Aβ1-42 upregulation of ACE in SH-SY5Y cells
Four-hour incubation of SH-SY5Y cells with either monomeric or oligomeric Aβ1-42 did not result in significant changes in ACE level or activity compared to the values in DMEM control cultures (Figure 5A and C). However, after 24h, ACE protein level (p=0.0004, ANOVA) and activity (p=0.033) differed significantly between the three groups. Post hoc comparisons revealed that ACE protein level was significantly greater in cells incubated with oligomeric Aβ1-42 than in control cultures (p=0.00087) or in cells incubated with monomeric Aβ1-42 (p=0.0011) (Figure 5B and D): the increase was approximately 1.8-fold.
Figure 5.
In vitro induction of neuronal ACE by oligomeric Aβ1-42. Human SH-SY5Y cells, differentiated in retinoic acid, were incubated with either monomeric (M) or oligomeric/fibrillar (OF) Aβ1-42. Bar charts show (A-B) ACE levels and (C-D) ACE activity in SH-SY5Y cells after incubation for 4 and 24h respectively. ANOVA revealed significant differences in ACE protein (p=0.0004) and activity (0.033) levels at 24h. Post hoc comparisons revealed that ACE protein level was significantly greater in cells incubated with oligomeric Aβ1-42 than in control cultures (p=0.00087) or in cells incubated with monomeric Aβ1-42 (p=0.0011). Error bars indicate 1 SEM.
Brain and CSF ACE levels and activity and ACE1 variation
We evaluated four genetic markers covering the primary ACE1 transcript (refseq NM_ 000789.2) that included rs4343, rs4291, rs1800764, and the commonly studied ACE1 indel in intron 16 which is located near to and closely correlated with rs4343. All markers were in relatively strong linkage disequilibrium (data not shown). Genotypic means of unadjusted and NSE-adjusted ACE protein and activity levels were compared for each marker by ANOVA. The case-control status by genotype interaction terms were not significant in second order factorial ANOVA models (adjusted and unadjusted ACE levels and activity are given for the ACE1 indel in Table 3). In a combined case-control cohort, there was modest evidence of association of ACE activity in brain homogenates with the indel polymorphism (F2,134 = 3.6, P = 0.030) with homozygotes of the insertion allele having the highest levels (Figure 6). The remaining markers showed no evidence of genetic association, although similar trends were observed to those for the indel polymorphism (adjusted and unadjusted ACE levels and activity are given for SNPs rs1800764, rs4291, rs4343 in Table 4).
Table 3.
Analysis of genetic association between ACE1 indel polymorphism and ACE protein level and enzyme activity
| ACE1 indel | DD (mean ± SEM) | ID (mean ± SEM) | II (mean ± SEM) | |
|---|---|---|---|---|
| ACE protein: | ||||
| Unadjusted | Control | 7158.575 ± 666.5 | 9013.801 ± 600.7 | 7017.929 ± 1096.3 |
| AD | 8992.339 ± 614.4 | 8793.35 ± 438.0 | 7812.1 ± 673.5 | |
| Combined | 8115.32 ± 467.4 | 8868.08 ± 351.2 | 7626.79 ± 570.7 | |
| Adjusted | Control | 10733.43 ± 3035.2 | 13436.51 ± 3903.1 | 12290.59 ± 3285.3 |
| AD | 17673.58 ± 2537.6 | 13685.57 ± 1698.2 | 14366.76 ± 1684.6 | |
| Combined | 14354.38 ± 2010.0 | 13601.14 ± 1714.9 | 13882.32 ± 1482.6 | |
| ACE activity: | ||||
| Unadjusted | Control | 11241.59 ± 1902.8 | 13172.35 ± 2328.4 | 16497.86 ± 5791.2 |
| AD | 17803.04 ± 2058.8 | 18234.92 ± 1283.7 | 21675.78 ± 1638.6 | |
| Combined | 14664.96 ±1476.0 | 16518.80 ± 1189.4 | 20467.60 ± 1829.2 | |
| Adjusted | Control | 14774.38 ± 3521.3 | 17107.29 ± 3979.2 | 29133.75 ± 10689.0 |
| AD | 37717.25 ± 6584.6 | 28745.83 ± 3899.8 | 42175.98 ± 5450.7 | |
| Combined | 26744.57 ± 4153.1 | 24800.56 ± 2977.9 | 39132.80 ± 4883.1* |
= p<0.05, ANOVA comparison between genotypes.
Figure 6.
Bar charts show genetic associations between ACE1 indel polymorphism (rs1799752) and ACE enzyme activity, (A) before and (B) after adjustment for neuson specific enolase, in a case-control cohort.
Table 4.
Analysis of genetic association between ACE1 and ACE protein level and enzyme activity
| (A) rs1800764 | CC (mean ± SEM) | CT (mean ± SEM) | TT (mean ± SEM) | |
| Protein | ||||
| Unadjusted | Control | 6848.17 ± 941.3 | 8456.223 ± 635.2 | 8777.975 ± 677.4 |
| AD | 8020.553 ± 1130.3 | 9350.531 ± 415.9 | 8006.376 ± 781.6 | |
| Combined | 7434.36 ± 729.6 | 8937.77 ± 370.4 | 8220.29 ± 625.5 | |
| Adjusted | Control | 10322.92 ± 3462.7 | 14190.79 ± 3853.1 | 14020.02 ± 3352.4 |
| AD | 13477.97 ± 3643.7 | 14141.88 ± 2111.1 | 13918.17 ± 1871.1 | |
| Combined | 11900.45 ± 2479.9 | 14164.45 ± 2088.2 | 13941.67 ± 1601.7 | |
| Activity | ||||
| Unadjusted | Control | 14168.25 ± 3433.2 | 13770.33 ± 1896.1 | 13192.5 ± 6712.2 |
| AD | 18109.42 ± 1743.0 | 19328.71 ± 1605.5 | 22363.9 ± 1859.2 | |
| Combined | 16138.83 ± 1927.1 | 16763.31 ± 1278.1 | 20247.42 ± 2167.4 | |
| Adjusted | Control | 20527.48 ± 7284.6 | 18051 ± 2848.6 | 24202.39 ± 12672 |
| AD | 30925.16 ± 6141.6 | 31634.81 ± 5318.9 | 40670.86 ± 5470.2 | |
| Combined | 25726.32 ± 4783.8 | 25365.36 ± 3263.9 | 36870.45 ± 5180.0 | |
| (B) rs4291 | AA | AT | TT | |
| Protein | ||||
| Unadjusted | Control | 6674.813 ± 930.4 | 9007.931 ± 625.6 | 7784.164 ± 834.8 |
| AD | 9621.446 ± 791.7 | 9127.483 ± 531.2 | 8265.006 ± 620.8 | |
| Combined | 7937.66 697.0 | 9069.10 404.1 | 8138.47 503.0 | |
| Adjusted | Control | 9488.098 ± 3444.6 | 16030.63 ± 4306.5 | 10633.72 ± 2383.5 |
| AD | 18701.01 ± 3910.5 | 11658.29 ± 1831.9 | 15348.46 ± 2231.2 | |
| Combined | 13436.49 2718.4 | 13793.62 2298.9 | 14107.74 625.5 | |
| Activity | ||||
| Unadjusted | Control | 12784.83 ± 2807.1 | 14433.57 ± 2296.0 | 13076.6 ± 4286.5 |
| AD | 17751.11 ± 2087.1 | 16793.96 ± 1555.4 | 22791.11 ± 1490.4 | |
| Combined | 14913.24 ±1877.5 | 15641.21 ± 1370.4 | 20234.66 ±1692.7 | |
| Adjusted | Control | 16285.31 ± 5769.2 | 19826.83 ± 3747.4 | 22050.34 ± 8271.9 |
| AD | 36095.67 ± 7123.8 | 22340.84 ± 3918.9 | 43185.43 ± 5596.7 | |
| Combined | 24775.46 ± 4895.5 | 21113.07 ± 2689.3 | 37623.56 ± 4854.2 | |
| (C) rs4343 | GG | AG | AA | |
| Protein | ||||
| Unadjusted | Control | 7078.823 ± 749.8 | 9237.298 ± 657.7 | 7292.015 ± 988.2 |
| AD | 8651.248 ± 899.3 | 9036.407 ± 452.7 | 7885.407 ± 869.0 | |
| Combined | 7873.97 ± 615.6 | 9115.93 ± 373.5 | 7695.52 ± 660.8 | |
| Adjusted | Control | 9459.306 ± 2561.1 | 16500.79 ± 4760.2 | 11496.72 ± 2953.8 |
| AD | 14183.05 ± 3029.0 | 15169.35 ± 2321.1 | 12784.28 ± 1800.7 | |
| Combined | 11663.7 ±1979.9 | 15696.38 ± 2320.2 | 12372.26 ±1516.4 | |
| Activity | ||||
| Unadjusted | Control | 12337.88 ± 2397.4 | 14206.42 ± 2400.5 | 14995.13 ± 5235.6 |
| AD | 17966.79 ± 2059.5 | 19039.76 ± 1484.5 | 22959.47 ± 1727.7 | |
| Combined | 14964.70 1656.7 | 17126.56 1336.3 | 20410.88 2118.2 | |
| Adjusted | Control | 15913.34 ± 4593.5 | 19518.65 ± 4063.5 | 25852.84 ± 9821.2 |
| AD | 32390.6 ± 6097.5 | 32735.51 ± 5510.9 | 40444.01 ± 5614.6 | |
| Combined | 23602.73 ±3991.1 | 27503.8 ± 3781.0 | 35774.83 ± 5022.3 | |
There was no evidence of association between any marker and ACE protein levels or activity in CSF. However, there was a trend towards lower ACE protein levels in AD cases with the ACE1 II than the DD genotype, with intermediate levels in the ID genotype (Figure 7); this pattern was repeated for the other markers.
Figure 7.
Bar charts show genetic associations between ACE1 SNPs (indel rs1799752, rs1800764, rs4291 and rs4343) and ACE protein level (A-D) and activity (E-H) in human post-mortem CSF. Error bars indicate 1 SEM.
Discussion
Our studies have revealed significantly increased ACE activity in the frontal cortex in AD, particularly in severe disease. Although ACE protein level showed little change in AD, once the measurements were corrected for damage to neurons, the principal source of ACE within the brain, adjusted ACE protein levels were found to increase with Braak tangle stage, a pathological indicator of progression of disease. These findings indicate that neuronal production of ACE is upregulated in AD. Our in vitro studies suggest that this upregulation is at least partly a reaction to elevated levels of Aβ. ACE enzyme activity was also elevated in post-mortem CSF from AD patients; however, in the same samples of CSF, ACE protein level was significantly reduced. Variations in ACE1 were found to account for some of the variation in ACE levels in CSF, mirroring previous findings in plasma. However, ACE activity in CSF, and ACE protein and activity in the frontal cortex, were not associated with ACE1 genotype.
ACE activity is increased in AD in post-mortem human tissue and CSF
Our study supports previous reports that ACE protein level and activity are increased in post-mortem AD brain tissue, particularly after adjustment for neuronal loss [25, 26]. NSE is a neuron-specific protein that has been used by others to adjust for neuronal loss [32]. In our cohort, NSE levels were significantly reduced in Braak stages V-VI. Since ACE is exclusively neuronally expressed within the brain [31], we adjusted ACE protein and enzyme levels to mean NSE levels in the controls as an indicator of ACE production per functioning neuron. Our finding that NSE-adjusted levels of ACE increased progressively with Braak tangle stage suggests that expression of ACE is upregulated as a consequence of disease progression and that the increase is not a primary pathogenic factor in the development of the disease.
In human APP transgenic mouse models of AD, the levels of other Aβ-degrading enzymes, IDE and neprilysin, increase as Aβ accumulates [40, 41] and we previously showed an association between parenchymal Aβ load and ACE activity [27]. In vitro, several Aβ-degrading enzymes are induced in neurons, microglia and cerebrovascular cells by exposure to Aβ [40, 42, 43]. We have now extended those observations to reveal that exposure for 24h to oligomeric (although not monomeric) Aβ1-42 also increases ACE level and activity in SH-SY5Y neuronal cells. Induction of Aβ-degrading enzymes such as ACE with progression of AD is likely to be a homeostatic feedback response to increasing Aβ levels but other AD-associated abnormalities, such as inflammation, may also influence ACE expression.
In CSF, ACE activity is increased in AD but the protein level is reduced
Our finding of increased ACE activity in post-mortem CSF in AD is in keeping with the recent demonstration of elevated ACE activity in CSF from living patients with MCI or AD compared to healthy controls [28]. ACE protein level was, however, reduced in post-mortem CSF in AD which is in agreement with two previous studies [30, 44]. A third study found no change in CSF ACE in AD [45]. Differences in the findings of these studies may be attributable to case selection, cohort size and variations in the methods used to measure ACE. Other factors to consider are potential differences in the ACE content of ventricular CSF (as obtained post-mortem) and lumbar CSF (as sampled in vivo), and the contribution of post-mortem changes in the level of ACE in the CSF.
The disparity between ACE levels and activity in the CSF may relate to post-translational modifications that influence ACE activity. ACE activity may be modulated by glycosylation: deglycosylation was shown to increase the catalytic activity of somatic ACE extracted from bovine lung [46]. It may be of relevance that reduced glycosylation has been described for at least one other protein in AD – reduced O-linked N-acetylglucosamine glycosylation of tau [47]. ACE requires zinc as a co-factor for normal function [48] and increased levels of intracellular zinc in AD [49] may also influence ACE activity.
Differences in the relative amounts of soluble and membrane-associated ACE in the tissue homogenates and CSF could also contribute to variations in the level and activities of ACE in the two types of specimen, particularly as the catalytic properties of membrane-associated and soluble forms of ACE vary in microenvironments of different pH and ionic composition [50].
It is clear that further work is needed to clarify how ACE activity and protein level inter-relate across different physiological compartments, as highlighted by our own studies in CSF and brain tissue and other studies in plasma. Nielsen and colleagues found no evidence of association between AD and ACE levels (i.e. protein concentration) in either CSF or plasma [29] however, a recent study reported significant reductions in plasma ACE activity in AD cases compared with controls [51] in a two-year follow-up study. The differences in plasma ACE in these two studies may be due, in part, to differences in case selection and the fact the relationship between ACE level and activity may not be entirely straightforward. The observation by Vardy and colleagues of a progressive decline in plasma ACE activity with advancing disease supports our finding that ACE activity is related to the stage of disease. The change in plasma ACE activity in AD however appears to be in the opposite direction from our findings in post-mortem CSF, although the elevated ACE activity in AD CSF did not quite reach significance, possibly due to limited numbers of control samples. These data suggest that there may not be simple correlations between ACE level and activity in different tissues and body fluids.
Influence of ACE1 genotypic variations
Previous studies have shown that ACE serum and plasma levels in healthy volunteers are influenced by variation in the rs1799752 indel polymorphism in intron 16 of ACE1 [17, 52]. The trend that we found between the ACE1 indel and ACE level within the CSF mirrors what was previously found in plasma, with highest ACE protein levels in DD homozygotes, intermediate levels in ID hetereozygotes and lowest levels in II homozygotes [17, 52]. Similar trends were observed on analysis of other SNPs; the lack of statistical significance may reflect the relatively small cohort sizes. Higher plasma [53–55] and tissue [36] ACE activity levels are associated with the DD genotype in several other diseases including hypertension [56], diabetes [57], renal disease [54], and rheumatoid arthritis [58].
Relevance to ACE inhibitors in a clinical setting
Clinical trial and observational data have suggested an association between the use of ACE-inhibitors and reduced cognitive decline in both MCI and AD [5, 15]. Elevated ACE may affect cognition and increase the risk of AD by exacerbation of atherosclerotic vascular disease which has itself been linked to AD, and through downstream inflammatory or anti-cholinergic effects mediated by AngII [5]. Interference with such mechanisms is consistent with the clinical benefits of ACE-inhibitors in AD and MCI patients [5, 15] and with more recent findings of an extensive retrospective analysis of the U.S. Veterans Affairs Health system, including data on approximately 6 million subjects over the five years of study. Here, angiotensin receptor antagonists, which prevent the action of AngII at its receptors, were found to be more protective against the development and progression of dementia including Alzheimer's disease, than ACE-inhibitors or other antihypertensive medications (Professor Benjamin Wolozin, personal communication).
Conclusions
Our present data suggest that elevation of ACE activity in advanced AD is a secondary phenomenon and may in part be induced by an increase in the amount of Aβ. It remains possible that ACE may contribute to Aβ degradation and that the elevation of ACE activity in AD is a physiological response to Aβ accumulation. Our findings indicate that peripheral measurements of ACE are not a reliable indicator of ACE level or activity within the CNS. Direct analysis of brain tissue will be needed to clarify the roles and regulation of ACE activity in the brain.
Acknowledgments
This work was supported by grants from the Alzheimer's Research Trust, James Tudor Foundation, BRACE (Bristol Research into Alzheimer's and Care of the Elderly) and the Sigmund Gestetner Foundation.
Disclosure Statement
None of the authors have any actual financial or personal conflicts. Research used human tissue from the South West Dementia Brain Bank (Human Tissue Authority licence number 12273), University of Bristol, UK, with local Research Ethics Committee approval.
References
- 1.Turner AJ, Hooper NM. The angiotensin-converting enzyme gene family: genomics and pharmacology. Trends Pharmacol Sci. 2002;23:177–183. doi: 10.1016/s0165-6147(00)01994-5. [DOI] [PubMed] [Google Scholar]
- 2.Pang S, Chubb AJ, Schwager SL, Ehlers MR, Sturrock ED, Hooper NM. Roles of the juxtamembrane and extracellular domains of angiotensin-converting enzyme in ectodomain shedding. Biochem J. 2001;358:185–192. doi: 10.1042/0264-6021:3580185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin ET, Turner AJ, Hooper NM. Secretase-mediated cell surface shedding of the angiotensin-converting enzyme. Protein Pept Lett. 2004;11:423–432. doi: 10.2174/0929866043406544. [DOI] [PubMed] [Google Scholar]
- 4.Sayed-Tabatabaei FA, Oostra BA, Isaacs A, van Duijn CM, Witteman JCM. ACE Polymorphisms. Circ Res. 2006;98:1123–1133. doi: 10.1161/01.RES.0000223145.74217.e7. [DOI] [PubMed] [Google Scholar]
- 5.Kehoe PG, Wilcock GK. Is inhibition of the renin-angiotensin system a new treatment option for Alzheimer's disease? Lancet Neurol. 2007;6:373–378. doi: 10.1016/S1474-4422(07)70077-7. [DOI] [PubMed] [Google Scholar]
- 6.Hemming ML, Selkoe DJ. Amyloid beta-protein is degraded by cellular angiotensin-converting enzyme (ACE) and elevated by an ACE inhibitor. J Biol Chem. 2005;280:37644–37650. doi: 10.1074/jbc.M508460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu J, Igarashi A, Kamata M, Nakagawa H. Angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide (A beta); retards A beta aggregation, deposition, fibril formation; and inhibits cytotoxicity. J Biol Chem. 2001;276:47863–47868. doi: 10.1074/jbc.M104068200. [DOI] [PubMed] [Google Scholar]
- 8.Oba R, Igarashi A, Kamata M, Nagata K, Takano S, Nakagawa H. The N-terminal active centre of human angiotensin-converting enzyme degrades Alzheimer amyloid beta-peptide. Eur J Neurosci. 2005;21:733–740. doi: 10.1111/j.1460-9568.2005.03912.x. [DOI] [PubMed] [Google Scholar]
- 9.Toropygin IU, Kugaevskaya EV, Mirgorodskaya OA, Elisseeva YE, Kozmin YP, Popov IA, Nikolaev EN, Makarov AA, Kozin SA. The N-domain of angiotensin-converting enzyme specifically hydrolyzes the Arg-5-His-6 bond of Alzheimer's A. 2007. [DOI] [PubMed]
- 10.Zou K, Yamaguchi H, Akatsu H, Sakamoto T, Ko M, Mizoguchi K, Gong JS, Yu W, Yamamoto T, Kosaka K, Yanagisawa K, Michikawa M. Angiotensin-converting enzyme converts amyloid beta-protein 1–42 (Abeta(1–42)) to Abeta(1–40), and its inhibition enhances brain Abeta deposition. J Neurosci. 2007;27:8628–8635. doi: 10.1523/JNEUROSCI.1549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J, Ho L, Chen L, Zhao Z, Zhao W, Qian X, Humala N, Seror I, Bartholomew S, Rosendorff C, Pasinetti GM. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J Clin Invest. 2007;117:3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckman EA, Adams SK, Troendle FJ, Stodola BA, Kahn MA, Fauq AH, Xiao HD, Bernstein KE, Eckman CB. Regulation of steady-state beta -amyloid levels in the brain by neprilysin and endothelin-converting enzyme, but not angiotensin-converting enzyme. J. Biol. Chem. 2006 doi: 10.1074/jbc.M605827200. M605827200. [DOI] [PubMed] [Google Scholar]
- 13.Hemming ML, Selkoe DJ, Farris W. Effects of prolonged angiotensin-converting enzyme inhibitor treatment on amyloid beta-protein metabolism in mouse models of Alzheimer disease. Neurobiol Dis. 2007;26:273–281. doi: 10.1016/j.nbd.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kehoe PG. The renin-angiotensin-aldosterone system and Alzheimer's disease? Journal of the Renin-Aldosterone-Angiotensin-System. 2003;4:80–93. doi: 10.3317/jraas.2003.017. [DOI] [PubMed] [Google Scholar]
- 15.Kehoe PG, Wilcock GK. The Renin Angiotensin System in Alzheimer's Disease – Updates Highlight a Clinical and Biological Dichotomy? Current Alzheimer Research. 2006;3:171–173. [Google Scholar]
- 16.Kehoe PG, Russ C, McIlory S, Williams H, Holmans P, Holmes C, Liolitsa D VD, Powell J, McGleenon B, Liddell M, Plomin R, Dynan K, Williams N, Neal J, Cairns NJ, Wilcock G, Passmore P, Lovestone S, Williams J, Owen MJ. Variation in DCP1, encoding ACE, is associated with susceptibility to Alzheimer disease. Nat Genet. 1999;21:71–72. doi: 10.1038/5009. [DOI] [PubMed] [Google Scholar]
- 17.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elkins JS, Douglas VC, Johnston SC. Alzheimer disease risk and genetic variation in ACE: a meta-analysis. Neurology. 2004;62:363–368. doi: 10.1212/01.wnl.0000106823.72493.ff. [DOI] [PubMed] [Google Scholar]
- 19.Kehoe PG, Katzov H, Feuk L, Bennet AM, Johansson B, Wiman B, de Faire UC, Wilcock N. J., Brookes G. K., Blennow A. J., Prince K., J. A. Haplotypes extending across ACE are associated with Alzheimer's disease. Hum Mol Genet. 2003;12:859–867. doi: 10.1093/hmg/ddg094. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann DJ, Cortina-Borja M, Warden DR, Smith AD, Sleegers K, Prince JA, van Duijn CM, Kehoe PG. Large meta-analysis establishes the ACE insertion-deletion polymorphism as a marker of Alzheimer's disease. Am J Epidemiol. 2005;162:305–317. doi: 10.1093/aje/kwi202. [DOI] [PubMed] [Google Scholar]
- 21.Narain Y, Yip A, Murphy T, Brayne C, Easton D, Evans JG, Xuereb J, Cairns N, Esiri MM, Furlong RA, Rubinsztein DC. The ACE gene and Alzheimer's disease susceptibility. Journal of Medical Genetics. 2000;37:695–697. doi: 10.1136/jmg.37.9.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Wetten S, Li L, St Jean PL, Upmanyu R, Surh L, Hosford D, Barnes MR, Briley JD, Borrie M, Coletta N, Delisle R, Dhalla D, Ehm MG, Feldman HH, Fornazzari L, Gauthier S, Goodgame N, Guzman D, Hammond S, Hollingworth P, Hsiung GY, Johnson J, Kelly DD, Keren R, Kertesz A, King KS, Lovestone S, Loy-English I, Matthews PM, Owen MJ, Plumpton M, Pryse-Phillips W, Prinjha RK, Richardson JC, Saunders A, Slater AJ, St George-Hyslop PH, Stinnett SW, Swartz JE, Taylor RL, Wherrett J, Williams J, Yarnall DP, Gibson RA, Irizarry MC, Middleton LT, Roses AD. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch Neurol. 2008;65:45–53. doi: 10.1001/archneurol.2007.3. [DOI] [PubMed] [Google Scholar]
- 23.Thornton-Wells TA, Moore JH, Martin ER, Pericak-Vance MA, Haines JL. Confronting complexity in late-onset Alzheimer disease: application of two-stage analysis approach addressing heterogeneity and epistasis. Genet Epidemiol. 2008;32:187–203. doi: 10.1002/gepi.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertram L, McQueen M, Mullin K, Blacker D, Tanzi R. The AlzGene Database. Alzheimer Research Forum. 2007. Accessed [10th September 2007]* [DOI] [PubMed]
- 25.Arregui A, Perry EK, Rossor M, Tomlinson BE. Angiotensin converting enzyme in Alzheimer's disease increased activity in caudate nucleus and cortical areas. J Neurochem. 1982;38:1490–1492. doi: 10.1111/j.1471-4159.1982.tb07930.x. [DOI] [PubMed] [Google Scholar]
- 26.Barnes NM, Cheng CH, Costall B, Naylor RJ, Williams TJ, Wischik CM. Angiotensin converting enzyme density is increased in temporal cortex from patients with Alzheimer's disease. Eur J Pharmacol. 1991;200:289–292. doi: 10.1016/0014-2999(91)90584-d. [DOI] [PubMed] [Google Scholar]
- 27.Miners JS, Ashby E, Van Helmond Z, Chalmers KA, Palmer LE, Love S, Kehoe PG. Angiotensin-converting enzyme (ACE) levels and activity in Alzheimer's disease, and relationship of perivascular ACE-1 to cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2008;34:181–193. doi: 10.1111/j.1365-2990.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 28.He M, Ohrui T, Maruyama M, Tomita N, Nakayama K, Higuchi M, Furukawa K, Arai H. ACE activity in CSF of patients with mild cognitive impairment and Alzheimer disease. Neurology. 2006;67:1309–1310. doi: 10.1212/01.wnl.0000238102.04582.ec. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen HM, Londos E, Minthon L, Janciauskiene SM. Soluble adhesion molecules and angiotensin-converting enzyme in dementia. Neurobiol Dis. 2007 doi: 10.1016/j.nbd.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Zubenko GS, Volicer L, Direnfeld LK, Freeman M, Langlais PJ, Nixon RA. Cerebrospinal fluid levels of angiotensin-converting enzyme in Alzheimer's disease, Parkinson's disease and progressive supranuclear palsy. Brain Res. 1985;328:215–221. doi: 10.1016/0006-8993(85)91032-7. [DOI] [PubMed] [Google Scholar]
- 31.Savaskan E, Hock C, Olivieri G, Bruttel S, Rosenberg C, Hulette C, Muller-Spahn F. Cortical alterations of angiotensin converting enzyme, angiotensin II and AT1 receptor in Alzheimer's dementia. Neurobiol Aging. 2001;22:541–546. doi: 10.1016/s0197-4580(00)00259-1. [DOI] [PubMed] [Google Scholar]
- 32.Harada H, Tamaoka A, Ishii K, Shoji S, Kametaka S, Kametani F, Saito Y, Murayama S. Beta-site APP cleaving enzyme 1 (BACE1) is increased in remaining neurons in Alzheimer's disease brains. Neurosci Res. 2006;54:24–29. doi: 10.1016/j.neures.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Velloso EP, Vieira R, Cabral AC, Kalapothakis E, Santos RA. Reduced plasma levels of angiotensin-(1–7) and renin activity in preeclamptic patients are associated with the angiotensin I- converting enzyme deletion/deletion genotype. Braz J Med Biol Res. 2007;40:583–590. doi: 10.1590/s0100-879x2007000400018. [DOI] [PubMed] [Google Scholar]
- 34.Winkelmann BR, Nauck M, Klein B, Russ AP, Bohm BO, Siekmeier R, Ihnken K, Verho M, Gross W, Marz W. Deletion polymorphism of the angiotensin I-converting enzyme gene is associated with increased plasma angiotensin-converting enzyme activity but not with increased risk for myocardial infarction and coronary artery disease. Ann Intern Med. 1996;125:19–25. doi: 10.7326/0003-4819-125-1-199607010-00004. [DOI] [PubMed] [Google Scholar]
- 35.Jalil JE, Piddo AM, Cordova S, Chamorro G, Braun S, Jalil R, Vega J, Jadue PL, Lavandero S, Lastra P. Prevalence of the angiotensin I converting enzyme insertion/deletion polymorphism, plasma angiotensin converting enzyme activity, and left ventricular mass in a normotensive Chilean population. Am J Hypertens. 1999;12:697–704. doi: 10.1016/s0895-7061(99)00040-0. [DOI] [PubMed] [Google Scholar]
- 36.Danser AH, Schalekamp MA, Bax WA, van den Brink AM, Saxena PR, Riegger GA, Schunkert H. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation. 1995;92:1387–1388. doi: 10.1161/01.cir.92.6.1387. [DOI] [PubMed] [Google Scholar]
- 37.Kehoe PG, Katzov H, Andreasen N, Gatz M, Wilcock GK, Cairns NJ, Palmgren J, de Faire U, Brookes AJ, Pedersen NL, Blennow K, Prince JA. Common variants of ACE contribute to variable age-at-onset of Alzheimer's disease. Hum Genet. 2004;114:478–483. doi: 10.1007/s00439-004-1093-y. [DOI] [PubMed] [Google Scholar]
- 38.Miners JS, Kehoe PG, Love S. Neprilysin and insulin-degrading enzyme levels are increased in AD. (submitted to J Neuropathol Exp Neurol, 2009) [DOI] [PubMed]
- 39.van Helmond Z, Heesom K, Love S. Characterisation of two antibodies to oligomeric Abeta and their use in ELISAs on human brain tissue homogenates. J Neurosci Methods. 2009;176:206–212. doi: 10.1016/j.jneumeth.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Leal MC, Dorfman VB, Gamba AF, Frangione B, Wisniewski T, Castano EM, Sigurdsson EM, Morelli L. Plaque-associated overexpression of insulin-degrading enzyme in the cerebral cortex of aged transgenic tg2576 mice with Alzheimer pathology. J Neuropathol Exp Neurol. 2006;65:976–987. doi: 10.1097/01.jnen.0000235853.70092.ba. [DOI] [PubMed] [Google Scholar]
- 41.Vepsalainen S, Hiltunen M, Helisalmi S, Wang J, van Groen T, Tanila H, Soininen H. Increased expression of Abeta degrading enzyme IDE in the cortex of transgenic mice with Alzheimer's disease-like neuropathology. Neurosci Lett. 2008;438:216–220. doi: 10.1016/j.neulet.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 42.Tucker HM, Kihiko-Ehmann M, Wright S, Rydel RE, Estus S. Tissue plasminogen activator requires plasminogen to modulate amyloid-beta neurotoxicity and deposition. J Neurochem. 2000;75:2172–2177. doi: 10.1046/j.1471-4159.2000.0752172.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee JM, Yin KJ, Hsin I, Chen S, Fryer JD, Holtzman DM, Hsu CY, Xu J. Matrix metalloproteinase-9 and spontaneous hemorrhage in an animal model of cerebral amyloid angiopathy. Ann Neurol. 2003;54:379–382. doi: 10.1002/ana.10671. [DOI] [PubMed] [Google Scholar]
- 44.Zubenko GS, Marquis JK, Volicer L, Direnfeld LK, Langlais PJ, Nixon RA. Cerebrospinal fluid levels of angiotensin-converting enzyme, acetylcholinesterase, and dopamine metabolites in dementia associated with Alzheimer's disease and Parkinson's disease: a correlative study. Biol Psychiatry. 1986;21:1365–1381. doi: 10.1016/0006-3223(86)90328-8. [DOI] [PubMed] [Google Scholar]
- 45.Konings CH, Kuiper MA, Scheltens P, Grijpma AM, van Pelt W, Wolters EC. Re-evaluation of cerebrospinal fluid angiotensin-converting enzyme activity in patients with ‘probable’ Alzheimer's disease. Eur J Clin Chem Clin Biochem. 1993;31:495–497. [PubMed] [Google Scholar]
- 46.Orth T, Voronov S, Binevski P, Saenger W, Kost O. Glycosylation of bovine pulmonary angiotensin-converting enzyme modulates its catalytic properties. FEBS Lett. 1998;431:255–258. doi: 10.1016/s0014-5793(98)00767-4. [DOI] [PubMed] [Google Scholar]
- 47.Robertson LA, Moya KL, Breen KC. The potential role of tau protein O-glycosylation in Alzheimer's disease. J Alzheimers Dis. 2004;6:489–495. doi: 10.3233/jad-2004-6505. [DOI] [PubMed] [Google Scholar]
- 48.Guy JL, Lambert DW, Warner FJ, Hooper NM, Turner AJ. Membrane-associated zinc peptidase families: comparing ACE and ACE2. Biochim Biophys Acta. 2005;1751:2–8. doi: 10.1016/j.bbapap.2004.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuajungco MP, Lees GJ. Zinc and Alzheimer's disease: is there a direct link? Brain Res Brain Res Rev. 1997;23:219–236. doi: 10.1016/s0165-0173(97)00002-7. [DOI] [PubMed] [Google Scholar]
- 50.Grinshtein SV, Levashov AV, Kost OA. Unusual behavior of membrane somatic angiotensin-converting enzyme in a reversed micelle system. Biochemistry (Mosc) 2001;66:34–41. doi: 10.1023/a:1002825527927. [DOI] [PubMed] [Google Scholar]
- 51.Vardy ERLC, Rice PJ, Bowie PCW, Holmes JD, Catto AJ, Hooper NM. Plasma angiotensin-converting enzyme in Alzheimer's disease. Journal of Alzheimer's Disease. 2008 doi: 10.3233/JAD-2009-1002. In Press. [DOI] [PubMed] [Google Scholar]
- 52.Danilov S, Savoie F, Lenoir B, Jeunemaitre X, Azizi M, Tarnow L, Alhenc-Gelas F. Development of enzyme-linked immunoassays for human angiotensin I converting enzyme suitable for large-scale studies. J Hypertens. 1996;14:719–727. doi: 10.1097/00004872-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Jalil JE, Ocaranza MP, Oliveri C, Cordova S, Godoy I, Chamorro G, Braun S, Fardella C, Michel JB, Lavandero S. Neutral endopeptidase and angiotensin I converting enzyme insertion/deletion gene polymorphism in humans. J Hum Hypertens. 2004;18:119–125. doi: 10.1038/sj.jhh.1001646. [DOI] [PubMed] [Google Scholar]
- 54.Isbir SC, Tekeli A, Ergen A, Yilmaz H, Ak K, Civelek A, Zeybek U, Arsan S. Genetic polymorphisms contribute to acute kidney injury after coronary artery bypass grafting. Heart Surg Forum. 2007;10:E439–444. doi: 10.1532/HSF98.20071117. [DOI] [PubMed] [Google Scholar]
- 55.Zhang YL, Zhou SX, Lei J, Zhang JM. [Association of angiotensin I-converting enzyme gene polymorphism with ACE and PAI-1 levels in Guangdong Chinese Han patients with essential hypertension] Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:1681–1684. [PubMed] [Google Scholar]
- 56.Jimenez PM, Conde C, Casanegra A, Romero C, Tabares AH, Orias M. Association of ACE genotype and predominantly diastolic hypertension: a preliminary study. J Renin Angiotensin Aldosterone Syst. 2007;8:42–44. doi: 10.3317/jraas.2007.006. [DOI] [PubMed] [Google Scholar]
- 57.Yang M, Qiu CC, Xu Q, Xiang HD. Association of angiotensin converting enzyme gene I/D polymorphism with type 2 diabetes mellitus. Biomed Environ Sci. 2006;19:323–327. [PubMed] [Google Scholar]
- 58.Uppal SS, Haider MZ, Hayat SJ, Abraham M, Sukumaran J, Dhaunsi GS. Significant association of insertion/deletion polymorphism of the angiotensin-converting enzyme gene with rheumatoid arthritis. J Rheumatol. 2007;34:2395–2399. [PubMed] [Google Scholar]