Abstract
It is not known whether the metabolic syndrome is associated with poor exercise capacity among patients who have established coronary heart disease. We evaluated the association of the metabolic syndrome with treadmill exercise capacity and heart rate recovery among patients who had coronary heart disease. We measured treadmill exercise capacity (METs) and heart rate recovery (beats per minute) in 943 subjects who had known coronary heart disease. Of these, 377 (40%) had the metabolic syndrome as defined by criteria of the National Cholesterol Education Program. Participants who had the metabolic syndrome were more likely to have poor exercise capacity (METs <5, 33% vs 18%, p <0.0001) and poor heart rate recovery (≤ 16 beats/min, 34% vs 21%, p <0.0001) than those who did not have the metabolic syndrome. In ordinal logistic regression analyses, the metabolic syndrome was associated with decreased exercise capacity (odds ratio [OR] 2.2, 95% confidence interval [CI] 1.7 to 2.8, p <0.0001) and decreased heart rate recovery (OR 1.8, 95% CI 1.4 to 2.3, p <0.0001). These associations remained strong after adjusting for potential confounding variables (OR 1.6, 95% CI 1.2 to 2.1, p = 0.003 for decreased exercise capacity; OR 1.4, 95% CI 1.1 to 1.9, p = 0.02 for decreased heart rate recovery). The metabolic syndrome is independently associated with poor exercise capacity and poor heart rate recovery in patients who have established coronary heart disease. Decreased exercise capacity may contribute to the adverse outcomes associated with the metabolic syndrome.
Exercise capacity and heart rate recovery are objective measurements of cardiac function that are easily obtained by treadmill testing.1,2 In patients who have established coronary heart disease (CHD) and those who do not, exercise capacity is commonly used in clinical practice as a well-validated predictor of outcomes.3,4 Heart rate recovery has also been described as a predictor of mortality in patients who have suspected CHD.2 Despite the high prevalence of the metabolic syndrome and the established value of exercise capacity and heart rate recovery as prognostic factors in patients who have CHD, association of the metabolic syndrome with these parameters is not known. Previous studies have been limited by small samples, lack of control groups, or use of a nonstandard definition for the metabolic syndrome.5–7 Our study evaluated whether the metabolic syndrome is independently associated with decreased exercise tolerance and decreased heart rate recovery in a study of 943 older adults who had CHD.
METHODS
Participants
The Heart and Soul Study is a prospective cohort study of psychosocial factors and health outcomes in patients who have stable CHD. Details regarding our recruitment procedures have been published.8 Between September 2000 and December 2002, we enrolled 1,024 participants who had stable CHD. Of these, 81 were unable to perform the exercise treadmill test, leaving 943 participants for this cross-sectional analysis. Our protocol was approved by the appropriate institutional review boards, and all participants provided written, informed consent.
Metabolic syndrome
Our predictor variable was the presence of the metabolic syndrome. We defined the metabolic syndrome according to the definition of the National Cholesterol Education Program, Adult Treatment Panel III (NCEP-ATP III) based on the presence of ≥3 of the following components: (1) fasting glucose level ≥110 mg/dl or taking medication for diabetes (insulin or oral hypoglycemic therapy), (2) systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg, (3) triglyceride level ≥150 mg/dl, (4) high-density lipoprotein level <40 mg/dl in men and <50 mg/dl in women, and (5) waist circumference >102 cm in men and >88 cm in women.9
Cardiac evaluation
Participants were instructed to fast for ≥4 hours before exercise, except for taking their usual medications. We performed a symptom-limited, graded exercise treadmill test according to standard Bruce’s protocol.10 To achieve maximal heart rate, participants who were unable to continue the standard Bruce’s protocol were switched to lower settings on the treadmill and encouraged to exercise for as long as possible. Continuous, 12-lead electrocardiographic monitoring was performed throughout testing, and exercise capacity was calculated as total METs achieved at peak exercise (1 MET = 3.5 ml of oxygen uptake per kilogram of body weight per minute).11 For analysis purposes, we grouped participants into 3 categories (<5 METs, 5 to 8 METs, >8 METs) according to previously published criteria.3 After achieving maximal workload, subjects were immediately placed supine. Heart rate was measured exactly 1 minute after termination of exercise, and heart rate recovery was calculated as maximal heart rate during exercise minus heart rate 1 minute into recovery. For analytic purposes, we organized heart rate recovery by quartiles: ≤16, 17 to 24, 25 to 32, and >32 beats/min.
Other measurements
Age, ethnicity, smoking status, alcohol use, and physical activity were determined by questionnaire. Medical history was determined by self-report with the exception of diabetes and hypertension. Diabetes was defined as self-reported diabetes, use of diabetic medication, or a fasting glucose level ≥126 mg/dl. Hypertension was defined as use of antihypertensive medication, systolic blood pressure ≥130 mm Hg, or diastolic blood pressure ≥85 mm Hg.
All participants underwent echocardiography at rest with an Acuson Sequoia ultrasound system (Acuson, Mountain View, California) and a 3.5-MHz transducer. A complete 2-dimensional echocardiogram at rest, including imaging and Doppler in all standard views and subcostal imaging of the inferior vena cava, was performed. We obtained 2-dimensional parasternal short-axis and apical 2- and 4-chamber views during inspiration and performed planimetry with a computerized digitization system to determine end-diastolic and end-systolic left ventricular volumes and left ventricular ejection fractions. Repeat echocardiography was performed immediately after exercise, and inducible ischemia was defined as the presence of ≥1 new wall motion abnormality at peak exercise.
Participants were instructed to bring their medication bottles to the study appointment, and study personnel recorded all current medications. Body mass index was calculated as weight in kilograms divided by height squared in meters. We measured angina frequency with the Seattle Angina Questionnaire.12
Statistical analysis
Differences in baseline characteristics between participants who had the metabolic syndrome and those who did not were compared with t tests for continuous variables that were normally distributed, Wilcoxon’s tests for continuous variables that were not normally distributed, and chi-square tests for dichotomous variables. We used analysis of covariance to compare mean exercise capacity (METs) and heart rate recovery (beats per minute) after adjusting for variables that were associated with the metabolic syndrome (at p <0.05).
To determine an independent association of the metabolic syndrome with exercise capacity and heart rate recovery, we used ordinal logistic regression, a method that allows the outcome variable to have >2 categories. We divided exercise capacity categories according to previously published criteria7 and divided categories of heart rate recovery by quartile. Ordinal logistic regression calculates a single odds ratio (OR) for the association between the predictor variable (the metabolic syndrome) and each combination of higher versus lower risk outcome categories (e.g., lowest quartile of heart rate recovery vs 3 higher quartiles, 2 lower quartiles vs 2 higher quartiles, 3 lower quartiles vs highest quartile). Variables associated with the metabolic syndrome (at p <0.05) were entered into the adjusted models. Because diabetes and hypertension are traditional cardiac risk factors that overlap with the metabolic syndrome, these variables were entered in sequential analyses to determine an independent association between the metabolic syndrome and exercise capacity. We verified the proportional odds assumptions for all models. Analyses were performed with SAS 8 (SAS Institute, Cary, North Carolina).
RESULTS
Of the 943 participants, 377 (40%) met the NCEP-ATP III criteria for the metabolic syndrome. Compared with those who did not have the metabolic syndrome, those who did were less likely to be men, to drink alcohol, and to be physically active (Table 1). Participants who had the metabolic syndrome were more likely to be taking renin-angiotensin system inhibitors, β blockers, diuretics, and aspirin. They had higher heart rates at rest and greater body mass index levels than did those who did not have the metabolic syndrome.
TABLE 1.
Characteristics of 943 Participants Who Had Coronary Heart Disease According to the Presence/Absence of the Metabolic Syndrome
| Variables | Metabolic Syndrome (n = 377) | No Metabolic Syndrome (n = 566) | p Value |
|---|---|---|---|
| Age (yrs) | 66 ± 10 | 67 ± 11 | 0.08 |
| Men | 294 (78%) | 494 (87%) | 0.0002 |
| White | 227 (60%) | 346 (61%) | 0.75 |
| Current smoker | 71 (19%) | 112 (20%) | 0.70 |
| Regular alcohol use (≥4/wk) | 52 (14%) | 136 (24%) | 0.0001 |
| Myocardial infarction | 191 (51%) | 309 (55%) | 0.32 |
| Stroke | 52 (14%) | 75 (13%) | 0.79 |
| Congestive heart failure | 71 (19%) | 84 (15%) | 0.11 |
| Kidney disease | 34 (9%) | 50 (9%) | 0.92 |
| Revascularization | 222 (59%) | 343 (61%) | 0.61 |
| Current angina pectoris (at least weekly) | 142 (38%) | 193 (34%) | 0.26 |
| Physically active | 216 (57%) | 400 (71%) | <0.0001 |
| Calcium channel blocker | 102 (27%) | 125 (22%) | 0.08 |
| Renin-angiotensin system inhibitor | 224 (59%) | 260 (46%) | <0.0001 |
| β Blocker | 244 (65%) | 305 (54%) | 0.001 |
| Statin | 253 (67%) | 361 (64%) | 0.29 |
| Diuretic | 135 (36%) | 141 (25%) | 0.0003 |
| Aspirin | 317 (84%) | 423 (75%) | 0.0006 |
| Heart rate at rest (beats/min) | 69 ± 12 | 67 ± 12 | 0.001 |
| Left ventricular ejection fraction (%) | 62 ± 8 | 62 ± 10 | 0.24 |
| Ischemia during treadmill testing | 88 (23%) | 140 (25%) | 0.66 |
| Body mass index (kg/m2) | 31 ± 6 | 27 ± 4 | <0.0001 |
| Low-density lipoprotein (mg/dL) | 102 ± 35 | 104 ± 32 | 0.33 |
| Diabetes mellitus* | 196 (52%) | 96 (17%) | <0.0001 |
| Hypertension† | 370 (98%) | 508 (90%) | <0.0001 |
| Waist circumference (cm) | |||
| Men | 109 ± 16 | 96 ± 11 | <0.0001 |
| Women | 104 ± 14 | 89 ± 12 | <0.0001 |
| High-density lipoprotein cholesterol (mg/dl) | |||
| Men | 38 ± 10 | 48 ± 13 | <0.0001 |
| Women | 45 ± 11 | 63 ± 17 | <0.0001 |
| Triglycerides (mg/dl) | 163 (108–228) | 90 (65–123) | <0.0001 |
Values are mean ± SD, numbers of patients (percentages), or medians (interquartile ranges).
Definition of diabetes (self-reported diabetes, use of diabetes medications, or fasting plasma glucose level ≥ 126 mg/dl) differs from criterion used for the metabolic syndrome (fasting glucose level ≥110 mg/dl or use of diabetes medications).
Definition of hypertension (blood pressure ≥130/85 mm Hg or taking antihypertensive medication) differs from criterion used in the metabolic syndrome (blood pressure ≥130/85 mm Hg).
Among the 377 participants who had the metabolic syndrome, 291 (77%) had high blood pressure (≥130/85 mm Hg), 290 (77%) had high fasting glucose levels (≥110 mg/dl) or diabetes (diabetes medication), 282 (75%) had a large waist circumference (>102 cm in men, >88 cm in women), 262 (70%) had low levels of high-density lipoprotein cholesterol (<40 mg/dl in men, <50 mg/dl in women), and 215 (57%) had high levels of triglycerides (≥150 mg/dl; Table 1).
Participants who had the metabolic syndrome had lower mean exercise capacity than did those who did not have the metabolic syndrome (6.4 ± 2.9 vs 7.9 ± 3.5 METs, p <0.0001), and this association remained strong after adjusting for potential confounding variables (Table 2). Participants who had the metabolic syndrome also had lower heart rate recovery (23 ± 12 vs 27 ± 13 beats/min, p <0.0001), although the strength of association between the metabolic syndrome and heart rate recovery was attenuated after adjustment for potential confounding variables (p = 0.08).
TABLE 2.
Exercise Capacity and Heart Rate Recovery by Presence/Absence of the Metabolic Syndrome
| Exercise Capacity (METs) |
Heart Rate Recovery (beats/min) |
|||||
|---|---|---|---|---|---|---|
| Metabolic Syndrome | No Metabolic Syndrome | p Value | Metabolic Syndrome | No Metabolic Syndrome | p Value | |
| Unadjusted mean ± SD | 6.4 ± 2.9 | 7.9 ± 3.5 | <0.0001 | 23 ± 12 | 27 ± 13 | <0.0001 |
| Adjusted mean (95% CI)* | 6.2 (5.8–6.7) | 6.9 (6.5–7.3) | 0.002 | 24 (22–26) | 26 (25–28) | 0.02 |
| Adjusted mean (95% CI)† | 7.2 (6.6–7.8) | 7.8 (7.2–8.3) | 0.02 | 25 (22–27) | 26 (24–29) | 0.08 |
Adjusted for gender, alcohol use, physical activity, use of renin-angiotensin system inhibitor, β blocker, diuretic, or aspirin, heart rate at rest, and body mass index.
Adjusted for variables above plus hypertension and diabetes.
CI = confidence interval.
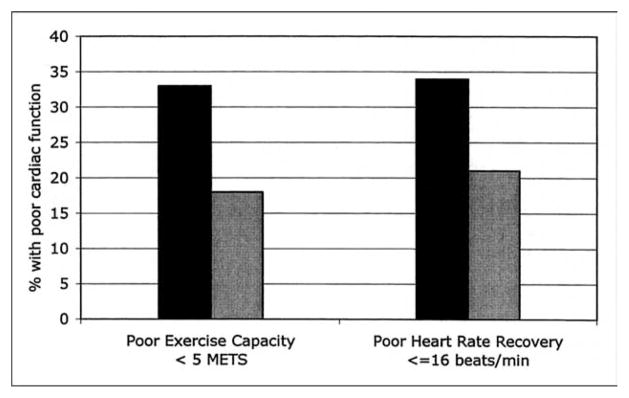
Participants who had the metabolic syndrome were more likely to have poor exercise capacity (<5 METs) and poor heart rate recovery (lowest quartile ≤16 beats/min) than those who did not have the metabolic syndrome (p <0.0001; Figure 1). In ordinal logistic regression analysis, the metabolic syndrome was associated with decreased exercise capacity (adjusted OR 2.2, 95% confidence interval 1.7 to 2.8, p <0.0001) and with lower heart rate recovery (OR 1.8, 95% confidence interval, 1.4 to 2.3; p <0.001). These associations remained strong after adjustment for potential confounding variables (Table 3). An association of the metabolic syndrome with poor exercise capacity and poor heart rate recovery was similar in participants who had diabetes mellitus and those who did not (p >0.20 for interaction).
FIGURE 1.
Proportion of participants who had poor exercise capacity and poor heart rate recovery according to presence (n = 377) (black bars) or absence (n = 566) (gray bars) of the metabolic syndrome (p <0.0001 for the 2 measurements).
TABLE 3.
Association of the Metabolic Syndrome to Poor Exercise Capacity and Poor Heart Rate Recovery
| Lower Exercise Capacity |
Lower Heart Rate Recovery |
|||
|---|---|---|---|---|
| Odds Ratio* (95% CI) | p Value | Odds Ratio* (95% CI) | p Value | |
| Unadjusted model | 2.2 (1.7–2.8) | <0.0001 | 1.8 (1.4–2.3) | <0.0001 |
| Adjusted model† | 1.7 (1.3–2.3) | 0.0002 | 1.5 (1.1–1.9) | 0.004 |
| Adjusted model‡ | 1.6 (1.2–2.1) | 0.003 | 1.4 (1.1–1.9) | 0.02 |
Odds ratios from ordinal logistic regression, representing the independent association between the predictor variable (presence of metabolic syndrome) and each combination of higher risk versus lower risk outcome categories (e.g., lowest quartile of heart rate recovery vs higher 3 quartiles, lower 2 quartiles of heart rate recovery vs higher 2 quartiles, lowest 3 quartiles of heart rate recovery vs highest quartile). Exercise capacity was divided into 3 categories (<5 METs, 5 to 8 METs, >8 METs) according to the method of Myers et al.7
Adjusted for gender, alcohol use, physical activity, use of renin-angiotensin system inhibitor, β blocker, diuretic, or aspirin, heart rate at rest, and body mass index.
Adjusted for above variables plus hypertension and diabetes.
Abbreviation as in Table 2.
DISCUSSION
We found that the metabolic syndrome is independently associated with low exercise capacity and poor heart rate recovery. These findings are very important, because every fourth American in the United States has the metabolic syndrome, and exercise capacity and heart rate recovery are well-established predictors of mortality.2,3,13 Our findings persisted even after adjusting for obesity, physical activity, β-blocker use, diabetes mellitus, and hypertension, parameters that are believed to be primarily responsible for worse outcome in patients who have the metabolic syndrome. Moreover, exercise-induced ischemia did not account for our findings, because the presence of inducible ischemia was not associated with exercise capacity. Thus, our study provides evidence for an independent relation between the metabolic syndrome and measurements of exercise capacity that are associated with adverse outcomes.
These findings suggest that poor heart rate recovery and limited exercise capacity may contribute to the adverse outcomes associated with the metabolic syndrome.14,15 Previous small studies found poor heart rate recovery and decreased exercise capacity in patients who had insulin resistance as defined by a hyperinsulinemic euglycemic clamp test, in patients who had abnormal glucose tolerance, and in patients who had the metabolic syndrome.5,6,16 Because insulin resistance is considered a pathogenic factor for the metabolic syndrome and its complications, results from these studies support our findings.17
The 0.5-MET difference in mean exercise capacity between participants who had the metabolic syndrome and those who did not is clinically relevant. Myers et al3 found that every MET decrease in exercise capacity decreased the likelihood of survival by 12%. Thus, a 0.5-MET lower mean exercise capacity translates into substantially increased mortality for participants who have the metabolic syndrome. Likewise, previous studies have demonstrated an association between poor heart rate recovery and increased mortality, although some concerns exist about limited reproducibility.2,18 –20 We found a higher heart rate at rest in patients who had the metabolic syndrome, and adjusting for higher heart rate at rest did not eliminate the association between the metabolic syndrome and poor heart rate recovery.
The metabolic syndrome consists of multiple components, some of which are major risk factors for cardiovascular morbidity and mortality. Controversy exists as to whether the metabolic syndrome itself is a distinct risk factor for cardiovascular disease, whether it is solely the sum of single components, or whether there is 1 risk factor such as insulin resistance or diabetes that is responsible for all core features of the metabolic syndrome.21 A factor analysis by Anderson et al22 indicated that multiple factors of the metabolic syndrome that are linked to adiposity account for the unique feature of this syndrome.22 Contradictory to this assumption, another study that examined the role of the metabolic syndrome as a predictor for cardiovascular disease using the Framingham data concluded that most of the risk associated with the metabolic syndrome is captured almost completely by the traditional risk factors of the Framingham algorithm that are associated with the metabolic syndrome.21 We found that the metabolic syndrome was associated with poor exercise capacity and heart rate recovery, independent of diabetes, hypertension, and obesity.
Another potential link that connects exercise capacity, heart rate recovery, and the metabolic syndrome is the obesity gene leptin. Obesity commonly is accompanied by increased plasma leptin levels,23 which have been related to higher heart rates at rest in healthy men.24 Interestingly, the baseline heart rate in our collective was significantly higher in the group with the metabolic syndrome. However, it is unclear whether leptin also modulates heart rate during and after exercise, thus potentially affecting heart rate recovery, or if the effect is confined to the resting state. Wolk et al25 examined stable patients who had congestive heart failure and found a positive correlation between plasma leptin levels and ventilatory efficiency during exercise.25 As a consequence, higher leptin levels would lead to a hyperventilatory response to exercise. Whether this possible mechanism explains the decreased exercise capacity in our patient collective with metabolic syndrome remains to be evaluated.
The primary targets of therapy for the metabolic syndrome are therapeutic lifestyle changes, such as adjusting nutritional habits and increasing physical activity. Because insulin resistance remains an important aspect of the metabolic syndrome, medication with insulin sensitizers may also be beneficial. A growing body of evidence suggests that diabetes medications such as metformin or acarbose may benefit patients who are in a prediabetic state.26,27 The Diabetes Prevention Program reported a decrease in new onset of type 2 diabetes with metformin.26 The Study to Prevent NonInsulin Dependent Diabetes trial associated acarbose with a decreased risk for developing cardiovascular disease in patients who had impaired glucose tolerance.27 However, no randomized trials have evaluated the effects of lifestyle changes or pharmacologic therapies on cardiovascular morbidity in patients who have the metabolic syndrome. Such a trial would help guide therapies for these patients.
Our study has several limitations. First, exercise capacity and heart rate recovery were derived from a symptom-limited treadmill exercise test, which relied on a participant’s effort to reach maximal exercise. This concern represents a general limitation of an exercise test, which we attempted to overcome by employing experienced personnel to motivate participants. Second, the metabolic syndrome has been associated with greater CHD severity, as evaluated by electron-beam computed tomographically or angio-graphically based estimates of atherosclerotic burden.28 –30 Thus, the association of the metabolic syndrome with poor exercise capacity and heart rate recovery may represent a difference in CHD severity. However, because the metabolic syndrome was not associated with angina, left ventricular ejection fraction, or inducible ischemia in our sample, it is unlikely that increased CHD severity explained the observed differences in exercise capacity and heart rate recovery. Third, our results are based on cross-sectional data, so the causal direction between the metabolic syndrome and exercise capacity cannot be determined.
In summary, we found that the metabolic syndrome is associated with poor exercise capacity and poor heart rate recovery in patients who have coronary disease. Poor exercise capacity may contribute to the adverse outcomes associated with the metabolic syndrome.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs (Epidemiology Merit Review Program), Washington DC; the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), Princeton, New Jersey, the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), New York, New York; and the Ischemia Research and Education Foundation, San Francisco, California. Dr. Whooley was supported by a Research Career Development Award from the Department of Veterans Affairs Health Services Research and Development Service.
References
- 1.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC. Physical activity and public health: a recommendation from the Centers of Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402– 407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 2.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 3.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793– 801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 4.Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, Blumenthal RS. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women. JAMA. 2003;290:1600 –1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 5.Panzer C, Lauer MS, Brieke A, Blackstone E, Hoogwerf B. Association of fasting plasma glucose with heart rate recovery in healthy adults. Diabetes. 2002;51:803– 807. doi: 10.2337/diabetes.51.3.803. [DOI] [PubMed] [Google Scholar]
- 6.Lind L, Andren B. Heart rate recovery after exercise is related to the insulin resistance syndrome and heart rate variability in elderly men. Am Heart J. 2002;144:666 – 672. doi: 10.1067/mhj.2002.124836. [DOI] [PubMed] [Google Scholar]
- 7.Kullo IJ, Hensrud DD, Allison TG. Relation of low cardiorespiratory fitness to the metabolic syndrome in middle-aged men. Am J Cardiol. 2002;90:795–797. doi: 10.1016/s0002-9149(02)02617-6. [DOI] [PubMed] [Google Scholar]
- 8.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NCEP Expert Panel. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486 –2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) J Am Coll Cardiol. 2002;40:1531–1540. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 11.American College of Sports Medicine. Guidelines for Exercise Testing and Prescription. 6. Baltimore: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 12.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 13.Chang JA, Froelicher VF. Clinical and exercise test markers of prognosis in patients with stable coronary artery disease. Curr Probl Cardiol. 1994;19:533–587. doi: 10.1016/0146-2806(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 14.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709 –2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 15.Sattar N, Gaw A, Scherbakova O, Ford I, O’Reilly DS, Haffner SM, Isles C, Macfarlane PW, Packard CJ, Cobbe SM, Shepherd J. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414 – 419. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 16.Whaley MH, Kampert JB, Kohl HW, III, Blair SN. Physical fitness and clustering of risk factors associated with the metabolic syndrome. Med Sci Sports Exerc. 1999;31:287–293. doi: 10.1097/00005768-199902000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Reaven GM. Banding Lecture 1988: role of insulin resistance of human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 18.Shetler K, Marcus R, Froelicher VF, Vora S, Kalisetti D, Prakash M, Do D, Myers J. Heart rate recovery: validation and methodologic issues. J Am Coll Cardiol. 2001;38:1980 –1987. doi: 10.1016/s0735-1097(01)01652-7. [DOI] [PubMed] [Google Scholar]
- 19.Yawn BP, Ammar KA, Thomas R, Wollan PC. Test-retest reproducibility of heart rate recovery after treadmill exercise. Ann Fam Med. 2003;1:236 –241. doi: 10.1370/afm.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palatini P. Recovery of heart rate after exercise. N Engl J Med. 2000;342:662– 663. [PubMed] [Google Scholar]
- 21.Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome (Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on scientific issues related to definition) Circulation. 2004;109:433– 438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 22.Anderson PJ, Critchley JA, Chan JC, Cockram CS, Lee ZS, Thomas GN, Chan JC. Factor analysis of the metabolic syndrome: obesity vs insulin resistance as the central abnormality. Int J Obesity. 2001;25:1782–1788. doi: 10.1038/sj.ijo.0801837. [DOI] [PubMed] [Google Scholar]
- 23.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 24.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Choe I, Winnicki M, Palatini P, Sivitz WI, Somers VK. Leptin interacts with heart rate but not sympathetic nerve traffic in healthy male subjects. J Hypertens. 2001;19:1089 –1094. doi: 10.1097/00004872-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Wolk R, Johnson BD, Somers VK. Leptin and the ventilatory response to exercise in heart failure. J Am Coll Cardiol. 2003;42:1644 –1649. doi: 10.1016/j.jacc.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393– 403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance. The STOP-NIDDM Trial. JAMA. 2003;290:486 – 494. doi: 10.1001/jama.290.4.486. [DOI] [PubMed] [Google Scholar]
- 28.Wong ND, Sciammarella MG, Polk D, Gallager A, Miranda-Peats L, Whitcomb B, Hachamovitch R, Friedman JD, Hayes S, Berman DS. The metabolic syndrome, diabetes, and subclinical atherosclerosis assessed by coronary calcium. J Am Coll Cardiol. 2003;41:1547–1553. doi: 10.1016/s0735-1097(03)00193-1. [DOI] [PubMed] [Google Scholar]
- 29.Solymoss BC, Bourassa MG, Lesperance J, Levesque S, Marcil M, Varga S, Campeau L. Incidence and clinical characteristics of the metabolic syndrome in patients with coronary artery disease. Coron Artery Dis. 2003;14:207–212. doi: 10.1097/01.mca.0000065744.52558.9f. [DOI] [PubMed] [Google Scholar]
- 30.Seibaek M, Sloth C, Vallebo L, Hansen T, Urhammer SA, Burchardt H, Torp-Pedersen C, Pedersen O, Hildebrandt P. Glucose tolerance status and severity of coronary artery disease in men referred to coronary arteriography. Am Heart J. 1997;133:622– 629. doi: 10.1016/s0002-8703(97)70163-7. [DOI] [PubMed] [Google Scholar]